Abstract
Remote ischemic preconditioning (RIPC) is characterized by the cyclical application of limb blood flow restriction and reperfusion and has been shown to protect vital organs during a subsequent ischemic insult. Blood flow restriction exercise (BFRE) similarly combines bouts of blood flow restriction with low-intensity exercise and thus could potentially emulate the protection demonstrated by RIPC. One concern with BFRE, however, is the potential for an augmented rise in sympathetic outflow due to greater activation of the exercise pressor reflex. Because of the use of lower workloads, however, we hypothesized that BFRE would elicit an attenuated increase in sympathetic outflow [assessed via plasma norepinephrine (NE) and mean arterial pressure (MAP)] and middle cerebral artery velocity (MCAv) when compared with conventional exercise (CE). Fifteen subjects underwent two leg press exercise interventions: 1) BFRE-220 mmHg bilateral thigh occlusion at 20% 1 rep-max (1RM), and 2) CE-65% 1RM without occlusion. Each condition consisted of 4 × 5-min cycles of exercise, with 3 × 10-reps in each cycle. Five minutes of rest and reperfusion (for BFRE) followed each cycle. MAP increased with exercise (P < 0.001) and was 4–5 mmHg higher with CE versus BFRE (P ≤ 0.09). Mean MCAv also increased with exercise (P < 0.001) and was higher with CE compared with BFRE during the first bout of exercise only (P = 0.07). Plasma NE concentration increased with CE only (P < 0.001) and was higher than BFRE throughout exercise (P ≤ 0.02). The attenuated sympathetic response, combined with similar cerebrovascular responses, suggest that cyclical BFRE could be explored as an alternative to CE in the clinical setting.
Keywords: KAATSU, exercise for stroke rehabilitation, exercise for cardiac rehabilitation, vascular occlusion training
ischemic preconditioning is a therapy that has demonstrated utility in protecting vital organs in the face of ischemia-reperfusion (IR) injury. First described in 1986 by Murray et al. (43), this technique was characterized by brief cycles of sublethal ischemia applied to a coronary artery, which then protected the heart from subsequent IR injury. Consequently, a number of investigators reported that this cyclical application of sublethal ischemia and reperfusion could also be administered to a “remote” limb, rather than a coronary vessel, and still provide cardioprotection within this IR injury setting (23, 49). This phenomenon of preconditioning from a distance became known as “remote ischemic preconditioning” (RIPC) and served as the foundation for the clinical trials that followed (5, 16).
Since this initial characterization, use of RIPC has expanded into multiple avenues of clinical investigation in patient populations. These applications include coronary artery bypass graft surgery (13, 16), aortic valve surgery (45, 57), and percutaneous coronary intervention (1, 9, 53). However, all of these applications have a common limitation: the IR injury is known or planned before initiation of the preconditioning stimulus, thus potentially limiting the use of RIPC in clinical practice. One potential solution to this constraint is to incorporate RIPC into current therapies utilized by patients at high risk of ischemic injury. Recently, two clinical trials have demonstrated that daily RIPC can be used prophylactically to decrease the incidence of stroke and increase cerebral blood flow in patients with significant carotid artery stenosis (37, 38); this is a novel implementation of RIPC that warrants further exploration. Another important target population for such an intervention would be individuals undergoing cardiac or stroke rehabilitation. Exercise, including resistance exercise, is a cornerstone of rehabilitation for cardiac (6, 22, 35) and stroke patients (2, 17, 56) and thus would be an ideal target for implementation of an RIPC-like stimulus.
Blood flow restriction exercise (BFRE) is a unique exercise paradigm that is characterized by limiting blood flow to the working muscles by the use of a restrictive cuff (30). This technique was originally developed to augment the muscle hypertrophic response to resistance exercise (30). A hallmark of resistance BFRE is the use of much lighter workloads than typically prescribed: intensities of 20–30% of 1 repetition maximum (1RM) (30) compared with 65–70% 1RM for conventional resistance exercise (3). Accordingly, BFRE has been adapted to numerous special populations that can benefit from the use of these lighter workloads, including the elderly (21, 25, 52, 59), patients at risk for osteoarthritis (51), and individuals with ischemic heart disease (31). Another potential application of BFRE that has yet to be explored is stroke rehabilitation. To our knowledge the cerebrovascular responses to this novel mode of exercise training have not been comprehensively assessed. Traditionally, the occlusive stimulus used during BFRE is applied continuously, during both the exercise and rest phases. Because of the parallels between BFRE and RIPC, this form of exercise training could potentially emulate the protection facilitated by RIPC, if performed in the same manner, with cyclical blood flow restriction and reperfusions. This is the approach utilized in the present investigation. Because of stimulation of type III (mechano-sensitive; via cuff compression) and type IV (metabo-sensitive; via cuff restriction) afferent nerves with application of the restrictive stimulus, there is some concern that BFRE could result in greater activation of the exercise pressor reflex, leading to an unsafe rise in arterial pressure (54). Conversely, because of the use of lower workloads and the cyclical nature of the occlusive stimulus used in the present study, we hypothesized that BFRE would elicit an attenuated increase in sympathetic outflow which would be manifest in a blunted rise in plasma catecholamines and arterial pressure.
METHODS
Subjects
Young healthy volunteers participated in this study conducted at the University of North Texas Health Science Center (UNTHSC) in Fort Worth, TX. All experimental procedures were conducted in accordance with a protocol approved by the UNTHSC Institutional Review Board (IRB no. 2014–149). Before participation, all subjects underwent a medical history evaluation, including seated and standing 12-lead electrocardiogram (ECG) and blood pressure measurements and were cleared to participate by a physician. Subjects did not routinely use any nicotine products (including tobacco cigarettes, electronic cigarettes, chewing tobacco). Before each experiment, subjects abstained from caffeine, alcohol, dietary supplements, medications, and exercise for 24 h and fasted for at least 8 h (overnight). Female subjects completed a urine pregnancy test to ensure they were not pregnant. All subjects underwent a familiarization session in which they were shown all equipment and experimental procedures that would be performed in the subsequent experimental sessions. Each subject gave written informed consent to participate in this study. Fourteen of these subjects also participated in an aerobic BFRE study reported in a companion paper (54a). The RIPC protocol that was performed in both studies was identical.
Maximal Exercise Testing
All subjects underwent a 1RM test on a leg press machine (VR3, Cybex, Medway, MA) to determine the load required for the submaximal intensities used in the subsequent experimental sessions. As the maximum load of this leg press machine is 184 kg, individuals who had a 1RM greater than 184 kg were excluded from participation in this study. After a 1-h rest period, peak oxygen uptake (Vo2peak) was assessed on a treadmill (TMX428CP, TrackMaster, Newton, KS) in accordance with the Bruce Protocol (3). Testing was terminated when subjects reached volitional fatigue. Expired gases were collected and analyzed via a metabolic cart (TrueOne, ParvoMedics, Sandy, UT). To ensure a relatively homogenous subject pool in regard to cardiorespiratory fitness, only subjects with Vo2peak values between 30 and 50 ml·kg−1·min−1 were included for participation in this study.
Experimental Protocols
At least 2 wk after completion of the maximal exercise testing session subjects reported back to the laboratory for three experimental sessions (randomized) separated by at least 1 mo each. The three sessions were the following: 1) blood flow restriction resistance exercise (BFRE), 2) conventional resistance exercise (CE) and, 3) remote ischemic preconditioning (RIPC). Female subjects were tested in the early follicular phase of their menstrual cycle (first 4 days determined by self-report) and completed a urine pregnancy test at the start of each visit to the laboratory to ensure they were not pregnant. All sessions were performed in the morning in a thermoneutral laboratory (temperature = 23.1 ± 0.1°C, humidity = 49.6 ± 2.4%, barometric pressure = 744.0 ± 0.9 mmHg).
Instrumentation.
Upon arrival to the laboratory, subjects were encouraged to empty their bladder to ensure optimal comfort and to limit the potential confounding effects of increased sympathetic nervous system activation with bladder distension (11). Subjects were instrumented with a standard lead II ECG (shielded leads, cable, and amplifier, AD Instruments, Bella Vista, NSW, Australia) for measurement of R-R intervals and calculation of heart rate (HR). Noninvasive arterial pressure and stroke volume [via the pulse contour method (18)] were measured via finger photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). This arm was also placed in a sling for stability to ensure accurate detection of the blood pressure waveform during exercise. The Finometer was placed on the right arm for most subjects and was on the same arm within each subject for all experiments. Bilateral transcranial Doppler ultrasound (ST3, Spencer Technologies, Seattle, WA) was used to measure middle cerebral artery velocity (MCAv) and posterior cerebral artery velocity (PCAv). The MCAv and PCAv signals were always obtained on opposite sides of the head, which was variable between subjects, but the same within each subject across all experiments. Cerebral oxygen saturation of the frontal cortex (ScO2) was measured via near-infrared spectroscopy (NIRS; OxiplexTS, ISS, Chaimpaign-Urbana, IL). This technique quantifies oxygenated hemoglobin (HbO2) and deoxygenated hemoglobin (dHb) (33), allowing for calculation of total hemoglobin (THC; HbO2 +dHb) and ScO2 as [(HbO2/THC) × 100]. Cerebral oxygenation measurements were only measured on one side of the forehead, and were always selected to be the same side as the TCD-derived MCAv measurement to ensure that regional oxygenation and perfusion were measured on the same side. A venous catheter was inserted into an antecubital vein of the arm contralateral to the blood pressure measurements for collection of venous blood samples. For most subjects, this was the same arm for all three experiments. During the blood flow restriction protocols (BFRE and RIPC sessions), 5-cm wide inflatable cuffs (SC5, D.E. Hokanson, Bellevue, WA) were placed around both upper thighs and connected to an inflation system (E20 Rapid Cuff Inflation System, D.E. Hokanson).
Blood flow restriction-resistance exercise session.
This session was used to determine the effects of BFRE on sympathetic, hemodynamic, and cerebrovascular responses. After instrumentation, a 15-min baseline period commenced during which subjects were seated on the leg press machine with their feet placed flat on the ground. During the final minute of rest, subjects placed their feet onto the leg press platform (knee at a ~90° angle), and 10 s before the commencement of exercise, the bilateral thigh cuffs were rapidly inflated to a target pressure of 220 mmHg. The exercise session was 40 min in duration, divided into 4 × 10-min blocks (Fig. 1). Each 10-min block consisted of a 5-min exercise period with cuffs inflated and a 5-min recovery/reperfusion period with cuffs deflated. The exercise period consisted of 3 sets of 10 repetitions with a load corresponding to 20% of 1RM. Each repetition was performed at a pace of 4 s/repetition (2 s concentric contraction, 2 s eccentric contraction; timed via a metronome), and each set was separated by 1-min rest periods. The thigh cuffs remained inflated throughout the 5-min exercise period, including the 1-min rest periods. At the end of the 5-min exercise period, the thigh cuffs were rapidly deflated, and subjects placed their feet back on the floor and rested quietly for a 5-min reperfusion period. An additional 5-min recovery period followed completion of the 40 min of exercise. Three blood samples (10 ml) were collected throughout the protocol: 1) 5-min into baseline (“pre”), 2) at the end of the second exercise block (“mid”), and 3) at the end of the final reperfusion period (“post”).
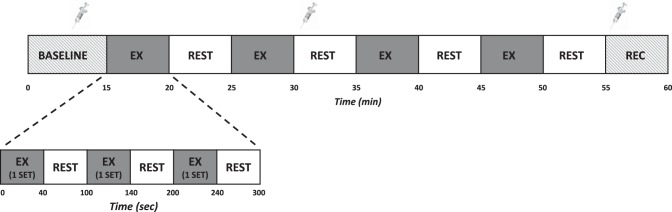
Fig. 1.
Experimental timeline consisting of a 15-min baseline, 40-min of exercise (4 sets of exercise (EX) and 4 rest/reperfusion periods), and a 5-min recovery period (REC). One 5-min exercise period consisted of 3 sets of exercise with a 1-min rest period between each set. Each set consisted of 10 repetitions performed at a load corresponding to 20% of 1 repetition maximum (1 RM) for blood flow restriction exercise (BFRE) or 65% of 1 RM for conventional exercise (CE). For the BFRE condition, the occlusive stimulus (cuff inflation) was maintained throughout the entire 5 min of the exercise period. Blood sampling is denoted by symbols of syringes.
Conventional resistance exercise session.
This session was included to compare sympathetic, hemodynamic, and cerebrovascular responses with BFRE exercise to resistance exercise as conventionally prescribed (65% of 1RM). This session was performed in exactly the same manner as the BFRE session but without use of the inflatable thigh cuffs and with a higher leg press load of 65% of 1RM per conventional guidelines (3). The timing of the exercise periods and rest periods were identical to the BFRE condition (see Fig. 1), and blood samples were collected at the same time points (pre, mid, and post).
Remote ischemic preconditioning session.
This session served as a control condition to isolate the effects of repeated occlusions and reperfusions independent of exercise. After instrumentation, subjects completed a 15-min seated baseline. The thigh cuffs were then rapidly inflated to 220 mmHg for 5 min followed by rapid deflation and reperfusion for 5 min. This inflation/deflation protocol was repeated four times over 40 min, followed by a 5-min recovery period. Blood samples were collected at the same time points as the BFRE session (pre, mid, and post).
Data Analysis
All continuous waveform data [ECG, arterial pressure, stroke volume, MCAv, PCAv, ScO2, THC, end-tidal CO2 (etCO2)] were recorded at 1,000 Hz (PowerLab/Labchart, AD Instruments, Bella Vista, NSW, Australia) and analyzed offline via specialized software (WinCPRS, Absolute Aliens, Turku, Finland). R-wave detection was performed on the ECG signal and used to determine the timing of each cardiac cycle. Mean arterial pressure (MAP), mean MCAv, and mean PCAv were calculated as the area under the curve for the arterial pressure and cerebral blood velocity waveforms. Cardiac output was calculated as HR multiplied by stroke volume, and total peripheral resistance (TPR) was subsequently calculated as MAP divided by cardiac output. For the baseline period, minutes 5–10 were averaged. All variables were calculated for each 5-min block of exercise and the first 4 min of each intervening reperfusion period; subjects were moving their feet into position for the subsequent exercise block during the final 1 min of each reperfusion period, so this was not included in the analysis. The data were evaluated in this way so that each occlusion and reperfusion period could be analyzed independently and compared with the matching time point during the CE condition with no occlusive stimulus and with the RIPC condition without the exercise stimulus.
Whole blood was collected in EDTA tubes treated with glutathione (1.23 mg glutathione/1 ml whole blood) as a preservative and centrifuged at 1,500 rpm for 15 min at 4°C. Plasma was separated and snap frozen in liquid nitrogen and then stored at −80°C until analyzed. Norepinephrine (NE) was measured in duplicate via enzyme-linked immunosorbent assay (BA E-6200, Rocky Mountain Diagnostics, Colorado Springs, CO). Only duplicate samples with a coefficient of variation <15% were included in the results. As a result, N = 9 for NE data. Hematocrit was also assessed with from the “pre” blood sample to ensure equivalent hydration status between each trial.
Statistics
Two-way repeated measures ANOVAs (factor 1: time, factor 2: condition; BFRE, CE, RIPC) were used to compare the effects of each condition over time. A one-way (condition only) repeated measures ANOVA was used to compare baseline hematocrit between conditions. Tukey post hoc tests were performed when a significant interaction was indicated by the ANOVAs. Exact P values are reported for all comparisons. Unless otherwise stated, all data are presented as means ± SE.
RESULTS
Twenty-four subjects were recruited to participate in this study. Of these 24 subjects, 2 were excluded due to Vo2peak < 30 ml·kg−1·min−1, 3 were excluded due to a 1RM > 184 kg, 2 were excluded due to medication use, and 2 withdrew due to scheduling or personal reasons.
As a result, 15 subjects completed all three experimental conditions (8M/7F, age 28 ± 1 yr, height 170 ± 3 cm, weight 71 ± 3 kg, BMI 24.6 ± 0.7 kg/m2). The average Vo2peak was 35.4 ± 1.7 ml·kg−1·min−1, and the average 1RM on the leg press machine was 120 ± 8 kg (264 ± 18 lbs). During the two exercise trials (CE and BFRE), all subjects completed all repetitions at the prescribed workloads (20% 1RM for BFRE and 65% 1RM for CE). The average workloads were 24 ± 2 kg for BFRE and 78 ± 5 kg for CE. There were no differences in baseline hematocrit between the three conditions (P = 0.14).
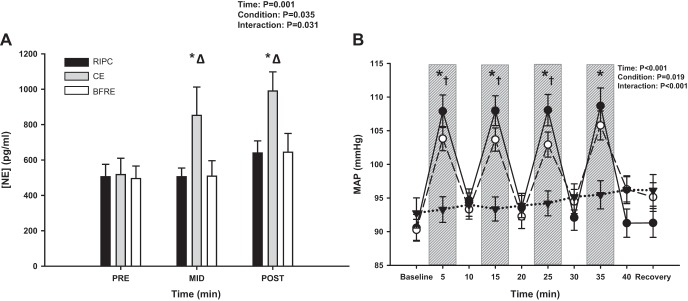
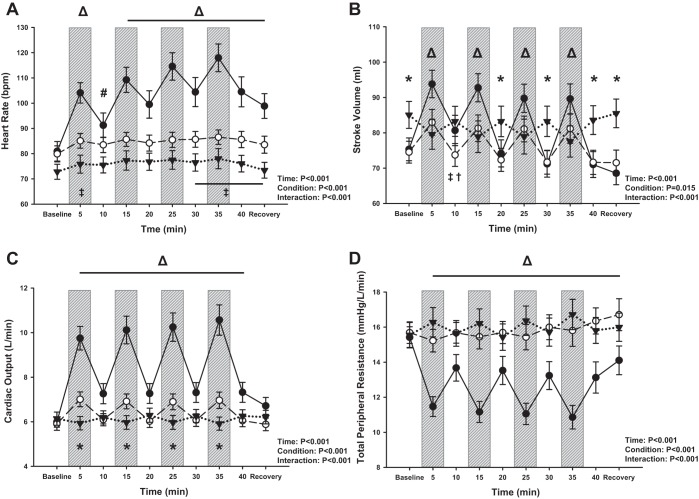
In support of our hypothesis, the increase in plasma NE concentration with exercise was higher with CE compared with BFRE and RIPC at both the “mid” (P = 0.02) and “post” (P = 0.02) time points (Fig. 2A). Neither BFRE nor RIPC elicited an increase in NE concentration (Pre vs. Post: BFRE, P = 0.24; RIPC, P = 0.31). HR increased with exercise for both CE and BFRE (P < 0.001), remained elevated with BFRE (P ≥ 0.99 vs. start of exercise), and decreased with CE during each 5-min rest period. (P < 0.001 vs. preceding exercise period). Stroke volume, cardiac output, and MAP also increased from baseline with each 5-min exercise bout (P ≤ 0.03; Figs. 2B and 3, A–C) and decreased during the subsequent 5-min rest periods for both CE and BFRE. As expected, these responses were augmented with CE compared with BFRE during each 5-min exercise period. We observed a higher stroke volume at baseline with RIPC compared with the two exercise conditions. This was unexpected as subjects were in the upright seated posture for all three experimental conditions but may be due to a slightly lower resting HR (73 ± 3 beats/min) compared with the two exercise conditions (BFRE: 80 ± 3 beats/min; CE: 81 ± 4 beats/min), although this was not statistically distinguishable (P ≥ 0.11). There were, however, no differences in stroke volume between CE and BFRE at baseline (P = 0.94). With RIPC, stroke volume decreased from baseline during each occlusion period (P = 0.07 for occlusion period 1; P ≤ 0.02 for remaining 3 occlusion periods) and returned to baseline values during the subsequent reperfusion periods (P ≥ 0.99 vs. baseline; Fig. 3B). This pattern of response suggests a restriction of venous return with each occlusion. Neither cardiac output nor MAP changed over time with RIPC (P ≥ 0.19), but HR increased slightly above baseline during the final two occlusion periods (P = 0.07 for occlusion 3 and P = 0.03 for occlusion period 4; Fig. 3A). TPR decreased from baseline throughout exercise for the CE condition (P < 0.001; Fig. 3D) and was lower for each leg press period compared with the preceding recovery period (P < 0.001). TPR was higher for both BFRE and RIPC versus CE throughout exercise (P ≤ 0.04), and there were no differences between BFRE and RIPC at any time points (P ≥ 0.14). Based on the observation that CE appeared to induce greater increases in MAP than BFRE during the first three exercise periods, a separate two-way repeated measures ANOVA was performed to compare CE versus BFRE without the RIPC condition. This analysis also revealed that CE elicited a higher MAP response during the first three exercise periods compared with BFRE (P ≤ 0.03).
Fig. 2.
Plasma norepinephrine concentration ([NE]; A, N = 9) responses to conventional exercise (CE), blood flow restriction exercise (BFRE), and remote ischemic preconditioning (RIPC). NE increased with exercise for CE (*P ≤ 0.002 vs .“pre” time point) and was higher with CE compared with BFRE and RIPC at both the “mid” and “post” time points (ΔP ≤ 0.02). Mean arterial pressure (MAP; B) responses to conventional exercise (CE; ●), blood flow restriction exercise (BFRE; ○), and remote ischemic preconditioning (RIPC; ▼). Exercise bouts (and occlusion periods for BFRE and RIPC) denoted by vertical gray bars. MAP increased with each exercise bout for both CE and BFRE and was higher with CE compared with BFRE during the first 3 exercise blocks (P ≤ 0.09). A two-way repeated measures ANOVA was performed to compare differences across time (factor 1) and between conditions (factor 2). Tukey post hoc tests were performed when an interaction effect was present. *P < 0.001 for CE and BFRE vs. RIPC; †P ≤ 0.09 for CE vs. BFRE.
Fig. 3.
Hemodynamic responses to conventional exercise (CE; ●), blood flow restriction exercise (BFRE; ○), and remote ischemic preconditioning (RIPC; ▼). Exercise bouts (and occlusion periods for BFRE and RIPC) denoted by vertical gray bars. A: heart rate increased with exercise and was higher with CE compared with BFRE and RIPC during all 4 exercise bouts/occlusion periods (Δ, P < 0.001). B: stroke volume increased with each exercise bout/occlusion period for both CE and BFRE (P ≤ 0.007) and decreased with RIPC during each occlusion period (P ≤ 0.07). C: cardiac output increased with each exercise bout/occlusion period for CE and BFRE (P < 0.001) and was higher with CE compared with BFRE and RIPC from the start of exercise through the last reperfusion period (ΔP ≤ 0.004). D: total peripheral resistance decreased with exercise for CE and was lower with CE compared with BFRE and RIPC throughout the intervention (Δ, P ≤ 0.04). A two-way repeated measures ANOVA was performed to compare differences across time (factor 1) and between conditions (factor 2). Tukey post hoc tests were performed when an interaction effect was present. *P ≤ 0.006 for CE and BFRE vs. RIPC; ΔP ≤ 0.04 for CE vs. RIPC and BFRE; #P < 0.001 for CE vs. RIPC; †P = 0.02 for CE vs. BFRE; ‡P ≤ 0.09 for BFRE vs. RIPC.
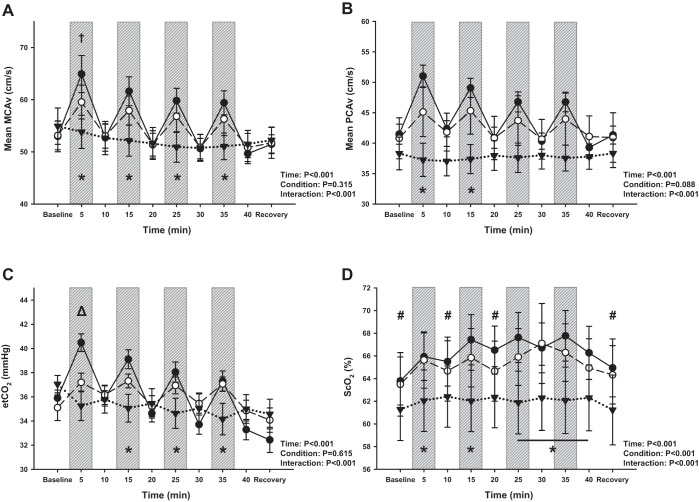
Mean MCAv and mean PCAv (Fig. 4, A and B) increased from baseline with each exercise bout for CE (P < 0.001) and returned back to baseline values during the subsequent rest periods. For BFRE mean MCAv also increased from baseline with exercise (P ≤ 0.002) and returned back to baseline values during the subsequent rest periods. For mean PCAv, N = 8 due to the difficulties associated with acquiring and maintaining the PCA signal throughout each of the three experiments performed in this study. Mean PCAv increased with exercise with BFRE and remained elevated from baseline during all but the third exercise period (P ≤ 0.002 for exercise periods 1 and 2; P = 0.08 for exercise period 4; P = 0.15 for exercise period 3). While mean MCAv was initially slightly higher with CE compared with BFRE during the first exercise period (P = 0.07), there were no differences between exercise conditions for the remainder of the intervention (P ≥ 0.27). This difference at the start of exercise was likely driven by etCO2, which was also higher with CE compared with BFRE at this same time point (P = 0.006; Fig. 4C). There were no differences in mean PCAv responses between CE and BFRE at any time points (P ≥ 0.13), although both were higher than RIPC during the first two exercise periods (P ≤ 0.03). Interestingly, mean MCAv progressively decreased over time with RIPC (P ≤ 0.05 vs. baseline by the second reperfusion period), likely driven by etCO2 (P ≤ 0.07 by the second occlusion period). Although there was a statistically distinguishable difference in ScO2 during baseline between CE and RIPC (P = 0.07), the absolute differences were small, 63.8 ± 2.1% vs. 61.2 ± 2.7%. The differences in ScO2 responses between trials (condition effect, P < 0.001) is likely driven by the exercise conditions (BFRE vs. RIPC, P = 0.007; CE vs. RIPC, P < 0.001), and there was no difference in the overall ScO2 response between CE and BFRE (P = 0.55; Fig. 4D).
Fig. 4.
Regional cerebral blood velocity [middle cerebral artery velocity (MCAv) and posterior cerebral artery velocity (PCAv)], cerebral oxygenation (ScO2), and end-tidal CO2 (etCO2) responses to conventional exercise (CE; ●), blood flow restriction exercise (BFRE; ○), and remote ischemic preconditioning (RIPC; ▼). Exercise bouts (and occlusion periods for BFRE and RIPC) denoted by vertical gray bars. MCAv (A, N = 13) increased with each exercise period for both CE and BFRE and was higher with CE and BFRE compared with RIPC (*P ≤ 0.08). Mean MCAv was also initially higher with CE compared with BFRE during the first exercise bout/occlusion period (†P = 0.07). Mean PCAv (B, N = 8) increased with exercise for CE and BFRE, although there were no differences between exercise conditions (P ≥ 0.13). Both CE and BFRE were higher than RIPC during the first two exercise periods (*P ≤ 0.03). etCO2 (C) increased with exercise for CE and BFRE and was initially higher with CE than BFRE (P = 0.006). Frontal lobe ScO2 (D, N = 14) was different between conditions (overall condition P < 0.001) with CE and BFRE higher than RIPC at several time points (P < 0.06). A two-way repeated measures ANOVA was performed to compare differences across time (factor 1) and between conditions (factor 2). Tukey post hoc tests were performed when an interaction effect was present. *P ≤ 0.08 for CE and BFRE vs. RIPC; ΔP ≤ 0.006 for CE vs. RIPC and BFRE; #P ≤ 0.07 for CE vs. RIPC; †P = 0.07 for CE vs. BFRE.
DISCUSSION
In this investigation we explored the sympathetic, hemodynamic, and cerebrovascular responses to an acute bout of cyclical BFRE and compared these responses to CE and cyclical blood flow restriction and reperfusion without exercise (i.e., RIPC). The major findings were the following: 1) in support of our hypothesis, we observed an attenuated increase in sympathetic activity with BFRE compared with CE, as indicated by lower plasma NE concentration; 2) also in support of our hypothesis, HR, MAP, stroke volume, cardiac output, and TPR responses were attenuated with BFRE compared with CE; and 3) the similar cerebrovascular responses between CE and BFRE suggest equivalent cerebro-metabolic demand between conditions.
The lower plasma NE with BFRE supports our hypothesis that there is an attenuated increase in sympathetic activity with BFRE compared with CE. This is likely due to the lower workloads used in the BFRE condition (20% 1RM vs. 65% 1RM). This finding is in contrast to previous work by Madarame et al. who reported higher NE responses to BFRE compared with CE in both healthy (32) and clinical (31) populations. However, both exercise conditions in these studies were performed at the same relative workload (20–30% 1RM), which is not consistent with the comparisons made in our study and thus could explain these divergent findings. More commonly, BFRE is prescribed at a lower relative workload than CE (28), which is why it is often adapted to elderly populations (4, 46, 59) or other individuals with musculoskeletal limitations (4, 36, 51). As such, we opted to compare CE with BFRE at the most commonly performed relative workloads (65% 1RM for CE vs. 20% 1RM for BFRE). While it is somewhat surprising that the combination of resistance exercise with thigh occlusion (the BFRE condition) did not elicit a greater increase in NE than thigh occlusion alone (the RIPC condition), we speculate that this is due to both the low workloads (20% 1RM) and cyclical nature of the stimulus used in the BFRE trial. Exercise performed at this workload is likely of insufficient intensity to exacerbate the exercise pressor reflex response resulting from the occlusive stimulus alone. Additionally, this attenuated sympathetic response may be related to the width of the occlusive cuffs (5 cm) and the occlusive pressure (220 mmHg). We speculate that wider cuffs and/or higher occlusive pressures would confer a greater restriction to arterial inflow (29) and a greater stimulation of type III and IV afferents, subsequently eliciting a magnified sympathetic response (reflected by elevated NE release) (54). It should be noted, however, that although higher cuff pressures are required for complete arterial restriction with a narrower cuff, when restrictive pressures are set relative to cuff width and individual subject responses, cuff widths of different sizes can still induce a similar degree of blood flow restriction (42). From the perspective of clinical application, however, this attenuated sympathoexcitatory response with BFRE is desirable in patient populations who are at an increased risk for adverse cardiovascular events.
We also observed an attenuated increase in HR, stroke volume, and MAP with BFRE compared with CE, which was accompanied by large reductions in TPR with CE that were not present with either BFRE or RIPC. Similarly, Poton and Polito (48) reported an attenuated increase in HR and arterial pressures with low-intensity resistance BFRE (20% 1RM; unilateral knee extension) in comparison to a high-intensity condition performed without blood flow restriction (80% 1RM). The investigators also included a condition where subjects performed low-intensity exercise at the same workload as the BFRE condition (20% 1RM), but without blood flow restriction. While the high-intensity condition elicited the greatest increase in arterial pressures, the low-intensity BFRE condition also induced greater increases in HR and systolic arterial pressure during the final set of exercise compared with the low-intensity condition that was performed without blood flow restriction (48). In contrast to our findings, however, there were no differences in stroke volume between conditions, whereas we observed an attenuated increase in stroke volume with BFRE compared with CE. This discrepancy may be due to differences in the exercise modality; Poton and Polito used unilateral knee extension compared with the bilateral leg press utilized in our investigation. While not assessed in the present investigation, we speculate that application of blood flow restriction to both thighs, rather than just one, would result in a greater decrease in venous return and subsequently stroke volume. A reduction in venous return with our BFRE condition is supported by data in the RIPC (occlusion only) condition that demonstrates a decrease in stroke volume during all of the occlusion periods (Fig. 3B). Although there were no differences in the absolute stroke volumes between BFRE and RIPC, the pattern of responses was quite different between conditions with stroke volume decreasing with each occlusion period with RIPC while increasing during the same period with BFRE. This pattern of responses suggests that engagement of the skeletal muscle pump, even with this low-intensity exercise, was sufficient to overcome the restriction in venous return resulting from the occlusions. We also observed a pronounced decrease in TPR from the start of exercise through the end of recovery with CE that was not present in the other two conditions. This is likely related to the higher workloads used in the CE condition as greater increases in metabolic demand would enhance the functional sympatholysis within the exercising muscle, causing a reduction in TPR, and subsequently eliciting increases in regional muscle blood flow.
Although we observed an attenuated increase in arterial pressures with BFRE compared with CE in young healthy subjects, we acknowledge that these responses to BFRE may be different in clinical populations. As highlighted by Spranger et al. (54), several clinical populations including patients with hypertension, heart failure, and peripheral artery disease exhibit augmented activation of the exercise pressor reflex, which could be further exacerbated by blood flow restriction training. In support of this perspective, one recent study demonstrated that low-intensity (20% 1RM) leg press performed with blood flow restriction elicited a greater increase in arterial pressures compared with high-intensity exercise (65% 1RM) in hypertensive women (47). Importantly, however, this study used a restrictive pressure that resulted in complete arterial occlusion (an uncommon practice for BFRE), which may have augmented the arterial pressure response. Studies investigating the acute hemodynamic responses to RIPC in either healthy or clinical populations are surprisingly scarce. One study by Li et al. did assess the effects of five cycles of unilateral arm RIPC (5 min occlusion/5 min reperfusion) on HR and arterial pressure in healthy subjects and subjects with unilateral middle cerebral artery stenosis (24). While there were no changes in HR and arterial pressure in either group during the RIPC stimulus, the authors did report a reduction in MAP and HR 30 min after the final cuff deflation period in the healthy subjects that was not present in the patient population. As such, caution should be taken when generalizing our findings to clinical populations; additional investigations are necessary to determine if our cyclical BFRE paradigm would be appropriate in patient groups of interest, such as individuals in cardiac and stroke rehabilitation.
The similarity in cerebrovascular responses between the two exercise trials suggests an equivalent cerebro-metabolic demand. Although mean MCAv was initially higher at the start of CE, this is likely due to the greater metabolic production of CO2 (reflected in the etCO2 response), which would stimulate an increase in cerebral blood flow (44). While several studies have assessed the cerebral blood flow responses to resistance exercise using small muscle groups such as hand grip (12, 20, 26), few studies have measured the cerebrovascular responses to resistance exercise of large muscle mass such as the leg press modality used in the present investigation. Dickerman et al. demonstrated that one repetition of maximal leg press resulted in a decrease in MCAv (10), potentially due to performance of the Valsalva maneuver, and subsequent increases in intracranial pressure. In contrast, two other investigations reported increases in MCAv during one set of 10 repetitions of leg press performed at a workload corresponding to estimated 10-rep max (40, 50). Importantly subjects were instructed to breathe throughout each leg press, hence avoiding the Valsalva maneuver and increases in intracranial pressure and subsequently facilitating the observed increases in MCAv. Studies assessing the cerebrovascular responses to an acute bout of RIPC or BFRE are very limited. In the aforementioned RIPC study, Li et al. reported that MCAv and ScO2 did not change in response to five cycles of unilateral arm RIPC in either healthy subjects or in patients with cerebral artery stenosis (24). In one of the only studies assessing cerebral responses to BFRE, Ganesan et al. assessed cerebral oxygen saturation (but not cerebral blood flow) in subjects performing three sets of bilateral knee extensions to volitional fatigue at 50% 1RM with and without blood flow restriction (14). Since the cerebral NIRS signal is predominantly obtained from venous blood (75%), we interpret an increase in deoxyhemoglobin (dHb) and decrease in oxyhemoglobin (HbO2) as an increase in tissue oxygen extraction, particularly if there is no difference in oxygen supply (i.e., MCAv). So, while ScO2 increased with exercise in both trials, the BFRE condition resulted in higher dHb and lower HbO2 compared with the nonocclusive conditions, suggesting a greater oxygen extraction with the BFRE condition. As ratings of perceived exertion were also higher with the BFRE condition, the higher cerebral oxygen extraction may be related to this response. This interpretation is limited, however, as cerebral blood flow (oxygen supply) was not assessed. In contrast, while we did not measure ratings of perceived exertion in our study, we did not observe any differences in ScO2 responses between the two exercise conditions. It is possible that the perceived exertion with low-intensity BFRE is comparable to high-intensity CE, resulting in these similar ScO2 responses, although this interpretation is speculative.
There are several methodological considerations that must be addressed when interpreting these findings. First, we utilized transcranial Doppler ultrasound for measurements of cerebral blood velocity, rather than performing direct measures of cerebral blood flow. This technique is dependent on the assumption that the diameter of the insonated artery does not change. Two recent studies, however, have demonstrated that periods of hypercapnia (+9 mmHg) or hypocapnia (−13 mmHg) can change MCA diameter (assessed via high resolution MRI) (8, 55). The etCO2 responses elicited by the interventions used in the present investigation, however, are lower in magnitude (+5 mmHg) than reported in these studies. Furthermore, even if this degree of hypercapnia did elicit an increase in MCA diameter, measurement of velocity in the MCA would actually result in an underestimation of cerebral blood flow responses. While the effects of perturbations in arterial CO2 on PCA diameter are unknown, this potential limitation is mitigated by the observation that there were no differences in mean PCAv responses between the two exercise conditions.
Second, our ScO2 measurement is assessed indirectly via cerebral NIRS. This measurement approach is potentially contaminated by skin blood flow, which would be expected to increase during exercise (39). The spatially resolved NIRS sensor used in this investigation, however, minimizes this limitation by using multiple emitters at varying distances from the detector, allowing blood flow from the skin, muscle, and fat layers to be mathematically removed from the final signal. This technique is also limited by our ability to only assess changes in oxygenation of the frontal lobe tissue. While global measurements of cerebral oxygenation may be more informative, these measurements require invasive (such as arterial-venous blood sampling across the brain) and/or impractical techniques (such as functional MRI) under the dynamic conditions of the exercise protocols used in the present investigation. Furthermore, by combining our NIRS-derived ScO2 measurements with simultaneous measurements of cerebral blood velocity within the same region (MCA), this allows high temporal resolution for estimation of oxygen extraction, as previously described.
A third methodological consideration is the use of plasma NE as an index of sympathetic activation, rather than direct recordings of muscle sympathetic nerve activity (MSNA) via microneurography. While MSNA would have been a more direct assessment of sympathetic activity, the repeated measures design that was employed in this study, combined with the technical difficulties of maintaining a nerve recording during exercise, required the use of plasma NE as an index of sympathetic drive. Despite this limitation, however, there is a direct relationship between venous NE and sympathetic neural activity (15), so we are confident that plasma NE is representative of general increases in sympathetic outflow within this investigation. Furthermore, we cannot exclude the potential role of pain resulting from the restrictive stimulus of cuff inflation. This discomfort could potentially increase sympathetic outflow independent of the exercise bouts. While the effect of pain was not directly assessed in our investigation, all subjects were able to tolerate this stimulus and no experiments were terminated due to the effects of pain.
An additional consideration is the length of time between each of the three experiments. Although the 1-mo intervening period between experiments allowed for control of menstrual cycle phase in our female subjects, this length of time could have also increased the variability in the key outcomes. This potential confound was minimized, however, with our randomized, counterbalanced design, and the robust results (reflected in the statistical comparisons) supports this contention.
When relating our findings to other similar investigations, the width of the occlusive cuff that was used (5 cm in the present study) should be considered, as previously discussed. As highlighted by Loenneke et al. (29), differences in cuff width can have profound influences on the degree of arterial occlusion that is achieved. In a recent review, Spranger et al. also indicated that these differences in cuff width can also directly affect the magnitude of exercise pressor reflex, with wider cuffs resulting in greater activation of type III and IV afferent nerves (54), presumably due to greater vascular occlusion and compression of the muscle mass. Based on these considerations, comparison of our results with other investigations should be performed judiciously if different cuff sizes and occlusive pressures were used. More work is clearly required to determine optimal occlusive pressures and cuff widths to elicit the key target outcomes with BFR. Indeed, these outcomes themselves, in addition to the target population of interest, may dictate variations in the degree of occlusion required. For example, while some RIPC literature has used cuff widths or pressures that would likely result in complete arterial occlusion (19, 37, 38), most of the BFRE literature suggests that submaximal occlusions are likely sufficient to induce muscular hypertrophy (7, 27). Based on these differences between BFRE and RIPC protocols, it is possible that the submaximal occlusions that were used in this investigation may not confer the same degree of cardio- and cerebro-protection as the maximal occlusions more commonly applied with RIPC. Because this is the first investigation to model BFRE around the RIPC paradigm, future work should seek to determine if an optimal pressure can be identified that elicits the beneficial effects of both RIPC and BFRE.
Additionally, when relating the findings of our study to other BFRE investigations, the total training volume utilized in the BFRE condition (3 sets of 10 repetitions) should be considered. While we selected this model to match the total volume between the CE and BFRE conditions, it should be noted that many BFRE studies focusing on muscular hypertrophy have either performed BFRE to failure or have used four sets (first set of 30 repetitions, then 3 sets of 15 repetitions) (34, 58, 59). As such, it is possible that the BFRE protocol used in our investigation would elicit an attenuated muscular hypertrophic response compared with these other models, although this is speculative, and remains to be tested experimentally.
One final consideration in our experimental design is that we did not include a condition with a load corresponding to 20% of 1RM performed without blood flow restriction. The reasons for this are twofold. First, a primary comparison of interest was between BFRE and RIPC to determine the effects of combining low-intensity exercise with RIPC. Second, conventional guidelines recommend resistance exercise loads of 65% 1RM or greater to achieve appreciable gains in muscle mass (3). In comparison, 20% 1RM (when not taken to failure) would not generally be utilized in practice as it may not elicit an optimal physiological benefit. When taken to failure, however, lower loads have been shown to induce a comparable degree of muscular hypertrophy as observed with heavier training loads (41). Furthermore, as previously indicated, one study has compared the hemodynamic responses to unilateral knee extensions performed under three different conditions: low intensity (20% 1RM), low intensity with blood flow restriction (20% 1RM), and high intensity (80% 1RM). As expected, the low-intensity BFRE condition elicited a greater increase in sympathetic activity (manifest by a higher HR and arterial pressure) in comparison to the low-intensity condition without blood flow restriction. Also as expected, and similar to the results observed in our investigation, the BFRE condition elicited a lower arterial pressure response compared with the high-intensity condition (48). These observations support those reported in our investigation in that BFRE elicited an attenuated increase in sympathetic drive compared with CE likely due to the use of lower workloads.
Perspectives and Significance
In this investigation we demonstrated an attenuated increase in sympathetic activity and hemodynamic responses with a novel BFRE paradigm modeled after RIPC. The similarity in cerebrovascular responses also suggests equivalent cerebro-metabolic demand between this model and resistance exercise as conventionally prescribed. Based on these observations, this cyclical BFRE model could potentially be adapted to clinical populations including patients participating in cardiac- or stroke-rehabilitation programs, where individuals might benefit from the RIPC stimulus superimposed with resistance exercise. As previously mentioned, recent clinical trials have already demonstrated that daily RIPC can be used as a preventative strategy to decrease the incidence of stroke and increase cerebral blood flow in patients with significant carotid artery stenosis (37, 38). As many patients participating in rehabilitation programs are already engaging in exercise, this setting would be an ideal target for implementation of an RIPC-like stimulus, such as the novel BFRE paradigm explored in this investigation.
A question that remains to be answered, however, is whether the beneficial adaptations to resistance training performed conventionally or with blood flow restriction, are still present when using this novel cyclical BFRE paradigm. For example, muscular hypertrophy and increased strength are well documented with BFRE when performed as traditionally prescribed with continuous vascular occlusion/cuff inflation (21, 27, 58). As we only explored the acute responses to a single cyclical BFRE session, future work is required to determine if exercise training with this novel BFRE paradigm elicits favorable cardiovascular and musculoskeletal adaptations, including muscle hypertrophy, increased bone density, improved vascular function, and reduced resting arterial pressure and heart rate. Similarly, as most RIPC protocols utilize cuff pressures that result in complete cessation of arterial inflow, further work is also needed to confirm that the cardio- and cerebro-protective effects associated with RIPC can also be extended to the submaximal occlusions that were used in this investigation.
GRANTS
This study was supported, in part, by training fellowships awarded to Justin Sprick through a National Institutes of Health-supported Neurobiology of Aging Training Grant (T32 AG020494, principal investigator: M. Singh), and a Ruth L. Kirchstein National Research Service Award F31 Predoctoral Fellowship (1F31HL134242; principal investigator: J. D. Sprick), a UNTHSC Faculty Research Pilot Grant (principal investigator: C. A. Rickards), and a Texas Chapter of the American College of Sports Medicine (TACSM) Student Research Development Award (principal investigator: J. D. Sprick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.D.S. and C.A.R. conceived and designed research; J.D.S. and C.A.R. performed experiments; J.D.S. analyzed data; J.D.S. and C.A.R. interpreted results of experiments; J.D.S. prepared figures; J.D.S. drafted manuscript; J.D.S. and C.A.R. edited and revised manuscript; J.D.S. and C.A.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank our subjects for their time and cooperation, Drs. Albert Yurvati, Sibi Thomas, and Angela Njoku for assistance with subject medical reviews, and Hannah Colby, Jace Coon, Eric Chang, Travis Schaefer, and Tyler Petree for assistance with data collection.
REFERENCES
- 1.Ahmed RM, Mohamed HA, Ashraf M, Maithili S, Nabil F, Rami R, Mohamed TI. Effect of remote ischemic preconditioning on serum troponin T level following elective percutaneous coronary intervention. Catheter Cardiovasc Interv 82: E647–E653, 2013. doi: 10.1002/ccd.24825. [DOI] [PubMed] [Google Scholar]
- 2.Aidar FJ, de Oliveira RJ, de Matos DG, Mazini Filho ML, Moreira OC, de Oliveira CE, Hickner RC, Reis VM. A Randomized Trial Investigating the Influence of Strength Training on Quality of Life in Ischemic Stroke. Top Stroke Rehabil 23: 84–89, 2016. doi: 10.1080/10749357.2015.1110307. [DOI] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription, edited by Pescatello LS, Arena R, Riebe D, Thompson PD. Baltimore, MD: Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Buford TW, Fillingim RB, Manini TM, Sibille KT, Vincent KR, Wu SS. Kaatsu training to enhance physical function of older adults with knee osteoarthritis: Design of a randomized controlled trial. Contemp Clin Trials 43: 217–222, 2015. doi: 10.1016/j.cct.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47: 2277–2282, 2006. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 6.Coke LA, Staffileno BA, Braun LT, Gulanick M. Upper-body progressive resistance training improves strength and household physical activity performance in women attending cardiac rehabilitation. J Cardiopulm Rehabil Prev 28: 238–245, 2008. doi: 10.1097/01.HCR.0000327180.29122.83. [DOI] [PubMed] [Google Scholar]
- 7.Counts BR, Dankel SJ, Barnett BE, Kim D, Mouser JG, Allen KM, Thiebaud RS, Abe T, Bemben MG, Loenneke JP. Influence of relative blood flow restriction pressure on muscle activation and muscle adaptation. Muscle Nerve 53: 438–445, 2016. doi: 10.1002/mus.24756. [DOI] [PubMed] [Google Scholar]
- 8.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- 9.Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv 6: 246–251, 2013. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 10.Dickerman RD, McConathy WJ, Smith GH, East JW, Rudder L. Middle cerebral artery blood flow velocity in elite power athletes during maximal weight-lifting. Neurol Res 22: 337–340, 2000. doi: 10.1080/01616412.2000.11740679. [DOI] [PubMed] [Google Scholar]
- 11.Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension 14: 511–517, 1989. doi: 10.1161/01.HYP.14.5.511. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes IA, Mattos JD, Campos MO, Machado AC, Rocha MP, Rocha NG, Vianna LC, Nobrega AC. Selective α1-adrenergic blockade disturbs the regional distribution of cerebral blood flow during static handgrip exercise. Am J Physiol Heart Circ Physiol 310: H1541–H1548, 2016. doi: 10.1152/ajpheart.00125.2016. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher SM, Jones DA, Kapur A, Wragg A, Harwood SM, Mathur R, Archbold RA, Uppal R, Yaqoob MM. Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney Int 87: 473–481, 2015. doi: 10.1038/ki.2014.259. [DOI] [PubMed] [Google Scholar]
- 14.Ganesan G, Cotter JA, Reuland W, Cerussi AE, Tromberg BJ, Galassetti P. Effect of blood flow restriction on tissue oxygenation during knee extension. Med Sci Sports Exerc 47: 185–193, 2015. doi: 10.1249/MSS.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension 5: 552–559, 1983. doi: 10.1161/01.HYP.5.4.552. [DOI] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 370: 575–579, 2007. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 17.Ivey FM, Prior SJ, Hafer-Macko CE, Katzel LI, Macko RF, Ryan AS. Strength Training for Skeletal Muscle Endurance after Stroke. J Stroke Cerebrovasc Dis 26: 787–794, 2017. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen JR, Wesseling KH, Settels JJ, Schreuder JJ. Continuous cardiac output monitoring by pulse contour during cardiac surgery. Eur Heart J 11, Suppl I: 26–32, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Jones H, Nyakayiru J, Bailey TG, Green DJ, Cable NT, Sprung VS, Hopkins ND, Thijssen DH. Impact of eight weeks of repeated ischaemic preconditioning on brachial artery and cutaneous microcirculatory function in healthy males. Eur J Prev Cardiol 22: 1083–1087, 2015. doi: 10.1177/2047487314547657. [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen LG, Perko G, Payne G, Secher NH. Effect of limb anesthesia on middle cerebral response to handgrip. Am J Physiol 264: H553–H559, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Karabulut M, Abe T, Sato Y, Bemben MG. The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol 108: 147–155, 2010. doi: 10.1007/s00421-009-1204-5. [DOI] [PubMed] [Google Scholar]
- 22.Kawauchi TS, Umeda II, Braga LM, Mansur AP, Rossi-Neto JM, Guerra de Moraes Rego Sousa A, Hirata MH, Cahalin LP, Nakagawa NK. Is there any benefit using low-intensity inspiratory and peripheral muscle training in heart failure? A randomized clinical trial. Clin Res Cardiol 106: 676–685, 2017. doi: 10.1007/s00392-017-1089-y. [Erratum in Clin Res Cardiol 106: 764–765, 2017. doi:. ]. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106: 2881–2883, 2002. doi: 10.1161/01.CIR.0000043806.51912.9B. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Ma C, Shao G, Esmail F, Hua Y, Jia L, Qin J, Ren C, Luo Y, Ding Y, Borlongan CV, Ji X. Safety and Feasibility of Remote Limb Ischemic Preconditioning in Patients With Unilateral Middle Cerebral Artery Stenosis and Healthy Volunteers. Cell Transplant 24: 1901–1911, 2015. doi: 10.3727/096368914X683520. [DOI] [PubMed] [Google Scholar]
- 25.Libardi CA, Chacon-Mikahil MP, Cavaglieri CR, Tricoli V, Roschel H, Vechin FC, Conceição MS, Ugrinowitsch C. Effect of concurrent training with blood flow restriction in the elderly. Int J Sports Med 36: 395–399, 2015. doi: 10.1055/s-0034-1390496. [DOI] [PubMed] [Google Scholar]
- 26.Linkis P, Jørgensen LG, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean flow velocity. J Appl Physiol (1985) 78: 12–16, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Lixandrão ME, Ugrinowitsch C, Laurentino G, Libardi CA, Aihara AY, Cardoso FN, Tricoli V, Roschel H. Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur J Appl Physiol 115: 2471–2480, 2015. doi: 10.1007/s00421-015-3253-2. [DOI] [PubMed] [Google Scholar]
- 28.Loenneke JP, Abe T, Wilson JM, Ugrinowitsch C, Bemben MG. Blood flow restriction: how does it work? Front Physiol 3: 392, 2012. doi: 10.3389/fphys.2012.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loenneke JP, Fahs CA, Rossow LM, Sherk VD, Thiebaud RS, Abe T, Bemben DA, Bemben MG. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol 112: 2903–2912, 2012. doi: 10.1007/s00421-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loenneke JP, Wilson GJ, Wilson JM. A mechanistic approach to blood flow occlusion. Int J Sports Med 31: 1–4, 2010. doi: 10.1055/s-0029-1239499. [DOI] [PubMed] [Google Scholar]
- 31.Madarame H, Kurano M, Fukumura K, Fukuda T, Nakajima T. Haemostatic and inflammatory responses to blood flow-restricted exercise in patients with ischaemic heart disease: a pilot study. Clin Physiol Funct Imaging 33: 11–17, 2013. doi: 10.1111/j.1475-097X.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 32.Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N. Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc 40: 258–263, 2008. doi: 10.1249/mss.0b013e31815c6d7e. [DOI] [PubMed] [Google Scholar]
- 33.Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol 58: 541–560, 1999. doi: 10.1016/S0301-0082(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 34.Martín-Hernández J, Marín PJ, Menéndez H, Ferrero C, Loenneke JP, Herrero AJ. Muscular adaptations after two different volumes of blood flow-restricted training. Scand J Med Sci Sports 23: e114–e120, 2013. doi: 10.1111/sms.12036. [DOI] [PubMed] [Google Scholar]
- 35.Marzolini S, Oh PI, Brooks D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: a meta-analysis. Eur J Prev Cardiol 19: 81–94, 2012. doi: 10.1177/1741826710393197. [DOI] [PubMed] [Google Scholar]
- 36.Mattar MA, Gualano B, Perandini LA, Shinjo SK, Lima FR, Sá-Pinto AL, Roschel H. Safety and possible effects of low-intensity resistance training associated with partial blood flow restriction in polymyositis and dermatomyositis. Arthritis Res Ther 16: 473, 2014. doi: 10.1186/s13075-014-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, Li G, Ren C, Luo Y, Ling F, Jia J, Hua Y, Wang X, Ding Y, Lo EH, Ji X. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 79: 1853–1861, 2012. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 38.Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, Shi J, Duan Y, Sun Z, Yu Y, Jia J, Ji X. Ischemic Conditioning Is Safe and Effective for Octo- and Nonagenarians in Stroke Prevention and Treatment. Neurotherapeutics 12: 667–677, 2015. doi: 10.1007/s13311-015-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyazawa T, Horiuchi M, Komine H, Sugawara J, Fadel PJ, Ogoh S. Skin blood flow influences cerebral oxygenation measured by near-infrared spectroscopy during dynamic exercise. Eur J Appl Physiol 113: 2841–2848, 2013. doi: 10.1007/s00421-013-2723-7. [DOI] [PubMed] [Google Scholar]
- 40.Moralez G, Romero SA, Rickards CA, Ryan KL, Convertino VA, Cooke WH. Effects of dehydration on cerebrovascular control during standing after heavy resistance exercise. J Appl Physiol (1985) 112: 1875–1883, 2012. doi: 10.1152/japplphysiol.01217.2011. [DOI] [PubMed] [Google Scholar]
- 41.Morton RW, Oikawa SY, Wavell CG, Mazara N, McGlory C, Quadrilatero J, Baechler BL, Baker SK, Phillips SM. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J Appl Physiol (1985) 121: 129–138, 2016. doi: 10.1152/japplphysiol.00154.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouser JG, Dankel SJ, Jessee MB, Mattocks KT, Buckner SL, Counts BR, Loenneke JP. A tale of three cuffs: the hemodynamics of blood flow restriction. Eur J Appl Physiol 117: 1493–1499, 2017. doi: 10.1007/s00421-017-3644-7. [DOI] [PubMed] [Google Scholar]
- 43.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 44.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol (1985) 107: 1370–1380, 2009. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 45.Pinaud F, Corbeau JJ, Baufreton C, Binuani JP, De Brux JL, Fouquet O, Angoulvant D, Furber A, Prunier F. Remote ischemic preconditioning in aortic valve surgery: Results of a randomized controlled study. J Cardiol 67: 36–41, 2016. doi: 10.1016/j.jjcc.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Pinto RR, Karabulut M, Poton R, Polito MD. Acute resistance exercise with blood flow restriction in elderly hypertensive women: haemodynamic, rating of perceived exertion and blood lactate. Clin Physiol Funct Imaging; Epub ahead of print, 2016. [DOI] [PubMed] [Google Scholar]
- 47.Pinto RR, Polito MD. Haemodynamic responses during resistance exercise with blood flow restriction in hypertensive subjects. Clin Physiol Funct Imaging 36: 407–413, 2016. doi: 10.1111/cpf.12245. [DOI] [PubMed] [Google Scholar]
- 48.Poton R, Polito MD. Hemodynamic response to resistance exercise with and without blood flow restriction in healthy subjects. Clin Physiol Funct Imaging 36: 231–236, 2016. doi: 10.1111/cpf.12218. [DOI] [PubMed] [Google Scholar]
- 49.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87: 893–899, 1993. doi: 10.1161/01.CIR.87.3.893. [DOI] [PubMed] [Google Scholar]
- 50.Romero SA, Cooke WH. Hyperventilation before resistance exercise: cerebral hemodynamics and orthostasis. Med Sci Sports Exerc 39: 1302–1307, 2007. doi: 10.1249/mss.0b013e3180653636. [DOI] [PubMed] [Google Scholar]
- 51.Segal NA, Williams GN, Davis MC, Wallace RB, Mikesky AE. Efficacy of blood flow-restricted, low-load resistance training in women with risk factors for symptomatic knee osteoarthritis. PM R 7: 376–384, 2015. doi: 10.1016/j.pmrj.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu R, Hotta K, Yamamoto S, Matsumoto T, Kamiya K, Kato M, Hamazaki N, Kamekawa D, Akiyama A, Kamada Y, Tanaka S, Masuda T. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol 116: 749–757, 2016. doi: 10.1007/s00421-016-3328-8. [DOI] [PubMed] [Google Scholar]
- 53.Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE, Investigators C; CONDI Investigators . Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 35: 168–175, 2014. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 54.Spranger MD, Krishnan AC, Levy PD, O’Leary DS, Smith SA. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Heart Circ Physiol 309: H1440–H1452, 2015. doi: 10.1152/ajpheart.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54a.Sprick JD, Rickards CA. Combining remote ischemic preconditioning and aerobic exercise: a novel adaptation of blood flow restriction exercise. Am J Physiol Regul Integr Comp Physiol 313, 2017. doi: 10.1152/ajpregu.00111.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 56.Wist S, Clivaz J, Sattelmayer M. Muscle strengthening for hemiparesis after stroke: A meta-analysis. Ann Phys Rehabil Med 59: 114–124, 2016. doi: 10.1016/j.rehab.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Xie JJ, Liao XL, Chen WG, Huang DD, Chang FJ, Chen W, Luo ZL, Wang ZP, Ou JS. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing heart valve surgery: randomised controlled trial. Heart 98: 384–388, 2012. doi: 10.1136/heartjnl-2011-300860. [DOI] [PubMed] [Google Scholar]
- 58.Yasuda T, Fujita S, Ogasawara R, Sato Y, Abe T. Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: a pilot study. Clin Physiol Funct Imaging 30: 338–343, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Yasuda T, Fukumura K, Uchida Y, Koshi H, Iida H, Masamune K, Yamasoba T, Sato Y, Nakajima T. Effects of Low-Load, Elastic Band Resistance Training Combined With Blood Flow Restriction on Muscle Size and Arterial Stiffness in Older Adults. J Gerontol A Biol Sci Med Sci 70: 950–958, 2015. doi: 10.1093/gerona/glu084. [DOI] [PubMed] [Google Scholar]