Abstract
Background
Performance on figure copy tests has been shown to predict progressive cognitive decline in Parkinson’s disease (PD). Historically, the interlocking pentagons from the Mini Mental State Exam (MMSE) have been the figure copy test most commonly used during cognitive screening evaluations. However, the wire cube from the Montreal Cognitive Assessment (MoCA) is increasingly being used.
Objective
To evaluate which of these figure copy tests is more sensitive for cognitive impairment in PD.
Methods
Sixty-three PD patients from UK and USA completed the MMSE and MoCA. Logistic regression and sensitivity/specificity analyses were used to evaluate the utility of each figure copy test for detecting global cognitive impairment.
Results
The wire cube was a significant indicator of cognitive impairment (OR = 4.79, 95% CI = 1.63–14.07, p = 0.004), with a sensitivity/specificity of 0.74/0.63 in our sample. In contrast, interlocking pentagons were not a significant indicator of cognitive impairment (OR = 1.88, 95% CI = 0.54–6.50, p = 0.32), with a sensitivity/specificity of 0.26/0.84.
Conclusion
The wire cube is more sensitive to cognitive impairment in PD, most likely related to its greater complexity. The results have implications for clinicians who may have time for just one figure copying task as part of a brief screen for cognitive impairment in busy clinics and for researchers applying the PD mild cognitive impairment diagnostic criteria necessitating two tests of visuospatial function to be administered.
Keywords: Parkinson’s, Cognitive, Cube, Pentagons, Dementia
1. Introduction
Decline in visuospatial abilities is a hallmark feature of cognitive impairment in PD [1] and longitudinal studies have shown that Parkinson’s disease (PD) patients who cannot accurately copy the interlocking pentagons are at an increased risk for incipient dementia [2,3]. As such, figure copy tasks are an important component of cognitive screens in PD.
The interlocking pentagons have been the most frequently used figure copy task as it is part of the Mini Mental State Exam (MMSE) [4,5] and the Addenbrooke’s Cognitive Examination (ACE) [6]. However, clinical use of the interlocking pentagons task is declining due to MMSE copyright enforcement [7–9], publication of a new version of ACE (ACE-III) with all MMSE items replaced [10], and also because of mounting evidence that the Montreal Cognitive Assessment Scale (MoCA) [11], which uses the wire cube, is a sensitive screen for cognitive impairment in PD [12–17]. As such, the wire cube may increasingly supplant the interlocking pentagons in cognitive screens.
The purpose of this study is to determine whether this transition is valid by comparing the sensitivity of these two figure copy tests to global cognitive impairment in PD. The results have implications for test selection – both for clinicians who may have time for just one figure copying task as part of a brief screen for cognitive impairment in busy clinics, and also for researchers applying the new PD-MCI criteria that requires two tests from the visuospatial domain to be administered [18].
2. Methods
Forty-nine PD patients were recruited from Leeds Teaching Hospitals NHS Trust (LTHT), UK, and fourteen from the University of California – San Francisco (UCSF) Memory and Aging Center, USA. Subjects completed the MMSE and MoCA consecutively during the same session whilst on. The order of assessments was randomly allocated and the scores of the orientation tasks in the first assessment were used to auto-complete the corresponding section in the second assessment. The study was approved by LTHT and UCSF ethics committees on human research and written consent was obtained from all subjects.
Patients were identified with normal cognition, PD-NC (n = 32), mild cognitive impairment, PD-MCI (n = 23), and dementia, PD-D (n = 8) using the previously validated MoCA ranges of ≥26, 21–25, and <21, respectively [12,13]. To avoid circular reasoning when predicting MoCA-defined global cognitive impairment using the MoCA cube item, total MoCA score was out of a maximum of 29 with the cube score excluded. We subtracted rather than added the cube point in order to increase sensitivity to cognitive impairment when classifying subjects. Compared to classifications based on MoCA scores out of 30, four additional cognitively intact subjects were classified as PD-MCI and no additional PD-MCI subjects were classified as PD-D.
Demographic and clinical variables were compared between the patients at different levels of cognitive impairment using one-way ANOVA with Games-Howell post hoc analysis for continuous variables, Chi-square for gender and Kruskal–Wallis test for Hoehn and Yahr (HY) stage. Using logistic regression and sensitivity/specificity calculations, we determined how well the cube and pentagon scores distinguished PD-NC from cognitively impaired PD (i.e. PD-MCI and PD-D combined), and PD-NC from PD-MCI.
3. Results
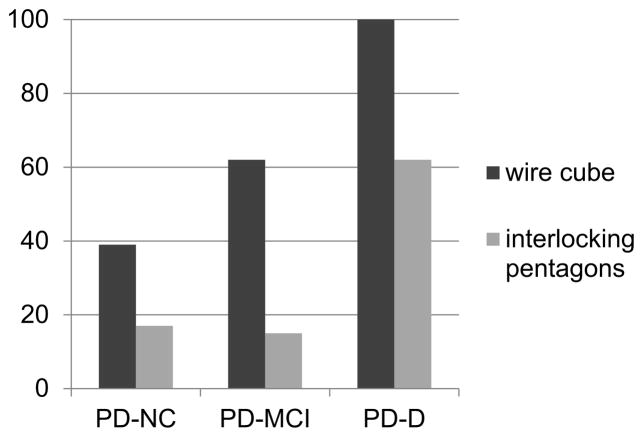
The groups did not differ in terms of gender, age, years of education, duration of disease, levodopa equivalent daily dose or HY stage (all p ≥ 0.1) (Table 1). The wire cube was copied incorrectly by 66% of PD patients and the pentagons were copied incorrectly by 21% (χ2 = 5.60, p = 0.018). Regarding the individual cognitive groups, the cube copy was impaired in 39% PD-NC, 63% PD-MCI and 100% PD-D (χ2 = 10.43, p = 0.005). The pentagon copy was impaired in 16% PD-NC, 13% PD-MCI and 63% PD-D (χ2 = 9.92, p = 0.007) (Fig. 1).
Table 1.
Demographic characteristics and screening test scores.
| Group | N | Age (years) | Male (%) | HY (stage) | Education (years) | LEDD (mg) | MMSE score | MoCA score |
|---|---|---|---|---|---|---|---|---|
| PD-NC | 32 | 68.6 (8.8) | 63 | 2.5 (0.6) | 13.0 (2.4) | 700 (475) | 29.3 (0.7) | 27.8 (1.2) |
| PD-MCI | 23 | 70.9 (8.2) | 65 | 2.6 (0.8) | 12.9 (1.4) | 725 (314) | 28.1 (1.5) | 24.4 (1.4) |
| PD-D | 8 | 70.5 (5.9) | 75 | 3.1 (1.3) | 12.8 (1.8) | 450 (397) | 24.6 (2.1) | 17.6 (2.6) |
PD-NC, PD patients with MoCA score ≥26; PD-MCI, PD patients with MoCA score 21–25; PD-D, PD patients with MoCA score <21; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; HY, Hoehn and Yahr stage; LEDD, levodopa equivalent daily dose.
Fig. 1.
The percentage of patients according to global cognitive status who incorrectly copied each figure.
Wire cube failure significantly predicted cognitive impairment (OR = 4.79, 95% CI = 1.63–14.07, p = 0.004), with a sensitivity of 0.74 and a specificity of 0.63. Even when the cognitively impaired group was restricted to those with PD-MCI but not PD-D, cube copy remained a significant predictor (OR = 3.13, 95% CI = 1.02–9.55, p = 0.046), with a sensitivity of 0.65. In contrast, incorrect copying of the interlocking pentagons was not a significant predictor of cognitive impairment (OR = 1.88, 95% CI = 0.54–6.50, p = 0.32), with a sensitivity of 0.26 and a specificity of 0.84. When the cognitively impaired group was limited to those with PD-MCI, the sensitivity of the interlocking pentagons dropped to 0.13.
4. Discussion
Visuospatial dysfunction is an early feature of PD-MCI [1], predicts a more aggressive rate of cognitive decline [2,3,19] and is severely affected in PD-D [1,20,21]. Historically, the most frequently used figure-copy test of visuospatial function has been interlocking pentagons incorporated into the MMSE and the ACE. However, use of the wire cube is increasing, in part because the MoCA is a recommended test of global cognition in PD [18].
The reason why one test of visuospatial function – copying a wire cube – predicted cognitive impairment but another – copying interlocking pentagons – did not may be explained by the relative complexity of the wire cube, evidenced by studies that have found a ceiling effect of interlocking pentagon scores when testing cognition in PD [22]. The wire cube is more complex because it is a 2D drawing of a 3D object. To perceive the 12 separate lines of the drawing as a 3D cube requires visual suppression and there is evidence that PD subjects lack the attentive resource [23] and perceptual flexibility [24] needed. Attention and visual perception difficulties in PD worsen with increasing cognitive dysfunction [25], mediated primarily by acetylcholine deficiency secondary to Lewy-body related infiltration of major brainstem nuclei [26]. This may explain the progressive difficulty of cube copying across the cognitive groups in this study and links with larger studies in PD subjects that have found significant correlations between cube copying and poor performance in a range of cognitive domains in addition to visuospatial function, including attention and memory [27].
Three limitations of this study are acknowledged. Firstly, using MoCA cut off scores to define MCI and dementia rather than considering functional dependence or performing detailed neuropsychological evaluations [18]. However, the MCI [14,15] and dementia [15] cut off scores have been validated previously and we did not include the cube item for classification. Secondly, our sample size is relatively small because assessments are from recruits to a trial of medical equipment and results may need to be confirmed in a larger sample. Thirdly, this is a cross-sectional rather than longitudinal study.
Despite limitations, this study suggests that copying a wire cube is a more sensitive detector of cognitive impairment in PD than copying interlocking pentagons; a finding that has a clear, practical use for clinicians when working under time limitations such as outpatient clinics.
Acknowledgments
This study was supported by the National Institute of Health K23AG037566 and P50AG023501, the Michael J. Fox Foundation, and the Hellman Family Foundation. We thank our participants for their time and efforts.
References
- 1.Johnson DK, Galvin JE. Longitudinal changes in cognition in Parkinson’s disease with and without dementia. Dement Geriatr Cogn Disord. 2011;31(2):98–108. doi: 10.1159/000323570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams-Gray CH, et al. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 3.Williams-Gray CH, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 4.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 5.Shulman KI, et al. IPA survey of brief cognitive screening instruments. Int Psychogeriatr. 2006;18(2):281–294. doi: 10.1017/S1041610205002693. [DOI] [PubMed] [Google Scholar]
- 6.Mioshi E, et al. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21(11):1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 7.Newman JC, Feldman R. Copyright and open access at the bedside. N Engl J Med. 2011;365(26):2447–2449. doi: 10.1056/NEJMp1110652. [DOI] [PubMed] [Google Scholar]
- 8.Powsner S, Powsner D. Cognition, copyright, and the classroom. Am J Psychiatry. 2005;162(3):627–628. doi: 10.1176/appi.ajp.162.3.627-a. [DOI] [PubMed] [Google Scholar]
- 9.Martin R, O’Neill D. Taxing your memory. Lancet. 2009;373(9680):2009–2010. doi: 10.1016/S0140-6736(09)60349-4. [DOI] [PubMed] [Google Scholar]
- 10.Zadikoff C, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23(2):297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 11.Gill DJ, et al. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23(7):1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 12.Addenbrooke’s Cognitive Examination – ACE-III English Version A. Available from: http://neura.edu.au/sites/./ACE-III Administration (UK).pdf [cited 26.02.13]
- 13.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoops S, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalrymple-Alford JC, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 16.Lessig S, et al. Changes on brief cognitive instruments over time in Parkinson’s disease. Mov Disord. 2012;27(9):1125–1128. doi: 10.1002/mds.25070. [DOI] [PubMed] [Google Scholar]
- 17.Nazem S, et al. Montreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57(2):304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvan I, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson DK, et al. Onset of Mild Cognitive Impairment in Parkinson Disease. Alzheimer Dis Assoc Disord. 2015 doi: 10.1097/WAD.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janvin CC, et al. Cognitive profiles of individual patients with Parkinson’s disease and dementia: comparison with dementia with lewy bodies and Alzheimer’s disease. Mov Disord. 2006;21(3):337–342. doi: 10.1002/mds.20726. [DOI] [PubMed] [Google Scholar]
- 21.Uc EY, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009;73(18):1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lord S, et al. Cognition and gait show a selective pattern of association dominated by phenotype in incident Parkinson’s disease. Front Aging Neurosci. 2014;6:249. doi: 10.3389/fnagi.2014.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett KM, Castiello U. Three-dimensional covert attentional functions in Parkinson’s disease subjects. Exp Brain Res. 1996;112(2):277–288. doi: 10.1007/BF00227646. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Santos M, et al. Perceptual, cognitive, and personality rigidity in Parkinson’s disease. Neuropsychologia. 2015;69:183–193. doi: 10.1016/j.neuropsychologia.2015.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011;26(14):2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 26.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 27.Bu XY, et al. Usefulness of cube copying in evaluating clinical profiles of patients with Parkinson disease. Cogn Behav Neurol. 2013;26(3):140–145. doi: 10.1097/WNN.0000000000000006. [DOI] [PubMed] [Google Scholar]