Abstract
Multiple vaginal parities have been reported to be an important risk factor for stress urinary incontinence (SUI). Simulated birth trauma with single vaginal distention (VD) has been used to induce the SUI condition in animals; however, the effect of multiple simulated birth traumas on the urethral continence function has not been well characterized. Therefore, we examined the effects of multiple VDs on urethral functions in vivo and the changes in gene expressions of several molecules in the urethra using female SD rats, which were divided into three groups; sham, VD-1 (single VD), and VD-3 groups (3 times of VDs every 2 wk). Two weeks after the final VD, leak point pressure (LPP) and urethral responses during sneezing were evaluated. Also, changes in mRNA levels of urethral molecules were quantified with RT-PCR. The VD-1 group did not show any change in LPP with only a tendency of decrease in amplitudes of the urethral responses during sneezing (A-URS); however, the VD-3 group showed a significant decrease in LPP and urethral responses such as baseline urethral pressure and A-URS accompanied with SUI episodes during sneezing. Nicotinic receptor subtypes and transforming growth factor (TGF)-β1 were significantly increased in both VD-1 and VD-3 groups while TNF receptor (TNFR)-1, IL-6, collagens, and matrix metalloproteinases-9 were significantly increased only in the VD-3 group. These data indicate that rats with multiple simulated birth traumas exhibit profound impairment of the urethral continence function and that these functional changes are associated with those in cytokines, extracellular matrix molecules, and nicotinic receptor subtypes in the urethra.
Keywords: stress urinary incontinence, multiple simulated birth trauma injuries, urethral closure reflex, molecular changes, rats
stress urinary incontinence (SUI) is the common type of urinary incontinence with a significantly negative impact on quality of life in women. SUI is defined as involuntary leakage of urine due to an increase in abdominal pressure during events such as sneezing, coughing, or laughing in the absence of bladder contraction. The prevalence of SUI was estimated at approximately 152 million in the world (8). Although the etiology of SUI in women seems to be multifactorial, vaginal parity, the aging process, and estrogen deficiency have been demonstrated to have major impacts on the development of SUI. Among them, vaginal parity, which causes combined muscular, nerve and connective tissue injury, can induce the long-term, insidious damage of pelvic floor nerves, and musculature even after the SUI condition following the first childbirth spontaneously recovers (9, 23, 33). Moreover, multiparities with three or more live births reportedly increase the risk of SUI (24, 32).
The urethral continence function depends on the contractile responses of smooth and striated muscles of the urethra, which are controlled by peripheral nervous pathways such as pelvic nerves, hypogastric nerves, and pudendal nerves (5, 7). The activity of striated muscles comprising the external urethral sphincter (EUS) is also modulated by somatic and visceral reflexes via afferent pathways through the dorsal nerve of the clitoris and the cavernous nerve and efferent axons traveling through the pudendal nerve and the lumbosacral trunk that converge in the motor branch of the lumbosacral plexus (5, 30). We have previously demonstrated in rats that the bladder-to-urethral reflex, which triggers both smooth and striated muscle sphincter contractions by afferent nerve activation in the pelvic nerve, is predominantly organized in the spinal cord and contributes to urinary continence during passive abdominal or intravesical pressure elevation (11) and that the additional continence reflex is recruited during sneezing via a pudendal nerve-mediated somatic reflex directly driven by sneezing without activating pelvic or hypogastric nerves (13, 14).
In preclinical studies, rats with vaginal distention (VD) are experimentally used as a SUI animal model. Previous studies demonstrated that single VD in rats induces the EUS damage (19), lower urinary tract ischemia (6), and bladder overdistension and pelvic and perineal nerves stretching in association with behavioral signs of SUI (28). In addition, rats with single VD showed the SUI condition evident as a decrease in leak point pressure (LPP) and urine leakage during sneezing; however, the SUI condition in this model is usually restored spontaneously within 2 wk after single VD (12, 29, 35). A previous study also reported that repeated VDs are required to maintain the SUI condition for a duration of 8 wk in rats (30). Taken together, we hypothesized that rats with multiple simulated birth traumas would be a useful model of long-lasting urethral dysfunction and SUI.
The present study was therefore performed to characterize the functional changes in urethral continence mechanisms during sneezing using rats with multiple simulated birth traumas induced by three times of VDs. In addition, changes in extracellular matrix (ECM)-related molecules such as collagen and matrix metalloproteinases (MMPs; collagen-degrading enzyme), nerve degeneration and inflammatory responses such as urethral transforming growth factor-β (TGF-β) upregulation have been reported in SUI animal models induced by single VD with or without ovariectomy (18, 20, 21, 31). Therefore, we examined the effect of multiple VDs on gene expressions of various molecules in urethra tissues, including cytokines such as TNF-α, TNF receptor-1 (TNFR1), TNFR2, IL-6, IL-1β, and TGF-β1; ECM molecules such as MMP-2, MMP-9, collagen 1a, collagen 3a, tissue inhibitors of metalloproteinase-1 (TIMP-1), and TIMP-2; as well as α1A-adrenoceptors and nicotinic receptor subunits (α, β, γ, δ, and ε), which are, respectively, involved in contractions of the smooth and striated muscle sphincter.
MATERIALS AND METHODS
Animals.
A total of 54 adult female Sprague-Dawley rats, weighing 252–330 g, were used. All experiments were conducted in accordance with institutional guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Experimental protocol.
The study design is illustrated in Fig. 1. Female rats were randomly divided into three groups: 1) sham group, 2), single vaginal distention (VD-1) group, and 3) three-times vaginal distention (VD-3) group, which underwent VDs every 2 wk for three times. Two weeks after the final VD, an in vivo assay such as measurements of LPP with the intravesical pressure-clamp method, urethral closure responses during sneezing, and LPP during sneezing as well as a molecular assay by real-time PCR were conducted. The number of animals per group was six in each of in vivo and molecular studies.
Fig. 1.
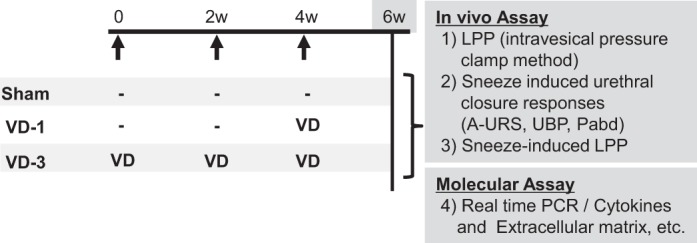
Study design. VD-1, single vaginal distention; VD-3, multiple vaginal distention; LPP, leak point pressure; A-URS, amplitudes of the urethral responses during sneezing; Pabd, abdominal pressure; UBP, urethral baseline pressure; −: sham operation was performed.
VD.
While the rats were under pentobarbital (45 mg/kg ip; Ovation, Deerfield, IL) anesthesia, a modified 12-Fr Foley balloon catheter (5 ml; Rusch, Salt Lake City, UT) with the tip cut off was inserted into the vagina, and then the vaginal orifice was closed with a suture. The balloon catheter was then inflated with 4 ml of water to distend the vagina for 4 h according to previous methods with slight modification (12).
Measurement of LPP.
LPP was measured using the intravesical pressure-clamp method as described previously (4) with slight modification. Briefly, while the rats were under isoflurane anesthesia, the spinal cord was transected at the Th8-9 thoracic spine level to prevent the spino-bulbo-spinal voiding reflex while the bladder-to-urethral spinal continence reflex was preserved (4). The rat bladder was exposed through an abdominal incision in a supine position, and then the ureters were cut bilaterally and the distal ends were ligated. A polyethylene catheter (PE-90) was then inserted into the bladder through the dome and secured with a ligature. Feces were removed from the distal colon through a small incision of the colon wall. After the surgery, isoflurane anesthesia was turned off and replaced with urethane anesthesia (1.2 g/kg ip; Sigma). The polyethylene tube was connected to a saline reservoir via three-way stopcocks, and intravesical pressure was recorded with data-acquisition software (Chart; AD Instruments, Castle Hill, NSW, Australia) on a computer system equipped with an analog-to-digital converter (Power Lab; AD Instruments). The saline reservoir was mounted on a metered vertical pole and elevated upward in 2.5-cm steps from zero to increase intravesical pressure until visual identification of leakage of fluid from the urethral orifice. The intravesical pressure inducing fluid leakage from the urethra was regarded as LPP. LPP was measured twice, and the bladder was emptied before starting the second measurement. The average of two consecutive LPPs was used as a data point in each animal.
Measurement of sneeze-induced urethral closure responses and LPP.
Rats were anesthetized with isoflurane, and the bladder was exposed through an abdominal incision. The ureters were cut bilaterally, and the distal ends were ligated. The visceral branches of the pelvic nerves were transected bilaterally near the internal iliac vessels to prevent reflex bladder contraction (10, 22, 25). Feces were removed from the distal colon through a small incision in the colon wall, and then a handmade balloon catheter connected to a pressure transducer was inserted through the rectal wall incision into the abdominal cavity to measure the abdominal pressure (Pabd). Thereafter, isoflurane was turned off and replaced with urethane (0.72 g/kg, intraperitoneally), and then the abdomen was closed with sutures. During the experiments, additional doses of urethane (2.5 mg/10 μl per injection) were administered as required through a PE-10 polyethylene catheter implanted into the jugular vein to obtain the sufficient level of anesthesia, which was confirmed by negative reflex responses to toe pinch. The final dose of urethane ranged from 1.0 to 1.2 g/kg among animals used. Thereafter, rats were placed in a supine position for experimental testing. A 3.5-Fr nylon catheter with a side-mounted microtransducer located 1 mm from the catheter tip (SPR-524; Millar Instruments, Houston, TX) was inserted into the middle urethra (10 to 15 mm from the urethral orifice), at which the highest urethral baseline pressure (UBP) was obtained. The microtransducer-tipped catheter was connected to a pressure transducer (Transbridge 4M; World Precision Instruments), and urethral responses were recorded with data-acquisition software (sampling rate 400 Hz; Chart; AD Instruments) on a computer system equipped with an analog-to-digital converter (Power Lab; AD Instruments). The catheter position was monitored throughout the experiments to confirm that the location of the transducer had not been changed. After UBP became stable, approximately 20 min after the catheter insertion, sneezes were induced by an insertion of a rat’s whisker into the nostril; and the changes of urethral responses during sneezing were examined by measuring amplitudes of the urethral responses during sneezing (A-URS), which were determined as the maximal pressure change from the baseline in centimeters per H2O during sneezing (Fig. 2). The averaged UBP value was obtained from a plateau section of pressure recordings just before the sneeze response (Fig. 2). To evaluate the intensity of the induced sneeze, which varied with each sneeze event, Pabd increases during sneezing were also measured via an intra-abdominal balloon catheter. The average of each parameter recorded during 10 sneezing events with approximately 30-s intervals was used for data analysis.
Fig. 2.
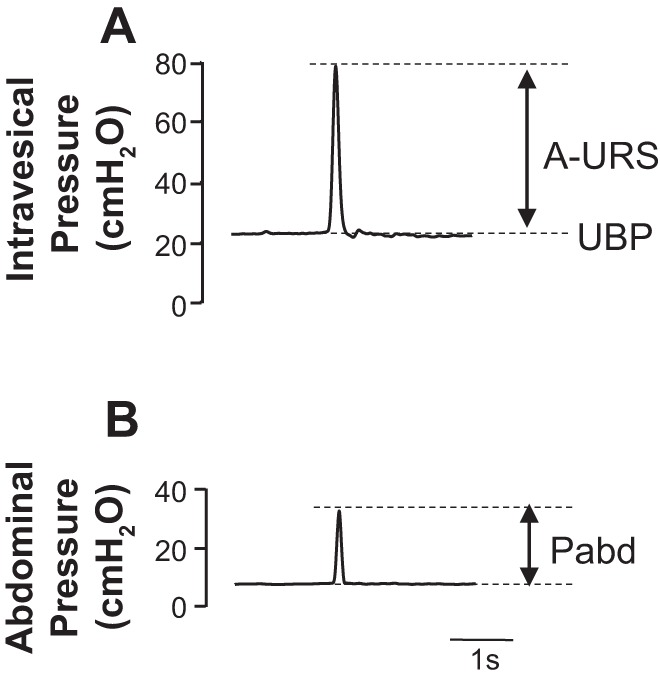
Representative traces of sneeze-induced urethral continence reflexes. Shown are the urethral pressure responses (A) and abdominal pressure changes (B) during sneezing, respectively. Pabd, abdominal pressure.
Quantification of mRNAs for cytokines, ECM molecules, TIMPs, α1A-adrenoceptors, nicotinic receptor subunits in the urethral tissue.
The urethral tissue was carefully dissected and isolated under the microscope to separate it from adjacent tissues such as the vagina in sham rats and rats with VD-1 or VD-3 (single or three times of VD, respectively) at 2 wk after the last VD or sham operation. The tissues were rapidly frozen and stored at −80°C until RNA extraction. One microgram of total RNA extracted from the whole urethra was reverse transcribed into cDNA using ThermoScript RT-PCR system (Invitrogen, Carlsbad, CA) according to the manufacturer’s manual. Quantitative PCR was performed with an MX3000P real-time PCR system (Stratagene, La Jolla, CA) in a 25-μl volume using SYBR Green PCR Master Mix (Qiagen, Valencia, CA). Real-time PCR was run by 40 cycles (denaturation at 95°C for 15 s; primer annealing at 55°C for 60 s; elongation at 72°C for 30 s). Relative expression data were quantified using the 2−ΔΔCT method, where CT is the cycle threshold. All target mRNA expression levels were normalized to that of the constitutive GAPDH RNA. Primer sequences used for real-time PCR are described in Table 1. All primers for PCR were designed based on the National Center of Biotechnology Information database sequence of rat reference mRNA and checked for specificity with BLAST software from the National Center for Biotechnology Information (NCBI) web site.
Table 1.
Primers used for real-time RT-PCR
| Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Size, bp |
|---|---|---|---|
| TNF-α | GAAGCTGTCTTCAGGCCAAC | CCAGATGGGGATAGCTGGTA | 67 |
| TNFR1 | TGACCCTCTCCTCTACGGA | CCATCCACCACAGCATACA | 135 |
| TNFR2 | TAGGACTGGCGAACTGCTT | AACTGGGTGCTGTGGTCAAT | 150 |
| IL-6 | GCCAGAGTCATTCAGAGCAA | CATTGGAAGTTGGGGTAGGA | 100 |
| IL-1β | GTCACTCATTGTGGCTGTGG | GGGATTTTGTCGTTGCTTGT | 327 |
| TGF-β1 | CCCCTGGAAAGGGCTCAACAC | TCCAACCCAGGTCCTTCCTAAAGTC | 136 |
| MMP-2 | CTGATAACCTGGATGCAGTCGT | CCAGCCAGTCCGATTTGA | 135 |
| MMP-9 | AAGCCTTGGTGTGGCACGAC | TGGAAATACGCAGGGTTTGC | 117 |
| Collagen 1a | ATCAGCCCAAACCCCAAGGAGA | CGCAGGAAGGTCAGCTGGATAG | 128 |
| Collagen 3a | TGATGGGATCCAATGAGGGAGA | GAGTCTCATGGCCTTGCGTGTTT | 143 |
| TIMP-1 | TCCTGGTTCCCTGGCATAAT | GGCAAAGTGATCGCTCTGGT | 194 |
| TIMP-2 | CTACATCTCCTCCCCGGATGA | GGTGCCCATTGATGCTCTTC | 68 |
| α1A | CGAGTCTACGTAGTAGCC | GTCTTGGCAGCTTTCTTC | 203 |
| nAChR (α) | AGCTCACCGCTGTCCTCCT | GGATCAGTTGCAGTCCCACA | 171 |
| nAChR (β) | CCGTGTCACTGCTGAATCTGT | CTCAAAGGACACCACGACAT | 106 |
| nAChR (γ) nAChR (δ) |
TGTCATCAACATCATCGTCCC TGTGGAGAGAAGACCTCG |
CGAGGAAAAGGAAGACGG AGCCTCTTGGAGATAAGCAAC |
140 77 |
| nAChR (ε) | AACTGTCTGACTGGGTGCGT | GAAGATGAGCGTAGAACCGAC | 95 |
| GAPDH | GGCCAAAAGGGTCATCATCT | GTGATGGCATGGACTGTGGT | 201 |
MMP, matrix metalloproteinases; TIMP, tissue inhibitors of metalloproteinase; nAChR, nicotinic acetylcholine receptors; TNFR, TNF receptor; TGF-β1, transforming growth factor-β1.
Statistical analysis.
All data are represented as means ± SE. Statistical significance was evaluated in each VD group vs. sham group using one-way ANOVA followed by Dunnett's multiple comparison test. Thereafter, an unpaired t-test was used for the comparison between two VD groups (VD-1 vs. VD-3). All data were analyzed using the statistical software package Prism (Graphpad Software, San Diego, CA). P < 0.05 was considered significant.
RESULTS
Effects of single or multiple VDs on LPP in rats.
The VD-1 group at 2 wk after single VD did not show any significant change in LPP compared with the sham group. On the other hand, the VD-3 group with three times of VDs with 2-wk intervals showed a significant decrease in LPP by approximately 72.6%, compared with the sham group at 2 wk after the last VD (Fig. 3). In addition, the decrease in LPP of the VD-3 group was statistically significant compared with the VD-1 group (Fig. 3).
Fig. 3.
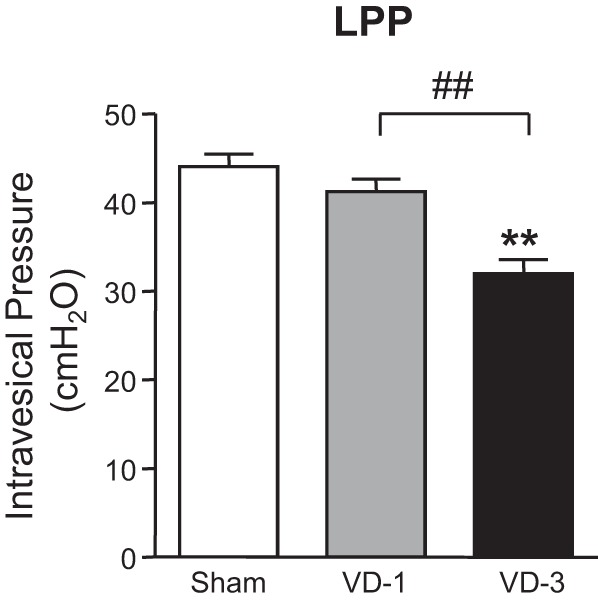
Effects of single (VD-1) and multiple simulated birth traumas (VD-3) on leak point pressure (LPP). Data are expressed as means ± SE of 6 animals per group. **P < 0.01, compared with the sham group (one-way ANOVA followed by Dunnett's multiple comparison test). ##P < 0.01, compared with the VD-1 group (unpaired t-test).
Effects of single or multiple VDs on sneeze-induced urethral continence reflex in rats.
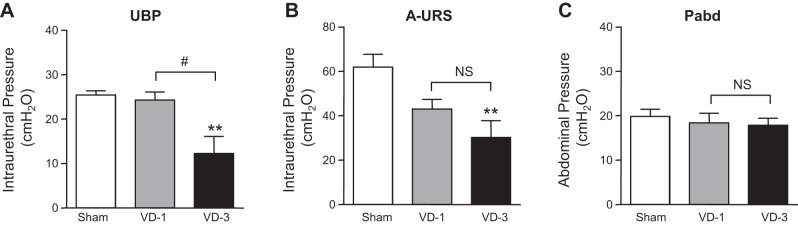
The VD-1 group showed a tendency to decrease the A-URS but without statistical significance, compared with the sham group (Fig. 4B). On the other hand, the VD-3 group showed significant decreases in UBP and A-URS compared with the sham group (Fig. 4, A and B). In addition, the decrease in UBP of the VD-3 group was statistically significant compared with the VD-1 group (Fig. 4A). No significant change in abdominal pressure (Pabd) during sneezing indicates that the intensity of induced sneezing was not different among groups (Fig. 4C). In sneeze-induced LPP measurements during sneezing, any of rats in the sham or VD-1 group had no urinary incontinence during sneezing (Table 2). However, five of six rats in the VD-3 group showed incontinence episodes evident as fluid leakage from the urethral orifice during sneezing, and the sneeze-induced LPP in one continent rat was lower (43.5 cmH2O) than that in the sham or VD-1 group (Table 2).
Fig. 4.
Effects of single (VD-1) and multiple simulated birth traumas (VD-3) on amplitudes of urethral baseline pressure (UBP; A), urethral responses during sneezing (A-URS; B), and abdominal pressure (Pabd). Data are expressed as means ± SE of 6 animals per group. **P < 0.001, compared with the sham group (one-way ANOVA followed by Dunnett's multiple comparison test). #P < 0.05, compared with the VD-1 group (unpaired t-test). NS, not significant (unpaired t-test).
Table 2.
Effect of single VD and multiple VDs on sneeze-induced leak point pressure in anesthetized rats
| Group | Rats with SUI Episodes | s-LPP, cmH2O |
|---|---|---|
| Non-VD (sham) | 0/6 | >80 |
| VD-1 | 0/6 | >80 |
| VD-3 | 5/6 | 43.5 |
SUI, stress urinary incontinence; s-LPP, sneeze-induced leak point pressure; VD-1, single vaginal distention; VD-3, multiple vaginal distention.
Effects of single or multiple VDs on gene expression of cytokines, ECM molecules, TIMPs, α1A-adrenoceptors, and nicotinic receptor subunits in the urethra.
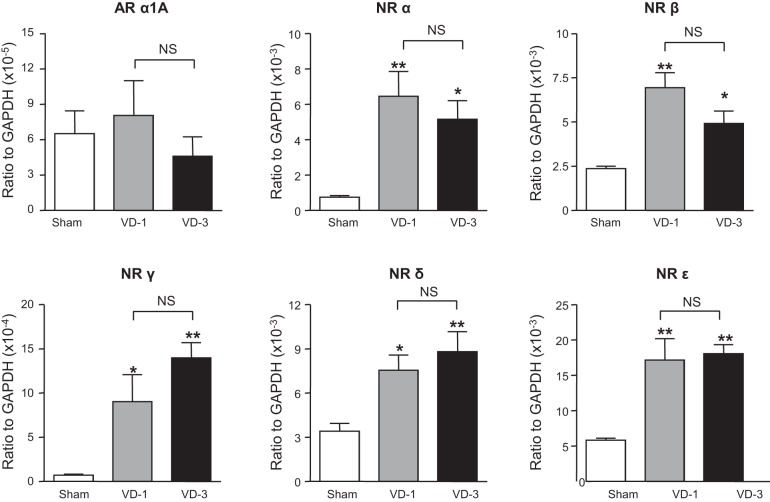
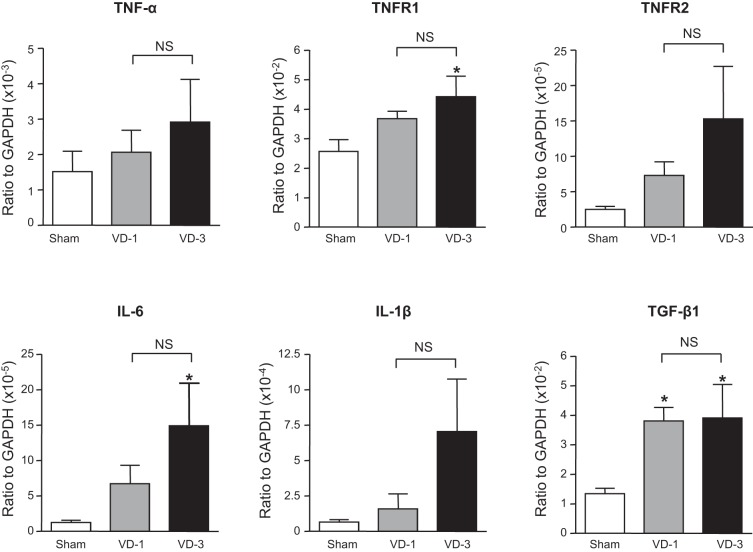
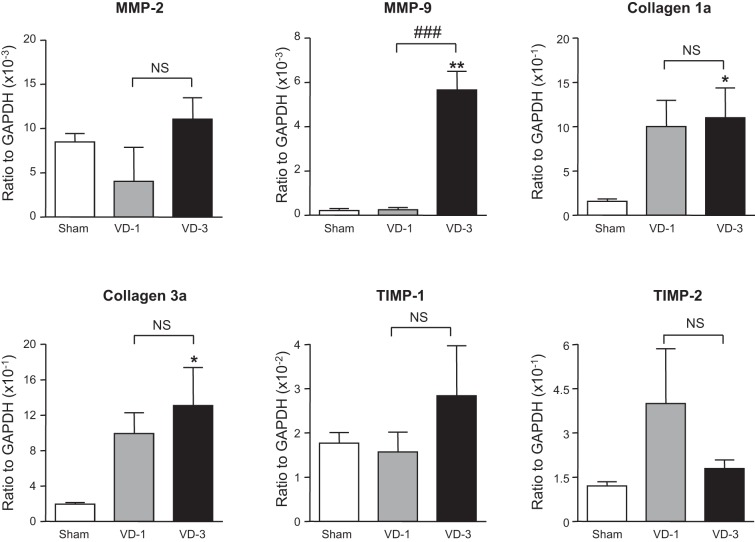
mRNA levels of TGF-β1 and nicotinic receptor α-, β-, γ-, δ-, and ε-subunits were significantly increased in the urethra of both VD-1 and VD-3 groups compared with the sham group although there was no statistical difference in the expression of these transcripts between VD-1 and VD-3 groups (Figs. 5 and 6). In addition, mRNA levels of IL-6, TNFR1, collagen 1a, collagen 3a, and MMP-9 were significantly increased in the urethra only in the VD-3 group compared with the sham group (Figs. 6 and 7). Furthermore, the increase in MMP-9 of the VD-3 group was statistically significant compared with the VD-1 group (Fig. 7). On the other hand, no significant changes in mRNA levels of α1A-adrenoceptors, TNF-α, TNFR2, IL-1β, MMP-2, TIMP-1, or TIMP-2 were observed in either VD-1 or VD-3 group compared with the sham group (Figs. 5–7).
Fig. 5.
Expression levels of α1A-adrenoceptors (AR) and 5 different nicotinic receptor (NR) subtypes in the urethra after single (VD-1) and multiple simulated birth traumas (VD-3). All target mRNA expression levels were normalized to that of a housekeeping gene (GAPDH) RNA. *P < 0.05, **P < 0.01, compared with the sham group (one-way ANOVA followed by Dunnett's multiple comparison test). NS, not significant (unpaired t-test).
Fig. 6.
Expression levels of cytokines and their receptors in the urethra after single (VD-1) and multiple simulated birth traumas (VD-3). All target mRNA expression levels were normalized to that of a housekeeping gene (GAPDH) RNA. TNRF1, TNF receptor-1; TGF-β1, transforming growth factor-β1. *P < 0.05, compared with the sham group (one-way ANOVA followed by Dunnett's multiple comparison test). NS, not significant (unpaired t-test).
Fig. 7.
Expression levels of matrix metalloproteinases (MMPs), collagens, and tissue inhibitors of metalloproteinase (TIMPs) in the urethra after single (VD-1) and multiple simulated birth traumas (VD-3). All target mRNA expression levels were normalized to that of a housekeeping gene (GAPDH) RNA. *P < 0.05, **P < 0.01, compared with the sham group (one-way ANOVA followed by Dunnett's multiple comparison test). ###P < 0.001, compared with the VD-1 group (unpaired t-test). NS, not significant (unpaired t-test).
DISCUSSION
We demonstrated that multiple, simulated birth trauma injuries impaired two different kinds of urethral closure mechanisms preventing urinary leakage, which are, respectively, induced during intravesical pressure elevation or sneezing in rats (13). The former mechanism is considered to be induced by a bladder-to-urethral continence reflex during passive elevation of abdominal pressure (13), which increases bladder afferent activity, because this reflex were totally abolished by cutting the pelvic nerves bilaterally (11). Therefore, in this study, for LPP measurements during passive intravesical pressure elevation, the pelvic nerves were left intact whereas the spinal cord was acutely transected to suppress the voiding reflex. On the other hand, the latter sneeze-induced urethral responses were significantly reduced after bilateral transection of pudendal nerves whereas transection of bilateral pelvic and hypogastric nerves had no effect (14). These results indicate that the sneeze-induced continence mechanism is largely dependent on direct activation of pudendal nerves innervating the external urethral sphincter without bladder afferent activation although the possibility of augmentation of sphincter muscle contractions through pudendal afferent-to-pudendal efferent reflex pathways cannot be excluded (5, 30). Therefore, for the sneeze experiments in this study, the spinal cord was kept intact whereas the pelvic nerves were transected to suppress the voiding reflex.
In this study, the VD-1 group at 2 wk after single VD showed no urinary incontinence during sneezing in association with a tendency toward a decrease in A-URS without statistical significance. Our previous studies showed that at 4 days after single VD, all VD rats were incontinent during sneezing with significant reductions in A-URS and UBP (25). Thus, although the incontinence episode during sneezing was not observed in the VD-1 group at 2 wk after VD in this study, the tendency of A-URS reduction may reflect the remaining damage in the external urethral sphincter function, which is in line with the findings in a previous study showing there are still tissue edema and muscle fiber disruption and mild waviness at 10 days after single VD despite the recovery of urethral function (29). On the other hand, the VD-3 group at 2 wk after the third VD showed an apparent functional deterioration of urethral closure reflexes evident as significant decreases in LPP during passive intravesical pressure elevation as well as in UBP and A-URS during sneezing. In addition, these functional deteriorations in the urethral continence function resulted in incontinence episodes during sneezing in the majority of animals (5 of 6 rats) in the VD-3 group. Previous clinical studies have shown that multiparities with three or more live births reportedly increases the risk of SUI (24, 32), whereas the SUI condition that is seen in approximately 30% of mothers after their first vaginal delivery is usually short lasting in most women (23). Thus rats with three-times VDs seem to be appropriate as an animal model of longer-lasting SUI induced by multiple simulated birth traumas.
The urethral sphincter contains smooth and striated muscles, which are innervated by hypogastric sympathetic and pudendal somatic nerves, and their contractile responses are mediated by α1A-adrenoreceptors and nicotinic receptors, respectively. In this study, we found significant increases in nicotinic receptor subtypes (α-, β-, γ-, δ-, and ε-subunits) but not in α1A-receptors in the urethral tissues from both VD-1 and VD-3 rats. We previously reported that increased urethral pressure during sneezing (i.e., A-URS) is mediated by striated muscle contractions of the external urethral sphincter and the pelvic floor due to activation of the pudendal nerves and the somatic nerves innervating the pelvic floor muscles, respectively (14). Thus the decrease in A-URS after multiple VDs in this study indicates impaired striated muscle-mediated continence mechanisms. In contrast, UBP seems to reflect urethral smooth muscle activity rather than striated muscle activity since in our previous study the increase in UBP was blocked by α-adrenoceptor antagonists such as prazosin or by hypogastric nerve transection (10, 14). Thus UBP is likely to represent the baseline coapting urethral function including urethral smooth muscle activity. Thus the reductions in A-URS and UBP in the VD-3 group shown in this study may indicate the impaired activity of both striated and smooth muscles in the urethra. Therefore, the upregulation of nicotinic receptor subtypes after multiple VDs might be a compensation mechanism against the impaired striated muscle urethral sphincter function following the pudendal nerve damage due to VD although α1A-adrenoreceptors in the smooth muscle urethral sphincter seem to be less affected after VD.
Alterations in the metabolism of ECM molecules, such as collagen and elastin, after neuromuscular damage due to vaginal childbirth have been focused in the research on SUI and pelvic organ prolapse (2, 3). Collagen is synthesized by fibroblasts and other connective tissue cells, and the most abundant collagen subtypes in the pelvic organs are type 1 and type 3 (1, 2, 26, 36). The breakdown of collagen is regulated by MMPs, and MMP-2 and MMP-9 are especially responsible for degrading the denatured fibrillar collagens type 1, type 3, and type 5 in addition to elastin, which is another primary constituent of the pelvic floor connective tissue (27, 34, 37). In the present study, rats with multiple VDs showed a significant increase in mRNA levels of collagen type 1a and type 3a in the urethra, which may be interpreted as a remodeling process of urethral tissues after neuromuscular damage due to VD. The upregulation of MMP-9 has been considered to play a role in the breakdown of EMC molecules such as collagen in the vagina and periurethral tissue of SUI patients (38). In this study, a high increase in mRNA of MMP-9 (26.1-fold vs. sham group and 23.0-fold vs. VD-1 group) was also observed in the VD-3 group. Although it was not in the vagina or urethra, previous studies reported that MMP-9 was increased in the tissues such as denervated muscle and injured nerves (15) and that the decrease in MMP-9 activity improved skeletal muscle regeneration (39). The significant increase in MMP-9 in association with upregulation of collagen transcripts in the urethra from VD-3 rats may suggest that the turnover of collagen synthesis and breakdown is accelerated after multiple VDs and that the ECM remodeling due to MMP-9 overexpression is involved in the impairment of the urethral continence function evident as decreases in UBP and A-URS after multiple VDs. In addition, the changes in LPP, UBP, and MMP-9 in the VD-3 groups were statistically significant compared with the VD-1 group, suggesting that the MMP-9-mediated ECM remodeling could contribute to the progression of urethral continence dysfunction after multiple simulated birth traumas. Further investigation is required to clarify the interaction among MMP-9, ECM molecule expression, and urethral continence function.
This study also showed the significant increases in TNFR1 and IL-6 in the urethra only after multiple VDs, whereas TGF-β1, which is known to play a pivotal role in tissue remodeling during inflammatory processes (3, 18), was increased after both single and multiple VDs. Previous studies have shown the upregulation of TGF-β1 and Smad2-mediated TGF signaling pathways in association with increased MMP-9 expression in urethral tissues from rats with single VD and ovariectomy (18, 20). Because both TGF-β1 and IL-6 are also known to stimulate the expression of MMP-9 (16, 17), the elevation of these cytokines could be involved in ECM remodeling and deterioration of the urethral closure mechanisms after multiple VDs.
Conclusions.
The results of the present study indicate that an animal model with multiple simulated birth traumas exhibits a relatively long-lasting SUI condition in association with changes in the expression of nicotinic receptor subunits and ECM-related and inflammation-related molecules in the urethra. Thus this model would be suitable for the study of different aspects of the pathophysiological basis of childbirth-related SUI such as functional changes in the urethral continence mechanisms, altered receptor expression, ECM remodeling, and inflammatory responses in the urethra.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK 107450.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.Y., Y.S., and N.Y. conceived and designed research; S.Y. and Y.S. performed experiments; S.Y., J.K., and T.S. analyzed data; S.Y., Y.S., J.K., T.S., T.K., and N.Y. interpreted results of experiments; S.Y. prepared figures; S.Y. drafted manuscript; S.Y. and N.Y. approved final version of manuscript; T.K., M.M., and N.Y. edited and revised manuscript.
REFERENCES
- 1.Bergman A, Elia G, Cheung D, Perelman N, Nimni ME. Biochemical composition of collagen in continent and stress urinary incontinent women. Gynecol Obstet Invest 37: 48–51, 1994. doi: 10.1159/000292520. [DOI] [PubMed] [Google Scholar]
- 2.Campeau L, Gorbachinsky I, Badlani GH, Andersson KE. Pelvic floor disorders: linking genetic risk factors to biochemical changes. BJU Int 108: 1240–1247, 2011. doi: 10.1111/j.1464-410X.2011.10385.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen B, Yeh J. Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol 186: 1768–1772, 2011. doi: 10.1016/j.juro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 16: 359–363, 2005. doi: 10.1007/s00192-004-1263-4. [DOI] [PubMed] [Google Scholar]
- 5.Cruz Y, Pastelín C, Balog BM, Zaszczurynski PJ, Damaser MS. Somatomotor and sensory urethral control of micturition in female rats. Am J Physiol Renal Physiol 307: F1207–F1214, 2014. doi: 10.1152/ajprenal.00255.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol (1985) 98: 1884–1890, 2005. doi: 10.1152/japplphysiol.01071.2004. [DOI] [PubMed] [Google Scholar]
- 7.de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol 5: 327–396, 2015. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griebling TL. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence, and bladder outlet obstruction. BJU Int 108: 1138–1139, 2011. doi: 10.1111/j.1464-410X.2011.10498.x. [DOI] [PubMed] [Google Scholar]
- 9.Gunnarsson M, Mattiasson A. Female stress, urge, and mixed urinary incontinence are associated with a chronic and progressive pelvic floor/vaginal neuromuscular disorder: An investigation of 317 healthy and incontinent women using vaginal surface electromyography. Neurourol Urodyn 18: 613–621, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Kaiho Y, Kamo I, Chancellor MB, Arai Y, de Groat WC, Yoshimura N. Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol 292: F639–F646, 2007. doi: 10.1152/ajprenal.00226.2006. [DOI] [PubMed] [Google Scholar]
- 11.Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol 287: F434–F441, 2004. doi: 10.1152/ajprenal.00038.2004. [DOI] [PubMed] [Google Scholar]
- 12.Kamo I, Kaiho Y, Canon TW, Chancellor MB, de Groat WC, Prantil RL, Vorp DA, Yoshimura N. Functional analysis of active urethral closure mechanisms under sneeze induced stress condition in a rat model of birth trauma. J Urol 176: 2711–2715, 2006. 10.1016/j.juro.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 13.Kamo I, Kaiho Y, Miyazato M, Torimoto K, Yoshimura N. Two kinds of urinary continence reflexes during abrupt elevation of intravesical pressure in rats. Low Urin Tract Symptoms 1, s1: S40–S43, 2009. doi: 10.1111/j.1757-5672.2009.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamo I, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol 285: R356–R365, 2003. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- 15.Kherif S, Dehaupas M, Lafuma C, Fardeau M, Alameddine HS. Matrix metalloproteinases MMP-2 and MMP-9 in denervated muscle and injured nerve. Neuropathol Appl Neurobiol 24: 309–319, 1998. doi: 10.1046/j.1365-2990.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci 46: 840–848, 2005. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 17.Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KM, Dannenberg AJ, Falcone DJ. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunol 192: 349–357, 2014. doi: 10.4049/jimmunol.1301906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li GY, Cui WS, Zhou F, Gao ZZ, Xin H, Liu T, Li WR, Gong YQ, Bai GY, Guo YL, Xin ZC. Pathology of urethral fibromuscular system related to parturition-induced stress urinary incontinence and TGF-β1/Smad pathway. Mol Cell Biochem 364: 329–335, 2012. doi: 10.1007/s11010-012-1234-x. [DOI] [PubMed] [Google Scholar]
- 19.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology 52: 143–151, 1998. doi: 10.1016/S0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin G, Shindel AW, Banie L, Deng D, Wang G, Hayashi N, Lin CS, Lue TF. Molecular mechanisms related to parturition-induced stress urinary incontinence. Eur Urol 55: 1213–1222, 2009. doi: 10.1016/j.eururo.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin YH, Liu G, Li M, Xiao N, Daneshgari F. Recovery of continence function following simulated birth trauma involves repair of muscle and nerves in the urethra in the female mouse. Eur Urol 57: 506–512, 2010. doi: 10.1016/j.eururo.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzo J, Vazquez MI, Cruz MR, Hernandez ME, Carrillo P, Pacheco P. Fertility ratio in male rats: effects after denervation of two pelvic floor muscles. Physiol Behav 68: 611–618, 2000. doi: 10.1016/S0031-9384(99)00219-X. [DOI] [PubMed] [Google Scholar]
- 23.Meyer S, Schreyer A, De Grandi P, Hohlfeld P. The effects of birth on urinary continence mechanisms and other pelvic-floor characteristics. Obstet Gynecol 92: 613–618, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell ES, Woods NF. Correlates of urinary incontinence during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Climacteric 16: 653–662, 2013. doi: 10.3109/13697137.2013.777038. [DOI] [PubMed] [Google Scholar]
- 25.Miyazato M, Kaiho Y, Kamo I, Chancellor MB, Sugaya K, de Groat WC, Yoshimura N. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol 295: F264–F271, 2008. doi: 10.1152/ajprenal.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimni ME. Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin Arthritis Rheum 13: 1–86, 1983. doi: 10.1016/0049-0172(83)90024-0. [DOI] [PubMed] [Google Scholar]
- 27.Norton PA. Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol 36: 926–938, 1993. doi: 10.1097/00003081-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Palacios JL, Juárez M, Morán C, Xelhuantzi N, Damaser MS, Cruz Y. Neuroanatomic and behavioral correlates of urinary dysfunction induced by vaginal distension in rats. Am J Physiol Renal Physiol 310: F1065–F1073, 2016. doi: 10.1152/ajprenal.00417.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan HQ, Kerns JM, Lin DL, Sypert D, Steward J, Hoover CR, Zaszczurynski P, Butler RS, Damaser MS. Dual simulated childbirth injury delays anatomic recovery. Am J Physiol Renal Physiol 296: F277–F283, 2009. doi: 10.1152/ajprenal.90602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauwels E, De Wachter S, Wyndaele JJ. Evaluation of different techniques to create chronic urinary incontinence in the rat. BJU Int 103: 782–785, 2009. doi: 10.1111/j.1464-410X.2008.08158.x. [DOI] [PubMed] [Google Scholar]
- 31.Prantil-Baun R, de Groat WC, Miyazato M, Chancellor MB, Yoshimura N, Vorp DA. Ex vivo biomechanical, functional, and immunohistochemical alterations of adrenergic responses in the female urethra in a rat model of birth trauma. Am J Physiol Renal Physiol 299: F316–F324, 2010. doi: 10.1152/ajprenal.00299.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rortveit G, Hannestad YS, Daltveit AK, Hunskaar S. Age- and type-dependent effects of parity on urinary incontinence: the Norwegian EPINCONT study. Obstet Gynecol 98: 1004–1010, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Snooks SJ, Setchell M, Swash M, Henry MM. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet 2: 546–550, 1984. doi: 10.1016/S0140-6736(84)90766-9. [DOI] [PubMed] [Google Scholar]
- 34.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516, 2001. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumino Y, Yoshikawa S, Mimata H, Yoshimura N. Therapeutic effects of IGF-1 on stress urinary incontinence in rats with simulated childbirth trauma. J Urol 191: 529–538, 2014. doi: 10.1016/j.juro.2013.08.109. [DOI] [PubMed] [Google Scholar]
- 36.van der Rest M, Garrone R. Collagen family of proteins. FASEB J 5: 2814–2823, 1991. [PubMed] [Google Scholar]
- 37.Woessner JF., Jr The family of matrix metalloproteinases. Ann N Y Acad Sci 732: 11–21, 1994. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang QF, Song YF, Zhu ZY. [Expression of matrix metalloproteinases-9 and tissue inhibitors of matrix metalloproteinases-1 in connective tissue of vaginal wall of women with stress urinary incontinence]. Zhonghua Fu Chan Ke Za Zhi 41: 810–813, 2006. [PubMed] [Google Scholar]
- 39.Zimowska M, Olszynski KH, Swierczynska M, Streminska W, Ciemerych MA. Decrease of MMP-9 activity improves soleus muscle regeneration. Tissue Eng Part A 18: 1183–1192, 2012. doi: 10.1089/ten.tea.2011.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]