Sympathetic activation evoked by a cold pressor test elicits heterogeneous extra- and intracranial blood vessel responses in young individuals that may serve an important protective role. The extra- and intracranial responses to the cold pressor test are blunted in older individuals.
Keywords: brain blood flow, elderly, sympathetic nerve activity
Abstract
We determined how the extra- and intracranial circulations respond to generalized sympathetic activation evoked by a cold pressor test (CPT) and whether this is affected by healthy aging. Ten young [23 ± 2 yr (means ± SD)] and nine older (66 ± 3 yr) individuals performed a 3-min CPT by immersing the left foot into 0.8 ± 0.3°C water. Common carotid artery (CCA) and internal carotid artery (ICA) diameter, velocity, and flow were simultaneously measured (duplex ultrasound) along with middle cerebral artery and posterior cerebral artery mean blood velocity (MCAvmean and PCAvmean) and cardiorespiratory variables. The increases in heart rate (~6 beats/min) and mean arterial blood pressure (~14 mmHg) were similar in young and older groups during the CPT (P < 0.01 vs. baseline). In the young group, the CPT elicited an ~5% increase in CCA diameter (P < 0.01 vs. baseline) and a tendency for an increase in CCA flow (~12%, P = 0.08); in contrast, both diameter and flow remained unchanged in the older group. Although ICA diameter was not changed during the CPT in either group, ICA flow increased (~8%, P = 0.02) during the first minute of the CPT in both groups. Whereas the CPT elicited an increase in MCAvmean and PCAvmean in the young group (by ~20 and ~10%, respectively, P < 0.01 vs. baseline), these intracranial velocities were unchanged in the older group. Collectively, during the CPT, these findings suggest a differential mechanism(s) of regulation between the ICA compared with the CCA in young individuals and a blunting of the CCA and intracranial responses in older individuals.
NEW & NOTEWORTHY Sympathetic activation evoked by a cold pressor test elicits heterogeneous extra- and intracranial blood vessel responses in young individuals that may serve an important protective role. The extra- and intracranial responses to the cold pressor test are blunted in older individuals.
the cold pressor test (CPT) has been widely employed for the assessment of human autonomic function (13, 17), peripheral vascular reactivity (7, 45, 53, 72), and cardiovascular risk stratification (6, 38, 61). However, the cerebrovascular responses to the CPT remain poorly understood, particularly in healthy aging and chronic disease. This issue is compounded by the controversy surrounding the sympathetic regulation of the extra- and intracranial blood vessels (1, 58). During the CPT, signals from activated cutaneous thermoreceptor and nociceptor afferents are rapidly integrated within the central nervous system (principally the hypothalamic and medullary regions) and lead to the activation of cortical sites (10). This activation elevates peripheral vascular resistance, heart rate (HR), and blood pressure (23) on account of the characteristic autonomic efferent response, consisting of a robust increase in sympathetic nerve activity (SNA) (e.g., increased plasma noradrenaline (19) and muscle sympathetic nerve activity; see Ref. 65) and potentially a decrease in cardiac parasympathetic nerve activity (e.g., decreased HR variability; see Ref. 16). Cerebral blood flow may be affected by several mechanisms during the CPT, including neurovascular coupling, a hydraulic pressure effect even in the absence of a change in vascular resistance, and local autoregulatory mechanisms, and by the sympathetic modulation of extra- and intracranial blood vessels.
In animal studies, innervation of the CCA, ICA, and intracranial vasculature by postganglionic sympathetic nerve fibers has been identified (12, 37, 42); electrical stimulation of sympathetic nerves can evoke cerebral vasoconstriction (2, 66), and norepinephrine causes vasoconstriction in cerebral microvessels (36, 59). In humans, the spillover of noradrenaline from the brain into the internal jugular vein has been reported (43); clinically indicated upper thoracic sympathectomy increases ICA diameter and flow (26), and stellate ganglion blockade reportedly increases cerebral perfusion (62), although this is not been a universal finding (27). The effect of CPT-evoked sympathoexcitation on cerebral perfusion has principally been evaluated in terms of intracranial artery mean blood flow velocity and usually within the middle cerebral artery (MCAvmean). Intriguingly, both reductions (3, 41) and elevations (46, 47, 56, 73) in cerebral perfusion have been reported during the CPT, which are possibly due to differences in the partial pressure of arterial carbon dioxide (). With respect to the regulation of extracranial blood flow during the CPT, an increase in common carotid artery (CCA) diameter by ∼8% is reported in young healthy individuals (28, 34, 53). In contrast, CCA diameter is reduced during the CPT in patients with coronary artery disease, which is possibly due to the greater sensitivity of the α-adrenergic receptors (53). Unfortunately, to date, no assessment of internal carotid artery (ICA) diameter or volumetric flow during the CPT has been made, but these are essential to understand the implications for cerebral blood flow (as opposed to blood flow to the head and scalp via the external carotid artery). It would seem unlikely that the same responses were observed in the CCA and ICA during the CPT. In accordance with Poiseuille’s Law, small changes in diameter have a major effect on flow [e.g., flow-α (diameter/2)4]. Accordingly, if the ICA were to dilate to a degree similar to that of the CCA (e.g., ∼8%), brain blood flow would increase markedly. Given that the brain seems to be particularly effective at protecting itself from overperfusion (68) and that the ICA (and vertebral arteries) are known to be integral to the regulation of cerebral blood flow through modifying vascular resistance (14, 22, 29, 39, 40), it seems reasonable to expect that different responses occur in the CCA and ICA during the CPT.
Increased age is associated with a multitude of structural, functional, and regulatory alterations throughout the cardiovascular system (30, 31), including the brain (5, 54). Age-related increases in arterial stiffness (28, 33), impairments in endothelial vasodilator function, and altered α- and β-adrenergic receptors signaling within the peripheral vasculature have been identified in humans (4, 11). However, the extent to which age modifies the cerebral blood flow responses to sympathetic stimulation remains unclear.
The purpose of this study was twofold: 1) to comprehensively describe the extra- (CCA, ICA) and intracranial (MCA) blood flow responses to the CPT and 2) to ascertain the influence of age on these cerebrovascular responses to the CPT. To achieve these goals, in both younger and older subjects, simultaneous measurements of CCA and ICA diameter, velocity, and flow were made along with MCAvmean and posterior cerebral artery mean blood flow velocity (PCAvmean) during the CPT under conditions of controlled isocapnia. We hypothesized that there would be less of an increase in ICA diameter compared with the CCA during the CPT in young individuals. In addition, we anticipated that the extra- and intracranial responses to the CPT would be blunted in older individuals.
MATERIALS AND METHODS
Ethical Approval
All experimental protocols and procedures were approved by the University of British Columbia Research Ethics Board (H15-01951) and conformed to the Declaration of Helsinki. Prior to participation, a detailed verbal and written explanation of the study was provided, and each participant completed written, informed consent.
Participants
Nineteen study participants, 10 young [2 women: 23 ± 2 yr, 176 ± 7 cm, 73 ± 9 kg (means ± SD)] and nine older subjects (2 women: 66 ± 3 yr, 176 ± 8 cm, 78 ± 13 kg), took part in the study. As determined by a written screening questionnaire and oral confirmation, no study participants had a history of cardiovascular, cerebrovascular, or respiratory disease. None of our participants were active smokers, but one of the older participants had a history of smoking. Participants were not taking prescription or over-the-counter medications, except for two of the older male study participants, who were using either Tamsulosin (0.4 mg/day) due to enlarged prostates or Ciclesonide (400 µg/day) due to mild asthma, and the two young women who were taking oral contraceptives and were tested on days 1 and 2 of their self-reported menstrual cycle. The two older women were both postmenopausal and not taking hormone replacements. Participants abstained from alcohol, caffeine, and exercise for ≥12 h before the experimental session.
Experimental Measures
Cardiorespiratory measures.
Heart rate (HR) was assessed using a three-lead electrocardiogram (ADI BioAmp ML132) and beat-to-beat blood pressure using a finger photoplethysmography (Finometer PRO; Finapres Medical Systems, Amsterdam, The Netherlands). Mean arterial pressure (MAP) was calculated from the Finometer reconstructed brachial waveform after values were back-calibrated to the average of three automated brachial blood pressure measurements made over 3 min (Tango+; SunTech, Morrisville, NC). Stroke volume (SV) was estimated using the Modelflow method (Finapres Medical Systems), which simulates aortic flow waveforms from an arterial pressure signal using a nonlinear three-element model of the aortic input impedance. Cardiac output (CO) was calculated as SV × HR and total peripheral resistance (TPR) as MAP/CO. Both the partial pressures of end-tidal CO2 () and O2 () were sampled at the mouth and recorded by a calibrated gas analyzer (model ML206; ADInstruments, Colorado Springs, CO). A pneumotachograph (model HR 800L; Hans Rudolph, Shawnee, KS) connected to a bacterial filter was used to assess minute ventilation (VE). All cardiorespiratory variables were sampled continuously at 1,000 Hz using an analog-to-digital converter (Powerlab, 16/30; ADInstruments), and data were interfaced with LabChart (version 7) and analyzed offline.
Cerebrovascular measurements.
Transcranial Doppler Ultrasound (2 MHz, TCD; Spencer Technologies, Seattle, WA) was used to simultaneously assess the right MCAvmean and left PCAvmean, in accordance with standard guidelines (67). A 2-MHz wavelength provides the optimal resolution-to-penetration depth ratio for imaging the deep cerebral vessels. The transmitted ultrasound beam contacts the red blood cells within the target vessel, and a portion of the signal is reflected back to the transducer. The difference between the emitted and received frequency signals (i.e., Doppler shift) is processed through a fast Fourier transformation to produce a velocity trace, and an envelope surrounding this is then exported in real time into LabChart (version 7) for offline analyses. For anatomic reasons, in two older individuals the orientation was switched such that the left MCAvmean and right PCAvmean were insonated. Despite sides being switched in one individual, a clear image was impossible; therefore, PCAvmean is based on n = 8. The bilaterally placed probes were secured in place by being attached to a headpiece (model M600 bilateral head frame; Spencer Technologies). The MCA and PCA were insonated through the middle transtemporal window, using previously described locations and standardization techniques (67). Blood velocity and vessel diameter of the left common carotid artery (CCA; right CCA, n = 3) and right internal carotid artery (ICA; left ICA, n = 3) were measured using a 10-MHz multifrequency linear array vascular ultrasound (Terason T3200; Teratech, Burlington, MA). Because of anatomic reasons, a clear image of the target artery was not possible in three study participants, and therefore, the side of insonation was switched. Only in two study participants were the ICA and MCA insonated contralaterally. B-mode imaging was used to measure arterial diameter, whereas pulse-wave mode was used to simultaneously measure peak blood velocity. Extracranial blood flow measurements were made in accordance with recent technical recommendations (60). All CCA and ICA recordings were screen-captured and stored as video files for offline analysis (70). A minimum of 10 consecutive cardiac cycles were used to determine extracranial blood flow measurements. In two older study participants, ICA images were of insufficient quality; thus ICA analysis in this cohort is based on n = 7. Volumetric blood flow was calculated using the following formula:
Cerebrovascular conductance (CVC) was calculated for intracranial arteries and extracranial arteries using the following formula:
Several indices of CCA and ICA stiffness were calculated in accordance with recently published methods (33, 34). β-Stiffness index = ln(SBP-DBP)/[(DIAsys-DIAdia)/DIAdia], elastic modulus = [(SBP-DBP)·DIAdia]/(DIAsys-DIAdia), arterial compliance = (DIAsys-DIAdia)/(SBP-DBP), and arterial distensibility = (DIAsys-DIAdia)/[(SBP-DBP)·DIAdia], where SBP is systolic blood pressure, DBP is diastolic blood pressure, DIAmax is maximum diameter, and DIAmin is minimum diameter.
Study Protocol
Study participants visited the laboratory on a single occasion. Prior to instrumentation, all study participants were carefully familiarized with the study design and measurements. Thereafter, the carotid, internal carotid, and vertebral arteries were scanned in each participant to exclude individuals with any stenosis. After instrumentation and a resting period of ≥5 min, a 3-min baseline was recorded before the start of the CPT. The CPT consisted of a 3-min immersion of the left foot into ice-cold water (0.8 ± 0.3°C), followed by a 3-min recovery. The foot, rather than the hand, was chosen to keep the upper body still and facilitate the acquisition of high-quality ultrasound images. Throughout the CPT, isocapnia was maintained using an end-tidal forcing system (Air-force; GE Foster, Kelowna, BC, Canada) described in detail elsewhere (49). Briefly, , , and inspiratory and expiratory tidal volume were sampled on a breath-by-breath basis and with the help of a feedback control, and using independent gas solenoid valves for O2, CO2, and N2, desired end-tidal gases were maintained at baseline values. To assess whether there were any age-related alterations in thermal perception that may have subsequently contributed to any differences in CPT responses, each study participant was asked to provide a rating of the perceived pain experienced at the onset and the end of the CPT using a Borg scale ranging from 0 (no pain) to 10 (worst pain).
Data and Statistical Analysis
Baseline (BL) values for the cardiovascular, respiratory, and cerebrovascular variables measured were taken as an average over the last minute of the resting phase before the CPT. Thereafter, the last 20 s of each minute was averaged during the CPT (CPT1, CPT2, and CPT3) and throughout recovery (RE1, RE2, and RE3). A repeated two-way ANOVA was used to test for differences in the cardiovascular, respiratory, and cerebrovascular responses with respect to experimental phase (BL, CPT1, CPT2, CPT3, RE1, RE2, and RE3) and age (young and older). Data were expressed in absolute terms and as a percentage change from baseline, thus permitting us to compare the extra- and intracranial responses to the CPT and to ascertain the influence of age on these cerebrovascular responses. A repeated two-way ANOVA was used to determine whether perceived pain responses to the CPT were different with respect to experimental phase (CPT1 and CPT2) and age (young and older). Finally, the existence of differences in arterial stiffness between experimental phases (BL and CPT) and age (young and older) was evaluated using a repeated two-way ANOVA. Tukey post hoc tests were used to examine significant main effects and interactions. Data are given as means ± SD unless otherwise indicated. Statistical significance was set at P < 0.05. Statistical analyses were performed using the SAS Enterprise Guide (4.3; SAS Institute, Cary, NC).
RESULTS
Cardiovascular and respiratory variables during baseline, CPT, and recovery in young and older participants are presented in Table 1. During the CPT, MAP increased from baseline in both groups (P < 0.01), but absolute values were higher in the older group throughout (P = 0.03). In both groups, HR was increased similarly at CPT1 (P < 0.01 vs. baseline) and declined thereafter. The was successfully kept at baseline values during the CPT by the end-tidal forcing system. Rating of perceived pain was not different between young and older groups at the onset (young: 5.8 ± 1.4; older: 4.6 ± 2.6) or the end of the CPT (young: 4.4 ± 2.1; older: 5.4 ± 2.6).
Table 1.
Cardiovascular and respiratory parameters at BL, at each minute of a 3-min CPT (CPT1, CPT2, and CPT3) and at each minute during a 3-min RE (RE1, RE2, and RE3)
| Experimental Phase |
P Values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BL | CPT1 | CPT2 | CPT3 | RE1 | RE2 | RE3 | Age | Phase | Age × phase | |
| MAP, mmHg | ||||||||||
| Y (n = 10) | 93 ± 7 | 109 ± 9 | 108 ± 10 | 104 ± 9 | 96 ± 7 | 93 ± 7 | 94 ± 9 | 0.026 | <0.001 | 0.79 |
| O (n = 9) | 101 ± 7 | 114 ± 10 | 114 ± 9 | 112 ± 8 | 104 ± 6 | 102 ± 5 | 103 ± 5 | |||
| HR, beats/min | ||||||||||
| Y (n = 10) | 73 ± 12 | 81 ± 18 | 77 ± 17 | 73 ± 15 | 67 ± 12 | 69 ± 13 | 68 ± 11 | 0.105 | <0.001 | 0.268 |
| O (n = 9) | 64 ± 9 | 68 ± 9 | 67 ± 6 | 65 ± 6 | 62 ± 8 | 61 ± 9 | 61 ± 9 | |||
| SV, ml | ||||||||||
| Y (n = 10) | 96 ± 42 | 93 ± 42 | 90 ± 41 | 90 ± 42 | 94 ± 43 | 94 ± 42 | 95 ± 42 | 0.271 | 0.046 | 0.855 |
| O (n = 9) | 93 ± 30 | 89 ± 28 | 88 ± 28 | 86 ± 26 | 89 ± 27 | 90 ± 27 | 87 ± 27 | |||
| CO, l/min | ||||||||||
| Y (n = 10) | 7.4 ± 1.1 | 8.0 ± 2.0 | 7.3 ± 1.6 | 7.2 ± 1.3 | 6.8 ± 1.1 | 7.0 ± 0.9 | 6.9 ± 1.0 | 0.005 | 0.002 | 0.505 |
| O (n = 9) | 5.6 ± 2.0 | 5.5 ± 1.5 | 5.4 ± 1.4 | 5.1 ± 1.3 | 5.1 ± 1.5 | 5.2 ± 1.5 | 5.0 ± 1.6 | |||
| TPR, mmHg·min−1·ml−1 | ||||||||||
| Y (n = 10) | 12.8 ± 1.9 | 14.4 ± 3.7 | 15.3 ± 3.1 | 14.9 ± 2.8 | 14.3 ± 2.6 | 13.5 ± 2.2 | 13.8 ± 2.3 | 0.003 | <0.001 | 0.806 |
| O (n = 9) | 20.4 ± 7.3 | 22.9 ± 7.6 | 22.9 ± 6.8 | 23.6 ± 6.3 | 22.3 ± 6.8 | 21.5 ± 6.5 | 22.6 ± 6.8 | |||
| VE, l/min | ||||||||||
| Y (n = 10) | 14.6 ± 3.8 | 18.4 ± 5.4 | 18.2 ± 5.0 | 18.1 ± 4.8 | 16.1 ± 3.7 | 14.8 ± 3.1 | 15.1 ± 3.2 | 0.038 | <0.001 | 0.273 |
| O (n = 9) | 12.1 ± 3.6 | 14.5 ± 4.5 | 13.7 ± 3.4 | 13.2 ± 3.9 | 11.7 ± 3.6 | 11.4 ± 3.6 | 11.7 ± 3.3 | |||
| , mmHg | ||||||||||
| Y (n = 10) | 41.5 ± 2.8 | 41.2 ± 2.4 | 41.3 ± 2.9 | 41.4 ± 2.9 | 41.3 ± 2.7 | 41.4 ± 2.7 | 41.0 ± 2.4 | 0.201 | 0.212 | 0.047 |
| O (n = 9) | 40.1 ± 2.5 | 39.65 ± 3.0 | 39.3 ± 3.0 | 39.1 ± 2.8 | 39.9 ± 2.7 | 39.7 ± 2.5 | 40.2 ± 2.7§ | |||
Values are means ± SD. BL, baseline; CPT, cold pressor test; RE, recovery; MAP, mean arterial pressure; HR, heart rate; SV, stroke volume; CO, cardiac output; VE, ventilation; , end-tidal partial pressure of CO2; Y, young; O, old. P values represent 2-way repeated ANOVA results (age: young and old; phase: BL, CPT1, CPT2, CPT3, RE1, RE2, and RE3).
P < 0.05 vs. CPT3.
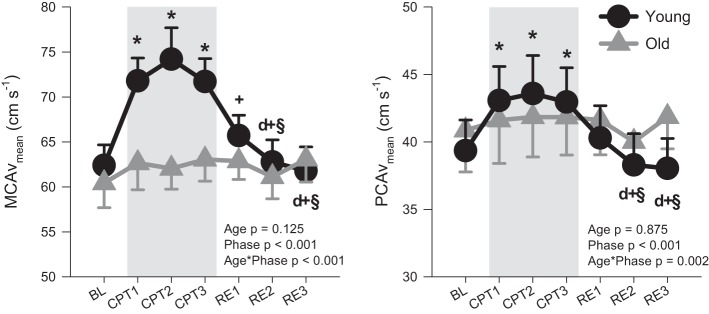
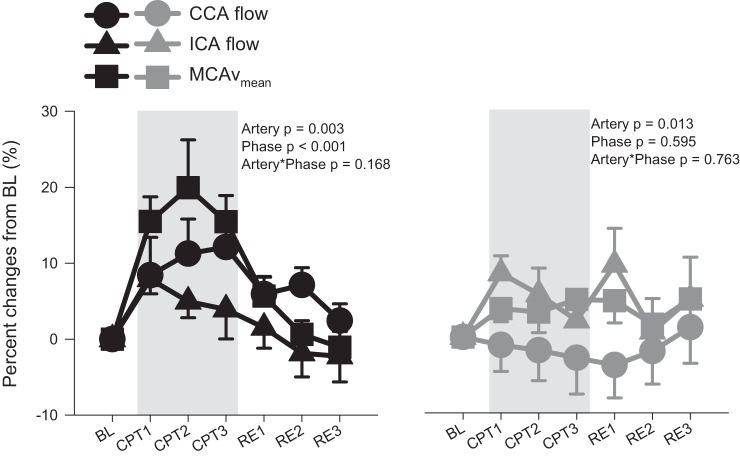
During the CPT, MCAvmean and PCAvmean increased in the younger participants (by 19 ± 19 and 11 ± 12% at CPT2, respectively), whereas no changes from baseline were observed in the older participants (Fig. 1). The CCA diameter increased in the young during CPT (by 5 ± 3% at CPT1), whereas no change from baseline in CCA diameter was observed in the older participants (P < 0.01; Fig. 2). No changes from baseline in CCA velocity were observed in either age group, whereas CCA flow tended (P = 0.08) to be increased in the young group. Both ICA diameter and ICA velocity were unchanged from baseline during the CPT, whereas ICA flow was increased from baseline at CPT1 (P = 0.03). During the CPT, the percentage increases in CCA flow and MCAvmean were significantly greater than ICA flow in the young group (CCA vs. ICA, P = 0.02; CCA vs. MCA, P = 0.70; ICA vs. MCA, P < 0.01; Fig. 3). However, in the older group, the percentage increases in ICA flow and MCAvmean were significantly greater than CCA flow (CCA vs. ICA, P = 0.02; CCA vs. MCA, P = 0.05; ICA vs. MCA, P = 0.83). In the young group, CPT evoked a greater velocity response in the MCA compared with the PCA (17 ± 14 vs. 10 ± 10%, P < 0.01), whereas no difference was seen in the older group (4 ± 7 vs. 3 ± 7%, P = 0.72).
Fig. 1.
Middle cerebral artery mean blood flow velocity (MCAvmean) and posterior cerebral artery mean blood flow velocity (PCAvmean) in young (n = 10; ●) and old (n = 9, n = 8 for PCAvmean;  ) at baseline (BL), during a 3-min cold pressor test (CPT1, CPT2, and CPT3), and followed by a 3-min recovery (RE1, RE2, and RE3). Values are means ± SE. P values represent repeated 2-way ANOVA results. *P < 0.05 vs. BL; dP < 0.05 vs. CPT1; +P < 0.05 vs. CPT2; §P < 0.05 vs. CPT3.
) at baseline (BL), during a 3-min cold pressor test (CPT1, CPT2, and CPT3), and followed by a 3-min recovery (RE1, RE2, and RE3). Values are means ± SE. P values represent repeated 2-way ANOVA results. *P < 0.05 vs. BL; dP < 0.05 vs. CPT1; +P < 0.05 vs. CPT2; §P < 0.05 vs. CPT3.
Fig. 2.
Common carotid (CCA) diameter, internal carotid (ICA) diameter, CCA velocity, ICA velocity, CCA flow, and ICA flow in young (n = 10; ●) and old (n = 9, n = 7 for ICA;  ) at baseline (BL), during a 3-min cold pressor test (CPT1, CPT2, and CPT3), and followed by a 3-min recovery (RE1, RE2, and RE3). Values are means ± SE. P values represent repeated 2-way ANOVA results. *P < 0.05 vs. BL; dP < 0.05 vs. CPT1; +P < 0.05 vs. CPT2; §P < 0.05 vs. CPT3.
) at baseline (BL), during a 3-min cold pressor test (CPT1, CPT2, and CPT3), and followed by a 3-min recovery (RE1, RE2, and RE3). Values are means ± SE. P values represent repeated 2-way ANOVA results. *P < 0.05 vs. BL; dP < 0.05 vs. CPT1; +P < 0.05 vs. CPT2; §P < 0.05 vs. CPT3.
Fig. 3.
%Change from baseline (BL) in common carotid (CCA) flow, internal carotid (ICA) flow, and middle cerebral artery mean blood flow velocity (MCAvmean) in young (n = 10; ●, ▲, and ■) and old (n = 9, n = 7 for ICA;  ,
,  ,
,  ), during a 3-min cold pressor test (CPT1, CPT2, and CPT3), and followed by a 3-min recovery (RE1, RE2, and RE3). Values are means ± SE. P values represent repeated 2-way ANOVA results.
), during a 3-min cold pressor test (CPT1, CPT2, and CPT3), and followed by a 3-min recovery (RE1, RE2, and RE3). Values are means ± SE. P values represent repeated 2-way ANOVA results.
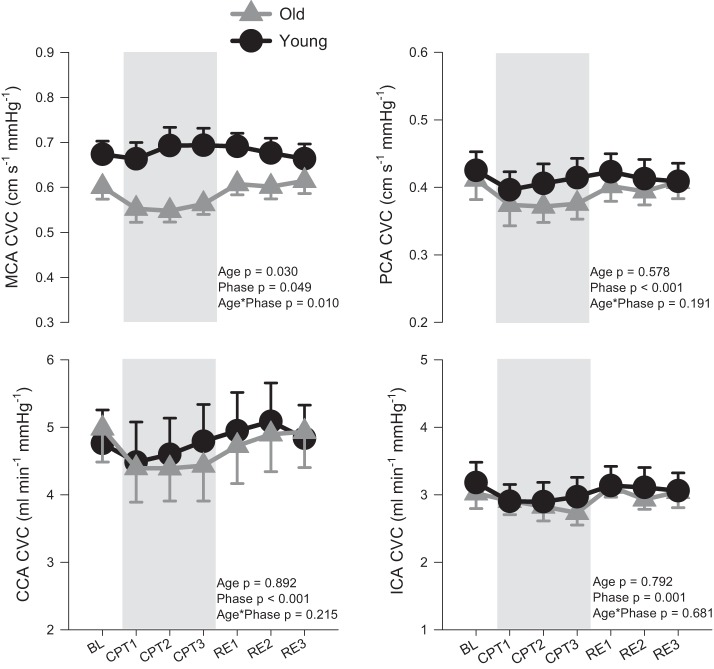
Figure 4 provides the CVC values for the MCA, PCA, CCA, and ICA during baseline, CPT, and recovery in young and older participants. A significant interaction between age and experimental phase was observed for MCA CVC. Although MCA CVC was numerically lower in the older group across all experimental phases, post hoc analyses showed only a trend toward an age difference at CPT2 (P = 0.07), with no significant differences from baseline in either group.
Fig. 4.
Middle cerebral artery cerebrovascular conductance (MCA CVC), posterior cerebral artery (PCA) CVC, common carotid (CCA) CVC, and internal carotid (ICA) CVC in young (n = 10; ●) and old (n = 9, n = 7 for ICA,  ) at baseline (BL), during a 3-min cold pressor test (CPT1, CPT2, and CPT3), and followed by a 3-min recovery (RE1, RE2, and RE3). Values are means ± SE. P values represent repeated ANOVA results.
) at baseline (BL), during a 3-min cold pressor test (CPT1, CPT2, and CPT3), and followed by a 3-min recovery (RE1, RE2, and RE3). Values are means ± SE. P values represent repeated ANOVA results.
Table 2 presents arterial stiffness indices for the CCA and ICA. Arterial stiffness in the CCA was greater in the older group compared with the young individuals, whereas ICA stiffness was not different. No index of arterial stiffness was altered during the CPT.
Table 2.
Arterial stiffness indices in young and old individuals at BL and during the CPT
|
P Value |
|||||
|---|---|---|---|---|---|
| Young | Old | Age | Phase | Age × phase | |
| CCA β-stiffness | |||||
| BL | 5.5 ± 1.1 | 7.6 ± 2.3 | 0.001 | 0.980 | 0.843 |
| CPT | 5.6 ± 1.1 | 7.5 ± 1.9 | |||
| ICA β-stiffness | |||||
| BL | 8.5 ± 4.2 | 6.7 ± 2.6 | 0.676 | 0.635 | 0.419 |
| CPT | 8.0 ± 4.9 | 8.6 ± 2.2 | |||
| CCA elastic modulus, mmHg | |||||
| BL | 555.9 ± 129.5 | 796.3 ± 198.2 | <0.001 | 0.131 | 0.910 |
| CPT | 636.9 ± 126.1 | 890.2 ± 219.1 | |||
| ICA elastic modulus, mmHg | |||||
| BL | 846.6 ± 384.4 | 698.4 ± 264.9 | 0.879 | 0.227 | 0.383 |
| CPT | 896.5 ± 496.2 | 1,000.8 ± 269.5 | |||
| CCA arterial compliance, cm/mmHg | |||||
| BL | 0.012 ± 0.003 | 0.010 ± 0.003 | 0.023 | 0.191 | 0.998 |
| CPT | 0.011 ± 0.002 | 0.009 ± 0.002 | |||
| ICA arterial compliance, cm/mmHg | |||||
| BL | 0.008 ± 0.003 | 0.011 ± 0.006 | 0.499 | 0.856 | 0.256 |
| CPT | 0.009 ± 0.005 | 0.009 ± 0.005 | |||
| CCA arterial distensibility, mmHg | |||||
| BL | 0.002 ± 0.000 | 0.001 ± 0.001 | 0.002 | 0.234 | 0.947 |
| CPT | 0.002 ± 0.000 | 0.001 ± 0.000 | |||
| ICA arterial distensibility, mmHg | |||||
| BL | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.467 | 0.562 | 0.343 |
| CPT | 0.003 ± 0.006 | 0.001 ± 0.000 | |||
Values are means ± SD. BL, baseline; CPT, cold pressure test; CCA, common carotid artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; P values represent 2-way repeated ANOVA results (age: young and old; phase: BL, CPT1, CPT2, CPT3, RE1, RE2, and RE3).
DISCUSSION
The first major novel finding of the present study is that in young individuals there is a differential response to the CPT within the extracranial blood vessels (CCA vs. ICA) and also discrepant responses between the extra- and intracranial circulations. The second major novel finding is that in older individuals there is a blunting of the extra- and intracranial responses to the CPT. The physiological and clinical significance of these findings are considered below.
Extra- and Intracranial Blood Flow Regulation During the Cold Pressor Test
In accord with earlier work in young individuals (53), we observed a significant increase in CCA diameter during the CPT. However, in contrast, and in accord with our hypothesis, we observed no change in ICA diameter during the CPT. Despite this lack of change in ICA diameter and only a transient increase in ICA flow during the first minute of CPT, we observed that the CPT evoked a marked and persistent increase in MCAvmean, a finding in contrast to Bramanti et al. (3) but in agreement with several previous studies (46, 47, 56, 73). This may imply a differential regulation of the extra- and intracranial arteries that could serve an important protective role. There is evidence that the extracranial arteries (at the level of the ICA and vertebral arteries) are integral to the regulation of cerebral blood flow through modifying vascular resistance (14, 22, 29, 39, 40). Furthermore, an MRI study reported decreased cerebral blood volume in response to sympathoexcitatory reflexes (69). In response to a sympathetically mediated hypertensive insult, the buffering function of the larger cerebral and large pial arterioles, but not the cerebral microcirculation, serves as a first line of defense in regulating cerebral perfusion pressure. Our data indicate that the responses of the CCA are different from the ICA and MCA during the CPT, at least in younger individuals.
Elevations in sympathetic vasoconstrictor activity and MAP produced by the CPT have at least three effects on cerebral blood flow. The first effect is the obvious hydraulic effect of MAP that increases flow even if vascular resistance is unchanged. The second effect, and the one commonly either neglected or misunderstood, is the autoregulatory effect of an increase in perfusion pressure to increase vascular resistance and minimize the increase in flow. A likely third effect is the influence of SNA on extra- and intracranial blood flow regulation. Thus, appreciation of the effects of the CPT on factors such as the hydraulic effect and potential shear patterns of elevations in MAP, as well as the concomitant changes in SNA and autoregulation, likely explains the apparent differential mechanisms of regulation between the CCA, ICA, and MCA.
Although the sympathetic regulation of the cerebral blood vessels in humans remains a controversial issue (1, 58), we did observe a decrease in MCA CVC (a finding consistent with other studies; see Refs. 21 and 52) and demonstrate for the first time that the CPT reduces CCA, ICA, and PCA CVC. These latter changes in CVC are possibly indicative of sympathetically mediated cerebral vasoconstriction or autoregulatory mediated. Bramanti et al. (3) demonstrated a reduction in MCAvmean during the CPT (by ∼23%), the magnitude of which was approximately halved following intrathecal administration of the α2-adrenergic receptor agonist clonidine. These findings support the role of a central noradrenergic mechanism in the cerebrovascular responses to the CPT. However, although not measured in this study, differences in may explain these conflicting findings. In the present study, a dynamic end-tidal forcing system was used in an attempt to maintain near baseline, thus permitting the effect of the generalized sympathetic activation associated with the CPT to be observed.
Along with MCAvmean, we determined the PCAvmean responses to CPT. There are known anatomic and physiological differences between anterior and posterior circulations. For example, the PCA may have less sympathetic innervation than the anterior cerebral portion (12, 20), and CO2 reactivity is reduced (51). We observed that the temporal pattern of response PCAvmean and MCAvmean was similar; however, interestingly, the magnitude of response was greater in the MCA compared with the PCA in the young (17 ± 14 vs. 10 ± 10%).
Blunting of the Extra- and Intracranial Responses During the CPT in Older Individuals
In contrast to the younger group, the changes in both the extra and intracranial resistance and flow were generally blunted in the older group during the CPT. This is significant because dysfunctional CCA and coronary artery responses to the CPT have been associated with atherosclerotic disease (45, 53, 72). Because the MAP “stimulus” or hydraulic effect was comparable, it seems reasonable that the differential extra- and intracranial responses in young and older individuals reflect some fundamental differences in potential shear patterns induced via the elevations in MAP as well as the influences of SNA, humoral factors, endothelial vasodilator function, autoregulation, and parasympathetic control. Rubenfire et al. (53) speculated that a β-adrenergic mechanism accounted for the increase of CCA diameter during the CPT in healthy individuals, whereas the reduction in CCA diameter in coronary artery disease patients was due to greater sensitivity of the α-adrenergic receptors. This shift from a β-adrenergic vasodilatory response to an α-adrenergic vasoconstrictor one may be related to underlying endothelial damage and dysfunction (71). Endothelial dysfunction is well established to occur within the peripheral vasculature of healthy elderly individuals, and its extension to the cerebral vasculature might explain the present findings. Age-related alterations in arterial stiffness may also have contributed to the cerebrovascular responses reported. CCA stiffness was elevated in the older individuals at baseline, but in accord with previous literature, none of the calculated arterial stiffness indices was modified by the CPT (28, 33). Unfortunately, on the basis of our data set, we cannot delineate the mechanism(s) for the blunting of the extra- and intracranial responses during the CPT in older individuals, but our findings provide direction for future studies.
Methodological Considerations
There are a number of methodological considerations that should be considered in the context of our study and related interpretation of the findings.
Discrepancies of flow and velocity during the CPT.
The assessment of cerebrovascular responses during a myriad of physiological interventions has been dominated by the use of transcranial Doppler over the last 30 yr. However, this approach operates on the assumption (also its primary limitation) that the insonated vessel (PCA and MCA) remains at a constant diameter. Older studies have partially corroborated that under various stimuli (e.g., orthostasis, CO2 changes), MCAvmean accurately reflected the magnitude of changes in MCA blood flow, as diameter remained unchanged (55); however, recent high-resonance imaging studies have challenged this assumption of constant vessel diameter during marked changes in or (8, 9, 64) or exercise-induced sympathetic activation (63). Furthermore, as recently reviewed (24), it is not known whether the MCA diameter changes during elevations in blood pressure. At least during hypertension (35) and hypotension (32), discrepancies between ICA flow and MCAvmean have been reported. Similarly, in the present study we observed that the percentage increase in ICA flow was less marked than MCAvmean during the CPT. The effects of CO2 and blood pressure on PCA diameter are unknown.
Flow vs. conductance.
To account for MAP in the analysis of extra- vs. intracranial cerebrovascular responses, CVC is commonly used. However, as outlined above, increases in MAP produced by the CPT may affect cerebral blood flow by several independent and interacting mechanisms (e.g., hydraulic effect, autoregulation, and shear stress). As such, CVC is not likely to accurately account for the CPT-induced elevations in MAP during the CPT, and consideration of these mechanisms will be needed to fully understand the apparent differential regulation of the CCA⃗ICA⃗MCA.
CPT recovery.
We included recovery data in our analyses to verify that the cardiovascular, respiratory, and cerebrovascular variables of interest returned to baseline following the CPT. In all instances the measured parameters did successfully recover. Interestingly, an elevated systolic blood pressure recovery from the CPT is an important predictor of a future elevation in systolic blood pressure (57). Whether there is any prognostic significance to the cerebrovascular response to or following the CPT remains to be investigated.
Study limitations.
Roatta et al. (52), reported that the MCAvmean increases during hand CPT were slightly but significantly greater on the contralateral side (+4.4%) compared with the ipsilateral side (+2.4%). However, because the aim of our study was to simultaneously assess CCA, ICA, MCA, and PCA responses to the CPT measurements were necessitated on both the contralateral and ipsilateral sides; thus, unfortunately, it was not practical to account for any potential lateralization of the cerebral hemodynamic response to the CPT. In addition, hydration status was not assessed, which may be a limitation, as this has recently been reported to modify the cerebrovascular response to the CPT (47). We cannot exclude the possibility that age-related differences in thermoreceptor sensitivity contributed to the CPT responses we observed (15), although ratings of perceived pain were not different in the young and old groups during the CPT. One older individual was taking the α1A-adrenoreceptor antagonist tamsulosin for an enlarged prostate. Although these receptors are present in the ureter, they are less well expressed in the peripheral vasculature (44, 50). This individual displayed cerebral perfusion responses that were similar to the rest of the older group, and their removal did not affect the results of the statistical analyses.
It should be noted that our findings can be directed only to young and older healthy volunteers and that the regulation of cerebral blood flow may further differ in patients with cerebrovascular disease. Nevertheless, to be able to interpret the pathophysiological significance of these observations, a clear understanding of the normal responses of the cerebral circulation must first be obtained before extension can be made to pathological groups. Given that risk factors for coronary artery disease are associated with the extracranial blood vessel responses (53), future studies should explore the cerebrovascular responses in individuals at risk or in those that have experienced cerebrovascular events.
Clinical Implications
The CPT has been widely employed for cardiovascular risk stratification (6, 38, 61). Likewise, an attenuated cerebrovascular reactivity is indicative of an increased risk for all cause and cardiovascular (inclusive of stroke) mortality (48). The magnitude of the vasomotor response in the extracranial (ICA and vertebral artery) and intracranial arteries (MCA and PCA) to a CPT perturbation may be indicative of cerebrovascular health (i.e., endothelial function), much like peripheral flow-mediated dilation is indicative of cardiovascular risk (18, 25). Thus future studies are needed to further explore vasomotor responses to CPT in individuals at risk of or who have experienced cerebrovascular events. Consequently, the CPT may serve as a simple diagnostic tool to predict cerebrovascular events and reduce related disabilities and mortality.
In conclusion, during the CPT, for the first time we reveal 1) differential mechanism(s) of regulation between the ICA compared with the CCA in young individuals, 2) a blunting of the extra- and intracranial responses in older individuals, and, 3) irrespective of age, the fact that there were discrepancies in the magnitude of change in CCA flow, ICA flow, and MCAvmean during the CPT.
GRANTS
This research was supported by a National Sciences and Engineering Research Council Discovery Grant (20150821-01 to P. N. Ainslie), a Canadian Research Chair in Cerebrovascular Physiology (950-230970 to P. N. Ainslie), the Swiss National Science Foundation (P2ZHP3_158576 to D. Flück), the National Institute for Health Research Efficacy and Mechanism Evaluation (12/10/19 to J. P. Fisher), and the British Heart Foundation (PG/15/45/31579 to J. P. Fisher.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.F., P.N.A., and J.P.F. conceived and designed research; D.F., A.R.B., K.W.W., L.E.M., and J.P.F. performed experiments; D.F. analyzed data; D.F., P.N.A., and J.P.F. interpreted results of experiments; D.F. prepared figures; D.F. and J.P.F. drafted manuscript; D.F., P.N.A., A.R.B., K.W.W., L.E.M., and J.P.F. edited and revised manuscript; D.F., P.N.A., A.R.B., K.W.W., L.E.M., and J.P.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the time and effort spent by our volunteer participants in the present study.
REFERENCES
- 1.Ainslie PN, Brassard P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Rep 6: 14, 2014. doi: 10.12703/P6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auer LM, Edvinsson L, Johansson BB. Effect of sympathetic nerve stimulation and adrenoceptor blockade on pial arterial and venous calibre and on intracranial pressure in the cat. Acta Physiol Scand 119: 213–217, 1983. doi: 10.1111/j.1748-1716.1983.tb07330.x. [DOI] [PubMed] [Google Scholar]
- 3.Bramanti P, Mariani CA, D’Aleo G, Malara A. The first in vivo experience of the effects of the continuous intrathecal infusion of clonidine on the locus coeruleus in the regulation of cerebral blood flow: a TCD study. Ital J Neurol Sci 18: 139–144, 1997. doi: 10.1007/BF02048481. [DOI] [PubMed] [Google Scholar]
- 4.Bühler FR, Kiowski W, van Brummelen P, Amann FW, Bertel O, Landmann R, Lütold BE, Bolli P. Plasma catecholamines and cardiac, renal and peripheral vascular adrenoceptor-mediated responses in different age groups of normal and hypertensive subjects. Clin Exp Hypertens 2: 409–426, 1980. doi: 10.3109/10641968009037122. [DOI] [PubMed] [Google Scholar]
- 5.Burgmans S, Gronenschild EH, Fandakova Y, Shing YL, van Boxtel MP, Vuurman EF, Uylings HB, Jolles J, Raz N. Age differences in speed of processing are partially mediated by differences in axonal integrity. Neuroimage 55: 1287–1297, 2011. doi: 10.1016/j.neuroimage.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll D, Davey Smith G, Willemsen G, Sheffield D, Sweetnam PM, Gallacher JE, Elwood PC. Blood pressure reactions to the cold pressor test and the prediction of ischaemic heart disease: data from the Caerphilly Study. J Epidemiol Community Health 52: 528–529, 1998. doi: 10.1136/jech.52.8.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corretti MC, Plotnick GD, Vogel RA. Correlation of cold pressor and flow-mediated brachial artery diameter responses with the presence of coronary artery disease. Am J Cardiol 75: 783–787, 1995. doi: 10.1016/S0002-9149(99)80411-1. [DOI] [PubMed] [Google Scholar]
- 8.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- 9.Coverdale NS, Lalande S, Perrotta A, Shoemaker JK. Heterogeneous patterns of vasoreactivity in the middle cerebral and internal carotid arteries. Am J Physiol Heart Circ Physiol 308: H1030–H1038, 2015. doi: 10.1152/ajpheart.00761.2014. [DOI] [PubMed] [Google Scholar]
- 10.Di Piero V, Ferracuti S, Sabatini U, Pantano P, Cruccu G, Lenzi GL. A cerebral blood flow study on tonic pain activation in man. Pain 56: 167–173, 1994. doi: 10.1016/0304-3959(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 11.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002. doi: 10.1161/01.CIR.0000028819.64790.BE. [DOI] [PubMed] [Google Scholar]
- 12.Edvinsson L, Owman C, Siesjö B. Physiological role of cerebrovascular sympathetic nerves in the autoregulation of cerebral blood flow. Brain Res 117: 519–523, 1976. doi: 10.1016/0006-8993(76)90760-5. [DOI] [PubMed] [Google Scholar]
- 13.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 285: 916–918, 1982. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraci FM, Heistad DD, Mayhan WG. Role of large arteries in regulation of blood flow to brain stem in cats. J Physiol 387: 115–123, 1987. doi: 10.1113/jphysiol.1987.sp016566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the Aging Skin. Adv Wound Care (New Rochelle) 2: 5–10, 2013. doi: 10.1089/wound.2011.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Florian JP, Simmons EE, Chon KH, Faes L, Shykoff BE. Cardiovascular and autonomic responses to physiological stressors before and after six hours of water immersion. J Appl Physiol (1985) 115: 1275–1289, 2013. doi: 10.1152/japplphysiol.00466.2013. [DOI] [PubMed] [Google Scholar]
- 17.Freeman R, Chapleau MW. Testing the autonomic nervous system. Handb Clin Neurol 115: 115–136, 2013. doi: 10.1016/B978-0-444-52902-2.00007-2. [DOI] [PubMed] [Google Scholar]
- 18.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 19.Halter JB, Stratton JR, Pfeifer MA. Plasma catecholamines and hemodynamic responses to stress states in man. Acta Physiol Scand Suppl 527: 31–38, 1984. [PubMed] [Google Scholar]
- 20.Hamel E, Edvinsson L, MacKenzie ET. Heterogeneous vasomotor responses of anatomically distinct feline cerebral arteries. Br J Pharmacol 94: 423–436, 1988. doi: 10.1111/j.1476-5381.1988.tb11544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwich D, Fowler KL, Wynn LJ, Fisher JP. Differential responses to sympathetic stimulation in the cerebral and brachial circulations during rhythmic handgrip exercise in humans. Exp Physiol 95: 1089–1097, 2010. doi: 10.1113/expphysiol.2010.054387. [DOI] [PubMed] [Google Scholar]
- 22.Heistad DD, Marcus ML, Abboud FM. Role of large arteries in regulation of cerebral blood flow in dogs. J Clin Invest 62: 761–768, 1978. doi: 10.1172/JCI109187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hines EA, Brown G. A standard test measuring the variability of blood pressure. Its significance as an index of the prehypertensive state. Ann Intern Med 7: 209–217, 1933. [Google Scholar]
- 24.Hoiland RL, Ainslie PN. CrossTalk proposal: The middle cerebral artery diameter does change during alterations in arterial blood gases and blood pressure. J Physiol 594: 4073–4075, 2016. doi: 10.1113/JP271981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 26.Jeng JS, Yip PK, Huang SJ, Kao MC. Changes in hemodynamics of the carotid and middle cerebral arteries before and after endoscopic sympathectomy in patients with palmar hyperhidrosis: preliminary results. J Neurosurg 90: 463–467, 1999. doi: 10.3171/jns.1999.90.3.0463. [DOI] [PubMed] [Google Scholar]
- 27.Kang CK, Oh ST, Chung RK, Lee H, Park CA, Kim YB, Yoo JH, Kim DY, Cho ZH. Effect of stellate ganglion block on the cerebrovascular system: magnetic resonance angiography study. Anesthesiology 113: 936–944, 2010. doi: 10.1097/ALN.0b013e3181ec63f5. [DOI] [PubMed] [Google Scholar]
- 28.Kawano H, Tanimoto M, Yamamoto K, Sanada K, Gando Y, Tabata I, Higuchi M, Miyachi M. Resistance training in men is associated with increased arterial stiffness and blood pressure but does not adversely affect endothelial function as measured by arterial reactivity to the cold pressor test. Exp Physiol 93: 296–302, 2008. doi: 10.1113/expphysiol.2007.039867. [DOI] [PubMed] [Google Scholar]
- 29.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL Jr. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol Heart Circ Physiol 234: H371–H383, 1978. [DOI] [PubMed] [Google Scholar]
- 30.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 31.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003. doi: 10.1161/01.CIR.0000048893.62841.F7. [DOI] [PubMed] [Google Scholar]
- 32.Lewis NC, Smith KJ, Bain AR, Wildfong KW, Numan T, Ainslie PN. Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (Lond) 129: 169–178, 2015. doi: 10.1042/CS20140751. [DOI] [PubMed] [Google Scholar]
- 33.Lim J, Pearman ME, Park W, Alkatan M, Machin DR, Tanaka H. Impact of blood pressure perturbations on arterial stiffness. Am J Physiol Regul Integr Comp Physiol 309: R1540–R1545, 2015. doi: 10.1152/ajpregu.00368.2015. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Cao TS, Duan YY, Yang YL, Yuan LJ. Effects of cold pressor-induced sympathetic stimulation on the mechanical properties of common carotid and femoral arteries in healthy males. Heart Vessels 26: 214–221, 2011. doi: 10.1007/s00380-010-0069-1. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Zhu YS, Hill C, Armstrong K, Tarumi T, Hodics T, Hynan LS, Zhang R. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension 62: 973–979, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Yang X, Gong H, Jiang B, Wang H, Xu G, Deng Y. Assessing the effects of norepinephrine on single cerebral microvessels using optical-resolution photoacoustic microscope. J Biomed Opt 18: 076007, 2013. doi: 10.1117/1.JBO.18.7.076007. [DOI] [PubMed] [Google Scholar]
- 37.Lowe RF, Gilboe DD. Demonstration of alpha and beta adrenergic receptors in canine cerebral vasculature. Stroke 2: 193–200, 1971. doi: 10.1161/01.STR.2.2.193. [DOI] [PubMed] [Google Scholar]
- 38.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 110: 74–78, 2004. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 39.McHedlishvili GI. Vascular Mechanisms Pertaining to the Intrinsic Regulation of the Cerebral Circulation. Circulation 30: 597–610, 1964. doi: 10.1161/01.CIR.30.4.597. [DOI] [PubMed] [Google Scholar]
- 40.Mchedlishvili GI, Mitagvaria NP, Ormotsadze LG. Vascular mechanisms controlling a constant blood supply to the brain (“autoregulation”). Stroke 4: 742–750, 1973. doi: 10.1161/01.STR.4.5.742. [DOI] [PubMed] [Google Scholar]
- 41.Micieli G, Tassorelli C, Bosone D, Cavallini A, Viotti E, Nappi G. Intracerebral vascular changes induced by cold pressor test: a model of sympathetic activation. Neurol Res 16: 163–167, 1994. doi: 10.1080/01616412.1994.11740219. [DOI] [PubMed] [Google Scholar]
- 42.Mineta Y, Morimoto M, Harano K, Totoki T. [Sympathetic postganglionic innervation of external carotid artery, internal carotid artery, common carotid artery and aorta in the dog—experimental study using HRP and WGA-HRP]. Masui 41: 547–553, 1992. [PubMed] [Google Scholar]
- 43.Mitchell DA, Lambert G, Secher NH, Raven PB, van Lieshout J, Esler MD. Jugular venous overflow of noradrenaline from the brain: a neurochemical indicator of cerebrovascular sympathetic nerve activity in humans. J Physiol 587: 2589–2597, 2009. doi: 10.1113/jphysiol.2008.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murata S, Taniguchi T, Takahashi M, Okada K, Akiyama K, Muramatsu I. Tissue selectivity of KMD-3213, an α1-adrenoreceptor antagonist, in human prostate and vasculature. J Urol 164: 578–583, 2000. doi: 10.1016/S0022-5347(05)67426-5. [DOI] [PubMed] [Google Scholar]
- 45.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation 77: 43–52, 1988. doi: 10.1161/01.CIR.77.1.43. [DOI] [PubMed] [Google Scholar]
- 46.Panerai RB, Dawson SL, Eames PJ, Potter JF. Cerebral blood flow velocity response to induced and spontaneous sudden changes in arterial blood pressure. Am J Physiol Heart Circ Physiol 280: H2162–H2174, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Perry BG, Bear TL, Lucas SJ, Mündel T. Mild dehydration modifies the cerebrovascular response to the cold pressor test. Exp Physiol 101: 135–142, 2016. doi: 10.1113/EP085449. [DOI] [PubMed] [Google Scholar]
- 48.Portegies ML, de Bruijn RF, Hofman A, Koudstaal PJ, Ikram MA. Cerebral vasomotor reactivity and risk of mortality: the Rotterdam Study. Stroke 45: 42–47, 2014. doi: 10.1161/STROKEAHA.113.002348. [DOI] [PubMed] [Google Scholar]
- 49.Querido JS, Ainslie PN, Foster GE, Henderson WR, Halliwill JR, Ayas NT, Sheel AW. Dynamic cerebral autoregulation during and following acute hypoxia: role of carbon dioxide. J Appl Physiol (1985) 114: 1183–1190, 2013. doi: 10.1152/japplphysiol.00024.2013. [DOI] [PubMed] [Google Scholar]
- 50.Rajpathy J, Aswathaman K, Sinha M, Subramani S, Gopalakrishnan G, Kekre NS. An in vitro study on human ureteric smooth muscle with the alpha1-adrenoceptor subtype blocker, tamsulosin. BJU Int 102: 1743–1745, 2008. doi: 10.1111/j.1464-410X.2008.08022.x. [DOI] [PubMed] [Google Scholar]
- 51.Reinhard M, Waldkircher Z, Timmer J, Weiller C, Hetzel A. Cerebellar autoregulation dynamics in humans. J Cereb Blood Flow Metab 28: 1605–1612, 2008. doi: 10.1038/jcbfm.2008.48. [DOI] [PubMed] [Google Scholar]
- 52.Roatta S, Micieli G, Bosone D, Losano G, Bini R, Cavallini A, Passatore M. Effect of generalised sympathetic activation by cold pressor test on cerebral haemodynamics in healthy humans. J Auton Nerv Syst 71: 159–166, 1998. doi: 10.1016/S0165-1838(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 53.Rubenfire M, Rajagopalan S, Mosca L. Carotid artery vasoreactivity in response to sympathetic stress correlates with coronary disease risk and is independent of wall thickness. J Am Coll Cardiol 36: 2192–2197, 2000. doi: 10.1016/S0735-1097(00)01021-4. [DOI] [PubMed] [Google Scholar]
- 54.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 60: 989–994, 2003. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 55.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. doi: 10.1161/01.STR.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 56.Sohn YH. Cerebral hemodynamic changes induced by sympathetic stimulation tests. Yonsei Med J 39: 322–327, 1998. doi: 10.3349/ymj.1998.39.4.322. [DOI] [PubMed] [Google Scholar]
- 57.Stewart JC, France CR. Cardiovascular recovery from stress predicts longitudinal changes in blood pressure. Biol Psychol 58: 105–120, 2001. doi: 10.1016/S0301-0511(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 58.Strandgaard S, Sigurdsson ST. Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Counterpoint: Sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol (1985) 105: 1366–1367, 2008. doi: 10.1152/japplphysiol.90597.2008a. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi R, Sakai T, Furuyama Y, Kondo Y, Inoue CN, Onuma S, Iinuma K. The vasocontractive action of norepinephrine and serotonin in deep arterioles of rat cerebral gray matter. Tohoku J Exp Med 190: 129–142, 2000. doi: 10.1620/tjem.190.129. [DOI] [PubMed] [Google Scholar]
- 60.Thomas KN, Lewis NC, Hill BG, Ainslie PN. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am J Physiol Regul Integr Comp Physiol 309: R707–R720, 2015. doi: 10.1152/ajpregu.00211.2015. [DOI] [PubMed] [Google Scholar]
- 61.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med 65: 46–62, 2003. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Umeyama T, Kugimiya T, Ogawa T, Kandori Y, Ishizuka A, Hanaoka K. Changes in cerebral blood flow estimated after stellate ganglion block by single photon emission computed tomography. J Auton Nerv Syst 50: 339–346, 1995. doi: 10.1016/0165-1838(94)00105-S. [DOI] [PubMed] [Google Scholar]
- 63.Verbree J, Bronzwaer A, van Buchem MA, Daemen M, van Lieshout JJ, van Osch M. Middle cerebral artery diameter changes during rhythmic handgrip exercise in humans. J Cereb Blood Flow Metab 37: 2921–2927, 2017. doi: 10.1177/0271678X16679419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 65.Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987. doi: 10.1161/01.HYP.9.5.429. [DOI] [PubMed] [Google Scholar]
- 66.Wagerle LC, Heffernan TM, Sacks LM, Delivoria-Papadopoulos M. Sympathetic effect on cerebral blood flow regulation in hypoxic newborn lambs. Am J Physiol Heart Circ Physiol 245: H487–H494, 1983. [DOI] [PubMed] [Google Scholar]
- 67.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 68.Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol 592: 841–859, 2014. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson TD, Shoemaker JK, Kozak R, Lee TY, Gelb AW. Reflex-mediated reduction in human cerebral blood volume. J Cereb Blood Flow Metab 25: 136–143, 2005. doi: 10.1038/sj.jcbfm.9600015. [DOI] [PubMed] [Google Scholar]
- 70.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol (1985) 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Young MA, Vatner SF. Enhanced adrenergic constriction of iliac artery with removal of endothelium in conscious dogs. Am J Physiol 250: H892–H897, 1986. [DOI] [PubMed] [Google Scholar]
- 72.Zeiher AM, Drexler H, Wollschlaeger H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol 14: 1181–1190, 1989. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]
- 73.Zvan B, Zaletel M, Pretnar J, Pogacnik T, Kiauta T. Influence of the cold pressor test on the middle cerebral artery circulation. J Auton Nerv Syst 74: 175–178, 1998. doi: 10.1016/S0165-1838(98)00163-5. [DOI] [PubMed] [Google Scholar]