Abstract
This study in α-chloralose-anesthetized cats discovered an excitatory peroneal nerve-to-bladder reflex. A urethral catheter was used to infuse the bladder with saline and record bladder pressure changes. Electrical stimulation was applied to the superficial peroneal nerve to trigger reflex bladder activity. With the bladder distended at a volume ~90% of bladder capacity, superficial peroneal nerve stimulation (PNS) at 1–3 Hz and threshold (T) intensity for inducing muscle twitching on the posterior thigh induced large-amplitude (40–150 cmH2O) bladder contractions. PNS (1–3 Hz, 1–2T) applied during cystometrograms (CMGs) when the bladder was slowly (1–3 ml/min) infused with saline significantly (P < 0.01) reduced bladder capacity to ~80% of the control capacity and significantly (P < 0.05) enhanced reflex bladder contractions. To determine the impact of PNS on tibial nerve stimulation (TNS)-induced changes in bladder function, PNS was delivered following TNS. TNS of 30-min duration produced long-lasting poststimulation inhibition and significantly (P < 0.01) increased bladder capacity to 140.5 ± 7.6% of the control capacity. During the post-TNS inhibition period, PNS (1–3 Hz, 1–4T) applied during CMGs completely restored bladder capacity to the control level and significantly (P < 0.05) increased the duration of reflex bladder contractions to ~200% of control. The excitatory peroneal nerve-to-bladder reflex could also be activated by transcutaneous PNS using skin surface electrodes attached to the dorsal surface of the foot. These results raise the possibility of developing novel neuromodulation therapies to treat underactive bladder and nonobstructive urinary retention.
Keywords: neuromodulation, underactivity, cat
underactive bladder (UAB) is a symptom complex suggestive of detrusor underactivity and characterized by prolonged urination time with or without a sensation of incomplete bladder empting, usually with hesitancy, reduced sensation on filling, and a slow stream (3, 21). The prevalence of UAB is ~25–40% in the >60-yr-old population (9, 24) and as many as 48% of older men and 45% of older women show detrusor underactivity during urological evaluation (1, 13). The impact of severe UAB on quality of life is significant, requiring intermittent self-catheterization or an indwelling suprapubic catheter to drain the bladder when urinary retention occurs (3, 15). Currently it is a therapeutic challenge for clinicians to successfully treat UAB, leaving the majority of UAB patients untreated; however, relative to overactive bladder syndrome (OAB), UAB has been the focus of relatively few basic science studies.
Tibial neuromodulation is an Food and Drug Administration-approved therapy for OAB (2). Our previous studies in cats of bladder responses to tibial nerve stimulation (TNS) revealed that brief periods of stimulation induce a transient inhibition of reflex bladder activity, whereas 30 min of TNS induces a more prolonged inhibition lasting at least two hours (23). Transcutaneous stimulation of distal branches of the tibial nerve on the plantar surface of the foot elicits a similar prolonged inhibition in cats (4) and has been shown to increase bladder capacity in healthy human subjects (5) and prevent bedwetting in children (10). In the present experiments, we discovered that electrical stimulation of the superficial peroneal nerve, which innervates the dorsal surface of the foot, elicits excitatory effects on the bladder and reverses the prolonged TNS inhibition. These observations raise the possibility that the excitatory peroneal-to-bladder reflex might be used in clinical applications to enhance bladder contractions and treat UAB or nonobstructive urinary retention (NOUR).
MATERIALS AND METHODS
The experimental protocol and animal use in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Surgical procedures.
A total of 10 cats (7 male and 3 female, 2.9–4.2 kg; Liberty Research, Waverly, NY) were used in this study. The animals were anesthetized with isoflurane (2–5% in oxygen) during surgery and then switched to α-chloralose anesthesia (initial 65 mg/kg iv and supplemented as needed) during data collection. The left cephalic vein was catheterized for administration of anesthetics and fluid. A tracheotomy was performed, and a tube was inserted to keep the airway patent. A catheter was inserted in the right carotid artery to monitor systemic blood pressure. Heart rate and blood oxygen were monitored by a pulse oximeter (9847V; NONIN Medical, Plymouth, MN) attached to the tongue. Through an abdominal incision, the ureters were isolated, tied, and cut for external drainage. A double-lumen catheter was inserted in the bladder via a small cut in the proximal urethra and secured by a ligature around the urethra. One lumen was connected to a pump to slowly (1–3 ml/min) infuse saline to induce bladder distention. The other lumen was attached to a pressure transducer to measure bladder pressure. The superficial peroneal nerve and tibial nerve on the right side were dissected via skin incisions at the ankle for implantation of tripolar cuff electrodes (NC223pt; MicroProbe, Gaithersburg, MD). The cuff electrodes were then connected to a dual-channel electrical stimulator (S88; Grass Medical Instruments, Quincy, MA) via constant-voltage stimulus isolators (SIU5; Grass Medical Instruments). The inner diameter of the cuff electrode is 2 mm with a 2-mm distance between each of three platinum fine wire electrodes. The two outside electrodes were connected to serve as the anode while the middle electrode was the cathode. After the surgery, the skin and muscle layers were closed by sutures. In three cats, the dorsal surface of the left foot was shaved to fully remove the fur using depilatory cream so that adhesive skin surface electrodes (1 cm diameter) could be attached to the foot for transcutaneous stimulation of the branches of the superficial peroneal nerve.
Stimulation protocol.
At the beginning of each experiment, uniphasic rectangular pulses (1-Hz frequency) were used to determine the intensity threshold (T) for superficial peroneal nerve stimulation (PNS) to induce observable twitches of the muscles in the posterior thigh. At the threshold intensity (1T) determined by 1-Hz PNS, the muscle twitches disappeared when PNS frequency was increased above 2 Hz. The decline in cutaneous afferent to flexor muscle spinobulbospinal reflexes with increasing frequency is well established (8). Based on our previous studies (22, 23), the intensity threshold for TNS to induce an observable toe twitch was determined by uniphasic rectangular pulses at 5-Hz frequency. A pulse width of 0.2 ms was used for both PNS and TNS.
Initially, multiple cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity. Bladder capacity was defined as the volume threshold to induce a reflex bladder contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Next, with the bladder distended at a volume ~90% of the bladder capacity (n = 9 cats), PNS of 30-s duration and 1–2T intensity was applied repeatedly at different frequencies (0.5–50 Hz) to determine the optimal PNS frequency for inducing a large bladder contraction. At the optimal frequency (1–3 Hz), PNS of 30-s duration was applied again at different intensities (0.25–2T) to determine the intensity-response relationship (n = 8 cats). After the frequency and intensity tests, multiple CMGs were performed without PNS to determine the control bladder capacity, which was followed by an additional two CMGs performed during 1T or 2T PNS (n = 8 cats). Finally, another control CMG was performed without PNS to determine any poststimulation effect.
To determine if PNS can remove the long-lasting poststimulation inhibition of bladder reflex activity induced by TNS, multiple control CMGs were performed again (n = 5 cats). At the end of the last control CMG, TNS of 3–4T intensity was applied for 30 min to induce a poststimulation inhibition that persists for at least 2 h as shown in our previous studies (10, 21). After the 30-min TNS, the following three CMGs were performed: 1) control CMG without stimulation, 2) CMG during PNS, and 3) control CMG again to determine any post-PNS effect.
At the end of the experiment with the bladder distended at a volume ~90% of the bladder capacity (n = 3 cats), electrical stimulation of 30-s duration and 2–4T intensity was repeatedly applied to the dorsal surface of the foot at different frequencies (0.5–50 Hz) to determine if a bladder contraction can be induced by transcutaneous stimulation of the superficial peroneal nerve. The threshold intensity for foot stimulation was determined as the minimal intensity to induce either toe twitches or muscle twitches on the posterior thigh.
During the experiment, the bladder was emptied after each CMG followed by a 2- to 3-min resting period to allow the bladder to recover. During the frequency and intensity tests, the 30-s PNS was applied at >60-s intervals, and different frequencies/intensities were applied in a random order to minimize the potential interaction between the repeated stimulation.
Data analysis.
Repeated measurements (2–3 CMGs) of control bladder capacity in the same animal were averaged. Next, the bladder capacity was measured during every CMG and normalized to the averaged control capacity in each cat. The amplitude, duration, and area under the curve of bladder contractions were also measured in each CMG and normalized to the averaged values obtained during control CMGs in each cat. For the frequency and intensity tests, the contraction amplitude and area under the contraction curve were measured and normalized to the maximal response in each cat. The data from different animals are presented as means ± SE. Statistical significance (P < 0.05) was determined by repeated-measures one-way ANOVA followed by Dunnett’s multiple comparison.
RESULTS
Peroneal-to-bladder reflex induced by PNS at different frequencies and intensities.
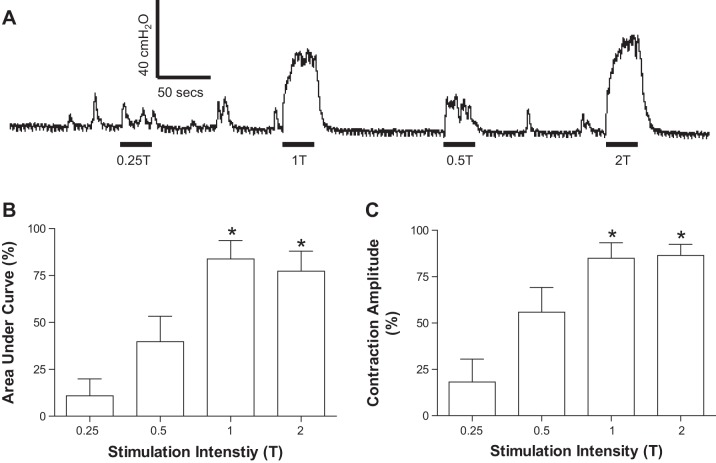
When the bladder was distended by saline to a volume ~90% of the bladder capacity, PNS applied for 30 s at frequencies between 1 and 3 Hz and at the threshold intensity (1T = 5.9 ± 2.1 V) for eliciting a muscle twitch on the posterior thigh produced large-amplitude (40–150 cmH2O) bladder contractions (Figs. 1A and 2A). As the PNS frequency was increased above 3 Hz or decreased below 1 Hz, the induced bladder contractions were gradually reduced in amplitude and area under the curve (Fig. 1, B and C). At an effective frequency, PNS at 1T intensity induced a maximal bladder contraction that was not further enhanced by PNS at 2T intensity (Fig. 2).
Fig. 1.
Bladder pressure responses to different frequencies (0.5–50 Hz) of superficial peroneal nerve stimulation (PNS). A: bladder pressure tracings. The black bar under the pressure tracing indicates the duration (30 s) of PNS (0.2 ms, 1T = 12 V). T, threshold intensity to induce muscle twitch on the posterior thigh. B: normalized area under the curve. C: normalized contraction amplitude. Bladder pressure response was normalized to the maximal response in each animal (n = 9 cats). *Significantly (P < 0.01) different from the response at 50 Hz (1-way ANOVA). PNS (0.2 ms, 1–2T, T = 0.9–20 V).
Fig. 2.
Bladder pressure responses to different intensities (0.25–2T) of PNS. A: bladder pressure tracings. The black bar under the pressure tracing indicates the duration (30 s) of PNS (1 Hz, 0.2 ms, T = 2.4 V). B: normalized area the under curve. C: normalized contraction amplitude. Bladder pressure response was normalized to the maximal response in each animal (n = 8 cats). *Significantly (P < 0.01) different from the response at 0.25T (1-way ANOVA). PNS (1–3 Hz, 0.2 ms, T = 0.9–20 V).
PNS reduced bladder capacity and enhanced reflex bladder contractions.
During repeated CMGs, continuous PNS at 1–3 Hz beginning at the start of bladder filling significantly (P < 0.01) reduced the bladder capacity to ~80% of the control capacity (Fig. 3) and significantly (P < 0.05) increased the area under the curve of the reflex bladder contractions (Fig. 4A). The responses evoked by 1T or 2T PNS were not significantly different. After terminating the PNS and after a 2- to 3-min recovery period, the next CMG revealed that bladder capacity (Fig. 3) and contraction area under the curve (Fig. 4A) returned to the original control, indicating that PNS did not induce a poststimulation effect.
Fig. 3.
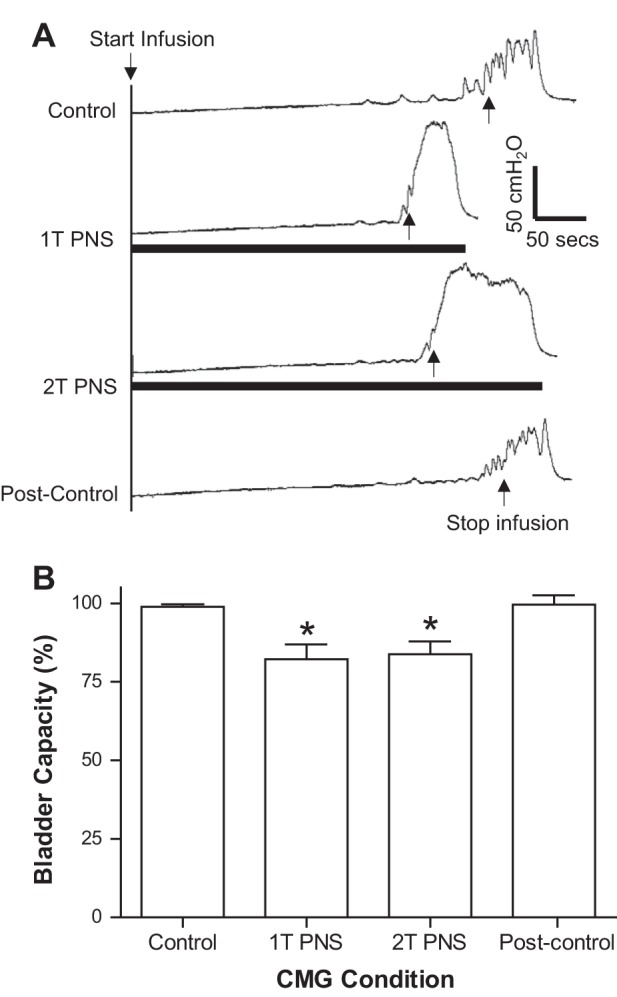
Effect of PNS on bladder capacity. A: repeated cystometrogram (CMG) tracings with/without PNS. The black bar under the bladder pressure tracing indicates the duration of PNS (1 Hz, 0.2 ms, T = 8 V). Infusion rate = 2 ml/min B: summarized results (n = 8 cats). Bladder capacity was normalized to the control capacity. *Significantly (P < 0.01) different from the control capacity (1-way ANOVA). PNS (1–3 Hz, 0.2 ms, T = 1.4–20 V).
Fig. 4.
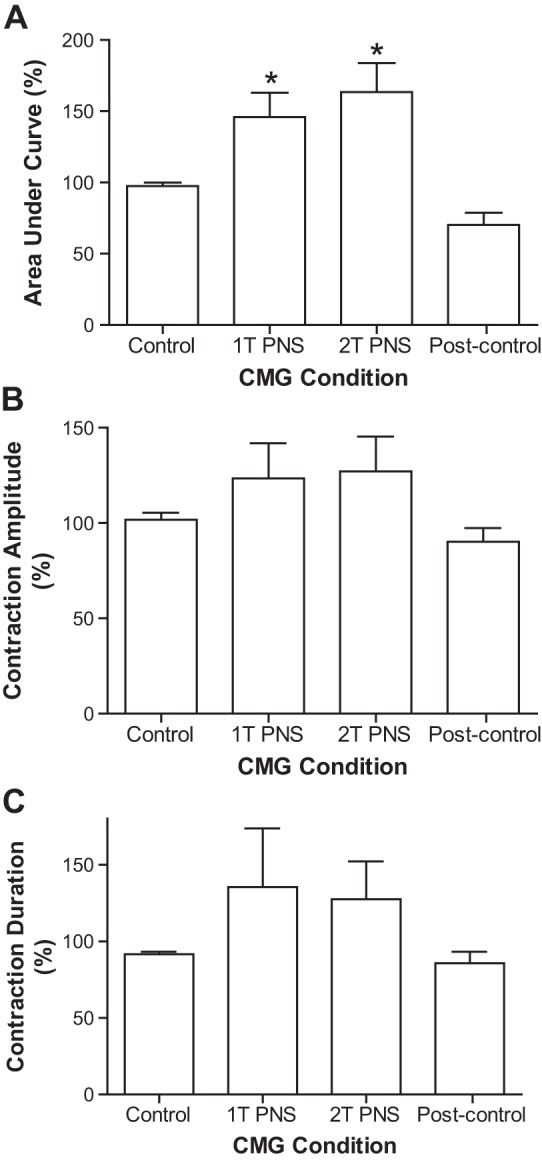
Effects of PNS on bladder contraction. A: area under the curve of the bladder contraction. B: contraction amplitude. C: contraction duration. Bladder contraction response was normalized to the control response. *Significantly (P < 0.05) different from the control response (1-way ANOVA, n = 6 cats). PNS (1–3 Hz, 0.2 ms, T = 1.4–20 V).
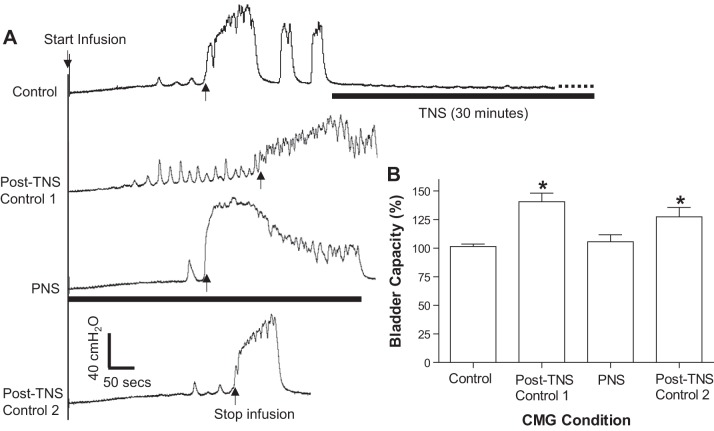
PNS removed post-TNS inhibition and prolonged reflex bladder contractions.
Our previous studies (11, 23) revealed that TNS for 30 min produces a prolonged poststimulation inhibition of reflex bladder activity evident as an increase in bladder capacity. The inhibition can last >2 h after termination of the TNS (23). In this study, after 30 min of TNS the bladder reflex activity was inhibited during the post-TNS period, during which a series of three CMGs were performed over a period of 1–2 h (Fig. 5A). The bladder capacity measured during the first post-TNS CMG was significantly (P < 0.01) increased to 140.5 ± 7.6% of the control capacity (Fig. 5B). PNS applied during the second CMG completely removed this post-TNS inhibition and restored the bladder capacity to the original control level (Fig. 5, A and B). PNS also significantly (P < 0.05) increased the duration of the bladder contractions (Figs. 5A and 6). The third CMG performed 2–3 min after termination of PNS indicated that the post-TNS inhibition reappeared (Fig. 5A), producing a significantly larger bladder capacity than during pre-TNS control CMG (Fig. 5B).
Fig. 5.
PNS removed the poststimulation inhibition induced by tibial nerve stimulation (TNS). A: repeated CMG tracings showing that 30 min TNS (5 Hz, 0.2 ms, 4T = 1.2 V) produced long-lasting poststimulation inhibition that was completely removed when PNS (1 Hz, 0.2 ms, 1T = 1.4 V) was applied. T, threshold intensity to induce observable toe twitch (TNS) or muscle twitch on the posterior thigh (PNS). The black bar under the bladder pressure tracing indicates the duration of TNS or PNS. Infusion rate = 3 ml/min B: summarized results (n = 5 cats). The bladder capacity was normalized to the control capacity. *Significantly (P < 0.01) different from the control or PNS (1-way ANOVA). TNS (5 Hz, 0.2 ms, 3–4T, T = 0.3–1.2 V), PNS (1–3 Hz, 0.2 ms, 1–4T, 1T = 1.4–12 V).
Fig. 6.
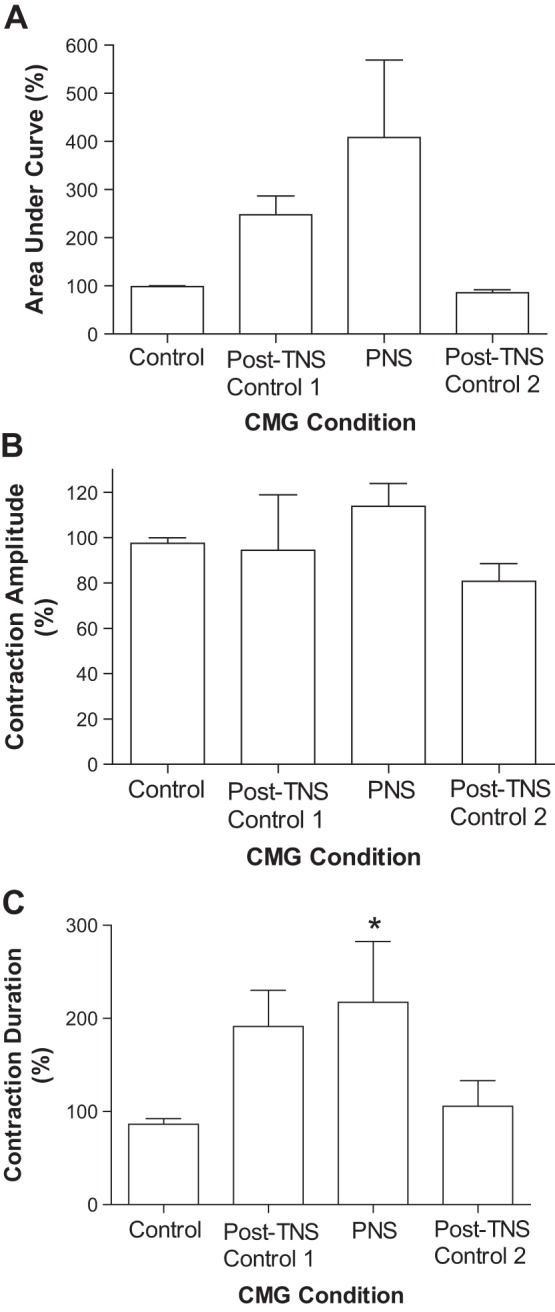
Effects of TNS and PNS on bladder contraction. A: area under the curve of the bladder contraction. B: contraction amplitude. C: contraction duration. Bladder contraction response was normalized to the control response. *Significantly (P < 0.05) different from control response (1-way ANOVA, n = 4 cats). TNS (5 Hz, 0.2 ms, 3–4T, T = 0.3–1.2 V), PNS (1–3 Hz, 0.2 ms, 1–4T, 1T = 1.4–8 V).
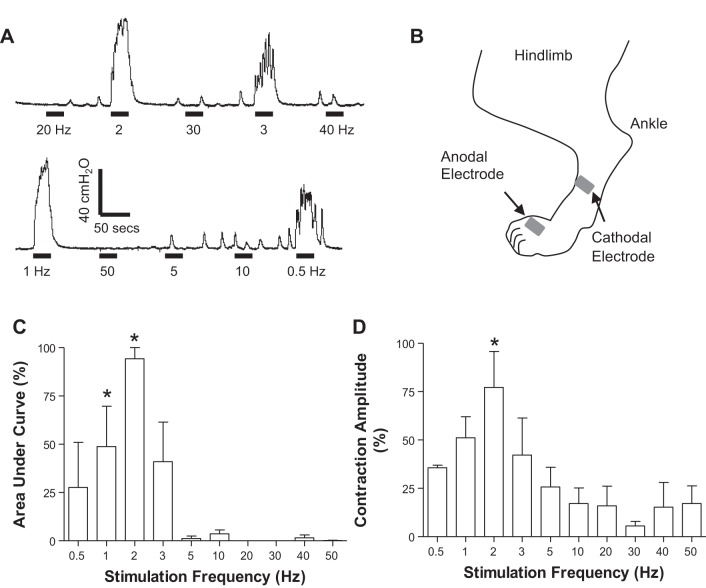
Peroneal-to-bladder reflex induced by transcutaneous foot stimulation.
When the bladder was distended at a volume ~90% of the bladder capacity, electrical stimulation (30 s, 2–4T, T = 3–7 V) applied to the dorsal skin surface of the left foot (Fig. 7B) activated the superficial peroneal nerve and induced large bladder contractions (50–100 cmH2O) at frequencies of 0.5–3 Hz (Fig. 7A). On average, the foot stimulation at 1–2 Hz produced significantly (P < 0.05) larger bladder contractions than the contractions elicited by other frequencies (Fig. 7, C and D).
Fig. 7.
Bladder pressure responses to different frequencies (0.5–50 Hz) of transcutaneous foot stimulation. A: bladder pressure tracings. The black bar under the pressure tracing indicates the duration (30 s) of foot stimulation (0.2 ms, 2T = 11.6 V). B: locations of the skin surface electrodes that were attached on the dorsal side of the foot to stimulate the superficial peroneal nerve and its branches. C: normalized area under the curve. D: normalized contraction amplitude. Bladder pressure response was normalized to the maximal response in each animal (n = 3 cats). *Significantly (P < 0.05) different from the response at 50 Hz (1-way ANOVA). Foot stimulation (0.2 ms, 2–4T, T = 3–7 V).
DISCUSSION
In anesthetized cats, PNS at frequencies of 1–3 Hz and at 1T for producing a muscle twitch on the posterior thigh elicits bladder contractions (Fig. 1), decreases bladder capacity during CMGs (Fig. 3), and reverses the prolonged increase in bladder capacity induced by 30-min TNS (Fig. 5). Activation of the superficial peroneal nerve by transcutaneous electrical stimulation on the dorsal surface of the foot also induces large bladder contractions (Fig. 7), raising the possibility that noninvasive electrical stimulation methods might be used clinically to trigger the excitatory peroneal-to-bladder reflex and promote voiding in patients with UAB or NOUR.
The superficial peroneal nerve contains three subtypes (Aβ, Aδ, and C) of cutaneous afferent axons. Our experiments showed for the first time that electrical stimulation of the Aβ afferent axons in this nerve induced or enhanced reflex bladder activity depending on the conditions of the experiment. The identification of the role of Aβ fibers in the initiation of bladder reflexes was based on the similarity of the stimulation thresholds for evoking these reflexes and the thresholds for evoking contractions of flexor muscles in the thigh. Previous studies by other investigators (8, 19, 20) revealed that reflex contractions of flexor muscles in chloralose-anesthetized cats can be elicited by stimulation of the fastest-conducting afferents (Aβ, 50–80 m/s) in various cutaneous nerves, including the superficial peroneal nerve. Because the initiation of bladder reflexes by PNS occurs at the same threshold intensities as those for evoking flexor reflexes, this indicates that Aβ afferents triggered the bladder reflexes in our experiments. On the other hand, it is known (17) that the thresholds for activating Aδ and C afferent axons in the superficial peroneal nerve of the cat are considerably higher (4–10T and 40–100T, respectively). As shown in Fig. 2, PNS at 0.5T induced a bladder response in some animals, but on average this was not significantly (P < 0.05) different from bladder baseline activity. If we count the 0.5T intensity as the threshold for activating Aβ afferents, then PNS at 2T (4 × 0.5T) might have activated some Aδ afferents. However, the activation of additional Aδ afferents did not significantly increase the bladder response (Fig. 2), indicating that activation of Aβ afferents alone is sufficient to fully trigger the excitatory peroneal-to-bladder reflex.
The PNS-evoked bladder reflexes in our experiments occurred over a narrow range of frequencies (1–3 Hz; Fig. 1) and were able to reduce bladder capacity during CMGs and increase the magnitude (area under the curve) of reflex bladder contractions induced by bladder filling. These results are different from those reported by Sato et al. (17) who also studied the effects of PNS on reflex bladder activity in the cat. They showed that activation of Aδ and C afferents in the superficial peroneal nerve at frequencies between 3 and 30 Hz elicited transient reflex contractions of a partially full bladder but inhibited reflex contractions of a full bladder when stimulated at frequencies between 2 and 10 Hz. Furthermore, they were unable to detect either excitatory or inhibitory effects of Aβ afferents on bladder activity at a stimulation frequency of 10 Hz. Their failure to detect an excitatory effect might be related to testing of 10 Hz stimulation, which is above the frequency (1–3 Hz) effective in our experiments. They also tested for excitatory reflexes when the bladder was distended with a small volume and quiescent. A larger bladder volume was used in our experiments, which activates bladder afferent firing. This afferent firing might be necessary to facilitate the Aβ afferent-evoked bladder reflex pathway. In addition, chloralose anesthesia was used in our experiments, whereas chloralose plus urethane was used in the experiments of Sato et al. (17), and this anesthetic combination may have suppressed the Aβ afferent-evoked bladder reflex.
Because the bladder is innervated by sympathetic and parasympathetic nerves, the effects of PNS on bladder function could be mediated by a change in activity of either of these autonomic pathways. Although lumbar sympathetic nerve activity is responsive to electrical stimulation of afferent axons in various hindlimb nerves (18), denervation experiments revealed that elimination of the sympathetic nerves did not notably change PNS-vesical reflex responses, whereas transection of the parasympathetic pathway in the pelvic nerves blocked the responses (17). Electrophysiological studies in which reflex firing was recorded in parasympathetic efferent nerves close to the bladder revealed that single-shock electrical stimulation of the peroneal nerve and other limb nerves, including branches of the tibial nerve, elicited long-latency (>100 ms) reflex firing that was similar to the long latency firing mediated by the spinobulbospinal micturition reflex pathway activated by electrical stimulation of bladder afferents in the pelvic nerve (6). Another study (17) revealed that trains of PNS also inhibited or enhanced activity in bladder parasympathetic nerves depending on the state of the bladder. Thus, modulation of bladder activity by PNS must be mediated by changes in the sacral parasympathetic outflow and very likely mediated by a supraspinal reflex pathway involving the periaqueductal gray (PAG) and the pontine micturition center (PMC).
The PNS excitatory effect on the bladder could occur on both afferent and efferent limbs of the spinobulbospinal micturition reflex pathway. PNS significantly reduced bladder capacity (Fig. 3), indicating modulation of the afferent limb to either enhance bladder afferent input to the PAG-PMC or reduce the threshold in the PMC circuitry for triggering a micturition reflex (7). At the same time PNS also produces stronger bladder contractions (Figs. 3A, 4, 5A, and 6), indicating that it also acts on the efferent limb to amplify the PMC output to the bladder. In contrast, the TNS inhibitory effect must occur primarily on the afferent limb because our previous study in cats showed that TNS increases bladder capacity but does not reduce the amplitude of bladder contractions induced by PMC stimulation (16).
The opposing effects of PNS and TNS on the bladder were clearly demonstrated in this study by the ability of PNS to eliminate post-TNS inhibition (Fig. 5). However, this reversal was only transient, and the post-TNS inhibition reappeared after the PNS was terminated (Fig. 5). The mechanisms responsible for the PNS reversal of TNS inhibition are not known. The reversal could be due to PNS activation of excitatory inputs to the bladder preganglionic neurons in the sacral spinal cord to counteract the TNS inhibition or because of a direct suppression of the inhibitory pathway activated by TNS. Whether a long-lasting post-PNS excitatory effect can be induced by a 30-min PNS is still unknown. However, because the 30-min TNS can induce a prolonged poststimulation inhibition lasting >2 h (Fig. 5) (11, 23), it is possible that a long-duration PNS might also induce a long-lasting poststimulation excitatory effect on bladder reflex.
The superficial peroneal nerve and tibial nerve innervate the dorsal and plantar surfaces of the foot and elicit opposite motor responses, producing dorsiflexion and plantar flexion of the foot, respectively. Therefore, it is possible that the dorsiflexion/plantar flexion induced by PNS/TNS or the afferent firing that induces these flexor and extensor reflexes has opposite effects on the bladder. The possibility that extensor and flexor reflex mechanisms might exert reciprocal modulatory effects on the lower urinary tract was raised by a previous study in rats and cats (14) showing that the external urethral sphincter (EUS) can be excited by triggering a flexor hindlimb reflex but inhibited by triggering an extensor reflex. The effects on EUS by PNS/TNS are currently unknown; however, because the EUS and bladder exhibit reciprocal activities during urinary storage and voiding, it would be interesting in future experiments to determine if TNS, which induces plantar flexion and bladder inhibition (11, 22, 23), excites the EUS, whereas PNS, which produces dorsiflexion and bladder excitation, inhibits the EUS. It will also be important to determine if PNS can facilitate voiding and increase voiding efficiency by targeting the bladder and EUS. This could not be tested in the present study because voiding was blocked by ligating the urethral outlet to measure bladder activity under isovolumetric conditions.
The somatovisceral interactions that underlie the antagonistic effects of PNS/TNS on reflex bladder function create an animal model that mimics some of the features of Fowler’s syndrome, an unusual clinical disorder characterized by NOUR (12). Patients with this disorder have a hyperactive EUS that triggers abnormal somatic afferent nerve activity that is conveyed through the pudendal nerve to the sacral spinal cord. It is believed that the pudendal afferent activity suppresses the bladder sensory and motor pathways, thereby reducing the patient’s ability to sense bladder filling and to voluntarily void (15). Electrical stimulation of somatic afferent axons in a sacral spinal nerve (a procedure termed sacral neuromodulation) suppresses the inhibition induced by pudendal afferents and restores bladder sensations and voiding (15). This pathophysiology and treatment are similar, respectively, to the TNS inhibition and the reversal of that inhibition by PNS in the cat model. Thus, post-TNS inhibition might be a useful animal model for certain types of UAB/NOUR conditions. Furthermore, the efficacy of PNS in reversing post-TNS inhibition raises the possibility that PNS might be useful clinically in treating some types of UAB/NOUR.
In summary, this study in anesthetized cats discovered an excitatory peroneal-to-bladder reflex that can reverse a long-lasting somatobladder inhibitory mechanism. This new reflex raises many scientific questions about the interactions of visceral and somatic reflex pathways and also provides potential opportunities to develop novel neuromodulation therapies for UAB/NOUR. The superficial peroneal nerve, which can be activated noninvasively by skin surface electrodes on the foot, makes the peroneal-to-bladder reflex pathway an attractive target for potential clinical applications.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-094905, DK-102427, and DK-111382.
DISCLOSURES
No conflicts of interests, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.Y., J.U., X.J., X.L., C.J., B.S., J.W., J.R.R., W.C.d.G., and C.T. conceived and designed research; M.Y., J.U., X.J., X.L., C.J., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; M.Y., J.U., X.J., X.L., C.J., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; M.Y., J.U., X.J., X.L., C.J., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; M.Y., J.U., X.J., X.L., C.J., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; M.Y., J.U., X.J., X.L., C.J., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; M.Y., J.U., X.J., X.L., C.J., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; M.Y., J.U., X.J., X.L., C.J., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology 69: 436–440, 2007. doi: 10.1016/j.urology.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Bartley J, Gilleran J, Peters K. Neuromodulation for overactive bladder. Nat Rev Urol 10: 513–521, 2013. doi: 10.1038/nrurol.2013. [DOI] [PubMed] [Google Scholar]
- 3.Chapple CR, Osman NI, Birder L, van Koeveringe GA, Oelke M, Nitti VW, Drake MJ, Yamaguchi O, Abrams P, Smith PP. The underactive bladder: a new clinical concept? Eur Urol 68: 351–353, 2015. doi: 10.1016/j.eururo.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Larson JA, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Post-stimulation inhibitory effect on reflex bladder activity induced by activation of somatic afferent nerves in the foot. J Urol 187: 338–343, 2012. doi: 10.1016/j.juro.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen ML, Chermansky CJ, Shen B, Roppolo JR, de Groat WC, Tai C. Electrical stimulation of somatic afferent nerves in the foot increases bladder capacity in healthy human subjects. J Urol 191: 1009–1013, 2014. doi: 10.1016/j.juro.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 6.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol 200: 87–108, 1969. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Groat WC, Wickens C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol (Oxf) 207: 66–84, 2013. doi: 10.1111/apha.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devanandan MS, Eccles RM, Lewis DM, Stenhouse D. Responses of flexor alpha-motoneurones in cats anaesthetised with chloralose. Exp Brain Res 8: 163–176, 1969. [DOI] [PubMed] [Google Scholar]
- 9.Faraj K, Doo F, Boura J, Vereecke A, Chancellor MB. A cross-sectional study in the USA of the epidemiology and quality of life of underactive bladder symptoms. Int Urol Nephrol 48: 1797–1802, 2016. doi: 10.1007/s11255-016-1382-0. [DOI] [PubMed] [Google Scholar]
- 10.Ferroni MC, Chaudhry R, Shen B, Chermansky CJ, Cannon GM, Schneck FX, Ost MC, Tai C, Stephany HA. Transcutaneous electrical nerve stimulation of the foot: results of a novel at-home, noninvasive treatment for nocturnal enuresis in children. Urology 101: 80–84, 2017. doi: 10.1016/j.urology.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Ferroni MC, Slater RC, Shen B, Xiao Z, Wang J, Lee A, Roppolo JR, de Groat WC, Tai C. Role of the brain stem in tibial inhibition of the micturition reflex in cats. Am J Physiol Renal Physiol 309: F242–F250, 2015. doi: 10.1152/ajprenal.00135.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler CJ, Christmas TJ, Chapple CR, Parkhouse HF, Kirby RS, Jacobs HS. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ 297: 1436–1438, 1988. doi: 10.1136/bmj.297.6661.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong SJ, Kim HJ, Lee YJ, Lee JK, Lee BK, Choo YM, Oh JJ, Lee SC, Jeong CW, Yoon CY, Hong SK, Byun SS, Lee SE. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: A comparison between men and women. Korean J Urol 53: 342–348, 2012. doi: 10.4111/kju.2012.53.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jolesz FA, Cheng-Tao X, Ruenzel PW, Henneman E. Flexor reflex control of the external sphincter of the urethra in paraplegia. Science 216: 1243–1245, 1982. doi: 10.1126/science.7200635. [DOI] [PubMed] [Google Scholar]
- 15.Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat Clin Pract Urol 5: 657–666, 2008. doi: 10.1038/ncpuro1251. [DOI] [PubMed] [Google Scholar]
- 16.Lyon TD, Ferroni MC, Kadow BT, Slater RC, Zhang Z, Chang V, Lamm V, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Pudendal but not tibial nerve stimulation inhibits bladder contractions induced by stimulation of pontine micturition center in cats. Am J Physiol Regul Integr Comp Physiol 310: R366–R374, 2016. doi: 10.1152/ajpregu.00490.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato A, Sato Y, Schmidt RF. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J Auton Nerv Syst 1: 229–241, 1980. doi: 10.1016/0165-1838(80)90019-3. [DOI] [PubMed] [Google Scholar]
- 18.Sato A, Schmidt RF. Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiol Rev 53: 916–947, 1973. [DOI] [PubMed] [Google Scholar]
- 19.Shimamura M, Akert K. Peripheral nervous relations of propriospinal and spino-bulbo-spinal reflex system. Jpn J Physiol 15: 638–647, 1965. doi: 10.2170/jjphysiol.15.638. [DOI] [Google Scholar]
- 20.Shimamura M, Yamauchi T. Neural mechanisms of the chloralose jerk with special reference to its relationship with the spino-bulbo-spinal reflex. Jpn J Physiol 17: 738–745, 1967. [DOI] [PubMed] [Google Scholar]
- 21.Smith PP, Birder LA, Abrams P, Wein AJ, Chapple CR. Detrusor underactivity and the underactive bladder: Symptoms, function, cause-what do we mean? ICI-RS think tank 2014. Neurourol Urodyn 35: 312–317, 2016. doi: 10.1002/nau.22807. [DOI] [PubMed] [Google Scholar]
- 22.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012. doi: 10.1152/ajprenal.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC. Prolonged poststimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol 300: F385–F392, 2011. doi: 10.1152/ajprenal.00526.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valente S, DuBeau C, Chancellor D, Okonski J, Vereecke A, Doo F, Lajiness M, Diokno A, Chancellor M. Epidemiology and demographics of the underactive bladder: a cross-sectional survey. Int Urol Nephrol 46, Suppl 1: S7–S10, 2014. doi: 10.1007/s11255-014-0811-1. [DOI] [PubMed] [Google Scholar]