Abstract
Intravesical prostaglandin E2 (PGE2) was previously used to induce overactive bladder (OAB) symptoms, as it reduces bladder capacity in rats and causes a “strong urgency sensation” in healthy women. However, the mechanism by which this occurs is unclear. To clarify how PGE2 reduces bladder capacity, 100 µM PGE2 was administered intravesically during open, single-fill cystometry with simultaneous measurement of sphincter EMG in the urethane-anesthetized female Wistar rat. PGE2 was also applied to the urethra or bladder selectively by use of a ligature at the bladder neck before (urethra) or during (bladder) closed-outlet, single-fill cystometry. Additional tests of urethral perfusion with PGE2 were made. PGE2 decreased bladder capacity, increased voiding efficiency, and increased sphincter EMG during open cystometry compared with saline controls. The number of nonvoiding contractions did not change with PGE2; however, bladder compliance decreased. During closed-outlet cystometry, PGE2 applied only to the bladder or the urethra did not decrease bladder capacity. Urethral infusion of PGE2 decreased urethral perfusion pressure. Taken together, these results suggest that intravesical PGE2 may decrease bladder capacity by targeting afferents in the proximal urethra. This may occur through urethral relaxation and decreased bladder compliance, both of which may increase activation of proximal urethra afferents from distension of the proximal urethra. This hypothesis stands in contrast to many hypotheses of urgency that focus on bladder dysfunction as the primary cause of OAB symptoms. Targeting the urethra, particularly urethral smooth muscle, may be a promising avenue for the design of drugs and devices to treat OAB.
Keywords: urinary urgency, overactive bladder, prostaglandin E2, bladder, urethra
overactive bladder (OAB) is a significant medical problem that negatively impacts quality of life and costs billions of dollars per year to treat (18, 28). Present treatments include lifestyle changes, physical therapy, medication, electrical stimulation, and surgery. Despite numerous advances in the last decade to treat the symptoms of OAB, a need for more effective therapies remains.
OAB is a set of symptoms that likely results from numerous etiologies. Because no particular animal model replicates the conditions of every individual, it may be useful to work with multiple models that each capture some aspect of disease that is representative of some portion of the population.
Intravesical infusion of prostaglandin E2 (PGE2) is one model of OAB, but the mechanism of action responsible for decreasing bladder capacity is unclear. PGE2 is a signaling molecule that is present throughout the body, including the lower urinary tract (23). Initial studies showed elevated levels of urinary PGE2 in men and women with OAB symptoms (8, 9), but these results were not reproduced in subsequent studies (10, 15). However, intravesical infusion of PGE2 (85 µM) in healthy women caused a much stronger desire to void at lower bladder volumes (27). Intravesical infusion of PGE2 in rats reduces bladder capacity (12, 17), which in animal models is used as an indicator of symptoms of OAB. A better understanding of the model should provide insight into why a particular OAB therapy works (or does not) in the model, as well as provide insight into OAB pathophysiology.
There are several hypotheses as to how PGE2 reduces bladder capacity. The first is that PGE2 causes bladder hypersensitivity, which decreases bladder capacity. This is based primarily on a study showing that systemic administration of capsaicin, causing degeneration of c fibers, prevented intravesical PGE2 from decreasing bladder capacity (17). However, this observation may not capture all the effects that PGE2 has on the lower urinary tract. Alternatively, intravesical PGE2 may cause detrusor overactivity (DO) (25, 26). However, the reported presence of DO often lacks formal analysis, and the presence of DO may be incorrectly attributed to nonspecific terms, such as hyperactive bladder.
In this study, we characterized the response of the lower urinary tract to PGE2 during open cystometry, as well as during closed-outlet (ligated urethra) cystometry with PGE2 applied selectively to either the isolated bladder or urethra. We use the term closed-outlet as opposed to isovolumetric cystometry because we were concerned with bladder filling, rather than rhythmic contractions of the bladder at a fixed volume. Intravesical PGE2 in healthy women caused a decrease in urethral closure pressure at rest (27), suggesting that urethral relaxation may contribute to OAB symptoms. Indeed, our results indicate that urethral relaxation and decreased bladder compliance contribute to the reduced bladder capacity observed in this model of OAB.
METHODS
All animal care and experimental procedures were reviewed and approved by the Duke University Institutional Animal Care and Use Committee.
Surgical Preparation and Equipment Setup
Female Wistar rats (n = 59) were anesthetized with urethane (1.2 g/kg sc, supplemented as necessary). Body temperature was monitored using an esophageal temperature probe and maintained at 36–38°C with a water blanket. Heart rate and arterial blood oxygen saturation levels were monitored using a pulse oximeter (Nonin Medical, 2500A VET).
For cystometrogram (CMG) measurements, the bladder was exposed via a midline abdominal incision. A PE-90 catheter (427420, BD Biosciences), the tip of which was heated to create a collar, was inserted into the bladder lumen through a small incision in the apex of the bladder dome, and a 6–0 silk suture (Teleflex Medical) was tied around the collar. In experiments in which the pudendal nerve was subsequently exposed, the abdominal wall was closed with 3–0 silk suture. The bladder catheter was connected via a three-way stopcock to an infusion pump (Braintree Scientific, BS-8000 or Harvard Apparatus PHD 4400) and to a pressure transducer (ArgoTrans, ArgonMedical Devices) connected to a bridge amplifier and filter (13–6615–50, Gould Instruments) for measuring intravesical pressure (IVP). The same type of equipment and setup was used to measure urethral pressure as well as in later experiments. Data were sampled at 1 kHz using a PowerLab system (AD Instruments).
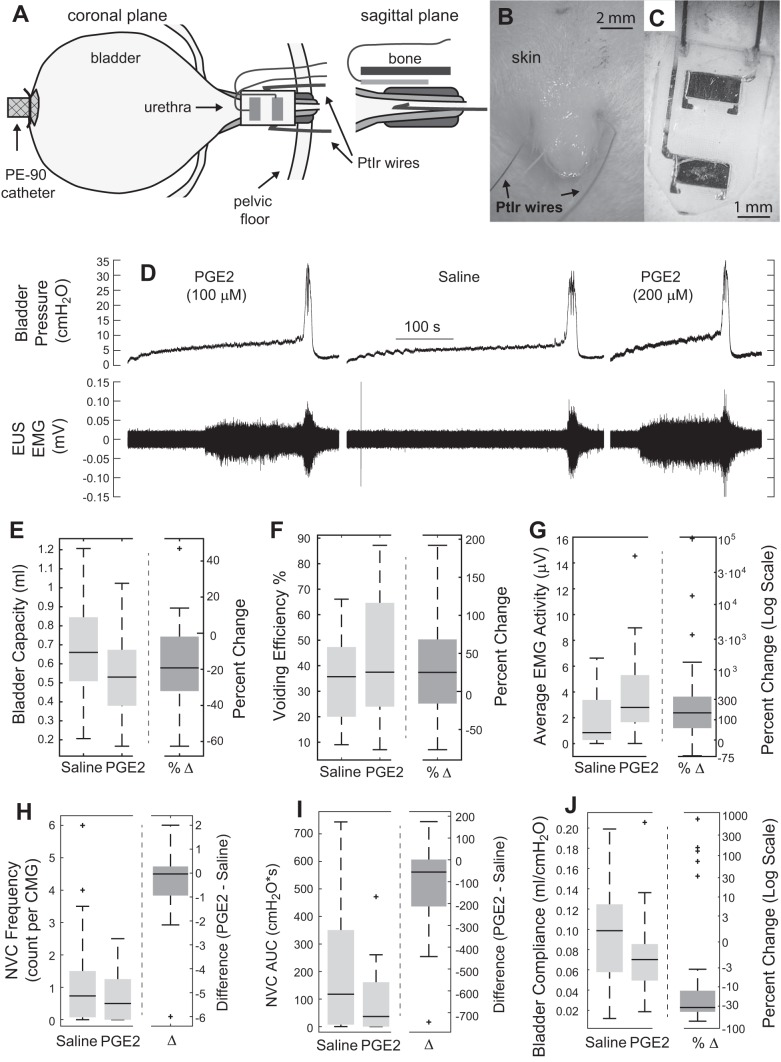
External urethral sphincter (EUS) EMG was measured using two different approaches (Fig. 1A). Most recordings used two PFA-coated platinum-iridium wires (0.0055-inch diameter, A-M Systems). These wires were inserted percutaneously using a needle to pierce the skin, one on each side of the urinary meatus (Fig. 1B). We hypothesized that a sheet-like interface would minimize the risk of damage from electrode placement compared with the percutaneous approach, which requires blind insertion of a needle into the tissue for electrode placement. Upon manufacturing and testing, we found that the sheet electrodes were easy to insert and that the signal quality was consistently higher than with the percutaneous approach. Because the observed changes in EMG with PGE2 remained the same (qualitatively) between the two approaches, all subsequent experiments used these new electrodes. These electrodes consisted of two platinum contacts bonded to a silicone backing with wires welded to each contact (Fig. 1C) (custom manufactured in laboratory and later manufactured by Micro-Leads). This sheet electrode was placed between the urethra and the pubic symphysis using an intra-abdominal approach. Blunt dissection of connective tissue between the pubic bone and the ventral portion of the urethra was performed to form a tissue pocket for insertion. Once the electrode had been placed on the ventral portion of the urethra, the wires were folded back over the pubic bone and sutured to the skin about halfway between the opening and the external meatus for strain relief. EUS EMG leads were connected through a preamplifier (HIP5, Grass Products) to an amplifier (P511, Grass Products). A subcutaneous needle served as ground. Signals were filtered (3 Hz–3 kHz) and sampled at 20 kHz.
Fig. 1.
Effects of intravesical administration of prostaglandin E2 (PGE2) on the bladder and external urethral sphincter (EUS) during open cystometry. A: illustration showing placement of the bladder catheter and electrodes for recording EUS electromyogram (EMG). 2 different electrode types were used (in different experiments), percutaneous wires (B) and flat metal contacts embedded in a silicone substrate (C) that was placed underneath the pubic bone. D: example cystometric traces from an experiment with 100 µM PGE2, followed by saline washout and 200 µM PGE2. Traces show a decreased bladder capacity from intravesical PGE2 compared with intravesical saline only, as well as increased EUS EMG activity during bladder filling. E: intravesical PGE2 decreased bladder capacity (P < 0.001, n = 33) and increased voiding efficiency (F) (P = 0.004, n = 33). Percent change was calculated as (PGE2 – saline)/saline. G: average EUS EMG activity over the last 20% of the filling period was increased during intravesical PGE2 compared with saline (P < 0.001, n = 29). H: frequency of nonvoiding contractions (NVCs) was not different between conditions. I: magnitude of NVCs was reduced in the intravesical PGE2 condition (P = 0.002, n = 32). J: intravesical PGE2 reduced bladder compliance during filling (P = 0.007, n = 32). Box plots were created using Matlab boxplot command in which center bars represent the median value, box edges are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points that are not considered to be outliers. Outliers are plotted individually. All percent change data are for reference and were not used for statistical calculations.
Results from open cystometry were collected as baseline data for subsequent experiments involving stimulation of the pelvic and pudendal nerves. In both cases, 100 µM PGE2 was determined to be sufficient to reduce bladder capacity. PGE2 (Sigma-Aldrich) was dissolved in ethanol to 10 mM concentration and stored in a −20°C freezer. On days of experiments, this stock solution was diluted in saline to the desired concentration. Data presented followed nerve cuff implantation but was before electrical stimulation (n = 27) or following stimulation but after a return to baseline (n = 6).
Additional experiments were conducted to examine further the effects of PGE2. These experiments involved isolating PGE2 to either the bladder only or the urethra only during closed-outlet single-fill cystometry. The bladder and urethra were isolated using a suture tied at the urethrovesical junction, identified by observation of nervous tissue that split distal to the major pelvic ganglion into pathways toward the bladder and urethra. This location is between the ureter and the ventral vaginal artery, rostral to the urethro-vaginal junction. When administering fluids to the urethra during closed-outlet cystometry, 32-g (n = 5) or PE-50 (n = 3) tubing was introduced from the meatus and advanced until the catheter tip abutted the suture, then withdrawn slightly. In one experiment in which the catheter was not withdrawn, the pressure data were excluded because of artifacts. EUS EMG was recorded in all experiments when administering PGE2 to the bladder but in only four of the eight experiments administering PGE2 to the urethra. In eight experiments in which PGE2 was administered to the bladder, a unilateral pelvic nerve transection was performed to record afferent (n = 4) or efferent (n = 4) activity. An additional six experiments were conducted with the nerve intact to confirm our observations.
In other experiments (n = 4), a PE-90 catheter was used for urethral perfusion. It was placed through the dome of the bladder and then sutured in place at the urethrovesical junction. The bladder was left open at the dome.
Experimental Procedures
Open cystometry.
The bladder was filled continuously with physiological saline at room temperature (2–8 ml/h) using an infusion pump with an open urethra for at least 45 min to allow postsurgical recovery. The bladder was subsequently emptied, and single-fill CMGs were recorded. For each CMG, the bladder was filled until a micturition event was observed, at which time the infusion pump was turned off. Approximately 1 min after the bladder pressure returned to baseline, the bladder was emptied via the catheter. Voided and residual volumes were recorded and used to calculate bladder capacity and voiding efficiency. The bladder remained empty for at least 3 min before the next trial. Following saline cystometry, continuous cystometry was repeated with PGE2 for an hour followed by single-fill CMG trials also with PGE2. At least two trials were collected under saline and PGE2 conditions.
Closed-outlet cystometry.
Following an initial period of continuous filling during open cystometry, the suture that had been placed around the bladder neck was tied off. The bladder was filled until a bladder contraction that matched the magnitude of previous voiding contractions developed. The pump was then stopped, and the bladder emptied. Following saline trials, multiple single fills were conducted with intravesical-only PGE2 administration (i.e., the urethra was not exposed to PGE2), emptying the bladder after each fill. After an hour of repeated fills, subsequent fills were used as trials for comparison to saline. At least two trials were collected from both conditions.
In other experiments, closed-outlet cystometry with saline was preceded by the infusion of fluid into the urethra. In the first two experiments, this consisted of infusing saline or PGE2 for 2 min at a rate of 1 ml/min. The trial then started 1 min after switching the urethral pump to a flow rate of 0.005 ml/min. In the other experiments (n = 6), each block of trials (intraurethral-only saline or PGE2) was preceded by 15 min of infusion at a rate of 0.1 ml/min. Each trial, except for the first in each block, was preceded by 3 min of infusion at 0.2 ml/min.
Urethral perfusion.
Trials consisted of 2 min of perfusing the urethra at 1 ml/min, followed by a pump rate of 0.005 ml/min for 10 min. PGE2 trials immediately followed the saline trials. In all but the first experiment, these trials were followed by saline-washout trials, as well as a single trial that occurred 5 min after euthanasia.
Data Analysis
All signals were collected using a PowerLab/16SP acquisition unit (AD Instruments) in conjunction with LabchartPro for visualization (versions 7 and 8, AD Instruments).
Bladder capacity was calculated as the sum of the residual and voided volumes. Voiding efficiency was calculated as the voided volume divided by the bladder capacity. For trials with a ligated urethra, the residual volume was used as the bladder capacity.
EUS EMG was high-pass filtered at 70 Hz using a first-order zero-phase Butterworth filter. The data were then rectified. The average value within the final 20% of the filling phase was calculated for comparison. Similar values calculated during the quiet period were subtracted out to remove baseline activity, except in the case of PGE2 to the urethra, in which urethral filling led to high baseline activity during the quiet period.
The number and size of nonvoiding contractions were quantified. Pressure traces were low-pass filtered using a first-order zero-phase Butterworth filter with a 5-Hz cutoff, and a line was fit to the pressure trace using Theil-Sen regression to minimize the impact that nonvoiding contractions (statistical outliers) had on the estimated slope. Residuals for each regression fit were calculated and normalized by the estimated standard deviation of all residuals (data from all trials across all animals). With the normalized residuals, nonvoiding contractions were classified as periods where the residuals exceeded two standard deviations from the mean for at least 2 s. Additionally, the residuals had to return to below threshold values. This meant that a contraction that led directly into the voiding contraction was not counted. For each trial, the area under the curve for nonvoiding contractions was calculated by multiplying the duration in which samples exceeded two standard deviations by the pressure at those points and summing the resulting per-contraction values.
The static urethral perfusion pressure was calculated as the average pressure from a period 3–8 min following the high-rate perfusion of fluid. When we measured pressure in the urethra during closed-outlet cystometry, the urethral pressure was prone to artifacts including increases in pressure following pumping fluid in the urethra or if the catheter touched the bladder neck suture. Thus urethral pressure was calculated as the average value of the lowest 25% of pressure points during filling.
For all statistical tests, trial values were averaged within animal stratified by trial type. A paired t-test was used for all statistical comparisons, with the exception of the Wilcoxon rank-sum test for comparing the number of nonvoiding contractions. P values <0.05 were considered statistically significant. All computations were performed in Matlab (Mathworks).
RESULTS
Open Cystometry
Open cystometry results are from 33 experiments. EMG data were not included in analysis for four experiments because of not recording EMG data (n = 3) and an incorrectly logged gain (n = 1). Pressure analysis was excluded because of a faulty pressure transducer (n = 1).
Intravesical instillation of 100 µM PGE2 decreased bladder capacity from 0.68 ± 0.04 ml to 0.54 ± 0.03 ml (P < 0.001, n = 33) and increased voiding efficiency from 31 ± 11% to 44 ± 5% (P = 0.003; Fig. 1). In addition to the decrease in bladder capacity, PGE2 generated an increase in EUS EMG activity during the latter portion of the filling phase (P < 0.001, n = 29). In an example experiment (Fig. 1D), saline washout resulted in an increase in bladder capacity and a reduction in EUS EMG activity. PGE2 did not change the number of nonvoiding contractions, but it decreased the magnitude of the contractions from 191 ± 36 to 84 ± 19 cmH2O × s (P = 0.002, n = 32). Bladder compliance was calculated by dividing the fill rate by the slope of bladder pressure vs. time. This slope was estimated by fitting a linear regression line to the middle 60% of the bladder pressure trace during filling. Note that bladder compliance is inversely proportional to the slope of bladder pressure over time during constant infusion, such that an increase in the observed slope during a cystometric trial corresponds to a reduction in bladder compliance. Bladder compliance decreased from 0.096 ± 0.009 to 0.073 ± 0.007 ml/cmH2O (P = 0.007, n = 32) following instillation of PGE2.
Closed-Outlet Cystometry
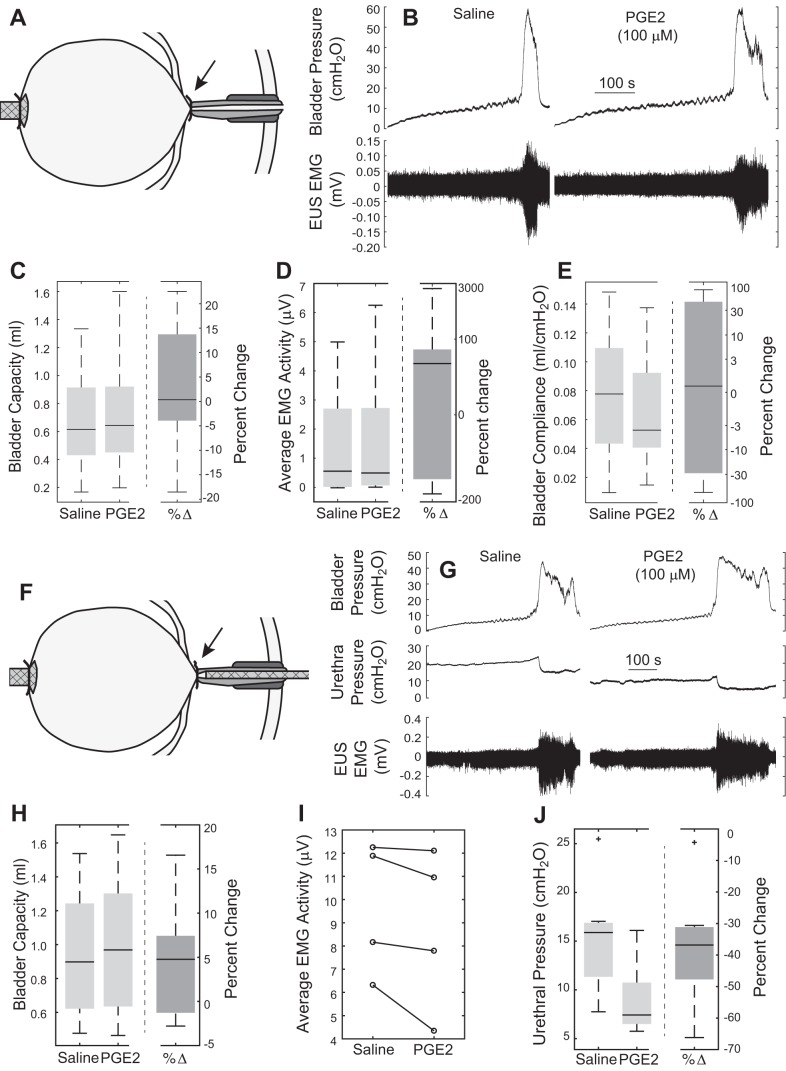
In other experiments, PGE2 was introduced to the bladder during closed-outlet (ligated urethra) cystometry. In contrast to effects during open cystometry, intravesical-only PGE2 administration did not reduce bladder capacity (Fig. 2C), even when stratified by the presence (n = 8) or absence (n = 6) of unilateral pelvic nerve transection. Average EUS EMG activity at the end of filling did not increase (Fig. 2D), nor did compliance decrease (Fig. 2E) following intravesical-only PGE2 administration.
Fig. 2.
Effects of administration of PGE2 to the bladder or urethra following ligation of the proximal urethra (closed-outlet cystometry). A: catheter for filling was placed in the dome of the bladder, and the bladder neck was ligated with suture. EMG electrodes were used but are not shown. B: example cystometric traces from an experiment using saline or PGE2 to fill the isolated bladder. No change was observed in bladder capacity (C), average EMG activity (D), or bladder compliance (E) (n = 14). F: catheter was used to administer PGE2 exclusively to the urethra following ligation of the proximal urethra. G: example cystometric traces and intraurethral pressure from an experiment infusing saline or PGE2 into the isolated urethra. H: no change in bladder capacity (n = 8) or increase in EUS EMG (I) was seen following PGE2 administration to the urethra, although there was a clear decrease in urethral pressure (P = 0.005, n = 7) (J). Arrows in A and F indicate the location of the ligature, just caudal to the ureters.
In other experiments with a ligated urethra, PGE2 was introduced into the urethra selectively via a separate catheter before the bladder filling with saline. Selective intraurethral PGE2 administration did not reduce bladder capacity during closed-outlet cystometry (Fig. 2H), despite a decrease in urethral pressure from 15.2 ± 2.1 to 9.1 ± 1.4 cmH2O (P = 0.005, n = 7, Fig. 2J). Furthermore, selective intraurethral PGE2 administration did not increase EUS EMG in any of the four experiments with EMG recordings (Fig. 2I).
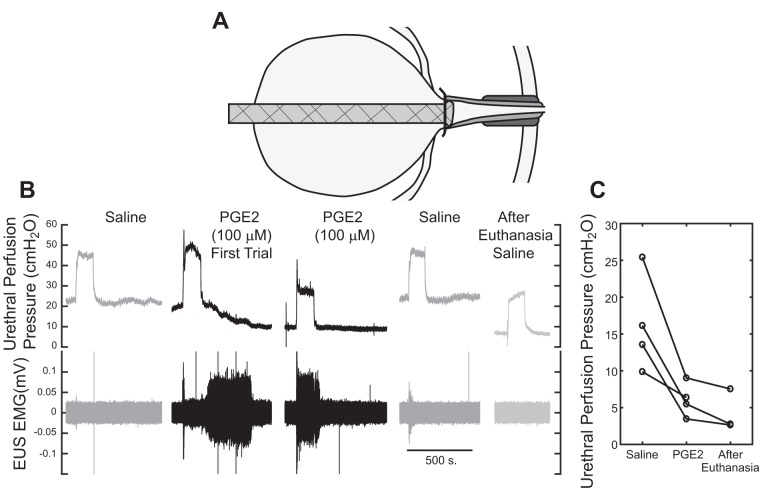
Urethral Perfusion
PGE2 (100 µM) was administered directly to the urethra via a catheter tied off at the bladder neck (Fig. 3A), whereas fluid from the ureters was allowed to drain via the dome of the bladder, keeping the bladder relatively empty and the system in continence mode. PGE2 reduced the urethral perfusion pressure, and the pressure increased following saline washout (Fig. 3B). On average, the urethral perfusion pressure decreased by 88% of the difference between saline pressures and pressures following euthanasia.
Fig. 3.
Effect of PGE2 administration through the urethra. A: catheter was placed through the dome of the bladder and tied off at the bladder neck. The ureters were allowed to empty via the incision in the bladder dome, keeping the bladder relatively empty. B: perfusion of PGE2 through the urethra reduced the steady-state urethral infusion pressure, and this effect was reversed following perfusion with saline after PGE2. A trial after euthanasia isolated the passive urethral perfusion pressure. C: PGE2 reduced the urethral perfusion pressure nearly to the level following euthanasia (n = 4).
DISCUSSION
Intravesically administered PGE2 in healthy women caused a “strong urgency sensation” resulting in reduced bladder capacity (27). This observation led to the use of intravesical PGE2 in animal models of OAB. Consistent with other rat studies (7, 12, 17), we observed a reduction in bladder capacity following intravesical PGE2 administration. A reduction in bladder capacity is commonly used as an indicator of OAB symptoms in animal models.
There are presently two hypotheses of how PGE2 reduces bladder capacity. The first is that PGE2 is an irritant, activating C-fiber afferents, and thereby reducing bladder capacity. The second is that PGE2 causes DO, leading to reduced bladder capacity. These theories are discussed below in the context of our results, and we propose a new theory based on increased activation of urethral afferents.
Decrease in Bladder Capacity
Intravesical PGE2 (100 µM) was sufficient to reduce bladder capacity in open cystometry (Fig. 1E), whereas PGE2 administered selectively to the bladder or urethra alone was insufficient to alter bladder capacity during closed-outlet cystometry (Fig. 2, C and H). The difference in results obtained by open cystometry vs. closed-outlet cystometry may reflect a contribution of the bladder neck in mediating the transition from continence to voiding modes.
Despite several different approaches, we were not able to administer successfully PGE2 to the bladder or the urethra only without the use of a suture. For example, an integrated catheter-plug structure at the bladder neck was used to administer fluid exclusively to the urethra and provide a separate catheter for bladder filling, but we had difficulty maintaining a seal during hours of single-fill cystometry. Using this approach, Yokoyama et al. (31) observed a 36% reduction in the bladder intercontraction interval (ICI) following intraurethral-only administration of PGE2 and only an 11% reduction following intravesical-only PGE2. These results are somewhat difficult to interpret, as the concentration of PGE2 (1,134 µM) was very high; it was not clear how well the plug isolated the bladder and urethra, and contrasting results were reported in a subsequent study (a 23% reduction in ICI following intraurethral-only administration and a 41% reduction in ICI following intravesical-only administration of PGE2) (32).
Impact of PGE2 on EUS EMG
An increase in tonic (nonbursting) EUS EMG was observed during the later portion of the bladder-filling phase in open cystometry. The increase in EUS EMG may be a continence (guarding) reflex (20) to counteract the reduction in urethral pressure resulting from PGE2-mediated urethral smooth muscle relaxation. The increase in EUS EMG during the latter portion of bladder filling was not observed during closed-outlet cystometry following PGE2 administration selectively to the bladder or urethra alone. The lack of change in EMG activity during selective intravesical PGE2 administration suggests that the increase in EUS EMG is evoked by exposure of the urethra to fluid during bladder filling and not by a bladder-to-urethra reflex. While we administered PGE2 to the urethra before closed-outlet cystometry, the suture prevented bladder filling from distending the relaxed urethra and thereby activating urethral afferents.
From our recordings, it is not possible to determine whether or not the increase in EUS EMG activity is from the EUS or whether it also includes contributions from other pelvic floor muscles. This is true for both the percutaneous electrodes and sheet electrode. With both electrode types, an increase in EUS EMG was observed during bladder filling following intravesical PGE2 administration, indicating that this effect was not dependent on electrode type. For the sheet electrode, the size of the electrode contacts (2-mm width) is similar in width to the urethra, and we expect that the primary recordings are from the EUS. Any part of the electrodes that were not in contact with the urethra should have been in closest contact with the vagina. We estimate that the vagina is ~5 mm in width and that the electrode contacts would not come into direct contact with the pelvic floor muscles. Although it is possible to record distant muscle activity attributable to volume conduction, we expect that this effect would be minimal.
PGE2 and Urethral Smooth Muscle Relaxation
Urethral perfusion with PGE2 reduced the perfusion pressure substantially, and the concentration that reduced bladder capacity during open cystometry (100 µm) led to a sizable reduction in the urethral perfusion pressure (Fig. 3C). Similarly, Schüssler et al. (27) noted that the maximum urethral closure pressure at rest decreased in healthy women who were given PGE2 intravesically. PGE2-evoked relaxation of urethral smooth muscle is believed to occur via EP2 and EP4 receptors, which increase cAMP levels (11), thereby inhibiting phosphorylation of myosin light chains. This is in contrast to nitric oxide, a well-known mediator of urethral relaxation, which generates an increase in cGMP, promoting dephosphorylation of the myosin light chains. Although not quantified, the time scales of action are notably different, with PGE2-mediated relaxation occurring over the course of minutes (Fig. 3B) as opposed to seconds with nitric oxide.
PGE2, DO, and Bladder Compliance
One possible cause of increased urgency from intravesical PGE2 is the emergence of DO. However, we did not observe an increase in the number of nonvoiding contractions during the filling phase (Fig. 1H). PGE2 can generate DO in awake animals (4, 14, 25, 26), and the reason for this discrepancy is unclear. One possible reason may be that the influence of PGE2 is different in the anesthetized preparation. Concentrations used in awake animals tended to be much lower (e.g., 10 or 20 µM) than in this study (100 µM) (7, 19). Additionally, the types of nonvoiding contractions shown in the conscious animal varied between frequent smaller contractions (4, 25) and larger less frequent contractions (14, 26), but the origin and significance of these differences are unclear. Perhaps most importantly, in healthy women receiving intravesical PGE2, only some of the cystometric fills showed DO (7/12), whereas urgency was a characteristic of every fill (27).
PGE2 decreased bladder compliance in the open cystometry preparation (Fig. 1J), and this may have contributed to the observed reductions in bladder capacity. The physiological relevance of poor bladder compliance is unclear (30), but maximum cystometric capacity was lower by 39% in men and by 35% in women, on average, for those with poor compliance (less than 10 ml/cmH2O) compared with those without poor compliance (2). We did not observe a decrease in bladder compliance following selective intravesical PGE2 administration during closed-outlet cystometry. The average bladder compliance was lower in the control condition compared with control condition in open cystometry, and this may have hindered the ability for PGE2 to further decrease bladder compliance.
PGE2 and Bladder Hypersensitivity
Strong urethral relaxation, in conjunction with a decrease in compliance, suggests that the sensation of urgency following intravesical PGE2 (27) may derive largely from the sensation of impending urine loss. An alternative hypothesis regarding the source of urinary urgency is bladder afferent hypersensitivity resulting in urinary urgency and/or pain (6, 24). The cause of this hypersensitivity may be increased C-fiber activation, increased Aδ-fiber activation, or even increased activation of higher-order neurons (possibly from organ cross talk, 29).
Systemic capsaicin pretreatment appears to prevent the decrease in bladder capacity from intravesical PGE2 administration (17), supporting the hypothesis of C-fiber-mediated afferent hypersensitivity. However, the study by Maggi et al. (17) used 10 µM PGE2 intravesically, and, in preliminary studies, we found 60 µM PGE2 to be insufficient to reduce bladder capacity. In initial testing, Maggi et al. (17) also found 10 µM to be insufficient to reduce bladder capacity, so all subsequent experimental results were collected following bilateral hypogastric nerve transection. In this specific preparation, 10 µM PGE2 reduced bladder capacity relative to saline controls. The hypogastric nerve increases urethral smooth muscle tone and reduces bladder smooth muscle tone, facilitating bladder filling (5). Nerve transection would thus be expected to relax urethral smooth muscle and prevent relaxation of bladder smooth muscle. That intravesical PGE2 at 10 µM only worked following hypogastric nerve transection suggests that the changes associated with transection, notably smooth muscle changes, led to the decrease in bladder capacity.
Contrary to the observations of Maggi et al (17), intravesical PGE2 (120 µM) given to capsaicin-desensitized spontaneously hypertensive rats reduced bladder capacity, although the reduction was not as large as observed in animals that were not pretreated (21). However, there are important differences in the preparations, including strain, sex, anesthesia, nerve status, and drug dosing. These results (21) suggest that both smooth muscle changes, as well as activation of capsaicin-sensitive afferents, are contributing factors to the PGE2-induced reduction in bladder capacity.
Furthermore, systemic capsaicin desensitization may lead to other changes that complicate interpretation of this result. Systemic capsaicin desensitization increased bladder capacity in urethane anesthetized rats (3, 16) and in awake female spontaneously hypertensive rats (21) but not in awake male albino rats (16), although large increases in bladder capacity at both 50- and 125-mg/kg doses suggest that this may be due to low statistical power. Furthermore, systemic resiniferatoxin desensitization increased bladder capacity (33), which may be mediated by a decrease in Aδ-fiber firing rates (1), rather than reflect C-fiber-mediated mechanisms.
That PGE2 can reduce urethral pressure and decrease bladder compliance suggests that the reduced bladder capacity may be due to a sensation of impending urine loss from smooth muscle changes. Evidence of urethral involvement in urgency comes from sling surgeries, showing resolution of urgency symptoms in a sizable subset of patients with mixed incontinence following sling placement (13, 22). PGE2 may also contribute to bladder hypersensitivity, but the pronounced effect of PGE2 on smooth muscle function should not be ignored.
In conclusion, intravesical PGE2 reduced bladder capacity, and we identified at least two contributing factors, a pronounced decrease in bladder compliance and a strong reduction in urethral perfusion pressure. On the other hand, we saw no increase in the number of nonvoiding contractions suggestive of DO. The decrease in bladder capacity was accompanied by an increase in EUS activity, suggestive of a guarding reflex attempting to maintain continence in the face of the decrease in urethral perfusion pressure. Selective intravesical or intraurethral PGE2 administration during closed-outlet cystometry did not change the bladder capacity despite relaxation of the urethra in the latter case. These results suggest that increased fluid impingement on the proximal urethra, whether from decreased compliance and/or increased urethral relaxation, is important for regulating bladder capacity. Targeting the urethra, particularly urethral smooth muscle, may be a promising avenue for new therapies to treat OAB.
GRANTS
Research described herein was funded by the National Institutes of Health Grants K12 DK100024 and by the GlaxoSmithKline Bioelectronics Research Program.
DISCLOSURES
J. A. Hokanson, C. L. Langdale, and W. M. Grill are inventors on patent applications owned by Duke University on peripheral nerve stimulation to treat bladder dysfunction and have rights to future compensation through a licensing agreement. A. Sridhar works for Galvani Bioelectronics, which has an interest in the field of peripheral nerve stimulation to treat bladder dysfunction.
AUTHOR CONTRIBUTIONS
J.A.H., C.L.L., A.S., and W.M.G. conceived and designed research; J.A.H. and C.L.L. performed experiments; J.A.H. and C.L.L. analyzed data; J.A.H., C.L.L., and W.M.G. interpreted results of experiments; J.A.H. prepared figures; J.A.H. drafted manuscript; J.A.H., C.L.L., and W.M.G. edited and revised manuscript; J.A.H., C.L.L., A.S., and W.M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gilda Mills and Danielle Degoski for assistance during the experiments.
REFERENCES
- 1.Aizawa N, Fukuhara H, Fujimura T, Homma Y, Igawa Y. Direct influence of systemic desensitization by resiniferatoxin on the activities of Aδ- and C-fibers in the rat primary bladder mechanosensitive afferent nerves. Int J Urol 23: 952–956, 2016. doi: 10.1111/iju.13181. [DOI] [PubMed] [Google Scholar]
- 2.Cho S-Y, Yi J-S, Oh S-J. The clinical significance of poor bladder compliance. Neurourol Urodyn 28: 1010–1014, 2009. doi: 10.1002/nau.20713. [DOI] [PubMed] [Google Scholar]
- 3.Chuang YC, Fraser MO, Yu Y, Beckel JM, Seki S, Nakanishi Y, Yokoyama H, Chancellor MB, Yoshimura N, de Groat WC. Analysis of the afferent limb of the vesicovascular reflex using neurotoxins, resiniferatoxin and capsaicin. Am J Physiol Regul Integr Comp Physiol 281: R1302–R1310, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Granato C, Korstanje C, Guilloteau V, Rouget C, Palea S, Gillespie JI. Prostaglandin E2 excitatory effects on rat urinary bladder: a comparison between the β-adrenoceptor modulation of non-voiding activity in vivo and micro-contractile activity in vitro. Naunyn Schmiedebergs Arch Pharmacol 388: 727–735, 2015. doi: 10.1007/s00210-015-1139-9. [DOI] [PubMed] [Google Scholar]
- 5.De Groat WC, Griffiths DJ, Yoshimura N. Neural control of the lower urinary tract, In: Comprehensive Physiology, edited by Terjung R. Hoboken, NJ: Wiley, 2015, p. 327–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Homma Y. Hypersensitive bladder: a solution to confused terminology and ignorance concerning interstitial cystitis. Int J Urol 21, Suppl 1: 43–47, 2014. doi: 10.1111/iju.12314. [DOI] [PubMed] [Google Scholar]
- 7.Ishizuka O, Mattiasson A, Andersson K-E. Prostaglandin E2-induced bladder hyperactivity in normal, conscious rats: involvement of tachykinins? J Urol 153: 2034–2038, 1995. doi: 10.1016/S0022-5347(01)67397-X. [DOI] [PubMed] [Google Scholar]
- 8.Kim JC, Park EY, Hong SH, Seo SI, Park YH, Hwang TK. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol 12: 875–880, 2005. doi: 10.1111/j.1442-2042.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JC, Park EY, Seo SI, Park YH, Hwang TK. Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol 175: 1773–1776, 2006. doi: 10.1016/S0022-5347(05)00992-4. [DOI] [PubMed] [Google Scholar]
- 10.Kim SW, Im YJ, Choi HC, Kang HJ, Kim JY, Kim JH. Urinary nerve growth factor correlates with the severity of urgency and pain. Int Urogynecol J Pelvic Floor Dysfunct 25: 1561–1567, 2014. doi: 10.1007/s00192-014-2424-8. [DOI] [PubMed] [Google Scholar]
- 11.Kurihara R, Ishizu K, Takamatsu H, Yoshino T, Masuda N. Study on physiological roles of stimulation of prostaglandin E2 receptor subtype EP2 in urethral function in rats. Low Urin Tract Symptoms 8: 125–129, 2016. doi: 10.1111/luts.12077. [DOI] [PubMed] [Google Scholar]
- 12.Langdale CL, Hokanson JA, Sridhar A, Grill WM. Stimulation of the pelvic nerve increases bladder capacity in the prostaglandin E2 rat model of overactive bladder. Am J Physiol Renal Physiol 2017, 2017. doi: 10.1152/ajprenal.00116.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JK-S, Dwyer PL, Rosamilia A, Lim YN, Polyakov A, Stav K. Persistence of urgency and urge urinary incontinence in women with mixed urinary symptoms after midurethral slings: a multivariate analysis. BJOG 118: 798–805, 2011. doi: 10.1111/j.1471-0528.2011.02915.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee T, Hedlund P, Newgreen D, Andersson K-E. Urodynamic effects of a novel EP(1) receptor antagonist in normal rats and rats with bladder outlet obstruction. J Urol 177: 1562–1567, 2007. doi: 10.1016/j.juro.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 15.Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor but not prostaglandin E2 increases in patients with interstitial cystitis/bladder pain syndrome and detrusor overactivity. BJU Int 106: 1681–1685, 2010. doi: 10.1111/j.1464-410X.2009.08851.x. [DOI] [PubMed] [Google Scholar]
- 16.Maggi CA, Conte B. Effect of urethane anesthesia on the micturition reflex in capsaicin-treated rats. J Auton Nerv Syst 30: 247–251, 1990. doi: 10.1016/0165-1838(90)90256-I. [DOI] [PubMed] [Google Scholar]
- 17.Maggi CA, Giuliani S, Conte B, Furio M, Santicioli P, Meli P, Gragnani L, Meli A. Prostanoids modulate reflex micturition by acting through capsaicin-sensitive afferents. Eur J Pharmacol 145: 105–112, 1988. doi: 10.1016/0014-2999(88)90221-X. [DOI] [PubMed] [Google Scholar]
- 18.Milsom I, Coyne KS, Nicholson S, Kvasz M, Chen CI, Wein AJ. Global prevalence and economic burden of urgency urinary incontinence: a systematic review. Eur Urol 65: 79–95, 2014. doi: 10.1016/j.eururo.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Pandita RK, Persson K, Andersson K-E. Effects of the K+ channel opener, ZD6169, on volume and PGE2-stimulated bladder activity in conscious rats. J Urol 158: 2300–2304, 1997. doi: 10.1016/S0022-5347(01)68239-9. [DOI] [PubMed] [Google Scholar]
- 20.Park JM, Bloom DA, McGuire EJ. The guarding reflex revisited. Br J Urol 80: 940–945, 1997. doi: 10.1046/j.1464-410X.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- 21.Patra PB, Thorneloe KS. Enhanced sensitivity to afferent stimulation and impact of overactive bladder therapies in the conscious, spontaneously hypertensive rat. J Pharmacol Exp Ther 338: 392–399, 2011. doi: 10.1124/jpet.111.180885. [DOI] [PubMed] [Google Scholar]
- 22.Porena M, Costantini E, Frea B, Giannantoni A, Ranzoni S, Mearini L, Bini V, Kocjancic E. Tension-free vaginal tape versus transobturator tape as surgery for stress urinary incontinence: results of a multicentre randomised trial. Eur Urol 52: 1481–1490, 2007. doi: 10.1016/j.eururo.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 23.Rahnama’i MS, van Kerrebroeck PE, de Wachter SG, van Koeveringe GA. The role of prostanoids in urinary bladder physiology. Nat Rev Urol 9: 283–290, 2012. doi: 10.1038/nrurol.2012.33. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds WS, Dmochowski R, Wein A, Bruehl S. Does central sensitization help explain idiopathic overactive bladder? Nat Rev Urol 13: 481–491, 2016. doi: 10.1038/nrurol.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo A, Castiglione F, Salonia A, Benigni F, Rigatti P, Montorsi F, Andersson KE, Hedlund P. Effects of the gonadotropin-releasing hormone antagonist ganirelix on normal micturition and prostaglandin E(2)-induced detrusor overactivity in conscious female rats. Eur Urol 59: 868–874, 2011. doi: 10.1016/j.eururo.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Schröder A, Newgreen D, Andersson K-E. Detrusor responses to prostaglandin E2 and bladder outlet obstruction in wild-type and Ep1 receptor knockout mice. J Urol 172: 1166–1170, 2004. doi: 10.1097/01.ju.0000134186.58854.2c. [DOI] [PubMed] [Google Scholar]
- 27.Schüssler B. Comparison of the mode of action of prostaglandin E2 (PGE2) and sulprostone, a PGE2-derivative, on the lower urinary tract in healthy women. A urodynamic study. Urol Res 18: 349–352, 1990. doi: 10.1007/BF00300786. [DOI] [PubMed] [Google Scholar]
- 28.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol 20: 327–336, 2003. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 29.Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol 290: F1478–F1487, 2006. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- 30.Wyndaele J-J, Gammie A, Bruschini H, De Wachter S, Fry CH, Jabr RI, Kirschner-Hermanns R, Madersbacher H. Bladder compliance what does it represent: can we measure it, and is it clinically relevant? Neurourol Urodyn 30: 714–722, 2011. doi: 10.1002/nau.21129. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama O, Miwa Y, Oyama N, Aoki Y, Ito H, Akino H. Antimuscarinic drug inhibits detrusor overactivity induced by topical application of prostaglandin E2 to the urethra with a decrease in urethral pressure. J Urol 178: 2208–2212, 2007. doi: 10.1016/j.juro.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama O, Yusup A, Oyama N, Aoki Y, Miwa Y, Akino H. Improvement in bladder storage function by tamsulosin depends on suppression of C-fiber urethral afferent activity in rats. J Urol 177: 771–775, 2007. doi: 10.1016/j.juro.2006.09.076. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Igawa Y, Ishizuka O, Nishizawa O, Andersson KE. Effects of resiniferatoxin desensitization of capsaicin-sensitive afferents on detrusor over-activity induced by intravesical capsaicin, acetic acid or ATP in conscious rats. Naunyn Schmiedebergs Arch Pharmacol 367: 473–479, 2003. doi: 10.1007/s00210-003-0748-x. [DOI] [PubMed] [Google Scholar]