Abstract
Hypoxic tissue conditions occur during a number of inflammatory diseases and are associated with the breakdown of barriers and induction of proinflammatory responses. At the same time, hypoxia is also known to induce several adaptive and tissue-protective pathways that dampen inflammation and protect tissue integrity. Hypoxia-inducible factors (HIFs) that are stabilized during inflammatory or hypoxic conditions are at the center of mediating these responses. In the past decade, several genes regulating extracellular adenosine metabolism and signaling have been identified as being direct targets of HIFs. Here, we discuss the relationship between inflammation, hypoxia, and adenosine and that HIF-driven adenosine metabolism and signaling is essential in providing tissue protection during inflammatory conditions, including myocardial injury, inflammatory bowel disease, and acute lung injury. We also discuss how the hypoxia-adenosine link can be targeted therapeutically in patients as a future treatment approach for inflammatory diseases.
Keywords: adenosine, adenosine receptors, hypoxia, HIF, inflammation, myocardial injury, inflammatory bowel disease, acute lung injury
hypoxia-inducible factors (HIFs) are found stabilized during a number of inflammatory conditions and diseases, including inflammatory bowel disease, pathogen infection, acute lung injury, myocardial injury, or during ischemia-reperfusion injury (45). What contributes to tissue hypoxia during inflammation? On the one hand, inflammation causes alterations in tissue metabolism and is characterized by a profound increase in local oxygen demand. On the other, ischemia, damage to tissue blood supply (trauma), thrombosis, or compression (interstitial hypertension) reduces the supply of oxygen and other metabolic substrates (44). Inflammatory bowel disease (IBD) serves as an elegant example of inflammation-associated hypoxia (Fig. 1) (45). In the gut, intestinal epithelial cells are at baseline conditions already hypoxic. However, with the onset of inflammation, as occurs with IBD, gut tissues become profoundly hypoxic (71). Organ transplant is a primary example of hypoxia-associated inflammation. The degree of ischemic injury in donor kidneys (78) or transplanted lungs (5, 28) is shown to correlate with increased expression of toll-like receptor (TLR) 4, an inflammation initiating receptor, and increased inflammatory cytokines; increased TLR4 and inflammatory cytokines correlates with loss of graft function as well. Relevant to this review, studies in adenosine metabolism/signaling gene-deficient mice show that short-term, low oxygen exposure increases vascular leakage, proinflammatory cytokine expression, and the accumulation of inflammatory cells in tissues (33, 43, 46, 119, 135). Suffice to say, hypoxia and inflammation are closely linked: on the cellular level, hypoxia can cause inflammation, and inflammation can cause hypoxia. Important to emphasize is that although hypoxia can be an inflammatory stimulus that encourages proinflammatory responses and breaks down tissue barriers, there are many examples in which stabilization of HIFs induces anti-inflammatory and tissue-protective responses. Indeed, extracellular adenosine metabolism and signaling is an essential HIF-driven pathway that dampens excessive inflammatory responses and induces barrier protection in conditions of low oxygen and inflammation. Of note, not all situations of hypoxia are associated with a primary role of inflammation. High-altitude pulmonary edema is the resultant of hypoxia pulmonary vasoconstriction and occurs in the absence of inflammation; inflammation follows the injury to the alveolar capillaries (7, 90, 126, 127). In this review, we discuss the relationship between inflammation, hypoxia, and adenosine and that HIF-driven adenosine metabolism and signaling is essential in providing tissue protection during inflammatory conditions, including myocardial injury, inflammatory bowel disease, and acute lung injury (Fig. 1). We also discuss how the hypoxia-adenosine link can be targeted therapeutically in patients as a future treatment approach for inflammatory diseases.
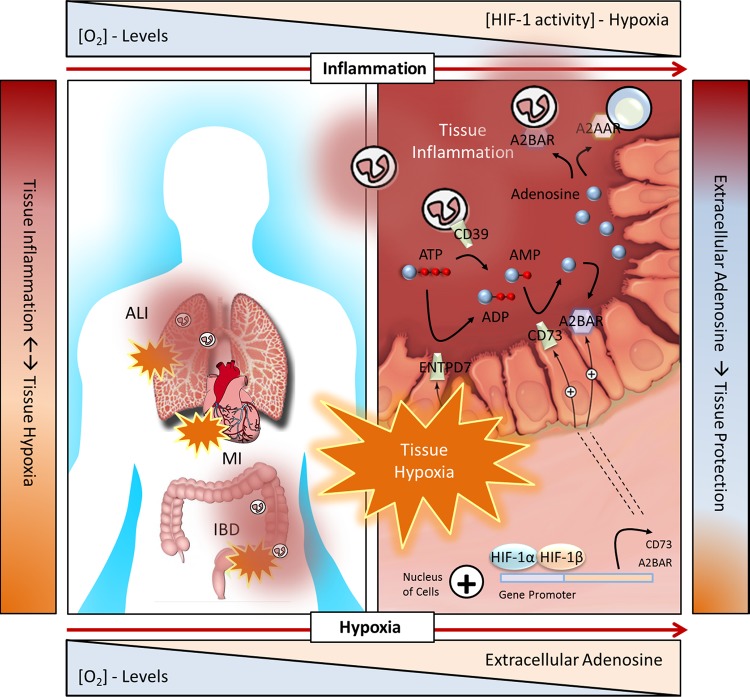
Fig. 1.
Adenosine metabolism and signaling in human health conditions and diseases characterized by hypoxia-associated inflammation or inflammation-associated hypoxia. Right: hypoxia-driven adenosine metabolism and signaling in intestinal inflammation. Inflammatory bowel disease (IBD) is characterized by excessive inflammation that severely damages the mucosa. Inflammation is associated with a severe shift in oxygen supply and demand, resulting in profound hypoxia. With inflammation and hypoxia, multiple cells release ATP/ADP, including inflammatory cells, platelets, and epithelial cells. Activation of the ATP/ADP receptor P2X7 on enteric neuron cells further promotes inflammation and tissue injury [not shown (58)]. Hypoxia results in an Sp1-dependent induction of CD39 [not shown (47)] and a hypoxia-inducible factor (HIF)-dependent induction of CD73 (128) and A2BAR (76). CD39 is largely restricted to immune cells and vascular endothelium. Epithelial cell expression of ectonucleoside triphosphate diphosphohydrolase (NTPDase) activity may be related to CD39 family member ENTPD7 (83) and alkaline phosphatases (97). Together, increased CD39, CD73, and A2BAR support increased adenosine metabolism and signaling. Adenosine signaling, particularly involving A2AAR (101) and A2BAR (53), dampens inflammatory responses, such as tissue infiltration of neutrophils, and promotes protection of the barrier. [Modified from Eltzschig et al. (48) with permission. Copyright 2012 Massachusetts Medical Society.]
Adenosine Signaling During Inflammation
Inflammation is devastating to tissues. Mounting an inflammatory response, however, is essential for eliminating harmful stimuli, such as pathogens or irritants, clearing out damaged cells from the initial cause of injury, and to initiate tissue repair. Equally essential are responses that exist to dampen potentially harmful, unrestricted inflammatory responses, minimizing contralateral damage to tissues and promoting tissue integrity. Therefore, once inflammation is initiated, bringing to an end the inflammatory response becomes as important as eliminating the inflammatory pathogen or insult. Adenosine signaling has emerged as being crucial to dampening immune responses. Nearly a century ago, observations by Drury and Szent-Gyorgyi (31) of an adenine compound from cardiac extracts to slow heart rate provided the first indications of extracellular adenosine as a signaling molecule. Adenosine belongs to a group of purinergic signaling molecules that also include ATP and ADP (42). Once released to the extracellular surface, ATP/ADP is rapidly hydrolyzed to adenosine by cell surface enzymes (42). Ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases), including E-NTPDase 1 (CD39), metabolize ATP/ADP to AMP, and ecto-5′-nucleotidase (CD73) generates extracellular adenosine from AMP. Adenosine can signal through one of four membrane-spanning adenosine receptors: A1AR, A2AAR, A2BAR, and A3AR, or re-enter the cell through equilibrative or concentrative nucleoside transporters (ENTs or CNTs, respectively). The movement of extracellular adenosine into the cell though ENTs or CNTs acts to dampen adenosine signaling (42, 56). Extracellular adenosine may also be deaminated by cell surface CD26-conjugated adenosine deaminase (69) as well as phosphorylated back to a nucleotide; both also serve in terminating adenosine signaling.
In the past several decades, pharmacological and genetic studies have contributed significantly to understanding adenosine signaling during inflammation. Two major concepts emerged: 1) adenosine signaling is central to dampening inflammatory responses and subsequently protects tissues; and 2) adenosine receptors, especially A2AAR and A2BAR, are involved in limiting immune responses. For example, in vivo studies from Cronstein et al. (20) showed the injection of mice with methotrexate raised extracellular adenosine levels and limited immune cell accumulation in inflamed tissues. The reduction in immune cell accumulation was completely reversed by an A2AAR antagonist. Studies in A2AAR−/− mice from the laboratory of Sitkovsky (105) confirmed these findings utilizing in vivo models of inflammation. It is now well recognized that inflammation and conditions of low oxygen increase extracellular ATP/ADP release, which substantially raises extracellular adenosine levels (48, 66). As discussed later in this review, raising extracellular adenosine levels is supported even more by transcriptional regulation of adenosine metabolizing and receptor genes via hypoxia-inducible factors (HIFs) (3, 43, 76, 98, 99, 128). The importance of extracellular adenosine metabolism is seen in CD39- and CD73-deficient mice, which show exaggerated inflammation, and in that inflammation is significantly suppressed in mice exposed to adenosine signaling terminating pathway member (e.g., ENTs, AK) inhibitors (43, 98, 99). Adenosine signaling via A2AAR has shown beneficial anti-inflammatory effects in models of ischemic or traumatic tissue damage in various tissues, including liver (27), kidney (26), intestine (101), heart (86, 141), skin (109), and lung (114).
In general, extracellular adenosine dampening inflammation via A2AAR involves its activation on immune cells. For example, A2AAR activity on macrophages limits their production of proinflammatory cytokines, such as interleukin-12 (IL-12) and tumor necrosis factor-α (TNF-α) (62, 77) and promotes their release of anti-inflammatory cytokine, interleukin-10 (IL-10) (102). Similarly, A2AAR activity on mature dendritic cells shifts their cytokine profile from proinflammatory to anti-inflammatory, reducing IL-12, interleukin-6 (IL-6), and interferon-α (IFN-α) production and increasing IL-10 production (107, 108, 122). A number of studies have defined that A2AAR activity on neutrophils (en route to sites of inflammation) prevents tissue injury by inhibiting the production of toxic oxygen species (18, 19, 21, 137) as well as modulates the production and release of proinflammatory cytokines (e.g., TNF-α), chemokines (94, 133), and prostaglandins (e.g., PGE2) (12, 111), and inhibits production of chemoattractant leukotrienes (e.g., LTB4) (79, 80). Considering T cells, activation of A2AAR on effector T cells inhibits their activation, differentiation, and effector functions, whereas A2AAR is implicated in enhancing the formation of T regulatory (Treg) cells and their expression of program cell death protein 1 (PD-1) and CTLA-4, both negative regulators of inflammation (104). Studies by Robson and colleagues (29) have shown an important regulatory loop between Tregs and effector T cells in that CD39/CD73 coordinated adenosine metabolism on Tregs activates A2AAR on effector T cells, dampening effector T cell activity. A2AAR activity on vascular endothelial cells has important anti-inflammatory effects. A2AAR activation inhibits endothelial cell release of inflammatory cytokines and expression of adhesion molecules, such as E-selectin and vascular cell adhesion molecule 1 (VCAM-1) (9), whereas A2BAR activity increases the vascular barrier (33, 87). Collectively, this limits the accumulation of neutrophils into tissues and subsequent tissue damage.
A2BAR also has important anti-inflammatory actions on immune cells. Similar to A2AAR, A2BAR activity on macrophages limits their production of inflammatory cytokines, such as TNF-α (77). A2BAR is also significant in protecting the epithelial barrier during inflammation (2, 60). The anti-inflammatory signaling molecule netrin-1’s engagement of A2BAR also dampens infiltration of neutrophils in models of lipopolysaccharide (LPS)-induced acute lung injury (96), hypoxia-induced lung inflammation (119), and acute experimental colitis (1). Taken together, extracellular adenosine metabolism and signaling is crucial in dampening inflammatory responses and involves shifting immune responses from proinflammatory to anti-inflammatory as well as protecting tissue integrity through inducing barrier protection.
Mechanisms of Hypoxia Adaptation
When oxygen becomes limited in tissues, such as occurs with inflammation, there is a need to be able to detect hypoxia as well as to initiate responses to adapt to the conditions. Recently, William Kaelin, Peter Ratcliffe, and Gregg Semenza were awarded the 2016 Albert Lasker Basic Medical Research Award for their exceptional discoveries that pioneered and has deepened our understanding of the fundamental pathways by which cells sense and respond to the presence of low oxygen. HIFs are αβ-heterodimeric transcription factors that are essential to hypoxia adaptation, having a central importance in orchestrating transcriptional programs that regulate tissue metabolism and maintenance of homeostasis in conditions of low oxygen.
When oxygen levels are sufficient in tissues, hypoxia-inducible factor-1 (HIF-1)-α and HIF-2α experience posttranscriptional iron- and oxygen-dependent hydroxylation, which occurs on two proline residues within the oxygen-dependent degradation (ODD) domain of the α-subunit. The α-subunit is recognized by the von Hippel-Lindau (VHL) tumor suppressor protein (pVHL) E3 ubiquitin ligase complex and sent for proteasome degradation (50, 68, 70, 103, 130). In hypoxic conditions, the hydroxylation of HIF-α subunits does not occur, which stabilizes HIF1α and HIF2α. With stabilization, HIF1α and HIF2α translocate to the nucleus and form a complex with the β-subunit and other coactivations and begin transcriptional regulation by binding to hypoxia-responsive elements (HRE) of target genes (123, 124). Over 2% of all human genes are direct target genes of HIFs and include genes that encode glycolytic enzymes, inhibit mitochondrial respiration, regulate apoptosis, modulate inflammation, and promote angiogenesis (92). Although HIF-1α and HIF-2α are closely related, knockout studies in mice have shown them to have nonredundant roles and distinct phenotypes (113). Tissue specificity and temporal expression of the isoforms are considered to account for the differences (64, 113, 118, 139). However, distinct transcriptional gene targets are more likely the source of the difference (65, 113). For example, the transcriptional gene regulation of glycolytic pathway enzymes appears to be driven by HIF-1α as opposed to HIF-2α (65). More so, the ability of HIF-2α to be involved in an oxygen-regulated translation initiation complex has been described (136). Hypoxia stimulates the formation of a complex involving HIF-2α, RNA-binding protein RBM4, and the cap-binding eIF4E2. The complex captures mRNAs and targets them for active translation, thus evading hypoxia-induced repression of protein synthesis. HIF-3α response in hypoxia is an area of active study (142).
The physical interaction of HIF-α subunits and pVHL is importantly controlled by prolyl hydroxylation. Prolyl hydroxylases (PHDs) were discovered to be essential molecular oxygen sensors after years of searching for the mechanism through which hypoxia stabilizes HIFs (50, 67, 68). In conditions of low oxygen, PHDs are inactive or have reduced activity since they require oxygen as a cofactor, which provides a clear connection between the availability of oxygen and the regulation of HIFs. In mammalian cells, three isoforms of PHD exists (PHD1, PHD2, and PHD3), which hydroxylate HIF-α and target HIF-α for proteasome degradation involving pVHL (50, 67, 68). Another level of control lies with asparaginyl hydroxylase factor inhibiting HIF (FIH), which is also oxygen-sensitive and iron dependent (84). Low oxygen levels block the hydroxylation of a conserved asparagine residue, facilitating the recruitment of transcriptional cofactors, such as p300, that are necessary for HIF-mediated gene transcription (85). Accordingly, both PHDs and FIH serve important roles as oxygen sensors by sensing the presence or absence of oxygen and controlling the activity of HIFs. HIF-driven gene programs have been shown to induce anti-inflammatory responses in a number of inflammatory disease models, for example, through the increased production and signaling effects of anti-inflammatory molecules, such as adenosine (3, 43, 76, 98, 99, 128) and netrin-1 (119).
Hypoxia Signaling During Inflammation
Inflammatory conditions are often characterized by hypoxia, whereas conditions of low oxygen are often characterized by the onset of tissue inflammation. Inflammation-associated hypoxia or hypoxia-associated inflammation is implicated in a number of human health conditions and diseases, including cancer, organ transplantation, and obesity as well as acute lung injury, IBD, and pathogen infection (45). Indeed, gut tissues of murine models of IBD, a disease characterized by excessive inflammation, are found to be profoundly hypoxic and show HIF stabilization (16, 71). In models of sepsis, proinflammatory factors, such as lipopolysaccharide (LPS), have been shown to directly stabilize HIFs (110). Here, LPS increases HIF-1α stability and decreases PHD in a Toll-like receptor (TLR)-dependent manner, specifically involving TLR4. As well, HIFs can also induce TLR expression, such as TLR2 and TLR6 (81). Several studies show evidence for HIF-1α stabilization during bacterial infections. For example, bacterial release of iron-binding siderophores, as such with Yersinia enterocolitica, can stabilize HIF by inhibiting PHD through forming chelate complexes with iron ions (61). Additionally, the citric acid cycle intermediate, succinate, can stabilize HIF-1α during LPS-induced inflammation (131).
Other studies show that NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells), a transcription factor important to inducing proinflammatory responses to pathogens, is intimately linked to hypoxia signaling. Studies by Taylor (22) suggested that PHDs may link oxygen sensing to NF-κB by IKK (IκB kinase complex). These studies identified that IKKβ (subunit of IκB kinase complex) contains a conserved prolyl hydroxylation site similar to HIF-1α (22). Hypoxia increases IKKβ stability through inhibition of IKKβ hydroxylation by PHD1, which directs NF-κB to disassociate from its inhibitor protein, thus promoting NF-κB to induce proinflammatory gene expression. Importantly, these studies demonstrated that oxygen-dependent PHDs are not restricted to regulating HIF-α to direct gene programs during inflammation and hypoxia. Additionally, similar to PHDs, the asparaginyl hydroxylase FIH has also been found to efficiently hydroxylate proteins of the IκB kinase family (15). Many more studies show that inflammatory stimuli can induce HIF-1α expression in a NF-κB-dependent manner, whereas, in turn, NF-κB activity in hypoxia can be regulated by HIF-1α (25).
Studies involving Type 1 regulatory cells (Tr1) and aryl hydrocarbon receptor (AHR) have provided additional insight into hypoxia signaling in modulating immune responses in inflamed tissues. Mascanfroni, Quintana, and colleagues (93) recently reported HIF-1α controls the early metabolic reprograming of Tr1 cells, whereas Tr1 cell metabolism is later controlled by AHR. AHR promotes HIF-1α degradation and increases CD39 expression. Extracellular ATP and hypoxia, both linked to inflammation, trigger AHR inactivation by HIF-1α and inhibit Tr1 cell differentiation. Conversely, CD39 promotes Tr1 cell differentiation by depleting extracellular ATP. Here, HIF-1β and HIF-1α interactions increase and the binding of AHR to HIF-1β decreases. AHR stability is reduced by being targeted to the proteasome, via HIF-1α, which decreases the recruitment of AHR to the CD39 promoter. Shift in control, i.e., HIF-1α to AHR, occurs with the upregulation of PHD proteins, promoted by AHR, which encourages HIF-1α degradation and the subsequent rise in CD39 expression and extracellular ATP depletion. Altogether, these data suggest that HIF-1α and AHR function to integrate immunological, metabolic, and environmental signals to regulate Tr1-type immune responses (93). Taken together, these findings support that hypoxia and inflammation share a close relationship. Although hypoxia itself is an inflammatory stimulus, there are many examples in which hypoxia signaling induces anti-inflammatory and tissue-protective responses, including that involving extracellular adenosine.
The Hypoxia-Adenosine Link
Similar to inflammation, an essential link exists between hypoxia, HIFs, and adenosine. As indicated earlier, it is well established that conditions of low oxygen or inflammation favor the release of extracellular ATP/ADP (48, 66). Indeed, cell membranes are damaged in these conditions, cell death is frequent, and nonlytic routes of ATP/ADP escape are exploited. Accordingly, extracellular adenosine levels increase at the cell surface. Considering that extracellular adenosine protects tissues in low oxygen, it was considered that adenosine metabolism and signaling genes were regulated by HIFs. Studies by the laboratory of Colgan (128) identified a HRE in the promoter of the CD73 gene and demonstrated CD73 to be a transcriptional target of HIF-1α. In 2004, with the successful generation of CD73-deficient mice, Thompson and colleagues (135) showed the extent to which CD73 gene regulation by HIF-1α was critical in low oxygen conditions. CD73-deficient mice suffer substantial vascular leakage and inflammatory cell accumulation in multiple organs with short-term exposure to low oxygen (49, 135). Extensions of these studies including CD39-deficient mice showed that extracellular adenosine production during hypoxia is immediate to the anti-inflammatory actions of neutrophils (49). CD39 is induced in hypoxia by transcription factor Sp1 (47). Sp1 is strongly implicated in hypoxic gene transcription and has been shown to play a protective role in regulation of CD39 during cardiac and hepatic ischemia (47, 59, 100).
CD73 is not the only adenosine pathway gene targeted by HIFs. Adenosine receptors, in particular A2AAR and A2BAR, are under the transcriptional control of HIFs (3, 76). Experimental studies provide evidence that A2AAR is a target gene of HIF-2α (3), whereas A2BAR is gene target of HIF-1α (76). Inasmuch as A2BAR protects the barrier in inflammation, pharmacological and genetic studies have demonstrated A2BAR is charged with protecting the barrier in low oxygen conditions. Indeed, A2BAR-deficient mice experience significant barrier leakage and increased tissue accumulation of immune cells when exposed to hypoxia (33). Genetic deletion studies of each adenosine receptor in mice indicate A2BAR as having a selective role in barrier protection in hypoxia (33). A2BAR expression is increased in murine studies of ischemia-reperfusion injury (60). As well, mice with gene deletion of CD73 and A2BAR experience more profound tissue injury with ischemia (41, 60). Ischemia results in a robust increase in HIFs, which is important to protecting tissues (40). Tissues experience significant destruction in the absence of HIF-1α with ischemia, whereas PHD2 absence substantially increases HIF-1α and attenuates tissue damage. Notably, HIF-1α-dependent ischemic protection is abolished in A2BAR-deficient mice (40). Studies with HIF activator, dimethyloxalylglycine (DMOG), provide another example in which the anti-inflammatory effects of HIF are routed through extracellular adenosine/adenosine signaling. Treatment of CD73 and A2BAR-deficient mice with DMOG provides no protection against ischemic injury (40).
HIFs have also been implicated in attenuating the termination of adenosine signaling. As such, HIFs directly repress the expression of ENT1 and ENT2 (43, 99) as well as reduce the intracellular conversion of adenosine to AMP via the adenosine kinase (98). Studies involving radiolabeled adenosine showed extracellular adenosine uptake slows in hypoxia and that ENT1 and ENT2 expression significantly decreases (43, 99). ENT1 and ENT2 are the main adenosine transporters that orchestrate the movement of adenosine from the cell surface to the intracellular space in vascular endothelial and epithelial cells. Accordingly, these studies demonstrated that ENT1 and ENT2 are direct transcriptional targets of HIF-1α, HIF-1α decreases ENT1 and ENT2 expression in hypoxia, and ENT1 and ENT2 repression attenuates inflammatory responses (43, 99). Interestingly, epithelial-targeted HIF-1α-deficient mice have increased expression of ENTs (43, 99). An elegant study by Schrader and colleagues (30) has shown that hypoxia-induced inhibition of adenosine kinase (AK) increases the release of extracellular adenosine. Studies with promoter constructs and a combination of HIF loss- and gain-of-function studies indicated AK is transcriptionally repressed by HIF-1α and inhibiting AK in vivo attenuates hypoxia-induced vascular leakage (98), thereby showing another adenosine pathway member targeted by HIF. Consistent with the role of HIFs in inducing extracellular adenosine and adenosine signaling, netrin-1 is increased by HIF-1α (119). As indicated earlier, netrin-1 can enhance A2BAR signaling to attenuate inflammatory responses (1, 96, 119). These studies show that by engaging A2BAR on neutrophils, HIF-1α-induced netrin-1 inhibits neutrophil transmigration, therefore dampening hypoxia-induced inflammation. Taken together, the above studies demonstrate that in hypoxia, HIFs coordinate the transcriptional control of multiple members of the adenosine pathway. HIFs increase extracellular adenosine-metabolizing enzymes and adenosine receptors, whereas expression of adenosine transport and intracellular metabolism genes are decreased. As such, this provides substantial support for adenosine signaling to protect tissues during conditions of low oxygen (Fig. 1). Additionally, studies involving erythrocyte metabolism and high-altitude adaptation have indicated an important role of the adenosine/purinergic pathway in driving adaptations to hypoxia. Exposure to hypoxia enhances erythrocyte glycolysis and higher ATP availability results in the accumulation of AMP and adenosine (24). Excellent studies by the laboratory of Xia (88) show soluble CD73 activity increases at high altitude and is associated with elevated erythrocyte 2,3-bisphosphoglycerate (2,3-BPG), a negative allosteric regulator of hemoglobin-O2 binding affinity, the expression of which increases through erythrocyte A2BAR activity involving AMP-activated protein kinase. Here, A2BAR activation was beneficial in inducing 2,3-BPG and triggering O2 release to prevent multiple tissue hypoxia, inflammation, and pulmonary leakage (88).
Examples for Hypoxia-Adenosine Link During Inflammatory Diseases
In the above paragraphs, we have discussed the associations of inflammation with adenosine, hypoxia with inflammation, and adenosine with hypoxia. Here, we focus on studies that bring together and emphasize the link between hypoxia and adenosine during inflammatory conditions and disease, specifically myocardial injury, inflammatory bowel disease, and acute lung injury. Together, these studies provide significant support that hypoxia-elicited, HIF-driven adenosine metabolism and signaling is protective to tissues, particularly by dampening inflammation and protecting tissue barriers.
Myocardial injury.
Myocardial injury is a significant health condition characterized by obstruction of blood supply to the heart. Damage occurs initially from the loss of blood supply and again with the sudden movement of blood back into cardiac tissues and reoxygenation. In many instances, profound inflammation occurs. Substantial evidence indicates that extracellular adenosine/adenosine signaling provides considerable cardioprotection during myocardial ischemia-reperfusion injury. For example, a robust induction of both CD39 and CD73 occurs in ischemic preconditioning. Deletion of CD39 or CD73 in mice results in larger infract sizes after closing off the coronary artery, whereas treatment of CD73-deficient mice with 5′-nucleotidase or CD39 mice with AMP or apyrase attenuates infarct sizes and provides additional cardioprotection benefit to wild-type mice during ischemic preconditioning (41, 75). The protection of CD73 to the heart has long been appreciated, as earlier studies in dogs revealed that inhibiting CD73 abolishes cardioprotection by ischemic preconditioning (73). Interestingly, the induction of CD39 expression by transcription factor Sp1 was shown to be central to providing cardioprotection in experimental studies of hypoxia and ischemia (47). Sp1 is strongly implicated in hypoxic gene transcription and shares similar target genes with HIFs (106).
Experimental studies in various models indicate that A1AR and A3AR are involved in providing protection before ischemia, A2AAR has promising protective actions during reperfusion, and more recent studies indicate that A2BAR is important as well in modulating myocardial reperfusion injury (95). Studies by Linden (141) indicated that A2AAR activity on T and B cells are involved in dampening ischemia-reperfusion injury in the heart. Other studies observed in a head-to-head comparison in mice deficient for each adenosine receptor that A2BAR-deficient mice are particularly susceptible to myocardial ischemia (41). Extension of these studies showed a robust increase in HIF-1α during ischemic preconditioning and that in vivo gene silencing of cardiac HIF-1α abolished cardioprotection, whereas pretreatment with HIF activator, DMOG, provided significant cardioprotection. Moreover, gene silencing of PHD2 resulted in significant HIF-1α activation and attenuated myocardial infarct sizes (40). Importantly, these studies demonstrated that HIF-dependent cardioprotection is through A2BAR. HIF-dependent cardioprotection was abolished in A2BAR-deficient mice (40). A2BAR providing cardioprotection is further supported by studies showing A2BAR agonist treatment significantly reduces infarct sizes after ischemia in wild-type mice but not A2BAR-deficient mice (41). More recently, the circadian rhythm protein period 2 (Per2) was identified as a target of HIF-dependent, A2BAR-elicited cardioprotection (36). A2BAR led to the stabilization of Per2 during myocardial ischemia, whereas Per2-deficient mice had larger infarct sizes. Ischemic hearts of Per2-deficient mice showed a limited ability to use carbohydrates for oxygen-efficient glycolysis, which was due to the stabilization of HIF-1α. The importance of the hypoxia-adenosine link in myocardial protection can be further supported by studies showing that hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release (30), and that ENT1-deficient mice are particularly more resilient to cardiac ischemia as opposed to wild-type mice (117). Further support includes studies of myocardial protection by remote ischemic preconditioning (RIPC) (112). RIPC is considered to protect the heart from subsequent ischemic myocardial injury. RIPC involves brief cycles of ischemia and reperfusion to an arm or a leg by inflating/deflating a cuff, which causes the release of protective molecules, such as adenosine. Myocardial protection by RIPC is mediated by adenosine receptor activity (112). Interestingly, the cardioprotection by RIPC is abrogated in HIF-1α-deficient mice and HIF-driven gene expression reduced (13).
Most of the above studies implicate adenosine and HIF in preventing myocardial injury during ischemic preconditioning. Certainly, the administration of adenosine or adenosine receptor antagonists, such as A2BAR agonist BAY 60-6583, or compounds that inhibit oxygen sensors, such as PHDs, thereby preventing HIF-α hydroxylation and leading to HIF stabilization, are attractive therapeutic approaches to minimizing myocardial injury. Both approaches would appear beneficial to administering to patients before undergoing major cardiac or thoracic surgery. PHD inhibitors are currently in active clinical trials for renal disease (Table 1) and appear to be safe for patients (8, 44, 51). The efficacy and safety of BAY 60-6583 in humans is unknown and remains mostly used in the context of experimental disease models (33, 35, 40, 57, 60, 121). Intravenous infusion of BAY 60-6583 is reported to have negligible effects on hemodynamics (82). Two multicenter clinical trials, Acute Myocardial Infarction Study of Adenosine (AMISTAD) I and II (91, 120), have examined the therapeutic potential of adenosine in the context of cardioprotection during reperfusion injury. Intravenous infusion of high-dose adenosine was shown to reduce myocardial infarct sizes. Patients administered adenosine with reperfusion therapy within the first few hours had significantly improved outcomes compared with placebo treatment (74). Importantly, these studies indicate that if adenosine reperfusion is accomplished within a small time period of onset of injury, the benefit of cardioprotection of adenosine is much greater. If reperfusion is delayed, it becomes rather difficult for adenosine to salvage the tissue. Therefore, the use of BAY 60-6583 or PHD inhibitors in situations such as a heart attack may have the greatest benefit when given in a defined time period during or after myocardial injury. Further experimental studies are needed to define such a therapeutic window. However, there is much hope for these compounds for the treatment of myocardial injury. Notably, adenosine has a considerably shorter half-life (1–2 s). Therefore, the use of BAY 60-6583 or PHD inhibitors, which are far more stable, would be considered to improve efficacy and reduce side effects due to adenosine’s nonselectivity [e.g., bradycardia, hypotension, rapid receptor desensitization (41)].
Table 1.
Therapeutic targets of the adenosine and hypoxia pathways and health conditions and diseases of current clinical trials
| Drug | Target | Phase | Condition | Indications | https://ClinicalTrials.gov Identifier (Status) |
|---|---|---|---|---|---|
| ATP | Phase 2 | Major depressive disorder | Antidepressant effect | NCT03138681 (recruiting) | |
| Acetogenins | ATP production inhibitor | Phase 2/Phase 3 | Breast cancer | Anti-proliferative, apoptosis effect | NCT02286778 (active, not recruiting) |
| Ticagrelor | P2Y12 antagonist | Phase 4 | Microvascular angina | Ameliorate coronary microvascular function | NCT02284048 (not yet recruiting) |
| Phase 4 | Migraine headache | Eliminate migraine headaches | NCT02518464 (recruiting) | ||
| Phase 3 | Acute ST-elevation myocardial infarction | Antiplatelet therapy | NCT01739556 (recruiting) | ||
| Phase 3 | Diabetes mellitus, Type 2 | Effect on cardiovascular death, myocardial infarction, or stroke | NCT01991795 (active, not recruiting) | ||
| Cangrelor | P2Y12 antagonist | Phase 1 | Partial obstruction of systemic to pulmonary artery shunt; complete obstruction of systemic to pulmonary artery shunt | Safety profile in neonatal participants at risk of thrombosis | NCT02765633 (recruiting) |
| Cangrelor; Ticagrelor | P2Y12 antagonist | Phase 4 | Acute coronary syndrome; microvascular obstruction; ST-segment elevation myocardial infarction; thrombolysis in myocardial infarction; unstable angina | Intravenous antiplatelet therapy | NCT02733341 (recruiting) |
| Clopidogrel; Ticagrelor | P2Y12 antagonist | Phase 4 | Coronary artery disease | Reduce later heart attacks | NCT02327624 (active, not recruiting) |
| Phase 4 | Patients who are scheduled to undergo a PCI (percutaneous coronary intervention) for CTO (chronic total occlusion) | Percutaneous coronary intervention and the recovery of vascular function at long-term | NCT02211066 (recruiting) | ||
| Phase 3 | Acute coronary syndrome | Prevention of fatal and nonfatal cardiovascular events, observation of coronary circulation effects due to chronic use | NCT02618733 (recruiting) | ||
| Phase 2 | Coronary artery disease; kidney dysfunction | Reducing ischemic events | NCT03039205 (not yet recruiting) | ||
| Phase 2 | Stroke; ischemic attack, transient | Combination with aspirin effect on reducing transient ischemic attack or minor stroke | NCT02506140 (recruiting) | ||
| Prasugrel; Ticagrelor | P2Y12 antagonist | Phase 4 | Acute coronary syndrome | Pleiotropic effects to antiplatelet therapy in type 2 diabetic patients with non-ST elevation acute coronary syndrome | NCT02487732 (active, not recruiting) |
| MEDI9447 with or without MEDI4736 | CD73 (MEDI9447); anti-PD-LI antibody (MEDI4736) | Phase 1 | Solid tumors | Safety profile in adult subjects with select advanced solid tumors (combat tumor immunosuppression) | NCT02503774 (recruiting) |
| Adenosine | Phase 2 | Cardiomyopathies | An adjunct to intermittent warm blood cardioplegia | NCT02681913 (recruiting) | |
| Phase 2 | Takotsubo cardiomyopathy; Takotsubo syndrome | Induce rapid recovery of left ventricle function | NCT02867878 (recruiting) | ||
| Phase 2/Phase 1 | Sinus bradycardia; atrioventricular block | Safety profile in children with heart transplant, effectiveness in treating fast heart rate | NCT02462941 (active, but not recruiting) | ||
| Neladenoson Bialanate (BAY 1067197) | A1AR agonist | Phase 2 | Heart failure | Optimal dose given in addition to standard therapy for heart failure | NCT02992288 (recruiting) |
| Regadenoson | A2AAR agonist | Phase 2 | Retinal artery occlusion | Induce vasodilatation in adjacent retinal areas to increase blood flow and retinal oxygenation | NCT03090087 (recruiting) |
| Phase 2 | Sickle cell anemia | Effective treatment for pain crises and acute chest syndrome in sickle cell disease | NCT01788631 (recruiting) | ||
| Phase 1 | Lung transplant | Ischemia reperfusion injury lowered or prevented. Regadenoson is an A2AAR drug | NCT03072589 (not yet recruiting) | ||
| Phase 1 | Blood-brain barrier defect | Transiently disrupt the blood-brain barrier, improve drug penetration | NCT02389738 (recruiting) | ||
| N/A | Sickle cell disease; sickle cell anemia | Prevent inflammation and injury caused by the sickle shaped cells | NCT01566890 (recruiting) | ||
| CF102 | A3AR agonist | Phase 2 | Nonalcoholic steatohepatitis | Safety and efficacy, improve liver pathology | NCT02927314 (not yet recruiting) |
| Phase 2 | Hepatocellular carcinoma | Efficacy and safety, tumor reduction, improved survival | NCT02128958 (recruiting) | ||
| Aminophylline | Nonselective adenosine receptor (AR) antagonist | Phase 1 | Premature obstetric labor | Combination with progesterone reduces the risk of preterm labor | NCT03152942 (not yet recruiting) |
| Phase 1 | Acute kidney injury | Mitigates this vasoconstriction, reduce ischemic kidney injury | NCT02983422 (not yet recruiting) | ||
| Vivarin (caffeine) | Nonselective AR antagonist | Phase 2/Phase 1 | Narcolepsy | Effects of caffeine consumption on daytime sleepiness and reaction time | NCT02832336 (recruiting) |
| Caffeine | Nonselective AR antagonist | Phase 2/Phase 1 | Pain | Assess caffeine on acupuncture analgesia | NCT02577770 (recruiting) |
| N/A | Schizophrenia | Improve cognitive function | NCT02832401 (not yet recruiting) | ||
| PBF-680 | A1AR antagonist | Phase 2 | Asthma | To attenuate “late asthmatic responses” as a primary efficacy outcome, assess airway inflammation-related outcomes | NCT02635945 (recruiting) |
| Phase 3 | Acute coronary syndrome | Prevention of fatal and nonfatal cardiovascular events, observation of coronary circulation effects due to chronic use | NCT02618733 (recruiting) | ||
| Istradefylline | A2AAR antagonist | Phase 3 | Idiopathic Parkinson’s disease | Safety and tolerability, nondopaminergic therapy | NCT02610231 (active, not recruiting) |
| Pentoxifylline | A2AAR antagonist | Phase 3 | Acute pancreatitis; gallstone pancreatitis; alcoholic pancreatitis; Post-ERCP/postprocedural pancreatitis; trauma acute pancreatitis; hypertriglyceridemia acute pancreatitis; idiopathic (unknown) acute pancreatitis; medication-induced acute pancreatitis; cancer acute pancreatitis; miscellaneous (i.e., acute or chronic pancreatitis) | Inhibition of the tumor necrosis factor-alpha pathway reduces inflammation | NCT02487225 (enrolling by invitation) |
| PBF-509 with or without PDR001 | A2AAR antagonist (PBF-509); anti-PD-1 antibody (PDR001) | Phase 2/Phase 1 | Non-small cell lung cancer (NSCLC) | Safety, tolerability, feasibility, and preliminary efficacy (combat tumor immunosuppression) | NCT02403193 (recruiting) |
| CPI-444 with or without atezolizumab | A2AAR antagonist (CPI-444); anti-PD-L1 antibody (atezolizumab) | Phase 1 | NSCLC; malignant melanoma; renal cell cancer; triple negative breast cancer; colorectal cancer; bladder cancer | Safety, tolerability, and anti-tumor activity (combat tumor immunosuppression) | NCT02655822 (recruiting) |
| EZN-2279; Adagen | Adenosine deaminase (ADA) | Phase 3 | ADA-SCID; adenosine deaminase deficiency; severe combined immunodeficiency | Safety, efficacy, and pharmacokinetics [enzyme (ADA) replacement therapy] | NCT01420627 (recruiting) |
| ADA gene transduced CD34+ cells | ADA | Phase 2 | Immunologic deficiency syndromes | Safety and the clinical efficacy of gene therapy (ADA), evaluate the immunological reconstitution and purine metabolism after gene therapy | NCT00598481 (active, not recruiting) |
| EF1αS-ADA | ADA | Phase 2/Phase 1 | Adenosine deaminase deficiency; severe combined immunodeficiencies (SCID) | Safety, feasibility, effectiveness of gene therapy for ADA-deficient SCID using lentiviral vector | NCT01380990 (recruiting) |
| EFS-ADA | ADA | Phase 2/Phase 1 | ADA-SCID | safety and effectiveness of using a lentiviral vector (based on HIV-1) for ADA-deficient SCID | NCT01852071 (active, not recruiting) |
| Pentostatin | ADA inhibitor | Phase 3 | Graft-vs.-host disease | Efficacy of ruxolitinib against best available therapy in participants with steroid-refractory chronic graft-vs.-host disease | NCT03112603 (recruiting) |
| Phase 2 | Renal cell carcinoma; Graft-vs.-Host disease; Engraftment Syndrome | Determine whether new, low-intensity transplant approach can yield objective partial or complete remission | NCT00923845 (active, not recruiting) | ||
| Phase 2 | Leukemia; lymphoma | Stop the growth of cancer cells, combination therapy | NCT00602836 (active, not recruiting) | ||
| Phase 2 | Acute lymphoblastic leukemia; acute myeloid leukemia; chronic lymphocytic leukemia; chronic myelogenous leukemia, BCR-ABL1 positive; graft vs. host disease; Hodgkin lymphoma; myelodysplastic/myeloproliferative neoplasm; non-Hodgkin’s lymphoma; plasma cell myeloma; Waldenstrom macroglobulinemia | Preventing graft rejection in patients who have undergone donor stem cell transplant | NCT00096161 (active, not recruiting) | ||
| Phase 2 | Hairy cell leukemia | Determine whether pentostatin or bendamustine is a more effective when combined with rituximab | NCT01059786 (recruiting) | ||
| Phase 2 | Graft-vs.-host disease | Effectiveness in treating chronic graft-vs.-host disease in patients refractory to treatment with steroids | NCT00074035 (active, not recruiting) | ||
| Phase 2/Phase 1 | Sickle cell disease | Novel immunosuppressive regimen without myeloablation to further decrease the transplant-related morbidity/mortality | NCT03077542 (recruiting) | ||
| Phase 2/Phase 1 | Sickle cell disease; thalassemia; stem cell transplantation; graft vs. host disease | Reduce the transplant failure rate | NCT02105766 (recruiting) | ||
| Phase 2/Phase 1 | Mesothelioma; adenocarcinoma of lung; pancreatic neoplasms | Safety, tolerability, and feasibility of recombinant anti-mesothelin immunotoxin in combination with immune-depleting regimen | NCT01362790 (active, not recruiting) | ||
| Phase 1 | Leukemia, B cell; lymphoma, Hodgkin’s lymphoma; non-Hodgkin’s lymphoma, B cell | Preventing graft rejection | NCT01087294 (recruiting) | ||
| Phase 1 | Chronic lymphocytic leukemia; B cell non-Hodgkin's lymphoma | Synergy between bendamustine and purine analogs in killing cancer cells | NCT01352312 (active, not recruiting) | ||
| Vadadustat (AKB-6548) | Hypoxia-inducible factor-prolyl hydroxylase (PHD) inhibitor | Phase 3 | Anemia; non-dialysis-dependent chronic kidney disease | Correction of anemia and maintenance of hemoglobin | NCT02648347 (recruiting) |
| Phase 3 | Anemia; dialysis-dependent chronic kidney disease | ↓ | NCT02865850 (recruiting) | ||
| Phase 3 | Anemia; non-dialysis-dependent chronic kidney disease | ↓ | NCT02680574 (recruiting) | ||
| Phase 3 | Anemia in subjects With DD-CKD | ↓ | NCT02892149 (recruiting) | ||
| Phase 2 | Anemia; dialysis-dependent chronic kidney disease | ↓ | NCT03054350 (active, not recruiting) | ||
| Phase 2 | Anemia; non-dialysis dependent chronic kidney disease | ↓ | NCT03054337 (active, not recruiting) | ||
| Phase 2 | Anemia; dialysis-dependent chronic kidney disease | ↓ | NCT03140722 (recruiting) | ||
| Daprodustat (GSK1278863) | PHD inhibitor | Phase 3 | Anemia | Maintain hemoglobin | NCT03029208 (recruiting) |
| Phase 3 | Anemia | ↓ | NCT02969655 (recruiting) | ||
| Phase 3 | Anemia | ↓ | NCT02876835 (recruiting) | ||
| Phase 3 | Anemia | ↓ | NCT02879305 (recruiting) | ||
| Phase 2 | Anemia | ↓ | NCT03029247 (not yet recruiting) | ||
| Roxadustat (FG-4592); also known as AP1517 | PHD inhibitor | Phase 3 | Anemia in chronic kidney disease in nondialysis patients | To treat anemia | NCT02021318 (recruiting) |
| Phase 3 | Anemia | ↓ | NCT02174627 (active, not recruiting) | ||
| Phase 3 | Anemia; end-stage renal disease (ESRD) | ↓ | NCT02278341 (active, not recruiting) | ||
| Phase 3 | Anemia | ↓ | NCT02174731 (recruiting) | ||
| Roxadustat (FG-4592); also known as AP1517 | PHD inhibitor | Phase 3 | Anemia in chronic kidney disease in nondialysis patients | To treat anemia | NCT01887600 (active, not recruiting) |
| Phase 3 | CKD anemia in stable dialysis patients | ↓ | NCT02273726 (recruiting) | ||
| Phase 3 | Anemia in incident dialysis patients | ↓ | NCT02052310 (recruiting) | ||
| Phase 1 | Normal renal function; impaired renal function | Evaluate pharmacokinetics in subjects with different degrees of renal function | NCT02965040 (recruiting) | ||
| ASP1517 | PHD inhibitor | Phase 3 | Chronic kidney disease | Oral dosing; treatment of anemia | NCT02988973 (recruiting) |
| Phase 3 | Peritoneal dialysis chronic kidney disease patients with anemia | ↓ | NCT02780726 (active, not recruiting) | ||
| Phase 3 | Chronic kidney disease | ↓ | NCT02964936 (recruiting) | ||
| Phase 3 | Hemodialysis chronic kidney disease patients With anemia | ↓ | NCT02952092 (recruiting) | ||
| Remote Ischemic Preconditioning | N/A | Myocardial infarction | Effective endogenous cardiac protection, assess major adverse cardiovascular events such as cardiovascular death, spontaneous myocardial infarction, unplanned revascularization and stroke | NCT02843464 (not yet recruiting) | |
| N/A | Myocardial infarction, acute | Effective endogenous cardiac protection | NCT03018873 (not yet recruiting) |
↓, Same as previous indication.
Inflammatory bowel disease.
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, represents a group of intestinal disorders caused by dysregulated immune responses and aberrant mucosal barrier protection. IBD is characterized by excessive inflammation of the bowel and profound hypoxia (17, 71). A substantial number of studies show HIFs are stabilized in experimental models of colitis (23, 71, 99, 116) and have almost uniformly beneficial influence on disease outcomes (16). For example, mice with deletions of HIF-1α in intestinal epithelial cells are more susceptible to intestinal inflammation and have more severe disease during experimental colitis (16, 44, 71, 132), whereas deletion of HIF-2α appears to be protective (140). HIF-1α deletion was associated with increased weight loss, increased intestinal inflammation, and more profound colonic shortening (71). Similarly, the deletion of VHL (71) or PHD1 (129) in mice appears to be protective during intestinal inflammation, again implicating HIFs in gut protection during IBD (16, 44, 132). Notably, CD73 expression was profoundly induced in VHL-deficient mice (71). Several studies demonstrate that pharmacological compounds that stabilize HIFs provided potent protection during intestinal inflammation (23, 44, 52, 116). In general, CD39- (54) and CD73 (89)-deficient mice develop increased disease severity during experimental colitis. For example, CD73-deficient mice experienced significant weight loss and colonic shortening with trinitrobenzene sulfonate (TNBS) colitis, and similarly, treatment of wild-type mice with CD73 inhibitor α,β-methylene ADP results in a similar increase in colitis severity (89). The onset of significant gut hypoxia during IBD was shown by the laboratory of Colgan (16, 71, 132) using murine IBD models and 2-nitroimidazole compounds. In addition, specimens from patients with IBD show prominent HIF-1α and HIF-2α activation (55). Similar to CD73 (128), A2BAR is directly induced by HIF-1α (76). Several studies implicate A2BAR signaling in IBD (1, 2, 53). A2BAR-deficient mice have an increased susceptibility to dextran sulfate sodium (DSS)-induced colitis; mice experience increased weight loss, colonic shortening, and disease activity indexes. Similarly, severity of colitis is increased in wild-type mice treated with the A2BAR antagonist PSB1115 (53). A2BAR-deficient mice as well as treatment of intestinal epithelial cells with PSB1115 show deficiencies in the anti-inflammatory cytokine IL-10, implicating A2BAR signaling as having anti-inflammatory activity in IBD. Both CD73 and A2BAR-deficient mice suffer extensive breakdown of tissue barriers with hypoxia (10, 33, 135). Studies have shown intestinal epithelial A2BAR provides significant protection during experimental colitis by enhancing barrier protection responses.
Indeed, mice with global or tissue-specific deletion of A2BAR experience increased severity of colitis, associated with a more acute onset of disease and epithelial barrier breakdown (1, 2, 53). In vitro and in vivo studies implicated A2BAR-driven phosphorylation of vasodilator-stimulated phosphoprotein (VASP) as a specific response to regain the barrier (2). These studies showed as well epithelial A2BAR signaling to selectively attenuate gut inflammation as opposed to vascular endothelial A2BAR (2). As well, other studies have demonstrated netrin-1-deficient mice to suffer significant disease severity during DSS colitis (1). Previous studies show HIF-1α-dependent induction of netrin-1 to attenuate hypoxia-elicited inflammation of mucosal surfaces by netrin-1 engaging A2BAR on neutrophils (119). Netrin-1-deficient mice show increased weight loss and colonic shortening during experimental colitis, whereas mice treated with exogenous netrin-1 show far less disease severity. These studies further showed that netrin-1-dependent attenuation of colitis is through suppression of neutrophil trafficking by A2BAR (119). Altogether, these studies provide substantial evidence that HIF-1-elicited adenosine metabolism and signaling is an essential pathway that provides considerable anti-inflammatory responses and barrier production during IBD.
Several studies have indicated and more are ongoing that provide the basis for therapeutic use of PHD inhibitors in IBD (44, 116). A concern of PHD inhibitors is that they are nonspecific for PHD isoforms, and many tissues express PHDs. As well, PHD inhibitors have the potential to inhibit other hydroxylases. The development of tissue-specific delivery of PHD inhibitors has the potential to improve upon these concerns and is an area of rising interest. Experimental studies have shown significant benefit of PHD inhibitor, AKB-4924, in murine models of colitis (72). Specifically, these studies demonstrated PHD inhibition in gut epithelial cells is immediate to providing therapeutic benefit in colitis. Oral administration of AKB-4924 therefore may be ideal as opposed to systemic administration of PHD inhibitors. Such an approach may limit possible adverse side effects. The oral PHD inhibitor roxadustat (FG-4592) is in clinical trials for renal disease (Table 1). Experimental studies also support the therapeutic benefit of the A2BAR agonist BAY 60-6583 in IBD (2). It would be worthwhile to assess the possible use of oral administration of BAY 60-6583 as a therapeutic strategy for IBD.
Acute lung injury.
Acute lung injury (ALI) is a lung disease characterized by pulmonary edema and severe hypoxia (138). ALI is caused by injuries or acute infections to the lungs. The past decade has provided considerable evidence of a link between hypoxia and adenosine signaling in dampening pulmonary edema and promoting resolution of lung inflammation (34, 35, 37–39, 121, 138). Patients with ALI often require mechanical ventilation due to difficulties in breathing, whereas ALI often occurs with mechanical ventilation. Studies using cyclic mechanical stretch, a model that mimics cyclic opening and closing of the airway during mechanical ventilation, identified that stretched pulmonary epithelial cells release extracellular adenosine (34, 138). Indeed, mechanical ventilation in mice increases pulmonary extracellular adenosine. Here, studies showed CD39- and CD73-deficient mice suffer severe ALI with mechanical ventilation. Similarly, drug inhibition of CD73 in mice induces significant pulmonary edema and inflammation, whereas treatment of CD39- or CD73-deficient mice with apyrase or 5′-nucleotidase significantly attenuates ventilator-induced ALI, implicating the role of extracellular adenosine in lung protection during ALI (34). Consistent with the continuing theme of A2BAR signaling to protect the barrier, studies show A2BAR is specifically associated with attenuating ventilator-induced ALI (35). Both A2BAR-deficient and wild-type mice treated with an A2BAR antagonist suffer pulmonary edema, inflammation, and attenuated gas exchange, whereas an A2BAR agonist attenuates ALI. Additional studies showed pulmonary inflammation involves A2BAR signaling from bone marrow-derived cells, whereas A2BAR on the lung tissue is responsible for attenuating pulmonary edema. A2BAR is also implicated in the protection of ALI induced by endotoxins (121). Other studies implicate ENT-dependent adenosine uptake enhances A2BAR signaling on pulmonary epithelial cells to dampen ALI (37). These studies revealed ENT2 to be selectively involved in attenuating pulmonary edema and improving gas exchange during ALI. Notably, increased extracellular adenosine was found in bronchoalveolar fluid, and ENT-dependent lung protection was abolished in mice with epithelial A2BAR deletion.
Recent studies identified HIF-1α as a transcriptional inducer of A2BAR during ventilation-induced ALI (38). Stretch-induced A2BAR was abolished with silencing of HIF-1α in pulmonary epithelial cells. Similarly, drug inhibition of HIF-1α inhibited the increase of A2BAR expression during cyclic stretch. Moreover, studies of ventilator-induced ALI revealed induction of A2BAR expression was abolished in conditional HIF-1α-deficient mice. An elegant study from the laboratory of Sitkovsky (134) considered that oxygenation may weaken hypoxia-elicited A2AAR-mediated anti-inflammatory responses, thereby further exacerbating ALI. The study is based on the observation that hypoxia induces A2AAR signaling that attenuates lung inflammation and tissue damage. In a bacterial infection ALI model, wild-type mice exposed to 100% oxygen, mimicking therapeutic oxygenation, had reduced survival rates compared with mice exposed to 21% oxygen. Indeed, five times more mice died after exposure to 100% oxygen. Intratracheal injection of an A2AAR agonist mimics the protective effects of hypoxia upon oxygenation with ALI, thereby decreasing lung injury and improving survival rates. Thus high oxygen percentages, as provided to patients suffering from ALI, may weaken hypoxia-driven, adenosine signaling-mediated protection of the lungs. Another study implicates HIF-2α in the control of the A2AAR in human pulmonary endothelial cells during hypoxia (3). Taken together, these studies emphasize that hypoxia is protective in ALI and involves the stabilization of HIFs and HIF-directed induction of adenosine metabolism and signaling.
Similar to IBD, patients with ALI are likely to benefit from therapeutic strategies that increase HIF stabilization and adenosine signaling, especially therapeutic approaches that are specifically directed to the lung. Recent studies have shown that inhaled BAY 60-6583 treatment during ALI in mice attenuates pulmonary edema, reduces inflammation, and protects against tissue damage of the lungs (63). Here, these studies found A2BAR of alveolar epithelial cells are central to lung protection during ALI. Inhaled drug delivery has a significant benefit to systemic delivery in that systemic side effects would be reduced and lower concentration of the drug could be used. PHD inhibitors have shown significant increases in survival and decreased lung inflammation during mechanical ventilation-induced ALI (32). These studies also implicate HIF-1α stabilization specifically in alveolar epithelial cells to be central to dampening lung inflammation during ALI. The delivery of PHD inhibitors by an inhaled approach may also provide significant benefit during ALI. However, future studies are needed to determine the benefit of such an approach.
Conclusions
Great progress has been made over the past decade to bring the adenosine pathway to the clinic. Indeed, many adenosine and several adenosine receptor agonists and antagonists are currently in clinical trials or are in use in preclinical studies in many conditions and diseases, such as Parkinson’s disease, sickle cell anemia, and cancer (Fig. 2 and Table 1). Recently completed clinical trials for adenosine metabolism and signaling are summarized in Fig. 3. In the present review, we discussed that adenosine metabolism and signaling is essential to dampening inflammation and protecting tissue barriers, and immediately involved with this are HIFs. Approaches to therapeutically target this response in myocardial injury, IBD, or ALI possibly include drugs that decrease proinflammatory responses induced through ATP/ADP receptors, such as P2Y12. Ticagrelor and Clopodogrel, both P2Y12 antagonists, are in clinical trials for several health conditions. Drugs that increase extracellular adenosine, such as adenosine analogs, dipyridamole, an ENT inhibitor, or pentostatin, an ADA inhibitor, may also have benefit. Pentostatin is currently in clinical trials for graft vs. host disease and different cancers; it is FDA approved for the treatment of hairy cell leukemia. Agents that increases A2AAR and A2BAR signaling serve as possible treatment targets as well, such as Regadenoson, an A2AAR agonist, and BAY 60-6583. Regadenoson is in clinical trials for a number of heart conditions as well as sickle cell disease. BAY 60-6583 use remains in experimental settings (33, 35, 40, 57, 60, 121). Adenosine receptor antagonists and CD73/CD39 inhibitors are being studied in clinical trials as potent anticancer drugs, specifically as agents that boost immune responses against tumors (4). On the contrary, increasing adenosine levels show benefit in certain leukemia and lymphomas and are related to adenosine inducing apoptosis in the cancer cells (115). Similarly, CF-102 (A3R agonist) has shown promise to patients with hepatocellular cancer in recent clinical trials and is suspected of inducing cancer cell apoptosis (6, 125). Adenosine metabolism and signaling can have very different roles in different tumors and cells of the tumor microenvironment (10, 11).
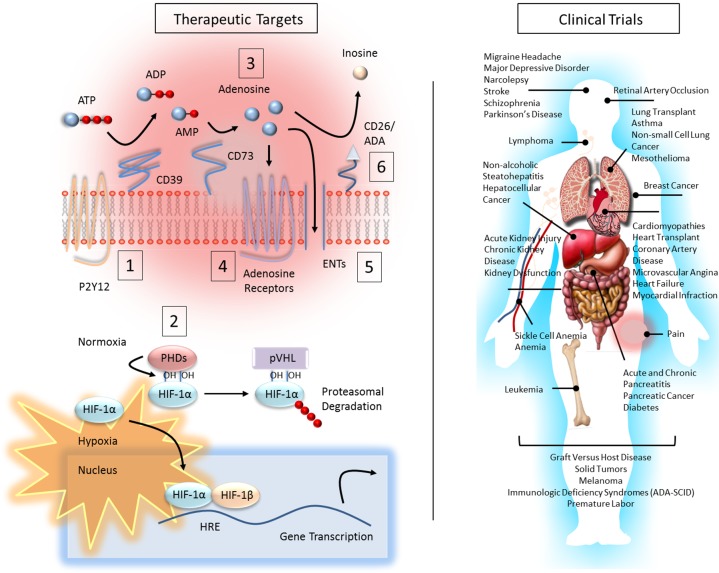
Fig. 2.
Therapeutic targets of the adenosine and hypoxia pathways and health conditions and diseases of current clinical trials. Left: multiple drugs exist for targeting adenosine metabolism and signaling and HIF activation. Many are in clinical trials (right panel and Table 1) or in use in preclinical studies. Dampening inflammation and protecting tissues barriers can be achieved by approaches that decrease proinflammatory responses (1), increase extracellular adenosine (2, 3, 5, 6), and increase adenosine signaling (2–6). Examples of drugs and drug targets include 1) Ticagrelor and Clopodogrel, P2Y12 (ATP/ADP receptor) antagonists; 2) DMOG and FG-4497, HIF stabilizers (PHD inhibitors); 3) adenosine and adenosine analogs [e.g., 5′-(N-ethylcarboxamido)], nonselective adenosine receptor agonists; 4) Regadenoson and BAY 60-6583, A2AAR and A2BAR agonists, respectively; 5) dipyridamole, ENT inhibitor; and 6) Pentostatin, ADA inhibitor. Adenosine receptor antagonists (e.g., A2AAR antagonists, PBF-509 and CPI-444) or CD73/CD39 inhibitors (e.g., MEDI9447) are currently in clinical trials in a number of solid tumors and are proposed as anticancer therapies that boost immune responses against tumor cells. On the contrary, Pentostatin, which increases adenosine levels, is currently in clinical trials for different solid tumors, leukemia, and lymphoma. In a similar manner, CF-102 an A3AR agonist has been shown to have therapeutic potential in hepatocellular cancer.
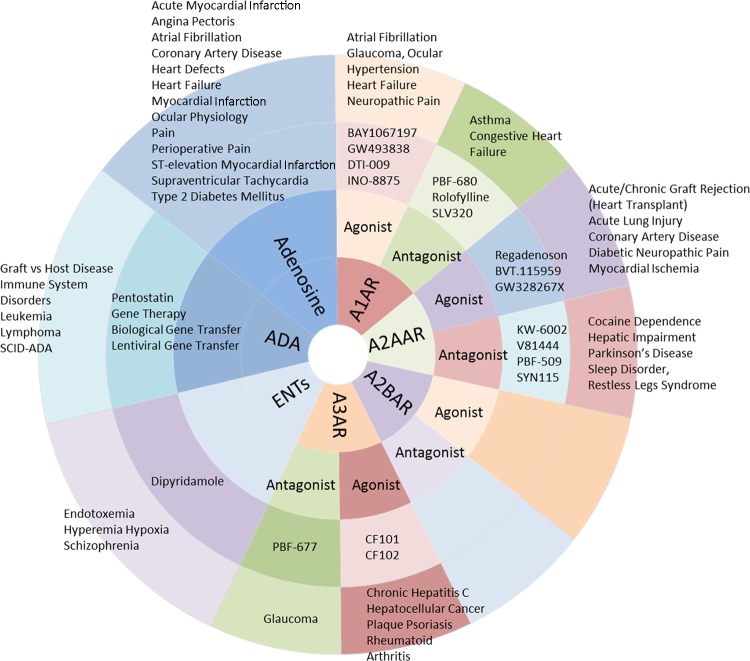
Fig. 3.
Summary of recently conducted clinical trials targeting adenosine metabolism and signaling.
Understanding the molecular basis of oxygen sensing has proven to have great implications for medicine. Recently, PHD inhibitors have made their way into active clinical trials for kidney disease; these agents are appearing to have great promise (44) (Table 1). Alternatively, given that HIFs increase CD73, A2AAR, and A2BAR expression, while decreasing ENT and AK expression, thereby increasing adenosine metabolism and signaling, the use of HIF stabilizers, such as PHD inhibitors (e.g., FG-4497), may serve as a promising therapeutic approach to targeting the adenosine pathway in inflammatory diseases. Adenosine signaling and PHDs have significant involvement in other tissues, which could produce limiting adverse side effects. Further advancement of cell- or tissue-specific delivery approaches of these agents may make all the difference in the future success of these therapies. As well, introducing more clinical trials that take into account the various challenges that have hampered the success of prior adenosine pathway drugs are certainly needed (14). We are optimistic for the future of these therapeutic targets and their potential as treatments of inflammatory conditions and diseases.
GRANTS
This research was supported by an International Anesthesia Research Society Mentored Research Award to J. L. Bowser, National Institutes of Health (NIH) Grants P50-CA-098258 and DK-056338 to J. L. Bowser, and NIH Grants R01-DK-097075, R01-HL-098294, POI-HL-114457, R01-DK-082509, R01-HL-109233, R01-DK-109574, R01-HL-119837, and R01-HL-133900 to H. K. Eltzschig.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.B. prepared figures; J.L.B. drafted manuscript; J.L.B., J.W.L., X.Y., and H.K.E. edited and revised manuscript; J.L.B., J.W.L., X.Y., and H.K.E. approved final version of manuscript.
REFERENCES
- 1.Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, Parnes JA, Furuta GT, Rivera-Nieves J, Eltzschig HK. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut 61: 695–705, 2012. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aherne CM, Saeedi B, Collins CB, Masterson JC, McNamee EN, Perrenoud L, Rapp CR, Curtis VF, Bayless A, Fletcher A, Glover LE, Evans CM, Jedlicka P, Furuta GT, de Zoeten EF, Colgan SP, Eltzschig HK. Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol 8: 1324–1338, 2015. doi: 10.1038/mi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci USA 106: 10684–10689, 2009. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 276: 121–144, 2017. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade CF, Kaneda H, Der S, Tsang M, Lodyga M, Chimisso Dos Santos C, Keshavjee S, Liu M. Toll-like receptor and cytokine gene expression in the early phase of human lung transplantation. J Heart Lung Transplant 25: 1317–1323, 2006. doi: 10.1016/j.healun.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle L, Fishman P. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappaB signal transduction pathways. Int J Oncol 33: 287–295, 2008. doi: 10.3892/ijo_00000008. [DOI] [PubMed] [Google Scholar]
- 7.Bärtsch P, Swenson ER. Clinical practice: acute high-altitude illnesses. N Engl J Med 368: 2294–2302, 2013. doi: 10.1056/NEJMcp1214870. [DOI] [PubMed] [Google Scholar]
- 8.Bernhardt WM, Wiesener MS, Scigalla P, Chou J, Schmieder RE, Günzler V, Eckardt KU. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol 21: 2151–2156, 2010. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouma MG, van den Wildenberg FA, Buurman WA. Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am J Physiol Cell Physiol 270: C522–C529, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Bowser JL, Blackburn MR, Shipley GL, Molina JG, Dunner K Jr, Broaddus RR. Loss of CD73-mediated actin polymerization promotes endometrial tumor progression. J Clin Invest 126: 220–238, 2016. doi: 10.1172/JCI79380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowser JL, Broaddus RR. CD73s protection of epithelial integrity: thinking beyond the barrier. Tissue Barriers 4: e1224963, 2016. doi: 10.1080/21688370.2016.1224963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadieux JS, Leclerc P, St-Onge M, Dussault AA, Laflamme C, Picard S, Ledent C, Borgeat P, Pouliot M. Potentiation of neutrophil cyclooxygenase-2 by adenosine: an early anti-inflammatory signal. J Cell Sci 118: 1437–1447, 2005. doi: 10.1242/jcs.01737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci USA 110: 17462–17467, 2013. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets–what are the challenges? Nat Rev Drug Discov 12: 265–286, 2013. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, Coles CH, Yu X, Hay RT, Ley SC, Pugh CW, Oldham NJ, Masson N, Schofield CJ, Ratcliffe PJ. Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH). Proc Natl Acad Sci USA 103: 14767–14772, 2006. doi: 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol 74: 153–175, 2012. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281–287, 2010. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest 85: 1150–1157, 1990. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med 158: 1160–1177, 1983. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest 92: 2675–2682, 1993. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol 135: 1366–1371, 1985. [PubMed] [Google Scholar]
- 22.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA 103: 18154–18159, 2006. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134: 156–165, 2008. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 24.D’Alessandro A, Nemkov T, Sun K, Liu H, Song A, Monte AA, Subudhi AW, Lovering AT, Dvorkin D, Julian CG, Kevil CG, Kolluru GK, Shiva S, Gladwin MT, Xia Y, Hansen KC, Roach RC. AltitudeOmics: red blood cell metabolic adaptation to high altitude hypoxia. J Proteome Res 15: 3883–3895, 2016. doi: 10.1021/acs.jproteome.6b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Ignazio L, Rocha S. Hypoxia Induced NF-κB. Cells 5: 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest 112: 883–891, 2003. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol 286: G285–G293, 2004. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 28.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, Wigle DA, Keshavjee S. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med 165: 211–215, 2002. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 29.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204: 1257–1265, 2007. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res 81: 154–164, 1997. doi: 10.1161/01.RES.81.2.154. [DOI] [PubMed] [Google Scholar]
- 31.Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol 68: 213–237, 1929. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckle T, Brodsky K, Bonney M, Packard T, Han J, Borchers CH, Mariani TJ, Kominsky DJ, Mittelbronn M, Eltzschig HK. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol 11: e1001665, 2013. doi: 10.1371/journal.pbio.1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111: 2024–2035, 2008. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckle T, Füllbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol 178: 8127–8137, 2007. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 35.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118: 3301–3315, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 18: 774–782, 2012. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckle T, Hughes K, Ehrentraut H, Brodsky KS, Rosenberger P, Choi DS, Ravid K, Weng T, Xia Y, Blackburn MR, Eltzschig HK. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J 27: 3078–3089, 2013. doi: 10.1096/fj.13-228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckle T, Kewley EM, Brodsky KS, Tak E, Bonney S, Gobel M, Anderson D, Glover LE, Riegel AK, Colgan SP, Eltzschig HK. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J Immunol 192: 1249–1256, 2014. doi: 10.4049/jimmunol.1100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 24: 298–306, 2009. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 40.Eckle T, Köhler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118: 166–175, 2008. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 41.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590, 2007. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 42.Eltzschig HK. Extracellular adenosine signaling in molecular medicine. J Mol Med (Berl) 91: 141–146, 2013. doi: 10.1007/s00109-013-0999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schönfeld C, Löffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med 202: 1493–1505, 2005. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13: 852–869, 2014. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med 364: 656–665, 2011. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198: 783–796, 2003. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eltzschig HK, Köhler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113: 224–232, 2009. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 367: 2322–2333, 2012. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood 104: 3986–3992, 2004. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 50.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 51.Flight MH. Deal watch: AstraZeneca bets on FibroGen’s anaemia drug. Nat Rev Drug Discov 12: 730, 2013. doi: 10.1038/nrd4135. [DOI] [PubMed] [Google Scholar]
- 52.Fraisl P, Aragonés J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov 8: 139–152, 2009. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 53.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol 182: 4957–4964, 2009. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman DJ, Künzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci USA 106: 16788–16793, 2009. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol 56: 209–213, 2003. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grenz A, Homann D, Eltzschig HK. Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal 15: 2221–2234, 2011. doi: 10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5: e137, 2008. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18: 600–604, 2012. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol 184: 4017–4024, 2010. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol 182: 3965–3968, 2009. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 61.Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS, Matteoli G, Bohn E, Autenrieth IB, Karhausen J, Neumann D, Colgan SP, Kempf VA. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 134: 756–767, 2008. doi: 10.1053/j.gastro.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Haskó G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabó C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J 14: 2065–2074, 2000. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 63.Hoegl S, Brodsky KS, Blackburn MR, Karmouty-Quintana H, Zwissler B, Eltzschig HK. Alveolar epithelial A2B adenosine receptors in pulmonary protection during acute lung injury. J Immunol 195: 1815–1824, 2015. doi: 10.4049/jimmunol.1401957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Påhlman S. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10: 413–423, 2006. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 65.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23: 9361–9374, 2003. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 509: 310–317, 2014. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 68.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 69.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science 261: 466–469, 1993. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 70.Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci USA 97: 10430–10435, 2000. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106, 2004. doi: 10.1172/JCI200421086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, Kotsakis A, McNamee EN, Eltzschig HK, Kominsky DJ, Colgan SP. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol 7: 114–123, 2014. doi: 10.1038/mi.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitakaze M, Hori M, Morioka T, Minamino T, Takashima S, Sato H, Shinozaki Y, Chujo M, Mori H, Inoue M. Infarct size-limiting effect of ischemic preconditioning is blunted by inhibition of 5′-nucleotidase activity and attenuation of adenosine release. Circulation 89: 1237–1246, 1994. doi: 10.1161/01.CIR.89.3.1237. [DOI] [PubMed] [Google Scholar]
- 74.Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. Eur Heart J 27: 2400–2405, 2006. doi: 10.1093/eurheartj/ehl094. [DOI] [PubMed] [Google Scholar]
- 75.Köhler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Müller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116: 1784–1794, 2007. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 76.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J 20: 2242–2250, 2006. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 77.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther 317: 172–180, 2006. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 78.Krüger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Krämer BK, Colvin RB, Heeger PS, Murphy BT, Schröppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci USA 106: 3390–3395, 2009. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krump E, Lemay G, Borgeat P. Adenosine A2 receptor-induced inhibition of leukotriene B4 synthesis in whole blood ex vivo. Br J Pharmacol 117: 1639–1644, 1996. doi: 10.1111/j.1476-5381.1996.tb15334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krump E, Picard S, Mancini J, Borgeat P. Suppression of leukotriene B4 biosynthesis by endogenous adenosine in ligand-activated human neutrophils. J Exp Med 186: 1401–1406, 1997. doi: 10.1084/jem.186.8.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS One 2: e1364, 2007. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol 43: 262–271, 2007. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kusu T, Kayama H, Kinoshita M, Jeon SG, Ueda Y, Goto Y, Okumura R, Saiga H, Kurakawa T, Ikeda K, Maeda Y, Nishimura J, Arima Y, Atarashi K, Honda K, Murakami M, Kunisawa J, Kiyono H, Okumura M, Yamamoto M, Takeda K. Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J Immunol 190: 774–783, 2013. doi: 10.4049/jimmunol.1103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 16: 1466–1471, 2002. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295: 858–861, 2002. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 86.Lasley RD, Jahania MS, Mentzer RM Jr. Beneficial effects of adenosine A(2a) agonist CGS-21680 in infarcted and stunned porcine myocardium. Am J Physiol Heart Circ Physiol 280: H1660–H1666, 2001. [DOI] [PubMed] [Google Scholar]
- 87.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med 188: 1433–1443, 1998. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu H, Zhang Y, Wu H, D’Alessandro A, Yegutkin GG, Song A, Sun K, Li J, Cheng NY, Huang A, Edward Wen Y, Weng TT, Luo F, Nemkov T, Sun H, Kellems RE, Karmouty-Quintana H, Hansen KC, Zhao B, Subudhi AW, Jameson-Van Houten S, Julian CG, Lovering AT, Eltzschig HK, Blackburn MR, Roach RC, Xia Y. Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation 134: 405–421, 2016. doi: 10.1161/CIRCULATIONAHA.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol 180: 4246–4255, 2008. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 90.Luks AM, Swenson ER, Bärtsch P. Acute high-altitude sickness. Eur Respir Rev 26: 160096, 2017. doi: 10.1183/16000617.0096-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol 34: 1711–1720, 1999. doi: 10.1016/S0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- 92.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105: 659–669, 2005. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 93.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, Pan F, Priel A, Clish CB, Robson SC, Quintana FJ. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med 21: 638–646, 2015. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McColl SR, St-Onge M, Dussault AA, Laflamme C, Bouchard L, Boulanger J, Pouliot M. Immunomodulatory impact of the A2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J 20: 187–189, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McIntosh VJ, Lasley RD. Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant? J Cardiovasc Pharmacol Ther 17: 21–33, 2012. doi: 10.1177/1074248410396877. [DOI] [PubMed] [Google Scholar]
- 96.Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Köhler D, Rosenberger P. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med 181: 815–824, 2010. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]
- 97.Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol 587: 3651–3663, 2009. doi: 10.1113/jphysiol.2009.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 111: 5571–5580, 2008. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 99.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136: 607–618, 2009. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 100.Mukhopadhyay D, Knebelmann B, Cohen HT, Ananth S, Sukhatme VP. The von Hippel-Lindau tumor suppressor gene product interacts with Sp1 to repress vascular endothelial growth factor promoter activity. Mol Cell Biol 17: 5629–5639, 1997. doi: 10.1128/MCB.17.9.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 177: 2765–2769, 2006. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 102.Németh ZH, Csóka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Haskó G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol 176: 5616–5626, 2006. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2: 423–427, 2000. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 104.Ohta A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol 7: 109, 2016. doi: 10.3389/fimmu.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414: 916–920, 2001. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 106.Pagès G, Pouysségur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene–a concert of activating factors. Cardiovasc Res 65: 564–573, 2005. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 107.Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood 101: 3985–3990, 2003. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 108.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J 15: 1963–1970, 2001. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 109.Peirce SM, Skalak TC, Rieger JM, Macdonald TL, Linden J. Selective A(2A) adenosine receptor activation reduces skin pressure ulcer formation and inflammation. Am J Physiol Heart Circ Physiol 281: H67–H74, 2001. [DOI] [PubMed] [Google Scholar]
- 110.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol 178: 7516–7519, 2007. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 111.Pouliot M, Fiset ME, Massé M, Naccache PH, Borgeat P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol 169: 5279–5286, 2002. doi: 10.4049/jimmunol.169.9.5279. [DOI] [PubMed] [Google Scholar]
- 112.Randhawa PK, Jaggi AS. Unraveling the role of adenosine in remote ischemic preconditioning-induced cardioprotection. Life Sci 155: 140–146, 2016. doi: 10.1016/j.lfs.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 113.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest 117: 862–865, 2007. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol 179: 1254–1263, 2007. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 115.Robak T, Korycka A, Lech-Maranda E, Robak P. Current status of older and new purine nucleoside analogues in the treatment of lymphoproliferative diseases. Molecules 14: 1183–1226, 2009. doi: 10.3390/molecules14031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134: 145–155, 2008. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rose JB, Naydenova Z, Bang A, Eguchi M, Sweeney G, Choi DS, Hammond JR, Coe IR. Equilibrative nucleoside transporter 1 plays an essential role in cardioprotection. Am J Physiol Heart Circ Physiol 298: H771–H777, 2010. doi: 10.1152/ajpheart.00711.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002. doi: 10.1097/01.ASN.0000017223.49823.2A. [DOI] [PubMed] [Google Scholar]
- 119.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol 10: 195–202, 2009. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 120.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW; AMISTAD-II Investigators . A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol 45: 1775–1780, 2005. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 121.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 184: 5271–5279, 2010. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, Davis ID, Cebon J, Maraskovsky E. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood 103: 1391–1397, 2004. doi: 10.1182/blood-2003-06-1959. [DOI] [PubMed] [Google Scholar]
- 123.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA 88: 5680–5684, 1991. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992. doi: 10.1128/MCB.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stemmer SM, Benjaminov O, Medalia G, Ciuraru NB, Silverman MH, Bar-Yehuda S, Fishman S, Harpaz Z, Farbstein M, Cohen S, Patoka R, Singer B, Kerns WD, Fishman P. CF102 for the treatment of hepatocellular carcinoma: a phase I/II, open-label, dose-escalation study. Oncologist 18: 25–26, 2013. doi: 10.1634/theoncologist.2012-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Swenson ER, Bärtsch P. High-altitude pulmonary edema. Compr Physiol 2: 2753–2773, 2012. doi: 10.1002/cphy.c100029. [DOI] [PubMed] [Google Scholar]
- 127.Swenson ER, Maggiorini M, Mongovin S, Gibbs JS, Greve I, Mairbäurl H, Bärtsch P. Pathogenesis of high-altitude pulmonary edema: inflammation is not an etiologic factor. JAMA 287: 2228–2235, 2002. doi: 10.1001/jama.287.17.2228. [DOI] [PubMed] [Google Scholar]
- 128.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110: 993–1002, 2002. doi: 10.1172/JCI0215337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 139: 2093–2101, 2010. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 130.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J 19: 4298–4309, 2000. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496: 238–242, 2013. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med (Berl) 85: 1295–1300, 2007. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 133.Thiel M, Chouker A. Acting via A2 receptors, adenosine inhibits the production of tumor necrosis factor-alpha of endotoxin-stimulated human polymorphonuclear leukocytes. J Lab Clin Med 126: 275–282, 1995. [PubMed] [Google Scholar]
- 134.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol 3: e174, 2005. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med 200: 1395–1405, 2004. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Uniacke J, Holterman CE, Lachance G, Franovic A, Jacob MD, Fabian MR, Payette J, Holcik M, Pause A, Lee S. An oxygen-regulated switch in the protein synthesis machinery. Nature 486: 126–129, 2012. doi: 10.1038/nature11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Varani K, Gessi S, Dionisotti S, Ongini E, Borea PA. [3H]-SCH 58261 labelling of functional A2A adenosine receptors in human neutrophil membranes. Br J Pharmacol 123: 1723–1731, 1998. doi: 10.1038/sj.bjp.0701758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vohwinkel CU, Hoegl S, Eltzschig HK. Hypoxia signaling during acute lung injury. J Appl Physiol (1985) 119: 1157–1163, 2015. doi: 10.1152/japplphysiol.00226.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wiesener MS, Jürgensen JS, Rosenberger C, Scholze CK, Hörstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 17: 271–273, 2003. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 140.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145: 831–841, 2013. doi: 10.1053/j.gastro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, French BA, Linden J. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation 111: 2190–2197, 2005. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 142.Zhang P, Yao Q, Lu L, Li Y, Chen PJ, Duan C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Reports 6: 1110–1121, 2014. doi: 10.1016/j.celrep.2014.02.011. [DOI] [PubMed] [Google Scholar]