ABSTRACT
Nitric oxide (NO) is a highly reactive gaseous free radical, which in plants was found to stimulate seed germination and ending of dormancy. Experiments were conducted to study the effect of NO inhibitors sodium tungstate (ST) and Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), NADPH oxidase inhibitor diphenyleneiodonium (DPI) and NO donor sodium nitroprusside (SNP) on untreated and magnetoprimed maize (Zea mays var: GSF-2) seeds. Treatment of maize seeds with these inhibitors inhibited germination related parameters like seedling length, fresh weight, dry weight and vigour indices and α-amylase activity of maize seeds under laboratory conditions, whereas NO donor (SNP) promoted all these parameters. Among 3 different inhibitors used ST was most effective and showed an inhibition in seedling length of 67% and 71% at 1 mM concentration for untreated (UT) and magnetically treated (MT) seeds respectively. Data presented here indicate the involvement of nitric oxide in enhanced germination and seedling growth of magnetoprimed maize seeds. ROS are continuosly produced by the cells of germinating seeds and play a positive role in germination of magnetoprimed maize seeds. ROS inhibitor (DPI) inhibited seedling length by 34% and 40% for control and MT seeds respectively. α-amylase activity was also inhibited by all the 3 inhibitors used. It is concluded that NO inhibitors and ROS inhibitor inhibited magnetic field induced promotion of seedling parameters and α- amylase activity of maize seeds.
KEYWORDS: Amylase, diphenyleneiodonium, magnetopriming, NO inhibitors, Nω-nitro-L-arginine methyl ester hydrochloride, seedling growth, sodium nitroprusside, sodium Tungstate
Introduction
The free radical nitric oxide (NO), has been demonstrated to be involved in many of the developmental processes in plants, animals and other organisms. NO synthesis and signaling have been well studied in both animals and plants. In mammals, endogenous NO regulates smooth muscle contraction, platelet aggregation, neurotransmission and immune response.1 NO production by plants was first observed by Klepper (1975) in Glycine max treated with herbicides.2 NO is an important signaling molecule exerting various physiologic functions in plants such as regulation of plant growth and development, including germination, flowering, fruit ripening and organ senescence.3 NO has a significant role in ending of dormancy and enhancement of seed germination.4
There are two pathways for nitric oxide synthesis in biological system, enzymatic pathway and the non-enzymatic pathway. The enzymatic pathway is catalyzed by cytosolic nitrate reductase (cNR), nitrite-NO reductase (Ni-NOR), and NO synthase (NOS) or NOS-like enzymes. Non-enzymatic pathway is nitrite dismutation to NO and nitrate at acidic pH values.5 Nitric oxide synthase (NOS), catalyzes the NADPH dependent oxidation of L-Arginine to L-Citrulline and NO. NO is mainly generated by this pathway. This mechanism is analogous to that used by animal NOS.6
Nitric oxide is an important signaling molecule, as it exerts very striking effect on seed germination stage. Positive effects of NO in germination stage have been observed, that was facilitated either by reducing seed dormancy or by the removal of germination inhibitory conditions.7 Sodium nitroprusside (SNP) is a donor of NO and SNP treated tomato seeds show a dose responsive germination pattern to its different concentrations. As the concentration of SNP increased germination percent, root length and shoot length were also enhanced, but after a particular concentration these parameters were inhibited.8 Root length and germination percent of SNP treated lupin seeds were more as compared with distilled water treated that was more effective after 18 and 24 h and ceased after 48 h.9 Furthermore, exogenous application of nitric oxide (SNP) also promoted seed germination and seedling growth in maize seeds.10
Previous researchers found an intimate but complex connection between magnetic fields and NO production in animal system. Enhanced level of NO was found in pulsed magnetic field treated macrophage cell lines of mouse.11 Similarly, HUVECs (human umbilical vein endothelial cells), exposed to oscillating magnetic field (20 Hz, 20 mT ) promoted NO production.12 It has been established that ELF-MF (Extra low frequency-magnetic field) exposure is able to increase NO production via NOS activation in the rat brain, leading to physiologic responses with intracellular signaling activation.13 Previous investigation also demonstrated that the exposure to ELF-MF might cause Ca2+ dependent NOS activation, and subsequently NO production.14 Unlike animal system, no investigation has been done in plant system, in relation to the involvement of nitric oxide in magnetic field induced signaling pathway. The present work is focused on the connection between magnetic fields and NO production in maize seeds.
The magnetic field has the ability to induce seed germination process via reactive oxygen species. Florez et al.15 reported higher percent germination and seedling length of magnetoprimed maize seeds. Pre-sowing exposure of seeds to magnetic field (30 and 85 mT) caused early emergence and germination of both broad bean and pea cultivars.16,17 Magnetic field has the ability to enhance the membrane permeability, the concentration of ions, free radicals and electrical charges of the seed and in this way activates the metabolic pathway.18,19 Level of Ca2+ ion inside the plant cells increases following exposure to magnetic fields. Ca2+ ions participate in many plant growth processes and responses to stress (heat, salt stress and wounding etc.). Electron paramagnetic resonance (EPR) signals of O2− and OH− radicals of the magnetoprimed soybean seeds have been recorded and enhanced level of O2− and OH− radicals were found.20
However, until now little is known about the influences of static magnetic field treatment on nitric oxide content and its signaling pathway in magnetoprimed seeds during germination. Therefore, the objective of the present study was to investigate the effect of magnetic field treatment (200 mT, 1 h) induced nitric oxide on seed germination, seedling growth and α- amylase activity in maize seeds.
Results
Seedling length
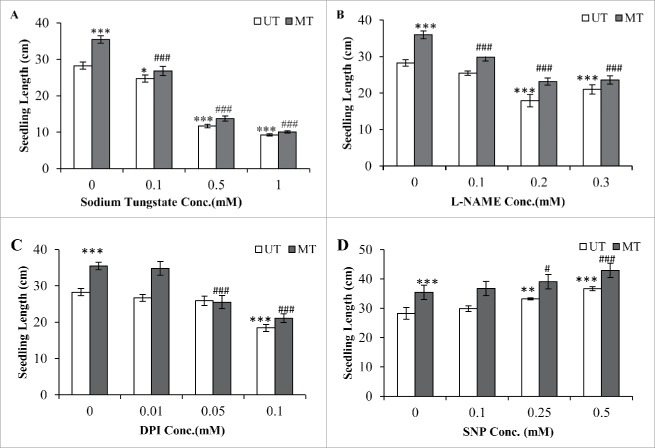
Seedling length of magnetically treated (MT) seeds was 25% more as compared with untreated (UT) seeds. All the NO inhibitors significantly affected the length of root and shoot as compared with untreated seeds. Sodium tungstate (ST) was most effective in inhibiting seedling growth showing a concentration response. At the highest concentration used (1.0 mM) inhibition was 67% and 71% in the UT and MT seeds respectively (Fig. 1A). L-NAME was less effective than ST in causing the inhibition. At 0.3 mM the maximum inhibition of 25% and 34% was recorded in UT and MT seeds (Fig. 1B). DPI used in the range of 0.01 to 0.1 mM showed an inhibition of 34% and 40% at 0.1 mM for UT and MT seeds respectively (Fig. 1C). SNP promoted seedling length in a dose responsive manner. Among 3 concentrations used, 0.5 mM was most effective showing a promotion of 29% and 21% for UT and MT seeds respectively (Fig. 1D).
Figure 1.
Effect of different concentration of ST (A), L-NAME (B), DPI (C) and SNP (D) on seedling length at 72 h after imbibition at 25°C of untreated control (UT) and magnetically treated (MT) Maize seeds. Vertical line above the bar indicates ± SEM. ***, ** and * indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with control (UT) and ###, ## and # indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with magnetically treated (MT) seed. Non-significant terms are not shown.
Seedling fresh weight and dry weight
A significant inhibition in fresh weight and dry weight of seedlings was recorded in all the 3 inhibitors. At the highest concentration used (1 mM) ST inhibited fresh weight 42% and 48% for UT and MT seeds respectively (Fig. 2A). Dry weight was inhibited by 44% and 37% for UT and MT seeds respectively at the same concentration (Fig. 3A).
Figure 2.
Effect of different concentration of ST (A), L-NAME (B), DPI (C) and SNP D) on seedling fresh weight at 72 h after imbibition at 25°C of untreated control (UT) and magnetically treated (MT) Maize seeds. Vertical line above the bar indicates ± SEM. ***, ** and * indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with control (UT) and ###, ## and # indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with magnetically treated (MT) seed. Non-significant terms are not shown.
Figure 3.
Effect of different concentration of ST (A), L-NAME (B), DPI (C) and SNP D) on seedling dry weight at 72 h after imbibition at 25°C of untreated control (UT) and magnetically treated (MT) Maize seeds. Vertical line above the bar indicates ± SEM. ***, ** and * indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with control (UT) and ###, ## and # indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with magnetically treated (MT) seed. Non-significant terms are not shown.
Inhibition of fresh weight by L-NAME was 19% and 26% respectively for UT and MT seeds at 0.3 Mm (Fig. 2B). Dry weight was inhibited by 28% and 42% respectively for UT and MT seeds at the same concentration (Fig. 3B).
DPI inhibited fresh weight by 42% (UT) and 48% (MT) and dry weight by 34% (UT) and 42% (MT) at 0.1 mM concentration (Figs. 2C & 3C).
Promotion of fresh weight by SNP was 25% and 23% respectively for UT and MT seeds at 0.5 mM. Dry weight was promoted by 24% and 19% respectively for UT and MT seeds at the same concentration (Figs. 2D & 3D).
Vigour index I and II
All the inhibitors lowered vigour index I and II. The trend was parallel to the inhibition of seedling length and dry weight by the inhibitors (Fig. 4, 5).
Figure 4.
Effect of different concentration of ST (A), L-NAME (B), DPI (C) and SNP (D) on Vigour index-I at 72 h after imbibition at 25°C of untreated control (UT) and magnetically treated (MT) Maize seeds. Vertical line above the bar indicates ± SEM. ***, ** and * indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with control (UT) and ###, ## and # indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with magnetically treated (MT) seed. Non-significant terms are not shown.
Figure 5.
Effect of different concentration of ST (A), L-NAME (B), DPI (C) and SNP (D) on Vigour index-II at 72 h after imbibition at 25°C of untreated control (UT) and magnetically treated (MT) Maize seeds. Vertical line above the bar indicates ± SEM. ***, ** and * indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with control (UT) and ###, ## and # indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with magnetically treated (MT) seed. Non-significant terms are not shown.
Amylase activity
α-amylase activity was estimated in the untreated and MT seeds after 72 h in the inhibitors solution. All the 3 inhibitors were effective in inhibiting the activity of α-amylase and showed a concentration response. Maximum inhibition of 78% (ST), 67% (L-NAME) and 73% (DPI) was obtained in the control at highest concentration used. In the MT seeds inhibition was 79% (ST), 73% (L-NAME) and 73% (DPI) at the highest concentration used as compared with MT treated seed (Fig. 6).
Figure 6.
Effect of different concentration of ST (A), L-NAME (B), and DPI (C) on α-amylase activity at 72 h after imbibition at 25°C of untreated control (UT) and magnetically treated (MT) Maize seeds. Vertical line above the bar indicates ± SEM. ***, ** and * indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with control (UT) and ###, ## and # indicate significance at P < 0.001, 0.01, and 0.05, respectively, compared with magnetically treated (MT) seed. Non-significant terms are not shown.
Discussion
Static magnetic field treatment positively affects seed germination and consequently induces seedling growth, seed vigour and crop yield.21 Germination characteristics of maize seeds exposed to magnetic field were enhanced in many studies.15,20,22,23 Previously a dose responsive enhancement of growth and germination parameters of soybean seeds was observed and found that 200 mT for 1 h was most effective among 50 mT, 100 mT, 150 mT and 200 mT, 250 mT and 300 mT for different time durations.24 Exogenous application of nitric oxide also has a strong stimulating effect on seed germination.7,25 The objective of the present study was to test whether magnetic field induced germination and seedling growth in maize is mediated by nitric oxide. Data presented by using specific inhibitors of NO production in vivo indicate that NO is involved in the signal transduction of magnetic field stimulated germination and seedling growth in maize.
NO is synthesized in plants by several enzymatic and non enzymatic pathways. One of the major pathway for the production of NO in plant is through the action of NAD(P)H- dependent nitrate or nitrite reductases.26 Sodium tungstate, an inhibitor of nitrate reductase activity also inhibits NO production by this pathway.27 The other enzymatic pathway for the production of NO in plants is through Nitric oxide synthase that catalyzes conversion of arginine to citrulline using NADPH and O2 and producing NO in the process.28 L-NAME inhibits the activity of NOS.28 Both of these inhibitors sodium tungstate and L-NAME have been shown to inhibit NO mediated physiologic processes in plants like prevention of ABA induced stomatal closure.29,30
Data presented here indicate prevention of germination in maize by the same inhibitors. The promotion of germination in magnetoprimed seeds is inhibited to a greater extent by the inhibitors indicating promotion of NO synthesis by magnetic field treatment. NO production is also closely related to the metabolism of ROS. ROS production is known to have a beneficial role during seed germination31 and release of dormancy. A close relationship between ROS, ABA and NO has been demonstrated in the germination of grass seeds.4 An inhibitor of NADPH oxidase enzyme that generates ROS like diphenyleneiodonium (DPI) inhibits germination and this inhibition is overcome by external H2O2 and NO.4 External H2O2 and NO also overcome the ABA induced negative effect on germination.4 Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity.32 DPI, an inhibitor of ROS also inhibits magnetic field induced promotion of germination of maize as presented here. Enhancement of ROS in soybean by direct EPR measurement of O2− and OH− radicals in the magneto primed soybean seeds has been demonstrated earlier in our laboratory.20
In contrast to the inhibitors SNP a donor of NO promotes MT induced growth of seedlings. The promotion is higher in MT seeds compared with untreated seeds. SNP has earlier been shown to promote seedling growth in wheat, lupin and Suaeda salsa seeds.9,31,33
Amylase is the major enzyme that is activated during germination in maize seeds and our data clearly shows an enhancement in this enzyme by MF and its inhibiotion by inhibitors of NO. In barley aleurone layers nitrate reductase activity is rapidly induced by nitrate treatment.34 NR activity in the aleurone layers contributes both to NO production and to an increased availability of nitrite for its non enzymatic conversion to NO.35 In wheat seeds NO activates β-amylase at early stages of seed germination and GA-dependent increased activity of α-amylase during later stages.36 Data presented here indicate, α-amylase activity promoted by magnetopriming of maize seeds is inhibited by all the three inhibitors, sodium tungstate, L-NAME and DPI. NO production by magnetic field treatment, thus contributes to the enhanced amylase activity in the maize seeds.
In conclusion exogenous application of inhibitors (sodium tungstate and L-NAME) and ROS inhibitor (DPI) negatively affects the promotion of germination, the hydrolytic activity of enzymes and seedling vigour in maize seeds promoted by magnetic field treatment. Thus signal transduction of magnetic field mediated through NO has the beneficial effects in magnetoprimed maize seeds. NO level and signal transduction is being worked out in detail.
Materials and methods
Seed material
The breeder seeds of maize (Zea mays var: GSF-2) were provided by the Jain seed agency in Indore, M.P., India. Seeds with uniform size and shape without any visible defects were selected. Moisture content of maize seeds was determined by oven drying seeds at 95°C to constant weight.37 Moisture content (%) was calculated by the formula [(W1-W2/W2)] × 100, where W1 was the initial weight of the seed and W2 was the final weight of the seed after drying. Moisture content was 6.14% in the seeds used for all treatments and experiments. Surface sterilized by soaking it in 0.01% HgCl2 for 10 minutes and washed thoroughly with distilled water. Surface sterilized seeds were placed in 15 cm Petri dishes containing filter paper moistened with 10 ml of treatment solution and grown in complete darkness at 26 ± 1°C for 8 d. After 8 days, germination data were taken.
Magnetic field generation
An electromagnetic field generator (Testron EM-20 Instruments, Delhi, India) was fabricated with a variable horizontal magnetic field strength (50–500 mT).38 Two cylindrical pole pieces with a diameter of 9 cm and a length of 16 cm were 5 cm apart from each other. The resistance of the coil was 16 V and there was 3000 turns per coil. A DC power supply (80 V/10 A) with a continuous variable output current was used for the electromagnet. The magnetic field strength produced in the pole gap was monitored by a digital gauss meter (Model DGM-30, Testron Instrument, Delhi, India), operating on the principle of the Hall Effect in semiconductors. The probe made of indium arsenide crystals is connected to the digital gauss meter by means of cable of suitable length. It was encapsulated in a non-magnetic sheet of 5 mm × 4 mm × 1 mm, which could measure 0– 2 T with full-scale range in increments of 5 mT.
Magnetic and chemical treatment
One hundred healthy maize seeds were placed in a cylindrical-shaped sample holder of 42 cm3 capacity, made from a non-magnetic thin transparent plastic sheet and exposed to a magnetic field of 200 mT (for 1 h). The required magnetic field strength was obtained by regulating the current in the coils of the electromagnet. A digital gauss meter was used to measure the strength of the magnetic field between the poles. The local geomagnetic field was <10 mT. All treatments in the experiments were run simultaneously along with control under similar conditions.
The sterilized seeds were placed in Petri dishes (15 cm diameter) containing filter paper moistened with 10 ml of treatment solutions and incubated at 26 ± 1°C in the dark. We investigated the effect of different concentration of nitric oxide inhibitors namely sodium tungstate (ST) and Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), NADPH oxidase inhibitor diphenyleneiodonium (DPI) and NO donor sodium nitroprusside (SNP) on untreated controls and magnetically treated seeds. The treatments were as follows: ST (0.1 mM, 0.5 mM and 1 mM), L-NAME (0.1 mM, 0.2 mM and 0.3 mM), DPI (0.01 mM, 0.05 mM and 0.1 mM) and SNP (0.1 mM, 0.25 mM and 0.5 mM).
Seed germination
Experiments were conducted using three replicates in three independent experiment with ten seeds in each Petri dishe containing moistened filter paper. Total 90 seeds were used in each experiment. Petri dishes were placed in a seed germinator (Remi Instruments, Mumbai, India) at 25°C in complete darkness for 8 d. After 8 days, ungerminated seeds were removed and only normal seedlings with well developed epicotyls, hypocotyls, and radicals were taken into account. Shoot length, root length, and whole seedling length were measured. Germination percentage was calculated based on normal seedlings. Subsequently, they were dried overnight in an oven at 60°C for 72 h and their dry weight was measured. Seedling vigor was calculated following Abdul-Baki and Anderson (1973)39 as:
Vigor index I = Germination% × Seedling length (Root + Shoot)
Vigor index II = Germination% × Seedling dry weight (Root + Shoot)
Determination of α-amylase content
α-amylase (EC 3.2.1.1) activity was assayed by the method described by Sawhney et al.40 in germinating seeds (100 mg) of maize at 72 h after imbibition. For the enzyme assay, 100 mg of seed tissue (after peeling) was ground in a prechilled mortar with 5 ml of 80% ice-cold acetone. The homogenates were centrifuged at 4°C for 10 min at 10,000 rpm and the supernatant was discarded and the pellets were dissolved in 0.02 M sodium phosphate buffer, and then centrifuged at 4°C for 20 min at 12,000 rpm. The supernatant was collected and used for α-amylase activity and protein content assays. The reaction mixture in a total volume of 5 ml contained 0.02 M (pH 6.4) sodium phosphate buffer, 0.01 % starch, 0.1 N HCl, I2KI (0.1%) and 200 microlitre enzyme extract. The absorbance was measured at 660 nm.
Statistical analysis
All experiments were conducted using three replicates at least. All of the germination parameters and biochemical parameters were performed within three independent experiments with three replicated measurements. Data are expressed as a mean ± SEM and were analyzed by one-way analysis of variance (ANOVA) followed by a post hoc Newman–Keuls multiple comparison test (*P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01, ###P < 0.001 ) using a Prism 4 software for Windows (GraphPad Software, La Jolla, CA, USA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Financial support by University Grant Commission-Junior Research Fellowship (UGC-JRF/ 2121330508; 22/12/2013(ii) EU-V) to Pinke Patel and Department of Science Technology Women Scientists-A Scheme (SR/WOS-/LS17/2017) to Dr. S. Kataria is thankfully acknowledged.
Author contributions
All the authors contributed in a significant way to this paper. Patel P. and Guruprasad K.N. had a major role in the design of the experiments, the coordination of the work and the writing of the paper. Patel P., Kataria S., and Baghel L. conceived, and performed the experiments and analyzed the data. Patel P., Guruprasad K.N. and Kataria S. read, corrected and approved the manuscript in its final form.
References
- 1.Schmidt HHWH, Walter U. NO at work. Cell. 1994;78:919–25. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 2.Klepper LA. Nitric oxide (NO) and nitrogen dioxide (NO2) emissions from herbicide treated soybean plants. Atoms Environ. 1979;13:537–42. doi: 10.1016/0004-6981(79)90148-3. [DOI] [Google Scholar]
- 3.Arasimowicz M, Floryszak-Wieczorek J. Nitric oxide as a bioactive signaling molecule in plant stress responses. Plant Sci. 2007;172:876–87. doi: 10.1016/j.plantsci.2007.02.005. [DOI] [Google Scholar]
- 4.Sarath G, Hou GC, Baird LM, Mitchell RB. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C-4 grasses. Planta. 2007;226: 697–708. doi: 10.1007/s00425-007-0517-z. [DOI] [PubMed] [Google Scholar]
- 5.Stohr C, Ullrich WR. Generation and possible roles of NO in plant roots and their apoplastic space. J Exp Bot. 2002;53:2293–2303. doi: 10.1093/jxb/erf110. [DOI] [PubMed] [Google Scholar]
- 6.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;35:36167–170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 7.Bethke PC, Libourel IGL, Jones RL. Nitric oxide in seed dormancy and germination In: Bradford K, Nonogaki H (eds) Seed development, dormancy and germination. Blackwell Publishing, Oxford: 2007; pp 153–75. doi: 10.1002/9780470988848.ch7. [DOI] [Google Scholar]
- 8.Hayat S, Yadav S, Alyemeni MN, Ahmad A. Effect of sodium nitroprusside on the germination and antioxidant activities of tomato (lycopersicon esculentum mill). Bulg J Agric Sci. 2014;20:140–44. [Google Scholar]
- 9.Kopyra M, Gwozdz E A. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol Biochem. 2003;4:1011–17. doi: 10.1016/j.plaphy.2003.09.003. [DOI] [Google Scholar]
- 10.Zhang-Shao Y, Ren-Xiao L, Cheng- Shun C. Effects of seed soaking with exogenous nitric oxide on the seed germination and the seedling growth of maize. Plant Physiol Commun. 2004;40:309–10. doi: 10.1016/j.plaphy.2003.09.003. [DOI] [Google Scholar]
- 11.Murabayashi S. Physical approach for immunomodulations. Tissue Culture Eng. (in Japanese). 1998;24:427–31. doi: 10.1088/1742-6596/344/1/012006. [DOI] [Google Scholar]
- 12.Sakamoto N, Ohashi T, Sato M. Effect of magnetic field on nitric oxide synthesis of cultured endothelial cells. Int J Appl Electromag Mech. 2002;14:317–22. [Google Scholar]
- 13.Salunke BP, Umathe SN, Chavan JG. Experimental evidence for involvement of nitric oxide in low frequency magnetic field induced obsessive compulsive disorder-like behavior. Pharmacol Biochem Behav. 2014; 122:273–8. doi: 10.1016/j.pbb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Cho SI, Nam YS, Chu LY, Lee JH, Bang JS, Kim HR, Kim HC, Lee YJ, Kim HD, Sul JD, Kim D, Chung YH, Jeong JH. Extremely low-frequency magnetic fields modulate nitric oxide signaling in rat brain. Bioelectromagnetics. 2012;33:568–74; Epub 2012 Apr 11. doi: 10.1002/bem.21715. [DOI] [PubMed] [Google Scholar]
- 15.Florez M, Carbonell MV, Martinez E. Exposure of maize seeds to stationary magnetic fields: Effects on germination and early growth. Environ Exp Bot. 2007;59:68–75. doi: 10.1016/j.envexpbot.2005.10.006. [DOI] [Google Scholar]
- 16.Podlesny J, Pietruszewski S, Podlesna A. Efficiency of the magnetic treatment of broad bean seeds cultivated under experimental plot Conditions. Int Agrophys. 2004;18: 65–71. [Google Scholar]
- 17.Podlesny J, Pietruszewski S, Podlesna A. Influence of magnetic stimulation of seeds on the formation of morphological features and yielding of the pea.Int Agrophys. 2005;19:1–8. [Google Scholar]
- 18.Iqbal1 M, Haq ZU, Jamil Y, Ahmad MR. Effect of presowing magnetic treatment on properties of pea. Int Agrophys. 2012;26:25–31. doi: 10.2478/v10247-012-0004-z. [DOI] [Google Scholar]
- 19.Pietruszewski S, Muszyñski S, Dziwulska A. Electromagnetic fields and electromagnetic radiation as non-invasive external stimulants for seeds (selected methods and responses) Int Agrophys 2007;21: 95–100. [Google Scholar]
- 20.Shine MB, Guruprasad KN, Anand A. Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics. 2012;33:428–37. doi: 10.1002/bem.21702. [DOI] [PubMed] [Google Scholar]
- 21.Pietruszewski S. Effect of magnetic seed treatment on yields of wheat. Seed Sci Technol. 1993;21:621–6. [Google Scholar]
- 22.Pittman UJ. Magnetism and plant growth III. Effect on germination and early growth of corn and beans. Can J Plant Sci. 1965;45:549–554. doi: 10.4141/cjps65-106. [DOI] [Google Scholar]
- 23.Aladjadjiyan A, Ylieva T. Influence of stationary magnetic field on the early stages of the development of tobacco seeds (Nicotiana tabacum L.). J Cent Eur Agric. 2003;4:132–7. [Google Scholar]
- 24.Shine MB, Guruprasad KN, Anand A, Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics. 2011;32:474–84. doi: 10.1002/bem.20656. [DOI] [PubMed] [Google Scholar]
- 25.Beligni MV, Lamattina L. Nitric oxide induces seed germination and de-etiolation, and inhibits hypocotyls elongation, three light-inducible responses in plants. Planta. 2000;210:215–21. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- 26.Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci. 1999;4:128–29. doi: 10.1016/S1360-1385(99)01393-X. [DOI] [PubMed] [Google Scholar]
- 27.Shrivastava N, Gonugunta VK, Puli MR, Raghavendra AS. Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta. 2009;229:757–65. doi: 10.1007/s00425-008-0855-5. [DOI] [PubMed] [Google Scholar]
- 28.Wojtaszek P. Nitric oxide in plants: to NO or not to NO. Phytochem. 2000;54:1–4. doi: 10.1016/S0031-9422(00)00056-X. [DOI] [PubMed] [Google Scholar]
- 29.Gonugunta VK, Shrivastava N, Puli MR, Raghavendra AS. Nitric oxide production occurs after cytosolic alkalinization during stomatal closure induced by abscisic acid. Plant Cell Environ. 2008;31:1717–24. doi: 10.1111/j.1365-3040.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 30.Neill SJ, Desikan R, Clarke A, Hancock JT. Nitric oxide is a novel component of abscisic acid signalling in stomatal guard cells. Plant Physiol. 2002;128:13–6. doi: 10.1104/pp.010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirovaa J, Sedlarovab M, Piterkovaa J, Luhovaa L, Petrivalsky M. The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci. 2011;181:560–72. doi: 10.1016/j.plantsci.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Zheng C, Jiang D, Liu F, Dai T, Liu W, Jing Q, Cao W. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ Exp Bot. 2009;67:222–227. doi: 10.1016/j.envexpbot.2009.05.002. [DOI] [Google Scholar]
- 33.Li W, Liu X, Ajmal Khan M, Yamaguchi S. The effect of plant growth regulators, nitric oxide, nitrate, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions, J Plant Res. 2005;118:207–214. doi: 10.1007/s10265-005-0212-8. [DOI] [PubMed] [Google Scholar]
- 34.Simontacchi M, Jasid S, Puntarulo S. Enzymatic sources of nitric oxide during seed germination, in: Lamattina L., Polacco J. (Eds.), Nitric Oxide in Plant Growth, Development and Stress Physiology, Springer, Berlin/Heidelberg, 2007; pp73–90. [Google Scholar]
- 35.Bethke PC, Badger MR, Jones RL. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell. 2004;16:332–41. doi: 10.1105/tpc.017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Shen WB, Zhang W, Xu LL. A rapid response of beta-amylase to Nitric oxide but not gibberellin in wheat seeds during the early stage of germination, Planta. 2005;220:708–16. doi: 10.1007/s00425-004-1390-7. [DOI] [PubMed] [Google Scholar]
- 37.Walters C. Understanding the mechanism and kinetics of seed ageing. Seed Sci Res. 1998;8:223:244. doi: 10.1017/s096025850000413X [DOI] [Google Scholar]
- 38.Vashisth A, Nagarajan S. Exposure of seeds to static magnetic field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.) Bioelectromagnetics. 2008;29:571–8. doi: 10.1002/bem.20426. [DOI] [PubMed] [Google Scholar]
- 39.Abdul-Baki AA, Anderson JD. Vigour determination in soybean by multiple criteria. Crop Sci. 1973;13:630–3. doi: 10.2135/cropsci1973.0011183x001300060013x. [DOI] [Google Scholar]
- 40.Sawhney S, Toky KL, Nanda KK. Changes in amylase activity during extension growth and floral induction in Impatiens balsamina a qualitative short day plant. Indian J Plant Physiol. 1970;13:198. [Google Scholar]