Abstract
Apolipoprotein AIV (ApoAIV) and cholecystokinin (CCK) are well-known satiating signals that are stimulated by fat consumption. Peripheral ApoAIV and CCK interact to prolong satiating signals. In the present study, we hypothesized that ApoAIV and CCK control energy homeostasis in response to high-fat diet feeding. To test this hypothesis, energy homeostasis in ApoAIV and CCK double knockout (ApoAIV/CCK-KO), ApoAIV knockout (ApoAIV-KO), and CCK knockout (CCK-KO) mice were monitored. When animals were maintained on a low-fat diet, ApoAIV/CCK-KO, ApoAIV-KO, and CCK-KO mice had comparable energy intake and expenditure, body weight, fat mass, fat absorption, and plasma parameters relative to the controls. In contrast, these KO mice exhibited impaired lipid transport to epididymal fat pads in response to intraduodenal infusion of dietary lipids. Furthermore, ApoAIV-KO mice had upregulated levels of CCK receptor 2 (CCK2R) in the small intestine while ApoAIV/CCK-KO mice had upregulated levels of CCK2R in the brown adipose tissue. After 20 wk of a high-fat diet, ApoAIV-KO and CCK-KO mice had comparable body weight and fat mass, as well as lower energy expenditure at some time points. However, ApoAIV/CCK-KO mice exhibited reduced body weight and adiposity relative to wild-type mice, despite having normal food intake. Furthermore, ApoAIV/CCK-KO mice displayed normal fat absorption and locomotor activity, as well as enhanced energy expenditure. These observations suggest that mice lacking ApoAIV and CCK have reduced body weight and adiposity, possibly due to impaired lipid transport and elevated energy expenditure.
Keywords: energy expenditure, fat absorption, fatty acid uptake, food intake, locomotor activity
obesity is a global epidemic, with more than 35% of adults in the US and ~13% of adults across the world identified as obese (61, 89). In parallel, the incidence of type 2 diabetes mellitus, cardiovascular disease, and certain types of cancers has increased (12, 17, 28). Stimulation of energy expenditure and brown adipose tissue (BAT) thermogenesis could have far-reaching health benefits in combatting obesity and obesity-related complications including diabetes and cardiovascular disease (5, 13, 71, 82). Consumption of a high-fat diet (HFD) promotes excess energy intake, leading to excess energy stored as fat and subsequent development of obesity (90). Cholecystokinin (CCK) and apolipoprotein AIV (ApoAIV) are found in the small intestine and brains (27, 45, 66). In response to dietary lipids, CCK is secreted by endocrine I cells in the small intestine, while ApoAIV, a major protein constituent of lymphatic triglyceride-rich lipoproteins, is produced by enterocytes lining the small intestine (27, 66). Their secretions are mediated by chylomicron formation induced by lipid meals (29, 68). A short-term feeding of HFD increases intestinal production of CCK and ApoAIV (33, 44). Thus dietary lipids are important regulators of the production of CCK and ApoAIV in the small intestine.
Both CCK and ApoAIV contribute to the control of food intake, lipid transport, and glucose homeostasis (2, 14, 23, 37, 68, 83). Since circulating ApoAIV and CCK cannot cross the blood-brain barrier (62, 74), CCK and ApoAIV are prominent short-term satiating proteins via a CCK 1 receptor (CCK1R) on the vagal nerves (23, 25, 47, 57). The satiety induced by the combination of ApoAIV and CCK is mediated via a CCK1R-dependent pathway (52). Peripherally administered ApoAIV and CCK interact to suppress food intake in a short-term manner and prolong satiating signals (52). Additionally, ApoAIV reduces food intake via a CCK-dependent pathway (47). The control of the endogenous interaction of ApoAIV and CCK with food intake remains unknown. CCK has been reported to reduce energy expenditure (31, 48, 51, 56). However, the effect of ApoAIV on energy expenditure remains unknown. Peripheral CCK or ApoAIV alone stimulates insulin secretion and reduces hepatic glucose production under hyperglycemic conditions (2, 14, 40, 50, 83). In studies involving ApoAIV knockout (ApoAIV-KO) mice, ApoAIV has been found to stimulate duodenal CCK synthesis and to improve lipid transport (1, 37). These observations collectively suggest that endogenous CCK and ApoAIV induced by dietary lipids are major controllers of energy homeostasis and lipid metabolism. Therefore, we hypothesized that HFD-induced ApoAIV and CCK control energy homeostasis. In the present study, we examined the effect of either a LFD or a HFD on energy homeostasis and metabolic parameters in mice lacking the genes for ApoAIV, CCK, and both of them.
MATERIALS AND METHODS
Animals.
Male ApoAIV and CCK double knockout (ApoAIV/CCK-KO) mice were generated from ApoAIV-KO and CCK knockout (CCK-KO) mice and did not produce functional ApoAIV and CCK. ApoAIV-KO, CCK-KO, ApoAIV/CCK-KO, and wild-type (WT) mice (C57BL/6J background) were generated in an AAALAC-accredited facility under conditions of controlled illumination (12:12-h light-dark cycle, lights from 0600 to 1800). All KO mice were backcrossed for >10 generations onto a C57BL/6J genetic background, and all mice were genotyped by PCR analysis of tail DNA (37, 39). WT mice in the experiment of lipid uptake by adipocytes were obtained from Jackson Laboratory (Bar Harbor, ME). All mice were housed individually beginning at 10 wk of age. Starting at 10 wk of age, all animals received free access to either a low-fat diet (LFD; 5% butter fat content) or a matched high-fat diet (HFD; 20% butter fat by weight; Research Diets, New Brunswick, NJ), in addition to water, for 20 wk. All animal protocols were approved by the Institutional Animal Care and Use Committee at Ohio University and the University of Cincinnati.
qPCR for relative mRNA measurement.
ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice (n = 6–8 mice per group) maintained on chow diets at 15 wk of age were fasted for 5 h with water access. The small intestine and epidiymal fat tissue of fasted mice were collected on dry ice. Total RNA was isolated, and first-strand cDNA was synthesized from 1 µg total RNA (91). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in a 27-µl final reaction volume with an Applied Biosystems RT-PCR instrument using SYBR green RT-PCR master mixes (Life Technologies, Warrington, UK). RT-PCR conditions were conducted as follows: 95°C for 3 min for one cycle, followed by 40 cycles of 95°C for 30 s and 60°C for 30 s. Threshold cycle readings for each of the unknown samples were used, and the results were analyzed in Excel using the ∆∆Ct method (46). Cyclophilin mRNA levels from each sample were used as internal controls to normalize the mRNA levels. The sequences of the primers (Integrated DNA Technologies, Coralville, IA) were as follows: mouse CCK1R, 5′-AAGAGGATGCGGACTGTCAC-3′ (forward) and 5′-CAGACGCGGGATTGTAGG-3′ (reverse); mouse CCK 2 receptor (CCK2R), 5′- GTCCAACAAATGTGGTCCGTGCTT-3′ (forward) and 5′-TTATCACCATCAAAGCGGAGCCCT-3′ (reverse); mouse CCK, 5′-CTAGCGCGATACATCCAGCAGGTC-3′ (forward) and 5′-ACTTAATAAATAGATACTCAAACC-3′ (reverse); mouse ApoAIV, 5′-ACCCAGCTAAGCAACAATGC-3′ (forward) and 5′-TGTCCTGGAAGAGGGTACTGA-3′ (reverse), and mouse cyclophilin, 5′-TTCATGTGCCAGGGTGGTGACT-3′ (forward) and 5′-TCAGTCTTGGCAGTGCAGAT-3′ (reverse).
Body weight, food intake, and energy expenditure.
Body weight was measured with a top-loading balance (±0.01 g; Adenturer SL, Ohau, Pine Brook, NJ). Before the start of data collection for meal patterns, food intake, and energy expenditure of ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice after 20 wk of a LFD or HFD, all mice (n = 5–8 per group) were acclimatized to individual metabolic cages in an Oxymax system (Columbus Instruments, Columbus, OH) for 3 days. Mice had free access to either a powdered LFD or HFD. Food intake and energy expenditure were recorded at 15-min intervals for 2 days using the manufacturer's software.
Fat absorption.
Following our published protocols (32, 48), cohorts of ApoAIV/CCK-KO and WT mice (n = 5 per group) were fed a LFD or a HFD for 18 wk. They were then fed the HFD mixed with sucrose polybehenate (Research Diets) for 4 days, and their fecal pellets were collected for analysis on the final day. Fatty acids in the fecal pellets were extracted, methylated, and analyzed by a gas chromatography system (Shimadzu GC 2010) equipped with a DB-23 Column (J&W Scientific, Folsom, CA) and Schimadzu Class EZStart 7.4 software. The percentage of fat absorption was determined based on the ratio of total fatty acids to behenic acid in the diet and in the feces.
Lipid uptake by adipocytes.
Intraduodenal infusion of dietary lipids was performed to determine lipid uptake by adipose tissue. For intraduodenal lipid infusions, ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice (n = 5 per group) maintained on a LFD for 16 wk received a continuous duodenal infusion of a lipid emulsion containing 4 μmol/h butter fat and labeled with [9,10-3H]oleic acid, 1 µCi/0.3 ml and [14C]cholesterol, 0.2 µCi/0.3 ml (Perkin Elmer, Boston, MA) 24 h after recovery from the duodenal cannulation. Because maximum transport of lymphatic lipids is observed at 2 h postinfusion (49), BAT, and inguinal and epididymal adipose tissue following the 2-h infusion was collected on dry ice. In accordance with our published protocols (37, 49), tissue lipids were extracted using the Folch method (20), and radioactivity in the plasma and tissues was measured by liquid scintillation counting.
Locomotor Activity.
Home cages were placed in SmartFrame stainless steel cage rack frames (Hamilton-Kinder, Poway, CA). Infrared photobeam interruption sensors mounted in the frames detected each animal’s movements. Activity counts in the form of beam interruptions were recorded for 4 days.
Plasma parameters.
Plasma insulin and leptin levels were determined using commercial ELISA kits (Millipore, St. Charles, MO). All samples were processed according to the manufacturer’s protocols. Briefly, 10-µl plasma samples with 1% dipeptidyl peptidase IV (DPPIV) inhibitor were added to each well of a microtiter plate precoated with anti-peptide monoclonal antibodies, and the detection antibody was added to the captured molecules. After incubation, absorbance was measured with a microplate reader (Synergy HT; BioTek Instruments, Richmond, VA), and the final concentrations were calculated using standards provided with the ELISA kits. Triacylglycerol and cholesterol in the plasma were determined using Randox triglyceride kits (Antrim, UK) and Infinity cholesterol kits (Thermo Electron, Noble Park, Victoria, Australia), respectively. Plasma glucose was determined using a Freestyle glucometer (Abbot Diabetes Care, Alameda, CA).
Data analysis and statistical analysis.
All values are presented as means ± SE. Parametric statistical analyses, one-way ANOVA, and two-way ANOVA were performed using GraphPad Prism (version 6.0, San Diego, CA), followed by a Sidak multiple comparisons test. All differences were considered significant if the P < 0.05.
RESULTS
Gene expressions, body weight, and plasma parameters.
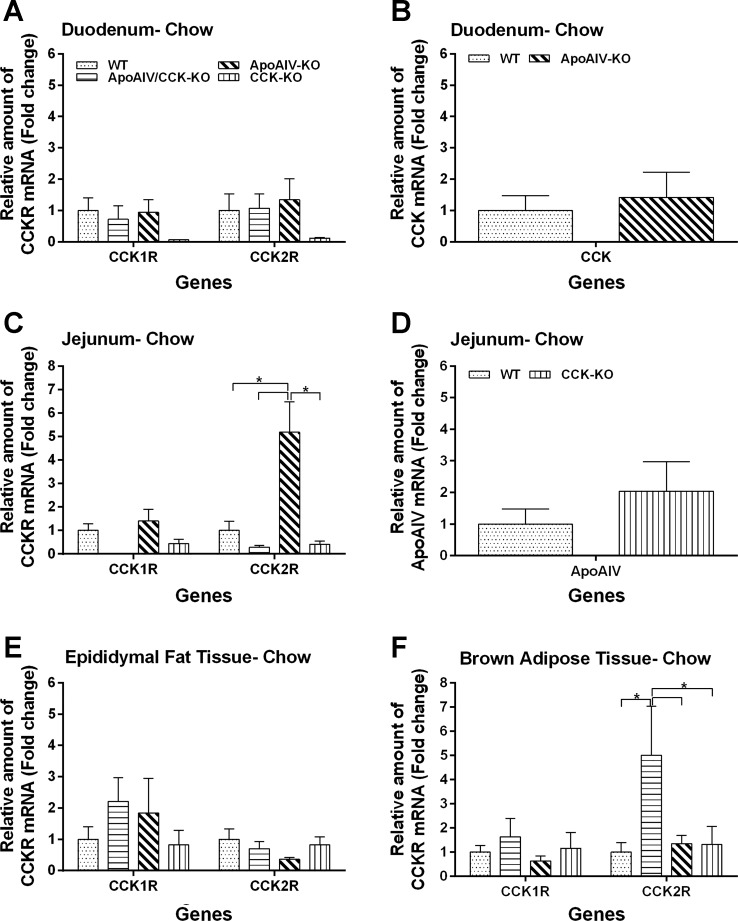
Two types of CCK receptor have been described: CCK1R and CCK2R. Peripheral CCK1R are present in the enteric neurons of the duodenal mucosa, predominantly involved in secretory and mucosal functions; myenteric plexus, predominantly involved in the control of motor activity; and nodose ganglia (8, 58, 65). Peripheral CCK2R is found in the small intestine, and adipose tissues (3, 54, 85). To understand whether mice with absence of ApoAIV, CCK, or both have compensation of CCK1R and CCK2R to control energy homeostasis, the gene expression of CCK1R, CCK2R, CCK and ApoAIV in the small intestine and adipose tissues was examined. Relative to WT mice, ApoAIV/CCK-KO and CCK-KO mice had comparable levels of CCK1R and CCK2R in the duodenum, jejunum, and epididymal fat tissues, except that ApoAIV/CCK-KO mice experienced a fivefold increase of CCK2R expression in the BAT (Fig. 1, A, C, E, and F). In addition, CCK-KO mice produced normal levels of jejunal ApoAIV compared with the control groups (Fig. 1B). Relative to their fasted control groups, ApoAIV-KO mice produced comparable levels of CCK1R and CCK2R in the small intestine, and adipose tissues, except for a fivefold increase of CCK2R expression in the jejunum (Fig. 1, A–E). Furthermore, ApoAIV-KO mice had normal levels of duodenal CCK (Fig. 1B). These findings suggest that the levels of CCK1R and CCK2R in BAT are upregulated in mice with absence of CCK or both ApoAIV and CCK, possibly due to the development of compensatory mechanisms.
Fig. 1.
Expression of apolipoprotein AIV (ApoAIV), cholecystokinin (CCK), and CCK receptors in the small intestine and epididymal fat tissues of ApoAIV/CCK-knockout (KO), ApoAIV-KO, CCK-KO, and wild-type (WT) mice. Duodenal CCK1R and 2R in 4 genotypes (A), duodenal CCK in ApoAIV-KO mice (B), jejunal CCK1R and 2R in four genotypes (C), jejunal ApoAIV in CCK-KO mice (D), CCK1R and 2R in epididymal fat tissue (E), and brown adipose tissue (F). Small intestine, epididymal fat tissue, and brown fat tissue in animals fed a chow diet were collected after a 5-h fast. Data are means ± SE for 5 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
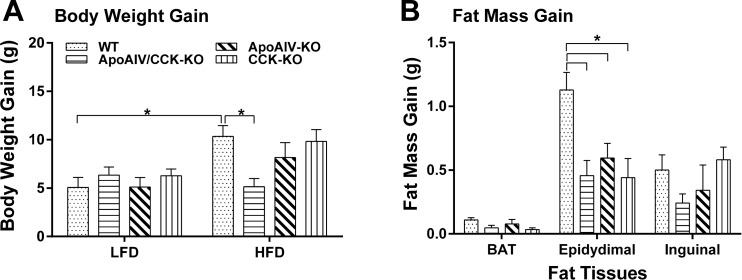
ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice maintained on a LFD for 20 wk (n = 7 per group) had comparable body weight; comparable mass of brown adipose tissue (BAT) and epididymal fat; and comparable plasma levels of lipids, insulin, and leptin (Table 1). When animals were maintained on a LFD, CCK-KO mice had increased inguinal fat mass relative to ApoAIV/CCK-KO mice, but these were not statistically significant. In contrast, the inguinal fat mass of ApoAIV-KO mice was significantly greater than that of ApoAIV/CCK-KO mice (P < 0.05, Table 1). Furthermore, ApoAIV-KO and CCK-KO mice fed a LFD had epididymal fat pads of larger mass relative to LFD-fed WT mice (P < 0.05, Table 1). Before a HFD, initial body weights in different genotypes fed a LFD were comparable, except for increased body weight in ApoAIV-KO mice (Table 2). After 10 wk on a HFD, WT mice had significantly increased body weight gain (10.34 ± 1.1 g), relative to LFD-fed WT mice (5.08 ± 1.0 g, Fig. 2A, P < 0.05). Relative to WT mice, ApoAIV/CCK-KO mice showed significantly reduced body weight and white fat mass, including both epididymal and inguinal fat mass, body weight gain, as well as significant reduction in plasma triacylglycerol, insulin, and leptin after chronic consumption of a HFD for 20 wk (Table 2 and Fig. 2, P < 0.05). In contrast, relative to WT mice, ApoAIV-KO and CCK-KO mice had similar body weight, body weight gain, fat mass, and levels of plasma lipids, glucose, insulin, and leptin, except for an increase in epididymal fat mass (Table 2 and Fig. 2, A and B). When compared with ApoAIV-KO mice, ApoAIV/CCK-KO mice exhibited significantly decreased body weight, epididymal fat mass, and plasma leptin (P < 0.05, Table 2). The HFD-fed ApoAIV/CCK-KO mice had body weight gain comparable to the LFD-fed ApoAIV/CCK-KO mice (Fig. 2, A and B). These findings indicate that ApoAIV/CCK-KO mice are resistant to gaining body weight and epididymal fat mass in response to chronic consumption of a HFD.
Table 1.
Body weight, adipose tissue weights, and plasma parameters in animals after a 20-wk period of low-fat diet
| WT | ApoAIV/CCK-KO | ApoAIV-KO | CCK-KO | |
|---|---|---|---|---|
| BW, g (initial) | 24.48 ± 0.9 | 22.55 ± 0.5 | 26.87 ± 0.5* | 22.81 ± 0.7 |
| BW, g (LFD) | 29.34 ± 0.7 | 28.57 ± 0.7 | 31.16 ± 0.6 | 29.16 ± 0.6 |
| BAT, g | 0.11 ± 0.0 | 0.10 ± 0.0 | 0.14 ± 0.0 | 0.18 ± 0.0 |
| Epididymal fat, g | 0.46 ± 0.0 | 0.35 ± 0.0 | 0.79 ± 0.1* | 0.85 ± 0.1* |
| Inguinal fat, g | 0.26 ± 0.0 | 0.17 ± 0.0 | 0.39 ± 0.1 | 0.31 ± 0.1 |
| Triacylglycerol, mg/dl | 63.81 ± 6.2 | 40.02 ± 7.9 | 61.34 ± 13.0 | 45.68 ± 7.1 |
| Cholesterol, mg/dl | 89.21 ± 5.6 | 55.53 ± 5.0* | 88.18 ± 3.6 | 62.34 ± 9.9 |
| Glucose, mg/dl | 151.85 ± 11.7 | 141.5 ± 10.8 | 157.00 ± 9.1 | 150.33 ± 6.1 |
| Insulin, ng/ml | 0.51 ± 0.1 | 0.44 ± 0.0 | 0.55 ± 0.1 | 0.57 ± 0.1 |
| Leptin, ng/ml | 2.34 ± 0.5 | 1.73 ± 0.4 | 1.98 ± 0.5 | 2.79 ± 0.6 |
Values represent means ± SE. ApoAIV, apolipoprotein AIV; CCK, cholecystokinin; BW, body weight; BAT, brown adipose tissue; WT, wild type; KO; knockout; LFD, low-fat diet. Plasma and tissues in ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice (n = 7 per group) were collected after a 5-h fast after a 20-wk LFD.
Significant difference (P < 0.05) compared with LFD-treated WT controls.
Table 2.
Body weight, adipose tissue weights, and plasma parameters in animals after a 20-week period of high-fat diet
| WT | ApoAIV/CCK-KO | ApoAIV-KO | CCK-KO | |
|---|---|---|---|---|
| BW, g (initial) | 24.1 ± 0.9 | 24.0 ± 0.4 | 26.9 ± 0.5* | 22.6 ± 0.8 |
| BW, g (HFD) | 35.8 ± 0.9 | 29.7 ± 0.8* | 36.5 ± 1.9 | 33.3 ± 1.5 |
| BAT, g | 0.22 ± 0.0 | 0.15 ± 0.0 | 0.23 ± 0.0 | 0.18 ± 0.0 |
| Epididymal fat, g | 1.54 ± 0.1 | 0.81 ± 0.1* | 1.36 ± 0.1 | 1.33 ± 0.2 |
| Inguinal fat, g | 0.80 ± 0.1 | 0.39 ± 0.1* | 0.66 ± 0.1 | 0.86 ± 0.1 |
| Triacylglycerol, mg/dl | 66.66 ± 11.9 | 39.41 ± 6.5* | 42.99 ± 5.2 | 50.46 ± 3.0 |
| Cholesterol, mg/dl | 149.36 ± 23.4 | 87.90 ± 20.6 | 139.5 ± 18.0 | 128.45 ± 9.9 |
| Glucose, mg/dl | 157.00 ± 9.1 | 175.83 ± 6.1 | 147.3 ± 6.6 | 126.85 ± 4.9 |
| Insulin, ng/ml | 0.96 ± 0.1 | 0.43 ± 0.1* | 0.79 ± 0.2 | 0.62 ± 0.2 |
| Leptin, ng/ml | 17.57 ± 2.7 | 7.26 ± 1.3* | 18.49 ± 2.9 | 10.51 ± 3.7 |
Values represent means ± SE. HFD, high-fat diet. Plasma and tissues in ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice (n = 8 per group) were collected after a 5-h fast after a 20-wk HFD.
Significant difference (P < 0.05) compared with HFD-treated WT controls.
Fig. 2.
Body weight gain and fat mass gain in ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice. Body weight gain (A) and fat mass gain (B) in animals before and after 20 wk of a high-fat diet (HFD). BAT, brown adipose tissue. Data are means ± SE for 8 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
Fat absorption and lipid transport to adipocytes.
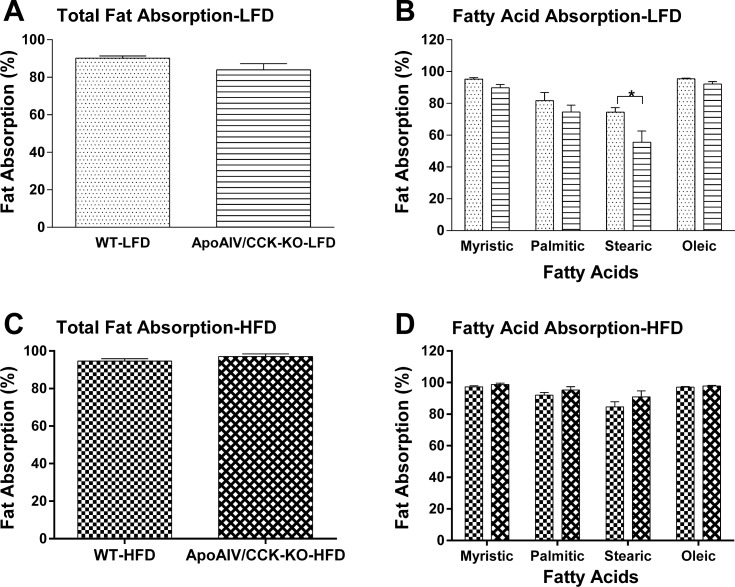
ApoAIV-KO and CCK-KO mice fed a LFD have comparable or reduced fat absorption (36, 37, 75). To investigate whether reduced fat absorption in the small intestine results in reduced fat mass in ApoAIV/CCK-KO mice, the efficiency of dietary fatty acid absorption in ApoAIV/CCK-KO and WT mice was measured using our Olestra method (32). When maintained on a LFD for 18 wk, ApoAIV/CCK-KO mice (83.9 ± 3.3%, Fig. 3A) displayed total fat absorption comparable to WT mice (90.2 ± 1.2%). Specifically, ApoAIV/CCK-KO mice experienced significantly lower absorption of stearic acid relative to WT mice (P < 0.05, Fig. 3B). Following a HFD for 18 wk, ApoAIV/CCK-KO mice (97.0 ± 1.4%, Fig. 3C) showed total fat absorption comparable to WT mice (94.61 ± 1.2%). There was no significant difference in the absorption of various fatty acids between WT and ApoAIV/CCK-KO mice (Fig. 3D). These findings suggest that the absence of both ApoAIV and CCK does not alter total fat absorption in mice, apart from impaired absorption of long-chain saturated fatty acids, such as stearic acid, when they are maintained on a LFD.
Fig. 3.
Fat absorption in ApoAIV/CCK-KO and WT mice. Total fat absorption (A) and fatty acid profiles (B) in fecal pellets of animals fed a low-fat diet (LFD) and total fat absorption (C) and fatty acid profiles (D) in fecal pellets of animals fed a HFD were determined using gas chromatography. Fecal pellets were collected on the 4th day after animals started to receive a 5% fat or 20% fat diet mixture with 5% Olestra and again during the 18th week on LFD or HFD. Data are means ± SE for 5 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
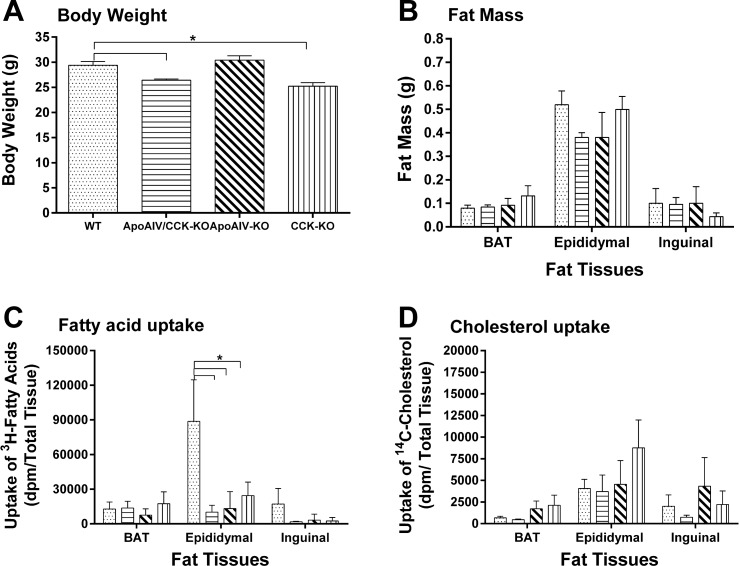
To determine whether impaired intestinal lipid transport to adipocytes results in reduced fat mass in ApoAIV/CCK-KO mice, different cohorts of ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice were used for the determination of lipid uptake in adipocytes. Relative to WT mice (29.40 ± 0.8 g), ApoAIV/CCK-KO and CCK-KO mice fed a LFD had lower body weight (26.40 ± 0.3 g and 25.20 ± 0.7 g, respectively) and comparable fat mass (Fig. 4, A and B). In addition, ApoAIV-KO mice had similar body weight (30.40 ± 0.9 g) and fat mass (Fig. 4, A and B). Since maximum transport of lymphatic lipids is observed at 2 h postinfusion (49), radioactive lipids in the tissues after 2-h infusion were counted in the present experiment. Following a 2-h infusion of dietary lipids, ApoAIV/CCK-KO, ApoAIV-KO, and CCK-KO mice showed reduced levels of radioactive triacylglycerol-derived fatty acid uptake by epididymal fat (Fig. 4C, P < 0.05). In contrast, the KO mice displayed comparable levels of [3H]triacylglycerol derived fatty acid uptake by inguinal fat and BAT (Fig. 4C). The KO mice experienced similar uptake of [14C]CHOL by white adipose tissues and BAT (Fig. 4D). These findings indicate that the absence of ApoAIV and CCK only influences triacylglycerol transport from the small intestine to epididymal fat depots when dietary lipids are infused into the duodenum.
Fig. 4.
Lipid uptake by adipocytes in ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice. Body weight (A), fat mass (B), uptake of [3H]triacylglycerol-derrived fatty acids (C), and cholesterol uptake (D) in animals fed a LFD. Five-hour fasted animals received an intraduodenal infusion of 4 µmol/h butter fat mixture, and tissues were collected at the end of the 2-h infusion. Data are means ± SE for 5 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
Energy expenditure, respiratory quotient, and food intake in ApoAIV/CCK-KO mice.
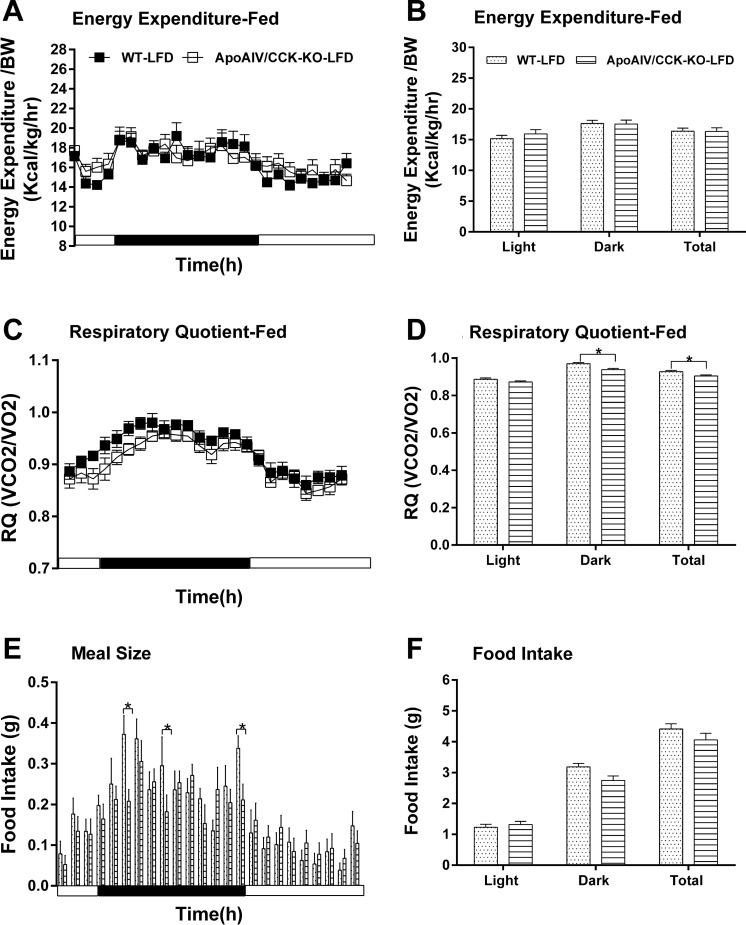
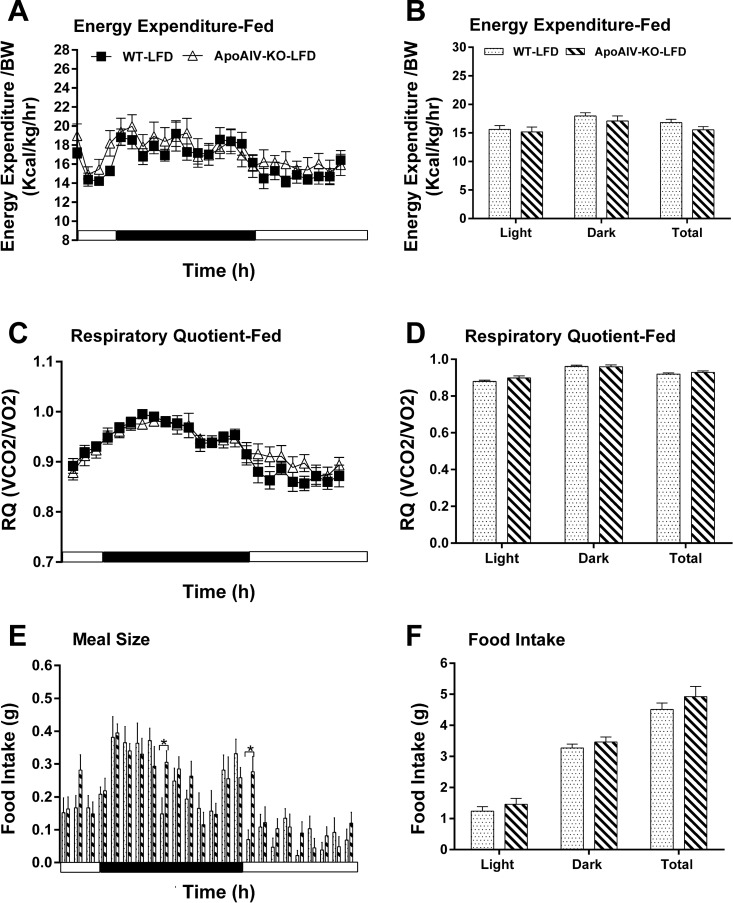
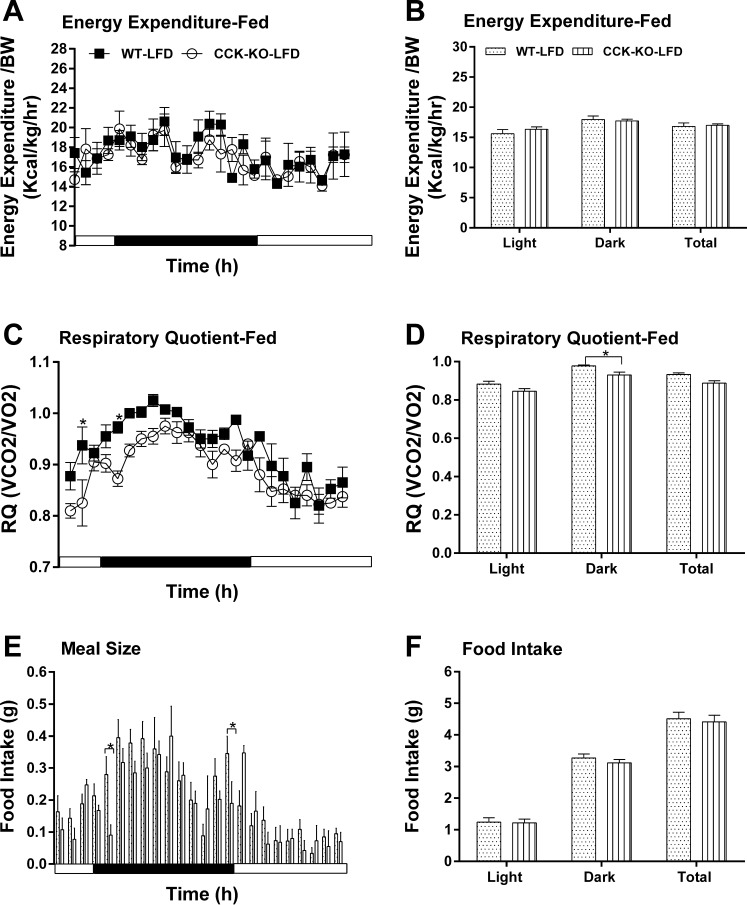
To investigate the effect of endogenous ApoAIV and CCK on the regulation of energy homeostasis, ApoAIV/CCK-KO, ApoAIV-KO, and CCK-KO mice were monitored after a 20-wk consumption of a LFD or HFD. Relative to the control group on a LFD, ApoAIV/CCK-KO, ApoAIV-KO, and CCK-KO mice fed a LFD had comparable hourly energy expenditure and average energy expenditure (Figs. 5, A and B, 6, A and B, and 7, A and B). The LFD-fed ApoAIV/CCK-KO and CCK-KO mice exhibited a reduced respiratory quotient (RQ) in the dark period (Figs. 5, C and D, and 7, C and D), suggesting that these KO mice utilized more fatty acids as energy substrates. In contrast, there was no significant difference in the RQ between ApoAIV-KO and WT mice (Fig. 6, C and D). When maintained on a LFD, ApoAIV/CCK-KO and CCK-KO mice exhibited reduced hourly food intake with respect to their control group at some time points, while ApoAIV-KO mice experienced increased food intake at some time points (Figs. 5E, 6E, and 7E). Relative to the WT control, no significant difference in total daily food intake was found in these KO mice (Figs. 5F, 6F, and 7F). Thus the deficiency of ApoAIV, CCK, or both ApoAIV and CCK genes does not alter daily total energy intake and expenditure in animals maintained on a LFD.
Fig. 5.
Energy expenditure and intake in ApoAIV/CCK-KO and WT mice fed a LFD. Hourly energy expenditure (A), total energy expenditure (B), hourly respiratory quotient (RQ; C), RQ (D), meal pattern (E), and food intake (F). Data are means ± SE for 8 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
Fig. 6.
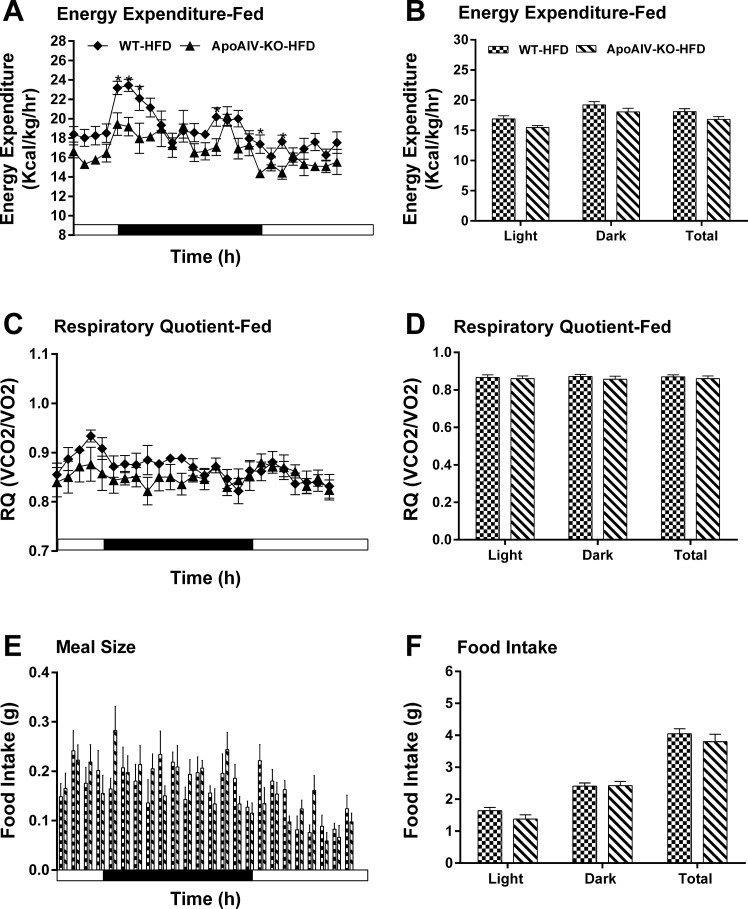
Energy expenditure and intake in ApoAIV-KO and WT mice fed a LFD. Hourly energy expenditure (A), total energy expenditure (B), hourly RQ (C), RQ (D), meal pattern (E), and food intake (F). Data are means ± SE for 8 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
Fig. 7.
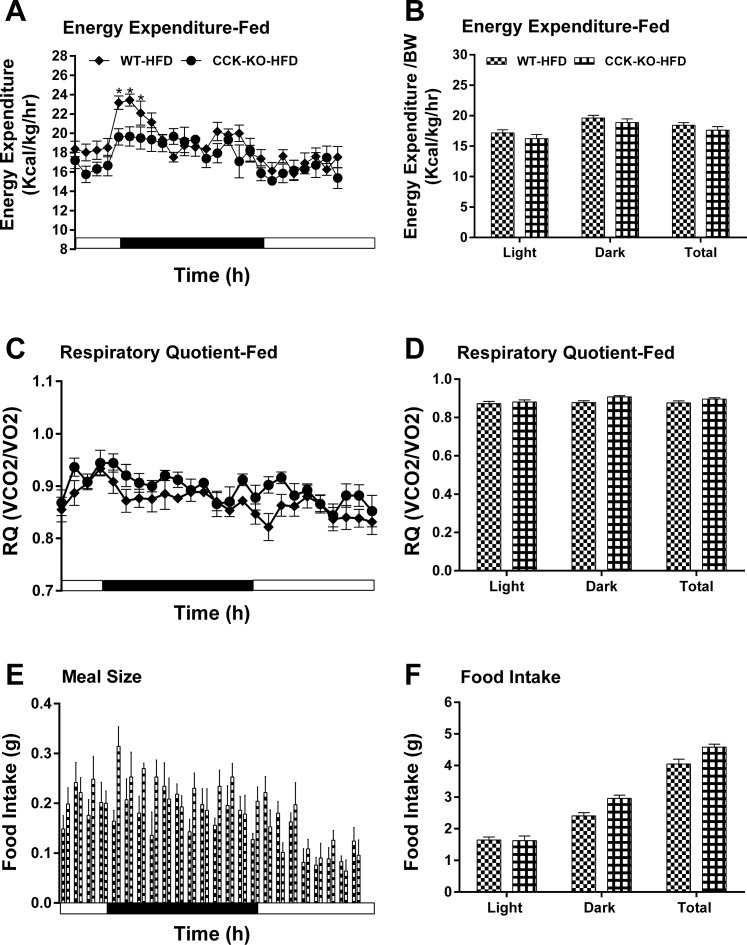
Energy expenditure and intake in CCK-KO and WT mice fed a LFD. Hourly energy expenditure (A), total energy expenditure (B), hourly RQ (C), RQ (D), meal pattern (E), and food intake (F). Data are means ± SE for 5 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
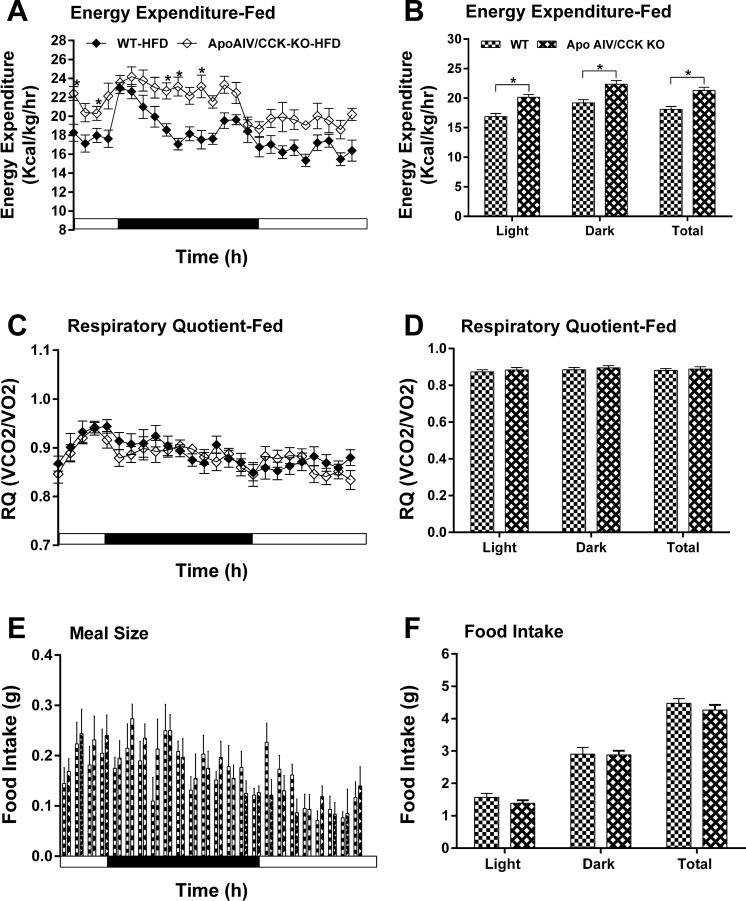
After chronic consumption of a HFD, ApoAIV/CCK-KO mice had increased hourly energy expenditure relative to their controls (Fig. 8, A and B), while ApoAIV-KO and CCK-KO mice fed a HFD showed lower energy expenditure relative to WT mice at some time points during the dark period (Figs. 9, A and B, and 10, A and B). Additionally, the RQ in these KO mice was normal (Figs. 8, C and D, 9, C and D, and 10, C and D). These findings indicate that a HFD alters energy expenditure in the KO mice. After chronic consumption of a HFD for 20 wk, ApoAIV/CCK-KO, ApoAIV-KO, and CCK-KO mice had comparable meal size and total food intake relative to their control groups (Figs. 8, E and F, 9, E and F, and 10, E and F).
Fig. 8.
Energy expenditure and intake in ApoAIV/CCK-KO and WT mice fed a HFD. Hourly energy expenditure (A), total energy expenditure (B), hourly RQ (C), RQ (D), meal pattern (E), and food intake (F). Data are means ± SE for 8 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
Fig. 9.
Energy expenditure and intake in ApoAIV-KO and WT mice fed a HFD. Hourly energy expenditure (A), total energy expenditure (B), hourly RQ (C), RQ (D), meal pattern (E), and food intake (F). Data are means ± SE for 5–6 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
Fig. 10.
Energy expenditure and intake in CCK-KO and WT mice fed a HFD. Hourly energy expenditure (A), total energy expenditure (B), hourly RQ (C), RQ (D), meal pattern (E), and food intake (F). Data are means ± SE for 5–6 animals per group. *Values represent significant differences relative to the WT mice (P < 0.05).
Locomotor activity in CCK/ApoAIV-KO mice.
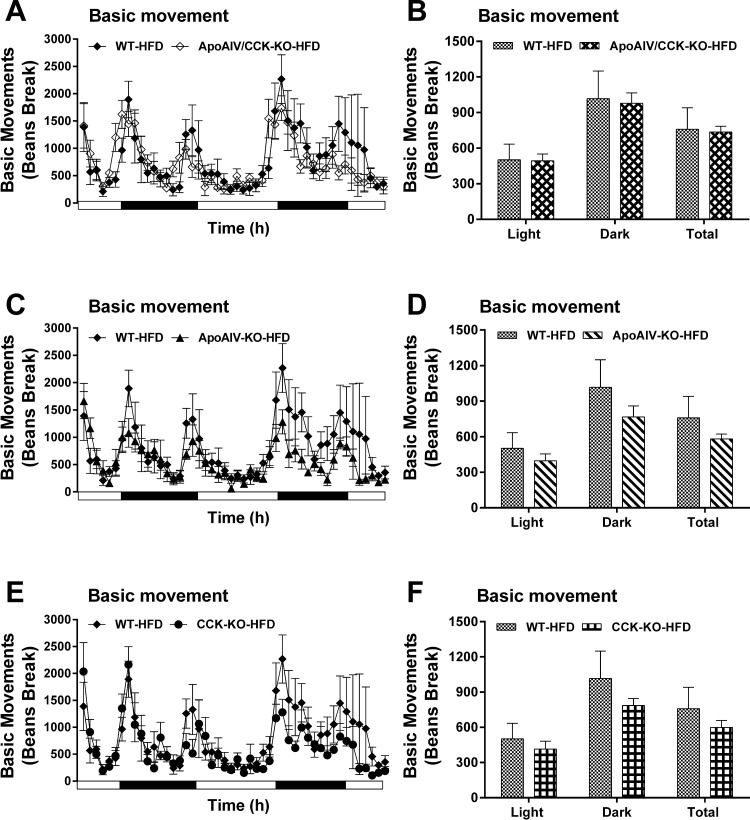
To investigate whether altered locomotor activity results in elevated or reduced energy expenditure in these HFD-fed KO mice, locomotor activity in ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice was monitored for 48 h after 19 wk of a HFD. Daily basic and cumulative movement in these KO mice were comparable to those movements in WT mice (Fig. 11, A–F). These findings suggest that HFD-fed mice without ApoAIV, CCK, or both ApoAIV and CCK genes have normal locomotor activity.
Fig. 11.
Locomotor activity in ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice fed a HFD. Basic movement (A) and average of basic movement (B) in ApoAIV/CCK-KO and WT mice. Basic movement (C) and average of basic movement (D) in ApoAIV-KO and WT mice. Basic movement (E) and average of basic movement (F) in CCK-KO and WT mice. ApoAIV/CCK-KO, ApoAIV-KO, CCK-KO, and WT mice were individually housed in home cages that were then placed in a SmartFrame system for 4 continuous days. Data are means ± SE for 6–8 animals per group.
DISCUSSION
These experiments tested the hypothesis that HFD-induced ApoAIV and CCK control energy homeostasis. Peripheral and central administration of CCK or ApoAIV produces short-term satiating signals that do not alter total food intake (23–25, 30). ApoAIV-KO mice fed a chow diet have normal food intake and increased body weight (37, 75). In the present experiment, both food intake and body weight remained at normal levels in ApoAIV-KO mice. In addition, CCK-KO mice fed a LFD had normal total food intake and body weight results confirmed by previous studies (36, 50, 51). The present study showed that ApoAIV/CCK-KO mice fed a LFD for 20 wk had normal total food intake and body weight (Table 1). Surprisingly, a significant reduction of body weight was found in CCK-KO and ApoAIV/CCK-KO mice fed a LFD for 16 wk (Fig. 4). The discrepancy in body weight of CCK-KO or ApoAIV/CCK-KO mice (Table 1 and Fig. 4) might have been due to their different feeding time and ages. Chronic feeding of a HFD alters intestinal and hypothalamic production of CCK and ApoAIV (33, 44, 46, 60). Past studies have demonstrated that the increase in body weight of CCK-KO mice and ApoAIV-KO mice is less than that of the WT controls for 10–12 wk, although maintenance on a HFD or Western diet increases body weight in both genotypes (37, 48, 75). However, ApoAIV-KO and CCK-KO mice displayed normal food intake and body weight after chronic consumption of a HFD for 20 wk, while ApoAIV/CCK-KO mice exhibited normal food intake but reduced gain of body weight compared with their controls. Thus HFD-induced ApoAIV and CCK regulated the gain of body weight. Moreover, ApoAIV and CCK activity were not primary factors in controlling total food intake.
CCK and ApoAIV alter plasma levels of glucose and insulin in a hyperglycemia condition (21, 83). CCK binds with CCK1R to assist glucose uptake by the pancreas to elevate insulin secretion (35). ApoAIV decreases circulating glucose by enhancing glucose-induced insulin release and attenuating hepatic glucose production (40, 83). In the present experiments, CCK-KO, ApoAIV-KO, and ApoAIV/CCK-KO mice had comparable levels of basal glucose and insulin in the plasma when maintained on LFD. Consistent with previous reports (50, 83), CCK-KO and ApoAIV-KO mice had comparable basal levels of insulin and glucose when fed a HFD. In contrast, lower levels of basal insulin were found in ApoAIV/CCK-KO mice with chronic consumption of a HFD, possibly due to the absence of insulin induction by ApoAIV and CCK. Leptin secretion is highly correlated to fat mass and is increased when animals are fed a HFD (55, 92). When maintained on a LFD, ApoAIV/CCK-KO mice had comparable levels of plasma leptin compared with the other genotypes, although they had reduced mass of epididymal and inguinal fat tissues. Leptin is also produced by the stomach (76, 92). It remains unknown whether higher production of gastric leptin or slower leptin degradation in the LFD-fed ApoAIV/CCK-KO mice results in comparable levels of plasma leptin relative to the other genotypes. The present study showed that a HFD increased the mass of white adipose tissues, including epididymal and inguinal fat tissues, and leptin content in WT mice, a finding also presented in previous studies (55). In the current study, when maintained on a HFD, ApoAIV-KO and CCK-KO mice displayed normal fat mass and leptin. In contrast, ApoAIV/CCK-KO mice experienced a smaller increase of white adipose tissues and plasma leptin compared with the controls and the ApoAIV-KO mice. Because insulin reduces lipolysis and enhances uptake of fatty acids in white adipose tissues (18, 22, 72), a lower level of insulin might induce diminished fat mass in HFD-fed ApoAIV/CCK-KO mice relative to HFD-fed WT mice.
Pancreatic lipase hydrolyzes dietary triacylglycerol to fatty acids in the intestinal lumen, and the absorbed fatty acids are subsequently resynthesized to triacylglycerol in the mucosal cells (80). Fatty acids are packaged into chylomicrons in the small intestine and transported to the bloodstream (80). Upon entering the circulation, lipoprotein lipase hydrolyzes the triglycerides in chylomicrons to free fatty acids, and half of the fatty acids are directly transported to adipose tissue (26, 63). CCK physiologically stimulates pancreatic lipase secretion to increase fat absorption (10, 73). CCK-containing neurons are present in the small intestine, and CCK increases intestinal smooth muscle contraction (9, 41). CCK and gastrin activate CCK2R, whereas only CCK activates CCK1R (85). In the present and previous studies (39), CCK-KO mice had normal levels of CCK1R and CCK2R in the small intestine and the epididymal fat tissue. CCK-KO mice have a slower transit time of lipids in the small intestine and delayed lipid transport from the small intestine to the lymph (36, 84). When fed a LFD, CCK-KO mice have normal fat absorption and delayed lipid transport (36). After a HFD feeding, CCK-KO mice have normal or reduced fat absorption, depending on their age (36, 48). Consistent with a previous report (36), LFD-fed CCK-KO mice had lower fatty acid uptake by epididymal fat and comparable uptake of fatty acids in the BAT and inguinal fat tissues. ApoAIV is required for chylomicron assembly and stabilizing expanding lipid interfaces (53, 87). In the previous and current experiments, ApoAIV-KO mice had normal levels of CCK and CCK1R in the small intestine and gallbladder (88, 91). In contrast, ApoAIV-KO mice had reduced expression of duodenal CCK (67). The ApoAIV-KO mice had upregulated levels of CCK1R in the nodose ganglia and upregulated levels of CCK2R in the jejunum, possibly due to the development of compensatory mechanisms (91). LFD-fed ApoAIV-KO mice have normal fat absorption and delayed chylomicron clearance (37, 38). The current study demonstrated that ApoAIV-KO mice fed a LFD had impaired fatty acid uptake by epididymal fat tissues. Because the effect of intestinal CCK2R in the regulation of lipid transport remains unknown, another experiment is required for the determination of lipid transport from the small intestine to epididymal fat tissue via a CCK2R-dependent pathway in ApoAIV-KO mice.
Fatty acids are used as an energy source in peripheral tissues, including BAT and muscle (13, 19, 70). Duodenal lipids enhance BAT thermogenesis via a CCK1R-dependent pathway in the small intestine (6). Peripheral administration of CCK acts on CCK1R and CCK2R to reduce whole-body energy expenditure (15, 34, 69). Central administration of CCK at lower doses decreases body temperature (59, 77). In contrast, higher doses of brain CCK elevate body temperature and energy expenditure (4, 79). CCK2R KO mice fed a LFD have increased energy expenditure (56). In the present study, when fed a LFD, ApoAIV-KO and CCK-KO mice had normal energy expenditure like CCK-KO and CCK1R-KO mice in the previous reports (51, 56). CCK-KO mice fed a LFD utilized more fatty acids as energy substrates than their control groups. A HFD stimulates peripheral release of CCK, reduces hypothalamic levels of CCK, and attenuates CCK signaling in the vagus nerves (16, 60, 78). Although HFD-fed CCK-KO mice had altered energy expenditure at some time points in present and previous results (36, 48), CCK-KO mice consumed a normal total daily energy expenditure compared with WT mice. Chronic feeding of a HFD attenuates intestinal and hypothalamic production of ApoAIV (33, 46). ApoAIV-KO mice fed the HFD had normal total energy expenditure with lower hourly energy expenditure at some time points, suggesting that HFD-induced ApoAIV enhanced energy expenditure.
Blocking CCK1R or CCK2R in WT and CCK2R-KO mice changes locomotor activity (81, 86). However, CCK-KO mice fed a HFD for 10 wk have normal locomotor activity (48). The present experiments showed that ApoAIV-KO and CCK-KO mice displayed normal locomotor activity after chronic consumption of a HFD for 20 wk. These findings suggest that the deficiency of global endogenous CCK or ApoAIV did not alter locomotor activity.
Three factors may have contributed to the reduced body weight of HFD-fed ApoAIV/CCK-KO mice: 1) reduced fat absorption and lipid transport to adipocytes; 2) increased energy expenditure; and 3) elevated locomotor activity. First, the present study showed that ApoAIV/CCK-KO mice displayed normal fat absorption and attenuated lipid transport from the small intestine to white adipose tissues. Second, ApoAIV/CCK-KO mice fed a LFD had normal energy expenditure and utilized more fatty acids as energy substrates than the control group. In contrast, maintenance on a HFD for 20 wk caused a greater increase in energy expenditure in ApoAIV/CCK-KO mice than in WT mice. Thus enhanced energy expenditure in HFD-fed mice lacking CCK and ApoAIV resulted in attenuation of body weight and fat mass. Furthermore, ApoAIV/CCK-KO mice had upregulated CCK2R expression in the BAT, suggesting that both ApoAIV and CCK interact with CCK2R to regulate BAT thermogenesis. Since the role of CCK2R in the control of BAT thermogenesis remains unknown, further study of BAT thermogenesis control induced by ApoAIV and CCK via a CCK2R-dependent pathway is warranted. Third, ApoAIV/CCK-KO mice exhibited normal locomotor activity following chronic consumption of a HFD for 20 wk. This finding suggests that locomotor activity was not responsible for the elevated energy expenditure in the ApoAIV/CCK-KO mice.
In summary, this study demonstrated that when maintained on a LFD, animals with global deficiency of ApoAIV, CCK, or both exhibited normal food intake, fat absorption, and energy expenditure, except for impaired lipid transport. After a 20-wk maintenance on a HFD, ApoAIV/CCK-KO mice experienced a profound reduction of body weight and white adipose tissues, possibly due to elevated energy expenditure, impaired lipid transport, and lower insulin-induced adiposity, despite normal food intake, fat absorption, and locomotor activity.
Perspectives and Significance
Obese subjects have normal or higher levels of basal CCK or ApoAIV in the plasma than control subjects (7, 11, 42, 43). Plasma ApoAIV is increased in human obesity and attenuated after a short-term weight loss (43, 64). In view of the observations in the current study, increased energy expenditure, impaired lipid transport, and reduced insulin-induced adiposity in the global deficiency of ApoAIV and CCK are particularly interesting factors in the reduction of body weight and white adipose tissues. Further investigation should be conducted to determine whether attenuated levels of ApoAIV and CCK via a CCK2R-dependent pathway enhance energy expenditure and reduce body weight.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-83550 and DK-97436.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.C.W., P.T., and C.C.L. conceived and designed research; J.W., D.L., S.C.B., N.C., and C.C.L. performed experiments; J.W., D.L., S.C.B., N.C., and C.C.L. analyzed data; J.W., S.C.B., S.C.W., P.T., and C.C.L. interpreted results of experiments; C.C.L. prepared figures; J.W. drafted manuscript; J.W. and C.C.L. edited and revised manuscript; C.C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Mouse Metabolic Phenotyping Center at the University of Cincinnati for excellent assistance with the Oxymax system.
REFERENCES
- 1.Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim Biophys Acta 1441: 4–13, 1999. doi: 10.1016/S1388-1981(99)00137-7. [DOI] [PubMed] [Google Scholar]
- 2.Ahrén B, Lundquist I. Effects of two cholecystokinin variants, CCK-39 and CCK-8, on basal and stimulated insulin secretion. Acta Diabetol Lat 18: 345–356, 1981. doi: 10.1007/BF02042819. [DOI] [PubMed] [Google Scholar]
- 3.Attoub S, Levasseur S, Buyse M, Goïot H, Laigneau JP, Moizo L, Hervatin F, Le Marchand-Brustel Y, Lewin JM, Bado A. Physiological role of cholecystokinin B/gastrin receptor in leptin secretion. Endocrinology 140: 4406–4410, 1999. doi: 10.1210/endo.140.10.7079. [DOI] [PubMed] [Google Scholar]
- 4.Balaskó M, Rostás I, Füredi N, Mikó A, Tenk J, Cséplő P, Koncsecskó-Gáspár M, Soós S, Székely M, Pétervári E. Age and nutritional state influence the effects of cholecystokinin on energy balance. Exp Gerontol 48: 1180–1188, 2013. doi: 10.1016/j.exger.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 6.Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One 7: e51898, 2012. doi: 10.1371/journal.pone.0051898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, Feinle-Bisset C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol 303: G129–G140, 2012. doi: 10.1152/ajpgi.00478.2011. [DOI] [PubMed] [Google Scholar]
- 8.Broberger C, Holmberg K, Shi TJ, Dockray G, Hökfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res 903: 128–140, 2001. doi: 10.1016/S0006-8993(01)02468-4. [DOI] [PubMed] [Google Scholar]
- 9.Buffa R, Solcia E, Go VL. Immunohistochemical identification of the cholecystokinin cell in the intestinal mucosa. Gastroenterology 70: 528–532, 1976. [PubMed] [Google Scholar]
- 10.Burton P, Hammond EM, Harper AA, Howat HT, Scott JE, Varley H. Serum amylase and serum lipase levels in man after administration of secretin and pancreozymin. Gut 1: 125–139, 1960. doi: 10.1136/gut.1.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler MG, Carlson MG, Schmidt DE, Feurer ID, Thompson T. Plasma cholecystokinin levels in Prader-Willi syndrome and obese subjects. Am J Med Genet 95: 67–70, 2000. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callow AD. Cardiovascular disease 2005–the global picture. Vascul Pharmacol 45: 302–307, 2006. doi: 10.1016/j.vph.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 14.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab 10: 99–109, 2009. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides 15: 731–755, 1994. doi: 10.1016/0196-9781(94)90104-X. [DOI] [PubMed] [Google Scholar]
- 16.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS One 7: e32967, 2012. doi: 10.1371/journal.pone.0032967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo RA. Current issues in the treatment of type 2 diabetes. Overview of newer agents: where treatment is going. Am J Med 123, Suppl: S38–S48, 2010. doi: 10.1016/j.amjmed.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract 93, Suppl 1: S52–S59, 2011. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- 19.Dulloo AG. A role for suppressed skeletal muscle thermogenesis in pathways from weight fluctuations to the insulin resistance syndrome. Acta Physiol Scand 184: 295–307, 2005. doi: 10.1111/j.1365-201X.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 21.Frame CM, Davidson MB, Sturdevant RA. Effects of the octapeptide of cholecystokinin on insulin and glucagon secretion in the dog. Endocrinology 97: 549–553, 1975. doi: 10.1210/endo-97-3-549. [DOI] [PubMed] [Google Scholar]
- 22.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia 45: 1201–1210, 2002. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol Gastrointest Liver Physiol 262: G1002–G1006, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto K, Fukagawa K, Sakata T, Tso P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest 91: 1830–1833, 1993. doi: 10.1172/JCI116395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84: 488–495, 1973. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res 37: 693–707, 1996. [PubMed] [Google Scholar]
- 27.Green PH, Glickman RM, Riley JW, Quinet E. Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J Clin Invest 65: 911–919, 1980. doi: 10.1172/JCI109745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo F, Moellering DR, Garvey WT. The progression of cardiometabolic disease: validation of a new cardiometabolic disease staging system applicable to obesity. Obesity (Silver Spring) 22: 110–118, 2014. doi: 10.1002/oby.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31: 1613–1625, 1990. [PubMed] [Google Scholar]
- 30.Inui A, Okita M, Inoue T, Sakatani N, Oya M, Morioka H, Ogawa T, Mizuno N, Baba S. Mechanism of actions of cholecystokinin octapeptide on food intake and insulin and pancreatic polypeptide release in the dog. Peptides 9: 1093–1100, 1988. doi: 10.1016/0196-9781(88)90095-2. [DOI] [PubMed] [Google Scholar]
- 31.Irwin N, Frizelle P, O’Harte FP, Flatt PR. (pGlu-Gln)-CCK-8[mPEG]: a novel, long-acting, mini-PEGylated cholecystokinin (CCK) agonist that improves metabolic status in dietary-induced diabetes. Biochim Biophys Acta 1830: 4009–4016, 2013. doi: 10.1016/j.bbagen.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127: 139–144, 2004. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Kalogeris TJ, Painter RG. Adaptation of intestinal production of apolipoprotein A-IV during chronic feeding of lipid. Am J Physiol Regul Integr Comp Physiol 280: R1155–R1161, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Kapás L, Obál F Jr, Penke B, Obál F. Cholecystokinin-octapeptide-induced hypothermia in rats: dose-effect and structure-effect relationships, effect of ambient temperature, pharmacological interactions and tolerance. Neuropharmacology 26: 131–137, 1987. doi: 10.1016/0028-3908(87)90200-0. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson S, Ahrén B. CCK-8-stimulated insulin secretion in vivo is mediated by CCKA receptors. Eur J Pharmacol 213: 145–146, 1992. doi: 10.1016/0014-2999(92)90245-Y. [DOI] [PubMed] [Google Scholar]
- 36.King A, Yang Q, Huesman S, Rider T, Lo CC. Lipid transport in cholecystokinin knockout mice. Physiol Behav 151: 198–206, 2015. doi: 10.1016/j.physbeh.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohan AB, Wang F, Li X, Bradshaw S, Yang Q, Caldwell JL, Bullock TM, Tso P. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am J Physiol Gastrointest Liver Physiol 302: G628–G636, 2012. doi: 10.1152/ajpgi.00225.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohan AB, Wang F, Li X, Vandersall AE, Huesman S, Xu M, Yang Q, Lou D, Tso P. Is apolipoprotein A-IV rate limiting in the intestinal transport and absorption of triglyceride? Am J Physiol Gastrointest Liver Physiol 304: G1128–G1135, 2013. doi: 10.1152/ajpgi.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am J Physiol Gastrointest Liver Physiol 276: G1302–G1309, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Xu M, Wang F, Kohan AB, Haas MK, Yang Q, Lou D, Obici S, Davidson WS, Tso P. Apolipoprotein A-IV reduces hepatic gluconeogenesis through nuclear receptor NR1D1. J Biol Chem 289: 2396–2404, 2014. doi: 10.1074/jbc.M113.511766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liddle RA. Cholecystokinin cells. Annu Rev Physiol 59: 221–242, 1997. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- 42.Lieverse RJ, Masclee AA, Jansen JB, Lamers CB. Plasma cholecystokinin and pancreatic polypeptide secretion in response to bombesin, meal ingestion and modified sham feeding in lean and obese persons. Int J Obes Relat Metab 18: 123–127, 1994. [PubMed] [Google Scholar]
- 43.Lingenhel A, Eder C, Zwiauer K, Stangl H, Kronenberg F, Patsch W, Strobl W. Decrease of plasma apolipoprotein A-IV during weight reduction in obese adolescents on a low fat diet. Int J Obes Relat Metab 28: 1509–1513, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Little TJ, Feltrin KL, Horowitz M, Meyer JH, Wishart J, Chapman IM, Feinle-Bisset C. A high-fat diet raises fasting plasma CCK but does not affect upper gut motility, PYY, and ghrelin, or energy intake during CCK-8 infusion in lean men. Am J Physiol Regul Integr Comp Physiol 294: R45–R51, 2008. doi: 10.1152/ajpregu.00597.2007. [DOI] [PubMed] [Google Scholar]
- 45.Liu M, Doi T, Shen L, Woods SC, Seeley RJ, Zheng S, Jackman A, Tso P. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol 280: R1382–R1387, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Liu M, Shen L, Liu Y, Woods SC, Seeley RJ, D’Alessio D, Tso P. Obesity induced by a high-fat diet downregulates apolipoprotein A-IV gene expression in rat hypothalamus. Am J Physiol Endocrinol Metab 287: E366–E370, 2004. doi: 10.1152/ajpendo.00448.2003. [DOI] [PubMed] [Google Scholar]
- 47.Lo CC, Langhans W, Georgievsky M, Arnold M, Caldwell JL, Cheng S, Liu M, Woods SC, Tso P. Apolipoprotein AIV requires cholecystokinin and vagal nerves to suppress food intake. Endocrinology 153: 5857–5865, 2012. doi: 10.1210/en.2012-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo CM, King A, Samuelson LC, Kindel TL, Rider T, Jandacek RJ, Raybould HE, Woods SC, Tso P. Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity. Gastroenterology 138: 1997–2005, 2010. doi: 10.1053/j.gastro.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, Yang Q, Lee D, Swift LL, Davidson NO, Tso P. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol 294: G344–G352, 2008. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]
- 50.Lo CM, Obici S, Dong HH, Haas M, Lou D, Kim DH, Liu M, D’Alessio D, Woods SC, Tso P. Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes 60: 2000–2007, 2011. doi: 10.2337/db10-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Woods SC, Tso P. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol 294: R803–R810, 2008. doi: 10.1152/ajpregu.00682.2007. [DOI] [PubMed] [Google Scholar]
- 52.Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, Davidson WS, Liu M, Raybould HE, Woods SC, Tso P. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol 293: R1490–R1494, 2007. doi: 10.1152/ajpregu.00329.2007. [DOI] [PubMed] [Google Scholar]
- 53.Lu S, Yao Y, Cheng X, Mitchell S, Leng S, Meng S, Gallagher JW, Shelness GS, Morris GS, Mahan J, Frase S, Mansbach CM, Weinberg RB, Black DD. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J Biol Chem 281: 3473–3483, 2006. doi: 10.1074/jbc.M502501200. [DOI] [PubMed] [Google Scholar]
- 54.Lucaites VL, Mendelsohn LG, Mason NR, Cohen ML. CCK-8, CCK-4 and gastrin-induced contractions in guinea pig ileum: evidence for differential release of acetylcholine and substance P by CCK-A and CCK-B receptors. J Pharmacol Exp Ther 256: 695–703, 1991. [PubMed] [Google Scholar]
- 55.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161, 1995. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 56.Miyasaka K, Ichikawa M, Ohta M, Kanai S, Yoshida Y, Masuda M, Nagata A, Matsui T, Noda T, Takiguchi S, Takata Y, Kawanami T, Funakoshi A. Energy metabolism and turnover are increased in mice lacking the cholecystokinin-B receptor. J Nutr 132: 739–741, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 272: R1245–R1251, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 526: 95–102, 1990. doi: 10.1016/0006-8993(90)90253-8. [DOI] [PubMed] [Google Scholar]
- 59.Morley JE, Levine AS, Lindblad S. Intraventricular cholecystokinin-octapeptide produces hypothermia in rats. Eur J Pharmacol 74: 249–251, 1981. doi: 10.1016/0014-2999(81)90538-0. [DOI] [PubMed] [Google Scholar]
- 60.Morris MJ, Chen H, Watts R, Shulkes A, Cameron-Smith D. Brain neuropeptide Y and CCK and peripheral adipokine receptors: temporal response in obesity induced by palatable diet. Int J Obes 32: 249–258, 2008. doi: 10.1038/sj.ijo.0803716. [DOI] [PubMed] [Google Scholar]
- 61.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311: 806–814, 2014. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oldendorf WH. Blood-brain barrier permeability to peptides: pitfalls in measurement. Peptides 2, Suppl 2: 109–111, 1981. doi: 10.1016/0196-9781(81)90020-6. [DOI] [PubMed] [Google Scholar]
- 63.Olivecrona G, Olivecrona T. Triglyceride lipases and atherosclerosis. Curr Opin Lipidol 6: 291–305, 1995. doi: 10.1097/00041433-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Pardina E, López-Tejero MD, Llamas R, Catalán R, Galard R, Allende H, Vargas V, Lecube A, Fort JM, Baena-Fustegueras JA, Peinado-Onsurbe J. Ghrelin and apolipoprotein AIV levels show opposite trends to leptin levels during weight loss in morbidly obese patients. Obes Surg 19: 1414–1423, 2009. doi: 10.1007/s11695-008-9793-3. [DOI] [PubMed] [Google Scholar]
- 65.Patterson LM, Zheng H, Ward SM, Berthoud HR. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res 305: 11–23, 2001. doi: 10.1007/s004410100402. [DOI] [PubMed] [Google Scholar]
- 66.Polak JM, Bloom SR, Rayford PL, Pearse AG, Buchan AM, Thompson JC. Identification of cholecystokinin-secreting cells. Lancet 306: 1016–1018, 1975. doi: 10.1016/S0140-6736(75)90297-4. [DOI] [PubMed] [Google Scholar]
- 67.Pressler JW, Haller A, Sorrell J, Wang F, Seeley RJ, Tso P, Sandoval DA. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes 64: 498–507, 2015. doi: 10.2337/db14-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raybould HE, Meyer JH, Tabrizi Y, Liddle RA, Tso P. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am J Physiol Regul Integr Comp Physiol 274: R1834–R1838, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Rezayat M, Ravandeh N, Zarrindast MR. Cholecystokinin and morphine-induced hypothermia. Eur Neuropsychopharmacol 9: 219–225, 1999. [DOI] [PubMed] [Google Scholar]
- 70.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77: 731–758, 1997. [DOI] [PubMed] [Google Scholar]
- 71.Rothwell NJ, Stock MJ. Diet-induced thermogenesis. Adv Nutr Res 5: 201–220, 1983. doi: 10.1007/978-1-4613-9937-7_9. [DOI] [PubMed] [Google Scholar]
- 72.Sadur CN, Eckel RH. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J Clin Invest 69: 1119–1125, 1982. doi: 10.1172/JCI110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt WE, Creutzfeldt W, Schleser A, Choudhury AR, Nustede R, Höcker M, Nitsche R, Sostmann H, Rovati LC, Fölsch UR. Role of CCK in regulation of pancreaticobiliary functions and GI motility in humans: effects of loxiglumide. Am J Physiol Gastrointest Liver Physiol 260: G197–G206, 1991. [DOI] [PubMed] [Google Scholar]
- 74.Shen L, Pearson KJ, Xiong Y, Lo CM, Tso P, Woods SC, Davidson WS, Liu M. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav 95: 161–167, 2008. doi: 10.1016/j.physbeh.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simon T, Cook VR, Rao A, Weinberg RB. Impact of murine intestinal apolipoprotein A-IV expression on regional lipid absorption, gene expression, and growth. J Lipid Res 52: 1984–1994, 2011. doi: 10.1194/jlr.M017418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, Attoub S, Lehy T, Henin D, Mignon M, Lewin MJ. Leptin secretion and leptin receptor in the human stomach. Gut 47: 178–183, 2000. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.South EH. Cholecystokinin reduces body temperature in vehicle- but not capsaicin-pretreated rats. Am J Physiol Regul Integr Comp Physiol 263: R1215–R1221, 1992. [DOI] [PubMed] [Google Scholar]
- 78.Spannagel AW, Nakano I, Tawil T, Chey WY, Liddle RA, Green GM. Adaptation to fat markedly increases pancreatic secretory response to intraduodenal fat in rats. Am J Physiol Gastrointest Liver Physiol 270: G128–G135, 1996. [DOI] [PubMed] [Google Scholar]
- 79.Szelényi Z, Barthó L, Székely M, Romanovsky AA. Cholecystokinin octapeptide (CCK-8) injected into a cerebral ventricle induces a fever-like thermoregulatory response mediated by type B CCK-receptors in the rat. Brain Res 638: 69–77, 1994. doi: 10.1016/0006-8993(94)90634-3. [DOI] [PubMed] [Google Scholar]
- 80.Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol Gastrointest Liver Physiol 250: G715–G726, 1986. [DOI] [PubMed] [Google Scholar]
- 81.Vasar E, Harro J, Lang A, Pôld A, Soosaar A. Differential involvement of CCK-A and CCK-B receptors in the regulation of locomotor activity in the mouse. Psychopharmacology (Berl) 105: 393–399, 1991. doi: 10.1007/BF02244435. [DOI] [PubMed] [Google Scholar]
- 82.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One 6: e17247, 2011. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang F, Kohan AB, Kindel TL, Corbin KL, Nunemaker CS, Obici S, Woods SC, Davidson WS, Tso P. Apolipoprotein A-IV improves glucose homeostasis by enhancing insulin secretion. Proc Natl Acad Sci USA 109: 9641–9646, 2012. doi: 10.1073/pnas.1201433109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang HH, Portincasa P, Liu M, Tso P, Samuelson LC, Wang DQ. Effect of gallbladder hypomotility on cholesterol crystallization and growth in CCK-deficient mice. Biochim Biophys Acta 1801: 138–146, 2010. doi: 10.1016/j.bbalip.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wank SA. Cholecystokinin receptors. Am J Physiol Gastrointest Liver Physiol 269: G628–G646, 1995. [DOI] [PubMed] [Google Scholar]
- 86.Weiland TJ, Voudouris NJ, Kent S. The role of CCK2 receptors in energy homeostasis: insights from the CCK2 receptor-deficient mouse. Physiol Behav 82: 471–476, 2004. doi: 10.1016/j.physbeh.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 87.Weinberg RB, Cook VR, DeLozier JA, Shelness GS. Dynamic interfacial properties of human apolipoproteins A-IV and B-17 at the air/water and oil/water interface. J Lipid Res 41: 1419–1427, 2000. [PubMed] [Google Scholar]
- 88.Whited KL, Lu D, Tso P, Kent Lloyd KC, Raybould HE. Apolipoprotein A-IV is involved in detection of lipid in the rat intestine. J Physiol 569: 949–958, 2005. doi: 10.1113/jphysiol.2005.097634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.World Health Organization. Obesity and Overweight World Health Organization. Fact Sheet No 311. http://www.who.int/mediacentre/factsheets/fs311/en/ [2015]. [last updated June 2016].
- 90.Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133: 1081–1087, 2003. [DOI] [PubMed] [Google Scholar]
- 91.Yoshimichi G, Lo CC, Tamashiro KL, Ma L, Lee DM, Begg DP, Liu M, Sakai RR, Woods SC, Yoshimatsu H, Tso P. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am J Physiol Gastrointest Liver Physiol 302: G1336–G1342, 2012. doi: 10.1152/ajpgi.00325.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]