Abstract
OBJECTIVES
Chronic obstructive pulmonary disease (COPD) is a common lung disease characterized by airflow limitation and systemic inflammation. Recently, neutrophil-to-lymphocyte ratio (NLR) has gathered increasing interest in the detection of inflammation in inflammatory diseases. This study aimed to investigate the role of NLR in COPD for identifying the detection of inflammation and recognition of acute exacerbation.
MATERIAL AND METHODS
The laboratory results of 103 COPD patients were included into the study, of which 47 patients were in acute exacerbation and 56 patients were at stable period, and there were 40 gender and age-matched healthy controls. Complete blood count (CBC), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were evaluated. NLR was calculated from CBC.
RESULTS
NLR values of patients with COPD (both acutely exacerbated and stable) were found significantly higher than those of the controls (p< 0.001, p< 0.05; respectively). In all patients with COPD, NLR values positively correlated with serum CRP (r= 0.641, p< 0.001) and ESR (r= 0.276, p= 0.005) levels and negatively correlated with forced vital capacity (r= −0.20, p= 0.043) and forced expiratory volume in the 1st second (r= −0.288, p= 0.003). For an NLR cutoff of 3.34, sensitivity for detecting exacerbation of COPD was 78.7% and specificity was 73.2% (AUC 0.863, p< 0.001).
CONCLUSION
Our results suggest that NLR may be considered as a reliable and simple indicator in the determination of increased inflammation in patients with COPD. Furthermore, NLR could be useful for the early detection of possible acute exacerbations in patients with COPD.
Keywords: Chronic obstructive pulmonary disease, neutrophil-to-lymphocyte ratio, inflammation
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is a preventable and treatable common disease characterized by progressive and permanent airflow limitation associated with increased chronic inflammatory response of the lungs and airways against harmful gases and particles [1]. Especially cigarette smoke and inhalation of toxic gases cause chronic inflammation in the airways and pulmonary parenchyma in patients with COPD [2]. Exacerbation in COPD is frequently caused by respiratory tract infections, and the number of acute phase proteins and inflammatory cells in circulation during exacerbation increases [3–5]. However, it is known that there is an increase in some inflammatory markers and a low-grade systemic inflammation in some patients with COPD at the stable state [6]. It has been established in previous studies that various inflammatory markers like C-reactive protein (CRP), fibrinogen and leucocyte count increase in stable patients of COPD and that this increase is associated with the negative results of the disease [7–10]. Moreover, it has been described that elevated CRP levels can be used in the diagnosis of acute exacerbation and to approximate the prognosis of the disease [11,12].
Leucocyte count and its subtypes are well-known markers of inflammation [11,13]. Since the physiological response of the leucocytes in circulation against stress precipitates an increase in netrophile count and decrease in the lymphocyte count, the ratio of these two sub-groups to one another is employed in the intensive care practice[14]. In various recent studies, neutrophile-to-lymphocyte ratio (NLR) has been evaluated for its probable role in the inflammation periods of chronic diseases [15–17]. It has been seen that NLR is a sign of poor prognosis in patients who have undergone cardiovascular involvement and an independent determinant of mortality in patients with acute coronary syndrome [18,19]. In addition, increased NLR in patients with COPD has been identified a marker that could be used to determine inflammation, detect acute exacerbation in early stages, and it has also been confirmed that it could be an independent marker for all-cause mortality [20–22].
The aim of this study was to evaluate the role of NLR in the identification of chronic inflammation and diagnosis of acute exacerbation in COPD and compare it with other traditional markers.
MATERIAL AND METHODS
Study Population
One hundred and eighty-two consecutive patients, who applied to the thoracic diseases polyclinic of our hospital with the diagnosis of COPD between June 1st, 2015 and September 1st, 2015, were enrolled to this retrospective study. Patients with COPD were determined using the data of our hospital’s digital archive system. COPD diagnosis was confirmed by the results of the pulmonary function test (PFT). Thirty-one patients with a chronic respiratory disease apart from COPD, a pulmonary-extrapulmonary malignancy or a systemic disease that could affect leucocyte values, 26 patients whose leucocyte count was > 12 × 103/μL or < 4 × 103/μL, and 22 patients with insufficient PFT values were excluded from the study. As a result, a total of one hundred and three patients were included into the study, and the patients were analysed in two groups of “acute exacerbation” and “stable” as regards their clinical picture. Patients who had not had any significant changes in their symptoms in the last 3 months and the ones who did not need additional inhaler treatment dosages or any other additional treatments were defined as “stable COPD” [23]. Patients who had deterioration in the symptoms of the respiratory tract that caused change in medical treatment beyond normal daily variations were classified as “acute exacerbation”[1]. The duration of the disease, history of smoking, clinical data, hemogram, erythrocyte sedimentation rate (ESR), CRP and PFT results were recorded from the digital archive system of the hospital.
The control group included 40 healthy cases that were in the same age group as the patients with COPD and that applied to the thoracic diseases polyclinic for routine check and tests.
The present study was carried out in accordance with the Helsinki Declaration, and consent was received from the local ethics committee of our university.
Pulmonary Function Tests
Pulmonary function parameters (forced vital capacity [FVC] and forced expiratory volume in the first second [FEV1]) were measured using standard spirometry device (Ultima CPX 790705-205; Medgraphics Corporation, St. Paul, MN, USA). FEV1/FVC ratio was calculated. The obtained results were expressed as the percentage of absolute and expected values. Spirometric values were evaluated as regards the standards specified by the European Respiratory Society [24].
Determination of Neutrophil-to-Lymphocyte Ratio
All blood samples were put in tubes with potassium EDTA for blood count. Hemoglobin, hematocrit, thrombocyte, and white blood cell and type (neutrophile, lymphocyte, eosinophile and monocyte) were identified with automatic blood count device (Siemens Advia 2120, Diagnostic Solutions, Milan, Italy) by electrical impedance method. NLR was obtained by dividing the neutrophile count in the hemogram blood to the lymphocyte count.
Statistical Analysis
IBM SPSS Statistics 21 (Statistical Product and Service Solutions 21.0 version, authorization code: d91314f638c364094170; Armonk, NY, USA) Package was used for the statistical analysis in this study. The results were given as mean ± standard deviation. p value < 0.05 was considered statistically significant. Student’s t-test was used for the comparison of two independent groups. OneWay Anova test was used for the comparison of multiple groups. Tukey test was used to test the significance of any observed difference. Chi-square (X2) test was carried out to compare gender distribution between the groups. Pearson correlation test was used to evaluate parametric values. ROC curve was drawn to show the specifity and sensitivity of NLR.
RESULTS
A total of 103 patients with COPD, of whom 47 (45.6%) were in the exacerbation state and 56 (54.4%) were at the stable period, and 40 healthy controls were evaluated in the study. No statistically significant difference was found among the three groups in terms of age and gender. Neutrophile, lymphocyte and NLR levels were detected statistically significantly high in both COPD groups when compared to the control group. Moreover, NLR was detected statistically significantly high in the COPD exacerbation group when compared to the stable patients with COPD. While no difference was determined between both COPD groups in terms of ESR, CRP levels were found to be statistically higher in the exacerbation group. When only COPD groups were included into the analysis, the duration of the disease was found statistically significantly high in the exacerbation group, and FVC and FEV1 levels were found low. Clinical and laboratory data of the three groups and the duration of the disease, smoking state, and PFT data of the patients are given in Table 1.
Table 1.
Clinical and laboratory findings of the study groups and the results of the pulmonary function test
| COPD (Exacerbation) (n= 47) | COPD (Stable) (n= 56) | Control (n= 40) | |
|---|---|---|---|
| Age (years) | 67.8 ± 10.1 | 65.6 ± 8.7 | 63.6 ± 8.7 |
| Gender (male, n (%) ) | 38 (80.9) | 44 (78.6) | 30 (75) |
| Cigarette (pack-year) | 42.76 ± 21.78 | 40.35 ± 19.79 | - |
| Duration of the disease (year) | 8.65 ± 2.82 a | 6.21 ± 2.73 | - |
| Laboratory findings | |||
| Leucocyte (103/μL) | 8.96 ± 2.03 *,b | 7.83 ± 1.67 | 7.05 ± 1.43 |
| Neutrophil (103/μL) | 6.66 ± 1.93 *,a | 4.77 ± 1.24*** | 3.88 ± 0.82 |
| Lymphocyte (103/μL) | 1.34 ± 0.53 *,a | 2.01 ± 0.75**** | 2.38 ± 0.58 |
| NLR | 5.78 ± 3.14 *,a | 2.67 ± 1.13**** | 1.68 ± 0.41 |
| Hemoglobin (g/dL) | 14.65 ± 2.13 | 14.58 ± 1.78 | 14.2 ± 1.86 |
| Platelet (103/μL) | 264.9 ± 103.7 | 248.6 ± 82.1 | 253.2 ± 69.6 |
| ESR (mm/saat) | 27.10 ± 21.41* | 20.05 ± 15.41** | 7.97 ± 5.80 |
| CRP (mg/L) | 48.18 ± 45.14*,a | 19.41 ± 28.42**** | 3.84 ± 1.18 |
| Pulmonary Function Tests | |||
| FVC, % | 46.95 ± 12.50 b | 56.96 ± 16.25 | - |
| FEV1, % | 34.55 ± 11.08 b | 43.05 ± 15.05 | - |
| FEV1/FVC | 55.31 ± 10.49 | 56.42 ± 10.77 | - |
NLR: Neutrophil-to-lymphocyte ratio; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; FVC: Forced vitalcapacity; FEV1: Forced expiration 1st second volume. The results were given as mean ± standard deviation.
p< 0.001,
p< 0.005,
p< 0.01,
p< 0.05; when compared to the controls
p< 0.001,
p< 0.005; when compared to the patients with stable COPD.
When all patients with COPD included into the study were incorporated into the correlation analysis, it was observed that there was a good degree of positive correlation between NLR and CRP levels and a poor degree of positive correlation between ESR levels (r= 0.641, p< 0.001; r= 0.276, p= 0.005 respectively) (Figure 1). Furthermore, it was detected that there was a poor degree of negative correlation between NLR and FVC levels and a poor-medium degree of negative correlation between FEV1 levels (r= −0.20, p= 0.043; r= −0.288, p= 0.003, respectively) (Figure 2).
Figure 1.
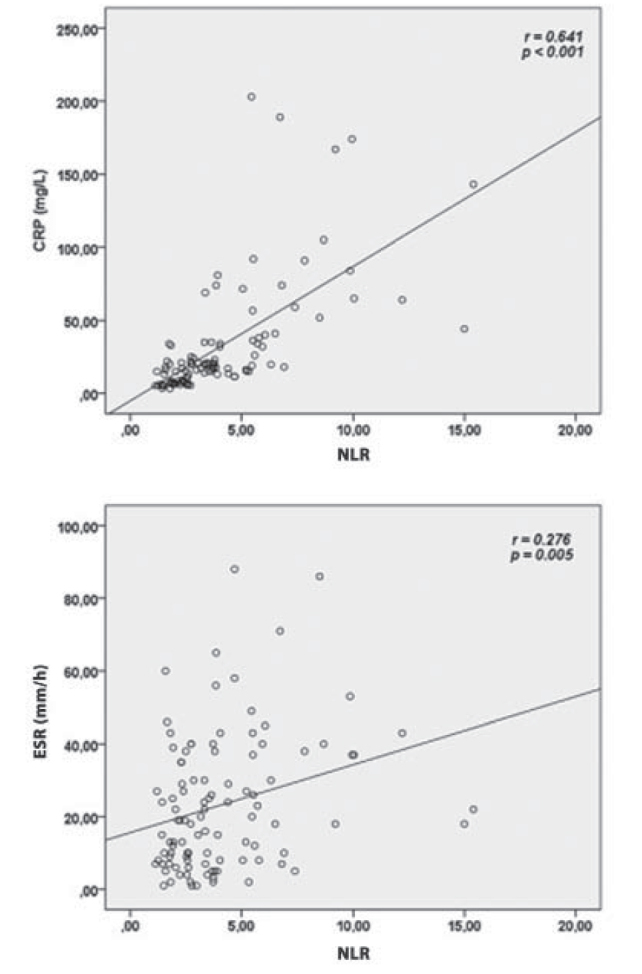
Correlations between neutrophile-to-lymphocyte ratio (NLR) and serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
Figure 2.
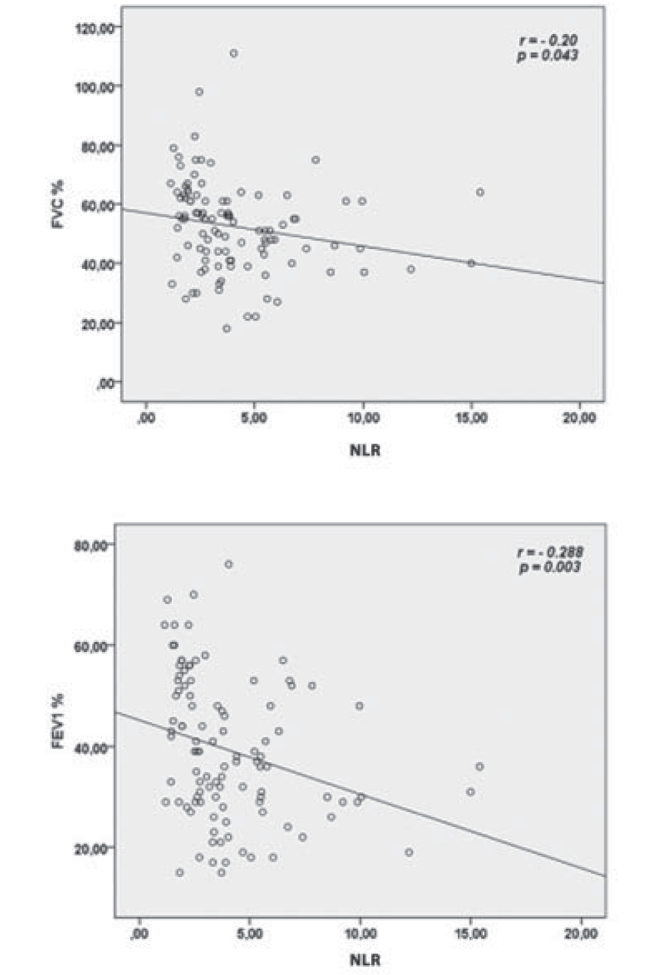
Correlations between neutrophile-to-lymphocyte ratio (NLR) and forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1).
When NLR was evaluated with ROC analysis to estimate exacerbation in patients with COPD, the area under the ROC curve was detected as 0.863 (95% Cl 0.796–0.931; p< 0.001). In addition, the sensitivity and specifity of NLR were found as 78.7% and 73.2%, respectively when the optimal cut-off value was specified as 3.34 in terms of estimating exacerbation (Figure 3).
Figure 3.
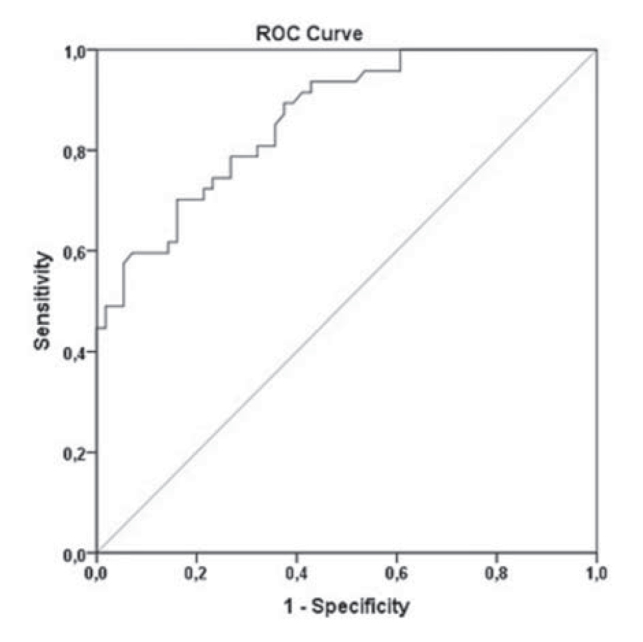
ROC curve indication of the ability of neutrophil-to-lymphocyte ratio in estimating exacerbations in patients with COPD.
DISCUSSION
It was detected in this retrospective study that NLR levels were higher in acute exacerbation or stable patients with COPD when compared with the healthy controls. Additionally, it was seen that there was a positive correlation between NLR levels and CRP and ESR levels, and that NLR had a significant specificity and sensitivity in terms of estimating exacerbation.
A set of markers that could be measured in the blood and that could show the presence/severity of an infection and inflammation has been already identified. These can be used as early and sensitive indicators in determining the infective picture. Parameters, such as serum CRP level, ESR value, leucocyte count, and neutrophile dominance in the leucocyte formula are quite frequently used parameters while following infection in clinical practice [25]. Pathological processes are not specifically localised to the lungs. COPD is a chronic inflammatory disease with high commorbidity and systemic involvement associated with conditions like metabolic syndrome, osteoporosis, diabetes, and cardiovascular diseases [26]. Levels of various inflammation markers like CRP, fibrinogen and leucocyte count have been detected to rise in COPD patients in the exacerbation period [3–5]. Additionally, it is known that acute phase proteins and other inflammatory cytokines increase even in stable patients with COPD and that there is a low-grade chronic inflammation [27]. This chronic inflammatory period seen in patients with COPD has an important role in the pathogenesis of the disease [6]. Furthermore, it has been established that these elevated inflammation markers are associated with the poor prognosis of the disease and with the increase in the commorbidity ratios [7–10]. The best known among these markers is CRP. Elevated systemic CRP levels have been found associated with the increase in disease severity, deterioration in health condition, hospitalization, and mortality rates in COPD [8,28,29]. In our study, CRP and ESR levels were found higher in both COPD groups when compared with the controls. Moreover, CRP levels were detected higher in patients of COPD at the exacerbation state when compared to the stable ones. It is known that the most frequent cause of exacerbation in patients with COPD is infections. In patients at exacerbation state, CRP values may have been notably elevated depending on infections. However, elevated CRP levels even in stable COPD patients suggest systemic inflammation in these patients.
The presence of chronic inflammation in central and peripheral airways along with an increase in various inflammatory cell types and proinflammatory mediators is a fundamental characteristic of COPD. Inflammation causes damage to the lung parenchyma and contribute to the evidencing of airway limitation. It is known that neutrophils, macrophages and CD8 T-lymphocytes are important inflammatory cells in COPD [30]. It is thought that neutrophils play a role as responsible key cells of lung damage in emphysema [31]. Neutrophil count in circulation rises in systemic inflammation. Elevated neutrophil count is associated with the progression of COPD [32]. Recently, NLR has caught the interest of many researchers as an inflammatory marker. NLR has been shown in various studies to be a prognostic marker in several inflammatory diseases, such as cardiovascular diseases, kidney disease and familial Mediterranean fever [15–19]. As regards our research, there are 4 studies in the literature which investigate the importance of NLR in patients with COPD. Günay et al. [20] have retrospectively analyzed 178 stable patients with COPD, 91 patients with COPD in the acute exacerbation period and 50 control cases. NLR value has been found notably higher in both COPD groups in their study when compared to the controls. Moreover, NLR has been detected statistically significantly higher in patients with COPD in the exacerbation period when compared to the stable ones. In addition, it has been observed in their study that there is a positive correlation between NLR and CRP levels. In a prospective study where 386 mild and severe COPD patients have been followed-up for 10 years, NLR has been detected as an independent marker for elevated all-cause mortality [22]. It has been expressed in a retrospective study where 140 stable patients with COPD and 50 controls have been included that NLR could be a simple, effective and practical indicator in the early detection of metabolic syndrome [33]. In another study where 100 patients with COPD first in the acute exacerbation period and then at the stable period and 50 controls were evaluated retrospectively, NLR has been shown to be a marker that could be used to detect elevated inflammation like CRP, leucocyte count and ESR [21]. It was detected similarly in our study that NLR levels were higher in patients with COPD at the exacerbation and table period when compared to the controls. When all patients with COPD were taken into consideration, a positive correlation was observed between NLR, ESR and CRP. Moreover, it was determined that NLR had high specifity and sensitivity in estimating acute exacerbation. Our results suggest that NLR could be used as an inflammatory biomarker in showing acute exacerbation and chronic inflammation.
It has been previously reported that the decrease in pulmonary functions is associated with the increase in the inflammatory markers [34]. However, the relation of NLR with pulmonary functions has not been sufficiently examined in the literature. Yasar et al. [33] have detected a negative correlation between NLR and FEV1 levels but a positive correlation between between dyspnea score. In our study, a negative correlation was detected between NLR level and both FVC and FEV1 levels. This results makes us think that the more severe the COPD is, the more severe the inflammation is and that NLR could be a practical marker to estimate inflammation severity.
Limitations to the Study
This is not a prospective study. It is a retrospective study that made use of the digital archive system of our hospital. Therefore, data like COPD evaluation test, mMRC dyspnea scale and previous exacerbation history could not be reached, and thus the patients could not be classified into recent COPD classification. In addition, as the echocardiography and arterial blood gas results of the patients were insufficient, these data were not analyzed in our study. Similarly, patients who lacked the data fundamental for the study were also excluded from the study. Furthermore, in terms of estimating exacerbation in patients with COPD, it would be more correct to evaluate NLR levels both at the stable and the exacerbation period in the same patient group to detect the significance of NLR since NLR levels could be varying like other inflammation markers while specifying cutoff values.
CONCLUSION
Overall, NLR levels in our study were detected higher in stable patients with COPD and in patients at the exacerbation period when compared to the controls, and they correlated with traditional inflammation indicators. NLR could be a useful and practical inflammation marker to estimate acute exacerbation in patients with COPD and to detect potential inflammation at the stable period.
Footnotes
Author Contributions: Concept - E.İ., FD.; Design - E.İ., FD.; Supervision - E.İ., F.D., Ö.Ö.; Resources - E.İ., M.K., Ö.Ö.; Materials - E.İ., Ö.Ö.; Data Collection and/or Processing - E.İ., Ö.Ö., M.K.; Analysis and/or Interpretation - E.İ., F.D., M.K.; Literature Search - E.İ., M.K.; Writing Manuscript - E.İ., F.D.; Critical Review - E.İ., F.D.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: updated. 2014. http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf.
- 2.Hogg JC, Chu F, Utokaparch S, et al. The nature of small airway obstruction in chronic obstructive pulmonary disease. New England Journal of Medicine. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. https://doi.org/10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–65. doi: 10.1056/NEJMra0800353. https://doi.org/10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 4.Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84:210–5. [PubMed] [Google Scholar]
- 5.Dentener MA, Creutzberg EC, Schols AM, et al. Systemic anti-inflammatory mediators in COPD: increase in soluble interleukin 1 receptor II during treatment of exacerbations. Thorax. 2001;56:721–6. doi: 10.1136/thorax.56.9.721. https://doi.org/10.1136/thorax.56.9.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. https://doi.org/10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahl M, Tybjaerg-Hansen A, Vestbo J, et al. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1008–11. doi: 10.1164/ajrccm.164.6.2010067. https://doi.org/10.1164/ajrccm.164.6.2010067. [DOI] [PubMed] [Google Scholar]
- 8.Dahl M, Vestbo J, Lange P, et al. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–5. doi: 10.1164/rccm.200605-713OC. https://doi.org/10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 9.Agustí A, Edwards LD, Rennard SI, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. https://doi.org/10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomsen M, Dahl M, Lange P, et al. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:982–8. doi: 10.1164/rccm.201206-1113OC. https://doi.org/10.1164/rccm.201206-1113OC. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353–61. doi: 10.1001/jama.2013.5732. https://doi.org/10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 12.Antonescu-Turcu AL, Tomic R. C-reactive protein and copeptin: Prognostic predictors in chronic obstructive pulmonary disease ex acerbations. Current Opinion in Pulmonary Medicine. 2009;15:120–5. doi: 10.1097/MCP.0b013e3283218603. https://doi.org/10.1097/MCP.0b013e3283218603. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. New England Journal of Medicine. 2003;348:138–50. doi: 10.1056/NEJMra021333. https://doi.org/10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 14.Zahorec R. Ratio of neutrophil to lymphocyte counts-Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 15.Ahsen A, Ulu MS, Yuksel S, et al. As a new inflammatory marker for familial Mediterranean fever: Neutrophil-to-lymphocyte ratio. Inflammation. 2013;36:1357–62. doi: 10.1007/s10753-013-9675-2. https://doi.org/10.1007/s10753-013-9675-2. [DOI] [PubMed] [Google Scholar]
- 16.Núñez J, Núñez E, Bodí V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. American Journal of Cardiology. 2008;101:747–52. doi: 10.1016/j.amjcard.2007.11.004. https://doi.org/10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Turkmen K, Erdur FM, Ozcicek F, et al. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodialysis International. 2013;17:391–6. doi: 10.1111/hdi.12040. https://doi.org/10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 18.Duffy BK, Gurm HS, Rajagopal V, et al. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993–6. doi: 10.1016/j.amjcard.2005.10.034. https://doi.org/10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–7. doi: 10.1016/j.amjcard.2008.05.006. https://doi.org/10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Günay E, Sarınç Ulaşlı S, Akar O, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation. 2014;37:374–80. doi: 10.1007/s10753-013-9749-1. https://doi.org/10.1007/s10753-013-9749-1. [DOI] [PubMed] [Google Scholar]
- 21.Taylan M, Demir M, Kaya H, et al. Alterations of the neutrophil-lymphocyte ratio during the period of stable and acute exacerbation of chronic obstructive pulmonary disease patients. Clin Respir J. 2015 Jun 19; doi: 10.1111/crj.12336. https://doi.org/10.1111/crj.12336. [DOI] [PubMed] [Google Scholar]
- 22.Sørensen AK, Holmgaard DB, Mygind LH, et al. Neutrophil-to-lymphocyte ratio, calprotectin and YKL-40 in patients with chronic obstructive pulmonary disease: correlations and 5-year mortality - a cohort study. J Inflamm (Lond) 2015;12:20. doi: 10.1186/s12950-015-0064-5. https://doi.org/10.1186/s12950-015-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. European Respiratory Journal. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. https://doi.org/10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. https://doi.org/10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Göçmen H, Çoban H, Yıldız A, et al. KOAH Akut Atakta Serum CRP Düzeyi ve Hematolojik Parametreler ile Hastalık Şiddeti Arasında Korelasyon Var mı? Solunum Hastalıkları. 2007;18:141–7. [Google Scholar]
- 26.Sevenoaks MJ, Stockley RA. Chronic Obstructive Pulmonary Disease, inflammation and comorbidity-a common inflammatory phenotype? Respir Res. 2006;7:70. doi: 10.1186/1465-9921-7-70. https://doi.org/10.1186/1465-9921-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schols AM, Buurman WA, Staal van den Brekel AJ, et al. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–24. doi: 10.1136/thx.51.8.819. https://doi.org/10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franciosi LG, Page CP, Celli BR, et al. Markers of disease severity in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2006;19:189–99. doi: 10.1016/j.pupt.2005.05.001. https://doi.org/10.1016/j.pupt.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. https://doi.org/10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockley RA. Progression of chronic obstructive pulmonary disease: impact of inflammation, comorbidities and therapeutic intervention. Curr Med Res Opin. 2009;25:1235–45. doi: 10.1185/03007990902868971. https://doi.org/10.1185/03007990902868971. [DOI] [PubMed] [Google Scholar]
- 31.Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002;121:151S–5S. doi: 10.1378/chest.121.5_suppl.151s. https://doi.org/10.1378/chest.121.5_suppl.151S. [DOI] [PubMed] [Google Scholar]
- 32.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of “overspill” of inflammatory mediators from the lungs? Review Evidence Thorax. 2010;65:930–6. doi: 10.1136/thx.2009.130260. https://doi.org/10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- 33.Yasar Z, Buyuksirin M, Ucsular FD, et al. Is an elevated neutrophil-to-lymphocyte ratio a predictor of metabolic syndrome in patients with chronic obstructive pulmonary disease? Eur Rev Med Pharmacol Sci. 2015;19:956–62. [PubMed] [Google Scholar]
- 34.Man SF, Connett JE, Anthonisen NR, et al. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–53. doi: 10.1136/thx.2006.059808. https://doi.org/10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]


