Abstract
Epithelial cells of the mucosa provide a first line of defense to prevent the inappropriate translocation of luminal antigens, and therefore contribute significantly to nonspecific innate immunity. In the gastrointestinal (GI) tract, barrier is provided by multiple components of the mucosa, including mucus production, epithelial junctional complexes, and the production of antimicrobial molecules. In recent years, it is better appreciated that tissue oxygen metabolism is key to homeostasis in the mucosa. The intestine, for example, maintains a low baseline Po2 level due to high rates of metabolism, countercurrent blood flow, and the presence of a steep oxygen gradient across the luminal aspect of tissue surface. As a result, hypoxia and hypoxia-inducible factor (HIF)-dependent signaling exists even in the healthy, unperturbed intestinal mucosa. In a number of examples, HIF has been demonstrated both to promote barrier function during homeostasis and to promote resolution of active inflammation. Hypoxia-elicited factors that contribute to innate responses in the mucosa include the transcriptional regulation of mucin genes, junction proteins, and autophagic flux. Here, we review current literature related to hypoxia and innate immunity in health and during mucosal inflammation.
Keywords: metabolism, inflammation, barrier, autophagy, mucosa, colitis, epithelium
epithelial cells provide a selective barrier to the outside world. Of the mucosal tissues, the gastrointestinal (GI) tract constitutes the largest surface area found in multicellular organisms, covering an area of nearly 300 m2 in adult humans. This epithelium provides a dynamic barrier that is intricately regulated to accommodate nutrient and fluid transport and to selectively exclude antigenic material from the lumen (36, 70). As a result of this multidimensional functionality, the intestinal mucosa exhibits a unique metabolic profile that is regulated by a plethora of stimuli ranging from changes in intestinal perfusion to shifts in enteric microbiota under healthy, steady-state conditions (13). It is also noteworthy that this metabolic profile significantly changes in diseases such as inflammatory bowel disease (IBD), which has become an area of significant research interest. Central to these host immune responses to microbial signals is the innate immune system, where multiple defects in innate immunity have been identified (18).
The establishment of a selectively permeable barrier occurs through interactions of the extracellular domains of multiple transmembrane adhesion domains between adjacent cells (adherens junction, tight junction, gap junction) or between the mucosal layer and extracellular matrix components (33). These interactions not only determine the physical integrity of the tissue, but further establish the physical organization of lipids and proteins within the plasma membrane, the so called “fence function” of the epithelium. Here, we discuss how oxygen metabolism impacts innate immune responses in health and during disease.
Oxygen Utilization in the Mucosa
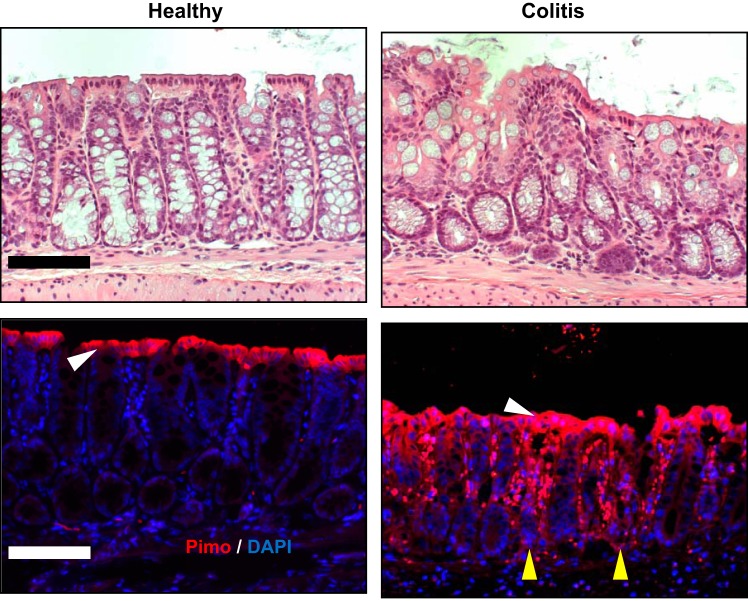
The most luminal aspect of the healthy colon exists at a Po2 of <10 mmHg (1, 26). This low basal Po2 reflects a combination of countercurrent blood flow within the villus and the presence of commensal microbes that deplete luminal O2 (73). Local Po2 has been tracked in vivo using 2-nitroimidazole dyes (e.g., pimonidazole), originally developed to image the low-O2 environment of growing tumors (11, 35), and indicates that microbes in the lumen drive a steep O2 gradient across the most apical compartment of the healthy mucosa (29) (Fig. 1). These nitroimidazoles form adducts with various macromolecules within cells and are retained at Po2 <10 mmHg. These nitroimidazoles have the added advantage that they image only viable, intact tissue (32). Localization of these nitroimidazole adducts in the mucosa has revealed in the healthy gut, particularly the colon, the existence of “physiologic hypoxia” (26). These studies have shown that a low Po2 is critical for the baseline expression of some innate immune factors (27) and epithelial tight junction (TJ) proteins (58). Conversely, lesions associated with active inflammation are profoundly hypoxic (termed “inflammatory hypoxia”) and are comparable to levels of hypoxia observed in some solid tumors (Po2 < 1 mmHg) (26). Multiple components likely contribute to this deep tissue hypoxia in active inflammation, including a significant contribution from O2 consumption by activated leukocytes (Fig. 2).
Fig. 1.
Localization of hypoxic regions of the healthy and colitic mucosa. Pimonidazole (Pimo, red) staining and nuclear (DAPI, blue) counterstain of colon from healthy and colitic mice. White arrowheads indicate presence/absence of brush-border epithelial cells; yellow arrowheads indicate crypt epithelial cells. Note superficial hypoxia in healthy tissue and deep, penetrating hypoxia in active inflammation. Scale bars = 100 μm. [Adapted from Campbell et al. (8) with permission from Elsevier. Copyright © 2014.]
Fig. 2.
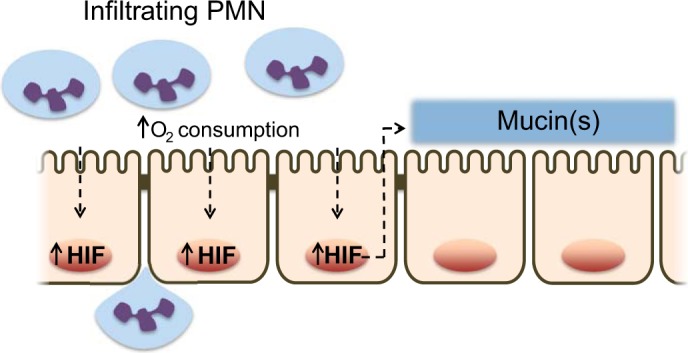
Mechanism and function for IEC stabilization of HIF by neutrophils: Neutrophils (PMN) consume oxygen in a respiratory burst via the NADPH oxidase complex (NOX2), with generation of antimicrobial superoxide anion (O2·−). The local depletion of oxygen results in HIF stabilization, nuclear translocation, and transactivation of HIF target genes, including barrier components such as mucins.
Given the low baseline Po2 and the substantial shifts in O2 availability during inflammation, a number of studies have shown that stabilization of hypoxia-inducible factor (HIF) in low O2 triggers the expression of genes that enable epithelial cells to function as an effective barrier (12). HIF is a member of the Per-ARNT-Sim family of basic helix-loop-helix transcription factors that recognizes hypoxia response elements (HREs) at target gene loci under low-oxygen conditions (56). Functional HIF exists as an α/β heterodimer, comprising both a constitutive subunit (HIF-1β), and a hypoxia-inducible ‘α’ component, stabilization of which is regulated in part by a family of oxygen- and iron-dependent prolyl hydroxylase (PHD) enzymes (61). To date, three regulatory subunits have been identified, namely HIF-1α, HIF-2α, and HIF-3α, with the highest level of sequence homology conserved between HIF-1α and HIF-2α (71). Evidence to date from genetic mouse models implies that HIF-1 and HIF-2 play nonredundant roles (56) despite their expression in many cell types, including intestinal epithelial cells (45).
Leukocytes and HIF-Dependent Signaling
Myeloid cells, including neutrophils, eosinophils, and macrophages, provide a central arm to innate immunity (24). HIF-1 has been implicated in the function of myeloid cells to clear infection and promote inflammatory resolution. HIF-1 is essential for myeloid cell glycolysis and also regulates key functions including bacterial uptake and antimicrobial activity (e.g., antimicrobial peptides and serine proteases) (14, 52, 53). These studies utilized conditional deletion of Hif1a in myeloid populations and revealed decreased bactericidal capacity of myeloid phagocytes lacking functional Hif1a. Subsequent studies have shown a role for both HIF-1 and HIF-2 in multiple neutrophil functions that may be temporally regulated during the inflammatory process (67). Studies utilizing PHD inhibitors (i.e., HIF stabilizers) have revealed potent antimicrobial activity in stimulating the killing of the pathogens Pseudomonas aeruginosa and Acinobacter baumanii (49) and that pulmonary infections with P. aeruginosa are significantly attenuated by select PHD inhibitors (60).
Mucosal inflammation initiated by recruitment of leukocytes into/across epithelial layers is coordinated by a series of cell-cell adhesion processes, recently summarized elsewhere (66). Far less is known about crosstalk pathways that elicit gene expression changes and induce tissue “memory” during the transmigration response. Campbell et al. (8) attributed epithelial gene expression changes during transmigration to the massive consumption of local O2 by polymorphonuclear leukocyte (PMN, neutrophil) NADPH oxidase. These studies revealed that O2 consumption by activated PMN resulted in the stabilization of HIF within the epithelium and a prominent induction of HIF target genes, most particular the induction of barrier protective mucins (Fig. 2). Utilizing murine models of colitis, these studies demonstrated that both the presence of PMNs as well as PMN-mediated hypoxia were necessary for mucosal protection elicited by “PMN imprinting” during inflammation (8). It is notable that consistent with previous reports (34), depletion of PMNs led to exacerbated tissue damage during colitis.
In a similar fashion as PMN, lymphocytes respond to low-oxygen conditions during inflammation and HIF-1 appears to be protective. It has been shown, for example, that chemically induced colitis is worsened in mice lacking T cell HIF-1α (22). Increased damage associated with a loss of T cell HIF-1 signaling was strongly associated with increased Th1 and Th17 responses. Others have shown that HIF-1 induces FoxP3, the key transcription factor influencing the development of regulatory T cells (Treg) and that HIF-1-deficent Tregs fail to control T cell-mediated colitis (10).
Hypoxia, HIF, and Barrier Regulation in the Mucosa
A major function of the mucosa is the provision of a physical barrier between the inside and outside world. In the GI mucosa, such a barrier coexists with trillions of microbes that provide both a symbiotic role with the host as well as a source of disease during dysbiosis. Various components of the barrier and their regulation by the hypoxic microenvironment provide a central role in mucosal innate immunity. Below are examples of such regulation and the contribution of hypoxia to these functions.
Mucus expression and hypoxia.
The mucus-gel layer forms a principal component to innate immunity in the mucosa. Mucus is composed of a mixture of goblet cell-derived glycoproteins that functions to prevent direct exposure of epithelial membranes to luminal contents. At least 10 distinct gel-forming and surface mucins are secreted by goblet cells (39). In healthy mucosa, mucus consists of an adherent layer that is devoid of bacteria and a thicker superficial layer that is many times the diameter of the epithelium (4, 65). Hypoxia and HIF regulate several components of the mucus layer. MUC3 is a HIF-1α target whose product, mucin-3, colocalizes with intestinal trefoil factor (ITF), another barrier-protective HIF target that coordinates mucin function in the intestine (40, 41) (also see Fig. 3). The 5′-flanking sequences of mammalian MUC5AC orthologs also bear evolutionarily conserved HIF binding regions (72).
Fig. 3.
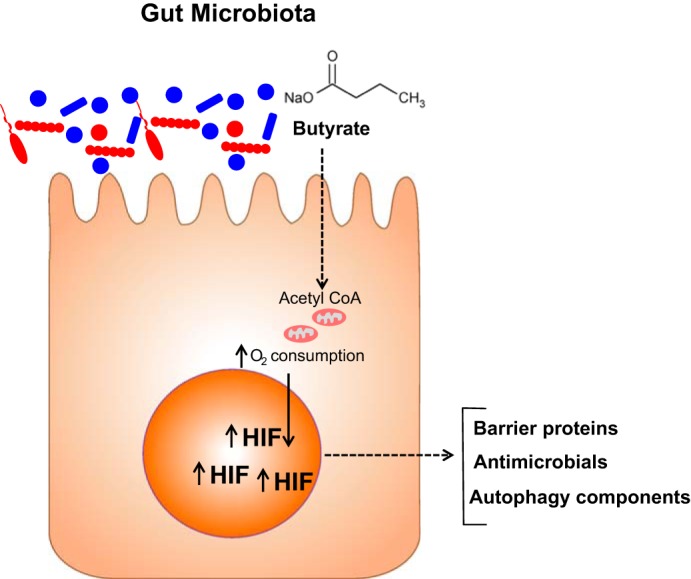
Oxidation of butyrate by intestinal epithelial cells stabilizes HIF and contributes to innate immunity. Microbial-derived SCFA such as butyrate is transported apically and through oxidative metabolism drives oxidative respiration to the extent that HIF is stabilized. The expression of a number of HIF target genes important in barrier regulation provides both homeostasis as well as resolution from injury.
The mucus-gel layer is also an important reservoir for secreted antimicrobial peptides (AMPs), such as cathelicidins and defensins (3). Defensins are a class of cysteine-rich AMPs that possess broad antimicrobial activity (17, 51). Human β defensin-1 (hBD1) is notable within the intestinal epithelium as a constitutively secreted protein that maintains the crypt in a sterile manner. Some AMPs are also induced by inflammatory mediators (73). Constitutive expression of hBD1 was shown to depend on basal HIF-1α signaling in multiple IEC lines and hBD1 expression correlated with other HIF target genes in human tissues (27). It is also notable that full functionality of hBD1 is dependent on disulfide bond reduction, consistent with the hypoxic microenvironment of the lower GI tract (62). Reduction of the hBD1 disulfide bonds is accomplished by thioredoxin, which colocalizes with hBD1 in the colonic mucus; oxidation of hBD1 is prevented by the low-Po2 environment of the lumen (25). Thus, at multiple levels hypoxia and HIF appear to provide important regulatory roles for the expression and function of the mucus layer.
Adherens junction and hypoxia.
Intercellular junctions depend in large part on initial cell-cell contact mediated by E-cadherin that drive the assembly of the adherens junction (AJ) complex in polarized epithelial cells (7). Following injury, epithelia depend on reassembly of the AJ proteins which necessitate resynthesis of E-cadherin, the reassembly of catenins, and reformation of functional AJ complex. “Chemical hypoxia” studies using ATP depletion models have shown an important role for high-energy phosphates in the regulation of barrier function (5, 69). One study identified cytosolic creatine kinase (CK) genes as HIF-2 selective targets expressed near the junctions of confluent intestinal epithelia. These studies showed that CK contributes fundamentally to the energy requirements of barrier regulation and barrier recovery (termed restitution) following injury (19). The CKs are often neglected in discussions of energy metabolism, as it is assumed that the diffusion of ATP and ADP are sufficient to provide compartmentalized availability of intracellular energy. Studies in intestinal epithelial cells revealed that the CK subunits (brain, muscle, and mitochondrial isoforms) are each expressed in cultured epithelial cell lines and in murine and human colonic epithelia (19). During periods of high energy demand (e.g., epithelial restitution), creatine (Cr) and phosphocreatine (PCr) remain at near equilibrium, thereby buffering concentrations of ATP and ADP at constant levels. During high energy expenditure (e.g., in active inflammation), it was demonstrated that Cr-supplemented feed promoted mucosal healing following damage in a chemically induced colitis models (19). Thus Cr-PCr may be central to energy homeostasis and tissue barrier function in both health and disease.
Hypoxia and tight junction expression.
Tight junctions (TJs) form the spine to the structural integrity of the barrier and constitute the physical basis for a permeability barrier to ions and macromolecules (9). TJ also prevent lipid diffusion between apical and basolateral membrane domains, the so-called “fence function” (9) found in polarized epithelial cells. TJs are composed of both membrane proteins that link to the energy-demanding actin cytoskeleton (23). Control of TJ expression is tightly regulated by a variety of stimuli (33) that alter the distribution of occludin, zonula occludens-1 (ZO-1), ZO-2, and cingulin (68, 69). TJ integrity can also be influenced by perturbations of the actin-based cytoskeleton (5, 48).
The claudins are a family of tetraspanning membrane proteins responsible for the selective permeability of TJs (9) and are categorized as “leaky” and “tight” with regard to their influence on barrier function when expressed in epithelia (2). Claudin-1 (CLDN1) is considered a tight claudin that has been shown to be dysregulated in a variety of human diseases (9). In a recent screen of TJ targets, claudin-1 (CLDN1) was identified to explain, at least in part, the appearance of an abnormal TJ morphology of HIF-deficient intestinal epithelial cell (IEC) lines (58). Using loss- and gain-of-function strategies, these studies revealed that HIF regulates CLDN1 expression basally at the gene promoter level. The reintroduction of CLDN1 into HIF-1β KD cells restored barrier function and morphological abnormalities. In vivo analysis also revealed an importance for HIF-mediated CLDN1 expression during experimental colitis. Such findings identify a functional link between HIF and selective TJ targets, providing new insight into a role for HIF in innate epithelial homeostasis (Fig. 3).
Host-Microbial Metabolism and Tissue Barrier
The GI tract is home to trillions of microbes that include bacteria, fungi, and viruses, collectively termed the microbiota. A finely regulated symbiosis exists within the mucosa. In addition to aiding in digestion, microbial communities produce short-chain fatty acids (SCFAs) that function as a fuel source within the mucosa. These SCFA include acetate, propionate, and butyrate. In the healthy gut, butyrate concentrations can exceed 30 mM (20), where it serves as a primary metabolic fuel for colonic epithelial cell metabolism. For this reason, shifts in microbial compositions can result in abnormal colonocyte function. It is likely that butyrate influences HIF expression in the colon. For example, it was recently shown that butyrate metabolism directly influences epithelial oxygen consumption and may shift intracellular oxygen availability. Intestinal epithelial cell lines stimulated with butyrate exhibit increased and sustained rates of oxygen consumption to the extent that HIF is stabilized and transactivates a number of HIF target genes (Fig. 3) (29). These studies also revealed that the presence of microbiota (e.g., tested in germ-free or broad spectrum antibiotic-treated mice) correlated with a loss of butyrate and a parallel attenuation of HIF stabilization and function. The HIF response was rescued by butyrate supplementation. These findings add to our understanding of host-microbe crosstalk pathways that promote innate immune function in the mucosa (Fig. 3).
A role for SCFA in the distal gut during inflammation is likely also important in the diseased mucosa (20, 55). For instance, recent studies investigating dysbiosis in patients with IBD identified lower levels of colonic butyrate and a decreased abundance of SCFA-producing organisms (e.g., certain Faecalibacterium and Roseburia genera) (16, 42, 64). Such findings have also revealed potential therapeutic approaches. For example, oral administration of butyrate or butyrate mimetics promotes resistance in murine colitis models (15, 37) and several trials have evaluated the efficacy of butyrate in the treatment of human disease (20). It is notable that SCFAs can also signal through G protein-coupled receptors (GPR) (31, 44). Gpr41 and Gpr43 mediate protective immunity in inflammatory mouse models (31, 44). Activation of the butyrate receptor (GPR109a), which also represents the niacin receptor, suppresses inflammation-associated colon cancer in preclinical models (63).
Autophagy and the Regulation of Barrier Function
Autophagy is a primitive cellular degradation pathway that facilitates cell survival during nutrient deprivation or metabolic stress (38). Variants in several genes that regulate the autophagy pathway have emerged as significant risk alleles for IBD, including autophagy-related 16 like 1 (ATG16L1) (21, 57) and immunity-related GTPase family M (IRGM) (46, 50). A number of transcription factors, including HIF-1 (6, 43), have been shown to regulate the expression of autophagy.
The role of selective autophagy in degrading intracellular bacteria, a process termed “xenophagy,” has defined an important crosstalk between mucosal homeostasis and innate immunity. Genomewide association studies have identified autophagy as a key pathway in IBD, with variants in autophagy-linked loci (ATG16L1, NOD2, IRGM, PARK7) conferring increased risk. These mutations result in intestinal-specific disease, reflecting the significance of the mucosal autophagic machinery. Numerous invasive bacteria have been identified as targets of xenophagy, including Salmonella, Listeria, and pathogenic forms of Escherichia coli associated with IBD pathogenesis (54). A role for hypoxia has been documented in the xenophagic process, wherein knockdown of HIF-1 results in increased survival of adherent invasive E. coli (47). The precise molecular components that elicit epithelial xenophagy are not yet elucidated. Host intracellular sensors that communicate intracellular bacterial detection with the autophagic machinery include pattern recognition receptors (PRRs) and NOD-like receptors (NLRs). Thus it is probable that IEC metabolism and HIF signaling by inflammatory hypoxia and/or microbial species may define a conserved innate response for host protection through autophagic bacterial capture.
Conclusions
Epithelial cells that line mucosal surfaces provide a first line of defense against invasive microbes and therefore provide a central role in innate immunity. These cells function in diverse and sometimes harsh conditions. A major function of the epithelium is to provide a selectively permeable barrier that prevents the free mixing of luminal and serosal constituents. The profound shifts in energy requirements during tissue injury have enlightened our overall understanding of tissue metabolism. Of particular relevance is the shift in tissue oxygenation toward hypoxia, and specifically HIF-target pathways that are strongly associated with tissue barrier function and metabolism that contribute fundamentally to the maintenance of barrier in health and the restitution response following injury. A better understanding of the gene targets and functional components of the HIF pathway will provide ample opportunities for the development of therapies to promote beneficial tissue metabolism and the resolution of inflammation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-50189, DK-104713, DK-095491, and DK-103712; Grant BX002182 from the Department of Veterans Affairs; and by the Crohn’s and Colitis Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P.C. and E.L.C. conceived and designed research; S.P.C. and E.L.C. performed experiments; S.P.C. and E.L.C. analyzed data; S.P.C. and E.L.C. interpreted results of experiments; S.P.C. and E.L.C. prepared figures; S.P.C. and E.L.C. drafted manuscript; S.P.C. and E.L.C. edited and revised manuscript; S.P.C. and E.L.C. approved final version of manuscript.
REFERENCES
- 1.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, Thom SR, Bushman FD, Vinogradov SA, Wu GD. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 147: 1055–63.e8, 2014. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16: 140–145, 2004. doi: 10.1016/j.ceb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Antoni L, Nuding S, Weller D, Gersemann M, Ott G, Wehkamp J, Stange EF. Human colonic mucus is a reservoir for antimicrobial peptides. J Crohn’s Colitis 7: e652–e664, 2013. doi: 10.1016/j.crohns.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Bacallao R, Garfinkel A, Monke S, Zampighi G, Mandel LJ. ATP depletion: a novel method to study junctional properties in epithelial tissues. I. Rearrangement of the actin cytoskeleton. J Cell Sci 107: 3301–3313, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29: 2570–2581, 2009. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt T, Rizvi A, Batta SP, Kataria S, Jamora C. Signaling and mechanical roles of E-cadherin. Cell Commun Adhes 20: 189–199, 2013. doi: 10.3109/15419061.2013.854778. [DOI] [PubMed] [Google Scholar]
- 8.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40: 66–77, 2014. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capaldo CT, Nusrat A. Claudin switching: physiological plasticity of the tight junction. Semin Cell Dev Biol 42: 22–29, 2015. doi: 10.1016/j.semcdb.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA 109: E2784–E2793, 2012. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobb LM, Nolan J, Butler SA. Distribution of pimonidazole and RSU 1069 in tumour and normal tissues. Br J Cancer 62: 915–918, 1990. doi: 10.1038/bjc.1990.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colgan SP, Curtis VF, Lanis JM, Glover LE. Metabolic regulation of intestinal epithelial barrier during inflammation. Tissue Barriers 3: e970936, 2015. doi: 10.4161/21688362.2014.970936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281–287, 2010. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112: 645–657, 2003. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cresci G, Nagy LE, Ganapathy V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN J Parenter Enteral Nutr 37: 763–774, 2013. doi: 10.1177/0148607113486809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, Steppe M, Haesebrouck F, Sas B, Ducatelle R, Vermeire S, Van Immerseel F. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 62: 1745–1752, 2013. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
- 17.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3: 710–720, 2003. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 18.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 13: 3–10, 2014. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, Miller L, Kominsky DJ, Jedlicka P, Colgan SP. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci USA 110: 19820–19825, 2013. doi: 10.1073/pnas.1302840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27: 104–119, 2008. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 21.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Günther S, Prescott NJ, Onnie CM, Häsler R, Sipos B, Fölsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 39: 207–211, 2007. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 22.Higashiyama M, Hokari R, Hozumi H, Kurihara C, Ueda T, Watanabe C, Tomita K, Nakamura M, Komoto S, Okada Y, Kawaguchi A, Nagao S, Suematsu M, Goda N, Miura S. HIF-1 in T cells ameliorated dextran sodium sulfate-induced murine colitis. J Leukoc Biol 91: 901–909, 2012. doi: 10.1189/jlb.1011518. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 177: 512–524, 2010. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 16: 343–353, 2015. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaeger SU, Schroeder BO, Meyer-Hoffert U, Courth L, Fehr SN, Gersemann M, Stange EF, Wehkamp J. Cell-mediated reduction of human β-defensin 1: a major role for mucosal thioredoxin. Mucosal Immunol 6: 1179–1190, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106, 2004. doi: 10.1172/JCI200421086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, Bowers BE, Bayless AJ, Saeedi BJ, Colgan SP. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol 6: 1110–1118, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671, 2015. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145: 396–406.e1, 2013. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 32.Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci 100: 1366–1373, 2009. doi: 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol 7: 35–60, 2012. doi: 10.1146/annurev-pathol-011811-120905. [DOI] [PubMed] [Google Scholar]
- 34.Kühl AA, Kakirman H, Janotta M, Dreher S, Cremer P, Pawlowski NN, Loddenkemper C, Heimesaat MM, Grollich K, Zeitz M, Farkas S, Hoffmann JC. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology 133: 1882–1892, 2007. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 35.Laughlin KM, Evans SM, Jenkins WT, Tracy M, Chan CY, Lord EM, Koch CJ. Biodistribution of the nitroimidazole EF5 (2-[2-nitro-1H-imidazol-1-yl]-N-(2,2,3,3,3-pentafluoropropyl) acetamide) in mice bearing subcutaneous EMT6 tumors. J Pharmacol Exp Ther 277: 1049–1057, 1996. [PubMed] [Google Scholar]
- 36.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol 14: 401–407, 2008. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonel AJ, Teixeira LG, Oliveira RP, Santiago AF, Batista NV, Ferreira TR, Santos RC, Cardoso VN, Cara DC, Faria AM, Alvarez-Leite J. Antioxidative and immunomodulatory effects of tributyrin supplementation on experimental colitis. Br J Nutr 109: 1396–1407, 2013. doi: 10.1017/S000711451200342X. [DOI] [PubMed] [Google Scholar]
- 38.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 469: 323–335, 2011. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol 1: 183–197, 2008. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, Wright NA. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut 47: 792–800, 2000. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 99: 1616–1627, 2006. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- 42.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63: 1275–1283, 2014. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 43.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40: 294–309, 2010. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 119: 1159–1166, 2009. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarroll SA, Huett A, Kuballa P, Chilewski SD, Landry A, Goyette P, Zody MC, Hall JL, Brant SR, Cho JH, Duerr RH, Silverberg MS, Taylor KD, Rioux JD, Altshuler D, Daly MJ, Xavier RJ. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn’s disease. Nat Genet 40: 1107–1112, 2008. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mimouna S, Bazin M, Mograbi B, Darfeuille-Michaud A, Brest P, Hofman P, Vouret-Craviari V. HIF1A regulates xenophagic degradation of adherent and invasive Escherichia coli (AIEC). Autophagy 10: 2333–2345, 2014. doi: 10.4161/15548627.2014.984275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molitoris BA, Leiser J, Wagner MC. Role of the actin cytoskeleton in ischemia-induced cell injury and repair. Pediatr Nephrol 11: 761–767, 1997. doi: 10.1007/s004670050385. [DOI] [PubMed] [Google Scholar]
- 49.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, von Köckritz-Blickwede M, Thienphrapa W, Corle C, Jeung SN, Kotsakis A, Shalwitz RA, Johnson RS, Nizet V. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med (Berl) 90: 1079–1089, 2012. doi: 10.1007/s00109-012-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D, Drummond H, Lees CW, Khawaja SA, Bagnall R, Burke DA, Todhunter CE, Ahmad T, Onnie CM, McArdle W, Strachan D, Bethel G, Bryan C, Lewis CM, Deloukas P, Forbes A, Sanderson J, Jewell DP, Satsangi J, Mansfield JC, Cardon L, Mathew CG; Wellcome Trust Case Control Consortium . Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet 39: 830–832, 2007. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human beta-defensins. Cell Mol Life Sci 63: 1294–1313, 2006. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol 178: 7516–7519, 2007. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 53.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest 115: 1806–1815, 2005. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pineton de Chambrun G, Colombel JF, Poulain D, Darfeuille-Michaud A. Pathogenic agents in inflammatory bowel diseases. Curr Opin Gastroenterol 24: 440–447, 2008. doi: 10.1097/MOG.0b013e3283023be5. [DOI] [PubMed] [Google Scholar]
- 55.Plöger S, Stumpff F, Penner GB, Schulzke JD, Gäbel G, Martens H, Shen Z, Günzel D, Aschenbach JR. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci 1258: 52–59, 2012. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 56.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest 117: 862–865, 2007. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, Shugart YY, Griffiths AM, Targan SR, Ippoliti AF, Bernard EJ, Mei L, Nicolae DL, Regueiro M, Schumm LP, Steinhart AH, Rotter JI, Duerr RH, Cho JH, Daly MJ, Brant SR. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 39: 596–604, 2007. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ, Bayless AJ, Kominsky DJ, Campbell EL, Kuhn KA, Furuta GT, Colgan SP, Glover LE. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell 26: 2252–2262, 2015. doi: 10.1091/mbc.E14-07-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaible B, McClean S, Selfridge A, Broquet A, Asehnoune K, Taylor CT, Schaffer K. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS One 8: e56491, 2013. doi: 10.1371/journal.pone.0056491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354, 2004. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 62.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469: 419–423, 2011. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 63.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139, 2014. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 15: 1183–1189, 2009. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 65.Strugala V, Allen A, Dettmar PW, Pearson JP. Colonic mucin: methods of measuring mucus thickness. Proc Nutr Soc 62: 237–243, 2003. doi: 10.1079/PNS2002205. [DOI] [PubMed] [Google Scholar]
- 66.Sumagin R, Parkos CA. Epithelial adhesion molecules and the regulation of intestinal homeostasis during neutrophil transepithelial migration. Tissue Barriers 3: e969100, 2015. doi: 10.4161/21688362.2014.969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson AA, Elks PM, Marriott HM, Eamsamarng S, Higgins KR, Lewis A, Williams L, Parmar S, Shaw G, McGrath EE, Formenti F, Van Eeden FJ, Kinnula VL, Pugh CW, Sabroe I, Dockrell DH, Chilvers ER, Robbins PA, Percy MJ, Simon MC, Johnson RS, Renshaw SA, Whyte MK, Walmsley SR. Hypoxia-inducible factor 2α regulates key neutrophil functions in humans, mice, and zebrafish. Blood 123: 366–376, 2014. doi: 10.1182/blood-2013-05-500207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol Renal Physiol 276: F737–F750, 1999. [DOI] [PubMed] [Google Scholar]
- 69.Tsukamoto T, Nigam SK. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J Biol Chem 272: 16133–16139, 1997. doi: 10.1074/jbc.272.26.16133. [DOI] [PubMed] [Google Scholar]
- 70.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 71.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE 2005: re12, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Young HW, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, Adachi R, Blackburn MR, Dickey BF, Evans CM. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol 37: 273–290, 2007. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol 309: C350–C360, 2015. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]