Abstract
Poly(vinyl alcohol) (PVA) is the most active synthetic mimic of antifreeze proteins and has extremely high ice recrystallization inhibition (IRI) activity. Addition of PVA to cellular cryopreservation solutions increases the number of recovered viable cells due to its potent IRI, but it is intrinsically nondegradable in vivo. Here we report the synthesis, characterization, and IRI activity of PVA containing degradable ester linkages. Vinyl chloroacetate (VClAc) was copolymerized with 2-methylene-1,3-dioxepane (MDO) which undergoes radical ring-opening polymerization to install main-chain ester units. The use of the chloroacetate monomer enabled selective deacetylation with retention of esters within the polymer backbone. Quantitative IRI assays revealed that the MDO content had to be finely tuned to retain IRI activity, with higher loadings (24 mol %) resulting in complete loss of IRI activity. These degradable materials will help translate PVA, which is nontoxic and biocompatible, into a range of biomedical applications.
Cell-based therapies are one of the fastest growing areas of the biopharmaceutical market, including heamatopetic stem cells for blood cancer treatment through to total organ transplants.1 A key challenge remains the logistics and distribution of these cells, which must be stored frozen (cryopreserved).2 The state-of-the-art for cryopreservation is addition of 5–20 wt % of toxic organic solvents such as dimethyl sulfoxide (DMSO) which can reduce cell function,3 methylate DNA,4 cause acute patient toxicity,5 or simply not work for some cells. To address this serious problem, new concepts in cryopreservation are needed. Extremophile fish species which survive in subzero polar oceans produce antifreeze (glyco)proteins which are potent ice recrystallization inhibitors (IRIs)6,7 but themselves are not suitable for cryopreservation due to unwanted ice shaping effects or toxicity.8,9 Ben et al. have developed small-molecule IRIs which can enhance the cryopreservation of cell lines.10,11 Gibson and co-workers and Matsumura and co-workers have identified synthetic polymers12,13 which are potent IRIs, including poly(vinyl alcohol)14−16 and poly(ampholytes)17 as well as amphiphilic metallohellicies.18 These IRI-active polymers dramatically improve cell viability postcryopreservation and have been applied to red blood cells,19−21 stem cells,22 and cell lines.23 A potential barrier to application for these polymers is that they are not all biodegradable, which is a desirable feature for them to be applied in vivo, but most vinyl-derived polymers cannot degrade.
Degradable polymers are most commonly accessed by ring-opening polymerization of cyclic esters,24,25N-carboxy anhydrides,26 or step-growth polymerizations.27 Polyesters in particular have emerged as promising biomaterials with tunable degradable rates and biocompatibility.28 The design of IRI-active polymers, however, has revealed very few structures which give this property, and it is not just a consequence of polyols.29,30 Vinyl polymerization typically results in all carbon (nondegradable) backbones. However, their copolymerization with cyclic ketene acetal (CKA) monomers, which undergo radical ring-opening polymerization, enables the installation of main-chain ester units and hence degradability into radical polymerizations.31 While copolymerizations of CKAs with vinyl monomers have been widely investigated, the use of less activated monomers (e.g., VAc) has been less studied.32 Dove, O’Reilly, and co-workers have reported the radical copolymerization of 2-methylene-1,3-dioxepane (MDO) using reversible addition–fragmentation chain-transfer (RAFT) polymerization to generate hydrophilic, degradable, and biocompatible vinyl-based biomaterials.33−36
Herein, our the aim was to synthesize degradable, ester-containing poly(vinyl alcohol) but with full retention of its potent IRI activity. We demonstrate a radical ring-opening polymerization strategy and that IRI activity can be retained if the MDO incorporation is controlled to achieve a balance between degradability and function.
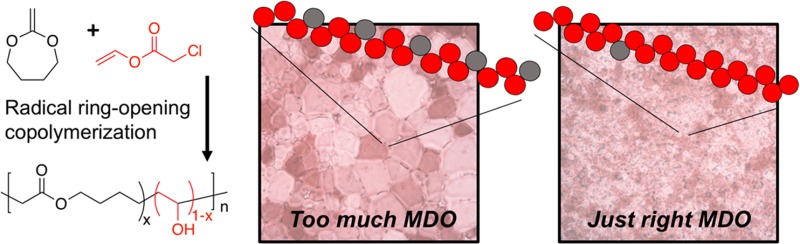
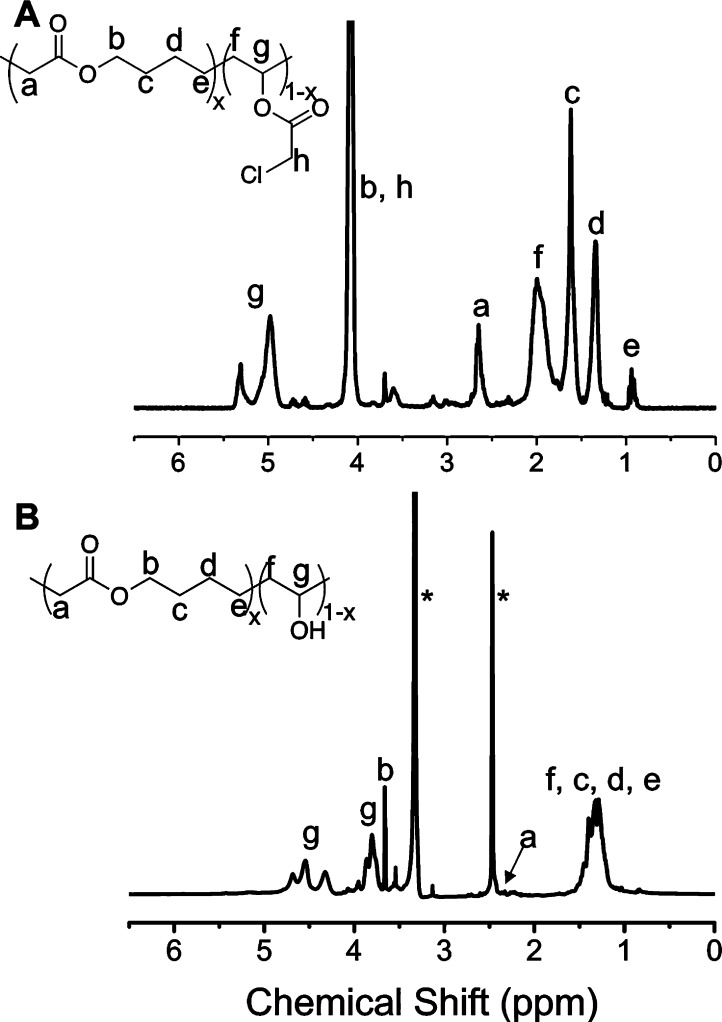
To obtain PVA featuring main-chain esters, MDO was selected as the radical ring-opening monomer.33,34 In place of the conventional vinyl acetate, vinyl chloroacetate (VClAc) was chosen as it can be selectively deprotected without hydrolyzing the alkyl esters within the backbone.37 The copolymerization of VClAc with MDO (Scheme 1) was performed in bulk at 60 °C using 2,2′-azobis(isobutyronitrile) (AIBN) as the radical initiator with or without the presence of O-hexyl-S-methyl-2-propionylxanthate as the chain transfer agent (CTA). The successful formation of poly(VClAc-co-MDO) was confirmed by 1H NMR spectroscopy analysis where characteristic signals at δ = 5.35–4.75 ppm and δ = 4.10 and 2.65 ppm were observed corresponding to the VClAc and MDO units, respectively. A panel of polymers was obtained containing 5 mol % and 24 mol % MDO (Table 1). SEC analyses confirmed the formation of polymers, and the addition of the chain transfer agent enabled some control over molecular weight; however, rather high dispersities were obtained, suggesting a poor control of the polymerization process (Table 1). This polymerization has also been shown to engage in branching at higher molecular weights, which again leads to greater dispersity values. This branching can be seen in the NMR spectra (Figure 1A), with peaks at around δ = 3.65 ppm being due to these side reactions. This characteristic was observed as a consequence of the poor stability of the propagating MDO radical and the subsequent likely chain transfer to VClAc monomer as previously reported by Dove and co-workers.34 Nevertheless, for the purposes of this study, narrow distributions were not essential, and current approved applications of PVA also use a disperse mixture.38 Kinetic analysis of the polymerization (Supporting Information) suggested a statistical copolymer, rather than blocky, is obtained.
Scheme 1. Radical Ring-Opening Copolymerization of VClAc and MDO and Subsequent Hydrolysis to Form Degradable PVA.
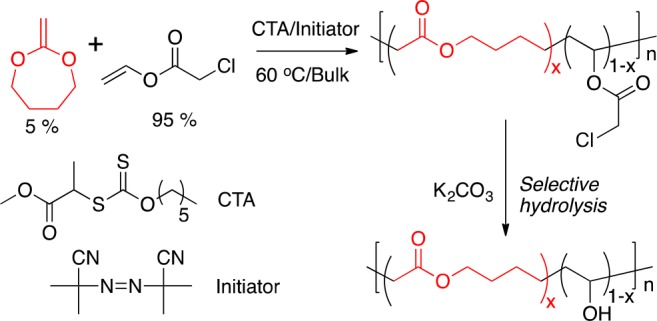
Table 1. Polymer Characterization.
| code | [M]:[CTA] | MDOa (%) | Mnb (g mol–1) | ĐM (−) |
|---|---|---|---|---|
| P1 | N/A | 5 | 12000 | 3.39 |
| P2 | 300 | 5 | 7300 | 2.48 |
| P3 | 400 | 5 | 8800 | 3.19 |
| P4 | 100 | 24 | 4600 | 2.10 |
Determined by 1H NMR spectroscopy in CDCl.3
Mn is the number-average molecular weight of the poly(VClAc-co-MDO) copolymers before any modification as observed by SEC in CHCl3.
Figure 1.
Polymer characterization. (A) 1H NMR of poly(VClAc-co-MDO) (P2) in CDCl3 and (B) 1H NMR of poly(VA-co-MDO) (P2) in DMSO.
Selective hydrolysis of the chloroacetate groups, to generate poly(VA-co-MDO), was achieved by using K2CO3, followed by dialysis. 13C NMR spectroscopy analysis confirmed retention of the MDO units including characteristic peaks at δ = 167 ppm. IR also confirmed formation of the hydroxyl group (see SI Figure S4). Degradation of a homopolymer of the MDO unit was carried out to determine the tolerance of the backbone to the deprotection conditions. While significant degradation was present after 2 h, after 10 min the homopolymer was almost completely unchanged (see SI Figure S2).
It is essential to confirm the presence of the esters in the main chain of the polymers, and hence the degradability. SEC analysis of PVA is challenging as a consequence of strong column interactions and tailing. Therefore, poly(VA-co-MDO) (P1) was subjected to NaOH hydrolysis and subsequently acetylated (Figure 2A) using acetic anhydride to generate PVAc, which is suitable for organic-phase SEC analysis. Aggressive reaction conditions were used to completely degrade all of the backbone MDO units, allowing the chain length after degradation to be determined. Such an analysis clearly shows a decrease in molecular weight, relative to the poly(VClAc-co-MDO) precursor (Figure 2B). To eliminate effects that result from the mass of the repeat units (chloroacetate vs acetate) this was also plotted as deconvoluted degree of polymerization (Figure 2C). This confirms that ester linkages are incorporated and that a degradable PVA was successfully synthesized. To demonstrate degradability in biologically relevant conditions, poly(VA-co-MDO) was degraded by immobilized lipase (broad spectrum esterase) in water at 37 °C for 24 h. The previously applied acetylation strategy could not be used, as the addition of pyridine could also degrade the polymers and mislead the analysis; therefore, we could not determine the degree to which degradation was a consequence of the lipase beads and not simply resulting from pyridine-catalyzed hydrolysis. 1H NMR spectroscopic analysis monitoring (ESI) failed, as a consequence of the overlapping of the MDO peaks at δ = 3.75 ppm in D2O by a peak which is present due to the leaching of material from beads in water. In order to show changes in unique resonances, MALDI-ToF MS was used. After 24 h incubation with immobilized lipase, there was a clear shift to lower molecular weight oligomer species where distributions with spacing of m/z 44.03 between neighboring peaks were observed, hence confirming degradability (Figure 2D).
Figure 2.
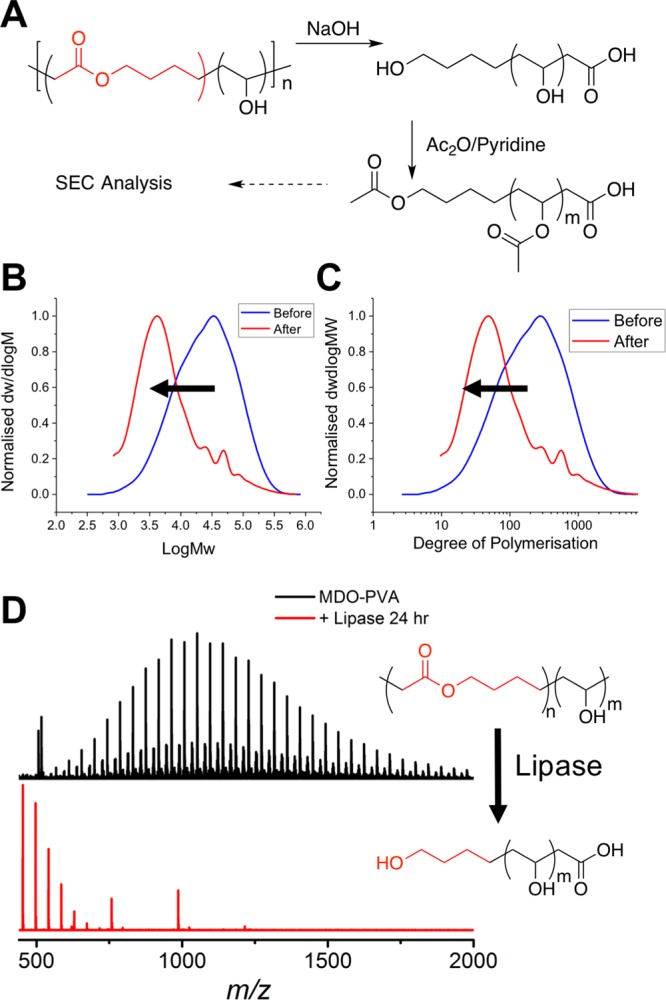
Degradability of poly(VA-co-MDO). (A) Scheme for basic hydrolysis followed by acetylation to enable SEC analysis; (B) SEC analysis of NaOH hydrolysis of poly(VA-co-MDO) as a function of molecular weight and (C) as a function of degree of polymerization; and (D) MALDI-ToF MS analysis of poly(VA-co-MDO) (P2) degradation products by lipase at 37 °C.
With the panel of polymers in Table 1, their IRI (ice recrystallization inhibition) activity could be evaluated. A crucial question was what impact the MDO incorporation into PVA would have, as copolymers of PVA show decreased IRI typically.15 A quantitative “splat” assay was employed; in short, a polynucleated wafer of small ice crystals was annealed at −8 °C for 30 min, before being imaged, and the average ice crystal size determined, relative to a PBS control. Smaller values indicate more IRI activity. Example ice wafers for P1-OH (Figure 3) show a clear dose-dependent IRI with near complete inhibition of ice growth at 1 mg mL–1 but significant ice growth at 0.1 mg mL–1, consistent with previously reported IRI of PVA.15,39 P1–P3 had similar activity at both concentrations and after degradation (in NaOH). Considering the average DP after polymerization (assuming statistical incorporation of MDO) the polymers should generate PVA with DP > 20, which would be expected to retain IRI at these concentrations.15
Figure 3.
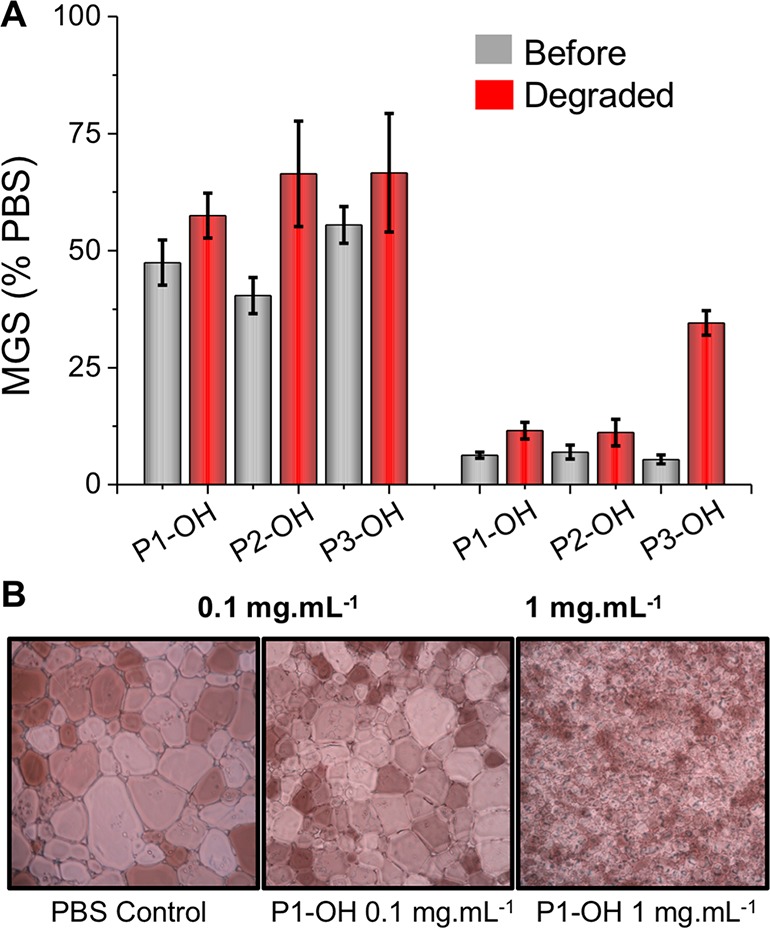
(A) IRI activity of polymers 1–3 before and after NaOH degradation. (B) Example ice wafers showing concentration effect on poly(VA-co-MDO) activity. MGS = mean grain size. Error bars are ± SD from minimum of 3 replicates.
P4-OH contained a higher density of MDO units, of 24 mol % compared to 5 mol % for P1–P3. Higher incorporation of MDO units results in increased degradation rates and releases shorter degraded units33 but must be balanced against retention of IRI. IRI activity for P4-OH (Figure 4) indicated that both before and after degradation there is significantly less activity than P1–P3 which contain less MDO. Even at 10 mg mL–1 MGS of 60% was obtained, compared to <20% for P1–P3 at just 1 mg mL–1. Degraded polymers without the MDO units also had low activity, as their chain length (DP 5 based on statistical incorporation of MDO) is too short for activity. These results highlight that in the design of degradable IRI active polymers there is a delicate balance between incorporation of degradable units but also the need to maintain IRI activity via unbroken continuous PVA blocks, estimated as being 10 units by Koop and co-workers.40
Figure 4.
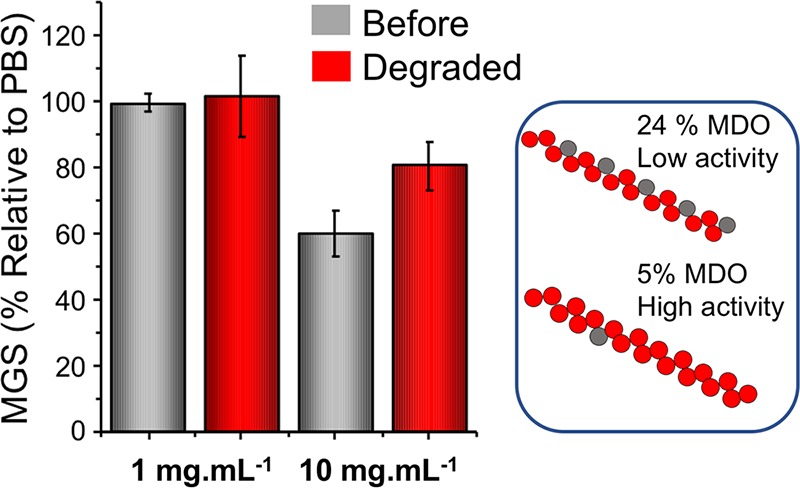
IRI activity of P4 containing 24 mol % MDO units. MGS = mean grain size. Error bars are ±SD from a minimum of 3 replicates.
In conclusion, we have presented the first synthesis of degradable poly(vinyl alcohol) by a new strategy of radical ring-opening copolymerization of chloro-vinyl acetate and 2-methylene-1,3-dioxepane. The resulting polymers were shown to contain main-chain ester linkages which can be degraded by basic hydrolysis or an esterase. Quantitative ice recrystallization inhibition assays demonstrated that control of the ester density was crucial to ensure that activity was retained, with polymers containing high (24%) ester linkages being ineffective but those with 5 mol % being highly active. These materials may find application in cryopreservation or other applications where higher molecular weights are required alongside degradability such as in agricultural or drug delivery fields.
Acknowledgments
The University of Warwick Institute of Advanced Study and Materials GRP are thanked for cofunding a scholarship to G.G.H. MIG and APD acknowledge the ERC for funding (No 638661 and 681559, respectively). The Royal Society are thanked for funding the cryomicroscope.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmacrolett.7b00905.
Synthetic, characterization, and ice growth assay details (PDF)
Author Contributions
∥ G.H. and C.S. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Wolfe R. A.; Merion R. M.; Roys E. C.; Port F. K. Trends in Organ Donation and Transplantation in the United States, 1998–2007. Am. J. Transplant. 2009, 9, 869–878. 10.1111/j.1600-6143.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Fowler A.; Toner M. Cryo-Injury and Biopreservation. Ann. N. Y. Acad. Sci. 2005, 1066, 119–135. 10.1196/annals.1363.010. [DOI] [PubMed] [Google Scholar]
- Iwatani M.; Ikegami K.; Kremenska Y.; Hattori N.; Tanaka S.; Yagi S.; Shiota K. Dimethyl Sulfoxide Has an Impact on Epigenetic Profile in Mouse Embryoid Body. Stem Cells 2006, 24 (11), 2549–2556. 10.1634/stemcells.2005-0427. [DOI] [PubMed] [Google Scholar]
- Kawai K.; Li Y.-S.; Song M.-F.; Kasai H. DNA Methylation by Dimethyl Sulfoxide and Methionine Sulfoxide Triggered by Hydroxyl Radical and Implications for Epigenetic Modifications. Bioorg. Med. Chem. Lett. 2010, 20 (1), 260–265. 10.1016/j.bmcl.2009.10.124. [DOI] [PubMed] [Google Scholar]
- Shu Z.; Heimfeld S.; Gao D. Hematopoietic Stem Cell Transplantation with Cryopreserved Grafts: Adverse Reactions after Transplantation and Cryoprotectant Removal Prior to Infusion HHS Public Access. Bone Marrow Transplant. 2014, 49 (4), 469–476. 10.1038/bmt.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding M. M.; Anderberg P. I.; Haymet A. D. J. Antifreeze” Glycoproteins from Polar Fish. Eur. J. Biochem. 2003, 270 (7), 1381–1392. 10.1046/j.1432-1033.2003.03488.x. [DOI] [PubMed] [Google Scholar]
- Davies P. L.Ice-Binding Proteins: A Remarkable Diversity of Structures for Stopping and Starting Ice Growth. Trends Biochem. Sci.; Elsevier, 2014; pp 548–555. [DOI] [PubMed] [Google Scholar]
- Chao H.; Davies P. L.; Carpenter J. F. Effects of Antifreeze Proteins on Red Blood Cell Survival during Cryopreservation. J. Exp. Biol. 1996, 199, 2071–2076. [DOI] [PubMed] [Google Scholar]
- Liu S.; Wang W.; von Moos E.; Jackman J.; Mealing G.; Monette R.; Ben R. N. In Vitro Studies of Antifreeze Glycoprotein (AFGP) and a C-Linked AFGP Analogue. Biomacromolecules 2007, 8 (5), 1456–1462. 10.1021/bm061044o. [DOI] [PubMed] [Google Scholar]
- Capicciotti C. J.; Kurach J. D. R.; Turner T. R.; Mancini R. S.; Acker J. P.; Ben R. N. Small Molecule Ice Recrystallization Inhibitors Enable Freezing of Human Red Blood Cells with Reduced Glycerol Concentrations. Sci. Rep. 2015, 5, 9692. 10.1038/srep09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcerzak A. K.; Capicciotti C. J.; Briard J. G.; Ben R. N. Designing Ice Recrystallization Inhibitors: From Antifreeze (Glyco)proteins to Small Molecules. RSC Adv. 2014, 4 (80), 42682–42696. 10.1039/C4RA06893A. [DOI] [Google Scholar]
- Gibson M. I. Slowing the Growth of Ice with Synthetic Macromolecules: Beyond Antifreeze(glyco) Proteins. Polym. Chem. 2010, 1 (8), 1141–1152. 10.1039/c0py00089b. [DOI] [Google Scholar]
- Biggs C. I.; Bailey T. L.; Ben Graham; Stubbs C.; Fayter A.; Gibson M. I. Polymer Mimics of Biomacromolecular Antifreezes. Nat. Commun. 2017, 8 (1), 1546. 10.1038/s41467-017-01421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T.; Lu S. S. Inhibition of Recrystallization of Ice Grains by Adsorption of Poly(vinyl Alcohol) onto Ice Surfaces. Cryst. Growth Des. 2003, 3 (5), 747–752. 10.1021/cg0340300. [DOI] [Google Scholar]
- Congdon T.; Notman R.; Gibson M. I. Antifreeze (Glyco)protein Mimetic Behavior of Poly(vinyl Alcohol): Detailed Structure Ice Recrystallization Inhibition Activity Study. Biomacromolecules 2013, 14 (5), 1578–1586. 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- Congdon T. R.; Notman R.; Gibson M. I. Influence of Block Copolymerization on the Antifreeze Protein Mimetic Ice Recrystallization Inhibition Activity of Poly(vinyl Alcohol). Biomacromolecules 2016, 17 (9), 3033–3039. 10.1021/acs.biomac.6b00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs C.; Lipecki J.; Gibson M. I. Regio-Regular Alternating Polyampholytes Have Enhanced Biomimetic Ice Recrystallization Activity Compared to Random Copolymers and the Role of Side Chain Verses Main Chain Hydrophobicity. Biomacromolecules 2017, 18 (1), 295–302. 10.1021/acs.biomac.6b01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. E.; Clarkson G.; Fox D. J.; Vipond R. A.; Scott P.; Gibson M. I. Antifreeze Protein Mimetic Metallohelices with Potent Ice Recrystallization Inhibition Activity. J. Am. Chem. Soc. 2017, 139 (29), 9835–9838. 10.1021/jacs.7b05822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller R. C.; Vatish M.; Mitchell D. A.; Gibson M. I. Synthetic Polymers Enable Non-Vitreous Cellular Cryopreservation by Reducing Ice Crystal Growth during Thawing. Nat. Commun. 2014, 5, 3244. 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E.; Lovett J. R.; Armes S. P.; Gibson M. I. Combining Biomimetic Block Copolymer Worms with an Ice-Inhibiting Polymer for the Solvent-Free Cryopreservation of Red Blood Cells. Angew. Chem., Int. Ed. 2016, 55 (8), 2801–2804. 10.1002/anie.201511454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller R. C.; Vatish M.; Mitchell D. A.; Gibson M. I. Glycerol-Free Cryopreservation of Red Blood Cells Enabled by Ice-Recrystallization-Inhibiting Polymers. ACS Biomater. Sci. Eng. 2015, 1 (9), 789–794. 10.1021/acsbiomaterials.5b00162. [DOI] [PubMed] [Google Scholar]
- Matsumura K.; Bae J. Y.; Kim H. H.; Hyon S. H. Effective Vitrification of Human Induced Pluripotent Stem Cells Using Carboxylated ??-Poly-L-Lysine. Cryobiology 2011, 63 (2), 76–83. 10.1016/j.cryobiol.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Deller R. C.; Pessin J. E.; Vatish M.; Mitchell D. A.; Gibson M. I. Enhanced Non-Vitreous Cryopreservation of Immortalized and Primary Cells by Ice-Growth Inhibiting Polymers. Biomater. Sci. 2016, 1079. 10.1039/C6BM00129G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounder R. J.; Dove A. P. Towards Poly(ester) Nanoparticles: Recent Advances in the Synthesis of Functional Poly(ester)s by Ring-Opening Polymerization. Polym. Chem. 2010, 1 (3), 260. 10.1039/b9py00327d. [DOI] [Google Scholar]
- Jérôme C.; Lecomte P. Recent Advances in the Synthesis of Aliphatic Polyesters by Ring-Opening Polymerization. Adv. Drug Delivery Rev. 2008, 60, 1056–1076. 10.1016/j.addr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Gibson M. I.; Cameron N. R. Experimentally Facile Controlled Polymerization of N -Carboxyanhydrides (NCAs), Including O -Benzyl-L-Threonine NCA. J. Polym. Sci., Part A: Polym. Chem. 2009, 47 (11), 2882–2891. 10.1002/pola.23364. [DOI] [Google Scholar]
- Okada M. Chemical Syntheses of Biodegradable Polymers. Prog. Polym. Sci. 2002, 27 (1), 87–133. 10.1016/S0079-6700(01)00039-9. [DOI] [Google Scholar]
- Brannigan R. P.; Dove A. P. Synthesis, Properties and Biomedical Applications of Hydrolytically Degradable Materials Based on Aliphatic Polyesters and Polycarbonates. Biomater. Sci. 2017, 5 (1), 9–21. 10.1039/C6BM00584E. [DOI] [PubMed] [Google Scholar]
- Gibson M. I.; Barker C. A.; Spain S. G.; Albertin L.; Cameron N. R. Inhibition of Ice Crystal Growth by Synthetic Glycopolymers: Implications for the Rational Design of Antifreeze Glycoprotein Mimics. Biomacromolecules 2009, 10 (2), 328–333. 10.1021/bm801069x. [DOI] [PubMed] [Google Scholar]
- Deller R. C.; Congdon T.; Sahid M. A.; Morgan M.; Vatish M.; Mitchell D. A.; Notman R.; Gibson M. I. Ice Recrystallisation Inhibition by Polyols: Comparison of Molecular and Macromolecular Inhibitors and Role of Hydrophobic Units. Biomater. Sci. 2013, 1 (5), 478–485. 10.1039/c3bm00194f. [DOI] [PubMed] [Google Scholar]
- Delplace V.; Nicolas J. Degradable Vinyl Polymers for Biomedical Applications. Nat. Chem. 2015, 7 (10), 771–784. 10.1038/nchem.2343. [DOI] [PubMed] [Google Scholar]
- Agarwal S. Chemistry, Chances and Limitations of the Radical Ring-Opening Polymerization of Cyclic Ketene Acetals for the Synthesis of Degradable Polyesters. Polym. Chem. 2010, 1 (7), 953–964. 10.1039/c0py00040j. [DOI] [Google Scholar]
- Hedir G.; Arno M.; Langlais M.; Husband J.; O’Reilly R.; Dove A. Poly(oligo (Ethylene Glycol) Vinyl Acetate)s: A Versatile New Class of Thermoresponsive and Biocompatible Polymers. Angew. Chem., Int. Ed. 2017, 56 (31), 9178–9182. 10.1002/anie.201703763. [DOI] [PubMed] [Google Scholar]
- Hedir G. G.; Bell C. A.; O’Reilly R. K.; Dove A. P. Functional Degradable Polymers by Radical Ring-Opening Copolymerization of MDO and Vinyl Bromobutanoate: Synthesis, Degradability and Post-Polymerization Modification. Biomacromolecules 2015, 16 (7), 2049–2058. 10.1021/acs.biomac.5b00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. A.; Hedir G. G.; O’Reilly R. K.; Dove A. P. Controlling the Synthesis of Degradable Vinyl Polymers by Xanthate-Mediated Polymerization. Polym. Chem. 2015, 6 (42), 7447–7454. 10.1039/C5PY01156F. [DOI] [Google Scholar]
- Hedir G. G.; Bell C. A.; Ieong N. S.; Chapman E.; Collins I. R.; O’Reilly R. K.; Dove A. P. Functional Degradable Polymers by Xanthate-Mediated Polymerization. Macromolecules 2014, 47 (9), 2847–2852. 10.1021/ma500428e. [DOI] [Google Scholar]
- Olijve L. L. C.; Hendrix M. M. R. M.; Voets I. K. Influence of Polymer Chain Architecture of Poly(vinyl Alcohol) on the Inhibition of Ice Recrystallization. Macromol. Chem. Phys. 2016, 217 (8), 951–958. 10.1002/macp.201500497. [DOI] [Google Scholar]
- DeMerlis C. C.; Schoneker D. R. Review of the Oral Toxicity of Polyvinyl Alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. 10.1016/S0278-6915(02)00258-2. [DOI] [PubMed] [Google Scholar]
- Phillips D. J.; Congdon T. R.; Gibson M. I. Activation of Ice Recrystallization Inhibition Activity of Poly(vinyl Alcohol) Using a Supramolecular Trigger. Polym. Chem. 2016, 7 (9), 1701. 10.1039/C5PY01948F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke C.; Koop T. Ice Recrystallization Inhibition and Molecular Recognition of Ice Faces by Poly(vinyl Alcohol). ChemPhysChem 2006, 7 (12), 2601–2606. 10.1002/cphc.200600533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.