Variation in O2 transport characteristics among Andean, Tibetan, and, when available, Ethiopian high-altitude residents supports the existence of genetic adaptations that improve the distribution of blood flow to vital organs and the efficiency of O2 utilization. Genome scans and whole genome sequencing studies implicate a broad range of gene regions. Future studies are needed using phenotypes of clear relevance for reproductive success for determining the mechanisms by which naturally selected genes are acting.
Keywords: Andes, Chronic Mountain Sickness, hypoxia, pregnancy, Tibet
Abstract
High altitudes (>8,000 ft or 2,500 m) provide an experiment of nature for measuring adaptation and the physiological processes involved. Studies conducted over the past ~25 years in Andeans, Tibetans, and, less often, Ethiopians show varied but distinct O2 transport traits from those of acclimatized newcomers, providing indirect evidence for genetic adaptation to high altitude. Short-term (acclimatization, developmental) and long-term (genetic) responses to high altitude exhibit a temporal gradient such that, although all influence O2 content, the latter also improve O2 delivery and metabolism. Much has been learned concerning the underlying physiological processes, but additional studies are needed on the regulation of blood flow and O2 utilization. Direct evidence of genetic adaptation comes from single-nucleotide polymorphism (SNP)-based genome scans and whole genome sequencing studies that have identified gene regions acted upon by natural selection. Efforts have begun to understand the connections between the two with Andean studies on the genetic factors raising uterine blood flow, fetal growth, and susceptibility to Chronic Mountain Sickness and Tibetan studies on genes serving to lower hemoglobin and pulmonary arterial pressure. Critical for future studies will be the selection of phenotypes with demonstrable effects on reproductive success, the calculation of actual fitness costs, and greater inclusion of women among the subjects being studied. The well-characterized nature of the O2 transport system, the presence of multiple long-resident populations, and relevance for understanding hypoxic disorders in all persons underscore the importance of understanding how evolutionary adaptation to high altitude has occurred.
NEW & NOTEWORTHY Variation in O2 transport characteristics among Andean, Tibetan, and, when available, Ethiopian high-altitude residents supports the existence of genetic adaptations that improve the distribution of blood flow to vital organs and the efficiency of O2 utilization. Genome scans and whole genome sequencing studies implicate a broad range of gene regions. Future studies are needed using phenotypes of clear relevance for reproductive success for determining the mechanisms by which naturally selected genes are acting.
high altitude poses a challenge for human adaptation due to the reduction in barometric pressure and consequently lower partial pressure of oxygen (Po2) in the atmosphere. The fall in Po2 (“hypoxia”) is approximately linear but due to the kinetics of O2 binding and resultant curvilinear shape of the hemoglobin-O2 dissociation curve, O2 levels in the arterial blood do not markedly fall until above ~8,000 ft (2,500 m). There are four major world regions where ~140 million persons reside at high altitude (104).1 The largest populations are the Andean (Aymara and Quechua) of South America and the Tibetan or Sherpa residents of central Asia, but considerable numbers of Amhara reside in the Ethiopian highlands of East Africa, and persons of largely European descent live in the Rocky Mountains of North America. The Rocky Mountains have been permanently inhabited for only ~150 years, but the other regions have been occupied for millennia (61, 71, 120) (referred to henceforth as “long term”), long enough for genetic adaptation to have occurred (41).
High altitudes comprise a natural laboratory for studying human biological adaptation as culture exerts little influence over the effects of hypoxia, even though it affects other characteristics, such as cold, diurnal temperature variation, and low humidity.2 This article summarizes studies appearing over the past ~25 years that have addressed the biological mechanisms by which populations have adapted to high altitudes and how they have been measured. Most concern Andeans or Tibetans, as Ethiopians have been less often studied. It begins by considering the meaning of the term “adaptation.” Then summarized are population comparisons of O2 transport traits and of genome scans for evidence of natural selection. Also reviewed are the relatively small number of efforts made to identify the physiological mechanisms by which the naturally selected genes may be influencing the adaptive process. The thesis is developed that short-term (acclimatization, developmental) and long-term (genetic) responses to high altitude exhibit a temporal gradient such that, although all influence O2 content, the latter also improve O2 delivery and metabolism (Fig. 1). The article concludes with some recommendations for future study.
Fig. 1.
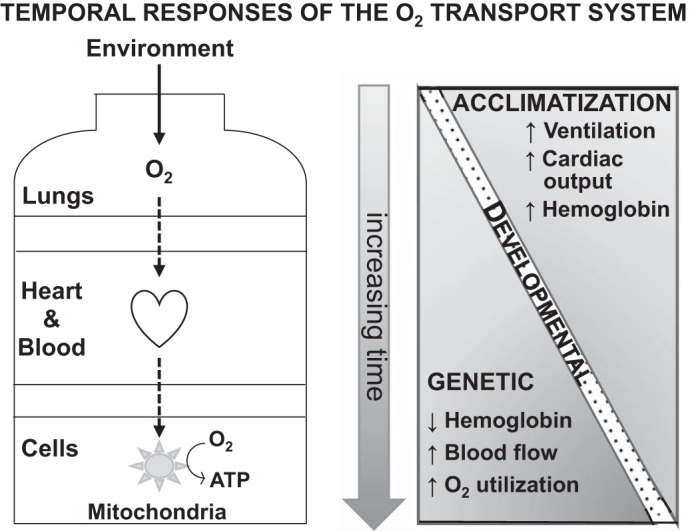
The O2 transport system comprises two pumps, the lungs and the heart, that bring in O2 from the atmosphere and circulates it via the blood throughout the body, and two diffusion steps. The diffusion steps transfer O2 across the alveolar membrane and from the blood into tissues where it is consumed in the mitochondria to generate chemical energy in the form of adenosine triphosphate (ATP). Acclimatization involves changes in these components over days and developmental responses take place across lifetimes in chiefly the components affecting arterial O2 content. Long-term high-altitude populations are distinguished from acclimatized newcomers in terms of their hemoglobin levels, regional blood flow, and O2 utilization components of the O2 transport system. [Adapted from Moore (99) with permission from Elsevier. Copyright © 2016 Elsevier.]
Meaning of Adaptation
The term “adaptation” has been used in differing ways but when referring to phenotypic traits that have been acted upon by natural selection, it has a specific meaning; namely, any features of structure, function, or behavior that increase the ability to survive and reproduce in a given environment (38). In physiology, “adaptation” has also been used to refer to traits that restore homeostasis and are therefore presumed to be beneficial. This, however, can lead to confusion because the presence of a trait does not necessarily mean that it is adaptive, and to assume that it is risks a teleological fallacy or the claim that the presence of a trait has a purpose, without evidence as to what that purpose is. For example, the assumption that because higher hemoglobin helps restore O2 content, it is beneficial, has come under question given that the expected gain in O2 delivery is offset by a reduction in blood flow due to increased viscosity (133).
Adaptations influence evolutionary process via their effects on fertility (the number of live offspring) and/or mortality (the number surviving through the reproductive period). The net effects of such adaptations determine “fitness,” also referred to as reproductive success. Natural selection is the process by which genetic traits increasing fitness become more prevalent over time. It is one of two directional forces in evolution, the other being gene flow (i.e., the movement of genes between populations) since the effects of the other two forces, mutation and genetic drift, are random. The distinction between gene flow and natural selection is that gene flow affects the whole genome in the same manner, whereas the effects of natural selection are confined to particular loci.
Adaptations acted upon by natural selection must be subject to genetic influences. The argument has been made that natural selection can only act on traits with high heritability (8), but this can be misleading because a trait with low heritability may already have been selected-for and become fixed in the population. It is also incorrect to compare heritability between populations as its calculation is specific to a given environment (126). Also problematic is judging whether an organism is adapted to one environment—high altitude—in terms of its characteristics (e.g., maximal O2 uptake) in another, e.g., low-altitude environment (154). Doing so is analogous to judging the adaptations of ice-age mammals in terms of their ability to survive in tropical forests, or to say that persons are not adapted to low altitude because their maximal O2 uptake declines with ascent.
“Adaptation” is being used here as distinct from “acclimatization” or the time-dependent rise in ventilation, hemoglobin concentration, heart rate, and redistribution of blood flow that serve to restore arterial O2 content and preserve O2 delivery to vital organs. Acclimatization processes have been well summarized elsewhere (66, 114, 142, 155) and will be referred to only for purposes of making comparisons between acclimatized newcomers and long-resident groups.
Support for genetic adaptation to high altitude having occurred comes from two sources: 1) studies of populations that have lived at high altitude for millennia showing differences from acclimatized newcomers in O2 transport traits or variation among these long-term populations that cannot be accounted for by developmental processes, and 2) direct evidence for the actions of natural selection on particular genes or gene regions. Connections between the two have been less common, that is, demonstrating the physiological mechanism(s) by which the naturally-selected gene is acting or has acted so as to influence reproductive success. Although difficult, such connections are important since, by definition, natural selection can only act on genes whose physiological effects influence reproductive success.
Evidence for Adaptation: Population Comparisons of O2 Transport Traits
The determinants of O2 transport have long been of interest for establishing the roles of genetic and developmental factors in high-altitude adaptation (Fig. 1). Summarized below are recent studies examining whether arterial O2 content, O2 distribution (blood flow), or O2 utilization (metabolism) differ between acclimatized newcomers and/or among long-term high-altitude populations and, if so, the contributions of genetic and developmental factors to the differences observed.
Arterial O2 content.
The level of resting ventilation is the primary determinant of arterial Po2, the other being the alveolar-arterial O2 gradient (Fig. 1). Alveolar, not total ventilation, is of chief interest since only the movement of air in the gas-exchange area of the lung can affect arterial Po2. Since changes in metabolic rate due to meal ingestion, subject anxiety, etc., affect total ventilation, the best measure of alveolar ventilation in healthy persons is the end-tidal (or arterial) Pco2 since it is inversely proportional to the amount of alveolar ventilation per unit CO2 production, which generally is a close approximation of metabolic rate. At sea level, end-tidal Pco2 averages ~40 mmHg, with values dropping with acclimatization to the low 30s or lower, depending on the altitude reached. Tibetans, Andeans, and acclimatized newcomers all have higher alveolar ventilation (lower end-tidal Pco2) than their sea-level counterparts, but Tibetan or acclimatized-newcomer lower end-tidal Pco2 values are lower than those of Andeans (Table 1) (8, 23, 176). Tibetans have a brisk ventilatory response to acute hypoxia, similar to that of acclimatized newcomers (176), whereas it is blunted in Andeans in proportion to the amount of indigenous (Quechua) ancestry (26). Surprisingly high end-tidal Pco2 values (38.2 mmHg) were reported in one study in Ethiopians at 3,622 m, with even greater values present at sea level (49.8 mmHg) (32). If confirmed, this would suggest that Ethiopians also increase their alveolar ventilation at high altitude but start from profoundly low levels.
Table 1.
Determinants of O2 transport and their average values in long-term highland groups and acclimatized newcomers studied at ~3,600–4,300 m
| Variable | Acclimatized Newcomer (AN) | Andeans (A) | Tibetans (T) | Ethiopians (E) | Summary |
|---|---|---|---|---|---|
| or a, mmHg | 30b (Refs. 176, 177) | Higher (= 32a) (Ref. 176) | Same (= 30) (Ref. 176) | Higher (= 38) (Ref. 32) | (AN = T) < A < E |
| A-aDO2c, mmHg | 7c–11d (Refs. 150, 177) | Lower (= 4c) (Ref. 150) | Lower (= 7c) | ? | AN > (A = T) |
| , % | 92 (Refs. 24, 27, 137) | Same (= 91) (Refs. 10, 70, 137, 176) | Lower (= 88) (Refs. 10, 70, 137, 176) | Higher (= 95) (Ref. 8) or Same (= 92) (Ref. 58) | (AN = A) > T |
| Hemoglobin, gm/dl | 17.6 (Refs. 24, 27) | Same (= 17) (Refs. 8, 83) | Lower (= 15e–18f) (Refs. 8, 133, 160, 176) | Lower? (= 15g–16h (Refs. 10, 129) | (AN = A) > (T = E) |
| , vol%i | 21 (Refs. 24, 27) | Same (= 21) | Same (= 21) | Lower (= 19, 20) | (AN = A = T) > E |
| Cardiac output | Same | Same | ? | ? | |
| Ppa hypoxic response | Present | Intermediate (Ref. 52) | Absent (Ref. 52) | Intermediate (Ref. 58) | AN > (A = E) > T |
| Brain blood flow, cm/s | 27j (Ref. 62) | Lower (−18%k) (Ref. 70) | Higher (= 29j,+6.2%k) (Refs. 62, 70) | ? | (AN < T) > A |
| Uterine blood flow, ml/min | 269 (Ref. 101) | Higher (= 452) (Ref. 101) | Higherl | ? | AN < (T = A) |
| O2 metabolismm | Higher | Higher | ? | AN < (T = A) |
A-aDO2, alveolar-to-arterial O2 diffusion gradient; AN, acclimatized newcomers of various populations; , arterial O2 content; or , arterial or end-tidal Pco2, respectively; Ppa, pulmonary arterial pressure; , arterial O2 saturation.
or are inversely related to alveolar ventilation.
Values are averaged from multiple studies whose original reference appears in reference(s) cited.
Measured at moderate exercise (~140 W) at 5,260 m.
Measured at moderate exercise (~140 W) at 3,658 m.
Hemoglobin concentration reported in Ref. 9.
Hemoglobin concentration reported in Ref. 128.
Estimated from above and hemoglobin values (without incorporation of dissolved O2).
Measured as internal carotid artery flow velocity at moderate exercise (~140 W) at 3,658 m.
Expressed as a percent of sea-level values measured using Doppler in Tibetans and the arterial-jugular difference in O2 content in Andeans.
The alveolar-arterial O2 gradient does not change appreciably with acclimatization. Both Andeans and Tibetans have lower values (131, 150, 177), but values are unknown for Ethiopians (Table 1). The lower gradient is largely the result of a greater total lung, especially residual, volumes due to developmental factors (24, 39) as even lifelong Coloradans or experimental animals born and raised at high altitude have greater lung volumes (36, 73). Genetic factors also contribute since Andean total lung volumes are even greater than those of lowlanders born and raised at high altitude (27), and the proportion of Native American ancestry-informative markers (AIMs) is directly related to residual volume (82). Lung growth is accelerated in Tibetans relative to Han born and raised at high altitude (153), but Ethiopians have not been studied. The accelerated lung growth begins in childhood in Tibetans and mid- to late-adolescence in Andeans (153). Greater lung volumes increase the surface area over which gas exchange takes place, thus lowering the alveolar-arterial O2 gradient and helping to maintain arterial O2 saturation (), particularly during exercise (83, 177).
If the position of the hemoglobin-O2 dissociation curve is unchanged, a given arterial Po2 determines the level of . The position of the hemoglobin-O2 dissociation curve changes little during acclimatization, with the leftward-shifting effects of respiratory alkalosis being offset by increased red blood cell 2,3-bisphosphoglyceric acid. Rightward as well as leftward shifts have been reported in native highlanders, but at present, there is little consensus that curve position is altered. has generally been reported to be higher in Andeans than Tibetans (8, 49, 98, 118, 159), and either similar or greater in Ethiopians (10, 58) (Table 1).
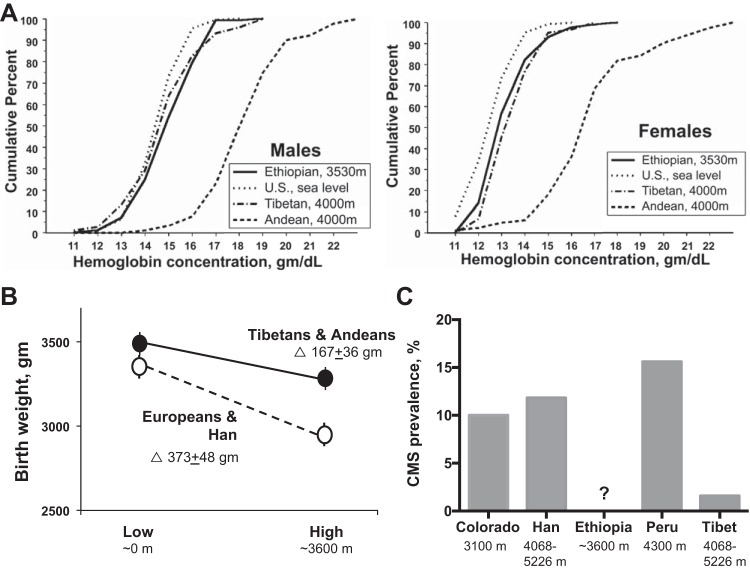
Hemoglobin levels rise with ascent in acclimatized newcomers due to a contraction in plasma volume and then an increase in red blood cell production, but vary among high-altitude populations. Figure 2A summarizes data from Beall and coworkers (10) showing higher levels in Andeans than Tibetans or Ethiopians, and only modest differences between the latter two groups and USA sea-level residents. Somewhat higher hemoglobin values were seen in other Tibetan studies (133, 176) (Table 1). It is important to recognize that hemoglobin values rise with increasing altitude in all groups [including Ethiopians (129)] although less so in Tibetans (160), with values in healthy Andeans and perhaps also Tibetans approximating those of acclimatized lowlanders (27) (Table 1). Factors other than altitude, such as lung disease resulting from occupational exposure (5) or the greater admixture present in Andean mining communities, are also likely to be contributing to the regional variation observed (126).
Fig. 2.
A: hemoglobin levels in Ethiopians and Tibetans are similar to those of US sea-level residents and below those seen Andean males or females at the altitudes shown[Reproduced from Beall et al. (10) with permission from the National Academy of Sciences. Copyright © 2002 National Academy of Sciences.]. Values among the high-altitude groups are, however, more similar if comparisons are made with acclimatized newcomers (see Table 2). B: Tibetans and Andeans have approximately half the altitude-associated reduction in infant birth weight compared with Europeans or Han Chinese [see text for details and (110) for original references]. C: prevalence of chronic mountain sickness (CMS) is markedly less in Tibetans than similarly aged men from various ancestry groups residing at the altitudes shown. Original references may be found in 110. [Adapted from Niermeyer et al. (110) with permission from SAGE Publications.]
Arterial O2 content () can be measured directly but usually is calculated as the product of hemoglobin (× 1.36, the ml of O2 bound per g) and , plus dissolved O2 (i.e., 0.0031 × ). Acclimatized newcomers reestablish sea-level values (~21 vol%) due to increased ventilation and hemoglobin. Andean is similar as the result of their narrower alveolar-arterial O2 gradient, maintained , and higher hemoglobin. Tibetan values are also ~21 vol%, and Ethiopian values being somewhat lower (Table 1).
O2 distribution (blood flow).
Cardiac output is similar for a given workload, but lower at maximal exercise at high than low altitude in acclimatized newcomers and highland Sherpa (139). Unclear is whether the lower maximum cardiac output is due to decreased left ventricular filling and/or function, increased right ventricular afterload, altered cardiac metabolism, or being suppressed by central nervous system factors (56, 139). Total blood volume is similar in low- and high-altitude residents (128), suggesting that decreased filling is unlikely, but slower left ventricular untwisting has been reported in Sherpa, suggesting that function may be impaired (139). Afterload is largely determined by pulmonary arterial (right heart) and systemic (left heart) pressure, which are modestly increased in most groups or decreased, respectively, at high altitude (139). Tibetans have less pulmonary vasoconstrictor response to hypoxia than Andeans, Ethiopians, or Coloradans (52, 54, 117, 159). However, the altitude-associated reduction in V̇o2max differs little between Andeans and Tibetans, suggesting that greater right ventricular afterload is not responsible for lowering cardiac output. The Tibetans’ lower pulmonary arterial pressures have been suggested to result from greater nitric oxide (NO) transfer from the airway wall to the pulmonary vascular lumen, but measured NO values were only weakly inversely correlated to estimated pulmonary arterial pressures (58), and studies have not been conducted in acclimatized newcomers or other high-altitude residents, suggesting that altitude not Tibetan ancestry was responsible. Pulmonary arterial pressure is not only due to hypoxic vasoconstriction but also pulmonary vascular remodeling (140), which has yet to be explored in high-altitude populations.
Alterations in regional blood flow greatly affect O2 distribution. Regional blood flows have been measured in the periphery (arm or leg), brain, and uterine circulations. The peripheral circulation is highly responsive to sympathetic nervous system stimulation, which is markedly increased in acclimatized lowlanders (37, 145). Compared with acclimatized newcomers, Tibetans exhibit parasympathetic dominance during exercise (178), which is retained after years at sea level (175). Sherpa also have greater leg vascular reserve (130), greater forearm flow-mediated vasodilation, and a trend toward greater brachial artery diameter than acclimatized lowlanders in one (21) but not another study (90). Of note, the Lewis study (90) found not only impaired flow, presumably NO- mediated, but also NO-independent vasodilation in Sherpa and acclimatized lowlanders relative to low-altitude values. Circulating NO metabolite levels were also similar between Sherpa and acclimatized lowlanders in the Horscroft report (60). During exercise, Andeans have lower leg blood flow and hence fractional leg O2 extraction than acclimatized lowlanders, due to blood being diverted to other tissues (94). Higher urinary nitrate, and lower cGMP and diastolic blood pressure levels in the longer-resident Amhara than the shorter-resident Oromo groups in Ethiopia were interpreted as indicating greater peripheral vasodilation, but no direct measures were obtained (31).
Brain blood flow has been measured as velocity through its major supply vessels, the internal carotid, middle cerebral, and vertebral arteries. A review of 10 studies indicated that Tibetans had ~20% higher resting middle cerebral flow velocities than Andeans, with Andean levels being lower than at low altitude but O2 delivery unchanged due to the Andeans’ higher hemoglobin (57, 70) (Table 1). Little difference was observed in middle cerebral artery blood flow velocity in Ethiopians at high vs. low altitude, but no comparisons with acclimatized newcomers were performed (3). Supporting higher Tibetan brain blood flows, Tibetans or Sherpa have greater common carotid artery diameters (90), internal carotid artery flow velocity during and up to maximal exercise (62), and microvascular flow in small (<25 μm) sublingual vessels (48) than acclimatized newcomers, although similar resting values for middle and posterior cerebral artery velocities have also been observed (135). Tibetans better preserved posterior (vertebral artery) relative to anterior (carotid artery) blood flow compared with low-altitude residents after prolonged residence at sea level (91). Suggesting possible functional sequelae, Han born and raised at high altitude but studied after years of residence at low altitude had slower psychomotor reaction times and decreased accuracy compared with sea-level Han (164) although no such studies were performed in lifelong high- vs. low-altitude residents.
Cerebral blood vessels are highly responsive to changes O2 or CO2 tensions, and bioactive molecules such as NO. At 5,300 m, Sherpa have better autoregulation of cerebral blood flow and fewer psychoneurological symptoms than acclimatized lowlanders (48). Andeans at high vs. low altitude had less middle cerebral artery vasodilator response to hypoxia or NO, and less vasoconstrictor response to hypocapnia (112), but unclear is how such responses compared with those of acclimatized newcomers. Ethiopians also had greater middle cerebral vasodilator response to a NO donor at high compared with low altitude (3), which the authors interpreted as indicating that the Ethiopians were better adapted than Andeans. The role of cerebral blood flow in protecting Ethiopians is complicated, however, since different NO donors were used in the Andean and Ethiopian studies, no functional measures were employed, and study conditions varied such that high-altitude Peruvians were compared with British sea-level residents and low-altitude Ethiopian values were acquired after ~10 min of high-O2 breathing (32).
During pregnancy, Tibetans and Andeans compared with acclimatized lowlanders demonstrate greater pelvic blood flow redistribution to favor the uteroplacental circulation, and higher uterine artery volumetric flow and O2 delivery as a result (30, 80, 108, 156) (Table 1). Andean protection was accompanied by greater pregnancy-associated increases in antioxidant levels and a more favorable balance of angiogenic to anti-angiogenic substances (35, 78), but was not due to developmental factors since Andeans also had higher uterine artery blood flow than Europeans born and raised at high altitude (76). Pregnant Andeans also have lower cortisol levels than acclimatized newcomers at high altitude (29), perhaps reflecting decreased sympathetic stimulation. Greater vascularity in placental tissues (144) as well as in the newborn skin microcirculation (46) has been noted, both of which would also serve to increase blood flow.
O2 utilization (metabolism).
O2 delivered to the tissue mitochondria fuels metabolism and the production of chemical energy or ATP (Fig. 1). However, ATP can be produced from alternate fuel sources and pathways, with some being more efficient than others. Specifically, utilization of carbohydrates (glucose, glycogen), rather than free fatty acids or lipids, can generate 25–50% more ATP per mole of O2 consumed (59). A switch to carbohydrates occurs after 3 wk of altitude acclimatization in males (19) but, interestingly, not females (18). Few studies have been performed in long-term high-altitude residents but those conducted also suggest a shift in fuel preference to carbohydrates. Using positron-emission tomography (PET) to measure heart metabolism, Hochachka and coworkers (59) found that Quechua and Sherpa males after descent to sea level had a greater reliance on carbohydrate metabolism and a 50–60% gain in the production of ATP per mole of O2 consumption relative to lowlanders (Table 1). 31P magnetic resonance spectroscopy studies in these same Sherpa upon arrival and after 4 wk at low altitude also showed a preference for carbohydrate that was maintained during acute hypoxia and moderate exercise in heart as well as chest wall skeletal muscle. The authors concluded that an increase in O2 efficiency represented a biochemical adaptation for defending against hypoxia in the hearts of these two high-altitude groups (56). A recent study by Horscroft and coworkers (60) has confirmed greater efficiency of O2 utilization, along with a lower capacity for fatty acid oxidation in skeletal muscle biopsies, and increased production of antioxidants in direct measurements of Sherpa vs. acclimatized newcomer skeletal muscle (Table 1). Glucose homeostasis is also altered at high altitude. Glucose uptake and consequently lower venous glucose levels have been observed in Andean, Tibetan, and acclimatized-newcomer men (60, 157) as well as pregnant Andeans (86). This was interpreted as reflecting greater placenta uptake to spare O2 for fetal consumption (172) although other tissues may also be involved.
Evidence of Adaptation: Genome Scans
The differences in O2 transport characteristics between high-altitude natives and newcomers that are not due to developmental factors, and the variation among long-resident groups provide indirect support for genetic adaptation to high altitude. Direct evidence comes from SNP-based genome scans and whole genome sequencing studies that have identified gene regions acted upon by natural selection (Table 2).
Table 2.
Autosomal gene regions acted upon by natural selection in high-altitude populations as identified in studies published from 2004 to 2017
| Andeans | Tibetans | Ethiopians |
|---|---|---|
| BRINP3 (34) | ANGPT1 (151) | ADRBK1 (1) |
| CBS (40) | ||
| EDNRA (14, 16) | CDH13 (151) | ARNT2 (9, 129) |
| EGLN1 (14, 16) | ECE1 (151) | ASF1B (1) |
| ELTD1 (40) | ||
| ET-1 (106) | EGLN1 (14, 61, 116, 134, 151, 163, 167) | BHLHE41 (64) |
| FAM213A (147) | EPAS1 (9, 14, 61, 116, 151, 163, 167) | CBARA1 (129) |
| NOS2 (14, 16, 34) | FOXO1 (151) | CUL3 (1) |
| PRKAA1 (14, 16) | HMOX2 (165) | CORO1B (1) |
| SFTPD (147) | KCTD12 (61) | HLA-DRA (1) |
| TP53 pathway (69) | LEPR (151) | MAPKAPK2 (1) |
| VEGFB (40) | ||
| TBX5 (34) | PPARA (134) | Multiple (CIC, LIPE, PAFAH1B3) (146) |
| PTGIS (61) | THRB (129) | |
| RUNX1 (151) | VAV3 (129) | |
| RYR1 (151) | ||
| TED (93) | ||
| VDR (61) |
Numbers in parentheses are reference citations. ADRBK1, adrenergic beta receptor kinase 1; ANGPT1, angiopoietin 1; ARNT2, aryl hydrocarbon receptor nuclear translocator 2; ASF1B, anti-silencing function 1B histone chaperone; BHLHE41, basic helix-loop-helix family member e41; BRINP3, BMP/retinoic acid inducible neural specific 3; CBARA1, mitochondrial calcium uptake 1; CBS, cystathionine beta-synthase; CDH13, cadherin 13; CIC, capicua transcriptional repressor; CUL3, cullin 3; CORO1B, coronin actin binding protein 1B; ECE1, endothelin converting enzyme 1; EDNRA, endothelin receptor type A; EGLN1, egl-9 family hypoxia-inducible factor 1; ELTD1, latrophilin and seven transmembrane domain-containing protein 1; EPAS1, endothelial PAS domain protein 1; ET-1, endothelin 1; FAM213A, family with sequence similarity 213 member A; FOXO1, forkhead box O1; HLA-DRA, major histocompatibility complex, class II, DR alpha; KCTD12, K+ channel tetramerization domain containing 12; LEPR, leptin receptor; LIPE, lipase E hormone sensitive type; MAPKAPK2, mitogen-activated protein kinase-activated protein kinase 2; NOS2, nitric oxide synthase 2; PAFAH1B3, platelet activating factor acetylhydrolase 1b catalytic subunit 3; PPARA, peroxisome proliferator-activated receptor alpha; PRKAA1, protein kinase AMP-activated, alpha 1 catalytic subunit; PTGIS, prostaglandin I2 synthase; RUNX1, runt related transcription factor 1; RYR1, ryanodine receptor 1; SFTPD, surfactant protein D; SNP, single-nucleotide polymorphism; TBX5, T-box 5; TED, Tibetan enriched deletion; THRB, thyroid hormone receptor beta; TP53, tumor protein p53; VAV3, vav guanine nucleotide exchange factor 3; VDR, vitamin D3 receptor; VEGFB, vascular endothelial growth factor B.
Particular attention has been devoted to those genes regulating or regulated by the hypoxia-inducible factor (HIF) pathway. As well reviewed elsewhere (132, 152), HIF is a dimer made up of one of three alpha chains (HIF-1alpha, -2alpha, or -3alpha) whose expression varies by tissue, and the constitutively expressed beta (ARNT) chain. At low altitudes, HIF is instantly degraded by a process requiring oxygen. Under conditions of hypoxia or in persons with gene-variants preventing HIF degradation (2) such degradation does not occur, resulting HIF being bound to genes containing a hypoxia response element (HRE) region and initiating gene transcription. More than 100 genes with HREs have been identified (e.g., erythropoietin, vascular growth factor), making the HIF pathway of interest in the study of genetic adaptation to high altitude. However, it is important to note that existing SNP data do not indicate that the HIF-pathway has been disproportionately acted upon by natural selection (16), and that not all oxygen-sensitive genes contain HREs. Hence HIF is not the only oxygen regulator.
Tibetans.
Tibetans have been the focus of the largest number of studies (Table 2). Several genome scans have identified gene regions in or near EPAS1, which controls the expression of HIF-2alpha, and EGLN1, which regulates the production of PHD2 that plays a key role in degrading HIF (Table 2). The identification of EPAS1 is reinforced by exome and whole genome sequencing studies (61, 167), with the Tibetan haplotype likely to have been derived from archaic Denisovan or Denisovan-related hominins (65). Other gene regions have been identified including several involved in vasoregulation (EDNRA), vascular development (ANGPT1, ECE1), heme metabolism (HMOX2), whole body metabolism (PPARA), and other biological processes.
The selected-for EPAS1 haplotype is associated with lower hemoglobin levels at high as well as at low altitudes (9, 93, 118, 134) and, together with the EGLN1 variants have an additional effect on lowering hemoglobin but even so, explain a small portion of the variance (143). The only coding-region variants thus far identified are two, closely linked missense mutations in exon 1 of EGLN1 [12C>G; 380G>C] that were calculated to have originated 8,000 years ago (92). Their functional effects are, however, unclear as they have been reported both to enhance the catalytic activity of PHD2 under hypoxic conditions, thus likely decreasing HIF activity, as well as to impair PHD2 function, which would be expected to potentiate HIF (92, 136). Suggesting that the Tibetan-selected EPAS1 variants acted at the transcriptional level and the EGLN1 variants at the posttranslational level to increase HIF-2a degradation was a recent study showing that the EPAS1 SNPs had weaker binding affinity to transcription factors, and lowered EPAS1 gene expression in umbilical artery endothelial cells (115). The selected-for gene regions may also be acting via other mechanisms as suggested by a recent Tibetan study that found as much as a 2 g lower hemoglobin associated with HMOX2 tag SNPs in males (but not females) that, when transfected into HeLa and HEK293T cell lines, increased HMOX2 transcriptional activity (165).
EPAS variants have been associated with lower pulmonary arterial pressures in Tibetans, although they only explain 0.4% of the pulmonary arterial pressure variance (115). A role for increased NO production in mediating pulmonary vasodilation was not supported given that the Tibetan variants upregulated iNOS but downregulated eNOS and did not influence NO metabolite levels. Rather, knockout mouse studies in EPAS1 heterozygotes (homozygotes being lethal) suggested that the lower pulmonary arterial pressures were not due to greater NO production but rather downregulation of ACE and VEGFC.
Several studies have also shown functional associations with PPARA variants. Ge and coworkers (47) showed that serum lactate and free fatty acids rose with increasing number of the naturally selected PPARA as well as EPAS1 alleles at 4,500 m, suggesting that selection may be acting to increase anaerobic glucose and decrease fatty acid metabolism. The greater efficiency of O2 utilization and increased production of antioxidants seen by Horscroft and coworkers (60) in Sherpa skeletal muscle was related to the presence of selected-for PPARA alleles. Of interest too is that fatty acid oxidation was identified as a pathway undergoing convergent evolution in Andeans and Himalayans (42).
Mitochondrial DNA variants have also been identified in Tibetans; specifically, the frequency of the M9 haplotype increases with altitude and, when the 3394C variant is present on the M9 background, raises complex I activity (53, 72). This haplotype contains ATP6, ATP8, Cyt b, ND2, COX2, tRNA, and 12 S rRNA genes, suggesting that mitochondrial as well as nuclear DNA may be involved in Tibetan genetic adaptation.
Andeans.
Andeans have received comparatively less attention, although the first genome scan was done in Bolivia (106). Only one region has been identified to date as common to both Andeans and Tibetans, EGLN1, although different regions are implicated (16). Other gene regions have been identified, including ones involved in vasoregulation (PRKAA1, NOS2), vascular growth (VEGFB, ELTD1), cerebral blood flow (CBS), and oxidative defense (FAM213A) (16, 40, 147) (Table 2). Of note, a recent whole genome sequencing study identified three gene regions—BRINP3, NOS2, and TBX5—only one of which (NOS2) had been identified in a SNP scan (16), and which are associated with cardiovascular development and function but not hypoxia-sensing (34).
Ethiopians.
The few studies of Ethiopians have generated a range of results (Table 2). One found the strongest signals in regions involved in pathogen defense, cell cycle control, DNA damage and repair (1). A range of pathways was implicated in another report, including those involved in central and peripheral nervous system function, cadherin and Wnt signaling, and found only marginal associations between hemoglobin and two HIF-1 pathway genes, THRB and ARNT2 (129). A third study found the strongest signal in BHLHE41, which has major roles in the HIF and circadian rhythm pathways (64).
Functional Measures of Adaptation
The studies reviewed above indicate that genetic factors distinguish the O2 transport characteristics of native highlanders from those of acclimatized newcomers, and that natural selection has operated on highland genomes. The question thus becomes what are the physiological traits on which natural selection has acted or, phrased differently, what are the physiological effects of the naturally selected genes? Since natural selection is driven by traits which confer reproductive success, functional measures of adaptation should be ones that influence the ability to live and reproduce in the high-altitude environment.
Suggesting places to look are the several adaptive challenges posed by high altitudes (Fig. 3). The largest number cluster in the perinatal period, which is that during which there is the greatest mortality before the end of the reproductive period. Others are the slowing of adolescent growth, as it might delay the age of menarche and shorten the reproductive period (44). A third is the reduction in V̇o2max, which is a measure of the integrated functioning of the O2 transport system (27). A fourth is Chronic Mountain Sickness (CMS), providing a clear instance of maladaptation (97). Likely less relevant are lung and heart diseases whose onset usually occurs after the completion of the reproductive period (13, 109).
Fig. 3.
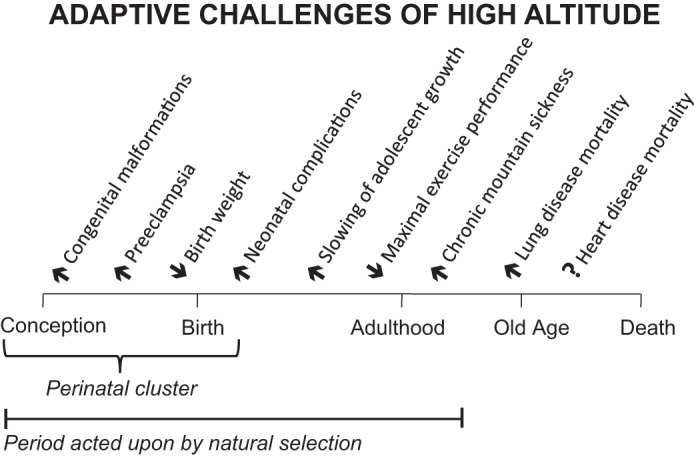
Adaptive challenges or those affecting reproductive success occur at high altitudes at multiple times across the lifespan. About half cluster during the perinatal period or that from gestation through the first week of postnatal life, with the remainder occurring during adolescence or adulthood (see text for references). There is increased mortality from chronic obstructive lung disease (105), but whether mortality from heart disease is affected as well is uncertain (13, 109).
Perinatal complications.
An altitude-associated reduction in birth weight, due to slowed fetal growth, was first described in Colorado 60 years ago and has since been seen in all highland groups (for review see 100, 110). However, Andeans and Tibetans demonstrate approximately half as much reduction in birth weight as acclimatized newcomers (Fig. 2B). The Andeans’ protection is related to the amount of Native American, specifically Andean, ancestry (12, 77, 138). Whether Ethiopian highlanders are also protected is as yet unknown.
One factor contributing to the Andeans’ and Tibetans’ protection is their greater pregnancy rise in uterine artery diameter (30, 80, 107, 156, 171), and resultant greater blood flow and uteroplacental O2 delivery (30, 80, 107, 156). Increased blood flow, not , is responsible for raising uteroplacental O2 delivery, since even though maternal ventilation is positively related to birth weight in each population, hemoglobin concentrations fell during pregnancy and was the same in natives and newcomers (80, 107). Not only maternal but also placental factors may also be involved as Tibetan (but not Andean) placentas weigh more, both absolutely and relative to fetal weight, than those of acclimatized newcomers (68, 173). Indicating functional consequences, low uterine artery blood flows are associated with fetal demise and decreased infant birth weights (20, 74, 80, 170).
Another factor lowering birth weights at high altitude is an increased incidence of preeclampsia. Preeclampsia is not only a major cause of maternal mortality (124); it also markedly raises both maternal and offspring mortality later in life (11). The increased incidence of preeclampsia at high altitude appears to occur both for acclimatized newcomers and Andean or Tibetan natives (81, 96, 113, 173), but the use of different diagnostic criteria prevents comparisons among regions. Suggesting that native women may be relatively protected is the lower frequency of hypertension during pregnancy reported for Tibetan than Han women, although it was unclear whether the hypertension was preexisting or developed during pregnancy (166). Pregnant Andeans compared with Europeans have lower sFlt-1 levels and sFlt-1/PLGF ratios (35), both of which are protective against preeclampsia (28). Andeans may also benefit from other factors, namely, higher levels of antioxidants (78), progesterone, estrone, 17-beta estradiol, and estriol (29). Cortisol levels are lower as well, with lower cortisol and higher estriol being associated with greater uterine artery diameter and blood flows (29).
To determine if genes were involved in the Andeans’ protection from altitude-associated fetal growth restriction, we examined the relationships between infant birth weight and 63 naturally selected SNPs in 16 gene regions (15). Several SNPs for each of two genes—PRKAA1 (which codes for the alpha-1 catalytic subunit of adenosine monophosphate kinase, AMPK) and EDNRA (that controls the expression of the vascular smooth muscle cell endothelin receptor A)—were related to heavier birth weights, but only those for PRKAA1 were related both to heavier birth weights and larger uterine artery diameters at high altitude. Gene expression patterns in peripheral blood mononuclear cells showed that the selected-for maternal PRKAA1 SNP genotype disproportionately affected the expression of genes in the mTOR pathway, which is of interest given that this pathway plays a crucial role mediating the effects of hypoxia, nutrient restriction, and other factors on fetal growth (125, 168, 169).
Mitochondrial genes have also been implicated in Tibetan protection, with mitochondrial respiratory state III oxidative activity, respiratory control ratios, oxidative phosphorylation, and O2 utilization being greater in the Tibetan compared with Han placentas (173). Although protective effects of Andean or Tibetan ancestry could involve maternal, fetal, and/or paternal genomes, even though the EPAS1 adaptive haplotype downregulated EPAS1 expression in Tibetan umbilical cord endothelial cells (115) or lymphocytes (118), EPAS1 expression was similar in Tibetan vs. Han placentas, suggesting that maternal (or paternal) factors were more important in altitude-associated protection from reductions in fetal growth.
Likely related to their heavier (more normal) birth weights, neonatal outcomes are also better in native than newcomer groups. Under comparable health-care conditions, Tibetans have less pre- and postnatal reproductive loss (107) and requirements for supplemental O2 than Han (166). The altitude-associated rise in perinatal mortality is reduced in the regions of Peru where populations have lived the longest (50). Neonatal oxygenation is markedly better in Tibetan than Han infants during wakefulness, feeding, active, or quiet sleep over the first 4 mo of life (111, 158). The fraction or partial pressure of exhaled NO did not differ between the two groups, suggesting that ventilatory rather than vascular factors were involved (158). Maturation of ventilatory control is especially important during infancy and childhood; nocturnal values in mixed Native American and European high-altitude residents of Bolivia showed that infants spent ~80%, children 34%, and adolescents 8% of the night with values below 90% (55). Unlike Tibetans, 1 wk to 4–6 mo Andeans do not appear to have higher values than Europeans, but existing data are limited by the small size and considerable admixture in the European sample. Across populations, Tibetan infants have higher than Andeans in all activities (110).
Growth, especially lung.
As noted above, developmental as well as genetic factors contribute to the larger lung volumes and smaller alveolar-arterial O2 gradients of native highlanders. Accelerated chest growth is associated with reduced upper and lower limb growth, resulting in larger lung volumes relative to body size (119). Slower Andean growth during mid to late adolescence (43) is associated with a later age of menarche (51), but there is no apparent reduction in Andean fertility at high altitudes (149).
Exercise performance: V̇o2max.
There is a clear fall in maximal exercise performance or V̇o2max in acclimatized newcomers, amounting to a ~25% reduction at 3,000–4,000 m (45). Independent of training effects, Tibetans and Andeans have less altitude-related fall than newcomers (22, 25, 84, 159, 175), but V̇o2max has not been measured in Ethiopians. Higher peak V̇o2 was related to lower hemoglobin levels in Tibetans (and Han), suggesting that one factor benefiting Tibetans may be a lower blood viscosity and improved regional blood flow (133). Other factors could also be involved including a metabolic shift to carbohydrates, higher maximum heart rate, smaller alveolar-arterial O2 gradients, greater tissue O2 extraction, and less sympathetic or greater parasympathetic stimulation (22, 159).
Although V̇o2max is a measure of the integrated functioning of the O2 transport system and hence has often been used as a measure of adaptation (e.g., 8, 154), there is no association to the best of our knowledge between V̇o2max and reproductive success. In fact, although exercise is strongly associated with a reduction in cardiovascular mortality, the greatest benefit is derived from low- rather than high-intensity exercise (85).
CMS.
CMS is a maladaptive syndrome present at high altitudes, defined as an elevated hemoglobin (>3 SD above the mean or ≥19 g/dl in females and ≥21 g/dl in males) in persons without other underlying conditions (e.g., chronic obstructive lung disease) and presenting with accompanying symptoms (e.g., headache, dizziness, breathlessness, fatigue, localized cyanosis, burning in the palms and soles, venous dilatation, muscle or joint pain, loss of appetite, lack of mental concentration and memory) (88). It has long been recognized at high altitudes (97) and frequently terminates in pulmonary hypertension and right or left heart failure. Hence factors that protect against it may be viewed as adaptive. Although deaths from pulmonary hypertension and heart failure occur typically after the completion of the reproductive period, anecdotal reports suggest that CMS affects fitness during adulthood as affected persons are no longer able to engage in normal, daily activities. However, to our knowledge, no actual fitness costs of CMS have been calculated.
Its prevalence varies by region (Fig. 2C), occurring in ~10% of Andean adult males over the age of 30 or postmenopausal females, a similar frequency of high-altitude Coloradans, ~6% of Han migrants, but only ~1% of Tibetans (89, 102, 161). To date, it has not been reported in Ethiopians (10, 32) but no detailed epidemiological study has been conducted. It begins in males in their early 20s as preclinical CMS or excessive erythrocytosis (> 2 SD above the mean or 18.3 g/dl in males with 3 or more of the CMS symptoms listed above) (79), with polycythemia, symptoms, and day- and night-time hypoxemia worsening with advancing age (4, 75, 103). Persons with clinical or preclinical CMS hypoventilate and have a blunted hypoxic ventilatory response compared with acclimatized newcomers, but these characteristics are also present in healthy Andeans, indicating that hypoventilation may be a necessary but not sufficient contributor. Sleep-disordered breathing is a prominent feature of the disease (75, 79, 87, 122, 141), and likely key to its development (121). Sleep not only affects breathing but also cerebral vascular responses (95). Unlike healthy Han residents of 3,600 m in whom the hypoxia and hypercapnia caused by sleep-disordered breathing raised internal carotid artery blood flow velocity, velocity fell during or immediately after an apneic or hypopneic episode in Han with CMS (141). An impaired vasodilator response may influence susceptibility to CMS more broadly. Andean men with CMS compared with healthy Andeans have a blunted middle cerebral artery vasodilator response to NO (3), greater carotid artery intimal thickness, and impaired flow-mediated brachial artery vasodilation (123). Another contributor may be early-life hypoxic exposures. Exaggerated neonatal hypoxia predisposes persons to develop pulmonary hypertension during acute-altitude exposure (127). CMS patients are more likely than healthy controls to have been small-for-gestational age (103), born to a preeclamptic mother, or to have experienced exaggerated neonatal hypoxia (75), suggesting that hypoxic or oxidative injury may disrupt pulmonary or cerebral vascular development in ways that predispose individuals to develop CMS later in life.
The role of genetic factors in CMS has received attention. As noted above, variants in or near EGLN1 and EPAS1 are related to hemoglobin levels in Tibetans but not in Andeans (17), suggesting that they may contribute to Tibetan protection but are not likely to explain Andean susceptibility. Among 19 genes, expression levels of PDP2 (which promotes entry of pyruvate into the Krebs cycle) were decreased in Tibetans with vs. without CMS in Ladakh (162). Unclear, however, was the basis for selecting these genes, whether other causes of hypoxemia were excluded, and whether the gene-expression comparisons were controlled for multiple testing. Whole genome sequencing studies in 10 men with vs. without CMS identified 11 gene regions that differed (174). Hypoxia upregulated the transcriptional responses for SENP1 and ANP32D (which play roles in regulating erythropoiesis and cellular metabolism, respectively) in fibroblasts from the CMS patients but not controls. Further, knocking out these two gene regions in Drosophila melanogaster markedly improved survival under conditions of severe hypoxia. Cole (33) replicated this study in a larger sample of CMS and control residents of 4,338 m in Peru and found an association between SENP1, but not ANP32D, and CMS. Another gene, SENP1, that codes for a protease that rescues HIF1alpha from degradation, has been suggested to play a role in reducing erythropoietin receptor levels in CMS and thereby increase susceptibility to the disease (148).
Summary, Conclusions, and Recommendations for Future Study
In terms of O2 transport characteristics, Tibetans, Andeans, and Ethiopians (if confirmed by additional study) differ with respect to the strategies employed for maintaining , but rely similarly on alterations in blood flow distribution for increasing O2 delivery and on metabolism for improving the efficiency of O2 utilization. With respect to , the Andeans’ narrower alveolar-arterial O2 gradient (but not higher ventilation) maintains close to acclimatized-newcomer levels and, together with an elevated hemoglobin, yield values similar to those of acclimatized newcomers or sea-level residents. Tibetan values are below Andeans’, despite their higher alveolar ventilation, but average is similar due to variation among studies in hemoglobin levels (8, 133, 160, 176). Ethiopian may be slightly lower but additional study is required given variation in the and hemoglobin values reported. Although brain blood flow is higher in Tibetans than Andeans, O2 delivery is similar due to the Andeans’ higher hemoglobin (70). Blood flow to the uterine circulation is greater during pregnancy in both Tibetans and Andeans compared with acclimatized newcomers, primarily as the result of larger uterine artery diameters and, in the Tibetan case, velocity. The factors responsible for these flow differences have been little explored but reduced sympathetic stimulation, lower oxidative stress, and greater vasodilator responses and/or vascularity may be involved. Simple mathematics reinforces the importance of blood flow in determining O2 delivery. Using near-term Andean values (80), a 50% reduction in O2 delivery would occur if flow fell from 123 to 61.5 ml/min, from 94% to 44%, or hemoglobin from 13.2 to 6.1 g/dl. Although any such reductions would be difficult, those for or hemoglobin are likely unsustainable. Therefore, blood-flow variation within the physiological range has a greater impact on O2 delivery than does or hemoglobin. As yet undefined are the physiological mechanisms regulating blood flow in high-altitude populations and the genetic factors involved. A role for greater vasodilator responses to NO has been suggested, but is not supported by recent data (60). Needed are well-controlled comparisons of vascular function, including measurements of vessel diameter, elastic properties, and hyperemic, endothelial-independent and -independent vasodilation as each can occur independently (21, 57, 67). In addition, additional studies are required to build on those conducted more than 20 years ago regarding O2 metabolism, and to extend recent findings in Tibetans (60) to Andean and Ethiopian populations.
Genome scans have made valuable contributions in recent years by pointing to the gene regions operated upon by natural selection. While studies are still accumulating, it is important to emphasize that our knowledge of the actual genes and the physiological mechanisms by which they are operating is still limited (99). Most studies have been based on SNPs, which only query ~20% of the human genome, the exact figure depending on the amount of linkage disequilibrium. Moreover, SNPs test for known polymorphisms, which themselves suffer from ascertainment bias since most were discovered in Europeans. Variation among the populations in the altitudes at which they reside and variation among individuals are likely to have affected study results. To date, only one whole genome (exon and intron) sequencing study has been performed in Tibetans (61) and one in Andeans (34); the results of the former appear to confirm those obtained using SNPs but this is less true for the latter.
As additional whole genome sequencing studies are performed (and replicated), our knowledge of the specific gene regions involved will increase. Especially important will be careful physiological studies that fully phenotype the genotypes identified. Further, as argued above, since natural selection can only act on phenotypes affecting reproductive success (fitness), it is urged that investigators measure phenotypes likely to impact fitness, and calculate the actual fitness costs for the phenotypes being studied. Finally, as in most physiological studies, subjects have usually been males and therefore greater inclusion of females is needed, not only to better represent the human condition but also for the additional variation provided and information to be derived regarding responsible mechanisms. Central to such efforts will be carefully designed studies that use multiple approaches for identifying the causal mechanisms, positive as well as negative controls, and replication or other means for validation. Although the conduct of such studies is challenging, the well-characterized nature of the O2 transport system, the presence of multiple long-resident populations, and relevance for understanding hypoxic disorders in all persons underscore the importance of understanding how evolutionary adaptation to high altitude has occurred.
GRANTS
Current grant support from the National Institutes of Health (NIH) (HD-088590) and prior grants from the NIH, American Heart Association, National Science Federation, and U.S. Department of Defense supporting our studies are gratefully acknowledged.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
L.G.M. analyzed data, prepared figures, drafted manuscript, edited and revised manuscript, and approved final version of manuscript.
ACKNOWLEDGMENTS
I thank the many students and colleagues who contributed to this work over many years.
Footnotes
Population estimates need to be updated as the 140 million figure was derived from nearly 30-yr-old census data (104), particularly as smaller population estimates have also been reported (7).
This would change if, as suggested, there was widespread use “oxygen conditioning” or oxygen supplementation to homes, schools, and hospitals (154). Such practices, at least in the short term, may be impractical due to the cost and infrastructure limitations of the developing world where most high-altitude populations reside. Caution should also be exerted given the harmful effects of oxidant injury, especially in children, that could result from O2 fluctuations upon leaving or reentering the oxygen-enriched environment (6).
REFERENCES
- 1.Alkorta-Aranburu G, Beall CM, Witonsky DB, Gebremedhin A, Pritchard JK, Di Rienzo A. The genetic architecture of adaptations to high altitude in Ethiopia. PLoS Genet 8: e1003110, 2012. doi: 10.1371/journal.pgen.1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang SO, Chen H, Gordeuk VR, Sergueeva AI, Polyakova LA, Miasnikova GY, Kralovics R, Stockton DW, Prchal JT. Endemic polycythemia in Russia: mutation in the VHL gene. Blood Cells Mol Dis 28: 57–62, 2002. doi: 10.1006/bcmd.2002.0488. [DOI] [PubMed] [Google Scholar]
- 3.Appenzeller O, Claydon VE, Gulli G, Qualls C, Slessarev M, Zenebe G, Gebremedhin A, Hainsworth R. Cerebral vasodilatation to exogenous NO is a measure of fitness for life at altitude. Stroke 37: 1754–1758, 2006. doi: 10.1161/01.STR.0000226973.97858.0b. [DOI] [PubMed] [Google Scholar]
- 4.Bailey DM, Rimoldi SF, Rexhaj E, Pratali L, Salinas Salmòn C, Villena M, McEneny J, Young IS, Nicod P, Allemann Y, Scherrer U, Sartori C. Oxidative-nitrosative stress and systemic vascular function in highlanders with and without exaggerated hypoxemia. Chest 143: 444–451, 2013. doi: 10.1378/chest.12-0728. [DOI] [PubMed] [Google Scholar]
- 5.Ballew C, Garruto RM, Haas J. High altitude hematology: paradigm or enigma. In: Human Population Biology, edited by Little MA, Haas JD. Oxford, UK: Oxford Univ. Press, 1989, p. 239–262. [Google Scholar]
- 6.Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, Schonwald A, Wilker RE, Stehle S, Kinane TB. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics 114: 805–816, 2004. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 7.Beall CM. Adaptation to high altitude: phenotypes and genotypes. Annu Rev Anthropol 43: 251–272, 2014. doi: 10.1146/annurev-anthro-102313-030000. [DOI] [Google Scholar]
- 8.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA 104, Suppl 1: 8655–8660, 2007. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107: 11459–11464, 2010. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci USA 99: 17215–17218, 2002. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335: 974, 2007. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett A, Sain SR, Vargas E, Moore LG. Evidence that parent-of-origin affects birth-weight reductions at high altitude. Am J Hum Biol 20: 592–597, 2008. doi: 10.1002/ajhb.20784. [DOI] [PubMed] [Google Scholar]
- 13.Bernabé-Ortiz A, Carrillo-Larco RM, Gilman RH, Checkley W, Smeeth L, Miranda JJ; CRONICAS Cohort Study Group . Impact of urbanisation and altitude on the incidence of, and risk factors for, hypertension. Heart 103: 827–833, 2017. doi: 10.1136/heartjnl-2016-310347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigham A, Bauchet M, Pinto D, Mao X, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, López Herráez D, Brutsaert T, Parra EJ, Moore LG, Shriver MD. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet 6: e1001116, 2010. doi: 10.1371/journal.pgen.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigham AW, Julian CG, Wilson MJ, Vargas E, Browne VA, Shriver MD, Moore LG. Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol Genomics 46: 687–697, 2014. doi: 10.1152/physiolgenomics.00063.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigham AW, Mao X, Mei R, Brutsaert T, Wilson MJ, Julian CG, Parra EJ, Akey JM, Moore LG, Shriver MD. Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Hum Genomics 4: 79–90, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigham AW, Wilson MJ, Julian CG, Kiyamu M, Vargas E, Leon-Velarde F, Rivera-Chira M, Rodriquez C, Browne VA, Parra E, Brutsaert TD, Moore LG, Shriver MD. Andean and Tibetan patterns of adaptation to high altitude. Am J Hum Biol 25: 190–197, 2013. doi: 10.1002/ajhb.22358. [DOI] [PubMed] [Google Scholar]
- 18.Braun B, Mawson JT, Muza SR, Dominick SB, Brooks GA, Horning MA, Rock PB, Moore LG, Mazzeo RS, Ezeji-Okoye SC, Butterfield GE. Women at altitude: carbohydrate utilization during exercise at 4,300 m. J Appl Physiol (1985) 88: 246–256, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT. Increased dependence on blood glucose after acclimatization to 4,300 m. J Appl Physiol (1985) 70: 919–927, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Browne VA, Toledo-Jaldin L, Davila RD, Lopez LP, Yamashiro H, Cioffi-Ragan D, Julian CG, Wilson MJ, Bigham AW, Shriver MD, Honigman B, Vargas E, Roach R, Moore LG. High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am J Physiol Regul Integr Comp Physiol 300: R1221–R1229, 2011. doi: 10.1152/ajpregu.91046.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruno RM, Cogo A, Ghiadoni L, Duo E, Pomidori L, Sharma R, Thapa GB, Basnyat B, Bartesaghi M, Picano E, Sicari R, Taddei S, Pratali L. Cardiovascular function in healthy Himalayan high-altitude dwellers. Atherosclerosis 236: 47–53, 2014. doi: 10.1016/j.atherosclerosis.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Brutsaert TD. Do high-altitude natives have enhanced exercise performance at altitude? Appl Physiol Nutr Metab 33: 582–592, 2008. doi: 10.1139/H08-009. [DOI] [PubMed] [Google Scholar]
- 23.Brutsaert TD. Population genetic aspects and phenotypic plasticity of ventilatory responses in high altitude natives. Respir Physiol Neurobiol 158: 151–160, 2007. doi: 10.1016/j.resp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Brutsaert TD, Araoz M, Soria R, Spielvogel H, Haas JD. Higher arterial oxygen saturation during submaximal exercise in Bolivian Aymara compared to European sojourners and Europeans born and raised at high altitude. Am J Phys Anthropol 113: 169–181, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 25.Brutsaert TD, Parra EJ, Shriver MD, Gamboa A, Palacios JA, Rivera M, Rodriguez I, León-Velarde F. Spanish genetic admixture is associated with larger V̇o2max decrement from sea level to 4338 m in Peruvian Quechua. J Appl Physiol (1985) 95: 519–528, 2003. doi: 10.1152/japplphysiol.01088.2002. [DOI] [PubMed] [Google Scholar]
- 26.Brutsaert TD, Parra EJ, Shriver MD, Gamboa A, Rivera-Ch M, León-Velarde F. Ancestry explains the blunted ventilatory response to sustained hypoxia and lower exercise ventilation of Quechua altitude natives. Am J Physiol Regul Integr Comp Physiol 289: R225–R234, 2005. doi: 10.1152/ajpregu.00105.2005. [DOI] [PubMed] [Google Scholar]
- 27.Brutsaert TD, Soria R, Caceres E, Spielvogel H, Haas JD. Effect of developmental and ancestral high altitude exposure on chest morphology and pulmonary function in Andean and European/North American natives. Am J Hum Biol 11: 383–395, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol 10: 531–540, 2014. doi: 10.1038/nrneph.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charles SM, Julian CG, Vargas E, Moore LG. Higher estrogen levels during pregnancy in Andean than European residents of high altitude suggest differences in aromatase activity. J Clin Endocrinol Metab 99: 2908–2916, 2014. doi: 10.1210/jc.2013-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D, Zhou X, Zhu Y, Zhu T, Wang J. [Comparison study on uterine and umbilical artery blood flow during pregnancy at high altitude and at low altitude]. Zhonghua Fu Chan Ke Za Zhi 37: 69–71, 2002. [PubMed] [Google Scholar]
- 31.Cheong HI, Janocha AJ, Monocello LT, Garchar AC, Gebremedhin A, Erzurum SC, Beall CM. Alternative hematological and vascular adaptive responses to high-altitude hypoxia in East African highlanders. Am J Physiol Lung Cell Mol Physiol 312: L172–L177, 2017. doi: 10.1152/ajplung.00451.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claydon VE, Gulli G, Slessarev M, Appenzeller O, Zenebe G, Gebremedhin A, Hainsworth R. Cerebrovascular responses to hypoxia and hypocapnia in Ethiopian high altitude dwellers. Stroke 39: 336–342, 2008. doi: 10.1161/STROKEAHA.107.491498. [DOI] [PubMed] [Google Scholar]
- 33.Cole AM, Petousi N, Cavalleri GL, Robbins PA. Genetic variation in SENP1 and ANP32D as predictors of chronic mountain sickness. High Alt Med Biol 15: 497–499, 2014. doi: 10.1089/ham.2014.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford JE, Ricardo Amaru R, Song J, Julian CG, Racimo F, Cheng JY, Guo X, Yao J, Ambale-Venkatesh B, Lima JA, Rotter JI, Stehlik J, Moore LG, Prchal JT, Nielsen R. Natural selection on genes related to cardiovascular health in high-altitude adapted Andeans. Am J Hum Genet; doi: 10.1016/j.ajhg.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dávila RD, Julian CG, Wilson MJ, Browne VA, Rodriguez C, Bigham AW, Shriver MD, Vargas E, Moore LG. Do anti-angiogenic or angiogenic factors contribute to the protection of birth weight at high altitude afforded by Andean ancestry? Reprod Sci 17: 861–870, 2010. doi: 10.1177/1933719110372418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeGraff AC Jr, Grover RF, Johnson RL Jr, Hammond JW Jr, Miller JM. Diffusing capacity of the lung in Caucasians native to 3,100 m. J Appl Physiol 29: 71–76, 1970. [DOI] [PubMed] [Google Scholar]
- 37.Dhar P, Sharma VK, Hota KB, Das SK, Hota SK, Srivastava RB, Singh SB. Autonomic cardiovascular responses in acclimatized lowlanders on prolonged stay at high altitude: a longitudinal follow up study. PLoS One 9: e84274, 2014. doi: 10.1371/journal.pone.0084274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobzhansky T. Adaptedness and fitness. In: Population Biology and Evolution, edited by Lewontin R. Syracuse, NY: Syracuse Univ. Press, 1968, p. 111. [Google Scholar]
- 39.Droma T, McCullough RG, McCullough RE, Zhuang JG, Cymerman A, Sun SF, Sutton JR, Moore LG. Increased vital and total lung capacities in Tibetan compared to Han residents of Lhasa (3,658 m). Am J Phys Anthropol 86: 341–351, 1991. doi: 10.1002/ajpa.1330860303. [DOI] [PubMed] [Google Scholar]
- 40.Eichstaedt CA, Antão T, Pagani L, Cardona A, Kivisild T, Mormina M. The Andean adaptive toolkit to counteract high altitude maladaptation: genome-wide and phenotypic analysis of the Collas. PLoS One 9: e93314, 2014. doi: 10.1371/journal.pone.0093314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 11: 1–51, 1998. doi: 10.1016/S0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- 42.Foll M, Gaggiotti OE, Daub JT, Vatsiou A, Excoffier L. Widespread signals of convergent adaptation to high altitude in Asia and America. Am J Hum Genet 95: 394–407, 2014. doi: 10.1016/j.ajhg.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisancho AR. Developmental responses to high altitude hypoxia. Am J Phys Anthropol 32: 401–407, 1970. doi: 10.1002/ajpa.1330320310. [DOI] [PubMed] [Google Scholar]
- 44.Frisancho AR. Human growth and pulmonary function of a high altitude Peruvian Quechua population. Hum Biol 41: 365–379, 1969. [PubMed] [Google Scholar]
- 45.Fulco CS, Rock PB, Cymerman A. Maximal and submaximal exercise performance at altitude. Aviat Space Environ Med 69: 793–801, 1998. [PubMed] [Google Scholar]
- 46.Gassmann NN, van Elteren HA, Goos TG, Morales CR, Rivera-Ch M, Martin DS, Cabala Peralta P, Passano Del Carpio A, Aranibar Machaca S, Huicho L, Reiss IK, Gassmann M, de Jonge RC. Pregnancy at high altitude in the Andes leads to increased total vessel density in healthy newborns. J Appl Physiol (1985) 121: 709–715, 2016. doi: 10.1152/japplphysiol.00561.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge RL, Simonson TS, Gordeuk V, Prchal JT, McClain DA. Metabolic aspects of high-altitude adaptation in Tibetans. Exp Physiol 100: 1247–1255, 2015. doi: 10.1113/EP085292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert-Kawai E, Coppel J, Court J, van der Kaaij J, Vercueil A, Feelisch M, Levett D, Mythen M, Grocott MP, Martin D; Xtreme Everest 2 Research Group . Sublingual microcirculatory blood flow and vessel density in Sherpas at high altitude. J Appl Physiol (1985) 122: 1011–1018, 2017. doi: 10.1152/japplphysiol.00970.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilbert-Kawai ET, Milledge JS, Grocott MPW, Martin DS. King of the mountains: Tibetan and Sherpa physiological adaptations to hypobaric hypoxia at high altitude: a review. Physiology (Bethesda) 29: 388–402, 2014. doi: 10.1152/physiol.00018.2014. [DOI] [PubMed] [Google Scholar]
- 50.Gonzales GF. Peruvian contributions to the study on human reproduction at high altitude: from the chronicles of the Spanish conquest to the present. Respir Physiol Neurobiol 158: 172–179, 2007. doi: 10.1016/j.resp.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Gonzales GF, Ortiz I. Age at menarche at sea level and high altitude in Peruvian women of different ethnic background. Am J Hum Biol 6: 637–640, 1994. doi: 10.1002/ajhb.1310060512. [DOI] [PubMed] [Google Scholar]
- 52.Groves BM, Droma T, Sutton JR, McCullough RG, McCullough RE, Zhuang J, Rapmund G, Sun S, Janes C, Moore LG. Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J Appl Physiol (1985) 74: 312–318, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Gu M, Dong X, Shi L, Shi L, Lin K, Huang X, Chu J. Differences in mtDNA whole sequence between Tibetan and Han populations suggesting adaptive selection to high altitude. Gene 496: 37–44, 2012. doi: 10.1016/j.gene.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Halperin BD, Sun S, Zhuang J, Droma T, Moore LG. ECG observations in Tibetan and Han residents of Lhasa. J Electrocardiol 31: 237–243, 1998. doi: 10.1016/S0022-0736(98)90139-X. [DOI] [PubMed] [Google Scholar]
- 55.Hill CM, Baya A, Gavlak J, Carroll A, Heathcote K, Dimitriou D, L’Esperance V, Webster R, Holloway J, Virues-Ortega J, Kirkham FJ, Bucks RS, Hogan AM. Adaptation to life in the high Andes: nocturnal oxyhemoglobin saturation in early development. Sleep 39: 1001–1008, 2016. doi: 10.5665/sleep.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hochachka PW, Clark CM, Holden JE, Stanley C, Ugurbil K, Menon RS. 31P magnetic resonance spectroscopy of the Sherpa heart: a phosphocreatine/adenosine triphosphate signature of metabolic defense against hypobaric hypoxia. Proc Natl Acad Sci USA 93: 1215–1220, 1996. doi: 10.1073/pnas.93.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoiland RL, Bain AR, Rieger MG, Bailey DM, Ainslie PN. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am J Physiol Regul Integr Comp Physiol 310: R398–R413, 2016. doi: 10.1152/ajpregu.00270.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoit BD, Dalton ND, Gebremedhin A, Janocha A, Zimmerman PA, Zimmerman AM, Strohl KP, Erzurum SC, Beall CM. Elevated pulmonary artery pressure among Amhara highlanders in Ethiopia. Am J Hum Biol 23: 168–176, 2011. doi: 10.1002/ajhb.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holden JE, Stone CK, Clark CM, Brown WD, Nickles RJ, Stanley C, Hochachka PW. Enhanced cardiac metabolism of plasma glucose in high-altitude natives: adaptation against chronic hypoxia. J Appl Physiol (1985) 79: 222–228, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Horscroft JA, Kotwica AO, Laner V, West JA, Hennis PJ, Levett DZH, Howard DJ, Fernandez BO, Burgess SL, Ament Z, Gilbert-Kawai ET, Vercueil A, Landis BD, Mitchell K, Mythen MG, Branco C, Johnson RS, Feelisch M, Montgomery HE, Griffin JL, Grocott MPW, Gnaiger E, Martin DS, Murray AJ. Metabolic basis to Sherpa altitude adaptation. Proc Natl Acad Sci USA 114: 6382–6387, 2017. doi: 10.1073/pnas.1700527114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu H, Petousi N, Glusman G, Yu Y, Bohlender R, Tashi T, Downie JM, Roach JC, Cole AM, Lorenzo FR, Rogers AR, Brunkow ME, Cavalleri G, Hood L, Alpatty SM, Prchal JT, Jorde LB, Robbins PA, Simonson TS, Huff CD. Evolutionary history of Tibetans inferred from whole-genome sequencing. PLoS Genet 13: e1006675, 2017. doi: 10.1371/journal.pgen.1006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang SY, Sun S, Droma T, Zhuang J, Tao JX, McCullough RG, McCullough RE, Micco AJ, Reeves JT, Moore LG. Internal carotid arterial flow velocity during exercise in Tibetan and Han residents of Lhasa (3,658 m). J Appl Physiol (1985) 73: 2638–2642, 1992. [DOI] [PubMed] [Google Scholar]
- 64.Huerta-Sánchez E, Degiorgio M, Pagani L, Tarekegn A, Ekong R, Antao T, Cardona A, Montgomery HE, Cavalleri GL, Robbins PA, Weale ME, Bradman N, Bekele E, Kivisild T, Tyler-Smith C, Nielsen R. Genetic signatures reveal high-altitude adaptation in a set of Ethiopian populations. Mol Biol Evol 30: 1877–1888, 2013. doi: 10.1093/molbev/mst089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huerta-Sánchez E, Jin X, Asan, Bianba Z, Peter BM, Vinckenbosch N, Liang Y, Yi X, He M, Somel M, Ni P, Wang B, Ou X, Huasang, Luosang J, Cuo ZX, Li K, Gao G, Yin Y, Wang W, Zhang X, Xu X, Yang H, Li Y, Wang J, Wang J, Nielsen R. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature 512: 194–197, 2014. doi: 10.1038/nature13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hultgren HN. High Altitude Medicine. Stanford, CA: Hultgren, 1997, p. 550. [Google Scholar]
- 67.Imray C, Chan C, Stubbings A, Rhodes H, Patey S, Wilson MH, Bailey DM, Wright AD; Birmingham Medical Research Expeditionary Society . Time course variations in the mechanisms by which cerebral oxygen delivery is maintained on exposure to hypoxia/altitude. High Alt Med Biol 15: 21–27, 2014. doi: 10.1089/ham.2013.1079. [DOI] [PubMed] [Google Scholar]
- 68.Jackson MR, Mayhew TM, Haas JD. The volumetric composition of human term placentae: altitudinal, ethnic and sex differences in Bolivia. J Anat 152: 173–187, 1987. [PMC free article] [PubMed] [Google Scholar]
- 69.Jacovas VC, Rovaris DL, Peréz O, de Azevedo S, Macedo GS, Sandoval JR, Salazar-Granara A, Villena M, Dugoujon JM, Bisso-Machado R, Petzl-Erler ML, Salzano FM, Ashton-Prolla P, Ramallo V, Bortolini MC. Genetic variations in the TP53 pathway in Native Americans strongly suggest adaptation to the high altitudes of the Andes. PLoS One 10: e0137823, 2015. doi: 10.1371/journal.pone.0137823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jansen GF, Basnyat B. Brain blood flow in Andean and Himalayan high-altitude populations: evidence of different traits for the same environmental constraint. J Cereb Blood Flow Metab 31: 706–714, 2011. doi: 10.1038/jcbfm.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeong C, Alkorta-Aranburu G, Basnyat B, Neupane M, Witonsky DB, Pritchard JK, Beall CM, Di Rienzo A. Admixture facilitates genetic adaptations to high altitude in Tibet. Nat Commun 5: 3281, 2014. doi: 10.1038/ncomms4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji F, Sharpley MS, Derbeneva O, Alves LS, Qian P, Wang Y, Chalkia D, Lvova M, Xu J, Yao W, Simon M, Platt J, Xu S, Angelin A, Davila A, Huang T, Wang PH, Chuang LM, Moore LG, Qian G, Wallace DC. Mitochondrial DNA variant associated with Leber hereditary optic neuropathy and high-altitude Tibetans. Proc Natl Acad Sci USA 109: 7391–7396, 2012. doi: 10.1073/pnas.1202484109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson RL Jr, Cassidy SS, Grover RF, Schutte JE, Epstein RH. Functional capacities of lungs and thorax in beagles after prolonged residence at 3,100 m. J Appl Physiol (1985) 59: 1773–1782, 1985. [DOI] [PubMed] [Google Scholar]
- 74.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol 295: R906–R915, 2008. doi: 10.1152/ajpregu.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julian CG, Gonzales M, Rodriguez A, Bellido D, Salmon CS, Ladenburger A, Reardon L, Vargas E, Moore LG. Perinatal hypoxia increases susceptibility to high-altitude polycythemia and attendant pulmonary vascular dysfunction. Am J Physiol Heart Circ Physiol 309: H565–H573, 2015. doi: 10.1152/ajpheart.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Julian CG, Hageman JL, Wilson MJ, Vargas E, Moore LG. Lowland origin women raised at high altitude are not protected against lower uteroplacental O2 delivery during pregnancy or reduced birth weight. Am J Hum Biol 23: 509–516, 2011. doi: 10.1002/ajhb.21167. [DOI] [PubMed] [Google Scholar]
- 77.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed 92: F372–F377, 2007. doi: 10.1136/adc.2006.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Julian CG, Vargas E, Browne VA, Wilson MJ, Bigham AW, Rodriguez C, McCord JM, Moore LG. Potential role for elevated maternal enzymatic antioxidant status in Andean protection against altitude-associated SGA. J Matern Fetal Neonatal Med 25: 1233–1240, 2012. doi: 10.3109/14767058.2011.636102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Julian CG, Vargas E, Gonzales M, Dávila RD, Ladenburger A, Reardon L, Schoo C, Powers RW, Lee-Chiong T, Moore LG. Sleep-disordered breathing and oxidative stress in preclinical chronic mountain sickness (excessive erythrocytosis). Respir Physiol Neurobiol 186: 188–196, 2013. doi: 10.1016/j.resp.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol 296: R1564–R1575, 2009. doi: 10.1152/ajpregu.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res 54: 20–25, 2003. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 82.Kiyamu M, Bigham A, Parra E, León-Velarde F, Rivera-Chira M, Brutsaert TD. Developmental and genetic components explain enhanced pulmonary volumes of female Peruvian Quechua. Am J Phys Anthropol 148: 534–542, 2012. doi: 10.1002/ajpa.22069. [DOI] [PubMed] [Google Scholar]
- 83.Kiyamu M, León-Velarde F, Rivera-Chira M, Elías G, Brutsaert TD. Developmental effects determine submaximal arterial oxygen saturation in Peruvian Quechua. High Alt Med Biol 16: 138–146, 2015. doi: 10.1089/ham.2014.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiyamu M, Elías G, León-Velarde F, Rivera-Chira M, Brutsaert TD. Aerobic capacity of Peruvian Quechua: a test of the developmental adaptation hypothesis. Am J Phys Anthropol 156: 363–373, 2015. doi: 10.1002/ajpa.22655. [DOI] [PubMed] [Google Scholar]
- 85.Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol 2012: 718789, 2012. doi: 10.5402/2012/718789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krampl E, Kametas NA, Nowotny P, Roden M, Nicolaides KH. Glucose metabolism in pregnancy at high altitude. Diabetes Care 24: 817–822, 2001. doi: 10.2337/diacare.24.5.817. [DOI] [PubMed] [Google Scholar]
- 87.Kryger M, Glas R, Jackson D, McCullough RE, Scoggin C, Grover RF, Weil JV. Impaired oxygenation during sleep in excessive polycythemia of high altitude: improvement with respiratory stimulation. Sleep 1: 3–17, 1978. doi: 10.1093/sleep/1.1.3. [DOI] [PubMed] [Google Scholar]
- 88.León-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, Zubieta-Calleja G. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 6: 147–157, 2005. doi: 10.1089/ham.2005.6.147. [DOI] [PubMed] [Google Scholar]
- 89.León-Velarde F, Ramos MA, Hernández JA, De Idiáquez D, Muñoz LS, Gaffo A, Córdova S, Durand D, Monge C. The role of menopause in the development of chronic mountain sickness. Am J Physiol Regul Integr Comp Physiol 272: R90–R94, 1997. [DOI] [PubMed] [Google Scholar]
- 90.Lewis NC, Bailey DM, Dumanoir GR, Messinger L, Lucas SJ, Cotter JD, Donnelly J, McEneny J, Young IS, Stembridge M, Burgess KR, Basnet AS, Ainslie PN. Conduit artery structure and function in lowlanders and native highlanders: relationships with oxidative stress and role of sympathoexcitation. J Physiol 592: 1009–1024, 2014. doi: 10.1113/jphysiol.2013.268615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu J, Liu Y, Ren LH, Li L, Wang Z, Liu SS, Li SZ, Cao TS. Effects of race and sex on cerebral hemodynamics, oxygen delivery and blood flow distribution in response to high altitude. Sci Rep 6: 30500, 2016. doi: 10.1038/srep30500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG Jr, Koivunen P, Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 46: 951–956, 2014. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lou H, Lu Y, Lu D, Fu R, Wang X, Feng Q, Wu S, Yang Y, Li S, Kang L, Guan Y, Hoh BP, Chung YJ, Jin L, Su B, Xu S. A 3.4-kb copy-number deletion near EPAS1 is significantly enriched in high-altitude Tibetans but absent from the Denisovan sequence. Am J Hum Genet 97: 54–66, 2015. doi: 10.1016/j.ajhg.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lundby C, Sander M, van Hall G, Saltin B, Calbet JA. Maximal exercise and muscle oxygen extraction in acclimatizing lowlanders and high altitude natives. J Physiol 573: 535–547, 2006. doi: 10.1113/jphysiol.2006.106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meadows GE, O’Driscoll DM, Simonds AK, Morrell MJ, Corfield DR. Cerebral blood flow response to isocapnic hypoxia during slow-wave sleep and wakefulness. J Appl Physiol (1985) 97: 1343–1348, 2004. doi: 10.1152/japplphysiol.01101.2003. [DOI] [PubMed] [Google Scholar]
- 96.Miller S, Tudor C, Nyima, Thorsten VR, Sonam, Droyoung, Craig S, Le P, Wright LL, Varner MW. Maternal and neonatal outcomes of hospital vaginal deliveries in Tibet. Int J Gynaecol Obstet 98: 217–221, 2007. doi: 10.1016/j.ijgo.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monge-C C, Arregui A, León-Velarde F. Pathophysiology and epidemiology of chronic mountain sickness. Int J Sports Med 13, Suppl 1: S79–S81, 1992. doi: 10.1055/s-2007-1024603. [DOI] [PubMed] [Google Scholar]
- 98.Moore LG. Comparative human ventilatory adaptation to high altitude. Respir Physiol 121: 257–276, 2000. doi: 10.1016/S0034-5687(00)00133-X. [DOI] [PubMed] [Google Scholar]
- 99.Moore LG. Human genetic adaptation to high altitudes: current status and future prospects. Quaternary Int. [Available online 6 October 2016]. doi: 10.1016/j.quaint.2016.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moore LG. Human genetic adaptation to high altitude. High Alt Med Biol 2: 257–279, 2001. doi: 10.1089/152702901750265341. [DOI] [PubMed] [Google Scholar]
- 101.Moore LG. Uterine blood flow as a determinant of feto-placental development. In: The Placenta and Human Developmental Programming, edited by Burton GJ, Barker DJP, Moffett A, Thornburg KL. Cambridge, UK: Cambridge Univ. Press, 2011, p. 126–146. [Google Scholar]
- 102.Moore LG, Asmus I, Curran L. Chronic mountain sickness: gender and geographic variation. In: Progress in Mountain Medicine and High Altitude Physiology, edited by Ohno H, Kobayashi T, Masuyama S, Nakashima M. Matsumoto, Japan: Press Committee of the 3rd World Congress on Mountain Medicine and High Altitude Physiology, 1998, p. 114–119. [Google Scholar]
- 103.Moore LG, Niermeyer S, Vargas E. Does chronic mountain sickness (CMS) have perinatal origins? Respir Physiol Neurobiol 158: 180–189, 2007. doi: 10.1016/j.resp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 104.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol 107, Suppl : 25–64, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 105.Moore LG, Rohr AL, Maisenbach JK, Reeves JT. Emphysema mortality is increased in Colorado residents at high altitude. Am Rev Respir Dis 126: 225–228, 1982. [DOI] [PubMed] [Google Scholar]
- 106.Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert T, Parra E, Vargas E. Maternal adaptation to high-altitude pregnancy: an experiment of nature. Placenta 25, Suppl A: S60–S71, 2004. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 107.Moore LG, Young D, McCullough RE, Droma T, Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol 13: 635–644, 2001. doi: 10.1002/ajhb.1102. [DOI] [PubMed] [Google Scholar]
- 108.Moore LG, Zamudio S, Zhuang J, Sun S, Droma T. Oxygen transport in Tibetan women during pregnancy at 3,658 m. Am J Phys Anthropol 114: 42–53, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 109.Mortimer EA Jr, Monson RR, MacMahon B. Reduction in mortality from coronary heart disease in men residing at high altitude. N Engl J Med 296: 581–585, 1977. doi: 10.1056/NEJM197703172961101. [DOI] [PubMed] [Google Scholar]
- 110.Niermeyer S, Andrade-M MP, Vargas E, Moore LG. Neonatal oxygenation, pulmonary hypertension, and evolutionary adaptation to high altitude (2013 Grover Conference series). Pulm Circ 5: 48–62, 2015. doi: 10.1086/679719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Niermeyer S, Yang P, Shanmina, Drolkar, Zhuang J, Moore LG. Arterial oxygen saturation in Tibetan and Han infants born in Lhasa, Tibet. N Engl J Med 333: 1248–1252, 1995. doi: 10.1056/NEJM199511093331903. [DOI] [PubMed] [Google Scholar]
- 112.Norcliffe LJ, Rivera-Ch M, Claydon VE, Moore JP, Leon-Velarde F, Appenzeller O, Hainsworth R. Cerebrovascular responses to hypoxia and hypocapnia in high-altitude dwellers. J Physiol 566: 287–294, 2005. doi: 10.1113/jphysiol.2005.086629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 180: 1161–1168, 1999. doi: 10.1016/S0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 114.Peacock AJ. ABC of oxygen: oxygen at high altitude. BMJ 317: 1063–1066, 1998. doi: 10.1136/bmj.317.7165.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peng Y, Cui C, He Y, Ouzhuluobu, Zhang H, Yang D, Zhang Q, Bianbazhuoma, Yang L, He Y, Xiang K, Zhang X, Bhandari S, Shi P, Yangla, Dejiquzong, Baimakangzhuo, Duojizhuoma, Pan Y, Cirenyangji, Baimayangji, Gonggalanzi, Bai C, Bianba, Basang, Ciwangsangbu, Xu S, Chen H, Liu S, Wu T, Qi X, Su B. Down-regulation of EPAS1 transcription and genetic adaptation of Tibetans to high-altitude hypoxia. Mol Biol Evol 34: 818–830, 2017. doi: 10.1093/molbev/msw280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peng Y, Yang Z, Zhang H, Cui C, Qi X, Luo X, Tao X, Wu T, Ouzhuluobu, Basang, Ciwangsangbu, Danzengduojie, Chen H, Shi H, Su B. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol 28: 1075–1081, 2011. doi: 10.1093/molbev/msq290. [DOI] [PubMed] [Google Scholar]
- 117.Petousi N, Croft QP, Cavalleri GL, Cheng HY, Formenti F, Ishida K, Lunn D, McCormack M, Shianna KV, Talbot NP, Ratcliffe PJ, Robbins PA. Tibetans living at sea level have a hyporesponsive hypoxia-inducible factor system and blunted physiological responses to hypoxia. J Appl Physiol (1985) 116: 893–904, 2014. doi: 10.1152/japplphysiol.00535.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petousi N, Robbins PA. Human adaptation to the hypoxia of high altitude: the Tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol (1985) 116: 875–884, 2014. doi: 10.1152/japplphysiol.00605.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pomeroy E, Wells JC, Stanojevic S, Miranda JJ, Moore LG, Cole TJ, Stock JT. Surname-inferred Andean ancestry is associated with child stature and limb lengths at high altitude in Peru, but not at sea level. Am J Hum Biol 27: 798–806, 2015. doi: 10.1002/ajhb.22725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rademaker K, Hodgins G, Moore K, Zarrillo S, Miller C, Bromley GR, Leach P, Reid DA, Álvarez WY, Sandweiss DH. Paleoindian settlement of the high-altitude Peruvian Andes. Science 346: 466–469, 2014. doi: 10.1126/science.1258260. [DOI] [PubMed] [Google Scholar]
- 121.Reeves JT, Weil JV. Chronic mountain sickness. A view from the crow’s nest. Adv Exp Med Biol 502: 419–437, 2001. doi: 10.1007/978-1-4757-3401-0_27. [DOI] [PubMed] [Google Scholar]
- 122.Rexhaj E, Rimoldi SF, Pratali L, Brenner R, Andries D, Soria R, Salinas C, Villena M, Romero C, Allemann Y, Lovis A, Heinzer R, Sartori C, Scherrer U. Sleep-disordered breathing and vascular function in patients with Chronic Mountain Sickness and healthy high-altitude dwellers. Chest 149: 991–998, 2016. doi: 10.1378/chest.15-1450. [DOI] [PubMed] [Google Scholar]
- 123.Rimoldi SF, Rexhaj E, Pratali L, Bailey DM, Hutter D, Faita F, Salinas Salmòn C, Villena M, Nicod P, Allemann Y, Scherrer U, Sartori C. Systemic vascular dysfunction in patients with chronic mountain sickness. Chest 141: 139–146, 2012. doi: 10.1378/chest.11-0342. [DOI] [PubMed] [Google Scholar]
- 124.Roberts JM, Hubel CA. Pregnancy: a screening test for later life cardiovascular disease. Womens Health Issues 20: 304–307, 2010. doi: 10.1016/j.whi.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 125.Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochem Soc Trans 37: 295–298, 2009. doi: 10.1042/BST0370295. [DOI] [PubMed] [Google Scholar]
- 126.Rupert JL, Hochachka PW. The evidence for hereditary factors contributing to high altitude adaptation in Andean natives: a review. High Alt Med Biol 2: 235–256, 2001. doi: 10.1089/152702901750265332. [DOI] [PubMed] [Google Scholar]
- 127.Sartori C, Allemann Y, Trueb L, Delabays A, Nicod P, Scherrer U. Augmented vasoreactivity in adult life associated with perinatal vascular insult. Lancet 353: 2205–2207, 1999. doi: 10.1016/S0140-6736(98)08352-4. [DOI] [PubMed] [Google Scholar]
- 128.Sawka MN, Young AJ, Rock PB, Lyons TP, Boushel R, Freund BJ, Muza SR, Cymerman A, Dennis RC, Pandolf KB, Valeri CR. Altitude acclimatization and blood volume: effects of exogenous erythrocyte volume expansion. J Appl Physiol (1985) 81: 636–642, 1996. [DOI] [PubMed] [Google Scholar]
- 129.Scheinfeldt LB, Soi S, Thompson S, Ranciaro A, Woldemeskel D, Beggs W, Lambert C, Jarvis JP, Abate D, Belay G, Tishkoff SA. Genetic adaptation to high altitude in the Ethiopian highlands. Genome Biol 13: R1, 2012. doi: 10.1186/gb-2012-13-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schneider A, Greene RE, Keyl C, Bandinelli G, Passino C, Spadacini G, Bonfichi M, Arcaini L, Malcovati L, Boiardi A, Feil P, Bernardi L. Peripheral arterial vascular function at altitude: sea-level natives versus Himalayan high-altitude natives. J Hypertens 19: 213–222, 2001. doi: 10.1097/00004872-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 131.Schoene RB, Roach RC, Lahiri S, Peters RM Jr, Hackett PH, Santolaya R. Increased diffusion capacity maintains arterial saturation during exercise in the Quechua Indians of Chilean Altiplano. Am J Hum Biol 2: 663–668, 1990. doi: 10.1002/ajhb.1310020609. [DOI] [PubMed] [Google Scholar]
- 132.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med 365: 537–547, 2011. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 133.Simonson TS, Wei G, Wagner HE, Wuren T, Qin G, Yan M, Wagner PD, Ge RL. Low haemoglobin concentration in Tibetan males is associated with greater high-altitude exercise capacity. J Physiol 593: 3207–3218, 2015. doi: 10.1113/JP270518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science 329: 72–75, 2010. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 135.Smirl JD, Lucas SJ, Lewis NC, duManoir GR, Smith KJ, Bakker A, Basnyat AS, Ainslie PN. Cerebral pressure-flow relationship in lowlanders and natives at high altitude. J Cereb Blood Flow Metab 34: 248–257, 2014. doi: 10.1038/jcbfm.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Song D, Li LS, Arsenault PR, Tan Q, Bigham AW, Heaton-Johnson KJ, Master SR, Lee FS. Defective Tibetan PHD2 binding to p23 links high altitude adaption to altered oxygen sensing. J Biol Chem 289: 14656–14665, 2014. doi: 10.1074/jbc.M113.541227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Soria R, Egger M, Scherrer U, Bender N, Rimoldi SF. Pulmonary artery pressure and arterial oxygen saturation in people living at high or low altitude: systematic review and meta-analysis. J Appl Physiol (1985) 121: 1151–1159, 2016. doi: 10.1152/japplphysiol.00394.2016. [DOI] [PubMed] [Google Scholar]
- 138.Soria R, Julian CG, Vargas E, Moore LG, Giussani DA. Graduated effects of high-altitude hypoxia and highland ancestry on birth size. Pediatr Res 74: 633–638, 2013. doi: 10.1038/pr.2013.150. [DOI] [PubMed] [Google Scholar]
- 139.Stembridge M, Ainslie PN, Shave R. Short-term adaptation and chronic cardiac remodelling to high altitude in lowlander natives and Himalayan Sherpa. Exp Physiol 100: 1242–1246, 2015. doi: 10.1113/expphysiol.2014.082503. [DOI] [PubMed] [Google Scholar]
- 140.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 141.Sun S, Oliver-Pickett C, Ping Y, Micco AJ, Droma T, Zamudio S, Zhuang J, Huang SY, McCullough RG, Cymerman A, Moore LG. Breathing and brain blood flow during sleep in patients with chronic mountain sickness. J Appl Physiol (1985) 81: 611–618, 1996. [DOI] [PubMed] [Google Scholar]
- 142.Swenson ER, Bartsch P (editors). High Altitude: Human Adaptation to Hypoxia. New York: Springer-Verlag, 2014, p. 496. doi: 10.1007/978-1-4614-8772-2 [DOI] [Google Scholar]
- 143.Tashi T, Scott Reading N, Wuren T, Zhang X, Moore LG, Hu H, Tang F, Shestakova A, Lorenzo F, Burjanivova T, Koul P, Guchhait P, Wittwer CT, Julian CG, Shah B, Huff CD, Gordeuk VR, Prchal JT, Ge R. Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E:C127S) in combination with EPAS1 (HIF-2α) polymorphism lowers hemoglobin concentration in Tibetan highlanders. J Mol Med (Berl) 95: 665–670, 2017. doi: 10.1007/s00109-017-1519-3. [DOI] [PubMed] [Google Scholar]
- 144.Tissot van Patot MC, Bendrick-Peart J, Beckey VE, Serkova N, Zwerdlinger L. Greater vascularity, lowered HIF-1/DNA binding, and elevated GSH as markers of adaptation to in vivo chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L525–L532, 2004. doi: 10.1152/ajplung.00203.2003. [DOI] [PubMed] [Google Scholar]
- 145.Tymko MM, Tremblay JC, Hansen AB, Howe CA, Willie CK, Stembridge M, Green DJ, Hoiland RL, Subedi P, Anholm JD, Ainslie PN. The effect of α1-adrenergic blockade on post-exercise brachial artery flow-mediated dilatation at sea level and high altitude. J Physiol 595: 1671–1686, 2017. doi: 10.1113/JP273183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Udpa N, Ronen R, Zhou D, Liang J, Stobdan T, Appenzeller O, Yin Y, Du Y, Guo L, Cao R, Wang Y, Jin X, Huang C, Jia W, Cao D, Guo G, Claydon VE, Hainsworth R, Gamboa JL, Zibenigus M, Zenebe G, Xue J, Liu S, Frazer KA, Li Y, Bafna V, Haddad GG. Whole genome sequencing of Ethiopian highlanders reveals conserved hypoxia tolerance genes. Genome Biol 15: R36, 2014. doi: 10.1186/gb-2014-15-2-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Valverde G, Zhou H, Lippold S, de Filippo C, Tang K, López Herráez D, Li J, Stoneking M. A novel candidate region for genetic adaptation to high altitude in Andean populations. PLoS One 10: e0125444, 2015. doi: 10.1371/journal.pone.0125444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Villafuerte FC. New genetic and physiological factors for excessive erythrocytosis and Chronic Mountain Sickness. J Appl Physiol (1985) 119: 1481–1486, 2015. doi: 10.1152/japplphysiol.00271.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vitzthum VJ. The home team advantage: reproduction in women indigenous to high altitude. J Exp Biol 204: 3141–3150, 2001. [DOI] [PubMed] [Google Scholar]
- 150.Wagner PD, Araoz M, Boushel R, Calbet JA, Jessen B, Rådegran G, Spielvogel H, Søndegaard H, Wagner H, Saltin B. Pulmonary gas exchange and acid-base state at 5,260 m in high-altitude Bolivians and acclimatized lowlanders. J Appl Physiol (1985) 92: 1393–1400, 2002. doi: 10.1152/japplphysiol.00093.2001. [DOI] [PubMed] [Google Scholar]
- 151.Wang B, Zhang YB, Zhang F, Lin H, Wang X, Wan N, Ye Z, Weng H, Zhang L, Li X, Yan J, Wang P, Wu T, Cheng L, Wang J, Wang DM, Ma X, Yu J. On the origin of Tibetans and their genetic basis in adapting high-altitude environments. PLoS One 6: e17002, 2011. doi: 10.1371/journal.pone.0017002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weitz CA, Garruto RM, Chin CT. Larger FVC and FEV1 among Tibetans compared to Han born and raised at high altitude. Am J Phys Anthropol 159: 244–255, 2016. doi: 10.1002/ajpa.22873. [DOI] [PubMed] [Google Scholar]
- 154.West JB. Are permanent residents of high altitude fully adapted to their hypoxic environment? High Alt Med Biol 18: 135–139, 2017. doi: 10.1089/ham.2016.0152. [DOI] [PubMed] [Google Scholar]
- 155.West JB, Schoene RB, Luks AM, Milledge JS. High Altitude Medicine and Physiology. Boca Raton, FL: CRC Press, 2012, p. 584. doi: 10.1201/b13633. [DOI] [Google Scholar]
- 156.Wilson MJ, Lopez M, Vargas M, Julian C, Tellez W, Rodriguez A, Bigham A, Armaza JF, Niermeyer S, Shriver M, Vargas E, Moore LG. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol 293: R1313–R1324, 2007. doi: 10.1152/ajpregu.00806.2006. [DOI] [PubMed] [Google Scholar]
- 157.Woolcott OO, Ader M, Bergman RN. Glucose homeostasis during short-term and prolonged exposure to high altitudes. Endocr Rev 36: 149–173, 2015. doi: 10.1210/er.2014-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu P, Shanminna, Liang K, Yue H, Qian L, Sun B. Exhaled nitric oxide is associated with postnatal adaptation to hypoxia in Tibetan and non-Tibetan newborn infants. Acta Paediatr 105: 475–482, 2016. doi: 10.1111/apa.13331. [DOI] [PubMed] [Google Scholar]
- 159.Wu T, Kayser B. High altitude adaptation in Tibetans. High Alt Med Biol 7: 193–208, 2006. doi: 10.1089/ham.2006.7.193. [DOI] [PubMed] [Google Scholar]
- 160.Wu T, Wang X, Wei C, Cheng H, Wang X, Li Y, Ge-Dong, Zhao H, Young P, Li G, Wang Z. Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs. Han. J Appl Physiol (1985) 98: 598–604, 2005. doi: 10.1152/japplphysiol.01034.2002. [DOI] [PubMed] [Google Scholar]
- 161.Wu TY, Li W, Li Y, Ge RL, Cheng Q, Wang S, Zhao G, Wei L, Jin Y, Don G. Epidemiology of Chronic Mountain Sickness: ten years’ study in Qinghai-Tibet. In: Progress in Mountain Medicine and High Altitude Physiology, edited by Ohno H, Kobayashi T, Masuyama S, Nakashima M. Matsumoto, Japan: Press Committee of the 3rd World Congress on Mountain Medicine and High Altitude Physiology, 1998, p. 120–125. [Google Scholar]
- 162.Xing G, Qualls C, Huicho L, Rivera-Ch M, Stobdan T, Slessarev M, Prisman E, Ito S, Wu H, Norboo A, Dolma D, Kunzang M, Norboo T, Gamboa JL, Claydon VE, Fisher J, Zenebe G, Gebremedhin A, Hainsworth R, Verma A, Appenzeller O. Adaptation and mal-adaptation to ambient hypoxia; Andean, Ethiopian and Himalayan patterns. PLoS One 3: e2342, 2008. doi: 10.1371/journal.pone.0002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu S, Li S, Yang Y, Tan J, Lou H, Jin W, Yang L, Pan X, Wang J, Shen Y, Wu B, Wang H, Jin L. A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol Biol Evol 28: 1003–1011, 2011. doi: 10.1093/molbev/msq277. [DOI] [PubMed] [Google Scholar]
- 164.Yan X, Zhang J, Gong Q, Weng X. Prolonged high-altitude residence impacts verbal working memory: an fMRI study. Exp Brain Res 208: 437–445, 2011. doi: 10.1007/s00221-010-2494-x. [DOI] [PubMed] [Google Scholar]
- 165.Yang D, Peng Y, Ouzhuluobu, Bianbazhuoma, Cui C, Bianba, Wang L, Xiang K, He Y, Zhang H, Zhang X, Liu J, Shi H, Pan Y, Duojizhuoma, Dejiquzong, Cirenyangji, Baimakangzhuo, Gonggalanzi, Liu S, Gengdeng, Wu T, Chen H, Qi X, Su B. HMOX2 functions as a modifier gene for high-altitude adaptation in Tibetans. Hum Mutat 37: 216–223, 2016. doi: 10.1002/humu.22935. [DOI] [PubMed] [Google Scholar]
- 166.Yangzom Y, Qian L, Shan M, La Y, Meiduo D, Hu X, Da Q, Sun B, Zetterström R. Outcome of hospital deliveries of women living at high altitude: a study from Lhasa in Tibet. Acta Paediatr 97: 317–321, 2008. doi: 10.1111/j.1651-2227.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- 167.Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan, Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J, Wang J. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329: 75–78, 2010. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 173: 451–462, 2008. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yung HW, Cox M, Tissot van Patot M, Burton GJ. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J 26: 1970–1981, 2012. doi: 10.1096/fj.11-190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol (1985) 79: 7–14, 1995. [DOI] [PubMed] [Google Scholar]
- 171.Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Ameller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol 582: 883–895, 2007. doi: 10.1113/jphysiol.2007.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zamudio S, Torricos T, Fik E, Oyala M, Echalar L, Pullockaran J, Tutino E, Martin B, Belliappa S, Balanza E, Illsley NP. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One 5: e8551, 2010. doi: 10.1371/journal.pone.0008551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhao XX, Gao WX, Gao YQ, Suo L, Chen J. [Placental mitochondrial respiratory function of native Tibetan at high altitude]. Zhonghua Yi Xue Za Zhi 87: 894–897, 2007. doi: 10.3760/j.issn:0529-5815.2007.13.012. [DOI] [PubMed] [Google Scholar]
- 174.Zhou D, Udpa N, Ronen R, Stobdan T, Liang J, Appenzeller O, Zhao HW, Yin Y, Du Y, Guo L, Cao R, Wang Y, Jin X, Huang C, Jia W, Cao D, Guo G, Gamboa JL, Villafuerte F, Callacondo D, Xue J, Liu S, Frazer KA, Li Y, Bafna V, Haddad GG. Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am J Hum Genet 93: 452–462, 2013. doi: 10.1016/j.ajhg.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou ZN, Zhuang JG, Wu XF, Zhang Y, Cherdrungsi P. Tibetans retained innate ability resistance to acute hypoxia after long period of residing at sea level. J Physiol Sci 58: 167–172, 2008. doi: 10.2170/physiolsci.RP009207. [DOI] [PubMed] [Google Scholar]
- 176.Zhuang J, Droma T, Sun S, Janes C, McCullough RE, McCullough RG, Cymerman A, Huang SY, Reeves JT, Moore LG. Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3,658 m. J Appl Physiol (1985) 74: 303–311, 1993. [DOI] [PubMed] [Google Scholar]
- 177.Zhuang J, Droma T, Sutton JR, Groves BM, McCullough RE, McCullough RG, Sun S, Moore LG. Smaller alveolar-arterial O2 gradients in Tibetan than Han residents of Lhasa (3,658 m). Respir Physiol 103: 75–82, 1996. doi: 10.1016/0034-5687(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 178.Zhuang J, Droma T, Sutton JR, McCullough RE, McCullough RG, Groves BM, Rapmund G, Janes C, Sun S, Moore LG. Autonomic regulation of heart rate response to exercise in Tibetan and Han residents of Lhasa (3,658 m). J Appl Physiol (1985) 75: 1968–1973, 1993. [DOI] [PubMed] [Google Scholar]