Abstract
Myogenic response, a phenomenon in which resistance size arteries and arterioles swiftly constrict or dilate in response to an acute elevation or reduction, respectively, in intravascular pressure is a key component of renal autoregulation mechanisms. Although it is well established that the renal system is functionally immature in neonates, mechanisms that regulate neonatal renal blood flow (RBF) remain poorly understood. In this study, we investigated the hypothesis that members of the transient receptor potential vanilloid (TRPV) channels are molecular components of renal myogenic constriction in newborns. We show that unlike TRPV1–3, TRPV4 channels are predominantly expressed in neonatal pig preglomerular vascular smooth muscle cells (SMCs). Intracellular Ca2+ concentration ([Ca2+]i) elevation induced by osmotic cell swelling was attenuated by TRPV4, L-type Ca2+, and stretch-activated Ca2+ channel blockers but not phospholipase A2 inhibitor. Blockade of TRPV4 channels reversed steady-state myogenic tone and inhibited pressure-induced membrane depolarization, [Ca2+]i elevation, and constriction in distal interlobular arteries. A step increase in arterial pressure induced efficient autoregulation of renal cortical perfusion and total RBF in anesthetized and mechanically ventilated neonatal pigs. Moreover, intrarenal arterial infusion of the TRPV4 channel blockers HC 067047 and RN 1734 attenuated renal autoregulation in the pigs. These data suggest that renal myogenic autoregulation is functional in neonates. Our findings also indicate that TRPV4 channels are mechanosensors in neonatal pig preglomerular vascular SMCs and contribute to renal myogenic autoregulation.
Keywords: mechanosensitive ion channels, neonatal pig, renal autoregulation, TRPV4 channels, vascular smooth muscle cells
healthy kidneys maintain constant renal blood flow (RBF) and glomerular filtration rate (GFR) despite physiological fluctuations in arterial pressure (10, 11, 40). This phenomenon known as “renal autoregulation” serves to preserve renal function and protect the kidneys from glomerular injury (10, 11, 40). Two primary mechanisms have been demonstrated to mediate renal autoregulation: 1) the myogenic response, and 2) tubuloglomerular feedback (TGF). The renal myogenic response is based on the intrinsic ability of afferent arterioles to swiftly constrict or dilate in response to an acute elevation or reduction, respectively, in renal perfusion pressure (10, 11, 40). Interlobular arteries have also been shown to contribute to renal myogenic autoregulation (27–29). TGF mechanism involves signal transduction events in which tubular flow rate-dependent changes in luminal sodium chloride concentration detected at the macula densa adjust the GFR by altering preglomerular vascular tone (10, 11, 40).
Renal autoregulation mechanisms have largely been described in adults. However, the morphological and functional properties of the renal system differ between maturational stages (31, 65). The autoregulation range in healthy adults is between ~80 and 180 mmHg (11). The mean arterial pressure (MAP) of term newborns is less than the lower limit of adult autoregulation range (44). Thus renal autoregulation may occur at a lower perfusion pressure in neonates. Angiotensin II-induced increase in perfusion pressure during selective inhibition of its renal vascular effects elicited RBF autoregulation in ~4- to 5-wk-old puppies (33). However, Buckley et al. (8) suggested that renal autoregulation is insignificant at birth. This suggestion was based on data showing that aortic compression decreased renal vascular resistance in 1- to 2-wk- and 1- to 2-mo-old pigs but not in 1- to 4-day-old pigs (8). Moreover, step increases in kidney perfusion did not induce consistent autoregulation in piglets of all age groups (8). Hence, further studies are required to characterize neonatal renal myogenic autoregulation.
In myogenic arteries/arterioles, the mechanical stretch on the vascular wall exerted by elevated intravascular pressure induces smooth muscle cell (SMC) depolarization that activates voltage-dependent Ca2+ channels (VDCCs) (15, 52). VDCC activation results in extracellular Ca2+ influx, an elevation in intracellular Ca2+ concentration ([Ca2+]i), and vasoconstriction (15, 52). The mechanism by which an elevation in intravascular pressure stimulates vascular SMC membrane depolarization involves activation of membrane-resident mechanosensitive ion channels (15, 52). Proposed mechanosensitive ion channels in vascular SMCs include members of the transient receptor potential (TRP) channels (17). TRPC6, TRPM4, TRPP2, and TRPV2 channels have been shown to participate in cerebral or retinal artery/arteriole myogenic constriction (19, 41, 43, 70). However, the physiological roles of TRP channels in the renal myogenic response remain unknown.
The TRPV subfamily of TRP channels is formed by six mammalian members (TRPV1–6). Unlike TRPV5 and TRPV6, TRPV1–4 channels are expressed in adult rat intralobar pulmonary arteries and aorta (74). Furthermore, TRPV1, TRPV4, TRPV5, and TRPV6 play unique functional roles in the kidneys. TRPV1 channels are expressed in renal tubules, pelvis, and nerves (34, 72). Activation of TRPV1 increased afferent renal nerve activity, GFR, and Na+ and water excretion in adult rats (37, 72, 76). Osmo- and mechanosensitive TRPV4 channels are expressed in adult mouse and rat nephrons but restricted to ascending thin limb, thick ascending limb, and distal convoluted and connecting tubules (14, 64). Both TRPV5 and TRPV6 are localized in adult mouse distal convoluted and connecting tubules and are involved in epithelial Ca2+ transport (30, 46, 67). Of note, TRPV channel expression and function in neuronal, inner ear, intestinal, renal, and endothelial cells (ECs) are age dependent (16, 36, 62, 66, 69).
Pig and human renal systems have similar anatomical features characterized by multipyramidal kidneys in contrast to unipyramidal kidneys of the mouse, rat, rabbit, dog, and sheep (22, 50). Pig and human also share similar renal maturational process (23, 45, 63). Hence, the newborn pig is an excellent large animal model of human neonatal renal physiology and pathophysiology. Here, we used pigs to investigate neonatal renal autoregulation. We tested the hypothesis that members of TRPV channels contribute to renal myogenic autoregulation mechanism in neonates.
METHODS
Animals.
All animal experimental procedures were approved and performed in accordance with the guidelines and regulations of the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center. Full-term male neonatal pigs (4–7 days old; Nichols Hog Farm, Olive Branch, MS) were used in this study.
Tissue preparation.
Neonatal pigs were euthanized by intramuscular injection of ketamine/xylazine (100/10 mg/kg im) followed by exsanguination. The kidneys were immediately removed, decapsulated, hemisected, and placed in ice-cold (4°C) modified Krebs’ solution (MKS) containing the following (in mM): 134 NaCl, 6 KCl, 2.0 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.4). The kidneys were dissected to isolate interlobar, arcuate, and interlobular arteries and afferent arterioles using a Zeiss SteREO Discovery.V12 stereomicroscope (Carl Zeiss, Thornwood, NY).
Isolation of renal vascular SMCs and ECs.
SMCs were isolated from renal arteries using a HEPES-buffered isolation solution containing the following (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.4). The arteries were incubated in isolation solution containing the following (in mg/ml): 1 papain, 1 dithioerythritol, and 1 mg/ml BSA for 16 min at 37°C. Arteries were then incubated in isolation solution containing the following 0.5 mg/ml liberase blendzyme 1, 1 mg/ml BSA, and 100 nM CaCl2 for 8 min at 37°C. Digested vessels were subsequently washed in isolation solution and triturated using fire-polished glass Pasteur pipettes to yield single SMCs.
Renal vascular ECs were isolated using the Dynabeads CD31 EC isolation kit (Life Technologies, Grand Island, NY). To obtain a high yield of cells, ECs were isolated from interlobar arteries by gentle rubbing of the vessel luminal surface with small-tip cotton swabs. The detached endothelial linings were then incubated in CD31-coated magnetic beads for ~20 min with gentle rotation. A DynaMag magnet (Life Technologies) was used to separate EC-bound Dynabeads. The beads were then washed five times in PBS containing 0.1% BSA. ECs were eluted from the magnetic beads using an RNA lysis buffer.
RT-PCR and quantitative RT-PCR.
For RT-PCR, total RNA was purified from intact arteries and SMCs using the RNAqueous-Micro Total RNA Isolation Kit (Life Technologies). For quantitative RT-PCR (qRT-PCR), total RNA was purified from renal vascular SMCs and ECs using the Single Cell Real-Time RT-PCR Assay Kit (Signosis, Santa Clara, CA). cDNAs were synthesized from the arterial and SMC RNA samples using a cDNA Synthesis Kit (SuperScript VILO; Life Technologies). cDNAs were amplified by PCR using oligonucleotide primer pairs (Table 1). PCR reactions were performed in an Eppendorf Mastercycler (Eppendorf, Westbury, NY) with the following reaction conditions: an initial denaturation at 98°C for 2 min, followed by 40 cycles (denaturation at 98°C for 10 s, annealing at 57°C for 30 s, and extension at 72°C for 30 s), with a final extension at 72°C for 10 min. Subsequently, PCR products were cleaned using the AxyPrep Mag PCR Clean-up Kit (Corning Life Sciences, Corning, NY) and resolved on a 2% agarose gel stained with SYBR Safe DNA gel stain (Life Technologies).
Table 1.
Oligonucleotide primer sequences
| Gene | Sequence | Accession | Length, bp |
|---|---|---|---|
| RT-PCR | |||
| ACTA2 | NM_001164650.1 | 456 | |
| Forward | 5′-CCCTGTGAAGCACCAGCCAGGA-3′ | ||
| Reverse | 5′-GTAGAGGGACAGCACCGCCTGA-3′ | ||
| vWR | NM_001246221.1 | 488 | |
| Forward | 5′-CGTGGAGAGTGCTGAGTGTT-3′ | ||
| Reverse | 5′-GGCCATCCCAGTCTATCTGC-3′ | ||
| TRPV1 | EF100781.1* | 167 | |
| Forward | 5′-TGTTCAAGTTCACCATCGGC-3′ | ||
| Reverse | 5′-CTCCTGTGCGATCTTGTTGAC-3′ | ||
| TRPV2 | XM_013980349.1# | 444 | |
| Forward | 5′- CAGGCTTGAGACGTCAGACA-3′ | ||
| Reverse | 5′-TCTGTACACTGGGCGTTGAC-3′ | ||
| TRPV3 | XM_005669116.2# | 211 | |
| Forward | 5′-CCAACATCGACAACCGGCAT-3′ | ||
| Reverse | 5′-CACCCCATTTTGTGCGTGAG-3′ | ||
| TRPV4 | NM_001130729.1 | 298 | |
| Forward | 5′-CTTCGCTGGCCAACCTGTTT-3′ | ||
| Reverse | 5′-TGCGGCTGCTTCTCTATGAC-3′ | ||
| Quantitative RT-PCR | |||
| 18S rRNA | NR_046261.1 | 102 | |
| Forward | 5′-CGAAAGCATTTGCCAAGAAT-3′ | ||
| Reverse | 5′-AGTCGGCATCGTTTATGGTC-3′ | ||
| TRPV4 | NM_001130729.1 | 105 | |
| Forward | 5′-CCGCTTACTACCAGCCTCTG-3′ | ||
| Reverse | 5′-GGACCCCAGTGAAGAGTGTG-3′ |
vWR, von Willebrand factor; TRPV, transient receptor potential vanilloid.
Partial sequence.
Predicted sequences.
Renal vascular SMC and EC RNA samples were analyzed by qRT-PCR using specific primer pairs (Table 1) and an Applied Biosystems SYBR Green Master Mix kit (Life Technologies, Grand Island, NY). Reactions were performed in triplicate in an Applied Biosystems StepOnePlus Real-Time PCR System (Life Technologies). 18S ribosomal RNA was used as a housekeeping gene control.
Western immunoblotting.
Tissues were homogenized in ice-cold RIPA buffer using a Bead Mill Homogenizer (Omni International, Kennesaw, GA). Protein concentrations were determined using a Bio-Rad protein assay kit and SmartSpec 3000 Spectrophotometer (Bio-Rad, Hercules, CA). Protein lysates were then mixed with LDS sample buffer containing a reducing agent (Life Technologies) and boiled at 70°C for 10 min. Proteins were separated by 4–20% ExpressPlus PAGE Gel (GenScript, Piscataway, NJ) using a Mini Trans Blot Cell (Bio-Rad) and transferred onto nitrocellulose membranes using a Pierce Fast Semi-Dry Blotter (Thermo Scientific). Nonspecific immunoreactive sites on the membranes were blocked with NAP blocking buffer (G-Biosciences, St. Louis, MO) for ~1 h at room temperature. The membranes were then incubated with primary antibodies overnight at 4°C. After several washes in Tris-buffered saline supplemented with 0.05% Tween 20 (TBS-T), the membranes were incubated in horseradish peroxidase-conjugated secondary antibodies for 45 min at room temperature and washed in TBS-T. Immunoreactive proteins were visualized using a chemiluminescent kit (G-Biosciences). Protein band intensities were analyzed by digital densitometry using Quantity One software (Bio-Rad).
Immunofluorescence.
Renal vascular SMCs attached to collagen-coated coverslips were fixed in 4% formaldehyde for ~20 min and permeabilized with 0.2% Triton X-100 for ~15 min at room temperature. After 1 h of incubation in PBS containing 5% BSA to block nonspecific immunoreactive sites, cells were treated overnight at 4°C with a TRPV4 antibody (1:50, each). The next day, cells were washed with PBS and incubated with Alexa 555-conjugated donkey anti-rabbit (1:500, each) for 1 h at room temperature. After several washes in PBS and mount, fluorescence images were acquired using a Zeiss laser-scanning confocal microscope.
Diameter measurement in pressurized microvessels.
Pressure-induced changes in interlobular artery luminal diameter were examined using the pressure myograph systems [Danish Myo Technology (DMT), Aarhus, Denmark; and Living Systems Instrumentation, St. Albans, VT]. Distal interlobular arteries were cannulated with a fabricated glass at each end in temperature-controlled chambers. The chambers were slowly and continuously perfused with MKS equilibrated with a 21 % O2-5 % CO2-74 % N2 gas mixture and maintained at 37°C. To alter intravascular pressure and prevent flow, a vessel “blind-sac” preparation was made by plugging the distal cannula. The proximal cannula was then connected to a software-controlled pressure interface (DMT) or pressure servocontroller (Living Systems Instrumentation) for maintenance of steady-state intravascular pressure. Microvessels were visualized using charge-coupled device cameras attached to inverted microscopes. Changes in vessel luminal diameter were continuously acquired using the vessel dimension analysis software (DMT or IonOptix, Milton, MA). The percentage of myogenic tone was calculated as (1 − active luminal diameter/passive luminal diameter) × 100. Passive luminal diameter was determined in Ca2+ free, EGTA (2 mM)-containing MKS.
Membrane potential measurement.
Renal vascular SMC membrane potential was measured by impaling borosilicate glass microelectrodes filled with 3 M KCl (tip resistances of 50–90 mΩ) into the adventitial side of pressurized interlobular arteries. Membrane potential was recorded using an A-M Systems Intracellular Electrometer Model 3100 (A-M Systems, Carlsborg, WA) and digitized using a pClamp 10 software (Axon Instruments). A successful intracellular recording was based on the following criteria: 1) recording originates from 0 ± 2 mV baseline, 2) a fast, negative change in potential upon microelectrode impalement, 3) stable membrane potential for at least 1 min following microelectrode impalement, and 4) a fast, positive potential change upon microelectrode withdrawal from the impaled cell.
Intracellular Ca2+ imaging.
Renal vascular SMCs on Cell-Tak (Corning Life Sciences, Corning, NY)-coated glass-bottom petri dishes were incubated with fura-2-acetoxymethyl ester (fura-2 AM; 10 μM), and 0.5% pluronic F-127 for ~1 h at room temperature in MKS. Cells were then washed for ~45 min to de-esterify fura-2 AM molecules before imaging. [Ca2+]i concentrations were determined using a fluorescence photometry system (Ionoptix). The fluorescence was collected simultaneously from cells located in the same field. Only one field was imaged per dish. To study pressure-induced changes in [Ca2+]i, vessel luminal diameter and [Ca2+]i changes were simultaneously measured in pressurized interlobular arteries (56). Before cannulation, microvessels were incubated in MKS containing fura-2 AM/pluronic acid at room temperature. Vessels were allowed to deesterify fura-2 AM before experimentation as described above.
Fura-2 AM fluorescence was recorded by exciting at wavelengths of 340 and 380 nm using a hyperswitch light source (Ionoptix). Background-subtracted fura-2 AM ratios were collected at 510 nm using a MyoCam-S CCD digital camera (Ionoptix) and analyzed with IonWizard software (Ionoptix) using the following equation (26): [Ca2+]i = Kd [(R − Rmin)/(Rmax − R)] δ, where R is the 340/380 nm ratio; Rmin and Rmax are the minimum and maximum fura 2 ratios determined in Ca2+-free + EGTA and Ca2+-replete solutions, respectively; δ represents the ratio of the 380 nm excitation in Ca2+-free and Ca2+-replete solution; and Kd is the apparent dissociation constant for fura-2 (224 nM (26). Rmin, Rmax, and δ were determined at the end of the experiments by perfusing the cells or pressurized arteries with 10 µM ionomycin and Ca2+-free (plus 10 mM EGTA) or 10 mM Ca2+ solution.
Renal blood flow autoregulation.
Neonatal pigs were acutely instrumented as we have previously described (55). Briefly, the pigs were anesthetized with ketamine/xylazine (20/2.2 mg/kg im) and maintained on α-chloralose (50 mg/kg, intravenously). The animals were maintained at 37°C, intubated via tracheostomy, and mechanically ventilated using a Bear Cub pediatric ventilator. Animals were continually monitored during experiments for anesthesia depth and redosed if necessary. Arterial blood gas, pH, and hematocrit were measured periodically with a GEM Premier 3000 Blood Gas Analyzer (Instrumentation Laboratory, Bedford, MA). Ventilation was adjusted to maintain Pco2, Po2, and pH at physiological ~30 mmHg, >85 mmHg, and 7.4, respectively. Urine was drained from the kidneys via a ureteral catheter. MAP was recorded via a right femoral artery catheter connected to a physiological pressure transducer (ADInstruments, Colorado Spring, CO). A femoral vein was catheterized for anesthetic and fluid administration. To administer pharmacological agents directly into the kidney, a catheter was inserted in the left femoral artery and advanced through the abdominal aorta until its tip was positioned at the junction of the aorta and left renal artery. The left kidneys were exposed retroperitoneally through flank incisions to permit access to the renal pedicles. RBF was measured with a flow probe (Transonic Systems, Ithaca, NY) placed around the main renal artery and connected to a flowmeter (Transonic Systems). Renal cortical perfusion was measured by placing a Laser-Doppler probe (PF 407) attached to a holder (Perimed, Jarfalla, Sweden) on the kidney surface. Data were acquired and analyzed using a PowerLab data acquisition system and LabChart software (ADInstruments).
To examine renal myogenic autoregulation, step decreases and increases in renal perfusion pressure were achieved by placing an inflatable vascular occluder cuff (4 mm; In Vivo Metrics, Healdsburg, CA) around the aorta immediately upstream of the main renal arteries. The cuff was inflated with a BasixCOMPAK inflation device (Merit Medical Systems, South Jordan, UT). MAP was increased by carefully tightening a ligature each around the celiac and mesenteric arteries. MAP was then adjusted back to the baseline using the occluder. Total RBF and renal cortical perfusion responses to a rapid change in perfusion pressure were measured by reducing the MAP by ~20 mmHg followed by an ~20-mmHg increase for 60 s. After a recovery time of ~10 min, the protocol was repeated at least three times. Autoregulatory index (AI) was calculated using the Semple and DeWardener equation below (53):
where RBF1 and MAP1 are the baseline values taken before the step increase in pressure and RBF2 and MAP2 are the values taken after the step increase in pressure. An AI of 0 indicates perfect autoregulation, and an AI ≤0.2 signifies effective RBF/renal cortical perfusion autoregulation, whereas an AI near or above 1.0 indicates ineffective autoregulation (11, 53).
Antibodies and reagents.
Rabbit polyclonal anti-TRPV4 (AP18990a; Western blot and blocking peptide: BP18990a), rabbit polyclonal anti-TRPV4 (ab39260; immunofluorescence), and mouse monoclonal anti-β-actin (MA515739) primary antibodies were purchased from Abgent (San Diego, CA), Abcam (Cambridge, MA), and Life Technologies, respectively. Horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies were purchased from Abcam. Unless otherwise specified, all reagents were purchased from Sigma-Aldrich (St. Louis, MO). HC 067047 and RN 1734 were purchased from EMD Millipore (Billerica, MA). Fura 2 AM, Pluronic F-127, ionomycin, GSMTX4, and liberase blendzyme 1 were obtained from Life Technologies, AnaSpec (Fremont, CA), Cayman Chemical (Ann Arbor, MI), Smartox Biotechnology (Saint Martin d’Hères, France), and Roche Life Science (Indianapolis, IN), respectively.
Statistical analysis.
Statistical analysis was performed using the InStat statistics software (GraphPad, Sacramento, CA). Data are presented as means ± SE. Student's t-test and Student-Newman-Keuls test were used for comparing paired or unpaired data and multiple data sets, respectively. Statistical significance implies a P < 0.05.
RESULTS
TRPV4 channels are predominantly expressed in neonatal pig renal vascular SMCs.
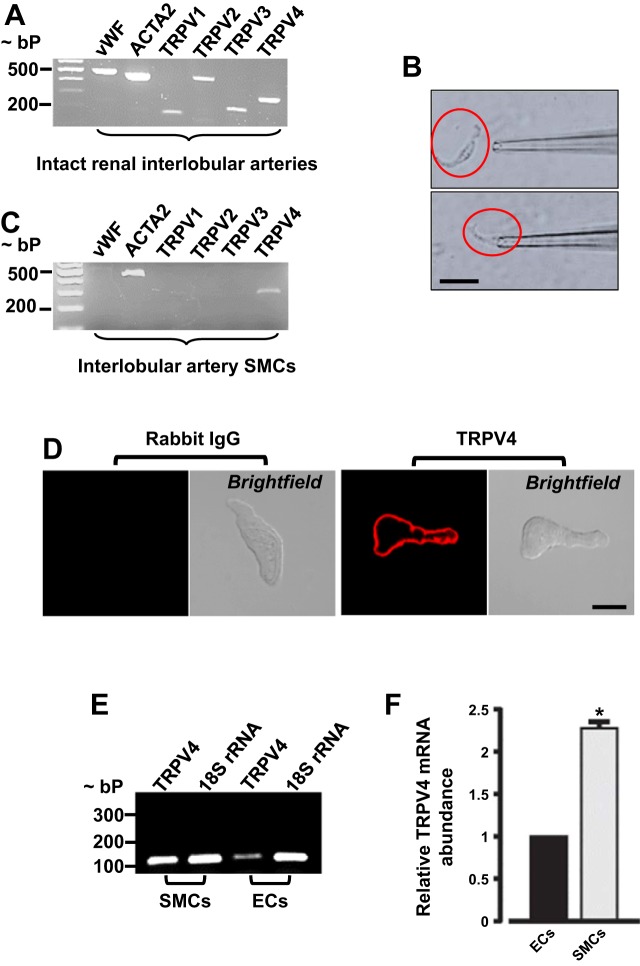
TRPV1–4 channels share structural and functional properties and are expressed in the vasculature (71, 74). Here, we determined whether TRPV1–4 channels are expressed in neonatal pig preglomerular vascular SMCs. Intact vessels contain both ECs and SMCs, as shown by PCR amplification of both von Willebrand factor and ACTA2, which are EC and SMC markers, respectively (Fig. 1A). Next, we determined TRPV1–4 expression in intact interlobular arteries. As shown in Fig. 1A, TRPV1–4 amplicons were detected in cDNA samples from intact interlobular arteries. To study TRPV isoforms that are expressed specifically in renal vascular SMCs, isolated interlobular artery SMCs (~100) were individually selected under the microscope using a micropipette (Fig. 1B). Only batches of SMCs that showed the absence of von Willebrand factor were used to determine TRPV isoform expression (Fig. 1C). Unlike intact vessels, only TRPV4 amplicons were detected in the cells (Fig. 1C). To examine cellular localization of TRPV4 channels, isolated SMCs were immunostained with a TRPV4 channel antibody. Confocal microscopy indicated that unlike normal rabbit IgG (negative control; Fig. 1D), TRPV4 channels are essentially localized to the plasma membrane of the cells (Fig. 1D). TRPV4 channels are functionally expressed in vascular ECs (5). Here, we quantified TRPV4 channel expression in EC and SMCs isolated from neonatal pig renal interlobar arteries. Quantitative RT-PCR indicated that TRPV4 mRNA expression is greater than twofold higher in neonatal pig renal interlobar artery SMCs when compared with ECs (Fig. 1, E and F). Together, these data suggest that TRPV4 channels are predominantly expressed in neonatal pig renal vascular SMCs.
Fig. 1.
Transient receptor potential vanilloid 4 (TRPV4) channels are predominantly expressed in neonatal pig renal vascular smooth muscle cells (SMCs). A: an agarose gel image showing the expression of von Willebrand factor [endothelial cell (EC) marker], ACTA2 (SMC marker), and TRPV1–4 channels in neonatal pig interlobular arteries. B: images showing selection of a neonatal pig renal vascular SMC using a patch pipette. C: an agarose gel image indicating that individually selected SMCs expressed only ACTA2 and TRPV4 channels. D: confocal microscopy images of neonatal pig renal vascular SMCs immunostained with normal rabbit IgG (negative control) and TRPV4 antibodies. An agarose gel image (E) and bar graphs (F) summarizing mean data (n = 3) for quantitative (q)RT-PCR experiments that compared TRPV4 mRNA expression in neonatal pig renal interlobar artery SMCs vs. ECs. Scale bar = 10 µM. *P < 0.05 vs. ECs.
TRPV4 channel protein expression levels in porcine kidney and renal preglomerular arteries are maturation dependent.
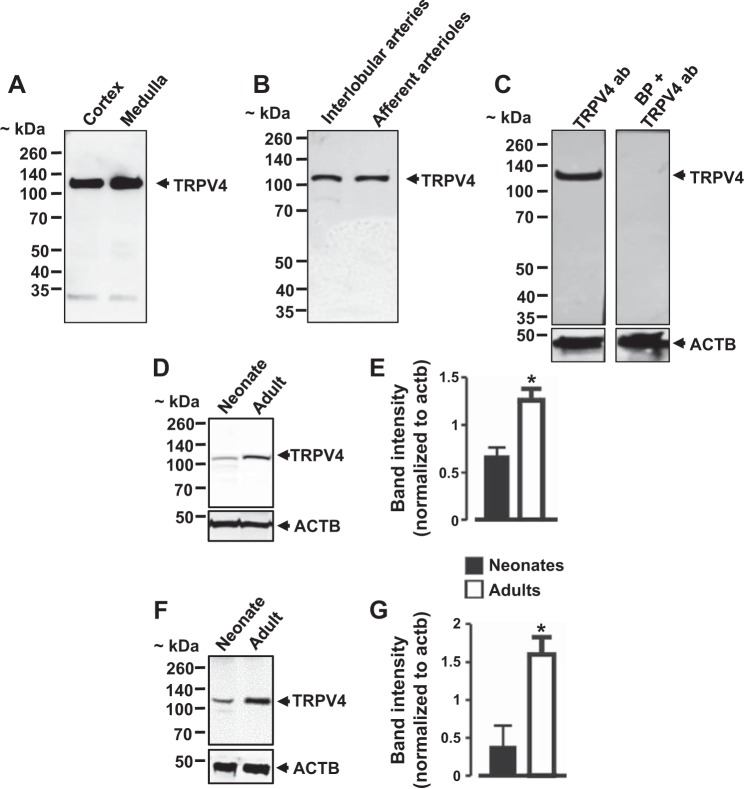
Western blotting using a rabbit polyclonal anti-TRPV4 antibody detected a prominent ~120-kDa immunoreactive band in neonatal pig renal cortex and medulla (Fig. 2A). Similarly, protein lysates from interlobular arteries and afferent arterioles were positive for the ~120-kDa band (Fig. 2B). A blocking peptide directed against the TRPV4 antibody completely blocked band detection, indicating that the ~120-kDa band corresponds to TRPV4 channels (Fig. 2C). To investigate whether renal TRPV4 channel protein expression is dependent on age, we obtained adult male (~6 mo old) pig kidneys from a local slaughterhouse. Thereafter, we compared TRPV4 channel protein expression in neonatal vs. adult pig kidneys and interlobular arteries. TRPV4 protein expression levels were ~54 and 74% higher in adult pig kidneys and interlobular arteries, respectively, when compared with neonatal pigs (Fig. 2, D–G). These findings demonstrate age-dependent changes in the protein expression levels of renal TRPV4 channels in pigs.
Fig. 2.
TRPV4 channel protein expression levels in porcine kidney and renal preglomerular vessels. A and B: Western blot images showing the expression of TRPV4 channels in neonatal pig renal cortex, medulla, and preglomerular vessels. C: a blocking peptide directed against TRPV4 antibody abolished TRPV4 immunoreactive band detection. Western blot images and bar graphs (n = 3 each) illustrating TRPV4 protein expression levels in neonatal and adult pig kidneys (D and E) and interlobular arteries (F and G). *P < 0.05 vs. neonates.
TRPV4 channels contribute to stretch-induced [Ca2+]i elevation and constriction in neonatal pig renal vascular SMCs.
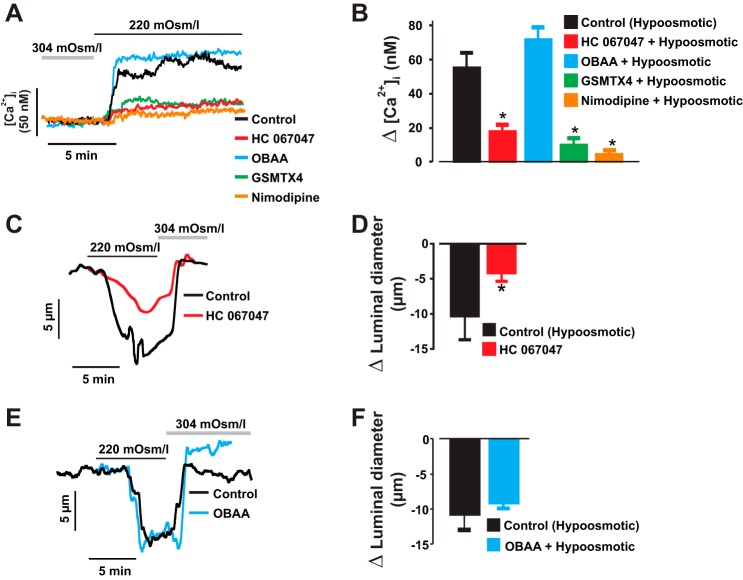
Transmembrane hyposmotic challenge causes vascular SMC swelling and stretch, leading to depolarization, [Ca2+]i elevation, and vasoconstriction (2, 9, 25). To test the hypothesis that cell swelling-induced neonatal renal vasoconstriction requires TRPV4 channels, we measured the [Ca2+]i concentration in renal vascular SMCs and constriction in interlobular arteries exposed to a hyposmotic solution. Phospholipase A2 (PLA2) contributes to hypotonic stress-induced activation of TRPV4 channels (68). Hence, we also examined whether PLA2 mediates cell swelling-induced [Ca2+]i elevation and constriction in the vessels. A change in cell bath solution from 304 to 220 mosmol/kgH2O increased the [Ca2+]i level by ~55 nM in interlobular artery SMCs. Hyposmotic-induced [Ca2+]i elevation was attenuated by stretch-activated channel blocker tarantula toxin GsMTx4, the selective TRPV4 channel blocker HC 067047 (20, 32), and the L-type Ca2+ channel blocker nimodipine (Fig. 3, A and B). By contrast, the PLA2 inhibitor OBAA did not alter hyposmotic-induced [Ca2+]i elevation in the cells (Fig. 3, A and B). Similarly, hyposmotic challenge reversibly stimulated neonatal pig renal artery constriction, an effect reduced by HC 067047 but not OBAA (Fig. 3, C–F). These findings suggest that cell swelling-induced TRPV4 channel activation stimulates neonatal renal vasoconstriction independently of PLA2.
Fig. 3.
TRPV4 channels contribute to stretch-induced intracellular Ca2+ concentration ([Ca2+]i) elevation and constriction in neonatal pig renal vascular SMCs. Traces (A) and bar graphs (B) showing changes in [Ca2+]i induced by transmembrane hyposmotic challenge (n = 5) in control and SMCs pretreated with GsMTx4 (300 nM; n = 4), nimodipine (1 µM; n = 4), HC 067047 (1 µM; n = 5), or OBAA (1 µM; n = 4). C–F, traces and bar graphs demonstrating that hyposmotic challenge-induced constriction of neonatal pig renal interlobular arteries is inhibited by HC 067047 (1 µM; n = 7 each) but not OBAA (1 µM; n = 5 each). Cells/vessels were pretreated with inhibitors/blockers 10–15 min before hyposmotic challenge. *P < 0.05 vs. control (hyposmotic).
TRPV4 channels contribute to pressure-induced membrane depolarization, [Ca2+]i elevation, and constriction in neonatal pig renal preglomerular arteries.
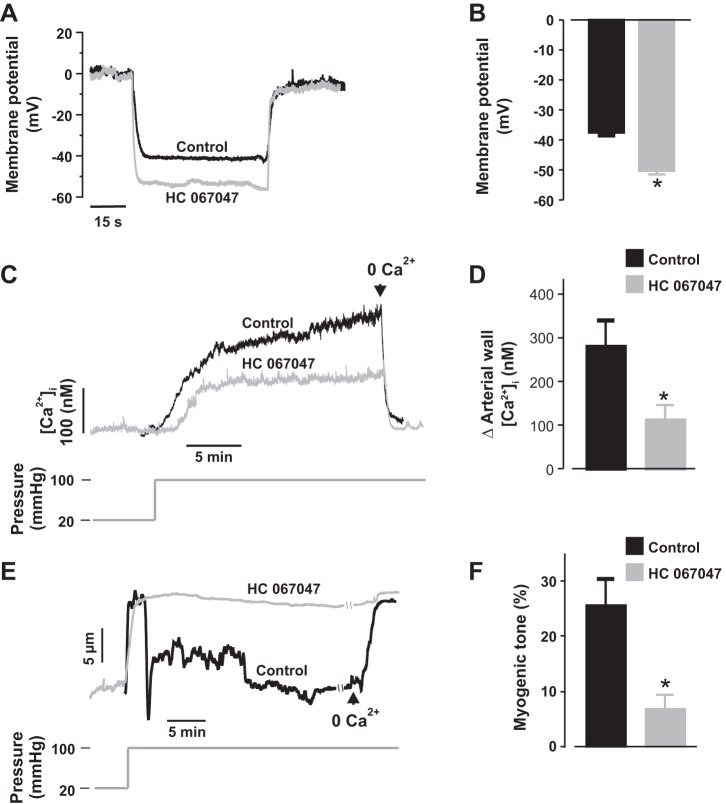
To further examine the role of TRPV4 channels in the mechanisms that underlie neonatal renal artery myogenic constriction, we first measured membrane potentials in newborn pig distal interlobular arteries that were pressurized to physiological renal arterial pressure (100 mmHg) following preincubation and continuous superfusion with modified Krebs’ solution containing DMSO (control) or HC 067047. An elevation in intravascular pressure from 20 to 100 mmHg depolarized the control arteries by ~37 mV (Fig. 4, A and B). However, pressure-induced membrane depolarization was significantly inhibited by HC 067047 (Fig. 4, A and B). Next, myogenic constriction and arterial wall [Ca2+]i concentration were recorded in the arteries. An increase in intravascular pressure from 20 to 100 mmHg elevated arterial wall [Ca2+]i concentration from ~113 to 396 nM in control arteries (~282 nM change; Fig. 4, C and D). In contrast, arterial wall [Ca2+]i concentration was elevated from ~102 to 214 nM in HC 067047-treated arteries (~113 nM change; Fig. 4, C and D). Correspondingly, pressure-induced constriction was reduced by ~73% in HC 067047-treated interlobular arteries (Fig. 4, E and F). These findings demonstrate that TRPV4 channels are required for pressure-induced membrane depolarization, [Ca2+]i elevation, and constriction in neonatal pig renal preglomerular arteries.
Fig. 4.
TRPV4 channels contribute to pressure-induced membrane depolarization, [Ca2+]i elevation, and constriction in neonatal pig renal preglomerular arteries. A and B: traces and bar graphs showing SMC membrane potentials in neonatal pig renal interlobular arteries that were pressurized to 100 mmHg in the presence of DMSO (control; n = 4) or HC 067047 (1 µM; n = 7). C and D: traces and bar graphs illustrating pressure-induced changes in [Ca2+]i concentration in neonatal pig renal interlobular arteries in the presence of DMSO (control; n = 4) or HC 067047 (1 µM; n = 5). E and F: traces and bar graphs showing myogenic tone in neonatal pig renal interlobular arteries that were pressurized to 100 mmHg in the presence of DMSO (control; n = 12) or HC 067047 (1 µM; n = 12). Vessels were pretreated and continuously superfused with DMSO or HC 067047. *P < 0.05 vs. control.
TRPV4 channel blockade reversed steady-state myogenic tone in neonatal pig renal preglomerular arteries.
Figure 4, E and F, indicates that blockade of TRPV4 channels before intravascular pressure elevation inhibits myogenic constriction. Here, we examined the effect HC 067047 on steady-state myogenic tone. HC 067047 concentration dependently overturned myogenic constriction in neonatal pig interlobular arteries pressurized to 100 mmHg (Fig. 5, A and B). These data support our hypothesis that TRPV4 channels contribute to neonatal pig renal myogenic constriction.
Fig. 5.
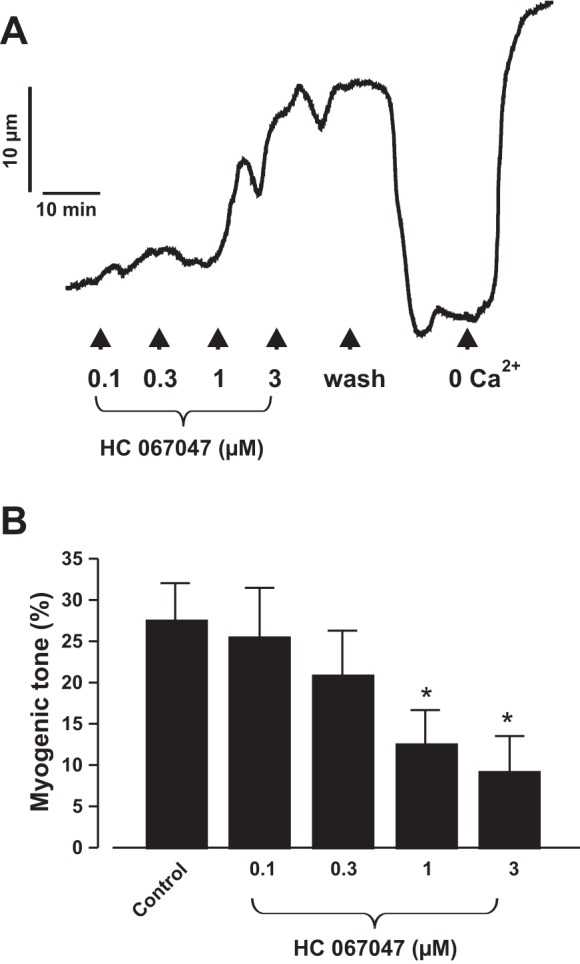
TRPV4 channel blockade reversed steady-state myogenic tone in pressurized neonatal pig renal preglomerular arteries. Traces (A) and bar graphs (B) (n = 8 each) summarizing the concentration-response effect of HC 067047 (1 µM) on steady-state myogenic tone in neonatal pig interlobular arteries pressurized to 100 mmHg. *P < 0.05 vs. control.
Blockade of TRPV4 channels does not alter voltage-gated Ca2+ channel-mediated [Ca2+]i elevation and vasoconstriction in neonatal pig renal preglomerular arteries.
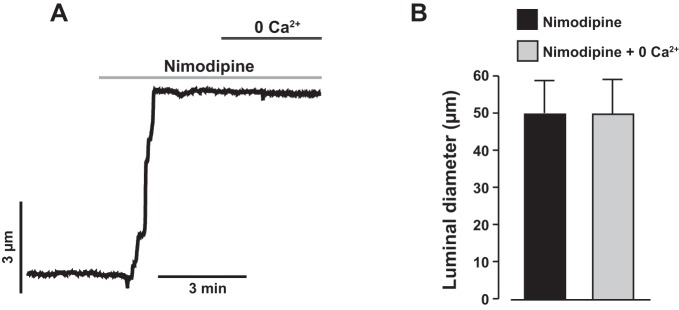
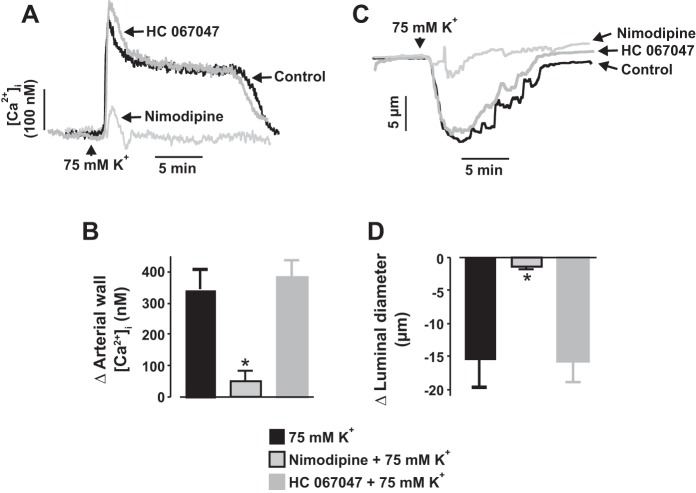
Nimodipine, essentially abolished stretch-induced [Ca2+]i elevation in neonatal pig renal vascular SMCs (Fig. 3, A and B). Nimodipine also reversed myogenic tone in neonatal pig renal interlobular arteries by evoking vasodilation (Fig. 6, A and B). Removal of extracellular Ca2+ in the presence of nimodipine did not cause any further dilation of the vessels (Fig. 6, A and B), indicating that myogenic constriction of neonatal pig renal preglomerular arteries occurs because of voltage-dependent L-type Ca2+ channel activation downstream of stretch-induced membrane depolarization. To confirm that inhibition of myogenic constriction by HC 067047 occurs independently of L-type Ca2+ channels, we studied its effect on depolarization-induced [Ca2+]i elevation and constriction. Depolarization by 75 mM K+ stimulated renal artery wall [Ca2+]i elevation and vasoconstriction that were blocked by nimodipine (Fig. 7, A–D). By contrast, HC 067047 did not alter depolarization-induced [Ca2+]i elevation and constriction in the arteries (Fig. 7, A–D). These findings demonstrate that HC 067047-induced inhibition of neonatal renal artery myogenic constriction is not mediated via L-type Ca2+ channels.
Fig. 6.
Myogenic constriction of neonatal pig renal interlobular arteries is dependent on L-type voltage-dependent Ca2+ channels. A: a trace showing that nimodipine (1 µM) abolished myogenic tone in neonatal pig renal interlobular arteries by blocking L-type Ca2+ channels and that the removal of extracellular Ca2+ in the presence of nimodipine did not cause any further dilation of the vessels. B: bar graphs summarizing mean data (n = 3) for luminal diameter of neonatal pig interlobular arteries in the presence of nimodipine and nimodipine + Ca2+ free/EGTA bath medium.
Fig. 7.
Blockade of TRPV4 channels does not alter L-type Ca2+ channel-mediated [Ca2+]i elevation and vasoconstriction in neonatal pig renal preglomerular arteries. Traces (A and C) and bar graphs (B and D) demonstrating that depolarization by 75 mM K+ stimulated renal artery wall [Ca2+]i elevation (n = 5) and vasoconstriction (n = 7) that were abolished by nimodipine ([Ca2+]i: n = 4; vasoconstriction: n = 5), but not HC 067047 ([Ca2+]i: n = 4; vasoconstriction: n = 5). *P < 0.05 vs. 75 mM K+.
TRPV4 channels contribute to renal autoregulation in neonatal pigs.
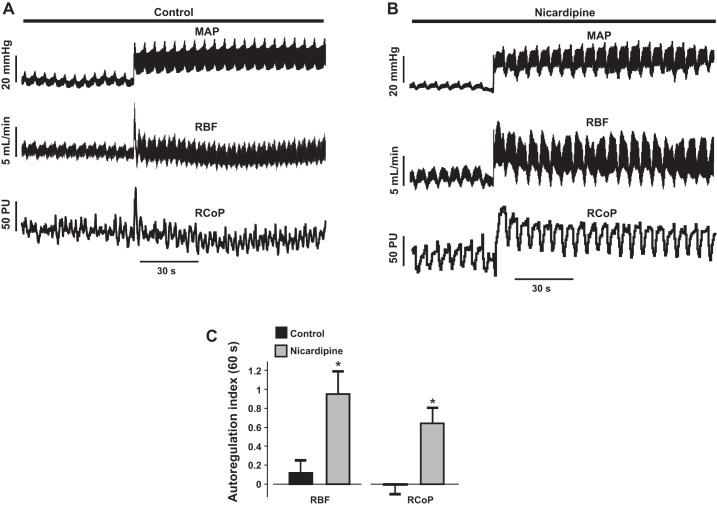
To investigate whether the myogenic mechanism observed in vitro can be recapitulated in vivo, we examined autoregulation of total RBF and renal cortical perfusion in anesthetized and mechanically ventilated neonatal pigs. As described in methods, MAP was increased by carefully tightening a ligature each around the celiac and superior mesenteric arteries. This procedure increased steady-state MAP from 86.9 ± 3.0 to 114.1 ± 2.3 mmHg (n = 15; P < 0.05). Following MAP elevation, step decreases and increases in MAP were made using a vascular occluder. We observed whether an increase in arterial pressure induces an efficient autoregulation of neonatal pig RBF and renal cortical perfusion. As shown in Fig. 8A, an increase in MAP by 20 mmHg resulted in autoregulation of total RBF and renal cortical perfusion within 10 s. The RBF and renal cortical perfusion autoregulation indexes following 60 s of increased MAP were ~0.1 and 0, respectively, indicating efficient autoregulation (11). Intrarenal arterial infusion of the L-type Ca2+ channel blocker nicardipine for ~30 min reduced basal MAP by 23.5 ± 7.4 mmHg (n = 4; P < 0.05) and abolished renal autoregulation in the pigs (Fig. 8, B and C). These data suggest that renal autoregulation is efficient in neonatal pigs. Our data also suggest that L-type Ca2+ channel-mediated myogenic constriction induces neonatal renal autoregulation.
Fig. 8.
Renal autoregulation is efficient in neonatal pigs, and dependent on L-type Ca2+ channels. A: traces illustrating that an increase in mean arterial pressure (MAP) by 20 mmHg induces autoregulation of renal blood flow (RBF) and renal cortical perfusion in neonatal pigs. B: traces showing that intrarenal arterial infusion of nicardipine (1 µg·kg−1·min−1; 30 min) abolishes RBF and renal cortical perfusion autoregulation in neonatal pigs. C: bar graphs summarizing mean (n = 4 each) RBF and renal cortical perfusion autoregulation indexes in the absence and presence of nicardipine. RCoP, renal cortical perfusion; *P < 0.05 vs. control.
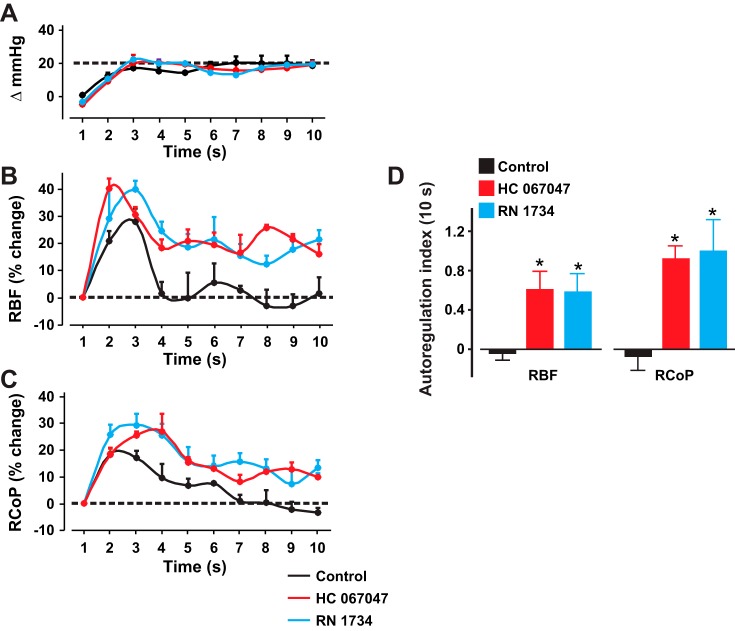
Next, we investigated whether TRPV4 channels contribute to myogenic autoregulation in the pigs. Intrarenal infusion of the TRPV4 channel blockers HC 067047 and RN 1734 for ~30 min did not alter basal MAP [change in MAP (mmHg): control: −4.3 ± 0.64 (n = 4) vs. HC 067047: −7.3 ± 1.05 (n = 5) vs. RN 1734: −5.5 ± 2.11 (n = 4) P > 0.05]. However, HC 067047 and RN 1734 inhibited RBF and renal cortical perfusion autoregulation in the pigs (Fig. 9, A–C). The effects of HC 067047 and RN 1734 on RBF and renal cortical perfusion autoregulation indexes following 10 s of MAP elevation are presented in Fig. 9D. Our data indicate that TRPV4 channel blockade attenuates myogenic renal autoregulation in neonatal pigs.
Fig. 9.
TRPV4 channels contribute to myogenic renal autoregulation in neonatal pigs. A–C: graphs showing steady-state MAP, RBF, and RCoP without (n = 4) and with intrarenal arterial infusion of HC 067047 (20 µg·kg−1·min−1; 30 min; n = 5) and RN 1734 (20 µg·kg−1·min−1; 30 min; n = 4). D: bar graphs illustrating RBF and RCoP autoregulation indexes in the absence (n = 4) and presence of HC 067047 (n = 5) and RN-1734 (n = 4). *P < 0.05 vs. control.
DISCUSSION
TRPV channels are highly expressed in the kidney, but their physiological functions in renal vascular bed are poorly understood. Similarly, mechanisms that control immature kidney perfusion are unclear. In this study, we show that unlike TRPV1–3, TRPV4 channels are expressed in neonatal pig preglomerular vascular SMCs. TRPV4 expression is higher in renal vascular SMCs than in ECs. A TRPV4 channel blocker attenuated cell swelling-induced [Ca2+]i elevation in renal vascular SMCs and vasoconstriction. Blockade of TRPV4 channels also reversed steady-state myogenic tone and inhibited pressure-induced membrane depolarization, [Ca2+]i elevation, and constriction in distal interlobular arteries. Moreover, intrarenal arterial infusion of TRPV4 channel blockers inhibited renal autoregulation in the pigs. These findings identify for the first time the physiological function of vascular SMC TRPV4 channels in neonatal renal microcirculation.
Data here show that TRPV1–3 are expressed in intact preglomerular arteries but not in SMCs that were uncontaminated with ECs. Hence, renal vascular TRPV1–3 may be restricted to ECs in neonatal pigs. We demonstrate that TRPV4 is predominantly expressed in neonatal pig renal vascular SMCs, suggesting that they may participate in SMC-dependent neonatal renal vasoregulation. In addition, localization of TRPV4 channels in the plasma membrane of freshly isolated neonatal renal vascular SMCs is consistent with a mechanotransduction function.
Vasoregulation by TRPV4 channels appears to depend on vascular bed type, vessel size, or the predominant location of the channels within the vasculature. Cerebral and mesenteric artery vasodilation induced by local [Ca2+]i signals, including Ca2+ sparks and sparklets has been associated with SMC and EC TRPV4 channels (18, 57, 58). Low intraluminal pressure stimulated rat cremaster arteriolar vasodilation via activation EC Ca2+ signaling mediated by myoendothelial junction TRPV4 channels (3). In pial arterioles, TRPV4 is expressed in ECs (35). By contrast, the channels are colocalized with astrocyte markers in parenchymal arteriole adventitia and mediate flow/pressure-induced myogenic constriction (35). Both flow- and arachidonic acid-induced dilation of human coronary arterioles are dependent on EC TRPV4 channels (7, 75). However, intravital microscopy of the lung showed that TRPV4 ablation attenuates hypoxia-induced constriction of mouse pulmonary arterioles (24). In adult mice, the TRPV4 agonist GSK1016790A relaxed large renal arteries precontracted with phenylephrine and vasa recta precontracted with norepinephrine (12). Whether pharmacological modulation or pressure-induced activation of TRPV4 channels directly regulates the diameter of resistance size renal vessels was previously unexplored. Our qRT-PCR data suggest that TRPV4 channels are expressed in neonatal pig renal vascular ECs, albeit at a much lower level than in SMCs. The physiological function of renal vascular EC TRPV4 in neonates is unclear and requires further investigation.
Both in vivo and in vitro studies have demonstrated that TRPV4 is a sensor of osmotic/mechanical stress, but conflicting evidence exists (14, 39, 49). Stretch-induced [Ca2+]i elevation was reduced in TRPV4-deficient urothelial cells (42). TRPV4 channels were hypotonic-gated, but not responsive to membrane stretch when transfected in HEK-293 cells (59). Genetic ablation of TRPV4 abolished cell-swelling-induced [Ca2+]i in renal tubules (60). Hypotonic stress has also been shown to induce [Ca2+]i elevation and whole cell currents in Chinese hamster ovary (CHO) cells overexpressing TRPV4 (38). By contrast, another study reported that cell inflation, but not hypotonic stress, significantly activated TRPV4 currents in CHO cells transiently expressing TRPV4 (61). Consistent with a previous report on pulmonary artery SMCs (73), we show in this study that hyposmotic challenge triggered TRPV4-dependent [Ca2+]i elevation in neonatal pig renal vascular SMCs. Conceivably, the functional role of TRPV4 as osmo-mechanosensitive channels is dependent on cell type.
Renal vascular SMC [Ca2+]i elevation induced by hyposmotic solution was inhibited by stretch-activated and L-type Ca2+ channel blockers, indicating that cell swelling stimulates stretch-activated channels and subsequent membrane depolarization in the cells. Cell swelling activates PLA2 in several cell types, including tumor cells, fibroblasts, and pancreatic islets (4, 47, 48). Swelling-induced channel activation in TRPV4-transfected HEK-293 cells has also been shown to be dependent on PLA2 (68). We show here that at the concentration that did not alter high K+-induced [Ca2+]i elevation and constriction, HC-067047-attenuated [Ca2+]i elevation, and vasoconstriction induced by hyposmotic challenge. These data suggest that cell swelling activates TRPV4 channels in neonatal pig renal vascular SMCs. The lack of effect of a PLA2 inhibitor on hyposmotic-induced [Ca2+]i elevation and vasoconstriction indicates that osmotic cell swelling-induced TRPV4 activation in neonatal pig renal vascular SMCs occurs independently of PLA2. Together, our data suggest that neonatal pig renal vascular SMC TRPV4 channels are mechanosensitive, and contribute to renal myogenic constriction. The identities and functions of mechanosensitive ion channels that underlie pressure-induced depolarization and successive [Ca2+]i elevation and constriction in renal vascular SMCs are understudied. So far, only epithelial Na+ channels (ENaC) have been shown to contribute to mechanotransduction in renal vascular SMCs. Knockdown of the β-subunits of ENaC (βENaC) attenuated stretch-induced currents in adult mouse renal vascular SMCs (13). Afferent arteriole myogenic constriction and renal autoregulation were also reduced in mice that lacked βENaC (21). Both ENaC and TRPV4 proteins interact with actin cytoskeleton (51). Whether ENaC and TRPV4 form a mechanosensitive signaling complex in renal vascular SMCs is unclear and requires additional studies.
Although isolated tissue experiments in this study utilized resistance arteries, our data indicate that TRPV4 channels are also expressed in afferent arterioles. Hence, the effect of TRPV4 channel blockers on neonatal pig renal autoregulation may reflect the contribution of both interlobular arteries and afferent arterioles. Attenuation of neonatal pig renal autoregulation by TRPV4 channel blockers is consistent with the data on myogenic constriction of isolated vessels and strongly support our hypothesis that mechanotransduction by TRPV4 channels in renal vascular SMCs contributes to neonatal pig renal myogenic autoregulation. Osmotic and mechanical stress trigger ATP release (6). Studies have also shown that hypotonicity- and stretch-induced ATP release by adult rat thick ascending limb and adult mouse urothelial cells, respectively, are dependent on TRPV4 channels (42, 54). Given that ATP release in the macula densa contributes to TGF-mediated autoregulation, it is possible that TRPV4 is also involved in TGF mechanisms. However, TRPV4 is absent in adult rat macula densa (64). Whether the absence of TRPV4 in the macula densa is consistent across species, and age is unknown. TGF mechanism may also be immature in neonates (1). Thus our current findings provide no insight into the functional role of TRPV4 channels in neonatal pig TGF mechanism.
TRPV4 protein expression levels and agonist-induced [Ca2+]i elevation in primary mesenteric artery ECs were higher in young (3 mo old) when compared with aged (22 mo old) rats, suggesting age-dependent regulation of the expression and function of vascular TRPV4 channels (16). Data here indicate that TRPV4 protein expression levels in pig kidneys and renal arteries are significantly higher in adults when compared with neonates. Perhaps, TRPV4 channels are also involved in renal myogenic autoregulation mechanism in adults. Hence, the functional significance of renal maturation-dependent expression of the channels requires further investigation.
In summary, we have uncovered a new physiological role for renal TRPV4 channels. We show that neonatal pig renal vascular SMC TRPV4 channels are activated by an elevation in intravascular pressure, and this reaction results in membrane depolarization, extracellular Ca2+ influx via L-type Ca2+ channels, and vasoconstriction. We also demonstrate that TRPV4-dependent myogenic constriction contributes to renal autoregulation in neonatal pigs.
GRANTS
This study is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-101668.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A. conceived and designed research; A.A. drafted manuscript; H.S., D.P.-N., A.T.M., and A.A. performed experiments; H.S., D.P.-N., and A.A. analyzed data; H.S., D.P.-N., and A.A. interpreted results of experiments; H.S., D.P.-N., and A.A. prepared figures; H.S., D.P.-N., A.T.M., and A.A. edited and revised manuscript; H.S., D.P.-N., A.T.M., and A.A. approved final version of manuscript.
REFERENCES
- 1.Al-Dahhan J, Haycock GB, Chantler C, Stimmler L. Sodium homeostasis in term and preterm neonates. I. Renal aspects. Arch Dis Child 58: 335–342, 1983. doi: 10.1136/adc.58.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anfinogenova Y, Brett SE, Walsh MP, Harraz OF, Welsh DG. Do TRPC-like currents and G protein-coupled receptors interact to facilitate myogenic tone development? Am J Physiol Heart Circ Physiol 301: H1378–H1388, 2011. doi: 10.1152/ajpheart.00460.2011. [DOI] [PubMed] [Google Scholar]
- 3.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18174–18179, 2012. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basavappa S, Pedersen SF, Jørgensen NK, Ellory JC, Hoffmann EK. Swelling-induced arachidonic acid release via the 85-kDa cPLA2 in human neuroblastoma cells. J Neurophysiol 79: 1441–1449, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf) 203: 99–116, 2011. doi: 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res 26: 959–969, 2001. doi: 10.1023/A:1012388618693. [DOI] [PubMed] [Google Scholar]
- 7.Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol 302: H634–H642, 2012. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley NM, Brazeau P, Frasier ID. Renal blood flow autoregulation in developing swine. Am J Physiol Heart Circ Physiol 245: H1–H6, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, Jaggar JH. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res 111: 1027–1036, 2012. doi: 10.1161/CIRCRESAHA.112.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12: 845–858, 2014. doi: 10.2174/15701611113116660149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Kaßmann M, Sendeski M, Tsvetkov D, Marko L, Michalick L, Riehle M, Liedtke WB, Kuebler WM, Harteneck C, Tepel M, Patzak A, Gollasch M. Functional transient receptor potential vanilloid 1 and transient receptor potential vanilloid 4 channels along different segments of the renal vasculature. Acta Physiol (Oxf) 213: 481–491, 2015. doi: 10.1111/apha.12355. [DOI] [PubMed] [Google Scholar]
- 13.Chung WS, Weissman JL, Farley J, Drummond HA. βENaC is required for whole cell mechanically gated currents in renal vascular smooth muscle cells. Am J Physiol Renal Physiol 304: F1428–F1437, 2013. doi: 10.1152/ajprenal.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen DM. The role of TRPV4 in the kidney, in TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, edited by Liedtke WB, Heller S. Boca Raton, FL: CRC/Taylor & Francis, 2007, chapt 29. [PubMed] [Google Scholar]
- 15.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Du J, Wang X, Li J, Guo J, Liu L, Yan D, Yang Y, Li Z, Zhu J, Shen B. Increasing TRPV4 expression restores flow-induced dilation impaired in mesenteric arteries with aging. Sci Rep 6: 22780, 2016. doi: 10.1038/srep22780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 19.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 20.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge Y, Gannon K, Gousset M, Liu R, Murphey B, Drummond HA. Impaired myogenic constriction of the renal afferent arteriole in a mouse model of reduced βENaC expression. Am J Physiol Renal Physiol 302: F1486–F1493, 2012. doi: 10.1152/ajprenal.00638.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giraud S, Favreau F, Chatauret N, Thuillier R, Maiga S, Hauet T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J Biomed Biotechnol 2011: 532127, 2011. doi: 10.1155/2011/532127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glauser EM. Advantages of piglets as experimental animals in pediatric research. Exp Med Surg 24: 181–190, 1966. [PubMed] [Google Scholar]
- 24.Goldenberg NM, Wang L, Ranke H, Liedtke W, Tabuchi A, Kuebler WM. TRPV4 is required for hypoxic pulmonary vasoconstriction. Anesthesiology 122: 1338–1348, 2015. doi: 10.1097/ALN.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 25.Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol 586: 5633–5649, 2008. doi: 10.1113/jphysiol.2008.161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 27.Hayashi K, Epstein M, Loutzenhiser R. Enhanced myogenic responsiveness of renal interlobular arteries in spontaneously hypertensive rats. Hypertension 19: 153–160, 1992. doi: 10.1161/01.HYP.19.2.153. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi K, Epstein M, Loutzenhiser R. Pressure-induced vasoconstriction of renal microvessels in normotensive and hypertensive rats. Studies in the isolated perfused hydronephrotic kidney. Circ Res 65: 1475–1484, 1989. doi: 10.1161/01.RES.65.6.1475. [DOI] [PubMed] [Google Scholar]
- 29.Heyeraas KJ, Aukland K. Interlobular arterial resistance: influence of renal arterial pressure and angiotensin II. Kidney Int 31: 1291–1298, 1987. doi: 10.1038/ki.1987.142. [DOI] [PubMed] [Google Scholar]
- 30.Hoenderop JG, Nilius B, Bindels RJ. Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Annu Rev Physiol 64: 529–549, 2002. doi: 10.1146/annurev.physiol.64.081501.155921. [DOI] [PubMed] [Google Scholar]
- 31.Hook JB, Bailie MD. Perinatal renal pharmacology. Annu Rev Pharmacol Toxicol 19: 491–509, 1979. doi: 10.1146/annurev.pa.19.040179.002423. [DOI] [PubMed] [Google Scholar]
- 32.Jin M, Berrout J, Chen L, O’Neil RG. Hypotonicity-induced TRPV4 function in renal collecting duct cells: modulation by progressive cross-talk with Ca2+-activated K+ channels. Cell Calcium 51: 131–139, 2012. doi: 10.1016/j.ceca.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jose PA, Slotkoff LM, Montgomery S, Calcagno PL, Eisner G. Autoregulation of renal blood flow in the puppy. Am J Physiol 229: 983–988, 1975. [DOI] [PubMed] [Google Scholar]
- 34.Kassmann M, Harteneck C, Zhu Z, Nürnberg B, Tepel M, Gollasch M. Transient receptor potential vanilloid 1 (TRPV1), TRPV4, and the kidney. Acta Physiol (Oxf) 207: 546–564, 2013. doi: 10.1111/apha.12051. [DOI] [PubMed] [Google Scholar]
- 35.Kim KJ, Iddings JA, Stern JE, Blanco VM, Croom D, Kirov SA, Filosa JA. Astrocyte contributions to flow/pressure-evoked parenchymal arteriole vasoconstriction. J Neurosci 35: 8245–8257, 2015. doi: 10.1523/JNEUROSCI.4486-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JC, Choe SY. Age-related changes in the distribution of transient receptor potential vanilloid 4 channel (TRPV4) in the central nervous system of rats. J Mol Histol 45: 497–505, 2014. doi: 10.1007/s10735-014-9578-z. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Wang DH. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol Res 57: 239–246, 2008. doi: 10.1016/j.phrs.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liedtke WB. TRPV channels' function in osmo- and mechanotransduction. In: TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, edited by Liedtke WB, Heller S. Boca Raton, FL: CRC/Taylor & Francis, 2007, chapt. 22. [PubMed] [Google Scholar]
- 40.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGahon MK, Fernández JA, Dash DP, McKee J, Simpson DA, Zholos AV, McGeown JG, Curtis TM. TRPV2 Channels contribute to stretch-activated cation currents and myogenic constriction in retinal arterioles. Invest Ophthalmol Vis Sci 57: 5637–5647, 2016. doi: 10.1167/iovs.16-20279. [DOI] [PubMed] [Google Scholar]
- 42.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284: 21257–21264, 2009. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narayanan D, Bulley S, Leo MD, Burris SK, Gabrick KS, Boop FA, Jaggar JH. Smooth muscle cell transient receptor potential polycystin-2 (TRPP2) channels contribute to the myogenic response in cerebral arteries. J Physiol 591: 5031–5046, 2013. doi: 10.1113/jphysiol.2013.258319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nascimento MC, Xavier CC, Goulart EM. Arterial blood pressure of term newborns during the first week of life. Braz J Med Biol Res 35: 905–911, 2002. doi: 10.1590/S0100-879X2002000800007. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen TW, Maaske CA, Booth NH. Some comparative aspects of porcine renal function. In: Swine in Biomedical Research, edited by Bukstad LK, McClellan RO. Seattle, WA: Frayn, 1965, p. 529–537. [Google Scholar]
- 46.Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol 14: 2731–2740, 2003. doi: 10.1097/01.ASN.0000094081.78893.E8. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen S, Lambert IH, Thoroed SM, Hoffmann EK. Hypotonic cell swelling induces translocation of the alpha isoform of cytosolic phospholipase A2 but not the gamma isoform in Ehrlich ascites tumor cells. Eur J Biochem 267: 5531–5539, 2000. doi: 10.1046/j.1432-1327.2000.01615.x. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen SF, Poulsen KA, Lambert IH. Roles of phospholipase A2 isoforms in swelling- and melittin-induced arachidonic acid release and taurine efflux in NIH3T3 fibroblasts. Am J Physiol Cell Physiol 291: C1286–C1296, 2006. doi: 10.1152/ajpcell.00325.2005. [DOI] [PubMed] [Google Scholar]
- 49.Plant TD, and Strotmann R. TRPV4. In: Transient Receptor Potential (TRP) Channels. Handbook of Experimental Pharmacology, edited by Flockerzi V, Nilius B. Heidelberg, Germany: Springer, 2007. [Google Scholar]
- 50.Reuter R. Dellmann’s Textbook of Veterinary Histology. Hoboken, NJ: Wiley-Blackwell, 2007. [Google Scholar]
- 51.Sasaki S, Yui N, Noda Y. Actin directly interacts with different membrane channel proteins and influences channel activities: AQP2 as a model. Biochim Biophys Acta 1838: 514–520, 2014. doi: 10.1016/j.bbamem.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond) 96: 313–326, 1999. doi: 10.1042/cs0960313. [DOI] [PubMed] [Google Scholar]
- 53.Semple SJ, De Wardener HE. Effect of increased renal venous pressure on circulatory autoregulation of isolated dog kidneys. Circ Res 7: 643–648, 1959. doi: 10.1161/01.RES.7.4.643. [DOI] [PubMed] [Google Scholar]
- 54.Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol 295: F1090–F1095, 2008. doi: 10.1152/ajprenal.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soni H, Adebiyi A. Pressor and renal regional hemodynamic effects of urotensin II in neonatal pigs. J Endocrinol 217: 317–326, 2013. doi: 10.1530/JOE-12-0556. [DOI] [PubMed] [Google Scholar]
- 56.Soni H, Buddington RK, Adebiyi A. Postnatal kidney maturation regulates renal artery myogenic constriction. J Perinat Med 0: 2014. doi: 10.1515/jpm-2013-0346. [DOI] [PubMed] [Google Scholar]
- 57.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal 7: ra66, 2014. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki M, Mizuno A. TRP channels and mechanical signals. In: Mechanosensing Biology. Tokyo, Japan: Springer, 2011, chapt. 7, p. 87–101. [Google Scholar]
- 61.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278: 22664–22668, 2003. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 62.Takumida M, Ishibashi T, Hamamoto T, Hirakawa K, Anniko M. Age-dependent changes in the expression of klotho protein, TRPV5 and TRPV6 in mouse inner ear. Acta Otolaryngol 129: 1340–1350, 2009. doi: 10.3109/00016480902725254. [DOI] [PubMed] [Google Scholar]
- 63.Terris JM. Swine as a model in renal physiology and nephrology: an overview. In: Swine in Biomedical Research, edited by Tumbleson ME. New York: Plenum, 1986, p. 1673–1709. [Google Scholar]
- 64.Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, Bachmann S, Cohen DM. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am J Physiol Renal Physiol 287: F17–F24, 2004. doi: 10.1152/ajprenal.00397.2003. [DOI] [PubMed] [Google Scholar]
- 65.Tóth-Heyn P, Drukker A, Guignard JP. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol 14: 227–239, 2000. doi: 10.1007/s004670050048. [DOI] [PubMed] [Google Scholar]
- 66.van Abel M, Huybers S, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ. Age-dependent alterations in Ca2+ homeostasis: role of TRPV5 and TRPV6. Am J Physiol Renal Physiol 291: F1177–F1183, 2006. doi: 10.1152/ajprenal.00038.2006. [DOI] [PubMed] [Google Scholar]
- 67.van de Graaf SF, Hoenderop JG, Bindels RJ. Regulation of TRPV5 and TRPV6 by associated proteins. Am J Physiol Renal Physiol 290: F1295–F1302, 2006. doi: 10.1152/ajprenal.00443.2005. [DOI] [PubMed] [Google Scholar]
- 68.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101: 396–401, 2004. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wanner SP, Garami A, Romanovsky AA. Hyperactive when young, hypoactive and overweight when aged: connecting the dots in the story about locomotor activity, body mass, and aging in Trpv1 knockout mice. Aging (Albany NY) 3: 450–454, 2011. doi: 10.18632/aging.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 71.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62: 381–404, 2010. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie C, Sachs JR, Wang DH. Interdependent regulation of afferent renal nerve activity and renal function: role of transient receptor potential vanilloid type 1, neurokinin 1, and calcitonin gene-related peptide receptors. J Pharmacol Exp Ther 325: 751–757, 2008. doi: 10.1124/jpet.108.136374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L555–L568, 2012. doi: 10.1152/ajplung.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- 75.Zheng X, Zinkevich NS, Gebremedhin D, Gauthier KM, Nishijima Y, Fang J, Wilcox DA, Campbell WB, Gutterman DD, Zhang DX. Arachidonic acid-induced dilation in human coronary arterioles: convergence of signaling mechanisms on endothelial TRPV4-mediated Ca2+ entry. J Am Heart Assoc 2: e000080, 2013. doi: 10.1161/JAHA.113.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y, Wang DH. Segmental regulation of sodium and water excretion by TRPV1 activation in the kidney. J Cardiovasc Pharmacol 51: 437–442, 2008. doi: 10.1097/FJC.0b013e318168d120. [DOI] [PMC free article] [PubMed] [Google Scholar]