Acute exercise in overweight/obese adults increased subcutaneous adipose tissue (SAT) mRNA expression of VEGFA, an important regulator of angiogenesis and capillary growth. In addition, subjects that regularly exercise had elevated SAT CD31 mRNA expression and elevated IL-6 mRNA in adipose tissue macrophages compared with nonexercisers. This study demonstrates that aerobic exercise may alter processes related to whole body metabolic outcomes in obesity, such as angiogenesis and immune response, in the SAT of overweight/obese adults.
Keywords: adipose tissue, angiogenesis, exercise, inflammation, metabolic health
Abstract
Alterations in the inflammatory state, metabolic function, and structure of subcutaneous adipose tissue (SAT) can impact the development of insulin resistance in obesity. Exercise can improve metabolic health in obesity, but the effects of exercise on SAT are not well known. The purpose of this study was to examine the effects of acute exercise and habitual exercise training on mRNA expression of markers of lipid metabolism, inflammation, fibrosis, and hypoxia/angiogenesis in SAT, as well as adipocyte cell size. We recruited overweight-to-obese adults who exercised regularly (ACTIVE: n = 8) or were sedentary (SED: n = 12). The groups were well matched for age (27 ± 1 vs. 24 ± 2 yr), body mass index (29 ± 1 vs. 27 ± 1 kg/m2), and body composition (30 ± 1 vs. 29 ± 1% body fat), but as expected, cardiorespiratory fitness was greater in ACTIVE vs. SED (V̇o2peak: 51 ± 3 vs. 42 ± 1 ml·kg fat-free mass−1·min−1; P = 0.01). Abdominal SAT biopsy samples were obtained before and 1 h after a single session of aerobic exercise (60 min at ~65% V̇o2peak). The exercise session increased SAT mRNA expression of VEGFA, an important regulator of angiogenic processes, in both groups. In addition, SAT from ACTIVE subjects had greater mRNA expression of the endothelial cell marker CD31 compared with SED, which may be a cumulative effect of the transient increases in VEGFA with regular exercise. We also magnetically sorted CD14+ immune cells from SAT samples and found that IL-6 expression was elevated in ACTIVE compared with SED. In conclusion, exercise initiates increases in factors related to angiogenic processes and may promote alterations in macrophage inflammation in SAT.
NEW & NOTEWORTHY Acute exercise in overweight/obese adults increased subcutaneous adipose tissue (SAT) mRNA expression of VEGFA, an important regulator of angiogenesis and capillary growth. In addition, subjects that regularly exercise had elevated SAT CD31 mRNA expression and elevated IL-6 mRNA in adipose tissue macrophages compared with nonexercisers. This study demonstrates that aerobic exercise may alter processes related to whole body metabolic outcomes in obesity, such as angiogenesis and immune response, in the SAT of overweight/obese adults.
regular exercise is known to have overall health benefits and is often prescribed to combat obesity-related metabolic complications that can lead to metabolic diseases, such as type 2 diabetes (T2D) and cardiovascular disease (CVD) (9). Elevated cardiorespiratory fitness is associated with reduced incidence of CVD and T2D (24, 57). While most of the focus on exercise adaptations that benefit metabolic health has centered on changes in skeletal muscle, exercise-induced changes in other tissues and organs may also contribute to the metabolic health effects of exercise (16). In particular, adaptations to exercise in adipose tissue may be important for mediating whole body metabolic outcomes since it is now well recognized that adipose tissue health and function play a prominent role in dictating obesity-related metabolic complications (3, 38, 39).
While the adipose tissue can actively secrete peptides (i.e., adipokines) that may have important systemic effects, complications associated with the regulation of lipid storage within adipocytes can also have profound metabolic consequences (48, 51, 52). Limitations in the lipid storage capacity of adipose tissue can expose peripheral tissues to high levels of fatty acids, which can impair insulin signaling (19, 40, 43) and disrupt other cellular processes (35, 42). Several factors within adipose tissue are postulated to be important for determining its lipid storage capacity, including lipase and esterification enzymes (2, 50), adipocyte size (32), extracellular matrix structure (12, 58), capillarization (10), and inflammation (15, 27). Several studies have reported beneficial effects of exercise on adipose tissue inflammation, fibrosis, and adipose tissue insulin sensitivity in humans, but these studies are confounded by reductions in body fat mass that accompanied their exercise interventions (6, 8, 36, 49). This limits the conclusions that can be drawn from these studies, as even modest weight loss can profoundly impact adipose tissue and whole body metabolic outcomes (4, 6, 8, 26). However, recent animal studies show promising evidence for beneficial direct effects of exercise on adipose tissue structure and metabolic function (5, 20, 21, 25, 45).
It remains unclear whether exercise (acute or chronic) causes beneficial adaptations in adipose tissue that can improve obesity-related metabolic complications in humans. Therefore, the purpose of this study was to examine the effects of acute exercise and habitual exercise training on the expression of factors related to adipose tissue fatty acid storage and release (e.g., lipolysis, esterification, lipogenesis, adipogenesis), extracellular matrix composition, angiogenesis, and inflammation in adipose tissue. We also examined the effects of exercise on adipose tissue cell size. We hypothesized that acute exercise would stimulate factors that may lead to improved adipose tissue metabolic health and function and that habitual exercisers would have markers of improved metabolic health in their adipose tissue compared with that of sedentary obese adults.
METHODS
Subjects
A total of 20 overweight-to-mildly obese adults [body mass index (BMI): 25–35 kg/m2] between the ages of 18 and 40 yr were recruited for the study. Subjects completed a comprehensive behavior survey/questionnaire and were categorized into two cohorts based on their self-reported habitual physical activity behavior. Twelve of the subjects were nonexercisers (SED: n = 12) while eight subjects exercised regularly (ACTIVE: n = 8) (i.e., at least 4 days/wk of moderate to vigorous intensity aerobic/cardiovascular exercise for 30–60 min/session). Participants were not taking any medications known to affect metabolic outcomes. All participants were nonsmokers and weight stable (within ±2 kg for ≥6 mo). None of the subjects had any history of metabolic or cardiovascular disease that would impact study outcomes. Written, informed consent was obtained from all subjects before initiating participation, and all study procedures were approved by the University of Michigan Institutional Review Board.
Preliminary Testing
Maximal oxygen consumption.
Subjects completed a graded exercise test that involved a 4-min warm-up, followed by an increase in exercise workload (increased speed and grade on treadmill or increased resistance on bike) every 1–2 min until the subject reached volitional fatigue. The participant had the choice to perform the test on either a stationary bike (Lode ergometer) or treadmill, and importantly, the modality of exercise chosen was the exercise modality used for the experimental procedures. Maximal oxygen consumption (V̇o2peak) and maximal heart rate were recorded and used to calculate the exercise workload during the experimental trial.
Oral glucose tolerance test.
Subjects arrived at the laboratory after an overnight fast for assessment of their glucose tolerance. Subjects who exercise regularly abstained from exercise for 3 days before the oral glucose tolerance test (OGTT). Subjects drank a flavored solution containing 75 g of glucose (Fisherbrand GTT Beverage), and blood was collected from an intravenous catheter inserted in an arm vein every 15 min for 3 h. Blood samples were centrifuged at 2,000 g at 4°C for 15 min, and plasma was aliquoted and stored at −80°C until analysis for glucose and insulin concentrations.
Index of whole body insulin sensitivity.
Plasma glucose and insulin concentrations measured before and during the OGTT were used to assess insulin sensitivity using the Matsuda composite index (28). The Matsuda composite index has been found to be a reasonably accurate marker for insulin sensitivity when compared with the hyperinsulinemic-euglycemic clamp (28). We also calculated homeostatic model assessment of insulin resistance (HOMA-IR) with the formula: [fasting serum insulin (µU/ml) × fasting plasma glucose (mmol/l)]/22.5 (29).
Body composition.
Body composition was assessed using hydrostatic weighing (7). Briefly, subjects were weighed underwater after exhaling all air except residual lung volume using a scale attached to a chair in the water. Water temperature was maintained at 37°C. Residual lung volume was estimated using appropriate equations based on age, stature, and body mass. The appropriate Siri equation (44) was then used to estimate percent body fat.
Experimental Protocol
All subjects arrived to the laboratory in the morning of testing after an overnight fast (~10 h fast). Furthermore, subjects who were regular exercisers refrained from exercise for 3 days before their experimental trial. After the subject rested quietly for 30 min, we collected a blood sample followed by collection of an abdominal subcutaneous adipose tissue sample using a needle aspiration method. This aspiration procedure involved injection of local anesthetic (2% lidocaine) in a region ~5 cm lateral to the umbilicus. Adipose tissue was removed using a 16-G needle with suction applied with a 10-ml syringe. A portion of the adipose tissue biopsy sample was cleaned with saline, blotted dry, and quickly frozen in liquid nitrogen, while a separate portion of cleaned sample was placed in phosphate buffered saline for separation of mature adipocytes and the stromalvascular fraction (SVF; see details below). Subjects then exercised on a treadmill or cycle ergometer for 1 h at a moderate intensity (~65% of their predetermined V̇o2peak). Exactly 1 h after cessation of exercise, we repeated the adipose tissue biopsy procedure.
Analytical Procedures
Plasma substrate measurements.
Plasma glucose (Thermo Scientific, Waltham, MA) and fatty acid (Wako Chemicals, Richmond, VA) concentrations were measured using commercially available colorimetric assay kits. Plasma insulin concentration was measured with a chemiluminescent immunoassay method (Siemens IMMULITE 1000, Flanders, NJ).
Isolation of mature adipocytes and resident immune cells.
To discriminate changes in the various adipose tissue cell populations in response to exercise, we separated mature adipocytes from the SVF. First, adipose tissue was digested in collagenase type I (1 mg/ml) in Hank’s balanced salt solution while gently rocking at 37°C for 1 h. The digested tissue was strained through a 300-µm nylon mesh filter to remove debris. The digested tissue was then allowed to sit until all of the mature adipocytes floated to the top of the digested tissue. The infranatant containing the SVF was then removed from beneath the floating adipocytes. The mature adipocytes were used for RNA expression and cell sizing analyses (described below). The SVF was centrifuged (500 g for 7 min) to pellet the cells and allow for the removal of the supernatant and any residual mature adipocytes. After three washes in PBS supplemented with 2% fetal bovine serum (to assist with maintaining cell viability), a commercially available immunomagnetic bead sorting kit (EasySep Human CD14 Positive Selection Kit, No. 18058; Stem Cell Technologies) was used to isolate the immune cell fraction from the SVF. The manufacturer reports a typical range of 97.8–99.7% CD14+ cells, measured by fluorescence-activated cell sorting (FACS), following isolation from peripheral blood mononuclear cells (https://www.stemcell.com/easysep-human-cd14-positive-selection-kit.html#section-data-and-publications), but we did not perform FACS analysis of these samples to verify the degree of CD14+ enrichment. The cell surface marker CD14 has been shown to be specific to the macrophage population (11, 56); therefore, we used this marker to identify and select the adipose tissue macrophage population.
Adipose tissue mRNA expression.
For isolation of RNA, sections of subcutaneous adipose tissue (~50 mg), mature adipocytes, and CD14+ cells were homogenized in RNA Stat-60 (Tel-Test, Friendswood, TX). RNA was isolated by chloroform extraction and quantified spectrophotometrically. Reverse transcription was performed with 0.25 μg of total RNA with the High-Capacity cDNA RT kit (4368813; Life Technologies, Grand Island, NY). Real-time quantitative PCR with predesigned PrimeTime qPCR Assays (IDT) was used to assess the mRNA expression levels of the genes listed in Table 1. The qPCR data were normalized to the expression of two housekeeping genes [peptidylprolyl isomerase A (PPIA) and β2-microglobulin (B2M)] using the −∆Ct method (41).
Table 1.
Quantitative PCR targets in the subcutaneous adipose tissue
| Gene Symbol | Protein Encoded |
|---|---|
| PPARG | Proliferator-activated receptor-γ (PPARγ) |
| SREBP1C | Sterol regulatory element-binding protein 1 (SREBP1c) |
| CIDEA | Cell death activator CIDE-A |
| GPAT1 | Glycerol-3-phosphate acyltransferase-1 (GPAT1) |
| DGAT1 | Diacylglyceride acyltransferase 1 (DGAT1) |
| ATGL | Adipose triglyceride lipase (ATGL) |
| HSL | Hormone-sensitive lipase (HSL) |
| IL-6 | Interleukin-6 |
| PLIN1 | Perilipin 1 (PLIN1) |
| COL6A1 | Collagen VI |
| COL1A1 | Collagen I |
| VEGFA | Vaascular endothelial growth factor (VEGF) |
| PECAM1 | Cluster of differentiation 31 (CD31) |
| MCP1 | Monocyte chemoattractant protein-1 (MCP-1) |
| TNFA | Tumor necrosis factor-α (TNF-α) |
| NLRP3 | NOD-like receptor family, pyrin domain containing 3 (NLRP3) |
| IL-1B | Interleukin-1β (IL-1β) |
| ITGAX | “M1” macrophage marker CD11c |
| MRC1 | “M2” macrophage marker CD206 |
| CD163 | “M2” macrophage marker CD163 |
| SLC2A1 | Glucose transporter 1 (GLUT1) |
Adipocyte size.
We quantified the distribution of adipocyte cell size by imaging mature adipocytes using a bright-field microscope. A portion of the isolated mature adipocytes (isolation described above) was added to an equal volume of 10% formalin, vortexed, and stored at 4°C. To image the cells, 10 µl of the suspension of fixed adipocytes were added to 20 µl of mounting medium on a microscope slide and covered with a coverslip. Quantification of cell size was then performed using the protocol described by Parlee et al. (33) using ImageJ software.
Statistical Analysis
We used a two-way ANOVA with Tukey post hoc analysis to assess significant differences in mRNA expression and adipocyte cell size between groups and in response to the single session of exercise. We used Grubb’s outlier test (14) to detect outliers in the data sets using GraphPad statistical software (https://www.graphpad.com/quickcalcs/). Using this test, we found one outlier value in each of the mRNA analyses for IL-1B, MCP1, and CD31 in the whole adipose tissue homogenate. For the CD14+ fraction, one outlier was removed from TNFA, IL-1B, NLRP3, CD11C, and IL-6. One outlier was removed from the mature adipocyte CIDEA mRNA data set. P < 0.05 was considered statistically significant.
RESULTS
Subject Characteristics
There were no differences in age, body mass, BMI, body fat percentage, fat mass, or fat free mass (FFM) between SED and ACTIVE (Table 2). In addition, there were no differences in markers of insulin sensitivity or glucose tolerance between the groups (Table 2). As expected, however, ACTIVE had a higher V̇o2peak compared with SED (52 ± 3 vs. 42 ± 2 ml·kg FFM−1·min−1, respectively; P = 0.01; Table 2).
Table 2.
Physical characteristics of subjects
| SED | ACTIVE | |
|---|---|---|
| Sex, M/F | 5/7 | 3/5 |
| Age | 27 ± 2 | 27 ± 1 |
| Body mass, kg | 82 ± 3 | 80 ± 3 |
| BMI, kg/m2 | 27 ± 1 | 29 ± 1 |
| Body fat, % | 31 ± 2 | 29 ± 2 |
| FM, kg | 26 ± 2 | 24 ± 3 |
| FFM, kg | 57 ± 3 | 57 ± 3 |
| V̇o2peak, ml·kg FFM−1·min−1 | 42 ± 2 | 52 ± 3* |
| Matsuda ISI | 8.2 ± 1.4 | 5.6 ± 0.3 |
| HOMA-IR | 1.3 ± 0.2 | 1.6 ± 0.1 |
| Glucose AUC | 868 ± 56 | 972 ± 60 |
| Insulin AUC | 8,647 ± 1,483 | 11,323 ± 1,101 |
Data are presented as means ± SD. MF, male/female; SED, sedentary; ACTIVE, exercised regularly; FM, fat mass; FFM, fat-free mass; ISI, insulin sensitivity index; HOMA-IR, homeostatic model assessment of insulin resistance; AUC, area under curve.
P < 0.05 compared with SED.
Adipose Tissue
Markers of angiogenesis, vascularization, and hypoxia.
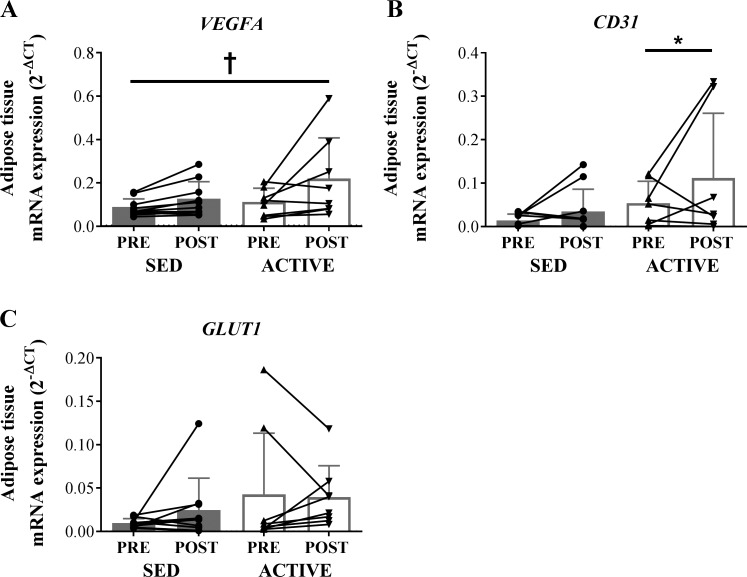
VEGFA mRNA expression was similar in ACTIVE and SED; however, the acute exercise session significantly increased (P < 0.05) VEGFA expression in both groups (Fig. 1A). In line with this finding, we found the mRNA expression of the endothelial cell marker CD31 was elevated (P < 0.05) in our regular exercisers (i.e., ACTIVE > SED; Fig. 1B). There was no effect of acute exercise on CD31. mRNA expression of GLUT1, a marker of elevated hypoxia, was not different between groups nor was it altered after acute exercise (Fig. 1C).
Fig. 1.
mRNA expression of factors related to angiogenesis, capillarization, and hypoxia in whole adipose tissue homogenate in sedentary (SED) compared with regular exercise (ACTIVE) before (PRE) and 1 h after (POST) exercise. Expression values were normalized to the mean of the housekeeping genes PPIA and B2M. For SED samples, n = 11 due to lack of RNA yield in 1 subject. One outlier was removed from the ACTIVE CD31 data set. A: VEGFA. B: CD31. C: GLUT1. Data are expressed as means ± SD. *P < 0.05 vs. SED, main effect of group. †P < 0.05, main effect of acute exercise.
Markers of inflammation.
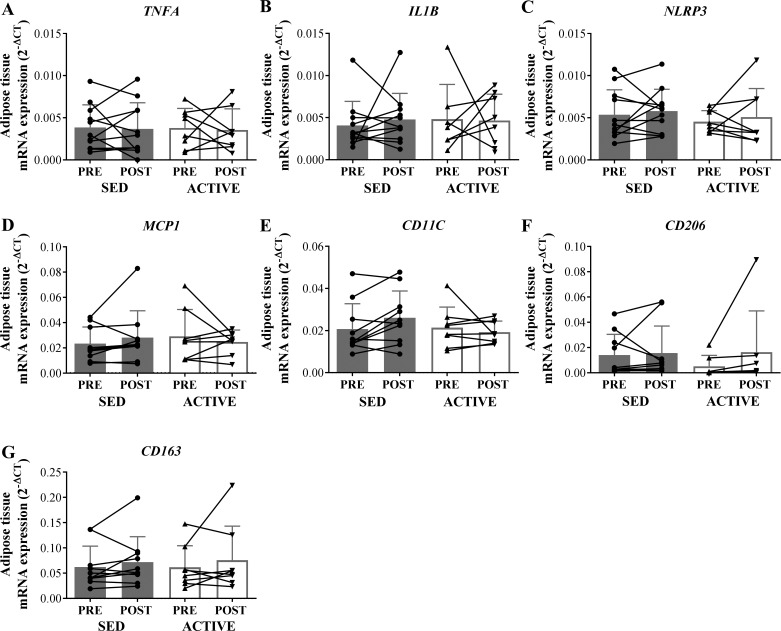
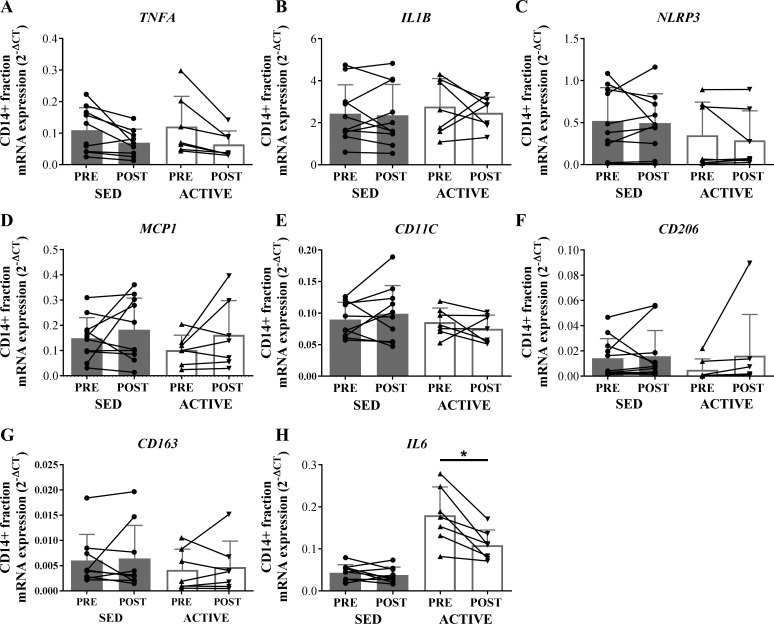
mRNA expression of all measured inflammation-related genes (TNFA, IL-1B, NLRP3, MCP1, CD11C, CD206, and CD163) did not differ between ACTIVE and SED before or 1 h after an acute exercise session in whole tissue homogenate (Fig. 2). In most of the whole tissue samples, the mRNA expression of IL-6 was undetectable or had cycle threshold values higher than 35; therefore, we did not use these data. The expression levels of IL-1B, NLRP3, MCP1, CD11C, CD206, and CD163 were also not different between groups in the CD14+ enriched adipose tissue SVF (Fig. 3). However, IL-6 expression in the CD14+ enriched fraction was fourfold greater in ACTIVE vs. SED (main effect for group; P < 0.05) (Fig. 3H). While the acute session of exercise did not significantly affect any of the inflammatory markers we measured in either the whole tissue homogenate (Fig. 2) or the CD14+ enriched fraction (Fig. 3), we did observe a trend for the exercise session to lower the expression of IL-6 (P = 0.055) in the CD14+ fraction (Fig. 3H).
Fig. 2.
mRNA expression of factors related to inflammation in whole adipose tissue homogenate in SED compared with ACTIVE before (PRE) and 1 h after (POST) exercise. Expression values were normalized to the mean of the housekeeping genes PPIA and B2M. One outlier determined from Grubb’s outlier test was removed from ACTIVE IL-1B and SED MCP1. A: TNFA. B: IL-1B. C: NLRP3. D: MCP1. E: CD11C. F: CD206. G: CD163. Data are expressed as means ± SD.
Fig. 3.
mRNA expression of factors related to inflammation in the CD14+ enriched stromal vascular fraction of adipose tissue in SED compared with ACTIVE before (PRE) and 1 h after (POST) exercise. For SED samples n = 11, and for ACTIVE samples, n = 7 due to lack of RNA yield from 1 sample in each group. In addition, n = 10 for SED for TNFA, IL-1B, NLRP3, CD11C, CD163, and IL-6 due to 1 an outlier determined from Grubb’s outlier test. Expression values were normalized to the mean of the housekeeping genes PPIA and B2M. A: TNFA. B: IL-1B. C: NLRP3. D: MCP1. E: CD11C. F: CD206. G: CD163. H: IL6. Data are expressed as means ± SD. *P < 0.05 vs. SED, main effect of group.
Markers of lipid metabolism.
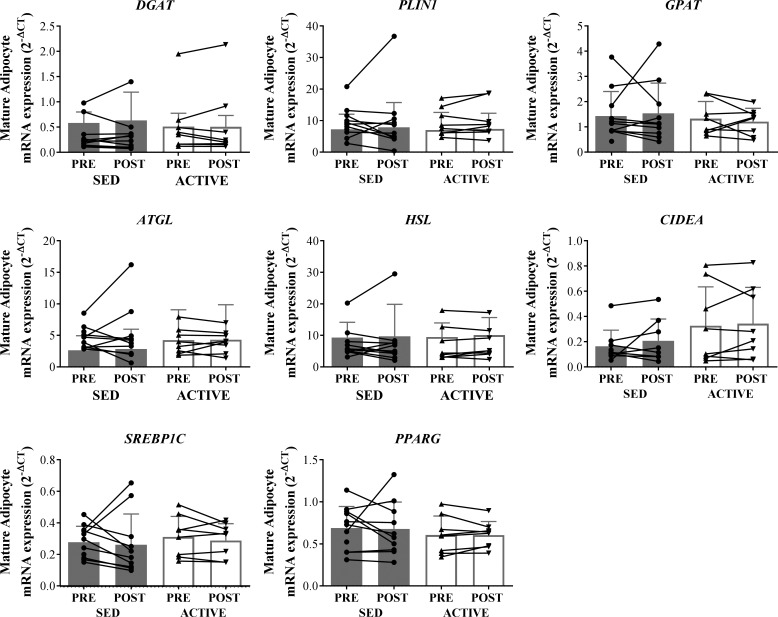
There were no effects of acute exercise on the expression of genes related to lipolysis (ATGL and HSL), esterification (GPAT and DGAT), lipid storage (PLIN1 and CIDEA), lipogenesis, or adipogenesis (SREBP1C and PPARG) in mature adipocytes extracted from adipose tissue samples collected 1 h after exercise compared with before exercise in either ACTIVE or SED. There were also no differences in the expression of any of these factors between groups (Fig. 4). In agreement with this data, there were no differences between groups (ACTIVE vs. SED) in circulating nonesterified fatty acids before, immediately after, and 1 h after the acute exercise (data not shown).
Fig. 4.
mRNA expression of factors related to related to lipolysis (ATGL and HSL), esterification (GPAT and DGAT), lipid storage (PLIN1 and CIDEA), or lipogenesis/adipogenesis (SREBP1C, PPARG) in mature adipocytes isolated from adipose tissue samples collected before (PRE) or 1 h after (POST) exercise in SED and ACTIVE. Expression values were normalized to the mean of the housekeeping genes PPIA and B2M. For samples, n = 11 for SED due to lack of RNA yield from 1 sample. For SED CIDEA samples, n = 10 due to an outlier determined from Grubb’s outlier test. Data expressed as means ± SD.
Extracellular matrix and adipocyte cell size.
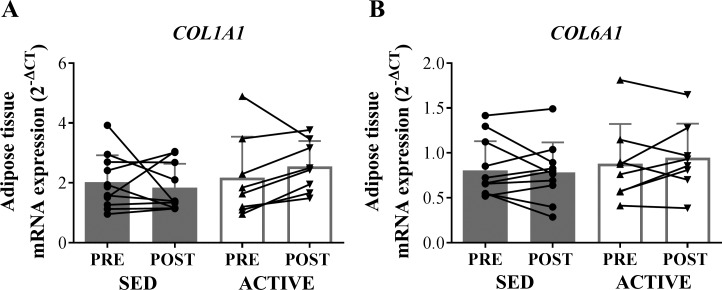
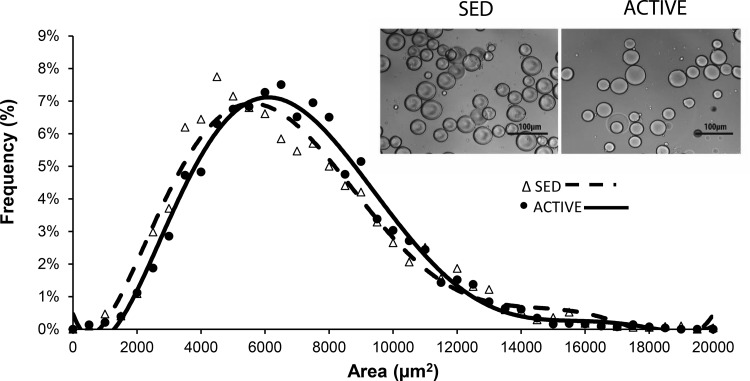
mRNA expression of COL6A1 and COL1A1 were similar in ACTIVE and SED subjects both before and 1 h after the exercise session (Fig. 5). Additionally, we found no differences in the proportion of small adipocytes (<5,000 µm2) or in the average adipocyte size between the groups. The frequency distribution of adipocyte cell sizes was very similar between ACTIVE and SED (Fig. 6).
Fig. 5.
mRNA expression of factors related to extracellular matrix in whole adipose tissue homogenate in SED compared with ACTIVE before (PRE) and 1 h after (POST) exercise. Expression values were normalized to the mean of the housekeeping genes PPIA and B2M. A: COL1A1. B: COL6A1. Data are expressed as means ± SD.
Fig. 6.
Distribution of frequencies of adipocyte size with representative images from each subject group.
DISCUSSION
The main findings of this study suggest that exercise may signal an increase in angiogenesis and capillarization in the subcutaneous adipose tissue of overweight and obese subjects. More specifically, a single session of aerobic exercise increased adipose tissue mRNA expression of VEGFA in both habitual exercisers and nonexercisers. An elevation in VEGFA after each exercise session may underlie a greater adipose capillarization in the habitual exercisers, as suggested by higher expression of the endothelial cell marker CD31 in adipose tissue from this group. Habitual exercisers also had higher mRNA expression levels of IL-6, a cytokine important for alternative macrophage activation, in the CD14+ enriched SVF of their adipose tissue. We did not find any other effects of acute or habitual exercise on other markers of inflammation or on our measured markers of adipose tissue fibrosis, adipogenesis, or adipocyte cell size. Overall, our data suggest that acute exercise may initiate angiogenic processes and exercise training may elevate IL-6 mRNA expression in macrophages in the subcutaneous adipose tissue of obese adults.
Inadequate adipose tissue vascularization and hypoxia have been implicated as primary mediators of metabolic complications in obesity (13, 60). Angiogenic processes are stimulated through the VEGF family of proteins and are considered to be one of, if not, the most important regulators of endothelial cell growth (47). While we did not observe differences in baseline expression of VEGFA between ACTIVE and SED, a single session of aerobic exercise increased VEGFA mRNA in both groups. Our ACTIVE participants habitually exercise at least 4 days per week; thus an elevation in VEGFA after each exercise session may lead to stable increases in capillarization, as evidenced by the elevated CD31 mRNA expression in ACTIVE compared with SED. To our knowledge, only one study has examined whether exercise training without weight loss can alter capillarization in adipose tissue in obese humans (54). Walton et al. (54) reported that 12 wk of aerobic exercise training in a group of insulin-sensitive obese adults was sufficient to increase CD31 mRNA expression and the density of capillaries in subcutaneous adipose tissue. Interestingly, they found no effect of exercise training on adipose tissue capillary density in their subjects who were “insulin resistant” (54). The average BMI for their insulin resistant cohort was ~35 kg/m2, compared with ~26 kg/m2 in their insulin sensitive cohort; therefore, it is possible that exercise has different effects on angiogenic processes in the adipose tissue of obese adults who are more obese and/or insulin resistant. Perhaps the greater fat mass in the insulin resistant cohort resulted in more inflammation, fibrosis, or other metabolic abnormalities that may have inhibited angiogenic processes.
Our finding that adipose tissue GLUT1 expression (a surrogate marker of hypoxic conditions and changes in oxygen tension Refs. 1, 55, 59) was not different between our habitual exercisers and nonexercisers provides crude data suggesting that adipose hypoxia may not differ between these groups despite potential differences in capillarization. Hypoxia-inducible factor-1α (HIF-1α) is also a major component of the response to hypoxia; however, tissue limitations in our study prevented us from measuring HIF-1α protein content and localization, so we could not assess differences in the abundance or functional activity of HIF-1α. We did not assess HIF1A mRNA expression, since it is the protein abundance and cellular localization of HIF1-α that are functionally linked to hypoxia, rather than its mRNA expression (17, 53).
The structure and composition of the extracellular matrix may dictate the ability of adipocytes to adequately expand and store energy in obesity (46). Interestingly, exercise may help modify adipose tissue extracellular matrix and/or prevent excessive deposition of extracellular matrix components (i.e., fibrosis). It has been reported that mice that exercised while on a high-fat diet exhibited lower adipose tissue fibrosis than did sedentary mice on the same high-fat diet (21). In a similar study by Kawanishi et al. (20), exercising mice had larger adipocytes than did their nonexercising counterparts on a high-fat diet, suggesting their lower adipose tissue fibrosis may have allowed their adipocytes to expand more freely to accommodate the excess nutrients. We did not find any differences in COL6A1 or COL1A1 between our regular exercisers and nonexercisers, suggesting that exercise training may not have the same effect in human subjects under weight-stable conditions. As the mice in Kawanishi et al. (20, 21) were exercising and gaining weight simultaneously, it is possible that these adaptations may occur only with the combined stimuli of exercise and nutrient excess.
Although adipocyte hypertrophy is the primary mechanism for storing excess energy in adipose tissue, adipose tissue may also expand through hyperplasia to accommodate the increased need for lipid storage. Several factors are released into the circulation during and/or after exercise (e.g., fatty acids, IGF-1, and cortisol) that are known activators of adipogenic processes and may upregulate the expression of factors critical for adipogenesis (e.g., PPARG and CEBPA). Stanford et al. (45) found that mice exposed to a short-term endurance exercise training program without weight loss greatly increased their abundance of small adipocytes, suggesting enhanced adipogenesis. However, we did not find any differences in the expression of critical regulators of adipogenesis or in adipocyte cell size between ACTIVE and SED. Our current data set in metabolically healthy overweight and obese adults suggests exercise may not have a potent effect on adipogenic processes in subcutaneous adipose tissue in weight-stable humans.
Some studies have reported that exercise training lowers adipose tissue inflammation in humans, which may lead to improved whole body metabolic outcomes (6, 36). Unfortunately, most of these exercise studies are confounded by weight loss, which is known to have a robust impact on lowering adipose tissue inflammation (4, 6, 8). However, recent evidence from some animal studies suggests beneficial effects of both acute and chronic exercise on inflammation and metabolic function of adipose tissue independent of weight loss and adiposity (25, 45). Our finding that ACTIVE had greater expression of IL-6 in the CD14+ SVF of their adipose tissue compared with that of SED suggests that habitual exercise training may alter the inflammatory state of the adipose tissue resident immune cells. In agreement with our findings, IL-6 expression and signaling within adipose tissue-has previously been found to be upregulated after an exercise session in mice that were on a high fat diet (25). In other work from this group, IL-6 expression was not increased in inguinal adipose tissue from mice fed a low-fat diet, which also suggests that changes in IL-6 expression may be impacted by obesity and/or different among fat depots. These findings are intriguing because IL-6 has been associated with improving or protecting adipose tissue metabolic health (30, 31, 53). However, as we did not find changes in the mRNA expression of IL-6 or other markers of inflammation in adipose tissue after a single session of exercise in CD14+ cells, it remains unclear why ACTIVE have greater IL-6 expression in their CD14+ fraction. Importantly, our data also suggest that outside of IL-6, exercise may not greatly impact the mRNA expression of many inflammatory factors in adipose tissue known to interfere with adipose tissue metabolic function in obese humans.
It is well known that most of the improvements in insulin sensitivity associated with exercise can be attributed to the most recent exercise session, as removal of exercise for as few as 48 h can return insulin sensitivity to levels similar to those of untrained individuals (18, 22, 23). Therefore, because we performed the OGTT on our ACTIVE subjects 3 days after their most recent exercise session, it is not surprising that glucose tolerance was similar between ACTIVE and SED. Our data do not necessarily indicate that exercise training does not have beneficial effects on adipose tissue that can translate into measurable improvements in metabolic health. It is important to note that, other than being overweight to mildly obese, our SED subjects were in generally good health, and thus, the effect of exercise training in our ACTIVE group may be difficult to detect when compared with our relatively healthy SED cohort. It is also possible that the metabolic benefits of exercise on adipose tissue are only measurable when combined with weight gain. Alterations in adipose tissue observed in studies from Kawanishi et al. (20, 21) occurred when exercise was combined with weight gain. Perhaps exercise training stimulus “primes” the adipose tissue for healthy adaptability to weight gain, but these adaptations only occur when nutrient excess also stimulates processes to upregulate lipid storage.
Findings from this study expand on the limited knowledge of the effects of exercise on adipose tissue structure and function in humans; however, this study has several limitations. The mRNA expression data in this study provide valuable information, especially when examining the acute responses 1 h after a session of exercise, before alterations in protein abundance would likely be measurable. Notably, many exercise training-induced adaptations result from the cumulative effect of transient increases in mRNAs that occur with each exercise bout, eventually leading to an accumulation of those proteins (16, 34). However, many metabolic processes and cellular functions are regulated at the level of protein translation and/or posttranslational modifications, without a direct effect of changes in mRNA levels (37). Unfortunately, limitations in tissue sample yield prevented us from making measurements at the protein level. It must also be acknowledged that some adipose tissue changes may not manifest until after 1-h recovery from exercise. We also acknowledge that the number of subjects in this study is small, and thus small changes in adipose tissue may not have been detected, especially considering the large amount of variability observed in adipose tissue outcomes in humans. Differences in the volume and intensity of exercise training regularly performed by our ACTIVE subjects may have contributed to some of the variability observed in this group. Future studies specifically designed to examine the effects of volume and/or intensity of exercise training on adipose tissue would be of high interest. Finally, the adipocyte isolation process could have affected the cells' gene expression, which limits direct comparison between adipose tissue homogenates and isolated adipocytes. However, importantly, the isolation process was identical for all samples, so we expect any stress-induced changes in gene expression to be comparable in pre- and postexercise samples. Nevertheless, this data set provides a foundation for further research focused on adipose tissue aspects that appear to respond to exercise (e.g., angiogenesis and macrophage polarization). Lastly, we studied the effects of a “conventional” exercise stimulus of 1 h at ~65% V̇o2peak, but the adipose tissue responses to different exercise modalities and intensities (e.g., high-intensity interval training, and resistance training) are likely to be very different.
In conclusion, our study suggests that exercise may upregulate adipose tissue angiogenic processes by upregulating VEGFA mRNA expression, which may lead to increased adipose tissue capillarization, as evidenced by elevated CD31 mRNA expression in habitual exercisers. Exercise training may also play a role in the alternative activation of immune cells, as evidenced by greater IL-6 mRNA expression in CD14+ adipose tissue SVF in habitual exercisers compared with that of sedentary individuals. Finally, our findings suggest that acute exercise may not affect mRNA expression of factors related to extracellular matrix and lipid metabolism within the hour after exercise. Further studies are needed to examine the effects of exercise on adipose tissue structure and function, especially to investigate whether any changes in adipose tissue translate to measurable improvements in metabolic health in obesity.
GRANTS
This work was primarily supported by National Institutes of Health (NIH) Grant R01 DK-077966, and the American Diabetes Association (ADA) Grant 1-16-ICTS-048, both awarded to J. F. Horowitz. Additional support for this project was provided by the Human Phenotyping Core of the University of Michigan Nutrition and Obesity Research Center (NIH Grant P30-DK089503).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.W.V.P. and J.F.H. conceived and designed research; D.W.V.P. and L.M.G. performed experiments; D.W.V.P. analyzed data; D.W.V.P., L.M.G., and J.F.H. interpreted results of experiments; D.W.V.P. prepared figures; D.W.V.P. drafted manuscript; D.W.V.P., L.M.G., and J.F.H. edited and revised manuscript; D.W.V.P., L.M.G., and J.F.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We are thankful to Alison Ludzki and Konstantinos Karabetsos for technical assistance and to Suzette Howton for assistance with subject recruitment and dietary control. We are particularly grateful to all of the study subjects for participation on this project.
REFERENCES
- 1.Airley R, Loncaster J, Davidson S, Bromley M, Roberts S, Patterson A, Hunter R, Stratford I, West C. Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res 7: 928–934, 2001. [PubMed] [Google Scholar]
- 2.Allister CA, Liu LF, Lamendola CA, Craig CM, Cushman SW, Hellerstein MK, McLaughlin TL. In vivo 2H2O administration reveals impaired triglyceride storage in adipose tissue of insulin-resistant humans. J Lipid Res 56: 435–439, 2015. doi: 10.1194/jlr.M052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arner P, Rydén M. Fatty acids, obesity and insulin resistance. Obes Facts 8: 147–155, 2015. doi: 10.1159/000381224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54: 2277–2286, 2005. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 5.Castellani L, Perry CG, Macpherson RE, Root-McCaig J, Huber JS, Arkell AM, Simpson JA, Wright DC. Exercise-mediated IL-6 signaling occurs independent of inflammation and is amplified by training in mouse adipose tissue. J Appl Physiol (1985) 119: 1347–1354, 2015. doi: 10.1152/japplphysiol.00551.2015. [DOI] [PubMed] [Google Scholar]
- 6.Catenacci VA, Wyatt HR. The role of physical activity in producing and maintaining weight loss. Nat Clin Pract Endocrinol Metab 3: 518–529, 2007. doi: 10.1038/ncpendmet0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clasey JL, Kanaley JA, Wideman L, Heymsfield SB, Teates CD, Gutgesell ME, Thorner MO, Hartman ML, Weltman A. Validity of methods of body composition assessment in young and older men and women. J Appl Physiol (1985) 86: 1728–1738, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 18: 1657–1669, 2004. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 9.Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ; American College of Sports Medicine . Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42: 2282–2303, 2010. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 10.Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta 1842: 463–472, 2014. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curat CA, Miranville A, Sengenès C, Diehl M, Tonus C, Busse R, Bouloumié A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes 53: 1285–1292, 2004. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 12.Divoux A, Clement K. Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev 12: e494–e503, 2011. doi: 10.1111/j.1467-789X.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 13.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clément K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124: 67–76, 2011. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 14.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics 11: 21, 1969. doi: 10.1080/00401706.1969.10490657. [DOI] [Google Scholar]
- 15.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2276–2283, 2007. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 16.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell 159: 738–749, 2014. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 17.He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab 300: E877–E885, 2011. doi: 10.1152/ajpendo.00626.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heath GW, Gavin JR 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol Respir Environ Exerc Physiol 55: 512–517, 1983. [DOI] [PubMed] [Google Scholar]
- 19.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kawanishi N, Mizokami T, Yano H, Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exerc 45: 1684–1693, 2013. doi: 10.1249/MSS.0b013e31828ff9c6. [DOI] [PubMed] [Google Scholar]
- 21.Kawanishi N, Niihara H, Mizokami T, Yano H, Suzuki K. Exercise training attenuates adipose tissue fibrosis in diet-induced obese mice. Biochem Biophys Res Commun 440: 774–779, 2013. doi: 10.1016/j.bbrc.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 22.King DS, Dalsky GP, Clutter WE, Young DA, Staten MA, Cryer PE, Holloszy JO. Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J Appl Physiol (1985) 64: 1942–1946, 1988. [DOI] [PubMed] [Google Scholar]
- 23.King DS, Dalsky GP, Clutter WE, Young DA, Staten MA, Cryer PE, Holloszy JO. Effects of lack of exercise on insulin secretion and action in trained subjects. Am J Physiol Endocrinol Physiol 254: E537–E542, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Kuk JL, Katzmarzyk PT, Blair SN, Church TS, Ross R. Cardiorespiratory fitness attenuates metabolic risk independent of abdominal subcutaneous and visceral fat in men. Diabetes Care 28: 895–901, 2005. doi: 10.2337/diacare.28.4.895. [DOI] [PubMed] [Google Scholar]
- 25.Macpherson RE, Huber JS, Frendo-Cumbo S, Simpson JA, Wright DC. Adipose tissue insulin action and IL-6 signaling after exercise in obese mice. Med Sci Sports Exerc 47: 2034–2042, 2015. doi: 10.1249/MSS.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 26.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las Fuentes L, He S, Okunade AL, Patterson BW, Klein S. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 23: 591–601, 2016. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Santibañez G, Lumeng CN. Macrophages and the regulation of adipose tissue remodeling. Annu Rev Nutr 34: 57–76, 2014. doi: 10.1146/annurev-nutr-071812-161113. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Matthews VB, Allen TL, Risis S, Chan MH, Henstridge DC, Watson N, Zaffino LA, Babb JR, Boon J, Meikle PJ, Jowett JB, Watt MJ, Jansson JO, Bruce CR, Febbraio MA. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53: 2431–2441, 2010. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- 31.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Brönneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Brüning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 15: 423–430, 2014. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, Cushman SW. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 50: 1707–1715, 2007. doi: 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 33.Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol 537: 93–122, 2014. doi: 10.1016/B978-0-12-411619-1.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poitout V. The ins and outs of fatty acids on the pancreatic beta cell. Trends Endocrinol Metab 14: 201–203, 2003. doi: 10.1016/S1043-2760(03)00086-9. [DOI] [PubMed] [Google Scholar]
- 36.Polak J, Klimcakova E, Moro C, Viguerie N, Berlan M, Hejnova J, Richterova B, Kraus I, Langin D, Stich V. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism 55: 1375–1381, 2006. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853, 2006. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 156: 20–44, 2014. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118: 2992–3002, 2008. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 42.Sharabi Y, Eldad A. Nonalcoholic fatty liver disease is associated with hyperlipidemia and obesity. Am J Med 109: 171, 2000. doi: 10.1016/S0002-9343(00)00434-4. [DOI] [PubMed] [Google Scholar]
- 43.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371: 1131–1141, 2014. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 44.Siri W. Techniques for measuring body composition. In: Body Composition from Fluid Space and Density, edited by Hanschel IJ. Washington, DC: National Academy of Science, 1961, p. 223–244. [Google Scholar]
- 45.Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes 64: 2361–2368, 2015. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 18: 470–477, 2013. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab 17: 61–72, 2013. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans 36: 935–940, 2008. doi: 10.1042/BST0360935. [DOI] [PubMed] [Google Scholar]
- 49.Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev 92: 157–191, 2012. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
- 50.Tuvdendorj D, Chandalia M, Batbayar T, Saraf M, Beysen C, Murphy EJ, Abate N. Altered subcutaneous abdominal adipose tissue lipid synthesis in obese, insulin-resistant humans. Am J Physiol Endocrinol Metab 305: E999–E1006, 2013. doi: 10.1152/ajpendo.00194.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801: 209–214, 2010. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab 21: 345–352, 2010. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 54.Walton RG, Finlin BS, Mula J, Long DE, Zhu B, Fry CS, Westgate PM, Lee JD, Bennett T, Kern PA, Peterson CA. Insulin-resistant subjects have normal angiogenic response to aerobic exercise training in skeletal muscle, but not in adipose tissue. Physiol Rep 3: e12415, 2015. doi: 10.14814/phy2.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455: 479–492, 2007. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watkins NA, Gusnanto A, de Bono B, De S, Miranda-Saavedra D, Hardie DL, Angenent WG, Attwood AP, Ellis PD, Erber W, Foad NS, Garner SF, Isacke CM, Jolley J, Koch K, Macaulay IC, Morley SL, Rendon A, Rice KM, Taylor N, Thijssen-Timmer DC, Tijssen MR, van der Schoot CE, Wernisch L, Winzer T, Dudbridge F, Buckley CD, Langford CF, Teichmann S, Göttgens B, Ouwehand WH, Bloodomics C; Bloodomics Consortium . A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood 113: e1–e9, 2009. doi: 10.1182/blood-2008-06-162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS Jr, Blair SN. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 282: 1547–1553, 1999. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 58.Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab 26: 357–366, 2015. doi: 10.1016/j.tem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood IS, Wang B, Lorente-Cebrián S, Trayhurn P. Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-D-glucose uptake in human adipocytes. Biochem Biophys Res Commun 361: 468–473, 2007. doi: 10.1016/j.bbrc.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes 33: 54–66, 2009. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]