Abstract
Insulin detemir (DET) is a basal insulin analog that, in contrast to other long-acting forms of insulin, has significant weight-gain-sparing effects in diabetic patients. We hypothesized that this effect of DET may be due to its enhanced catabolic action in the central nervous system. We investigated the long-term effects of single third ventricular (3V) microinjections of equimolar doses of DET and regular insulin in normal male rats on feeding, body weight, energy expenditure (EE), and respiratory quotient (RQ). Also, in acute testing, we assessed the ability of lower doses of DET to alter feeding, EE, and RQ when microinjected directly into the paraventricular nucleus (PVN). The anabolic peptide ghrelin served as a positive control in acute testing. 3V administration of both DET (0.5–2.0 mU) and regular insulin (2.0–8.0 mU) significantly reduced feeding and body weight over 48 and 120 h, respectively, with DET yielding greater inhibitory effects. DET also stimulated greater elevations of EE and reductions of RQ over 72 and 48 h postinjection, respectively. In acute (4 h) testing, microinjections of DET (0.5 mU) into the PVN reduced feeding, increased EE, and reduced RQ, while ghrelin (100 pmol) had the opposite effects. When administered sequentially into the PVN, DET (0.25 and 0.5 mU) reversed ghrelin-induced feeding, EE, and RQ effects. These data support the notion that the weight-sparing effect of DET is at least in part based on its central catabolic action and that enhanced EE and reduced RQ may participate in this effect.
Keywords: blood brain barrier, hypothalamus, paraventricular nucleus, insulin signaling, ghrelin, oxygen consumption, respiratory quotient
insulin detemir (DET) is a basal, long-acting insulin analog in wide clinical use (11). DET is unique in that its effect is achieved by addition of a fatty acid chain to the basic insulin molecule (26). This alteration promotes the binding of DET to blood albumin, which prolongs its life in the circulation and selectively enhances its uptake into hepatic and central nervous system (CNS) tissue (13, 25, 26). Clinical trials have demonstrated that DET has a significant weight-gain-sparing effect in diabetic patients, in contrast to other long-acting forms of insulin (14, 15). However, the mechanism underlying this effect remains unknown. Among the hypotheses proposed for the weight-sparing effect of DET is the possibility of enhanced CNS catabolic effects of this insulin analog (14, 15, 23).
It is well established that the effects of insulin administered centrally are catabolic, in distinction to its anabolic effects when administered peripherally. Numerous studies have shown that both acute and chronic CNS administration of regular insulin reduce feeding and body weight in rats (1, 6, 7, 17, 19, 21). In addition Begg et al. (5) recently demonstrated greater feeding and body weight gain inhibition in rats microinjected in the third ventricle (3V) with DET, in comparison with neutral protamine Hagedorn (NPH) insulin, a long-acting suspension of regular insulin. Since the weight regulatory effects of insulin are known to be mediated by the same hypothalamic signaling pathway utilized by leptin (30), it remains possible that activation of thermogenesis via sympathetic nervous system outflow may also be involved in insulin’s catabolic effects. Indeed, the results of the first study to demonstrate the weight-reducing effects of centrally administered insulin in rats identified enhanced energy expenditure (EE) as contributing to the effect (6). Consistent with these results, chronic 3V administration of insulin has been shown to activate brown adipose tissue (18). Thus the catabolic effects of DET may include not only feeding inhibition but also enhanced EE.
As noted above, the catabolic effects of DET may in part be due to its selective uptake into CNS tissue. Enhanced CNS access and prolonged tissue residency, however, may not represent the only mechanisms by which DET acts to inhibit body weight gain. Hennige et al. (13) demonstrated that in C57BL/6 mice insulin signaling occurred faster and was significantly enhanced in hypothalamic and cerebrocortical tissue, while total insulin concentration in the brain was increased severalfold, following intravenous administration of equivalent doses of DET and regular insulin. Insulin signaling was determined by tyrosine phosphorylation of insulin receptor and insulin receptor substrate-2 proteins. This result contrasted with the systemic effects of the two insulin forms, which showed no difference in the extent or time course of insulin signaling in several peripheral tissues. Thus DET may also act to enhance insulin receptor signaling in the brain.
The feeding- and body weight-inhibitory effects of centrally administered insulin are known to be based on activation of the insulin receptor substrate-phosphatidylinositol 3-kinase pathway in the arcuate nucleus (Arc) of the hypothalamus (19). However, these effects may not be restricted to the Arc. Both insulin and ghrelin receptors are present in the paraventricular nucleus (PVN) of the hypothalamus (4, 34), and insulin microinjected into the PVN inhibits feeding (17). Moreover, the PVN has been shown to be the site of insulin-induced activation of peripheral sympathetic pathway signaling (24, 31). Thus the PVN may be involved not only in feeding inhibition but also in metabolic alterations in response to insulin.
In the present study, we sought to determine the effects of DET and regular insulin at hypothalamic feeding-related sites, independent of the hormone’s peripheral or CNS uptake characteristics. Although the effects of central injections of DET have previously been compared with those of NPH insulin in rats (5), our intent was to compare the potency of regular insulin in the brain to that of this altered form of the insulin molecule (DET). To accomplish this, we compared the long-term feeding, body weight, and metabolic effects of equimolar doses of regular insulin and DET in response to single 3V microinjections of each peptide in intact rats. Based on earlier findings (5, 13), we hypothesized that central administration of DET would be more effective than regular insulin on these measures. Furthermore, in acute testing utilizing low doses of DET, we sought to determine whether DET, when microinjected directly into the PVN, would be capable of inducing both feeding inhibition and metabolic alterations. We used the anabolic peptide ghrelin as a positive control, and in sequential injection testing, also determined the ability of DET to inhibit ghrelin’s feeding-stimulatory and metabolic effects. To control for injection site, we compared PVN responses to the effects of the exact same doses of each peptide when administered into the 3V.
METHODS
Animals and maintenance.
Adult male Sprague-Dawley rats (Charles River, Wilmington MA) weighing 250–275 g upon receipt were housed in individual polypropylene living cages and maintained on laboratory chow (Purina 3001) and water ad libitum in a temperature-controlled (21 ± 1°C) room with a 12:12-h light-dark cycle (lights on at 0600). A total of 68 rats were used for this study, with 55 rats completing testing. Food intake and body weight were monitored at 24- to 48-h intervals throughout a 3-wk habituation period, and the animals were handled daily. During the two experimental periods (long-term and acute microinjection testing), food intake and body weight were measured daily in midafternoon, and food intake was corrected for spillage. However, when the rats were tested in the metabolic apparatus, food and water were not provided, since any consumption would alter the metabolic variables being measured. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Columbia University and Reed College.
Stereotaxic surgery.
For 3V microinjection, rats were stereotaxically implanted with 26-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) aimed at the 3V in the region the hypothalamus. Details of the surgical procedure have been published previously (16). Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg ip) and placed in a Kopf stereotaxic frame with the incisor bar set to −3.5 mm relative to the interaural line. Guide cannulas were implanted 4 mm dorsal to the target using the following coordinates relative to bregma (20): posterior: 1.4 mm, lateral: 0 mm, and ventral: 3.8 mm. The cannula apparatus was held in place with acrylic cement anchored to three stainless steel screws. Guide cannulas were fitted with removable 28-gauge stainless steel stylets to maintain patency. Rats were given a 2-wk recovery period before testing, during which the animals were habituated to the microinjection test procedure via repeated mock injections. For PVN microinjection, similar surgical procedures were used. Briefly, chronic indwelling 22-gauge guide cannulas were unilaterally implanted in all animals and aimed at the PVN. Cannulas were implanted 4 mm dorsal to the PVN, as described previously (8), according to the following coordinates relative to bregma: posterior: 1.8 mm, lateral: ±0.4 mm, and ventral: 4.0 mm. Groups with cannulas implanted into the 3V were used as controls for PVN microinjection effects. Following microinjection testing, the accuracy of all cannula placements was confirmed via histological assessment, as previously described (10). Only data from rats with correct cannula placements were utilized in analysis of results.
Dose determinations.
For long-term testing, the effects of 3V microinjections of DET and regular insulin were compared over 120 h (feeding and body weight) and 72 h (metabolic rate) postinjection periods. A ratio of 1:4 was used for determining equimolar doses of DET and regular insulin, respectively. In human peripheral testing, the potency of DET has been determined to be 25% of that of regular insulin (27). Thus one unit of DET is equivalent to 24 nmol, while one unit of regular insulin is equivalent to 6 nmol (27). As we wanted to replicate the dose conditions most likely in effect that promoted the weight-sparing effect of DET in humans, we chose to use this potency ratio to determine the molar concentration of our doses of insulin for microinjection into rats. Pilot 3V testing indicated that both forms of insulin were effective in the low-milliunit (mU) range; thus 3V doses of 0 (vehicle), 0.5, 1.0, and 2.0 mU of DET were compared with doses of 0, 2.0, 4.0, and 8.0 mU of regular insulin. Artificial cerebrospinal fluid (aCSF) was used as the vehicle, and all 3V microinjections were 1.0 µl in volume. DET was supplied as Levemir (100 U/ml) by Novo Nordisk, and regular human insulin was purchased (Humulin Regular; 100 U/ml; Eli Lilly, Indianapolis, IN). The diluents and stabilizers used in the two insulin solutions as received were identical in type and concentration, and the pH of the solutions was closely comparable (pH ~7.4). For acute testing, the effects of lower doses of DET and/or ghrelin over a 4-h postinjection period in both PVN and 3V groups were determined. A ghrelin dose known to robustly stimulate feeding when microinjected into the PVN (100 pmol) was selected (10). Acylated rat ghrelin was purchased from Phoenix Pharmaceuticals and dissolved in sterile saline. Following preliminary testing, two doses of DET were selected for PVN and 3V microinjection testing (0.25 and 0.5 mU).
Feeding and body weight test protocols.
For long-term testing, two separate groups of rats (n = 6) were administered single microinjections of the three equimolar doses of DET or regular insulin in ascending order at one week intervals. We chose to use independent groups for administration of the two forms of insulin due to the known long carry-over effects of CNS insulin administration (6). On test days, food was removed from the rats’ cages at 1000. Microinjections were administered at 1600, immediately after which the animals were restored to their home cages with food available. The food intake and body weight of the animals were measured before, and at 24-h intervals following, each dose for 5 successive days. For acute food intake testing, separate groups of rats with 3V or PVN cannula were used (n = 7 and 8, respectively). On test days, food was removed from the rats’ cages at 1400. The rats were microinjected with vehicle or DET 60 min before administration of vehicle or ghrelin at dark onset (1800) and then restored to their home cages with food available. Food intake was measured at 4 h postinjection. For the PVN group, six sequential microinjection tests were administered with the injection volume set at 0.2 µl: vehicle/vehicle, DET 0.25 mU/vehicle, DET 0.50 mU/vehicle, vehicle/100 pmol ghrelin, DET 0.25 mU/100 pmol ghrelin, and DET 0.50 mU/100 pmol ghrelin. An interval of 4 days separated each test. For the 3V group, the same test sequence was administered, with injection volume set to 1.0 µl.
Measurement of EE and respiratory quotient.
Additional groups of rats were tested for the effects of DET, regular insulin and ghrelin on EE and respiratory quotient (RQ). In all cases, rats were acclimated to rodent metabolic chambers before the start of testing. For long-term testing, two groups of 3V-implanted rats (n = 6) received single microinjections of 0, 0.5 and 1.0 mU of DET or 0, 2.0, and 4.0 mU of regular insulin in ascending order at 1-wk intervals. On test days, food was removed from the rats’ cages at 1000. Before the administration of each dose, the rats were placed in the metabolic chambers in mid-afternoon (1400), and mean 3-h baseline rates of O2 utilization and CO2 production were determined. The dose was then administered in a volume of 1 μl, and the animals were returned to their home cages with food available. Additional 3-h O2 and CO2 measurements were made in the metabolic chambers 24, 48, and 72 h later. Food was not available in the metabolic chambers during testing. For acute EE and RQ testing, two additional groups of male rats were implanted with 3V or PVN cannula (n = 7 and 9, respectively). On test days, food was removed from the animals’ cages at 1000, and they were injected at 1400 with the same doses of DET and ghrelin used in acute feeding testing. Animals were placed in metabolic chambers immediately following microinjections, and measures of O2 and CO2 were taken continuously for 4 h. Food was not available during testing. The first microinjection of vehicle/vehicle was used to establish baseline rates of O2 and CO2 utilization and emission, respectively. All metabolic testing was conducted in specially designed rodent metabolic chambers in which the flow of O2 and CO2 was continuously monitored with online detectors, as described previously (8). Oxyscan open-circuit indirect calorimeters (AccuScan Instruments, Columbus, OH) were used. Three variables were analyzed statistically following testing: whole animal EE, RQ, and volume of oxygen consumed (Vo2; metabolic rate). RQ was calculated as the volume of CO2 produced (Vco2) divided by the volume of O2 consumed (Vo2). EE was computed as EE (kJ) = liters O2 × (364 + 113 × RQ)/22.4.
Data analyses.
Food intake, body weight, EE, and RQ measures at the indicated intervals were analyzed with two and three-way repeated-measures ANOVA, followed by comparisons of individual means by post hoc analysis where appropriate (Newman-Keuls test). Specifically, three-way repeated-measures ANOVAs were carried out on absolute food intake, food intake difference from aCSF, and body weight change scores obtained in the long-term study (insulin type × dose × hours postinjection, Figs. 1, 2, and 3, respectively). The same design was used for separate analysis of Vo2, EE, and RQ scores obtained in the long-term study (Fig. 4). Food intake, Vo2, EE, and RQ scores obtained in the acute study were analyzed using two-way repeated measures ANOVAs (anatomical site × dose, Figs. 5, 6, and 7). Statistical significance was set at a level of P < 0.05 or smaller. Data are presented as group means ± SE.
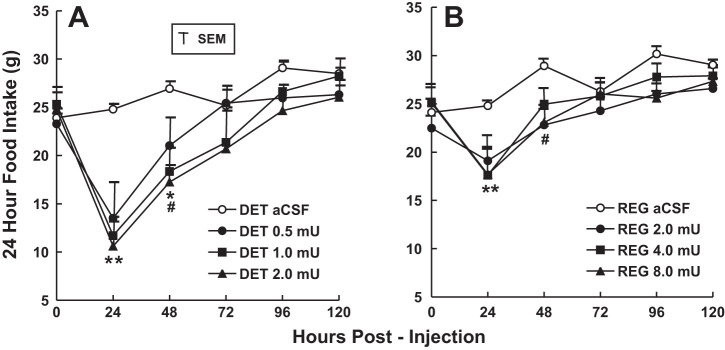
Fig. 1.
Absolute 24-h food intake over 5 days of groups of rats administered single 3rd ventricular (3V) microinjections of insulin detemir (A) or regular insulin (B) in ascending dose order. Two groups of rats (n = 6) received equimolar doses of either insulin detemir (DET) or regular insulin, with microinjection tests separated by 1 wk. **P < 0.01, all doses decreased vs. artificial cerebrospinal fluid (aCSF) condition; #P < 0.01, mid and high doses decreased vs. aCSF control condition; *P < 0.05, low dose decreased vs. aCSF control condition.
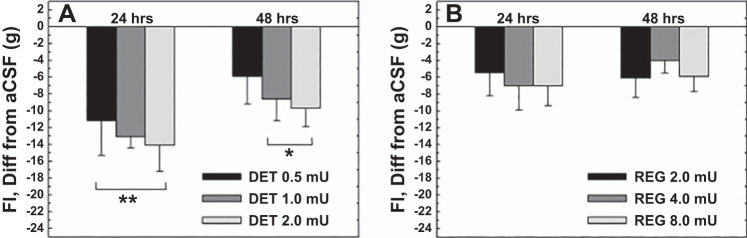
Fig. 2.
Reductions of 24-h food intake compared with control (aCSF) feeding (set to zero) by separate groups of rats (n = 6) administered either insulin DET (A) or regular insulin (B) over the 1st 48 h postmicroinjection. Reductions were significantly greater following administration of equimolar doses of insulin DET compared with regular insulin at all 3 doses for the 1st 24 h and at the mid and high doses at 48 h. **P < 0.01, all 3 doses different from regular insulin, 24 h; *P < 0.05, mid and high doses different from regular insulin, 48 h.
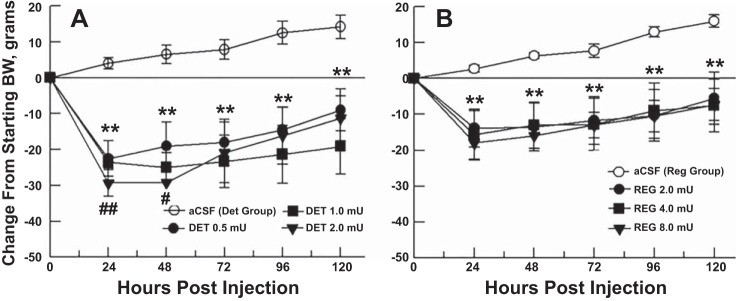
Fig. 3.
Changes in body weight of the groups, calculated from starting body weight (set to zero), in response to a single microinjection of aCSF (control), or 3 doses of insulin DET (A) or regular insulin (B) over 5 postinjection days. For both insulin groups (n = 6), body weight reductions were significantly different from control level at all 3 doses and all time points. **P < 0.01, all doses different from aCSF; ##P < 0.01, all doses different from regular insulin at same time point; #P < 0.05, mid and high doses different from regular insulin at same time point.
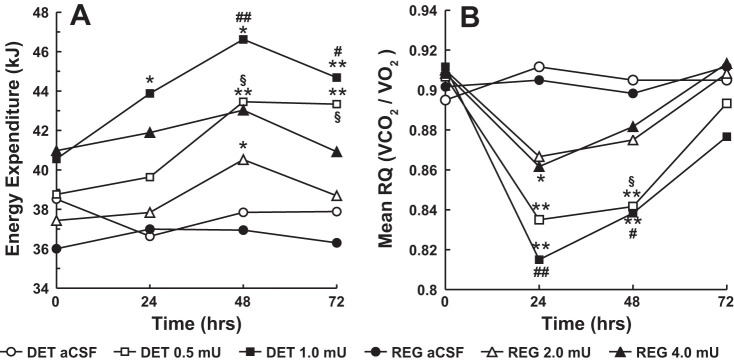
Fig. 4.
Energy expenditure (EE; A) and respiratory quotient (RQ; B) scores over 72 h postinjection for separate groups of rats (n = 6) microinjected into the 3V with single equimolar doses of DET (0, 0.5, and 1.0 mU) or regular insulin (0, 2.0 and 4.0 mU) in ascending order in a volume of 1.0 µl. aCSF served as the vehicle for the insulin and was used as the 0-mU dose. Doses were administered at 1-wk intervals. Following administration of each dose, rates of O2 utilization and CO2 production were measured for a 3-h period each 24 h over 24-72 h and used for calculation of EE and RQ values of the animals. Baseline (0 h) measurements were obtained in a 3-h session before microinjection of each dose. Food was not available in the metabolic chambers during testing. Group mean EE and RQ values are represented. A: *P < 0.05, different from aCSF baseline; **P < 0.01, different from aCSF baseline; #P < 0.05, different from 4.0 mU regular insulin; ##P < 0.01, different from 4.0 mU regular insulin; §P < 0.05, different from 2.0 mU regular insulin. B: *P < 0.05, different from aCSF baseline; **P < 0.01, different from aCSF baseline; #P < 0.05, different from 4.0 mU regular insulin; ##P < 0.01, different from 4.0 mU regular insulin; §P < 0.05, different from 2.0 mU regular insulin.
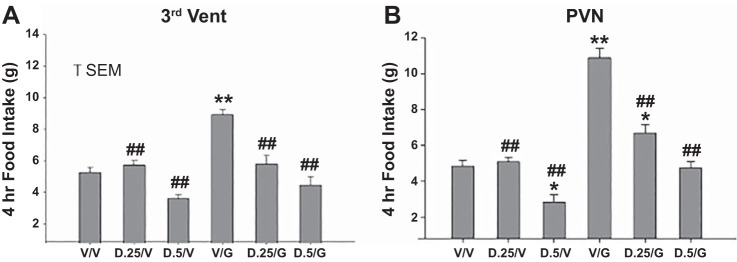
Fig. 5.
Feeding by groups of rats in response to two sequential microinjections of vehicle, 0.25 or 0.5 mU of DET and vehicle, ghrelin (100 pmol) and vehicle, or combinations of DET and ghrelin. Microinjections were administered to two separate groups at 3V (A) or paraventricular nucleus (PVN) (B) sites (n = 7 and 8, respectively). Two microinjections separated by 60 min were made, with feeding monitored for 4 h following the 2nd microinjection. Injection combinations were as follows: V/V, vehicle/vehicle; D.25/V, 0.25 mU DET/vehicle; D.5/V, 0.5 mU DET/vehicle; V/g, vehicle/ghrelin; D.25/g, 0.25 mU DET/ghrelin; D.5/g, 0.5 mU DET/ghrelin. Significance levels are as follows: *P < 0.05, different from V/V; **P < 0.01, different from V/V; ##P < 0.01, different from V/g.
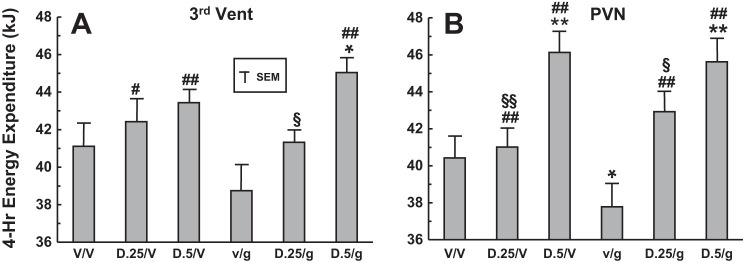
Fig. 6.
EE in groups of rats in response to two sequential microinjections of vehicle, 0.25 or 0.5 mU of DET and vehicle, ghrelin (100 pmol) and vehicle, or combinations of DET and ghrelin. Microinjections were administered to two separate groups at 3V (A) or PVN (B) sites (n = 7 and 9, respectively). Two microinjections separated by 60 min were made, with O2 utilization and CO2 production measured over 4 h following the 2nd microinjection, and used for determination of EE. Injection combinations were as follows: V/V, vehicle/vehicle; D.25/V, 0.25 mU DET/vehicle; D.5/V, 0.5 mU DET/vehicle; V/g, vehicle/ghrelin; D.25/g, 0.25 mU DET/ghrelin; D.5/g, 0.5 mU DET/ghrelin. Significance levels are as follows: *P < 0.05, different from V/V; **P < 0.01, different from V/V; #P < 0.05, different from V/g; ##P < 0.01, different from V/g; §P < 0.05, different from D.5/g; §§P < 0.01, different from D.5/V.
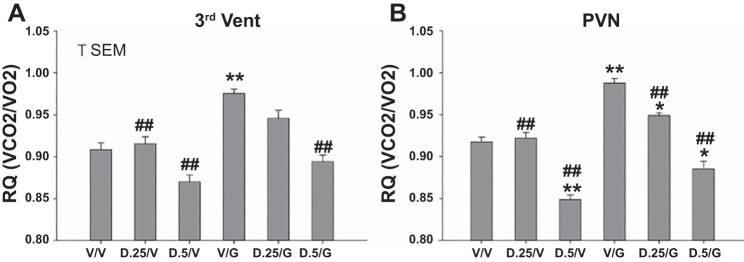
Fig. 7.
Respiratory quotient (RQ) of groups of rats in response to two sequential microinjections of vehicle, 0.25 or 0.5 mU of DET and vehicle, ghrelin (100 pmol) and vehicle, or combinations of DET and ghrelin. Microinjections were administered to two separate groups at 3V (A) or PVN (B) sites (n = 7 and 9, respectively). Two microinjections separated by 60 min were made, with RQ determined at 4 h following the 2nd microinjection. Injection combinations were as follows: V/V, vehicle/vehicle; D.25/V, 0.25 mU DET/vehicle; D.5/V, 0.5 mU DET/vehicle; V/g, vehicle/ghrelin; D.25/g, 0.25 mU DET/ghrelin; D.5/g, 0.5 mU DET/ghrelin. Significance levels are as follows: *P < 0.05, different from V/V; **P < 0.01, different from V/V; ##P < 0.01, different from V/g.
RESULTS
Long-term feeding and body weight effects of single 3V microinjections of DET or regular insulin.
Both DET and regular insulin significantly reduced 24-h feeding of the groups for 2 days following a single 3V microinjection (Fig. 1). All three doses of each form of insulin reduced feeding below control (aCSF) levels at 24 h postinjection (P < 0.01), as did the mid and high doses at 48 h postinjection (P < 0.01). No further significant effects were seen after 48 h, and intake gradually returned to control levels over the remaining 3 days. Feeding by the DET- injected group was reduced compared with feeding by the regular insulin group for all doses at 24 h (P < 0.01) and for the mid and high doses at 48 h (P < 0.05, see Fig. 2). A tendency for a dose-response effect of DET on feeding at 24 and 48 h proved nonsignificant in both cases (Fig. 2). Both DET and regular insulin reduced the body weight of the groups at all three doses over the entire 5-day postinjection period, in comparison with control (aCSF) body weight levels (P < 0.01, Fig. 3). Body weight reductions were greatest at 24 h postinjection and gradually decreased for the groups over the following 4 days. Body weight reductions were greater for the DET compared with regular insulin groups for all three doses at 24 h postinjection (P < 0.01) and remained greater for DET for the mid and high doses at 48 h postinjection (P < 0.05).
Long-term metabolic effects of single 3V microinjections of DET or regular insulin.
The metabolic effects of 3V microinjections of DET or regular insulin on EE over the 72-h observation period are shown in Fig. 4A. Although we observed a widespread of 0-h (baseline) EE values among the groups, there were no significant differences in baseline EE values between DET and regular insulin groups treated under the same experimental condition. Thus we were able to compare changes of EE from baseline, and differences of absolute EE scores, of DET and regular groups under the same treatment conditions. 3V microinjections of DET significantly elevated EE above levels seen following aCSF at 24, 48, and 72 h postinjection for both the 0.5- and 1.0-mU doses (P < 0.05 or 0.01, Fig. 4A). In contrast, microinjections of regular insulin at 2.0 and 4.0 mU failed to significantly elevate EE above aCSF levels, with the exception of the 2.0-mU dose at 48 h (P < 0.01). Elevations of EE at 24, 48, and 72 h stimulated by 1.0 mU DET were greater than those seen for regular insulin at the 4.0-mU dose (P < 0.05 or <0.01), while the elevation of EE at 48 and 72 h by 0.5 mU DET was greater than that stimulated by 2.0 mU of regular insulin (P < 0.05). 3V microinjections of both 0.5 and 1.0 mU DET decreased RQ at 24 and 48 h postinjection compared with levels following aCSF (P < 0.01). This effect was also seen following regular insulin at 2.0- and 4.0-mU doses, but only the latter dose decreased RQ relative to baseline (P < 0.05) at 24 h postinjection. DET at the 1.0-mU dose was more effective in decreasing RQ at 24 and 48 h postinjection than 4.0 mU of regular insulin (P < 0.01 and 0.05, respectively), while 0.5 mU DET decreased RQ at 48 h more than 2.0 mU of regular insulin (P < 0.05). In summary, a single 3V microinjections of DET at 0.5 and 1.0 mU significantly elevated EE and decreased RQ over postinjection intervals of 72 and 48 h, respectively, and these effects were significantly greater than those seen for 2.0 and 4.0 mU of regular insulin.
Acute effects of DET and ghrelin microinjections on feeding at 3V and PVN sites.
At both the 3V and PVN sites, 4-h feeding following administration of 0.25 or 0.5 mU DET plus vehicle was not altered in comparison with vehicle alone, with the exception of a significant reduction at the 0.5-mU dose of DET in the PVN group (P < 0.05). In contrast, feeding following administration of ghrelin alone at both sites was significantly elevated in comparison with vehicle alone (P < 0.01, Fig. 5). At both sites, feeding following administration of 0.25 or 0.5 mU DET, whether followed by vehicle or ghrelin, was reduced in comparison with feeding following vehicle plus ghrelin (P < 0.01). However, at the PVN site, feeding following 0.25 mU DET plus ghrelin remained greater than that following vehicle alone (P < 0.05). Overall, while ghrelin plus vehicle significantly elevated feeding in comparison with vehicle alone, both doses of DET significantly reduced ghrelin-induced feeding at the PVN and 3V sites. Also at the PVN site, DET at the 0.5-mU dose significantly reduced feeding compared with vehicle alone. Note that the profiles of feeding stimulation and inhibition induced by vehicle and peptide combinations were remarkably similar at the PVN and 3V microinjection sites.
Acute effects of DET and ghrelin microinjections on EE and RQ at 3V and PVN sites.
At both 3V and PVN sites, 4-h postinjection EE following administration of 0.25 or 0.5 mU DET plus vehicle was not altered in comparison with vehicle alone, with the exception of a significant elevation of EE at the 0.5-mU dose of DET plus vehicle in the PVN group (P < 0.01, Fig. 6). In contrast, EE following administration of vehicle plus ghrelin at the PVN site was significantly reduced in comparison with vehicle alone (P < 0.05). Also, at the PVN site, 0.5 mU DET followed by ghrelin elevated EE in comparison with vehicle alone (P < 0.01). Finally at the PVN site, microinjections of 0.25 and 0.5 mU DET, whether followed by vehicle or ghrelin, increased EE significantly in comparison with vehicle plus ghrelin administration (P < 0.01). At the 3V site, similar significant effects of DET plus vehicle or ghrelin in elevating EE compared with vehicle plus ghrelin were seen (P < 0.05 or 0.01), with the exception of the 0.25-mU dose of DET plus ghrelin. Finally, at both 3V and PVN sites, the 0.25-mU dose of DET plus vehicle elevated EE less than the 0.5-mU dose plus vehicle (P < 0.05 or 0.01), suggesting a dose-response effect. Overall, at the PVN site, while 0.5 mU DET plus vehicle significantly elevated EE in comparison with vehicle alone, ghrelin administration significantly decreased EE in comparison with vehicle alone, while administration of DET plus ghrelin significantly reversed the effects of ghrelin on EE. Again, the response profiles to vehicle and/or peptide administration at the 3V and PVN microinjection sites were quite similar. Separate analysis of Vo2 data for the groups resulted in almost identical significant differences between the groups (data not shown).
At both 3V and PVN sites, 4-h postinjection RQ following administration of 0.25 or 0.5 mU DET plus vehicle was not altered in comparison with vehicle alone, with the exception of a significant decrease of RQ at the 0.5-mU dose of DET plus vehicle in the PVN group (P < 0.01). In contrast, RQ following administration of ghrelin alone at both sites was significantly elevated in comparison with vehicle alone (P < 0.01, respectively, Fig. 7). At the PVN site, 0.5 mU DET followed by ghrelin decreased RQ in comparison with vehicle alone (P < 0.05), while the opposite effect was observed for 0.25 mU DET plus ghrelin at the PVN site (significant increase of RQ over vehicle alone, P < 0.05). Also at the PVN site, microinjections of 0.25 and 0.5 mU DET, followed by vehicle or ghrelin, partially or completely reversed RQ values significantly in comparison with vehicle plus ghrelin administration (P < 0.01). Similar significant effects of DET plus vehicle or ghrelin were seen at the 3V site (P < 0.01), with the exception of the 0.25-mU dose of DET followed by ghrelin, which failed to alter RQ level in comparison with vehicle alone or vehicle plus ghrelin. Overall, administration of ghrelin plus vehicle at both hypothalamic sites significantly elevated RQ, while microinjections of DET plus ghrelin were, with one exception, able to reverse ghrelin-induced increases of RQ. Also, 0.5 mU DET plus vehicle at the PVN site significantly decreased RQ in comparison with vehicle alone. Again, almost identical profiles of responsiveness to vehicle and/or peptide administration were seen for the two microinjection sites, with effects somewhat more robust at the PVN site.
DISCUSSION
Both DET and regular insulin decreased the food intake and body weight of normal male rats following single microinjections into the 3V, with significant decreases observed up to 48 and 120 h postinjection, respectively. In support of our initial hypothesis, central administration of equimolar doses of DET resulted in significantly greater reductions of feeding and body weight in comparison with regular insulin over the first 48 h postinjection, perhaps due to the ability of DET to enhance CNS receptor second messenger signaling, as reported by Hennige et al. (13). Since in this study both types of insulin were delivered directly into the 3V in equimolar amounts, differences in the systemic distribution and CNS uptake were not a factor in the enhanced effects of DET. Reductions of food intake and body weight induced by 3V microinjection of both types of insulin were large and relatively long lasting. This is consistent with previous reports. For example Niswender et al. (19), in results quite comparable with ours, observed significant decrements of 24-h food intake (−14 g) and body weight (−13 g) in male rats administered 4.0 mU of regular insulin into the 3V. Begg et al. (5) reported reductions of feeding and body weight in response to 3V microinjections of 8 mU DET of −5 and −6 g, respectively, 24 and 48 h following administration. Consistent with our results, reductions with DET were significantly greater than those seen by Begg et al. with NPH insulin at the same intervals.
In agreement with our earlier results (9), microinjections of ghrelin significantly increased feeding over a 4-h postinjection period at both 3V and PVN sites. In contrast, administration of relatively low doses of DET at the same sites had the opposite effect, resulting in a significant inhibition of feeding. Furthermore, pretreatment with DET at both the 3V and PVN sites significantly reversed ghrelin-induced feeding. An earlier study (19) identified the Arc as the site of action of insulin’s central anorectic effects. While we cannot rule out the involvement of the Arc in the feeding inhibitory effects of DET administered to the PVN, since diffusion from the PVN injection site remains a possibility, our results are consistent with the possibility that the neural pathway mediating the central anorectic effects of insulin includes the PVN.
The long-term effects of 3V administration of DET and regular insulin on feeding and body weight were paralleled by elevations of EE and reductions of RQ from 24 to 72 h postinjection, which were highly significant in the case of DET. Decreases of RQ are indicative of increased fat oxidation and, along with elevated EE, characterize insulin’s catabolic effects. Indeed, elevations of EE stimulated by DET showed little sign of diminishing at 72 h postinjection (Fig. 4) and may well underlie the slow recovery of body weight seen in the DET-injected animals despite a return of feeding to control levels at 72 h and beyond. Thus in our study 3V administration of DET stimulated both aspects of insulin’s well-known central catabolic effects and did so more effectively than regular insulin in equimolar doses at the same injection site. The extent to which each process may have contributed to body weight loss in response to administration of DET and regular insulin cannot be determined, however, based solely on measurements made in this study.
We have previously demonstrated a significant increase in RQ in rats microinjected with ghrelin in both the Arc and PVN (9). Consistent with the known anabolic and catabolic effects of central ghrelin and insulin, respectively (6, 9), microinjection of these peptides singly into PVN and 3V sites in this study was seen to decrease EE and elevate RQ in the case of ghrelin and generate the reverse effects for DET. In addition DET, when administered before ghrelin, significantly reversed the effects of ghrelin on EE and RQ at both microinjection sites. Insulin stimulation of sympathetic neural effects in the PVN has already been shown (31); however, our results demonstrate that these effects can occur simultaneously with PVN feeding-inhibition. Thus given the degree and duration of body weight reductions seen in the long-term study following 3V microinjection of both DET and regular insulin in our rats, it is likely that elevated EE and reduced RQ played some role in this effect. It should be noted, however, that the long-term weight loss with seen with DET can itself result in fat mobilization and a decreased RQ. Thus both the direct effects of insulin DET on fat metabolism, demonstrated in the acute study, and its indirect effects on body weight via feeding inhibition, can contribute to the decreased RQ seen in our study. Furthermore, given that we had no regular insulin group in the acute study, it is difficult to assess the precise extent to which the direct central effects of DET contributed to this decrease of RQ seen over days.
As noted above, our procedure permits a direct comparison of the effectiveness of each form of insulin specifically on hypothalamic tissue, ruling out differences of systemic availability and/or distribution between them. We have identified at least one potential explanatory mechanism for the enhanced effects of DET in brain tissue, that of enhanced insulin receptor second messenger signaling, as demonstrated by Hennige et al. (13). The question remains as to whether the effects of DET in the CNS seen in our study can help explain the ability of this form of insulin to inhibit weight gain when administered peripherally to human diabetic patients. Under human treatment conditions, the possibility remains that the ability of DET to exert enhanced beneficial CNS effects may also depend on its more effective transfer into the brain from the systemic circulation or its longer residency in brain tissue or interstitial fluid. Although no human data are available to address this issue, a recent paper by Begg et al. (5) demonstrated significantly longer elevations of DET in the CSF of rats following peripheral administration, in comparison with NPH insulin and insulin glargine (GLAR), another long-acting basal insulin. Concentrations of DET detected in rat CSF by Begg et al. (0.2–0.8 ng/ml) were roughly a tenth of those seen in the systemic circulation, and in the range of concentrations microinjected in this study when diluted by the volume of rat CSF. The authors cite their findings as conclusive evidence that DET transits from the systemic circulation into the brain, since insulin is known to cross the blood-brain barrier into the interstitial tissue, from which it eventually collects in the CSF (2, 3). Consistent with this finding, studies in diabetic and obese humans administered DET peripherally have demonstrated significant CNS effects, including enhanced blood flow in appetite-controlling brain regions (demonstrated by PET scanning) and restoration of cerebrocortical β-activity in obese subjects to lean levels (28, 29). Moreover Hallschmid et al. (12) found that, in contrast to regular insulin, peripherally administered DET in normal men resulted in a significant change in frontal cortex electroencephalogram activity and significantly reduced food intake. Zachariah et al. (32) demonstrated significantly reduced food intake and inhibited body weight gain in patients with type 1 diabetes treated with DET. Results from animal studies also support the notion of enhanced CNS effects of DET. Zafar et al. (33) reported decreased mRNA and protein expression of two hypothalamic appetite-stimulating peptides, neuropeptide Y and galanin, in streptozotocin-diabetic rats following 4 wk of treatment with subcutaneous injections of DET, in contrast to GLAR. During the 4-wk period, the DET-injected group showed significantly decreased food intake and body weight gain. Begg et al. (5) observed significantly inhibited feeding and body weight gain in rats subcutaneously injected with DET over a 6-wk treatment period, in contrast to GLAR. Finally Rojas et al. (22) reported significant decreases in food intake and body weight gain in normal rats fed a high-fat diet and injected subcutaneously with DET over a 4-wk treatment period, in contrast to GLAR.
Despite numerous feeding and body weight studies in animals and humans with DET, only two studies to date have addressed the ability of DET to alter metabolic activity in treated subjects. Rojas et al. (22), in the study cited above, measured EE and RQ over a 24-h period in their rats and observed no effects of DET or GLAR on these measurements, despite the ability of DET to inhibit body weight gain in the high-fat fed group. It should be noted that a relatively low peripheral doses of DET and GLAR were used in this study (0.5 and 0.2 U/kg, respectively), and the effects of once-daily subcutaneous administration of these doses on metabolic activity may have been transient. In a human clinical study cited above (32), measurements of total and resting EE, and diet-induced thermogenesis, were made at the end of a 16-wk period in type 1 diabetics treated with DET or NPH insulin. Despite significant feeding- and weight gain-inhibitory effects during treatment with DET, no alterations in total or resting EE or diet-induced thermogenesis were detected. The authors concluded that decreased caloric intake, but not increased EE, contributed to inhibited weight gain. However, EE measurements were made only at the conclusion of 16-wk treatment period, leaving open the possibility that the metabolic effects of DET may have occurred earlier during the course of treatment. Clearly more parametric studies of the metabolic effects of DET in basic and clinically effective settings need to be conducted.
In summary, both regular insulin and DET microinjected into the 3V of normal male rats reduced feeding and body weight, enhanced EE, and reduced RQ over a 5-day postinjection period, with the effects significantly greater for DET. In acute testing, microinjections of DET decreased feeding, increased EE, and reduced RQ when administered into the PVN and 3V, and reversed ghrelin-induced feeding and metabolic effects. Thus DET, when administered directly into the CNS, can activate both aspects of insulin’s central catabolic effects. The enhanced ability of DET to inhibit feeding and body weight regain in comparison with regular insulin may be due to its unique structure, which includes a fatty acid moiety known to enhance tissue absorption and prolong its duration of action. In addition, the significantly greater effects of DET vs. regular insulin when delivered directly into the brain in equimolar amounts suggest that this form of insulin may activate the insulin receptor more effectively in CNS tissue. Consistent with the above effects, clinical use of DET in diabetic patients has repeatedly been shown to have significant feeding-inhibitory and weight-gain-sparing effects. The important questions of whether the metabolic effects of DET in part underlie its ability to inhibit body weight gain, and the mechanism by which insulin receptor signaling specifically in the CNS is enhanced by DET, require further investigation.
GRANTS
This work was supported by a grant from Novo Nordisk (to J. R. Vasselli and F. X. Pi-Sunyer) and Obesity and Nutrition Research Center, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-26687. Additional support was provided by a Reed College faculty research grant (to P. J. Currie) and a Reed College Science Research Fellowship (to D. G. Wall).
DISCLOSURES
J. R. Vasselli and F. X. Pi-Sunyer have previously served as consultants for Novo Nordisk. All other authors have no conflicts of interest financial or, otherwise, to declare.
AUTHOR CONTRIBUTIONS
J.R.V. FXP-S and PJC conceived and designed research; J.R.V., D.G.W., C.S.J., C.D.C., and P.J.C. performed experiments; J.R.V., D.G.W., C.S.J., C.D.C., and P.J.C. analyzed data; J.R.V., F.X.P.-S., and P.J.C. interpreted results of experiments; J.R.V. prepared figures; J.R.V. drafted manuscript; J.R.V., F.X.P.-S., and P.J.C. edited and revised manuscript; J.R.V., F.X.P.-S., D.G.W., C.S.J., C.D.C., and P.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Angela Lu, Karin Chen, and Neville Cummins for expert technical assistance and Dr. Carol A. Maggio for a critical reading of the manuscript.
REFERENCES
- 1.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav 72: 423–429, 2002. doi: 10.1016/S0091-3057(01)00780-8. [DOI] [PubMed] [Google Scholar]
- 2.Baura GD, Foster DM, Porte D Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest 92: 1824–1830, 1993. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides 18: 1423–1429, 1997. doi: 10.1016/S0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 4.Baskin DG, Sipols AJ, Schwartz MW, White MF. Immunocytochemical detection of insulin receptor substrate-1 (IRS-1) in rat brain: colocalization with phosphotyrosine. Regul Pept 48: 257–266, 1993. doi: 10.1016/0167-0115(93)90355-C. [DOI] [PubMed] [Google Scholar]
- 5.Begg DP, May AA, Mul JD, Liu M, D’Alessio DA, Seeley RJ, Woods SC. Insulin detemir is transported from blood to cerebrospinal fluid and has prolonged central anorectic action relative to NPH insulin. Diabetes 64: 2457–2466, 2015. doi: 10.2337/db14-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull 12: 571–575, 1984. doi: 10.1016/0361-9230(84)90174-6. [DOI] [PubMed] [Google Scholar]
- 7.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 8.Currie PJ, Coscina DV. Regional hypothalamic differences in neuropeptide Y-induced feeding and energy substrate utilization. Brain Res 737: 238–242, 1996. doi: 10.1016/0006-8993(96)00738-X. [DOI] [PubMed] [Google Scholar]
- 9.Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol 289: R353–R358, 2005. doi: 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- 10.Currie PJ, Khelemsky R, Rigsbee EM, Dono LM, Coiro CD, Chapman CD, Hinchcliff K. Ghrelin is an orexigenic peptide and elicits anxiety-like behaviors following administration into discrete regions of the hypothalamus. Behav Brain Res 226: 96–105, 2012. doi: 10.1016/j.bbr.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frier BM, Russell-Jones D, Heise T. A comparison of insulin detemir and neutral protamine Hagedorn (isophane) insulin in the treatment of diabetes: a systematic review. Diabetes Obes Metab 15: 978–986, 2013. doi: 10.1111/dom.12106. [DOI] [PubMed] [Google Scholar]
- 12.Hallschmid M, Jauch-Chara K, Korn O, Mölle M, Rasch B, Born J, Schultes B, Kern W. Euglycemic infusion of insulin detemir compared with human insulin appears to increase direct current brain potential response and reduces food intake while inducing similar systemic effects. Diabetes 59: 1101–1107, 2010. doi: 10.2337/db09-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennige AM, Sartorius T, Tschritter O, Preissl H, Fritsche A, Ruth P, Häring H-U. Tissue selectivity of insulin detemir action in vivo. Diabetologia 49: 1274–1282, 2006. doi: 10.1007/s00125-006-0192-9. [DOI] [PubMed] [Google Scholar]
- 14.Hermansen K, Davies M. Does insulin detemir have a role in reducing risk of insulin-associated weight gain? Diabetes Obes Metab 9: 209–217, 2007. doi: 10.1111/j.1463-1326.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 15.Hollander PA. Insulin detemir for the treatment of obese patients with type 2 diabetes. Diabetes Metab Syndr Obes 5: 11–19, 2012. doi: 10.2147/DMSO.S26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacoby SM, Currie PJ. SKF 83566 attenuates the effects of ghrelin on performance in the object location memory task. Neurosci Lett 504: 316–320, 2011. doi: 10.1016/j.neulet.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 17.McGowan MK, Andrews KM, Grossman SP. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol Behav 51: 753–766, 1992. doi: 10.1016/0031-9384(92)90112-F. [DOI] [PubMed] [Google Scholar]
- 18.Müller C, Voirol MJ, Stefanoni N, Surmely JF, Jéquier E, Gaillard RC, Tappy L. Effect of chronic intracerebroventricular infusion of insulin on brown adipose tissue activity in fed and fasted rats. Int J Obes Relat Metab Disord 21: 562–566, 1997. doi: 10.1038/sj.ijo.0800441. [DOI] [PubMed] [Google Scholar]
- 19.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52: 227–231, 2003. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Plata-Salamán CR, Oomura Y, Shimizu N. Dependence of food intake on acute and chronic ventricular administration of insulin. Physiol Behav 37: 717–734, 1986. doi: 10.1016/0031-9384(86)90177-0. [DOI] [PubMed] [Google Scholar]
- 22.Rojas JM, Printz RL, Niswender KD. Insulin detemir attenuates food intake, body weight gain and fat mass gain in diet-induced obese Sprague-Dawley rats. Nutr Diabetes 1: e10, 2011. doi: 10.1038/nutd.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell-Jones D, Danne T, Hermansen K, Niswender K, Robertson K, Thalange N, Vasselli JR, Yildiz B, Häring HU. Weight-sparing effect of insulin detemir: a consequence of central nervous system-mediated reduced energy intake? Diabetes Obes Metab 17: 919–927, 2015. doi: 10.1111/dom.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaguchi T, Bray GA. Sympathetic activity following paraventricular injections of glucose and insulin. Brain Res Bull 21: 25–29, 1988. doi: 10.1016/0361-9230(88)90115-3. [DOI] [PubMed] [Google Scholar]
- 25.Smeeton F, Shojaee Moradie F, Jones RH, Westergaard L, Haahr H, Umpleby AM, Russell-Jones DL. Differential effects of insulin detemir and neutral protamine Hagedorn (NPH) insulin on hepatic glucose production and peripheral glucose uptake during hypoglycaemia in type 1 diabetes. Diabetologia 52: 2317–2323, 2009. doi: 10.1007/s00125-009-1487-4. [DOI] [PubMed] [Google Scholar]
- 26.Soran H, Younis N. Insulin detemir: a new basal insulin analogue. Diabetes Obes Metab 8: 26–30, 2006. doi: 10.1111/j.1463-1326.2005.00487.x. [DOI] [PubMed] [Google Scholar]
- 27.Sørensen AR, Stidsen CE, Ribel U, Nishimura E, Sturis J, Jonassen I, Bouman SD, Kurtzhals P, Brand CL. Insulin detemir is a fully efficacious, low affinity agonist at the insulin receptor. Diabetes Obes Metab 12: 665–673, 2010. doi: 10.1111/j.1463-1326.2010.01206.x. [DOI] [PubMed] [Google Scholar]
- 28.Tschritter O, Hennige AM, Preissl H, Porubska K, Schäfer SA, Lutzenberger W, Machicao F, Birbaumer N, Fritsche A, Häring H-U. Cerebrocortical beta activity in overweight humans responds to insulin detemir. PLoS One 2: e1196, 2007. doi: 10.1371/journal.pone.0001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Golen LW, IJzerman RG, Huisman MC, Hensbergen JF, Hoogma RP, Drent ML, Lammertsma AA, Diamant M. Cerebral blood flow and glucose metabolism in appetite-related brain regions in type 1 diabetic patients after treatment with insulin detemir and NPH insulin: a randomized controlled crossover trial. Diabetes Care 36: 4050–4056, 2013. doi: 10.2337/dc13-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115: 951–958, 2005. doi: 10.1172/JCI200524301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57: 435–441, 2011. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zachariah S, Sheldon B, Shojaee-Moradie F, Jackson NC, Backhouse K, Johnsen S, Jones RH, Umpleby AM, Russell-Jones DL. Insulin detemir reduces weight gain as a result of reduced food intake in patients with type 1 diabetes. Diabetes Care 34: 1487–1491, 2011. doi: 10.2337/dc11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zafar MI, Hu C, Liu D, Shafqat RA, Gao F. Insulin detemir causes lesser weight gain in comparison to insulin glargine: role on hypothalamic NPY and galanin. J Diabetes Res 2014: 458104, 2014. doi: 10.1155/2014/458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494: 528–548, 2006. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]