Abstract
Recent evidence suggests that a greater density of pericytes in renal cadaveric allografts is associated with better recovery following transplant. The physiological mechanism(s) through which pericyte density may be beneficial is not well understood. The goal of this study was to test the hypothesis that lower medullary pericyte density is associated with greater renal injury following ischemia reperfusion (IR) in a rat model, providing a basis for future studies to better understand pericytes in a pathological environment. To test our hypothesis, we determined the association between medullary pericyte density and renal injury in spontaneously hypertensive rats (SHR) following 45 min of warm bilateral IR. We found that there was a significant negative relationship between pericyte density and plasma creatinine (slope = −0.03, P = 0.02) and blood urea nitrogen (slope = −0.5, P = 0.01) in female but not male SHR. Pericyte density was negatively associated with medullary peritubular capillary (PT) congestion in both sexes following IR (male: slope = −0.04, P = 0.009; female: slope = −0.03, P = 0.0001). To further test this relationship, we used a previously reported method to reduce pericyte density in SHR. Medullary erythrocyte congestion in vasa recta (VR) and PT significantly increased following IR in both sexes when pericyte density was pharmacologically decreased (VR: P = 0.03; PT: P = 0.03). Our data support the hypothesis that pericyte density is negatively associated with the development of IR injury in SHR, which may be mediated by erythrocyte congestion in the medullary vasculature.
Keywords: ischemia reperfusion injury, sex difference, erythrocyte trapping, spontaneously hypertensive rats
pericytes are contractile cells that surround the endothelium of the microvasculature (23). Within the kidney, pericytes are present on capillaries in both the renal cortex and the renal medulla; however, the descending vasa recta capillaries (VR) of the renal outer medulla have the highest density of vascular pericytes (9, 32). The VR are specialized vessels that extend downward into the renal medulla and give rise to the peritubular (PT) capillary network providing blood to the region. Pericytes on the VR have been speculated to regulate medullary blood flow (23) and act as a support structure for endothelial cells by maintaining the integrity of capillaries (20). However, studying the renal medulla and VR pericytes in vivo is extremely difficult due to access, and as a result, the physiological role of these cells is not well understood (23).
A recent clinical study demonstrated improved recovery following renal ischemia in cadaveric allografts with higher pericyte densities (19). More specifically, transplanted renal allograft biopsies that recovered after surgery had greater peritubular interstitial pericyte density in the renal cortex when assessed by immunohistochemistry compared with kidneys with sustained impairment of function (19). Our goal was to determine whether this association is also observed in an experimental model. We hypothesize that lower pericyte density is associated with increased ischemia-reperfusion (IR)-induced injury in rats. Our data will provide a platform that will allow us to better delineate the potential pathways through which greater pericyte density may be beneficial following a period of ischemia.
A well-accepted experimental model of acute kidney injury (AKI) that mimics the ischemic period in cadaveric allografts is 45 min of warm bilateral IR (7, 31), which is the model used in this study. To more directly assess the role of pericytes following IR, we adapted a previously reported pharmacological approach, which demonstrated a reduction of pericyte density in the medulla of spontaneously hypertensive rats (SHR) (4). For this reason, we used SHR for all of our experiments.
MATERIALS AND METHODS
Animals
Male and female SHR from an in-house colony at Augusta University were studied (72 rats in total: 39 male and 33 female). An additional set of SHR was purchased from Harlan Laboratories for kidney volume measurements (23 rats: 12 male and 11 female; Indianapolis, IN). Rats were housed in temperature- (20–26°C) and humidity (30–70%)-controlled, 12:12-h light-cycled quarters. Rats were provided ad libitum access to water and standard 18% protein rodent chow (Harlan Teklad, 2918). All experiments were conducted in accordance with the National Institutes of Healthʼs “Guide for the Care and Use of Laboratory Animals”, and they were approved and monitored by the Augusta University Institutional Animal Care and Use Committee.
Warm Bilateral Renal Ischemia Reperfusion Model
Male and female rats were randomized to serve either as controls with no surgery or receive IR surgery at 13 wk of age. IR surgery was performed as previously described, but with minor modifications (6). Briefly, rats were anesthetized with ~2% isoflurane. Body temperature was measured via a rectal probe, and it was maintained at 37°C throughout the procedure by a servo-controlled heating table and UV heating lamp. The left and right kidneys were accessed by a flank incision. The kidneys were exposed, and the renal artery was carefully separated from the renal vein via manual blunt dissection. Both left and right renal arteries were then clamped with microserrefines (Fine Science Tools, Foster City, CA); a clamp was first applied to the left renal artery and then a second was applied to the contralateral renal artery within 5 min. After 45 min of warm ischemia, the clamps were simultaneously removed, and blood flow was restored to both kidneys. Sterile saline (~0.2 ml) was introduced into the peritoneal space to replace any fluids lost, and the wound was closed with 4-0 polypropylene suture for the muscle layer and wound clips for the skin. Each rat was monitored until consciousness was regained, and buprenorphine (0.9 mg/kg sc) was administered as an analgesic. Animals were allowed to recover for 24 h in conventional animal housing before being anesthetized with a ketamine-xylazine cocktail (50 and 10 mg/kg ip, respectively; Phoenix Pharmaceuticals, St. Joseph, MO). A midline abdominal incision was made, and blood was collected (~9 ml for males and 6 ml for females) from the abdominal aorta with an 18-gauge needle attached to a 10-ml syringe containing ~0.2 ml of 7.5% ethylenediaminetetraacetic acid. Both kidneys were then located and excised for histological analysis. Thoracotomy incision was made as a secondary form of euthanasia.
Determination of Pericyte Density
The right kidney of each rat was transversely bisected, and a single ~5-mm section of fresh tissue was taken from the cut end. The slice was fixed in 10% formalin for 24–48 h and then embedded in paraffin. Three 5-µm slices, 15 µm apart, were cut and mounted on a glass slide. The slide was then deparaffinized, rehydrated, and incubated with an epitope retrieval solution for 40 min in a steam bath (IHC World, Ellicott City, MD). The slide was then incubated with a 6% BSA/PBS blocking buffer for 30 min. Neural glial 2 (NG2) is a chondroitin sulfate proteoglycan that is present on the surface of pericytes (5); slides were incubated with NG2 primary antibody overnight (cat. no. AB5320; 1:100; EMD Millipore, Darmstadt, Germany). Slides were then rinsed and incubated with donkey anti-rabbit secondary antibody (cat. no. A21207; 1:200; Alexa Fluor 594 conjugate) for 90 min at room temperature. 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (1:1,000 for 5 min; Sigma-Aldrich, St. Louis, MO) was performed at the end of NG2 incubation. In addition to pericytes, NG2 is expressed on early postnatal skin, adult skin stem cells, adipocytes, vascular smooth muscle cells, neuronal progenitors, oligodendrocyte progenitors, and developing cartilage, bone, and muscle (2). The unique architecture of the VR allows for NG2 staining to be used to quantify pericytes in the renal medulla. Pericyte-bound VR run through the outer medulla in bundles (9, 24). NG2 staining in the renal medulla outlines these vessel bundles and the cell bodies of the pericytes, and pericyte cell bodies can be identified by the presence of blue DAPI staining of the nucleus; a representative image of renal medullary pericytes is shown in Fig. 1.
Fig. 1.
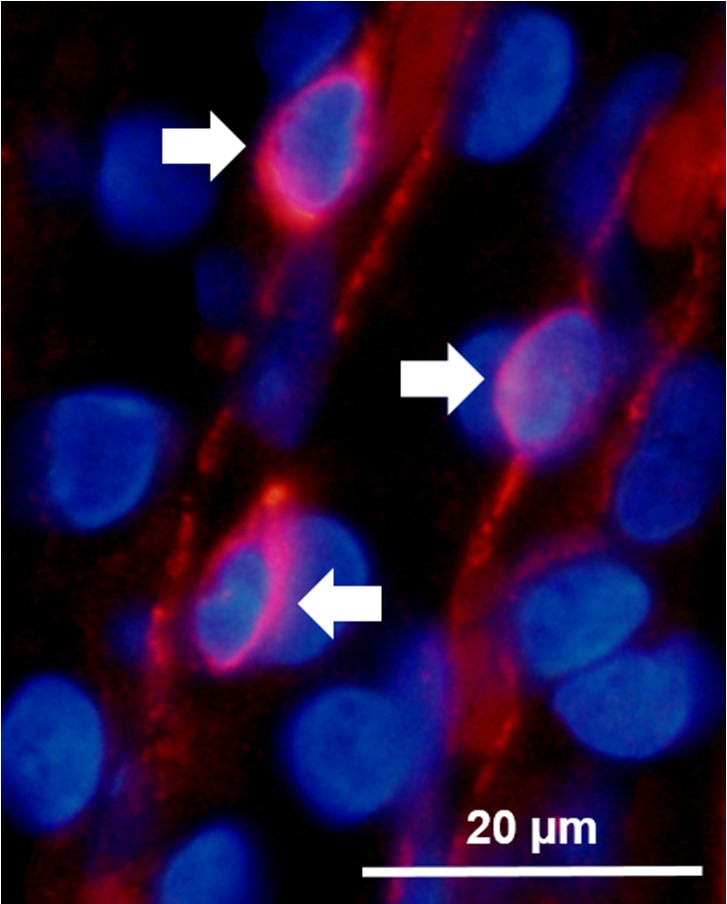
Representative image of fluorescently labeled neural glial 2 (NG2)-positive pericyte cell bodies overlapped with DAPI staining of nuclei, which was used for the quantification of pericytes. White arrows point to the three pericyte cells that are visible; image was taken from an ischemia-reperfusion (IR) male. Note the width of the vessels in relation to diameter of the light-red-stained red blood cells (RBCs) that can be seen in the image.
Slides were labeled 1 to 67 to remove group identifiers. A systematic approach was followed to ensure images of the medulla were consistent between samples. Specifically, six nonoverlapping images of the renal medulla were taken at ×40 magnification (BX40 Olympus optical microscope) using the pelvic wall as the starting point. This was done for all three 5-µm kidney slices on each sample slide. The number of pericyte cell bodies from each image was quantified and then divided by the area. This was done for each animal.
Assessment of Renal Injury
An arterial blood sample from each animal was collected at the time of sacrifice. Creatinine concentrations and blood urea nitrogen (BUN) in arterial plasma were quantified by a QuantiChrom creatinine assay kit (BioAssay Systems, Hayward, CA) and QuantiChrom urea assay kit (BioAssay Systems), respectively, according to the manufacturer’s instructions.
Calculate the % area of medullary protein casts.
Paraffin-embedded kidney tissue slices were stained with Masson’s trichrome, according to the manufacturer’s instructions (Thermo Scientific, Richard-Allan Scientific, Kalamazoo, MI). A representative image of the renal medulla was taken for each rat at ×10 magnification, covering the majority of the medulla (BX40 Olympus optical microscope) and analyzed with MetaMorph Software. The color threshold of a protein cast from a stained slide was determined, and the hue-saturation-intensity equally applied to all subsequent images. The percentage area covered at the inclusive threshold for each kidney was recorded.
Estimation of tubular injury.
Additional paraffin-fixed kidney slices were stained with hematoxylin and eosin, according to manufacturer’s instructions (Leica Biosystems; Buffalo Grove, IL). Group identifiers were removed from each slide, and a researcher blinded to the hypothesis scored each section. The percentage of tubules that showed signs of injury was determined for each sample using the following as criteria: tubular necrosis, a lack of brush border, tubular dilation, and protein cast formation.
Assessment of medullary vascular congestion.
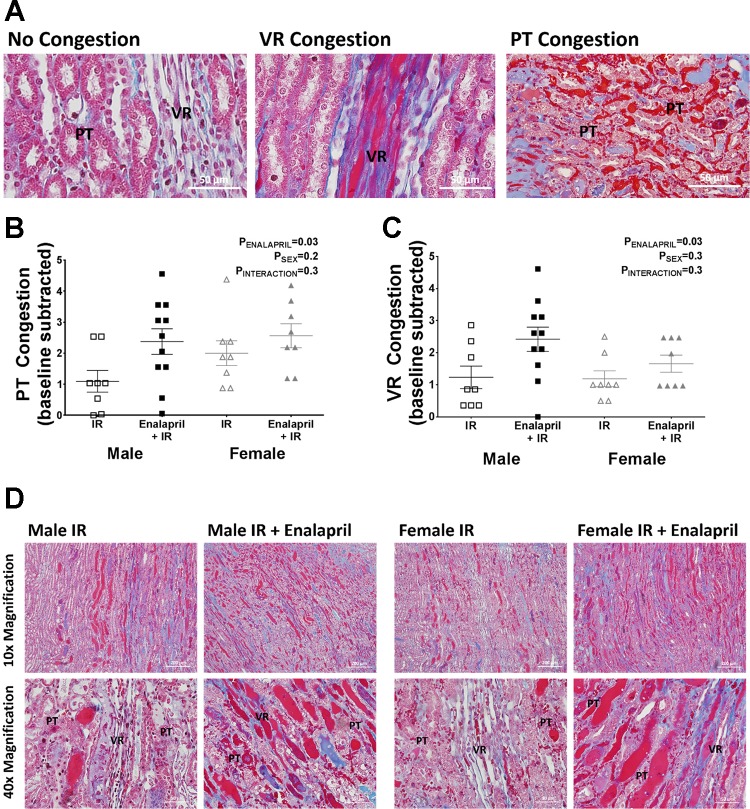
Paraffin-embedded kidney slices were stained with Masson’s trichrome, according to manufacturer’s instructions (Thermo Scientific, Richard-Allan Scientific) to assess the extent of medullary red blood cell (RBC) congestion. Medullary VR and PT congestion were assessed using a semiquantitative scoring method. Group identifiers were removed from each slide, and sections were scored by a researcher blinded to the hypothesis. A score of 0.0–5.0 was given to reflect the extent of RBC congestion in the VR and PT independently of one another, 0.0 representing conditions in which all vessels appear open (0% congestion) and 5.0 representing congestion in all vessels visualized (100% congestion). VR were identified as long vessels that are bundled together, and PT capillaries were identified as the vessels found in between tubular structures. Representative images of VR and PT are depicted in Fig. 4A. Mean vascular congestion scores for rats that did not undergo IR surgery were subtracted from their respective post-IR groups since kidneys were not flushed before harvest, and some residual RBCs were present.
Fig. 4.
Effect of transient enalapril treatment on medullary vascular congestion following IR. Incidence of RBC congestion was assessed in peritubular (PT) and vasa recta (VR) capillaries post-IR independently of one another, where a score of 0 indicates all visible vessels are open and 5 indicates all visible vessels are congested. PT capillaries were determined as vessels found between tubular structures and VR as bundles of long vessels. A: representative images show the distinction between these vessels and an example of congested and uncongested PT and VR. Scatterplots show the respective congestion scores from each individual animal in PT (B) and VR (C). Baseline levels for each respective group of rats were subtracted to eliminate any baseline congestion that occurred in animals with no surgery. D: representative images used for scoring the kidneys from each treatment group that had IR surgery are shown. RBCs and protein casts are stained in red. Values are expressed as means ± SE; n = 8–11 for scatterplots.
Depletion of Medullary Pericytes by Transient Enalapril Treatment
To further test our hypothesis that lower medullary pericyte density is associated with greater IR injury, we altered pericyte density through an experimental intervention by a method adapted from Baumann et al. (4), which has previously been demonstrated to reduce renal pericyte density in SHR. In the study, male SHR were treated with the angiotensin receptor blocker losartan from 4 to 8 wk of age, and at 12 wk of age, renal medullary pericyte density was decreased. We used a similar approach in the current study, in which a subset of rats were treated with either vehicle or the angiotensin-converting enzyme inhibitor enalapril (10 mg·kg−1 day−1 in tap water ad libitum) from 5 until 9 wk of age. Water intake and body weight were measured every 2–3 days, and fresh medicated water was prepared according to these measurements to ensure consistent and proper dosing with enalapril. All rats received tap water from 9 to 13 wk of age. At 13 wk of age, rats were anesthetized with a ketamine-xylazine cocktail before being euthanized for measurement of pericyte density and renal injury, as described above.
Blood Pressure Monitoring
To determine whether enalapril treatment from 5 to 9 wk of age significantly affected long-term blood pressure, systolic pressure was measured by tail cuff at 13 wk of age in vehicle- and enalapril-treated rats. Specifically, rats were placed in a medium-sized rodent restrainer to prevent excessive movement. The restrained rat was placed within a compartment with a controlled ambient temperature of 32°C (IITC Life Science, Woodland Hills, CA) that is housed in a quiet, isolated room. The tails of the rats were passed through a 7/16′′ cuff with a photoelectric sensor. The rats were acclimated to the tail cuff technique by following this procedure for three consecutive days before data collection. Systolic blood pressure of each rat was determined by the average of five independent readings with at least a 1-min break between each inflation of the pressure cuff.
Kidney Volume Measurements
An early response to IR is renal volume expansion. To determine whether a change in total renal volume was driving the change in pericyte density following IR, we determined medullary kidney volume, allowing for the calculation of total pericytes in the kidney. A separate set of rats were used for these experiments. Thirteen-week-old SHR were randomized to either serve as controls with no surgery or receive IR surgery as described above. After IR surgery, the rats were allowed to recover for 24 h. Rats were then anesthetized with ~2% isoflurane, and a midline incision was made to expose the abdominal organs. The left renal pedicle was ligated with 4-0 black braided silk, and the left kidney was extracted. Pericyte density was determined in the left kidney, as described above, using NG2 immunofluorescence. The right kidney was perfusion-fixed in situ to avoid volume changes associated with loss of perfusion pressure, and renal outer-medullary volume was measured by way of stereology. In brief, the abdominal aorta below the branch of the renal artery was cannulated with PE-60 polyethylene tubing. A suture was used to tie the abdominal aorta above the branch of the right renal artery to isolate perfusion fluid to the kidney. A Perfusion One pressure perfusion system (Leica Biosystems; Buffalo Grove, IL) attached to a pressure bulb was used to perfuse the right kidney with chilled heparinized saline (195 mmHg for males; 175 mmHg for females). We chose a perfusion pressure above the average systolic blood pressures measured by tail cuff (18). The abdominal vein was punctured immediately after starting perfusion to allow for venous outflow. Once the flow of fluid exiting the punctured vein was clear of blood (~3–5 min of perfusion), the system was switched to perfuse paraformaldehyde (4% weight/volume dissolved in 0.1 M phosphate buffer; 5–10 min of perfusion) to fix the kidney. The kidney was then removed and placed in a vial for incubation with 10% formalin for 2–3 days. After incubation, the kidney was cut into 1.08-mm slices using a home-made razor device. The slices were counted, and every odd slice was viewed under a microscope (×5 magnification). Outer-medullary kidney volume was estimated using a dot grid printed on translucent paper with dots 0.85 mm apart. The dots identified to be in the outer medulla were counted and used to estimate the outer-medullary volume (VOM) by the Cavalieri Principle (11, 12).
Kidneys with an even number of slices:
Kidneys with an odd number of slices:
where ΣP is the total number of dots counted, pn is the number of dots of the last slice, a(p) is the area associated with each grid dot, and T is section thickness.
Statistical Analysis
All data are presented as means ± SE. Statistical analyses were performed using GraphPad Prism version 7.0 software (GraphPad Software, La Jolla, CA). Multiple comparisons were carried out using one-way or two-way ANOVA where appropriate. Deming model II regression was used to determine the relationship between renal injury markers and pericyte density.
RESULTS
Lower Pericyte Density Is Associated with Increased Peritubular Capillary Congestion
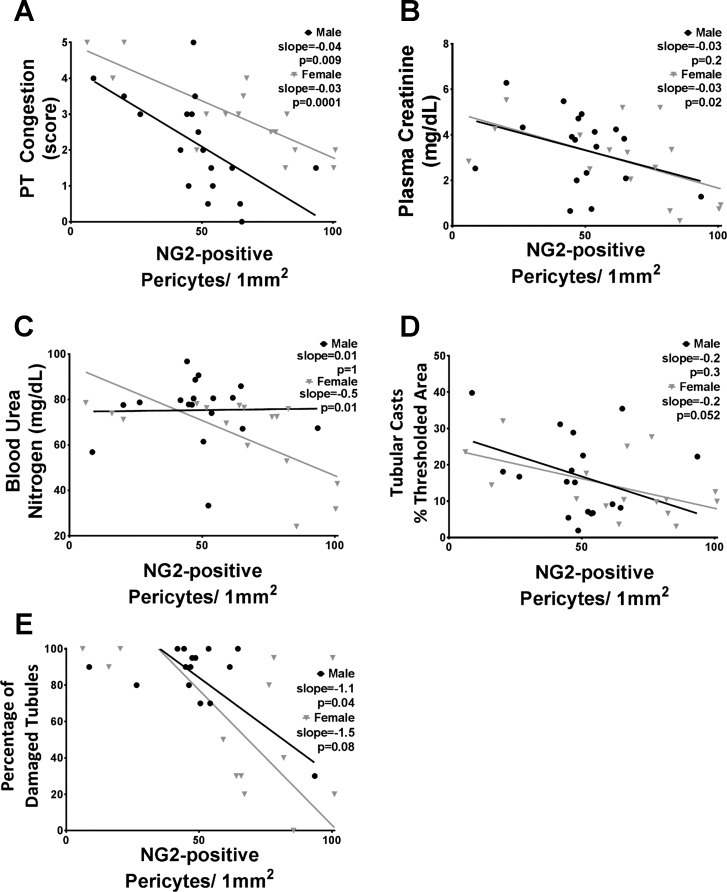
Pericyte density was assessed by positive immunofluorescence using an NG2 antibody along with visual identification of stained cells bordering the VR. A representative image of NG2-positive pericytes on two VR is shown in Fig. 1; NG2 stains the outline of the cell red, and DAPI stains the nucleus blue, confirming the presence of a cell body. We performed a bivariate correlation analysis comparing pericyte density to renal injury in all rats that received IR surgery. Both male and female SHR demonstrated a strong negative correlation between pericyte density and vascular congestion, where rats with lower pericyte density had higher PT congestion (Fig. 2A). Female SHR also exhibited a significant negative correlation between pericyte density and plasma creatinine, but statistical significance was not reached in males (Fig. 2B). Our data also indicate a significant negative correlation between pericyte density and BUN (Fig. 2C) among female, but not male, SHR, and pericyte density tended (P = 0.052) to negatively correlate with tubular cast formation only in females (Fig. 2D). Alternatively, only male SHR demonstrated a significant negative correlation between pericyte density and tubular damage (Fig. 2E). Lastly, the regression line slopes of males vs. females were not significantly different from one another in each correlation graph.
Fig. 2.
Correlation of renal injury markers and pericyte density determined by NG2 immunofluorescence. Data from each individual animal that underwent IR surgery are shown; males are represented by black circles and females by gray triangles. The markers that were compared with pericyte density include peritubular capillary (PT) congestion (A), plasma creatinine (B), blood urea nitrogen (C), percent area with tubular casts (D), and percentage of damaged tubules (E). Regression slopes and P values are shown; n = 13–18.
Transient Treatment with Enalapril Decreases Pericyte Density
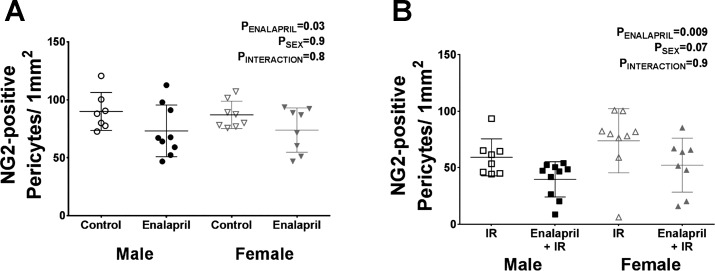
We confirmed a previous report that transient renin-angiotensin system (RAS) blockade in SHR lowers pericyte density (4). In our study, we observed a 19% ± 8% decrease in medullary pericyte density in male SHR and a 15% ± 8% decrease in female SHR following treatment with enalapril when compared with vehicle-treated control rats (Fig. 3A). Transient enalapril treatment also decreased pericyte density in rats receiving IR surgery, although the magnitude of the decrease was greater. Enalapril treatment resulted in a 33% ± 8% decrease in pericyte density in male SHR and a 29% ± 11% decrease in female SHR when compared with vehicle-treated IR rats (Fig. 3B).
Fig. 3.
Effect of transient enalapril treatment on pericyte density determined by NG2 immunofluorescence. Pericyte density was determined in vehicle- and enalapril-treated rats (A) and in treated rats following IR (B) to confirm that enalapril treatment decreases pericyte density. Values are expressed as means ± SE; n = 7–10.
Decreasing Pericyte Density Exacerbates Medullary Congestion 24 Hours After Ischemia Reperfusion
Representative histological images illustrate examples of uncongested and congested PT and VR (Fig. 4A). Reducing pericyte density by transient enalapril treatment markedly increased PT and VR congestion 24 h after IR in both sexes when compared with vehicle-treated SHR (Fig. 4, B and C). Representative histological images show the degree of vascular congestion 24 h post-IR (Fig. 4D). Only congested RBCs were included in the scoring of these images; tubular casts were excluded from scoring. Enalapril-treated male and female SHR had more vascular staining of RBCs when compared with their vehicle-treated groups after IR; RBC medullary vascular congestion increased from ~25% to 50% in males, and females had 15% more medullary vessels congested after enalapril treatment compared with vehicle-treated controls.
Outside of Vascular Congestion, Transient Enalapril Treatment Does Not Affect Renal Injury Parameters 24 Hours After Ischemia-Reperfusion
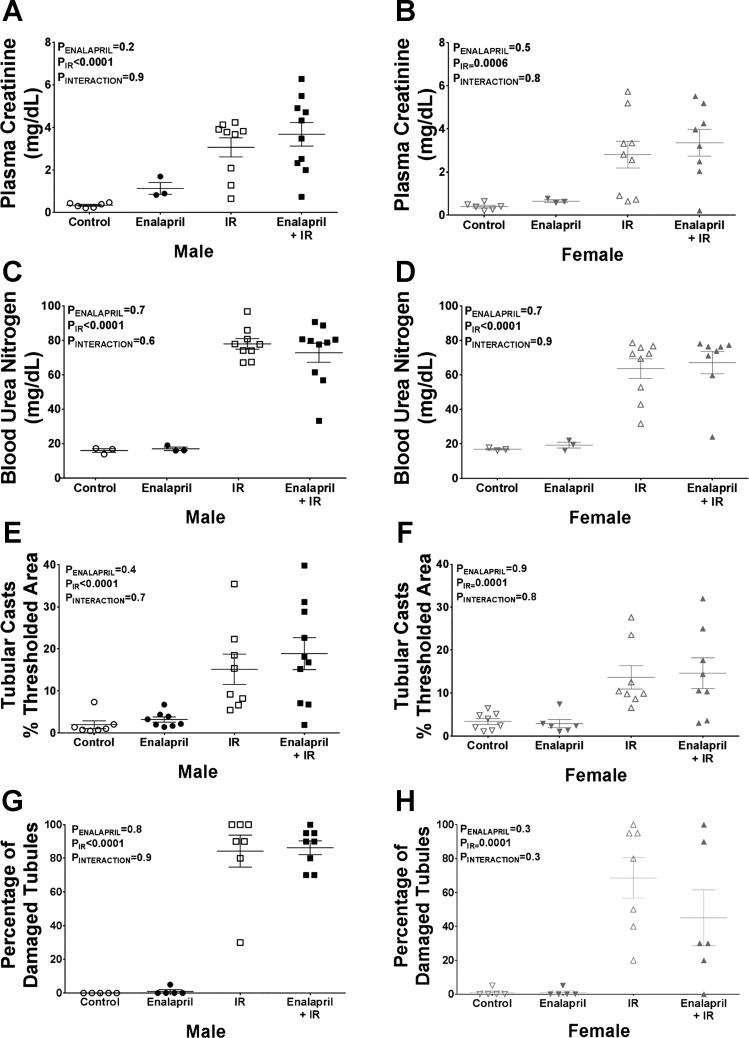
Plasma creatinine and BUN were measured as markers of kidney function, while medullary tubular injury was assessed by histological analysis. In male SHR, IR resulted in significant increases in plasma creatinine (nine-fold increase; Fig. 5A), BUN (five-fold, Fig. 5C), tubular cast formation (13% increase; Fig. 5E), and damaged tubules (85% increase; Fig. 5G). Transient enalapril treatment in males had no effect on renal injury. Similarly, IR increased plasma creatinine (seven-fold; Fig. 5B), BUN (four-fold; Fig. 5D), protein cast formation (11% increase; Fig. 5F), and tubular damage in female SHR (57% increase; Fig. 5H), and none of these measurements was altered by transient enalapril treatment.
Fig. 5.
Effect of transient enalapril treatment on renal injury in adulthood. Renal injury markers were measured to determine whether enalapril treatment had an effect on IR-induced injury in either sex. Plasma creatinine (male: A; female: B), blood urea nitrogen (BUN; male: C; female: D), tubular cast area (male: E; female: F), and the percentage of tubules damaged (male: G; female: H) are shown. Values are expressed as means ± SE; n = 3 for rats without IR due to limited number of samples for plasma creatinine and BUN assays; n = 5–10 for the rest of the groups.
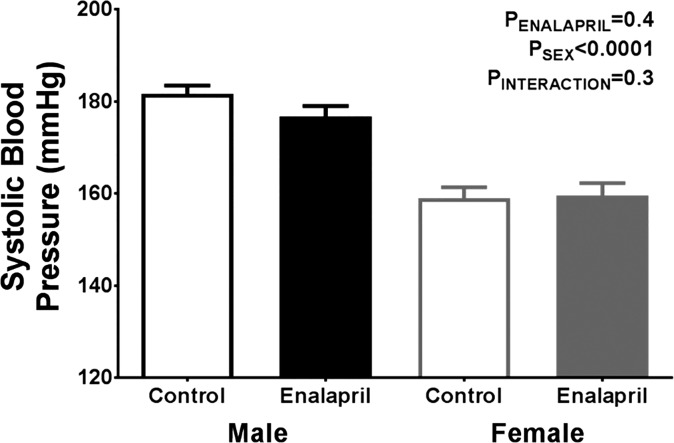
Transient Treatment with Enalapril Does Not Alter Blood Pressure at 13 Weeks of Age
As previous reports have indicated that transient inhibition of the RAS during maturation results in chronically lower blood pressures in adult SHR (1, 3, 25, 29), blood pressure was measured in vehicle- and enalapril-treated male and female SHR at 13 wk of age (4 wk following cessation of treatment). Males retained a 20-mmHg higher systolic pressure compared with females; however, we did not observe a significant effect of transient enalapril treatment on blood pressure in SHR of either sex (Fig. 6).
Fig. 6.
Effect of transient enalapril on blood pressure in adulthood. Tail cuff method was used to measure systolic blood pressure in vehicle and enalapril-treated rats at 13 wk of age. Values are expressed as means ± SE; n = 8–13.
Male Rats Lose More Pericytes After Ischemia-Reperfusion than Females
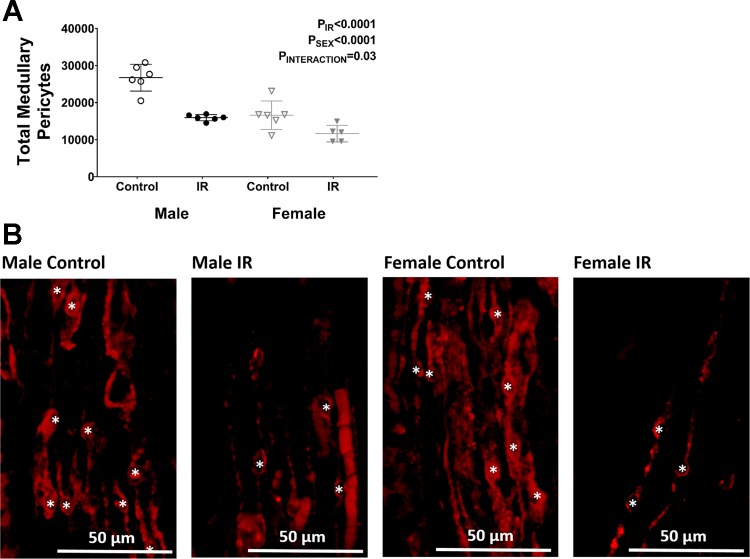
To determine whether changes in outer-medullary volume were responsible for decreases in the density of outer-medullary pericytes observed following IR, total pericyte numbers were calculated by multiplying volume × density in these kidneys. The total number of pericytes in the outer medulla decreased following IR in both sexes; however, males lost more pericytes than females (40% decrease in males and 30% decrease in females; Fig. 7A). Representative images from each group depicting this decrease in density is shown in Fig. 7B.
Fig. 7.
Quantification of pericyte number. A: total pericyte number was determined in untreated rats before and after IR by multiplying the volume of the outer medulla and pericyte density determined by neural glial 2 (NG2) immunofluorescence. B: representative images show the decrease in pericyte density following IR in both sexes. Asterisks indicate NG2-positive pericyte cell bodies used for quantification. Values are expressed as means ± SE; n = 5 or 6.
DISCUSSION
The major finding of this study is that lower outer-medullary pericyte density is associated with increased IR-induced injury in rats. The injury marker that was most negatively correlated with pericyte density was RBC congestion in PT. Our data are consistent with a recent study demonstrating improved recovery following renal ischemia in human cadaveric allografts with higher pericyte densities (19). Our study builds on these previous data, indicating that the beneficial effects of greater pericyte density are likely mediated by reduced vascular congestion in the outer medulla. Further, by demonstrating a protective effect of increased pericyte density in a rat model, our work provides an experimental platform that can be used to study the physiological mechanisms through which greater pericyte density provides protection following periods of ischemia.
Vascular congestion in the outer-medullary region is a hallmark of acute tubular necrosis in kidneys from cadaveric donors (30), and several studies from the 1980s demonstrate that medullary vascular congestion impairs recovery following IR in rat models (13, 14, 16, 17, 21, 36). Although the pathophysiological mechanisms that contribute to RBC congestion in the renal medulla remain incompletely understood (22), our data provide evidence supporting a novel role for pericytes to limit RBC congestion in the renal medulla after IR. Additional support for this hypothesis was provided by experimental intervention to lower pericyte density. Our data indicate even a modest reduction in pericyte density promoted significantly greater vascular congestion in the renal outer medulla of both male and female SHR following IR.
A second interesting finding of our study is that IR itself results in a loss of vascular pericytes in the renal outer medulla. We observed that kidneys are visibly larger following IR compared with controls, where male SHR have a 25% increase in kidney weight following IR and females have a 35% increase (data not shown), which we speculate may be partly indicative of RBC congestion. We doubt that volume expansion of renal tissue after IR was solely responsible for the reduced pericyte density; however, this may contribute to the variability that is observed between rats within the same treatment groups. We calculated total pericyte number by multiplying stereological measurements of outer-medullary volume with pericyte density following IR and found that IR resulted in a loss of outer-medullary pericytes in both sexes, although the decrease was greater in males (40% in males compared with 30% in females). We postulate that this reduction of pericytes may contribute to long-term injury following renal ischemia.
Several studies have reported a sex difference in IR-induced renal injury, where males have greater injury vs. females; however, the majority of the published data has been generated using normotensive animals (10, 27, 33, 34). Very few studies on IR-induced injury have been performed using SHR, and such studies, to date, have exclusively used males (15, 28). Moreover, male SHR have been reported to be more susceptible to IR-induced injury compared with male Dahl salt-sensitive, Wistar-Furth, and Sprague-Dawley rats (28). Given the importance of hypertension as a risk factor for AKI (8), more research needs to be performed on IR-induced injury in animals with elevated blood pressure, particularly females. In contrast to findings in normotensive animals, our study indicates that there is not a sex difference in IR-induced injury in SHR. We speculate that elevations in blood pressure compromise the protective mechanisms found in normotensive female animal models following IR, abolishing the sex difference. Future studies will be conducted to directly address this question.
The current study was not designed to investigate the physiological mechanisms through which pericytes may mediate enhanced recovery following IR, but rather, the primary goal of our study was to determine whether the beneficial effects of pericyte density observed in human allografts is evident in a rat model. Our results indicating that pericyte density is negatively associated with PT congestion following IR raises the question of how pericytes prevent RBC congestion. The PT capillaries in the outer medulla arise from the descending VR, which are densely covered in pericyte cell bodies. We speculate that following IR events, the cessation of blood flow results in stagnant RBCs in medullary vessels. Upon rapid restoration of blood flow with reperfusion, additional RBCs carried by plasma flow are introduced into these vessels, which may lead to congestion. Contraction of pericytes may help disrupt these aggregations and restore flow to these vessels following an ischemic event by physically disrupting this accumulation of RBCs. Perhaps surprisingly, given this hypothesis, we found that VR congestion following IR did not associate with pericyte density using correlation analysis; however, VR congestion did increase in enalapril-treated rats compared to untreated controls following IR. One potential explanation stems from the fact that VR are larger vessels than PT; thus, washout of congested RBCs may occur more easily in VR vessels independent of pericyte contraction, making them more likely to clear of congested blood. An alternative hypothesis is that renal pericytes promote reflow by stabilizing the vessels during ischemia. Pericytes have been shown to be important in maintaining endothelial integrity (20), and therefore, lower pericyte density may result in destabilization of endothelial cells and dysfunction of vessels post-IR, leading to congestion. Further studies will be required to better delineate the mechanisms through which greater pericyte density attenuates RBC congestion following renal ischemia.
To more directly test our hypothesis that lower pericyte density results in increased vascular congestion following IR, we used an experimental approach to decrease pericyte density in SHR before performing IR. Because of limited methods available to specifically target pericytes in vivo, we adapted a pharmacological method previously reported to reduce pericyte density in male SHR (4). In this previous study, the angiotensin receptor blocker losartan was used to decrease pericyte density. This study further performed an intervention to show that transient treatment with losartan induces a proapoptotic stimulus on medullary pericytes (4). We did not determine the cause of pericyte depletion in our current study. Nevertheless, transient enalapril treatment successfully decreased medullary pericytes in male and female SHR.
Although NG2 is not specific to pericytes (2), we show that NG2 staining of the renal medulla outlines the VR vessels, which are distinguishable by their unique anatomical features, allowing for easy detection of medullary pericytes based on their localization on the VR. The expression of proteins on pericytes, including NG2, depends on tissue localization, the pathological state, and the maturity of the animal (26). The expression of NG2 is reduced by cellular loss or shedding of the NG2 protein in response to hypoxia (37) and proinflammatory cytokines (35) in neuronal cells. While NG2 is a common marker used to detect renal pericytes in healthy adult murine animal models (2), less is known regarding the regulation of renal pericytes. NG2-positive pericytes decrease in aged mice, contributing to increased fibrosis in the kidney (32), and our studies further confirm that renal NG2-positive pericytes are lost in response to IR.
Contrary to our results, previous reports demonstrated that transient treatment with a RAS inhibitor early in life promotes prolonged reductions in arterial pressure in adult SHR when assessed by tail cuff (1, 25) and intra-arterial catheterization (3, 29). Moreover, the blood pressure phenotype in adult SHR has previously been shown to be dependent on the dose of inhibitor used to transiently block the RAS (29). Of the referenced studies, the only one that transiently treated rats with enalapril used a higher dose (25 mg·kg−1·day−1) (1) than what we used in our study (10 mg·kg−1·day−1). Regardless, we were able to confirm that transient blockade of the RAS with enalapril treatment in SHR decreased renal pericyte density independent of an effect on blood pressure, removing blood pressure as a potential confounding variable in the current study.
Our finding that decreasing pericyte density by transient enalapril treatment promoted significantly greater RBC congestion in both VR and PT but did not worsen other markers of renal function (plasma creatinine/BUN) and tubular injury may appear at odds with clinical data, in which lower pericyte density was associated with slower recovery of graft function (19). It should be noted, however, that in our study, we examined kidney function and injury at 24 h post-IR. Data from another report indicate that medullary congestion does not lead to greater renal injury and reduced renal function in rats within the first 2 h of reperfusion (14). Instead, persistent medullary congestion promotes tubular injury and reduces kidney function at later time points (4 wk post-IR) (13), which is speculated to be the result of reduced reperfusion of the renal medulla and prolonged ischemia to this region. As a result, reducing the prevalence of medullary congestion early on, will improve long-term recovery following IR (21). Therefore, we would not necessarily expect increased medullary congestion to alter renal function at 24 h post-IR; however, greater medullary congestion could still account for delayed graft function in human cadaveric allografts. Indeed, our data indicating no difference in functional measurements at 24 h may be somewhat advantageous, as it confirms that increased medullary congestion was independent of an initial renal functional decline immediately following IR and not simply a function of transient enalapril treatment worsening IR in rats.
In conclusion, our studies provide new evidence that pericyte density is negatively associated with IR-induced injury in male and female SHR, with the strongest relationship being an increase in PT congestion when pericyte density is reduced. Additionally, pericyte number decreases in response to IR, particularly in males. Given the importance of RBC congestion as a precursor to IR-induced injury, these findings provide a basis for better understanding the role of pericytes in IR injury.
GRANTS
This work was supported by the American Heart Association (Predoctoral Fellowship 15PRE25850008 to G. R. Crislip) and National Institutes of Health (1P01HL-134604-01 and R01HL-127091 to J. C. Sullivan; 1P01HL-134604-01 to P. M. O’Connor).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.R.C., P.M.O., and J.C.S. conceived and designed research; G.R.C. and Q.W. performed experiments; G.R.C. analyzed data; G.R.C., P.M.O., and J.C.S. interpreted results of experiments; G.R.C. prepared figures; G.R.C. drafted manuscript; G.R.C., P.M.O., and J.C.S. edited and revised manuscript; G.R.C., P.M.O., Q.W., and J.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the excellent technical assistance of Sarah Ray and Erica Ralph.
REFERENCES
- 1.Adams MA, Bobik A, Korner PI. Enalapril can prevent vascular amplifier development in spontaneously hypertensive rats. Hypertension 16: 252–260, 1990. [Erratum in Hypertension 17: 262, 1991.] doi: 10.1161/01.HYP.16.3.252. [DOI] [PubMed] [Google Scholar]
- 2.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Baumann M, Janssen BJ, Hermans JJ, Peutz-Kootstra C, Witzke O, Smits JF, Struijker Boudier HA. Transient AT1 receptor-inhibition in prehypertensive spontaneously hypertensive rats results in maintained cardiac protection until advanced age. J Hypertens 25: 207–215, 2007. doi: 10.1097/HJH.0b013e3280102bff. [DOI] [PubMed] [Google Scholar]
- 4.Baumann M, Janssen BJ, Rob Hermans JJ, Bartholome R, Smits JF, Struijker Boudier HA. Renal medullary effects of transient prehypertensive treatment in young spontaneously hypertensive rats. Acta Physiol (Oxf) 196: 231–237, 2009. doi: 10.1111/j.1748-1716.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 5.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol 7: 452–464, 2005. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boesen EI, Crislip GR, Sullivan JC. Use of ultrasound to assess renal reperfusion and P-selectin expression following unilateral renal ischemia. Am J Physiol Renal Physiol 303: F1333–F1340, 2012. doi: 10.1152/ajprenal.00406.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford C, Kennedy-Lydon T, Sprott C, Desai T, Sawbridge L, Munday J, Unwin RJ, Wildman SS, Peppiatt-Wildman CM. An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron, Physiol 120: 17–31, 2012. doi: 10.1159/000339110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fekete A, Vannay A, Vér A, Rusai K, Müller V, Reusz G, Tulassay T, Szabó AJ. Sex differences in heat shock protein 72 expression and localization in rats following renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 291: F806–F811, 2006. doi: 10.1152/ajprenal.00080.2006. [DOI] [PubMed] [Google Scholar]
- 11.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc 147: 229–263, 1987. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 12.Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology—reconsidered. J Microsc 193: 199–211, 1999. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 13.Hellberg PO, Bayati A, Källskog O, Wolgast M. Red cell trapping after ischemia and long-term kidney damage. Influence of hematocrit. Kidney Int 37: 1240–1247, 1990. doi: 10.1038/ki.1990.107. [DOI] [PubMed] [Google Scholar]
- 14.Hellberg PO, Källskog O, Wolgast M. Nephron function in the early phase of ischemic renal failure. Significance of erythrocyte trapping. Kidney Int 38: 432–439, 1990. doi: 10.1038/ki.1990.223. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov M, Mihailović-Stanojević N, Grujić Milanović J, Jovović Đ, Marković-Lipkovski J, Ćirović S, Miloradović Z. Losartan improved antioxidant defense, renal function and structure of postischemic hypertensive kidney. PLoS One 9: e96353, 2014. doi: 10.1371/journal.pone.0096353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsson J, Odlind B, Tufveson G, Wahlberg J. Effects of cold ischemia and reperfusion on trapping of erythrocytes in the rat kidney. Transpl Int 1: 75–79, 1988. doi: 10.1111/j.1432-2277.1988.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 17.Karlberg L, Källsko Ö, Nygren K, Wolgast M. Erythrocyte and albumin distribution in the kidney following warm ischemia. A study in rats. Scand J Urol Nephrol 16: 173–177, 1982. doi: 10.3109/00365598209179749. [DOI] [PubMed] [Google Scholar]
- 18.Kett MM, Alcorn D, Bertram JF, Anderson WP. Enalapril does not prevent renal arterial hypertrophy in spontaneously hypertensive rats. Hypertension 25: 335–342, 1995. doi: 10.1161/01.HYP.25.3.335. [DOI] [PubMed] [Google Scholar]
- 19.Kwon O, Hong SM, Sutton TA, Temm CJ. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol 295: F351–F359, 2008. doi: 10.1152/ajprenal.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemos DR, Marsh G, Huang A, Campanholle G, Aburatani T, Dang L, Gomez I, Fisher K, Ligresti G, Peti-Peterdi J, Duffield JS. Maintenance of vascular integrity by pericytes is essential for normal kidney function. Am J Physiol Renal Physiol 311: F1230–F1242, 2016. doi: 10.1152/ajprenal.00030.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason J, Welsch J, Torhorst J. The contribution of vascular obstruction to the functional defect that follows renal ischemia. Kidney Int 31: 65–71, 1987. doi: 10.1038/ki.1987.10. [DOI] [PubMed] [Google Scholar]
- 22.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 281: F948–F957, 2001. doi: 10.1152/ajprenal.0071.2001. [DOI] [PubMed] [Google Scholar]
- 23.Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol 9: 165–170, 2001. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- 24.Park F, Mattson DL, Roberts LA, Cowley AW Jr. Evidence for the presence of smooth muscle α-actin within pericytes of the renal medulla. Am J Physiol Regul Integr Comp Physiol 273: R1742–R1748, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Peng F, Lin J, Lin L, Tang H. Transient prehypertensive treatment in spontaneously hypertensive rats: a comparison of losartan and amlodipine regarding long-term blood pressure, cardiac and renal protection. Int J Mol Med 30: 1376–1386, 2012. doi: 10.3892/ijmm.2012.1153. [DOI] [PubMed] [Google Scholar]
- 26.Pitera JE, Woolf AS, Gale NW, Yancopoulos GD, Yuan HT. Dysmorphogenesis of kidney cortical peritubular capillaries in angiopoietin-2-deficient mice. Am J Pathol 165: 1895–1906, 2004. doi: 10.1016/S0002-9440(10)63242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prókai A, Fekete A, Bánki NF, Müller V, Vér A, Degrell P, Rusai K, Wagner L, Vannay A, Rosta M, Heemann U, Langer RM, Tulassay T, Reusz G, Szabó AJ. Renoprotective effect of erythropoietin in rats subjected to ischemia/reperfusion injury: gender differences. Surgery 150: 39–47, 2011. doi: 10.1016/j.surg.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Raman RN, Pivetti CD, Ramsamooj R, Matthews DL, Demos SG, Troppmann C. Factors influencing rat survival in a warm renal ischemia model: time to adapt the protocols. Transplant Proc 43: 1511–1514, 2011. doi: 10.1016/j.transproceed.2011.01.177. [DOI] [PubMed] [Google Scholar]
- 29.Rusai K, Jianxing C, Schneider R, Struijker-Boudier H, Lutz J, Heemann U, Baumann M. Renin inhibition mitigates anti-angiogenesis in spontaneously hypertensive rats. J Hypertens 29: 266–272, 2011. doi: 10.1097/HJH.0b013e328340aa72. [DOI] [PubMed] [Google Scholar]
- 30.Snoeijs MG, Vink H, Voesten N, Christiaans MH, Daemen JW, Peppelenbosch AG, Tordoir JH, Peutz-Kootstra CJ, Buurman WA, Schurink GW, van Heurn LW. Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol Renal Physiol 299: F1134–F1140, 2010. doi: 10.1152/ajprenal.00158.2010. [DOI] [PubMed] [Google Scholar]
- 31.Snoeijs MG, Winkens B, Heemskerk MB, Hoitsma AJ, Christiaans MH, Buurman WA, van Heurn LW. Kidney transplantation from donors after cardiac death: a 25-year experience. Transplantation 90: 1106–1112, 2010. doi: 10.1097/TP.0b013e3181f83b0b. [DOI] [PubMed] [Google Scholar]
- 32.Stefanska A, Eng D, Kaverina N, Duffield JS, Pippin JW, Rabinovitch P, Shankland SJ. Interstitial pericytes decrease in aged mouse kidneys. Aging (Albany NY) 7: 370–382, 2015. doi: 10.18632/aging.100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takayama J, Takaoka M, Sugino Y, Yamamoto Y, Ohkita M, Matsumura Y. Sex difference in ischemic acute renal failure in rats: approach by proteomic analysis. Biol Pharm Bull 30: 1905–1912, 2007. doi: 10.1248/bpb.30.1905. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka R, Tsutsui H, Ohkita M, Takaoka M, Yukimura T, Matsumura Y. Sex differences in ischemia/reperfusion-induced acute kidney injury are dependent on the renal sympathetic nervous system. Eur J Pharmacol 714: 397–404, 2013. doi: 10.1016/j.ejphar.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Wennström M, Janelidze S, Bay-Richter C, Minthon L, Brundin L. Pro-inflammatory cytokines reduce the proliferation of NG2 cells and increase shedding of NG2 in vivo and in vitro. PLoS One 9: e109387, 2014. doi: 10.1371/journal.pone.0109387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolgast M, Karlberg L, Källskog O, Norlén BJ, Nygren K, Ojteg G. Hemodynamic alterations in ischaemic acute renal failure. Nephron 31: 301–303, 1982. doi: 10.1159/000182671. [DOI] [PubMed] [Google Scholar]
- 37.Yang QK, Xiong JX, Yao ZX. Neuron-NG2 cell synapses: novel functions for regulating NG2 cell proliferation and differentiation. BioMed Res Int 2013: 402843, 2013. doi: 10.1155/2013/402843. [DOI] [PMC free article] [PubMed] [Google Scholar]