Noninvasive assessment of cardiac function during human spaceflight is an important tool to monitor astronaut health. This study demonstrated that pulse contour analysis of finger arterial blood pressure to estimate cardiac output failed to track the 46% increase measured by a rebreathing method. These results strongly suggest that alternative methods not dependent on pulse contour analysis are required to track cardiac function in spaceflight.
Keywords: astronaut, Modelflow, arterial blood pressure, stroke volume, aortic compliance
Abstract
Pulse contour analysis of the noninvasive finger arterial pressure waveform provides a convenient means to estimate cardiac output (Q̇). The method has been compared with standard methods under a range of conditions but never before during spaceflight. We compared pulse contour analysis with the Modelflow algorithm to estimates of Q̇ obtained by rebreathing during preflight baseline testing and during the final month of long-duration spaceflight in nine healthy male astronauts. By Modelflow analysis, stroke volume was greater in supine baseline than seated baseline or inflight. Heart rate was reduced in supine baseline so that there were no differences in Q̇ by Modelflow estimate between the supine (7.02 ± 1.31 l/min, means ± SD), seated (6.60 ± 1.95 l/min), or inflight (5.91 ± 1.15 l/min) conditions. In contrast, rebreathing estimates of Q̇ increased from seated baseline (4.76 ± 0.67 l/min) to inflight (7.00 ± 1.39 l/min, significant interaction effect of method and spaceflight, P < 0.001). Pulse contour analysis utilizes a three-element Windkessel model that incorporates parameters dependent on aortic pressure-area relationships that are assumed to represent the entire circulation. We propose that a large increase in vascular compliance in the splanchnic circulation invalidates the model under conditions of spaceflight. Future spaceflight research measuring cardiac function needs to consider this important limitation for assessing absolute values of Q̇ and stroke volume.
NEW & NOTEWORTHY Noninvasive assessment of cardiac function during human spaceflight is an important tool to monitor astronaut health. This study demonstrated that pulse contour analysis of finger arterial blood pressure to estimate cardiac output failed to track the 46% increase measured by a rebreathing method. These results strongly suggest that alternative methods not dependent on pulse contour analysis are required to track cardiac function in spaceflight.
cardiac output (Q̇) is elevated under resting conditions in spaceflight compared with baseline, upright seated values on Earth (18, 21, 24). It was not known until recently whether Q̇ remained elevated during long-duration spaceflight. Norsk et al. (17) observed 35–41% increases in stroke volume and Q̇ when comparing long-duration spaceflight on the International Space Station to a baseline of upright seated posture on Earth. This finding contrasts with our recent report of <10% increase in Q̇ in astronauts on the International Space Station compared with their seated baseline on Earth (13, 34). Methodological differences might explain this discrepancy. Norsk et al. (17) employed a rebreathing technique with uptake of a soluble tracer gas, while we used an estimate of Q̇ obtained from pulse contour analysis of the continuous finger arterial pressure waveform (33).
The pulse contour method, based on a three-element Windkessel model, is performed by commercial software referred to as “Modelflow.” This algorithm has been validated under many conditions on Earth with good agreement to standard methods including thermodilution, Doppler ultrasound, and rebreathing (11, 23, 29, 33). Under conditions where the aortic characteristic impedance and arterial compliance vary in a consistent way, such as steady-state conditions of postural change (11) or exercise (9), estimates of Q̇ derived from pulse contour analysis track those obtained through other methods. However, there are conditions where pulse contour analysis does not track other methods, including with sympathomimetic drug injection (8) and under heat stress (22). It is possible that the deviation between measured change during spaceflight by rebreathing and by analysis of the arterial pulse wave might be a consequence of unanticipated changes in parameters required for pulse contour calculation. In parabolic flight, which has brief periods of alternating hyper- and hypogravity, there was a systematic difference between rebreathing and pulse contour estimates as cardiac output changed over brief periods of time, but there did not appear to be differences introduced by the phase of gravity loading (16).
The purpose of the current study was to compare rebreathing and pulse contour estimates of Q̇ from astronauts during preflight baseline and during long-duration spaceflight on the International Space Station. It was hypothesized that the increase in Q̇ from seated posture baseline to spaceflight measured by rebreathing would be greater than the increase estimated by pulse contour analysis.
METHODS
Nine male astronauts (age: 37−53 yr; height: 168–185 cm; and weight: 70−92 kg) participated in the study “BP Reg” between December 2012 and May 2015. Written informed consent was obtained before participation in the study that was approved by five autonomous Ethics Review Boards: the Office of Research Ethics at the University of Waterloo, the National Aeronautics and Space Administration (NASA) Institutional Review Board, the NASA Human Research Medical Review Board, the European Space Agency Medical Review Board, and the Japanese Space Agency Medical Review Board (NASA MPA 7116301606HR; FWA 00019876). The experiments conformed to the principles of the Declaration of Helsinki.
No alcohol, blood pressure medications, over-the-counter cold medications, or allergy medications were consumed within 24 h of the test. Food and caffeine were not consumed in the 2 h before testing, and no exercise training was conducted on the test day before testing.
Data were recorded in baseline preflight testing sessions at Johnson Space Center ~2–3 mo before launch and near the end of spaceflight (days 119–166). During baseline testing, subjects were instrumented in the supine posture to measure heart rate (Heart Rate Module; FMS, Amsterdam, The Netherlands) and finger arterial blood pressure (Finometer Pro; FMS). Finger pressure was referenced by a height correction system to the level of the right atrium on the midaxillary line. The heart rate, arterial pressure, and estimated cardiac output from Modelflow (33) were recorded at 1,000 Hz (PowerLab; ADInstruments, Castle Hill, NSW, Australia). After a minimum of 3-min supine rest, a 2-min supine baseline was recorded before moving to a sitting position for the rebreathing maneuvers for measurement of cardiac output by a foreign gas technique using the Portable Pulmonary Function System (PPFS; Danish Aerospace, Copenhagen, Denmark) recorded at 100 Hz with the Agile Data Analyzer and Monitor (ADAM; Danish Aerospace) as described recently by Norsk et al. (17). Subjects breathed through a mouthpiece, while wearing a nose clip. At the start of the rebreathing maneuver, they expired to a normal end-expiratory point and then followed a visual display that prompted smooth breathing back and forth to completely empty the bag at a rate of 20 breaths/min for 25 s. The bag contained 1.5 liter of a gas mixture [1% Freon-22 (soluble tracer gas), 1% SF6 (nonsoluble tracer gas), 25% O2, and balance N2]. Infrared photoacoustic and magnetoacoustic gas analyzers followed the disappearance of Freon-22, the equilibrium of SF6, and the variations in O2 and CO2. Given the Bunsen solubility coefficient for Freon-22 (30) and its rate of disappearance, flow of blood through the pulmonary capillaries was determined to estimate Q̇ through linear regression of a semiogarithmic plot of end-expiratory values during a period of lung-bag equilibrium (10). Three separate rebreathing maneuvers were separated by 5 min for elimination of the foreign gas markers.
On the ISS, the same procedure was followed for the rebreathing maneuver with the flight models of the Pulmonary Function System equipment for gas analysis. Data were recorded at 100 Hz with the ADAM system. Inflight, the analog output of the finger arterial blood pressure was collected simultaneously during rebreathing using the Continuous Blood Pressure Device (NASA), and the analog output was routed through the Cardiolab (CADMOS, Centre National d’Etudes Spatiale, France) to the Pulmonary Function System and then recorded by ADAM at 100 Hz.
Pulse contour analysis of the finger arterial blood pressure waveforms was completed in all cases by processing the digital waveform through the BeatScope program (FMS) that accepts only 200-Hz data sets, thus requiring a down-sampling of data from the preflight testing and an up-sampling by a sample and hold method of the 100-Hz data from the inflight testing to have a 200-Hz data set. Beat-by-beat data were available at rest and throughout the rebreathing maneuvers (Fig. 1). For the purpose of analysis, the data were divided into regions associated with supine rest, seated rest between rebreathing maneuvers, and during the rebreathing.
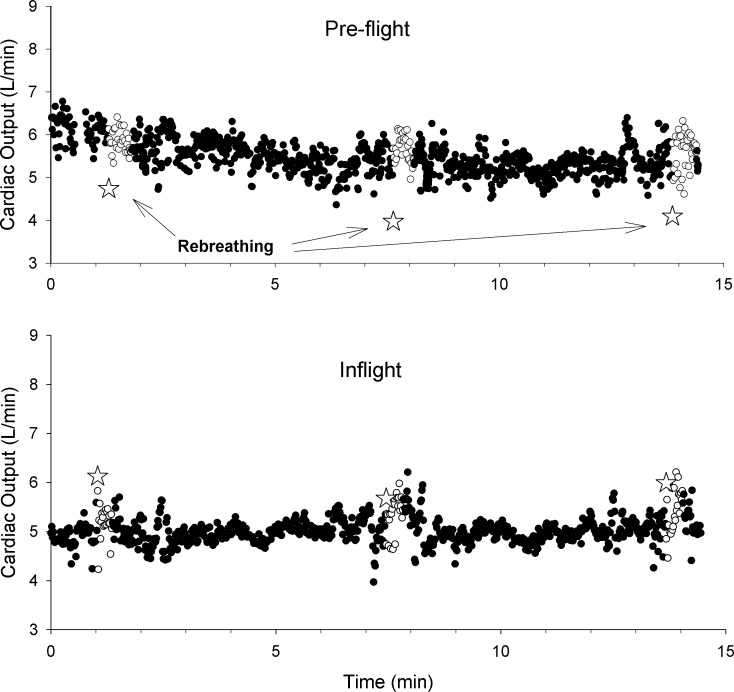
Fig. 1.
Individual beat values for cardiac output estimated by Modelflow for a single astronaut in preflight testing (top) in a seated position (●) as well as during the period of rebreathing (○). The star symbols indicate the estimate of cardiac output from the 3 different rebreathing tests. The same symbols apply for inflight testing (bottom).
Statistical analysis, including a test for normality, was completed with SigmaPlot (version 12.5; Systat Software). A one-way repeated-measures ANOVA compared cardiovascular variables as well as the Modelflow estimates from preflight baseline among supine, seated rest and during rebreathing. A two-way repeated measures ANOVA compared method (rebreathing and Modelflow) and spaceflight condition (preflight seated and inflight). Post-hoc testing was with a Holm-Sidak all pairwise multiple comparison. Significance was accepted for P < 0.05, and data are presented as means ± SD. Coefficient of variation for rebreathing Q̇ was computed for each individual as SD/mean × 100 and averaged across individuals for preflight and inflight conditions as an indication of between measurement variability. Effect size was calculated for the difference between rebreathing and Modelflow estimates of for the inflight condition (5). Bland-Altman statistics were calculated with SigmaPlot.
RESULTS
Baseline heart rate in preflight testing was lower in supine compared with upright posture, but there was no difference between preflight and inflight. None of systolic, diastolic, mean, or pulse pressures was different between any of the conditions (Table 1).
Table 1.
Heart rate and brachial arterial blood pressure recorded in supine rest and 30 s before the preflight seated and inflight rebreathing maneuvers
| Preflight (Supine) | Preflight (Seated) | Inflight | |
|---|---|---|---|
| HR, beats/min | 55.2 ± 8.4* | 62.1 ± 9.2 | 60.0 ± 10.6 |
| SBP, mmHg | 128.5 ± 16.9 | 133.8 ± 16.8 | 126.8 ± 16.4 |
| DBP, mmHg | 68.2 ± 6.8 | 70.5 ± 10.0 | 71.1 ± 3.6 |
| MAP, mmHg | 91.5 ± 7.5 | 91.2 ± 11.6 | 91.5 ± 5.8 |
| PP, mmHg | 60.4 ± 13.3 | 66.3 ± 10.5 | 57.2 ± 15.9 |
Values are means ± SD. HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure. Statistical analysis by one-way repeated-measures ANOVA, with Holm-Sidak post hoc.
P = 0.012, preflight supine vs. preflight seated.
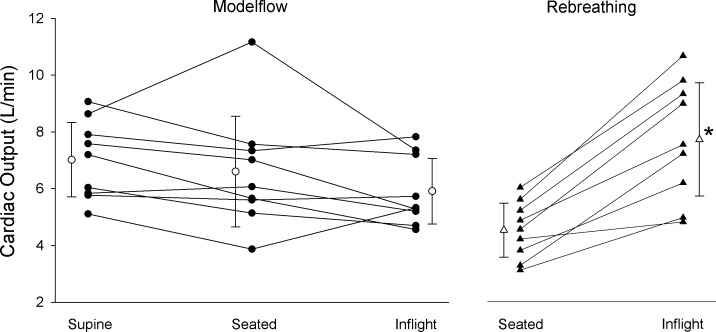
Cardiac output estimated by Modelflow had small beat-by-beat variations (shown for a single individual in Fig. 1) but differed systematically from the rebreathing estimates. In comparison to the supine baseline, there were no significant differences (P = 0.056) for either seated baseline or inflight (Fig. 2). The Modelflow Q̇ during rebreathing in preflight (6.61 ± 1.80 l/min) or inflight testing (5.79 ± 1.19 l/min) did not differ from the seated or inflight periods reported in Fig. 2. When Modelflow was compared with rebreathing in the baseline seated position and in spaceflight, there was a significant main effect of spaceflight (P = 0.028) and an interaction effect of spaceflight and method (P < 0.001). Figure 2 shows that the estimate of Q̇ by rebreathing increased by 46.6 ± 18.2% from seated baseline to inflight, while the estimate by Modelflow was unchanged. The average coefficient of variation based on the three repetitions of the rebreathing maneuver for each astronaut was 9.0 ± 2.7% for preflight and 5.6 ± 3.1% for inflight measurements. The effect size for the difference between increases detected by these methods from preflight to inflight was d = 2.45, which is considerably larger than “large, d = 0.8” (5). Bland-Altman analysis of the differences in methods (rebreathing and Modelflow) was conducted for each of preflight and inflight. In the preflight testing, there was a bias of 1.85 l/min, standard deviation of 1.72 l/min, and limits of agreement of −1.52 to 5.22 l/min. During inflight testing, the bias was −1.21 l/min with a standard deviation of 1.66 l/min and limits of agreement of −4.47 to 2.04 l/min.
Fig. 2.
Estimates of cardiac output by Modelflow are shown for each astronaut (● with connecting solid lines) and for the group average and standard deviation (○ with error bars) in supine and seated positions on the ground and inflight (no significant difference between conditions). Rebreathing estimates of cardiac output are shown for individual astronauts (▲) and group average (△ with error bars) for seated on the ground and inflight. Statistical analysis: a two-way repeated measures ANOVA comparing Modelflow and rebreathing for the seated and inflight conditions revealed significant interaction (Method × Spaceflight, P < 0.001) and significant difference within rebreathing of seated and inflight (*P < 0.001).
Calculated cardiac stroke volume determined by rebreathing increased from 77.8 ± 13.5 to 118.8 ± 25.7 ml (54.1 ± 29.7%). Stroke volume estimated from Modelflow was greater in supine baseline (127 ± 18 ml) compared with seated baseline (106 ± 25 ml, P = 0.038) and inflight (101 ± 25 ml, P = 0.018).
As a consequence of the greater inflight rebreathing Q̇ and no differences in mean arterial blood pressure, total systemic vascular resistance decreased from preflight [19.7 ± 4.9 mmHg/(l/min)] to inflight (14.0 ± 4.2, P < 0.001).
DISCUSSION
The current study confirmed the previous report of Norsk et al. (17) that Q̇ measured by rebreathing during long-duration spaceflight was significantly elevated, 46.6% in the current study, compared with the seated, upright posture on Earth. The major novel finding supporting our hypothesis was the lack of coherence between the changes in Q̇ determined by the rebreathing method compared with pulse contour analysis with the Modelflow algorithm. That is, while rebreathing Q̇ increased from preflight to inflight, there was no change with Modelflow Q̇.
Cardiac output increases during spaceflight relative to an upright posture on Earth because of a change in cardiac filling pressure. On Earth, cardiac filling is related to central blood volume and central venous pressure, which are greater in the supine than the upright posture (27, 28), resulting in greater stroke volume and Q̇. The current study did not measure rebreathing Q̇ in the supine posture. With spaceflight and parabolic flight, central venous pressure is reduced relative to supine posture on Earth (6, 15), but the absence of gravitational effects on internal organs allows increased transmural pressure with greater cardiac filling (15). Elevated Q̇ with spaceflight occurs even though total blood volume is reduced in compensation for the shift of blood volume from the legs with the removal of the head-to-foot gravitational vector (1). It is not known what happens to the distribution of Q̇ in spaceflight. The normal response to upright posture is a reduction in splanchnic blood flow (26), while brain blood flow (19) and resting leg blood flow (32) can be reduced. With long-duration spaceflight, blood flow is unchanged from preflight in the femoral and cerebral arteries compared with supine baseline (3). Portal vein volume increased by 45%, and it is speculated that splanchnic blood flow was elevated (4).
In the current study, the estimated Q̇ by Modelflow was not different among baseline supine, baseline seated, and inflight. However, with the lower heart rate in supine baseline compared with the other conditions, the calculated Modelflow stroke volume was greater while supine. Stroke volume is typically found to be different between supine and upright posture, but cardiac output is not always significantly changed due to compensation by heart rate (12, 13). Previously, Modelflow Q̇ was increased during spaceflight only at the second measurement point and by only 10% (13).
Our observation that Modelflow failed to track the rebreathing method during spaceflight contrasts with observations from parabolic flight (16) but is consistent with other observations where changes were induced in compliant vascular beds (22). Under parabolic flight, there are large, rapid shifts in blood volume within the venous system that affect cardiac stroke volume and Q̇ (18, 20). With the increase in Q̇ during the weightless phase of parabolic flight, there is a rapid response of systemic vascular resistance mediated by withdrawal of sympathetic neural input to peripheral arteries and arterioles to maintain mean arterial pressure close to the baseline (18, 20). In contrast to parabolic flight, heat stress caused increases in Q̇ measured by thermodilution that were two times greater than increases estimated by Modelflow (22). The authors considered the possibility that increased flow resulted in enhanced release of nitric oxide, potentially altering aortic impedance and compliance, which would affect Modelflow calculations. However, it was noted that deviations of Modelflow estimates compared with standard methods were associated with reduced systemic resistance related to dilation of the highly compliant skin vascular bed with thermal loading (22) and with isoprenaline infusion (8). These findings are consistent with the speculated increased compliance in the splanchnic vascular bed with spaceflight.
The estimation of Q̇ by pulse contour analysis derives the aortic flow rate from a measured arterial pressure waveform at the finger via a nonlinear, three-element Windkessel model. The model incorporates a pressure-dependent model of aortic characteristic impedance and arterial compliance and total systemic vascular resistance (33) determined from age- and sex-dependent aortic pressure-aortic area relationships developed from cadaver studies (14). The method assumes that compliance of the entire circulation is captured by the nonlinear aortic capacitance, which precludes blood volume redistribution through changes in capacitance of other regions, such as the skin vascular bed or splanchnic region. The model aortic impedance and compliance are dependent on age, body mass, and height of the subject, the latter of which does change during extended space flight (7). However, even if the aorta lengthened the same amount as reported for the spine (5 cm), this change would account for only ~6% increase in Q̇. If during the process of lengthening the aortic diameter simultaneously increased 5%, then an increase of 16% would be expected. In any case, there is no evidence to support this change, and a reduction in diameter might be predicted. The impact of model parameters is considered to be modest and inadequate to explain the discrepancy between rebreathing and Modelflow.
Pulse contour analysis by various methods has been conducted on data collected during parabolic flight with a corrected impedance method providing the smallest errors (2). However, neither this method nor any other investigated would resolve the current discrepancy between Modelflow and rebreathing Q̇, as all of the methods rely on the magnitude of the arterial pulse wave. In the current study, there was no difference between pulse pressure preflight compared with inflight, so all methods reliant on the integral of the pulse waveform or differences in systolic and diastolic pressure would fail to follow the increase observed by rebreathing.
Rebreathing estimates of Q̇ are based on the rate of uptake of a soluble tracer gas. The soluble gas of the current method, Freon-22, has a known solubility coefficient (30) and is taken up initially in lung tissues, rapidly reaching an equilibrium value. There is no known reason for different uptake by lung tissues, and inhomogeneity of ventilation, measured by an argon rebreathing method, is not different in upright posture in normal gravity and in spaceflight (31). The soluble gas also rapidly equilibrates with blood and is removed from the lungs in proportion to the blood flow through the alveoli (10). The solubility coefficient increases with hematocrit (30); thus an increase in hematocrit could appear as a more rapid removal of soluble gas. Hematocrit is not elevated during short-duration spaceflight (1) nor in samples collected within hours of return from space (25), although the latter are complicated by variable hydration conditions associated with landing and renewed orthostatic stress. The Modelflow estimates of Q̇ before and during rebreathing indicated that Q̇ was not artificially increased by the rebreathing maneuver. These considerations suggest that soluble gas uptake should be directly proportional to lung blood flow and that Q̇ was elevated during spaceflight.
There were no significant differences detected in arterial blood pressure between preflight (supine or seated) and inflight measurements made with the finger cuff device. These results were consistent with our previous observations of finger arterial pressure (13) but contrast with the arm cuff measurements by Norsk et al. (17). The current study did not measure arm cuff blood pressure and was not designed to specifically address this finding.
Conclusion.
The current study has revealed an important limitation with the finger pulse contour analysis method to estimate cardiac stroke volume and Q̇ during spaceflight. Previous observations with this method have probably underestimated the change in Q̇ with spaceflight (13). Future spaceflight research cannot rely on Modelflow estimates of Q̇ to reflect changes from preflight baseline data collections. It remains to be determined whether the changes in Modelflow Q̇ in response to experimental manipulations will reflect absolute changes in Q̇. Rebreathing and other methods that do not use pulse wave analysis should be incorporated into any assessments of cardiac function during spaceflight. These data also reinforce the findings of Shibasaki et al. (22) and suggest the need for caution in the interpretation of Modelflow estimates Q̇ under conditions when arterial compliance is acutely or chronically changed.
GRANTS
This work was supported by Canadian Space Agency Contract 9F053-111259 and Natural Sciences and Engineering Research Council of Canada Grants RGPIN-6473 (to R. L. Hughson) and RGPIN-05778-2015 (to S. D. Peterson). N. Yee was supported by a American Physiological Society Undergraduate Research Excellence Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.L.H. conceived and designed research; R.L.H. and D.K.G. performed experiments; R.L.H., S.D.P., N.J.Y., and D.K.G. analyzed data; R.L.H., S.D.P., N.J.Y., and D.K.G. interpreted results of experiments; R.L.H. prepared figures; R.L.H. and S.D.P. drafted manuscript; R.L.H., S.D.P., N.J.Y., and D.K.G. edited and revised manuscript; R.L.H., S.D.P., N.J.Y., and D.K.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the astronauts for commitment and attention to detail during the study. Excellent support was provided by Poul Knudsen of Danish Aerospace, the support team at CADMOS center for the development of microgravity applications and space operations, and the Project Team at the Canadian Space Agency.
REFERENCES
- 1.Alfrey CP, Udden MM, Leach-Huntoon C, Driscoll T, Pickett MH. Control of red blood cell mass in spaceflight. J Appl Physiol (1985) 81: 98–104, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Arai T, Limper U, Gauger P, Beck L. Pulse contour methods to estimate cardiovascular indices in micro- and hypergravity. Aviat Space Environ Med 84: 1178–1185, 2013. doi: 10.3357/ASEM.3683.2013. [DOI] [PubMed] [Google Scholar]
- 3.Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol 86: 157–168, 2001. doi: 10.1007/s004210100473. [DOI] [PubMed] [Google Scholar]
- 4.Arbeille P, Provost R, Zuj K, Vincent N. Measurements of jugular, portal, femoral, and calf vein cross-sectional area for the assessment of venous blood redistribution with long duration spaceflight (Vessel Imaging Experiment). Eur J Appl Physiol 115: 2099–2106, 2015. doi: 10.1007/s00421-015-3189-6. [DOI] [PubMed] [Google Scholar]
- 5.Becker LA. Effect Size Calculators (Online). https://www.uccs.edu/~lbecker/ [2000].
- 6.Buckey JC Jr, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancy CW Jr, Meyer DM, Blomqvist CG. Central venous pressure in space. J Appl Physiol (1985) 81: 19–25, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Chang DG, Healey RM, Snyder AJ, Sayson JV, Macias BR, Coughlin DG, Bailey JF, Parazynski SE, Lotz JC, Hargens AR. Lumbar spine paraspinal muscle and intervertebral disc height changes in astronauts after long-duration spaceflight on the International Space Station. Spine 41: 1917–1924, 2016. doi: 10.1097/BRS.0000000000001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyson KS, Shoemaker JK, Arbeille P, Hughson RL. Modelflow estimates of cardiac output compared with Doppler ultrasound during acute changes in vascular resistance in women. Exp Physiol 95: 561–568, 2010. doi: 10.1113/expphysiol.2009.050815. [DOI] [PubMed] [Google Scholar]
- 9.Faisal A, Beavers KR, Robertson AD, Hughson RL. Prior moderate and heavy exercise accelerate oxygen uptake and cardiac output kinetics in endurance athletes. J Appl Physiol (1985) 106: 1553–1563, 2009. doi: 10.1152/japplphysiol.91550.2008. [DOI] [PubMed] [Google Scholar]
- 10.Gabrielsen A, Videbaek R, Schou M, Damgaard M, Kastrup J, Norsk P. Non-invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clin Sci (Lond) 102: 247–252, 2002. doi: 10.1042/cs1020247. [DOI] [PubMed] [Google Scholar]
- 11.Harms MPM, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97: 291–301, 1999. doi: 10.1042/cs0970291. [DOI] [PubMed] [Google Scholar]
- 12.Houtman S, Colier WN, Hopman MT, Oeseburg B. Reproducibility of the alterations in circulation and cerebral oxygenation from supine rest to head-up tilt. Clin Physiol 19: 169–177, 1999. doi: 10.1046/j.1365-2281.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- 13.Hughson RL, Shoemaker JK, Blaber AP, Arbeille P, Greaves DK, Pereira-Junior PP, Xu D. Cardiovascular regulation during long-duration spaceflights to the International Space Station. J Appl Physiol (1985) 112: 719–727, 2012. doi: 10.1152/japplphysiol.01196.2011. [DOI] [PubMed] [Google Scholar]
- 14.Langewouters GJ, Wesseling KH, Goedhard WJ. The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J Biomech 17: 425–435, 1984. doi: 10.1016/0021-9290(84)90034-4. [DOI] [PubMed] [Google Scholar]
- 15.Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA, Levine BD. Effect of gravity and microgravity on intracranial pressure. J Physiol 595: 2115–2127, 2017. doi: 10.1113/JP273557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limper U, Gauger P, Beck LE. Upright cardiac output measurements in the transition to weightlessness during parabolic flights. Aviat Space Environ Med 82: 448–454, 2011. doi: 10.3357/ASEM.2883.2011. [DOI] [PubMed] [Google Scholar]
- 17.Norsk P, Asmar A, Damgaard M, Christensen NJ. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J Physiol 593: 573–584, 2015. doi: 10.1113/jphysiol.2014.284869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norsk P, Damgaard M, Petersen L, Gybel M, Pump B, Gabrielsen A, Christensen NJ. Vasorelaxation in space. Hypertension 47: 69–73, 2006. doi: 10.1161/01.HYP.0000194332.98674.57. [DOI] [PubMed] [Google Scholar]
- 19.Ogoh S, Sørensen H, Hirasawa A, Sasaki H, Washio T, Hashimoto T, Bailey DM, Secher NH. Dynamic cerebral autoregulation is unrelated to decrease in external carotid artery blood flow during acute hypotension in healthy young men. Exp Physiol 101: 1040–1049, 2016. doi: 10.1113/EP085772. [DOI] [PubMed] [Google Scholar]
- 20.Petersen LG, Damgaard M, Petersen JCG, Norsk P. Mechanisms of increase in cardiac output during acute weightlessness in humans. J Appl Physiol (1985) 111: 407–411, 2011. doi: 10.1152/japplphysiol.01188.2010. [DOI] [PubMed] [Google Scholar]
- 21.Prisk GK, Guy HJ, Elliott AR, Deutschman RA 3rd, West JB. Pulmonary diffusing capacity, capillary blood volume, and cardiac output during sustained microgravity. J Appl Physiol (1985) 75: 15–26, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat-stressed individuals. Am J Physiol Regul Integr Comp Physiol 300: R486–R491, 2011. doi: 10.1152/ajpregu.00505.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata S, Levine BD. Biological aortic age derived from the arterial pressure waveform. J Appl Physiol (1985) 110: 981–987, 2011. doi: 10.1152/japplphysiol.01261.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shykoff BE, Farhi LE, Olszowka AJ, Pendergast DR, Rokitka MA, Eisenhardt CG, Morin RA. Cardiovascular response to submaximal exercise in sustained microgravity. J Appl Physiol (1985) 81: 26–32, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Zwart SR, Block G, Rice BL, Davis-Street JE. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J Nutr 135: 437–443, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 290: H665–H673, 2006. doi: 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundblad P, Spaak J, Kaijser L. Time courses of central hemodynamics during rapid changes in posture. J Appl Physiol (1985) 116: 1182–1188, 2014. doi: 10.1152/japplphysiol.00690.2013. [DOI] [PubMed] [Google Scholar]
- 28.van Lieshout JJ, Harms MP, Pott F, Jenstrup M, Secher NH. Stroke volume of the heart and thoracic fluid content during head-up and head-down tilt in humans. Acta Anaesthesiol Scand 49: 1287–1292, 2005. doi: 10.1111/j.1399-6576.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol (1985) 94: 833–848, 2003. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- 30.Varene N, Choukroun ML, Marthan R, Varene P. Solubility of Freon 22 in human blood and lung tissue. J Appl Physiol (1985) 66: 2468–2471, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Verbanck S, Linnarsson D, Prisk GK, Paiva M. Specific ventilation distribution in microgravity. J Appl Physiol (1985) 80: 1458–1465, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Villar R, Hughson RL. Lower limb vascular conductance and resting popliteal blood flow during head-up and head-down postural challenges. Clin Physiol Funct Imaging 33: 186–191, 2013. doi: 10.1111/cpf.12008. [DOI] [PubMed] [Google Scholar]
- 33.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol (1985) 74: 2566–2573, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Xu D, Shoemaker JK, Blaber AP, Arbeille P, Fraser K, Hughson RL. Reduced heart rate variability during sleep in long-duration spaceflight. Am J Physiol Regul Integr Comp Physiol 305: R164–R170, 2013. doi: 10.1152/ajpregu.00423.2012. [DOI] [PubMed] [Google Scholar]