There is mounting evidence that IgG autoantibodies are involved in many autoimmune neurologic diseases including Guillain-Barré syndrome (GBS), chronic immune neuropathies, myasthenia gravis, immune myositis, and neuromyelitis optica variant of multiple sclerosis [2, 6]. Despite availability of current immunotherapies, a significant proportion of patients with neuroimmunological disorders are left with severe and permanent neurologic sequelae. Lowering the levels of pathogenic autoantibodies could be critical to treat these disorders, and has been postulated to be one of the mechanisms account for IVIg's beneficial effects [4]. Neonatal Fc receptor (FcRn) is pivotal in IgG hemostasis/catabolism. The blockage of FcRn can reduce in vivo IgG levels, and had been demonstrated to be effective in animal model of humoral autoimmune disorder [5]. However, it remains untested whether a reduction of pathogenic antibody via inhibiting FcRn can ameliorate autoantibody-mediated peripheral neuropathies in animal models. Anti-glycan/ganglioside antibodies (AGAs) are strongly associated with axonal GBS [7]. We previously showed that AGA inhibits axon regeneration in a murine model of AGA mediated-nerve injury [3], which echo the clinical observations correlating AGA and poor prognosis in GBS. Besides FcRn mediated IgG catabolism, recent studies suggest that AGA uptake at nerve terminal might also contribute to AGA clearance [1]. However, in the current study, we mainly focus on examining whether modulation of FcRn expression or IgG-FcRn interactions can alter Ab-mediated pathological effects in this model.
We first examined the effects of AGA in mice lacking FcRn. Serological studies showed that the levels of circulating AGA were significantly decreased in FcRn-null mice compared to wild type (WT) animals, the area under the curve (AUC) of AGA in FcRn-null is about 20% of the AUC in WT mice (Fig. 1a). Behavioral, electrophysiological, and morphometric studies showed that FcRn-null mice were completely resistant to AGA-mediated inhibition of axon regeneration (Fig. 1b-e). Next, efficacy of an antibody based competitive FcRn inhibitor [5], MST-HN {an Abdeg (antibodies that enhance IgG degradation) } was evaluated and our results show that there was highly significant decrease in circulating levels of AGA at all time points examined after Abdeg treatment compared to a control humanized mAb (Hulys10), the AUC of Abdeg-treated mice is 45-55% of that of Hulys10 treated animals (Fig. 2a). Behavior testing, electrophysiology, and morphometry showed that Abdeg significantly ameliorated the pathological effects of AGA (Fig. 2b-e).
Figure 1.
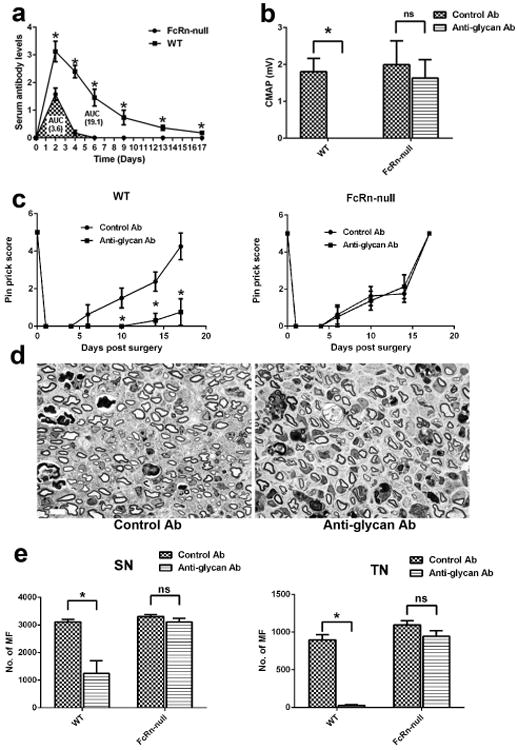
FcRn is required for the anti-glycan antibody-mediated inhibition of axon regeneration. (a) Level of circulating anti-glycan mAb were significantly lower in FcRn-null mice than WT mice. (b) Quantitative electrophysiology data indicate that anti-glycan mAb adversely affected the motor nerve regeneration and target (muscle) reinnervation in WT animals but not in FcRn -/- mice. n = 10. (c) Pinprick test showed that anti-glycan mAb significantly reduced sensory functional recovery after nerve injury compared to control Abs in WT mice, whereas the sensory functional recovery is similar in FcRn -/- mice treated with anti-glycan mAb and control Abs. n = 10. (d) Representative micrographs from sciatic nerve segments distal to the crush site showing similar regeneration of myelinated fibers (MF) in FcRn -/- mice treated with anti-glycan mAb and control Abs. Scale bar, 20 μm. (e) Morphometric analysis showing significant decrease in MF in anti-glycan mAb-treated WT animals at sciatic (SN) and tibial (TN) nerves compared with control Ab-treated sciatic and tibial nerves. Whereas, the difference in MF regeneration in FcRn -/- mice treated with anti-glycan mAb or control Ab is not significant. n = 10. *p < 0.05.
Figure 2.
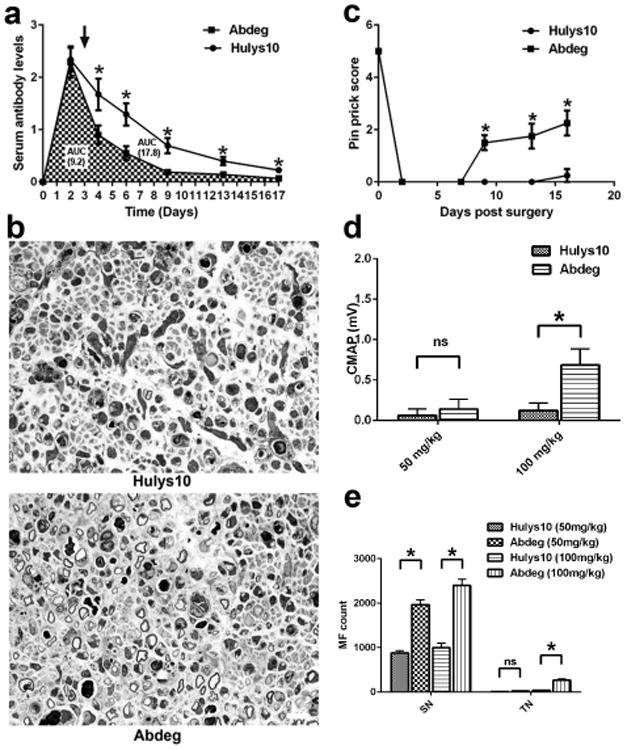
Abdeg (MST-HN) suppresses anti-glycan antibody-mediated inhibition. (a) Abdeg treatment significantly increased the clearance of anti-glycan mAb compared to control Ab (Hulys10). (b) Micrographs from sciatic nerves showing regeneration of MF in mice treated with Hulys10 (Top) or Abdeg (Bottom). Scale bar, 20 μm. (c-e) Pin prick behavior (c), electrophysiology (d), and myelinated fiber regeneration (e) show that animals receiving Abdeg have significant protection. n = 10. *p < 0.05
Our previous work supports that the antibody bound to ganglioside on nerve fiber (ABG)-activating FcγRs-macrophage axis drives Ab-mediated nerve damage in our model [8]. We have established that AGA bind with corresponding target antigens on nerve fibers [3, 8] and the bound antibodies are the primary initiators of inflammation via their interactions with activating FcγRs on macrophages in this model [8]. We examined the amount of endoneurial mouse IgGs in animals treated with Abdeg or Hulys10, and found that the IgGs level was significantly decreased in Abdeg treated nerves (Supplementary Fig. 1a & 1b). We further inspected the endoneurial innate immune effectors, i.e., macrophage/microglial numbers, and Fcγ common chain expression in mice treated with Abdeg by immunocytochemistry. Notably, we found comparable number of macrophage/microglia and expression of Fcγ common chain in distal segments of sciatic nerves of Abdeg- or Hulys10-treated animals (Supplementary Fig. 1c-e). These observations would support the assertion that FcRn modulation reduces the endoneurial IgG content including AGA and its binding to ganglioside, which in turn disrupts ABG-activating FcγRs-macrophage axis leading to reduced nerve damage (Supplementary Fig. 2). This observation would support that amelioration of Ab-mediated nerve injury with Abdeg-treatment is due to reduced endoneurial content of AGA.
We report that elimination of FcRn receptors or modulation of IgG-FcRn interactions with an Abdeg, significantly shortened the half-life of passively transferred AGA and reduced associated nerve injury in an animal model. Abdeg/MST-HN is a clinically relevant biologic drug, which enhanced the clearance of pathogenic AGA by competitive blockade of FcRn, resulting in reduced endoneurial content of AGA and ABG formation, and amelioration of nerve injury in our model. These results validate the concept that IgG-FcRn interactions can be modulated to increase the clearance of autoAbs to prevent tissue/neural injury. Our observations support that this stratgey can be used as autoAb-specific immunotherapy for humoral autoimmune disorders. Since this approach leads to nonspecific degradation of all IgGs, it is preferable to initially use it as short-term or pulse therapy clinically for monophasic disorders, such as GBS. The strategy of reducing pathogenic autoAb levels via Abdeg mediated-FcRn modulation offers advantages over IVIg. Abdegs could provide comparable efficacy at much lower infusion dosages, potentially cutting down infusion related costs and side effects. Further, recombinant Abdegs are less likely to have biologic variability and worldwide shortages inherent to IVIG manufacturing. This therapeutic approach has translational potential in treating autoimmune neuropathies as reagents which can modulate FcRn function in humans are beginning to emerge.
Supplementary Material
Supplementary Figure 1: Abdeg does not alter endoneurial innate immune responses, but decreases the amount of IgG in the endoneurium. (a) Immunofluorescence images showing decreased IgG deposition in Abdeg-treated nerve compared with control Ab (Hulys10)-treated nerve. Scale bar, 20 μm. (b) Quantification showing significant decrease of IgG level in Abdeg-treated nerves. n = 20. (c) Immunofluorescence images showing FcγRs (Fcγ common chain; Red) and macrophages (CD68; Green) in the injured sciatic nerves of animal tread with Hulys10 (Top) or Abdeg (Bottom). Scale bar, 20 μm. (d) Quantitative analysis of macrophage number in endoneurium and Fcγ common chain expression. n = 20. (e) Mean fluorescence intensity of activating FcγRs expression. n = 20.
Supplementary Figure 2: Schematic diagram showing the possible pathogenic mechanisms of anti-ganglioside Ab (AGA)-mediated nerve injury and how Abdeg treatment potentially interrupts ABG- FcγRs-macrophage axis in the endoneurium.
Acknowledgments
This study was supported by the National Institute of Neurological Disorders and Stroke (NIH/NINDS; Grants R01 NS42888, R01 NS54962, R21NS087467). We would like to thank Dr. Sally Ward (Texas A&M Health Science Center) for kindly providing us with Abdge (MST-HN) and Hulys10 antibodies.
References
- 1.Fewou SN, Rupp A, Nickolay LE, Carrick K, Greenshields KN, Pediani J, Plomp JJ, Willison HJ. Anti-ganglioside antibody internalization attenuates motor nerve terminal injury in a mouse model of acute motor axonal neuropathy. The Journal of clinical investigation. 2012;122:1037–1051. doi: 10.1172/JCI59110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs BC, van Doorn PA, Schmitz PI, Tio-Gillen AP, Herbrink P, Visser LH, Hooijkass H, van der Meche FG. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barre syndrome. Ann Neurol. 1996;40:181–187. doi: 10.1002/ana.410400209. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann HC, Lopez PH, Zhang G, Ngyuen T, Zhang J, Kieseier BC, Mori S, Sheikh KA. Passive immunization with anti-ganglioside antibodies directly inhibits axon regeneration in an animal model. J Neurosci. 2007;27:27–34. doi: 10.1523/JNEUROSCI.4017-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, Roopenian DC, Liu Z. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. The Journal of clinical investigation. 2005;115:3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 6.Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–2625. doi: 10.1093/brain/awf272. [DOI] [PubMed] [Google Scholar]
- 7.Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, et al. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc Natl Acad Sci U S A. 2004;101:11404–11409. doi: 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang G, Bogdanova N, Gao T, Song JJ, Cragg MS, Glennie MJ, Sheikh KA. Fcgamma receptor-mediated inflammation inhibits axon regeneration. PLoS One. 2014;9:e88703. doi: 10.1371/journal.pone.0088703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Abdeg does not alter endoneurial innate immune responses, but decreases the amount of IgG in the endoneurium. (a) Immunofluorescence images showing decreased IgG deposition in Abdeg-treated nerve compared with control Ab (Hulys10)-treated nerve. Scale bar, 20 μm. (b) Quantification showing significant decrease of IgG level in Abdeg-treated nerves. n = 20. (c) Immunofluorescence images showing FcγRs (Fcγ common chain; Red) and macrophages (CD68; Green) in the injured sciatic nerves of animal tread with Hulys10 (Top) or Abdeg (Bottom). Scale bar, 20 μm. (d) Quantitative analysis of macrophage number in endoneurium and Fcγ common chain expression. n = 20. (e) Mean fluorescence intensity of activating FcγRs expression. n = 20.
Supplementary Figure 2: Schematic diagram showing the possible pathogenic mechanisms of anti-ganglioside Ab (AGA)-mediated nerve injury and how Abdeg treatment potentially interrupts ABG- FcγRs-macrophage axis in the endoneurium.


