renal transforming growth factor-β (TGF-β) has been implicated in the pathogenesis of hypertension. Renal TGF-β1 protein expression increased along with blood pressure in male Dahl salt-sensitive (DS) rats in response to a high-sodium diet (6), and in male stroke-prone spontaneously hypertensive rats (SHR) when the drinking water contained 1% sodium (8). Moreover, a neutralizing antibody to TGF-β prevented the sodium-induced increase in mean arterial pressure (MAP) in the male DS rats. Less is known regarding the role renal TGF-β plays in hypertension in females since TGF-β protein expression did not increase with MAP in the DS female rats in response to a high-sodium diet (6), and no females were included in the above stroke-prone SHR study (8).
Rigor and reproducibility (R&R) are the foundation of science; however, there is a growing realization in the scientific community that practices optimizing R&R have not been adequately adopted, which has led to irreproducible findings and wasted resources (2). The National Institutes of Health (NIH) now expects research grants to address R&R in their proposals, and comprehensive educational programs in R&R must be included in all individual and institutional training and career development grant applications. As of January 2016, NIH requires all grant applicants to consider the biological variable of sex as an important aspect of R&R since one's sex impacts the incidence, age of onset, manifestation, severity and rate of progression, as well as response to treatment in diverse pathologies, including cardiovascular, renal, and metabolic diseases (1, 7).
Not reporting findings or interpreting data by sex has contributed to the lack of reproducibility. Mirabito et al. (5) found the angiotensin type 2 receptor plays a protective role in the pressure-natriuresis relationship in female, but not in male, rats. Ji et al. (3) showed that T cells from males but not from females increase the magnitude of ANG II-dependent hypertension in mice. These two examples illustrate how negative findings in one sex could mask positive effects in the other if the results were not separated by sex and how extrapolating positive results discovered in one sex could lead to erroneous conclusions in the other.
In a recent issue of the American Journal of Physiology-Renal Physiology, Tipton et al. (10) show that maturation from ~1 to 4 mo of age doubled renal TGF-β1 protein expression in the female, but not male, SHR. Thus, this study demonstrates a sex-specific role for renal TGF-β1 in the maturing female SHR kidney and supports a previous report showing that renal TGF-β protein expression was nearly threefold higher in the 12- vs. 4-mo-old female DS rat maintained on a low-sodium diet (4). The Sullivan laboratory previously found that the frequency of renal Foxp3+-CD4+ T-regulatory cells correlated with systolic blood pressure in female, but not in male, SHR (9). While hydrochlorothiazide-reserpine (HCTZ-Res) reduced systolic blood pressure in both male and female SHR, the frequency of T-regulatory cells was reduced only in the female rat.
In this current study (10), HCTZ-Res reduced both MAP and renal TGF-β1 protein expression in the female SHR, and HCTZ-Res treatment prevented the age-associated increase in MAP and renal TGF-β1 protein. The authors also reported that treating female SHR with a monoclonal antibody to TGF-β did not result in lowering MAP. These findings suggest that the rise in renal TGF-β1 is a consequence of the increased MAP rather than the cause. Further studies are needed, however, to confirm that the antibody treatment fully neutralized TGF-β. Research is also needed to compare the role of renal TGF-β in various male models of hypertension since renal TGF-β1 protein expression correlated with MAP in DS (6) and stroke-prone SHR (8) under high-sodium ingestion but not in the current study of male SHR on a normal sodium diet.
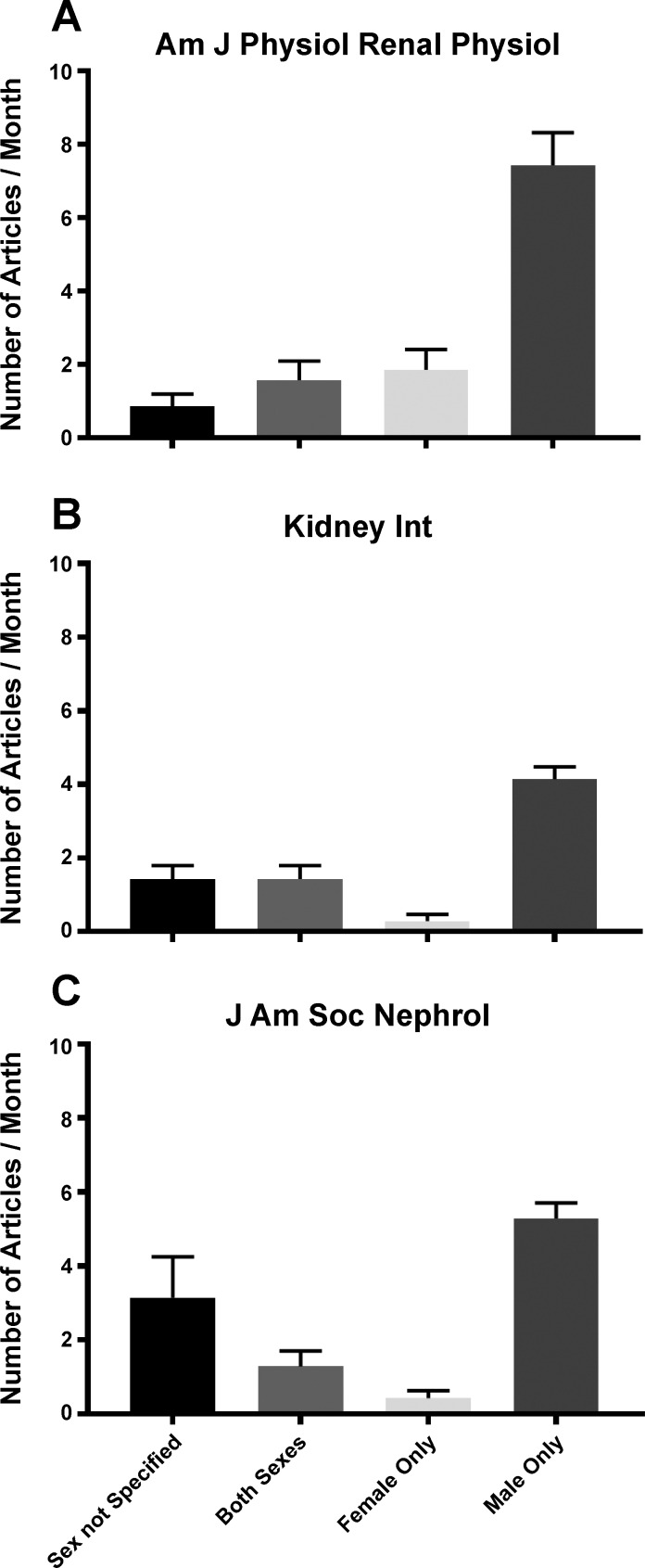
This article is important not only because it provides mechanistic insight into the role of renal TGF-β1 in hypertension, but also because the study improves our fundamental understanding of female renal pathophysiology. Few investigators study renal mechanisms in the female, even though kidney disease afflicts both men and women. A review of all papers published this year between January and July in the American Journal of Physiology-Renal Physiology, which publishes “information on kidney and urinary tract physiology, epithelial cell biology, and control of body fluid volume and composition” (http://ajprenal.physiology.org), showed that less than 15% of the animal studies included both sexes, and of these, half did not specify the n value by sex. Furthermore, there were five studies conducted solely in male animals to every study performed in females (Fig. 1A). This male:female (M:F) ratio has not improved over the past 10 years, since a review of all animal studies published in this journal in January 2007 revealed a similar 4.5:1 M:F ratio. Two other prominent journals, Kidney International (11:1 M:F; Fig. 1B) and the Journal of the American Society of Nephrology (16:1 M:F; Fig. 1C), which also publish key findings in renal physiology and pathophysiology, exhibited even greater male bias.
Fig. 1.
The number of articles published monthly between January–July 2017 in the American Journal of Physiology-Renal Physiology (Am J Physiol Renal Physiol) (A) Kidney International (Kidney Int) (B), and the Journal of the American Society of Nephrology (J Am Soc Nephrol) (C) as a function of the sex of the animals studied ± SE. In addition to reviewing the methods, figure and table legends for each article, the entire article was electronically searched for the terms: male, man, men, female, woman, women, sex, and gender.
The dearth of research in female animal models has contributed to the lack of reproducibility since the biological variable of sex is often not considered in the interpretation of results. The National Institute of Diabetes and Digestive and Kidney Diseases held a workshop this past July on “Sex and the Kidneys: Sex Differences in Renal Disease” to raise awareness of this problem. These journal statistics serve as one metric for assessing the efficacy of efforts led by NIH and others in the scientific community to improve consideration of biological sex in mechanisms of renal physiology and pathology. Hopefully, the intriguing findings arising from the Sullivan laboratory will inspire more investigators to study disease mechanisms in the female kidney.
GRANTS
This work was supported by the National Heart Lung and Blood Institute (R01 HL-119380 to K. Sandburg) and the National Center for Advancing Translational Sciences (TL1-TR-001431 to K. Sandberg and A. V. Pai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S., A.V.P. and T.M. drafted, edited and approved the manuscript; T.M. conducted the research, analyzed the data and prepared the figures.
REFERENCES
- 1.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol 37: 746–756, 2017. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begley CG, Ioannidis JP. Reproducibility in science: improving the standard for basic and preclinical research. Circ Res 116: 116–126, 2015. doi: 10.1161/CIRCRESAHA.114.303819. [DOI] [PubMed] [Google Scholar]
- 3.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64: 573–582, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17β-estradiol in the aging Dahl salt-sensitive rat. J Am Soc Nephrol 15: 1546–1556, 2004. doi: 10.1097/01.ASN.0000128219.65330.EA. [DOI] [PubMed] [Google Scholar]
- 5.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM. Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol. 307: F901–F907, 2014. doi: 10.1152/ajprenal.00288.2014. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SR, Dahly-Vernon AJ, Dunn KM, Chen CC, Ledbetter SR, Williams JM, Roman RJ. Renoprotective effects of anti-TGF-β antibody and antihypertensive therapies in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 303: R57–R69, 2012. doi: 10.1152/ajpregu.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reckelhoff JF, Samson WK. Sex and gender differences in cardiovascular, renal and metabolic diseases. Am J Physiol Regul Integr Comp Physiol 309: R1057–R1059, 2015. doi: 10.1152/ajpregu.00417.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tahira Y, Fukuda N, Endo M, Suzuki R, Ikeda Y, Takagi H, Matsumoto K, Kanmatsuse K. Transforming growth factor-β expression in cardiovascular organs in stroke-prone spontaneously hypertensive rats with the development of hypertension. Hypertens Res 25: 911–918, 2002. doi: 10.1291/hypres.25.911. [DOI] [PubMed] [Google Scholar]
- 9.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64: 557–564, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tipton AJ, Musall JB, Crislip GR, and Sullivan JC. Greater transforming growth factor-β in adult female SHR is dependent on blood pressure, but does not account for sex differences in renal T-regulatory cells. Am J Physiol Renal Physiol. doi: 10.1152/ajprenal.00175.2017 (July 5, 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]