Abstract
Oocyte meiotic spindles are associated with spindle-enriched mRNAs, phosphorylated ribosome protein S6, and phosphorylated variants of the key translational regulator, eukaryotic translation initiation factor 4E-binding protein 1 (eIF4E-BP1), consistent with translational control of localized mRNAs by eIF4E-BP1 in facilitating spindle formation and stability. Using specific kinase inhibitors, we determined which kinases regulate phosphorylation status of eIF4E-BP1 associated with meiotic spindles in mouse oocytes and effects of kinase inhibition on chromosome congression and spindle formation. Neither ataxia telangiectasia-mutated kinase nor mechanistic target of rapamycin inhibition significantly affected phosphorylation status of spindle-associated eIF4E-BP1 at the phosphorylation sites examined. Spindle-associated phospho-eIF4E-BP1, spindle formation, and chromosome congression were strongly disrupted by polo-like kinase I (PLK1) inhibition at both metaphase I (MI) and MII. In addition, direct inhibition of eIF4E-BP1 via 4EGI led to spindle defects at MI, indicating a direct role for eIF4E-BP1 phosphorylation in meiotic spindle formation. PLK1 also regulated microtubule dynamics throughout the ooplasm, indicating likely coordination between spindle dynamics and broader ooplasm cytoskeletal dynamics. Because diverse upstream signaling pathways converge on PLK1, these results implicate PLK1 as a major regulatory nexus coupling endogenous and exogenous signals via eIF4E-BP1 to the regulation of spindle formation and stability.
Keywords: eukaryotic initiation factor 4E-binding protein 1, translational control, oocyte meiotic spindle, phosphorylation, chromosome segregation, meiosis
oocytes accumulate a large store of maternal mRNA, much of which is stored in an inactive form and then translationally recruited in a temporally regulated manner to produce different proteins at different times once oocyte transcription has ceased (26, 43, 44). In some species, spatial localization of mRNA also contributes to localized production of proteins that direct embryo cell fate specification (70). Mammalian oocytes, however, are not known to localize mRNAs for translation. One exception is at the oocyte spindle, around which certain mRNAs are enriched at second meiotic metaphase (MII) (45). An RNA-rich domain and translational hotspots are also seen around the chromosomes after nuclear envelope breakdown following the resumption of meiosis (56). Xenopus oocyte spindles are also enriched for mRNAs, including mRNAs that encode proteins that are known to be important in spindle formation and function (4).
In addition to RNAs, proteins and phosphorylated variants of proteins are enriched at spindles (45). Phosphorylated ribosomal protein S6, a marker of active mRNA translation, is present around condensing chromosomes and on MII spindles in mouse oocytes (45). Phosphorylated variants of eukaryotic translation initiation factor 4E-binding protein 1 (eIF4E-BP1), a global inhibitor of cap-dependent RNA translation that is inactivated by phosphorylation, are enriched on oocyte spindles in a temporally and spatially dynamic manner (45).
Because eIF4E-BP1 binding to eIF4E and repressing cap-dependent mRNA translation (7) is the only known function of eIF4E-BP1, and because eIF4E-BP1 phosphorylation releases eIF4E to allow formation of the cap-dependent translation initiation complex, the above observations have been interpreted collectively to indicate ongoing translational control of spindle-associated mRNAs by eIF4E-BP1 phosphorylation to facilitate spindle formation and stability (45). Consistent with this model, injecting germinal vesicle (GV) oocytes with a dominant negative eIF4E-BP1 variant causes spindle defects at the MII stage (22). This provides a powerful means by which spindle formation and function could be controlled by and coordinated with cell cycle progression and other exogenous and endogenous signals and by which disruptions in the regulation could lead to aneuploidy. Understanding the mechanisms that regulate eIF4E-BP1 phosphorylation at the spindle should thus reveal key insight into mechanisms that enable the formation and ovulation of high-quality oocytes.
In somatic cells, eIF4E-BP1 phosphorylation is regulated by several upstream kinases in conjunction with cell cycle progression, hormone stimulation, and nutrient deprivation. Phosphorylation of eIF4E-BP1 at four sites increases in response to insulin stimulation, and at least two of these sites are phosphorylated by the mechanistic target of rapamycin (mTOR) (14, 17). Also during insulin stimulation, the ataxia telangiectasia mutated (ATM) kinase phosphorylates eIF4E-BP1 at S111 (68). The polo-like kinase 1 (PLK1) phosphorylates eIF4E-BP1 at S111 on the mitotic spindle in human HepG2 cells (49). Cyclin-dependent kinase 1 (CDK1) phosphorylates eIF4E-BP1 at up to four sites during mitosis (18, 51). Because mTOR, ATM, CDK1, and PLK1 are all present in the oocyte during meiosis, they are candidates to contribute to eIF4E-BP1 phosphorylation. Furthermore, these kinases are detected directly on oocyte meiotic spindles (10, 22, 25, 30). mTOR inhibition during MI disrupts spindle migration, formation of the actin cap, and formation of the spindle structure (56). ATM is expressed throughout oocyte maturation but has not been shown to phosphorylate eIF4E-BP1 during this time (30). In porcine oocytes, ATM inhibition inhibits germinal vesicle breakdown (GVBD) or PB1 (first polar body extrusion) (30). ATM−/− oocytes display meiotic failure and are infertile due to meiosis I arrest (66). CDK1 regulates meiotic resumption, kinetochore-microtubule spindle attachments, meiosis II metaphase, and polar body extrusion (2, 9, 36, 42). PLK1 becomes active immediately before GVBD, and its inhibition disrupts GVBD, bipolar meiotic spindle formation, microtubule-organizing center formation, chromosome segregation, and chromosome condensation (39, 53, 60).
To identify the specific kinases regulating eIF4E-BP1 phosphorylation in association with oocyte meiotic spindle formation, we examined the roles of individual upstream kinases in eIF4E-BP1 phosphorylation at the meiotic spindle during the first and second meiotic division of mouse oocytes. We found that PLK1 is the only upstream kinase tested that regulates eIF4E-BP1 phosphorylation at the spindle and that loss of the PLK1-mediated eIF4E-BP1 phosphorylation disrupts normal spindle formation and function. Additional roles for PLK1 are seen for microtubule polymerization. Inhibition of eIF4E-BP1 phosphorylation at the spindle reduces abundance of the key structural protein β-tubulin on the MI spindle, consistent with a role for eIF4E-BP1 phosphorylation in oocyte spindle formation. Collectively, these observations reveal PLK1 as a key regulatory nexus of mammalian oocyte spindle formation, stability, and function operating through eIF4E-BP1 and presumably its attendant control over the production of spindle-associated proteins.
MATERIALS AND METHODS
Oocyte isolation and culture.
(B6D2)F1 females were obtained from Jackson Laboratories at 7 wk age and used from 8 to 12 wk age. Germinal vesicle (GV) stage oocytes were collected from ovaries of females 46–48 h after intraperitoneal injection with 5 IU of equine chorionic gonadotropin (eCG; EMD Millipore: 367222). Ovaries were transferred to room temperature HEPES-buffered M2 media with 0.225M 3-isobutyl-1-methylxanthine (IBMX; Sigma: I7018), and cumulus-oocyte complexes (COCs) were released. Only GV stage oocytes with multiple rows of cumulus cells completely surrounding the oocyte were used. Denuded, abnormal, and dying oocytes were discarded. GV stage oocytes were cultured for 1 h in α-MEM (ThermoFisher: 12561072) with 20% fetal bovine serum (ThermoFisher: 16000044) and 0.225 M IBMX in 5% O2 and 5% CO2 that had been preequilibrated in a humidified atmosphere for ≥1 h. Attached cumulus cells were removed by pipetting using a narrow bore pipet with a 100-μm outer diameter. For in vitro maturation (IVM), GV stage COCs were released from IBMX treatment by washing and culturing in α-MEM with 20% FBS. In vivo-matured MII stage oocytes were collected from females superovulated with 5 IU eCG, followed 48 h later by 5 IU of human chorionic gonadotropin (hCG; Sigma-Aldrich: C1063), at 14–16 h post-hCG injection. MII stage COCs were released in M2 medium, and cumulus cells removed using hyaluronidase at 300 μg/ml (Sigma: H4272), and oocytes were cultured in potassium simplex optimized medium (KSOM) (19, 27) in a humidified atmosphere, as described above. For each experimental replicate, GV or MII oocytes were collected from 5 BDF1 mice, pooled, and randomly assigned to either DMSO or inhibitor treatment groups; therefore, each replicate represents five independent mice. All BDF1 mice in this study were from the same source and are genetically identical. All studies were approved by the Michigan State University Institutional Animal Care and Use Committee, consistent with National Institutes of Health’s Guide for the Care of Use of Laboratory Animals, and with the Association for Assessment and Accreditation of Laboratory Animal Care accreditation.
Inhibitor treatments.
The PLK1 inhibitor BI2536 (Selleckchem: S1109) was applied at a concentration of 500 nM, as in previous studies that showed effective PLK1 inhibition in mouse oocytes (3, 8, 53). The ATM inhibitor KU55933 (Selleckchem: S1092) was applied at a concentration of 10 μM, a concentration used previously in mouse embryos and porcine oocytes (38, 63). The mTOR inhibitor Torin 1 (Selleckchem: S2827), was applied at 1 μM, an effective concentration in mouse oocytes (67). The eIF4E-BP1 inhibitor 4EGI was applied at 100 μM, as described for mouse oocytes (56). All inhibitors except 4EGI were diluted in α-MEM with 20% fetal bovine serum at a 1:1,000 concentration. Vehicle-treated control received DMSO at a concentration of 1:1,000. 4EGI was diluted 1:250 in the same medium, and DMSO controls were treated with DMSO at 1:250. GV stage oocytes were washed through several droplets of media to remove IBMX and then treated for 7 h to permit progression to MI and an opportunity for spindle formation. For MII oocytes, inhibitors were diluted in KSOM medium, and oocytes were rinsed through several droplets. MII oocytes were treated for 3 h. The efficacy of the Torin 1 and KU55933 treatments was also verified in mouse embryonic fibroblasts (MEFs) to ensure that inhibitor treatment was successfully inhibiting mTOR and ATM, respectively. To validate Torin 1 activity, (B6D2)F1 MEFs were treated with 1 μM Torin 1 or 1:1,000 DMSO vehicle for 1 h and stained using oocyte immunofluorescence protocol (data not shown). To validate KU55933 activity, (B6D2)F1 mouse embryonic fibroblasts were pretreated with 10 μM KU55933 or 1:1,000 DMSO for 3 h and then treated with 10 μM KU55933 only, 50 μM etoposide only, or 10 μm KU55933 and 50 μM etoposide simultaneously and stained using oocyte immunofluorescence protocol (data not shown).
Oocyte fixation and immunofluorescence.
For fixation, zonae pellucidae were removed by treating in acidified Tyrode’s buffer (61) for ∼30 s, followed by immediate washing through M2 media for 1 min. To remove any bovine serum albumin (BSA), oocytes were rinsed briefly through 0.4% polyvinyl alcohol (PVA)-PBS before fixation in 4% paraformaldehyde/PBS (pH 7.0; Sigma-Aldrich: P6148). Fixed oocytes were rinsed with 0.4% PVA/PBS and either stored in 0.4% PVA-PBS at 4° or processed immediately.
Immunofluorescence detection of target proteins was performed in NUNC four-well dishes (Sigma Aldrich: 179830), using 750 μl of each solution. With the exception of overnight incubation with primary antibody, all steps were performed at room temperature. Oocytes were permeabilized for 30 min in PBS containing 0.1% Triton X-100 (Fisher Scientific: BP151) and then moved to blocking buffer [PBS with 0.1% BSA (Sigma-Aldrich: A9418), 0.01% Tween-20 (Sigma-Aldrich: P9416), and 0.02% (Sigma-Aldrich: S8032)] for 1 h. Primary and secondary antibodies were diluted in blocking buffer. Primary antibodies included phospho-S64-eIF4E-BP1 (Cell Signaling Technology: 9451S, diluted 1:50), phospho-S111-eIF4E-BP1 (Abgent: AP3473a, diluted 1:50), PLK1 (Sigma-Aldrich: SAB1404220, diluted 1:100), β-tubulin (Santa Cruz Biotechnology: sc-9935, diluted 1:200), nuclear mitotic apparatus protein 1 (NuMA; Santa Cruz Biotechnology: sc-51164, diluted 1:500), and pan-eIF4E-BP1 (Cell Signaling Technology: 9452, diluted 1:50). Torin 1- and KU55933-treated MEFs were stained for phospho-235/236-S6-kinase (Cell Signaling Technology: 4858P, diluted 1:100) and phospho-S15-p53 (Cell Signaling Technology: 9286, diluted 1:400). Specificity of the polyclonal phospho-S64-eIF4E-BP1 antibody has been established in earlier studies (12, 13, 15, 33, 65). Specificities of the PLK1, β-tubulin, NuMA, phospho-235/236-S6-kinase, and phospho-S15-P53, and pan-eIF4E-BP1 antibodies have all been previously validated by Western blotting (5, 16, 32, 52, 59, 69). Phospho-S111-eIF4E-BP1 (Abgent) has been used extensively by our laboratory in the past (45) and was further validated using a second, independent phosphor-S111-eIF4E-BP1 antibody (MyBioSource MBS9210664). Specificities of the primary antibodies were also confirmed by replicating staining patterns using another primary antibody from a different vendor (data not shown). Oocytes were incubated in primary antibodies overnight at 4°C. Oocytes were then washed three times in blocking buffer for 10 min per wash and incubated in secondary antibodies for 1 h at room temperature. Secondary antibodies included donkey anti-rabbit-Alexa 594 (Abcam: ab150076, diluted 1:300), donkey anti-goat-Alexa 594 (Abcam: ab150132, diluted 1:300), donkey anti-mouse-Alexa 488 (Abcam: ab150109, diluted 1:500), and donkey anti-goat-Alexa 488 (Abcam: ab150129, diluted 1:1000). Controls lacking primary antibody treatment were tested for each secondary antibody and yielded no detectable spindle signal (data not shown). After secondary antibody incubation, oocytes were washed three times in blocking buffer and mounted on slides in 11 μl Vectashield mounting solution with DAPI (Vector: H-1200) and on slides with coverslips, and the coverslip edges were sealed with nail polish.
Confocal microscopy and immunofluorescence image analysis.
Immunofluorecence confocal microscopy (IFCM) was performed on an Olympus FluoView FV1000 confocal laser scanning microscope with a ×40 1.25 NA oil objective, using ×2 zoom and a 1-μm step size. The Olympus software is the Olympus FluoView FV1000 Advanced Software (FV10-ASW) version 4.2. Signal detection was provided through the software using photomultiplier tube detection. For DAPI excitation, the sampled was excited with a 405-nm laser. For Alexa 488 excitation, each sample was excited with a 488-nm laser. For 594 excitation, each sampled was excited with a 543-nm laser. All settings were kept constant within groups. Spindle images were compiled as Z-projections of all slices containing signal at the spindle (5–10 1-μM slices). All image analysis was done using ImageJ (47).
Spindle and cytoskeleton protein analyses.
With 4EGI treatment, lagging chromosomes were quantified at 7 h of IVM as chromosomes not overlapping with other chromosomes at the metaphase plate of the MI spindle. The number of oocytes with and without lagging chromosomes was compared between control and inhibitor-treated groups. Spindles were analyzed only for lagging chromosomes and β-tubulin when oriented perpendicular to the confocal laser and only if DAPI stain intensity was equal between the treated and vehicle control groups in each experimental run, thereby minimizing potential imaging artifacts resulting from part of the spindle being out of the visualization plane. The intensity of β-tubulin on MI spindles was measured after immunofluorescence confocal microscopy and image analysis, using Image J to measure the average pixel intensity in the region of interest normalized to average background fluorescence in an adjacent region of same size. Since the MII spindle is stably arrested, a fair comparison can be made between the size of MII spindles, and this can be taken into account to calculate total β-tubulin. Therefore, in MII spindle studies, apparent spindle area when viewing the Z-stack (henceforth denoted “spindle area”) was also measured. For quantification of total β-tubulin intensity at the MII midzone, the average pixel intensity for the region containing the metaphase plate was quantified, normalized to background by dividing the average midzone intensity by the average intensity values for an equal-sized region over the nearby ooplasm (cytoplasm within oocyte), and then multiplied by the area. Quantification of total β-tubulin on the MII spindle was done the same way, but the area measured contained the entire MII spindle.
Chromosome congression was examined in oocytes treated with BI2536 either during IVM to the MI metaphase stage or in matured MII oocytes. Spindles were scored as displaying failed chromosome congression (FCC) following BI2536 treatment either if they had apparent metaphase plates but some chromosomes were outside of the apparent spindle structure or if they displayed no obvious metaphase plate due to nearly complete failure of chromosome congression. Spindles with lagging chromosomes contained within the image area demarcated by the spindle structure were not scored as having failed chromosome congression. NUMA intensity was quantified by measuring NuMA intensity at the spindle pole regions, normalized to the background by dividing by average intensity values for an equal-sized region over the nearby ooplasm, and the averaged together. The association of total eIF4E-BP1, phosphorylated eIF4E-BP1 variants, and PLK1 with spindles was examined by IFCM. The presence of eIF4E-BP1-P-S64, eIF4E-BP1-P-S111, and PLK1 was scored solely as present or absent, except in the case of MIIs treated with Torin 1 or DMSO. Intensity of phospho-eIF4E-BP1 variants and PLK1 was measured at MII spindle poles in both DMSO and Torin 1 treatment groups, and the signal was normalized to the background. Total eIF4E-BP1 was examined at spindle poles in 7–12 individual 1-μm confocal sections spanning each entire spindle and in Z-stacks of those sections combined to assess possible changes in at the spindle poles. Microtubule flaring was quantified at MII only and was the presence of microtubules attached to spindle poles at one end, but the other end was not oriented toward the spindle. Microtubule hyperpolarization was quantified at MI and MII and was the presence of microtubules present but not attached to the spindle.
Statistical analysis.
Statistical analyses were performed using Prism GraphPad version 7.01 for windows (www.graphpad.com; GraphPad Software, La Jolla CA). An unpaired t-test or Mann-Whitney U-test was used as indicated to calculate differences between groups. A difference of P < 0.05 was considered significant. Error bars shown represent standard deviation (SD).
RESULTS
Confirmation of role for eIF4E-BP1 in spindle formation.
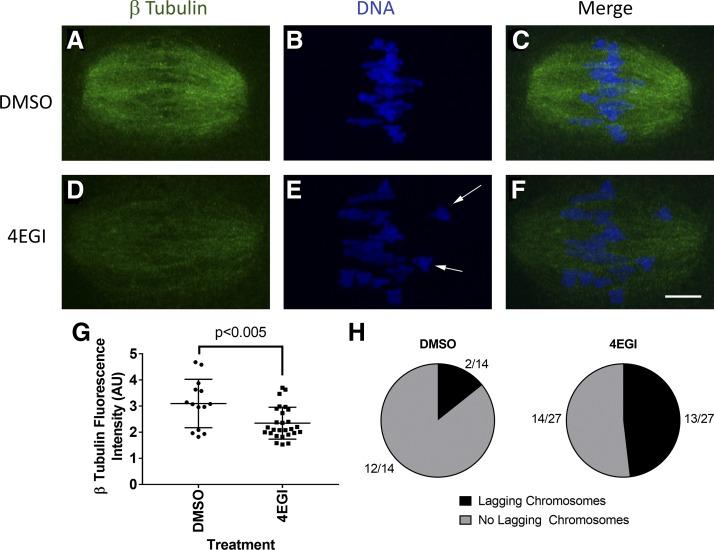
Preventing eIF4E-BP1 phosphorylation and release of eIF4E to bind eIF·4G could disrupt spindle formation and function. Consistent with this, a previous study (56) reported disruption in spindle formation and chromosome congression using the specific inhibitor 4EGI, which mimics hypophosphorylated eIF4E-BP1 and binds to eIF4E to inhibit formation of cap-dependent initiation complexes (37). To confirm this effect for the system used here, we cultured (B6D2)F1 mouse GV oocytes for 7 h in the presence of 4EGI and imaged MI spindles to assess any defects in β-tubulin and chromosome congression. The total amount of β-tubulin on MI spindles was decreased by an average of 34% (DMSO, n = 14; 4EGI, n = 27 oocytes; P < 0.005) with the 4EGI treatment, consistent with reduced production of β-tubulin for spindle formation (Fig. 1, A–G). The frequency of lagging chromosomes was increased in the 4EGI-treated oocytes by more than fourfold from 14% (n = 2 of 14) in the DMSO control to 54% (n = 13 of 24) with 4EGI treatment (Fig. 1H). These results confirm a role for eIF4E-BP1 in regulating mRNA translation initiation via eIF4E binding and the need for release of eIF4E-BP1 from eIF4E to allow efficient spindle formation and function.
Fig. 1.
Decreased β-tubulin intensity and lagging chromosomes on mouse oocyte MI (metaphase I) spindle after 7-h 4EGI treatment observed by immunofluorecence confocal microscopy (IFCM). Germinal vesicle (GV) oocytes were matured in DMSO or 4EGI for 7 h in vitro, fixed, and immunostained. A–C: MI oocytes matured in DMSO vehicle control. D–F: MI oocytes matured in 4EGI inhibitor. A and D show the β-tubulin innunoreactive signal. B and E show DNA observed by fluorescent DAPI staining. C and F are the merged images, in which β-tubulin is shown in green and DNA is shown in blue. White arrows denote lagging chromosomes. G: quantitative analysis of β-tubulin fluorescence intensity on MI spindle after 4EGI treatment (P = 0.003 using a one-tailed t-test with equal variance, error bars = SD; DMSO, n = 14, 4EGI, n = 27). H: no. of oocytes in each treatment group with lagging chromosomes present was quantified. Oocytes were collected from 5 BDF1 mice, pooled, and randomly assigned to either the DMSO or 4EGI treatment group; this was repeated for 2 replicates. Bar, 10 μm.
Upstream kinases affecting eIF4E-BP1 phosphorylation and formation of the first (MI) meiotic spindle.
To understand how eIF4E-BP1 is regulated and thereby couples spindle formation to other events in the cell, it is essential to determine the upstream kinases that control eIF4E-BP1 phosphorylation, localization, and activity. eIF4E-BP1 phosphorylation events observed in cultured cells and mitotic cell extracts may have little relevance to processes occurring during oocyte meiosis. Additionally, first and second meiotic divisions are markedly different, making it necessary to examine eIF4E-BP1 regulation during both divisions. We used a series of highly specific kinase inhibitors to test for involvement of individual upstream kinases in eIF4E-BP1 phosphorylation on the first meiotic spindle and attendant effects on spindle properties and chromosome congression.
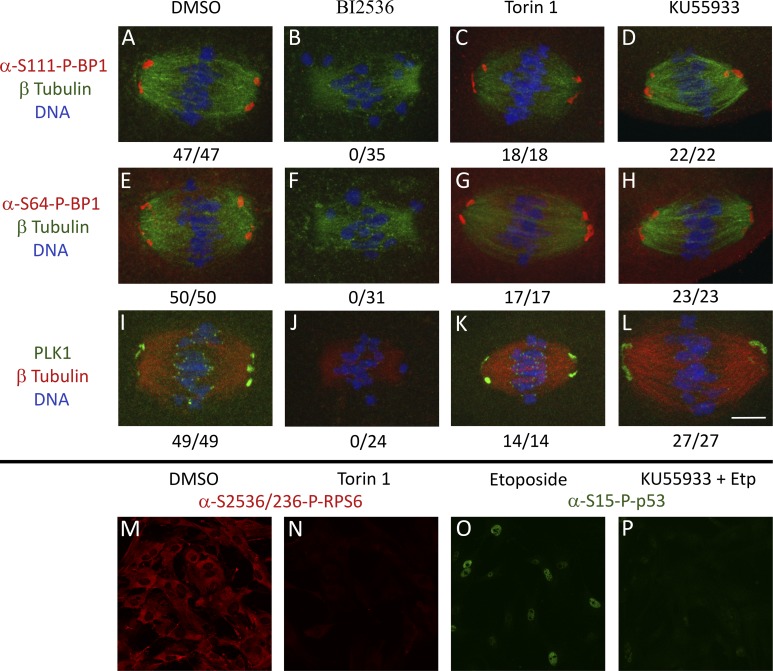
Our first target kinase for analysis was PLK1, which earlier studies showed is involved in mitotic and meiotic spindle formation (8, 62). PLK1 directly phosphorylates eIF4E-BP1 at S111 during mitosis on the spindle (49). Treatment of oocytes during IVM with BI2536 completely eliminated eIF4E-BP1-P-S111 at the spindle poles (n = 35 oocytes; Fig. 2B). Another site, eIF4E-BP1-P-S64, is phosphorylated by CDK1, which is activated by PLK1 in oocytes (18, 39). As with eIF4E-BP1-P-S111, spindle-associated eIF4E-BP1-P-S64 was completely eliminated with BI2536 treatment (n = 31 oocytes; Fig. 2F). No change in spindle-associated eIF4E-BP1-P-S111 or eIF4E-BP1-P-S64 was seen in DMSO negative control oocytes (n = 47 and n = 50 oocytes affected, respectively) (Fig. 2, A and E).
Fig. 2.
Localization of eukaryotic translation initiation factor 4E-binding protein 1 (eIF4E-BP1)-P-S64, eIF4E-BP1-P-S111, and polo-like kinase 1 (PLK1) signal mouse oocyte MI spindle after DMSO, BI2536, Torin 1, and KU55933 treatment observed by IFCM. A–L: GV oocytes were matured in DMSO, BI2536, Torin 1, or KU55933 for 7 h in vitro, fixed, and immunostained. A–D: eIF4E-BP1-P-S111 spindle localization in MI oocytes matured in DMSO, BI2536, Torin 1, or KU55933. E–H: eIF4E-BP1-P-S64 spindle localization in MI oocytes matured in DMSO, BI2536, Torin 1, or KU55933. I–L: PLK1 spindle localization MI oocytes matured in DMSO, BI2536, Torin 1, or KU55933. Quantification for no. of oocytes with target protein localized to spindle out of total oocytes quantified is shown below each image. Oocytes were collected from 5 BDF1 mice, pooled, and randomly assigned to either DMSO or inhibitor treatment groups; this was repeated for a minimum of 2 replicates. Bar, 10 μm. M–P: efficacy of Torin 1 and KU55933 inhibitor as tested in mouse embryonic fibroblasts (MEFs).
BI2536 treatment also resulted in a loss of PLK1 on all MI spindles (n = 24), but PLK1 remained present on all MI spindles in DMSO-treated controls (n = 49) (Fig. 2, J and I, respectively). The loss of PLK1 from the first meiotic spindle after inhibition indicates successful PLK1 inhibition, as PLK1 must be active to be tethered to the spindle structure (28, 29).
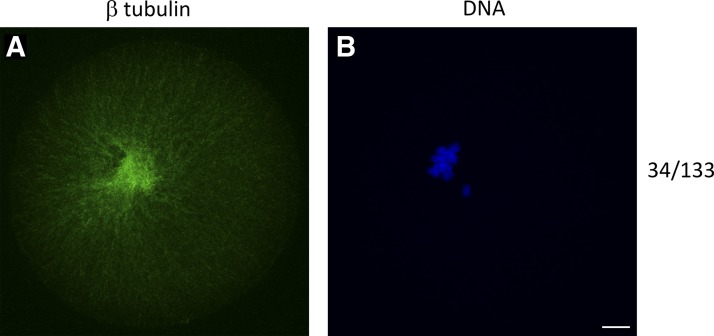
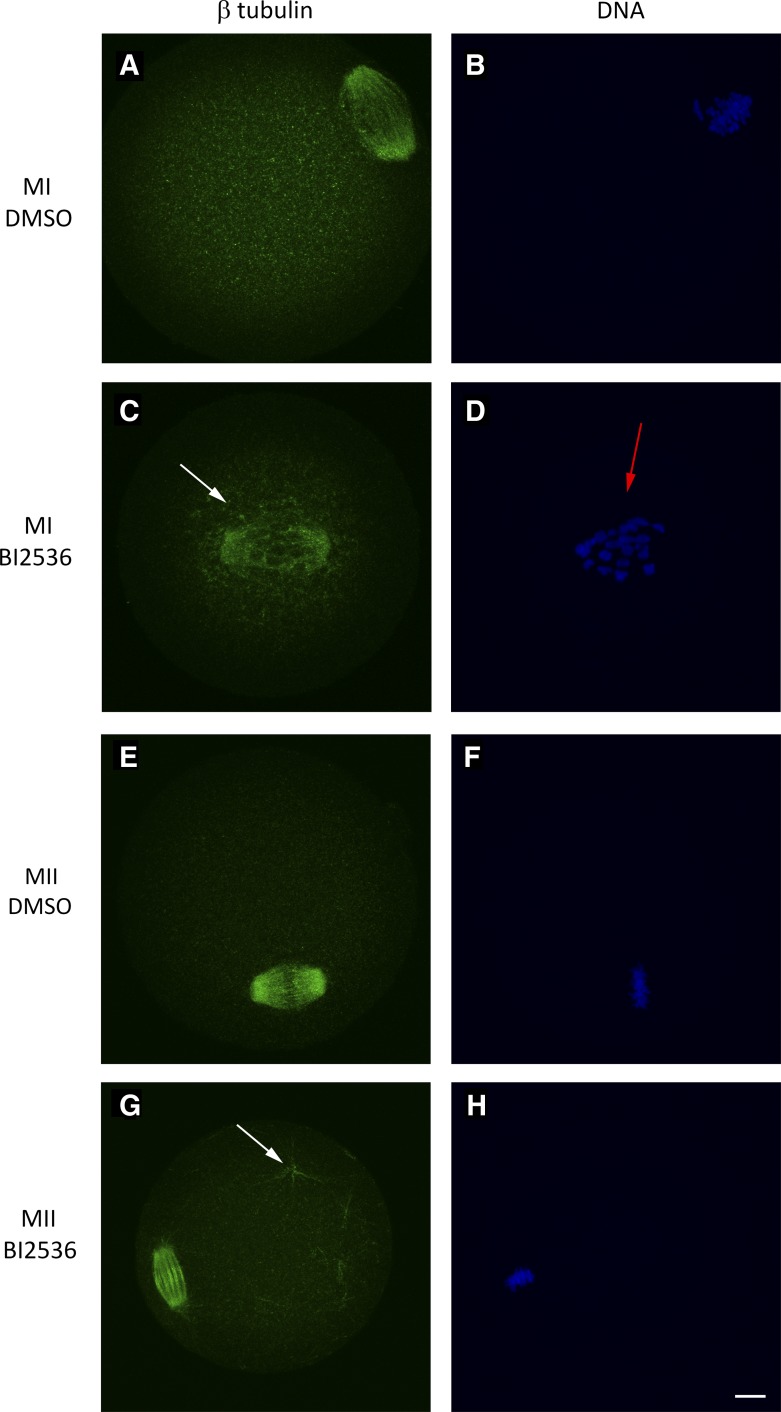
BI2536 treatment caused pronounced spindle defects after 7 h of IVM. We observed a complete failure of a bipolar spindle formation in 24.8% of the total MI oocytes quantified (n = 34 of 133), a phenotype not seen in the DMSO treatment group (n = 154) (Fig. 3 and Table 1). Nearly all (n = 98 of 99) of the remaining spindles displayed failed chromosome congression (FCC). No FCC was seen in the DMSO treatment group (n = 154; Table 1). As with the 4EGI treatment, BI2536 treatment also resulted in a significant decrease in average β-tubulin intensity on the MII spindle (Fig. 4). These results illustrate a strong negative effect of PLK1 inhibition on spindle formation and chromosome congression that is associated with loss of PLK1 from the spindle, loss of spindle-associated PLK1-mediated eIF4E-BP1-P-S111 phosphorylation, and loss of spindle-associated PLK1-regulated CDK1-mediated eIF4E-BP1-P-S64 phosphorylation.
Fig. 3.
Spindle failure phenotype on mouse MI oocyte after 7-h BI2536 treatment during in vitro maturation observed by IFCM. GV oocytes were matured in BI2536 for 7 h in vitro, fixed, and immunostained. A and B: MI oocytes matured in BI2536. A: β-tubulin innunoreactive signal. B: DNA observed by fluorescent DAPI staining. Quantification of oocytes with no bipolar MI spindle after BI2536 treatment shown on far right. Oocytes were collected from 5 BDF1 mice, pooled, and randomly assigned to either DMSO or inhibitor treatment groups; this was repeated for a minimum of 5 replicates. Bar, 10 μm.
Table 1.
Quantification of failed chromosome congression to MI spindles after 7 h of treatment
| Treatment | Total MI Oocytes | Spindle Failure | n, Spindles | Failed Chromosome Congression |
|---|---|---|---|---|
| DMSO | 154 | 0 | 154 | 0/154 |
| BI2536 | 133 | 34 | 99 | 98/99 |
| Torin 1 | 15 | 0 | 15 | 0/15 |
| KU55933 | 21 | 0 | 21 | 0/21 |
MI, metaphase I.
Fig. 4.
Decreased β-tubulin intensity on mouse oocyte MI spindle after 7-h BI2536 treatment. Average β-tubulin intensity was measured on MI spindle after DMSO, BI2536, Torin 1, and KU55933 treatments, and the signal was normalized to the background. Inhibitor treatment groups were all compared with the DMSO control. BI2536 treatment significantly decreased average β-tubulin fluorescence on MI spindle. P < 0.0001 determined using a Mann-Whitney nonparametric test. Error bars = SD (DMSO, n = 19; BI2536, n = 23; Torin 1, n = 9; KU55933, n = 16).
The second upstream kinase we tested was mTOR, which regulates eIF4E-BP1 in interphase cells in response to insulin stimulation, nutrient restriction, and other treatments (1, 17). mTOR phosphorylates eIF4E-BP1 at T36/45 during insulin stimulation and nutrient restriction (14, 17) and may also impact eIF4E-BP1-P-S111 and eIF4E-BP1-P-S64 indirectly through interactions with other upstream kinases or by regulating the priming T36/45 phosphorylation. eIF4E-BP1-P-S64 (n = 17) and eIF4E-BP1-P-S111 (n = 18) were unaffected at spindle poles by Torin 1 treatment (Fig. 2, C and G). PLK1 was also unaffected by Torin 1 treatment (n = 14/14) (Fig. 2K). mTOR inhibition did not yield any spindle defects or a decrease in average β-tubulin intensity (Fig. 4). Chromosomes still congressed (n = 15) to the metaphase plate by 7 h of IVM, and β-tubulin was still highly expressed and organized similarly to the DMSO vehicle controls (Table 1). The efficacy of the Torin 1 inhibitor was validated in MEFs (Fig. 2, M and N). These results indicate that mTOR inhibition has no significant effect on spindle formation and function during first meiosis and no effect on phosphorylation at p-S111 or p-S64 forms of eIF4E-BP1 associated with the spindle. The lack of an effect of Torin-1 indicates that mTOR activity is not required for a priming event to allow the CDK1-mediated eIF4E-BP1-S64 phosphorylation. These results indicate that mTOR is not a key regulator of eIF4E-BP1 phosphorylation on the first meiotic spindle.
The next upstream kinase we tested was ATM. ATM phosphorylates eIF4E-BP1 at S111 in other cell types (68). ATM inhibition by KU55933 treatment did not yield any abnormal spindle phenotypes or defects. Chromosomes still congressed to the metaphase plate by 7 h of IVM (n = 21), and β-tubulin was still expressed and organized similarly to the DMSO vehicle controls (Fig. 4 and Table 1). Both eIF4E-BP1-P-S64 (n = 22 of 22) and eIF4E-BP1-P-S111(n = 23 of 23) were unaffected at spindle poles by KU55933 treatment (Fig. 2, D and H). PLK1 likewise remained present at the spindle poles after KU55933 treatment (n = 27 of 27; Fig. 2L). However, ATM inhibition caused a partial diminishment of PLK1 staining at the kinetochores (Fig. 2L). The efficacy of the KU55933 inhibitor was validated in MEFs (Fig. 2, O and P). These results show that ATM does not directly regulate eIF4E-BP1 phosphorylation at the spindle pole but may have a slight effect by modulating PLK1 association with the kinetochores. The results also highlight key differences in ATM function between oocyte first meiosis and mitotic events in cultured cells.
Upstream kinases affecting eIF4E-BP1 phosphorylation and formation of the second (MII) meiotic spindle.
The above results revealed a major role for PLK1 in formation and function of the first meiotic spindle, and dramatic effects on spindle-associated phospho-eIF4E-BP1. Because the regulation of MI and MII spindle formation and their specific characteristics differ, we evaluated potential effects of PLK1 and other upstream kinases on stability and maintenance of the MII spindle in matured oocytes. Ovulated MII oocytes were treated for 3 h with each inhibitor or with DMSO as a negative control.
Similarly to effects on MI spindles during IVM, treatment with the PLK1 inhibitor BI2536 led to a complete loss of and eIF4E-BP1-P-S111 (n = 46) and eIF4E-BP1-P-S64 (n = 61) on the MII spindle (Fig. 5, B and F). There was no effect of DMSO treatment on eIF4E-BP1 phosphorylation in nearly all of the treated MII oocytes (n = 104 of 104 for P-S111 study, and n = 90 of 92 for P-S64 study) (Fig. 5, A and E). PLK1 staining remained present on spindle poles in all DMSO-treated control oocytes (n = 95) but was completely abolished in all BI2536 treated MII oocytes (n = 51) (Fig. 5, I and J).
Fig. 5.
Localization of eIF4E-BP1-P-S64, eIF4E-BP1-P-S111, and PLK1 signal mouse oocyte MII spindle after DMSO, BI2536, Torin 1, and KU55933 treatment observed by IFCM. A–L: MII oocytes were treated with DMSO, BI2536, Torin 1, or KU55933 for 3 h in vitro, fixed, and immunostained. A–D: eIF4E-BP1-P-S111 spindle localization in MII oocytes matured in DMSO, BI2536, Torin 1, or KU55933. E–H: eIF4E-BP1-P-S64 spindle localization in MII oocytes matured in DMSO, BI2536, Torin 1, or KU55933. I–L: PLK1 spindle localization in MII oocytes matured in DMSO, BI2536, Torin 1, or KU55933. M and N: MII oocytes treated with DMSO (M) or BI2536 (N) were stained for total eIF4E-BP1. This pan-eIF4E-BP1 staining was examined in each confocal section encompassing the spindle to determine whether void or difference in signal. Two representative Z-stacks are shown for each group. Quantification for no. of oocytes with target protein localized to spindle out of total oocytes quantified shown below each images. Oocytes were collected from 5 BDF1 mice, pooled, and randomly assigned to either DMSO or inhibitor treatment groups; this was repeated for a minimum of 2 replicates. Bar, 10 μm.
As previously shown, IFCM using an antibody to all eIF4E-BP1 forms revealed uniform staining across the ooplasm, including across the spindle pole region (Fig. 5, M and N) (45). The uniformity in distribution of total eIF4E-BP1 as compared with phosphorylated variants highlights the intense, selective phosphorylation of eIF4E-BP1 at the spindle poles. There was no diminishment of total eIF4E-BP1 fluorescence at the spindle pole region with BI2536 treatment, indicating that there is no large void in staining associated with treated spindle pole regions, which would be indicative of a large loss of total eIF4E-BP1 protein from the region and retention of dephosporylated eIF4E-BP1. However, because the pan-eIF4E-BP1 antibody yielded high uniform staining across the spindle pole region and surrounding ooplasm without the same intense foci seen with the phospho-specific antibodies, we cannot rule out that loss of eIF4E-BP1-P-S64 and eIF4E-BP1-P-S111 with PLK1 inhibition is due to release of previously phosphorylated peptide from the spindle pole region, rather than eIF4E-BP1 dephosphorylation and retention, or steady-state turnover without phosphorylation of newly acquired eIF4E-BP1 protein.
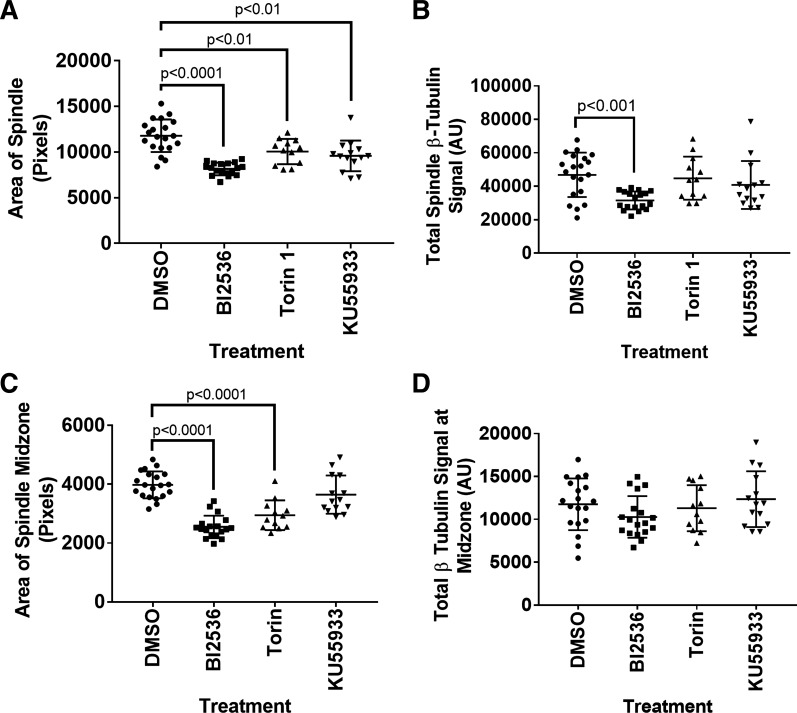
Because oocytes are arrested in the second metaphase when ovulated, we could also compare effects of the inhibitors on spindle area and use this to calculate the effects on total β-tubulin. All inhibitors resulted in a significant decrease in total MII spindle area, but only BI2536 resulted in a decrease in total MII spindle β-tubulin signal (DMSO, n = 20; BI2536, n = 18; Torin 1, n = 12; KU55933, n = 14; Fig. 6, A and B). We then quantified that spindle midzone area and found that it was significantly decreased after Torin 1 and BI2536 treatments (DMSO, n = 20; BI2536, n = 18; Torin 1, n = 12; KU55933, n = 14; Fig. 6C). We initially noted an increase in the average β-tubulin fluorescence at the spindle midzone, but when an area is included to calculate total β-tubulin, the change in total β-tubulin at the midzone is not significant after any of the treatments (DMSO, n = 20; BI2536, n = 18; Torin 1, n = 12; KU55933, n = 14; Fig. 6 C and D).
Fig. 6.
Total β-tubulin decreased on MII spindle after 3-h BI2536 treatment. Quantitative analysis of MII spindle characteristics after DMSO, BI2536, Torin 1, or KU55933 treatment. A: quantification of total MII spindle area. B: total MII spindle β-tubulin signal. C: quantification of MII spindle midzone area. D: total MII spindle midzone β-tubulin signal. P values listed in each graph were determined using a Mann-Whitney nonparametric test. Error bars = SD (DMSO, n = 20; BI2536, n = 18; Torin 1, n = 12; KU55933, n = 14).
To test whether loss of phospho-eIF4E-BP1 and PLK1 from the MII spindle poles could be an indirect effect of overall loss of spindle pole structures, we compared NuMA staining between treated and vehicle control MII oocytes (Fig. 7, A and B). NuMA is a commonly used marker of spindle poles. NuMA fluorescence intensity remained unchanged in BI2536-treated oocytes (n = 11) compared with vehicle controls (n = 10) (Fig. 7C), consistent with a previous study (20). There was also no apparent effect of BI2536 on the maintenance of chromosome congression at the metaphase plates of MII spindles, further indicating stable spindle structures throughout the 3 h of treatment.
Fig. 7.
Nuclear mitotic apparatus protein 1 (NuMA) signal unaffected at MII spindle poles after 3-h BI2536 treatment observed by IFCM. MII oocytes were treated with DMSO or BI2536 for 3 h in vitro, fixed, and immunostained. NuMA was quantified at each spindle pole (arrowheads) and normalized to nearby background signal (arrows). A: NuMA signal in MII spindle treated with DMSO. B: NuMA signal on MII spindle treated with BI2536. C: NuMA fluorescence intensity at MII spindle poles not significantly different between DMSO and BI2536-treated oocytes. P = 0.7045 determined using a Mann-Whitney nonparametric test. Error bars = SD (DMSO, n = 10; BI2536, n = 11). Bar, 10 μm.
We next tested whether treatment with mTOR inhibitor Torin 1 would affect eIF4E-BP1phosphorylation at the MII spindle. Torin had no effect on eIF4E-BP1-P-S111 presence (n = 33; Fig. 5C) or intensity on the MII spindle (Fig. 8A). Although eIF4E-BP1-P-S64 remained present on the MII spindle (n = 18 of 18; Fig. 5G), Torin 1 caused a (36%) decrease in the fluorescence intensity of eIF4E-BP1-P-S64 (Fig. 8B). Torin 1 had no effect on the presence (n = 32; Fig. 5K) or intensity of PLK1 on the MII spindle (Fig. 8C). As noted above, Torin 1 treatment also significantly decreased the MII spindle area and spindle midzone area (Fig. 6, A and C), indicating inhibitor efficacy in the oocyte. Torin 1 did not affect total β-tubulin signal on the MII spindle or at the spindle midzone (Fig. 6, B and D).
Fig. 8.
Torin 1 treatment has a slight effect on eIF4E-BP1-P-S64 but not -S111 phosphorylation on MII spindle. A: normalized eIF4E-BP1-P-S111 average fluorescence intensity on MII spindle poles after DMSO or Torin 1 treatment. B: normalized eIF4E-BP1-P-S64 average fluorescence intensity on MII spindle poles after DMSO or Torin 1 treatment (P < 0.01, using unpaired t-test with equal variance). eIF4E-BP1-P-S64 is significantly decreased after Torin 1 treatment. C: normalized PLK1 fluorescence average intensity on MII spindle poles after DMSO or Torin 1 treatment. The nos. of oocytes analyzed are indicated for each treatment and phosphorylation site. Data are from 2 replicates of staining containing both DMSO controls and Torin 1-treated oocytes plus additional identical DMSO controls from other inhibitor studies. Error bars = SD.
Next, we tested whether ATM inhibition affects eIF4E-BP1 phosphorylation on MII spindles. KU55933 treatment had no significant effect on eIF4E-BP1 phosphorylation at the sites tested (n = 32 and n = 35 for S111 and S64, respectively; Fig. 5, D and H) and no effect on PLK1 (n = 44; Fig. 5L). As noted above, KU55933 treatment did significantly decrease the overall area of the MII spindle, indicating inhibitor efficacy in the oocyte (Fig. 6A), but did not affect spindle midzone area (Fig. 6C). KU55933 treatment did not affect total β-tubulin at on the MII spindle or at the spindle midzone (Fig. 5, B and D). In addition, MT flaring was not observed with KU55933 treatment (n = 15; Table 2). An effect on PLK1 presence at kinetochores on MII spindles could not be evaluated because PLK1 does not localize in discrete foci at kinetochores on MII spindles, as it does during MI.
Table 2.
Quantification of MT flaring on MII spindles after 3 h of treatment
| Treatment | n, Spindles | MT Flaring |
|---|---|---|
| DMSO | 126 | 0 of 126 |
| BI2536 | 122 | 121 of 122 |
| Torin 1 | 18 | 0 of 18 |
| KU55933 | 15 | 0 of 15 |
MT, microtubule; MII, metaphase II.
Additional effects of PLK1 inhibitor on oocyte cytoskeleton.
In addition to the striking effects of PLK1 inhibition on spindle-associated eIF4E-BP1-P-S64 and eIF4E-BP1-P-S111 at both MI and MII, effects on spindle structures, effects on chromosome congression at MI, and microtubule flaring at MII spindle poles, we noted striking effects on cytoplasmic microtubules, specifically microtubule hyperpolarization, defined as additional microtubules present outside of the spindle structure. After 7 h of treatment with BI2536, 47.4% of MI oocytes displayed microtubule (MT) hyperpolarization (n = 47 of 99), a phenotype seen in only 0.65% of DMSO-treated control oocytes (n = 1 of 154) and not seen in the Torin 1 (n = 15) or KU55933 treatment groups (n = 21) (Fig. 9, A–D, and Table 3). These additional microtubules formed in close proximity to the MI spindle and were not seen throughout the rest of the ooplasm. This particular microtubule configuration was not observed in MII stage oocytes. However, hyperpolarization was also seen in 73% MII stage oocytes treated with BI2536 (n = 89 of 122) and in a single DMSO control (0.79%, n = 1 of 126), but not in the Torin 1 (n = 18) or KU55933 (n = 15) group. At the MII stage, MT hyperpolarization was observed as microtubules spread throughout the cytoplasm, often appearing in aster-like structures (Fig. 9, E–H, and Table 4).
Fig. 9.
Microtubule (MT) hyperpolarization and failed chromosome congression present in MI and MII oocytes after BI2536 treatment observed by IFCM. β-Tubulin immunoreactive signal is shown in green. DNA was stained with DAPI and is shown in blue. A–D: GV oocytes were matured in DMSO or BI2536 for 7 h in vitro, fixed, and immunostained, as described in materials and methods. A and B: MI oocytes cultured in DMSO. C and D: MI oocytes matured in BI2536. White arrow denotes MT hyperpolarization present proximal to MI spindle (C); red arrow shows failed chromosome congression (D). E–H: MII oocytes were treated for 3 h in DMSO or BI2536, fixed, and immunostained. E and F: MII oocytes treated with DMSO. G and H: MI oocytes treated with BI2536. White arrow in G denotes MT hyperpolarization present in ooplasm. Bar, 10 μm.
Table 3.
Quantification of MT hyperpolarization in MI oocytes after 7 h of treatment
| Treatment | n, Spindles | MT Hyperpolarization |
|---|---|---|
| DMSO | 154 | 1 of 154 |
| BI2536 | 99 | 47 of 99 |
| Torin 1 | 15 | 0 of 15 |
| KU55933 | 21 | 0 of 21 |
MT, microtubule; MI, metaphase I.
Table 4.
Quantification of MT hyperpolarization in MII oocytes after 3 h of treatment
| Treatment | n, Spindles | MT Hyperpolarization |
|---|---|---|
| DMSO | 126 | 1 of 126 |
| BI2536 | 122 | 89 of 122 |
| Torin 1 | 18 | 0 of 18 |
| KU55933 | 15 | 0 of 15 |
MT, microtubule; MII, metaphase II.
DISCUSSION
Meiosis in oocytes is a complex process involving intricate coordination between endogenous and exogenous signals that allow for alternate phases of meiotic progression and arrest. Many of these signals converge to regulate spindle formation stability and function, which are essential for successful chromosome congression and segregation. Here we provide additional data on the regulated phosphorylation of eIF4E-BP1 and its association at the MI and MII spindle poles, extending previous studies of MII oocytes (45). More importantly, we demonstrate an overwhelmingly predominant role for PLK1 in controlling the presence of phsopho-eIF4E-BP1 at the spindle pole, spindle formation, and chromosome congression. Without disrupting spindle poles themselves, PLK1 inhibition eliminates eIF4E-BP1-P-S64 and eIF4E-BP1-P-S111 from the spindle and disrupts spindle properties and chromosome congression. Inhibition of mTOR had only a slight effect on eIF4E-BP1-S64 on the spindle, and only at the MII stage. ATM inhibition had no significant effect on eIF4E-BP1 phosphorylation. The limited impacts of mTOR and ATM inhibition exclude a major role for these kinases in regulating eIF4E-BP1 phosphorylation at the spindle. We also found that PLK1 regulates microtubule dynamics throughout the ooplasm, likely indicating coordination between spindle dynamics and broader ooplasm cytoskeletal dynamics.
Earlier studies reported that PLK1 is required for meiosis in oocytes (53) and mitosis in zygotes (3). Other studies revealed that PLK1 phosphorylates eIF4E-BP1 in cultured cells (49). The only known role of eIF4E-BP1 is binding to eIF4E and inhibiting cap-dependent translation (35), a function that is released by eIF4E-BP1 phosphorylation (40). The results here provide the first direct evidence that PLK1 is the principal regulator of eIF4E-BP1 phosphorylation and function at the oocyte spindle, identifying a new role for PLK1 during meiosis. Because PLK1 itself is regulated by other major upstream kinases [e.g., Aurora kinases (AURKs), CDK1] that also affect spindle formation (39, 48, 50), these results further indicate that PLK1 may act as a major regulatory nexus, coupling the control of spindle formation and stability to other endogenous cellular cues or exogenous signals acting through upstream pathways.
An accumulating body of literature indicates that localized mRNAs associated with spindles in oocytes and mitotic cells contribute to spindle formation by supporting the production of spindle component proteins (4, 45, 56). Additional studies revealed a temporally and spatially dynamic pattern of eIF4E-BP1 phosphorylation at MII stage spindles (45). However, the exact role of localized protein synthesis at the spindle has been difficult to resolve due to the difficulty in dissecting specific effects on spindles from broader effects in the ooplasm. In support of a role for localized protein synthesis, specific mRNAs enriched at the spindle have been identified along with spindle-enriched proteins encoded by some of these mRNAs (45), a localized RNA-rich domain coinciding with locally enriched protein synthesis activity has been reported (4, 56), and spindles contain phosphorylated S6 kinase, a marker of active protein translation (4, 45). Previous studies with the 4EGI inhibitor supported a role for localized translation in formation and function of meiotic spindles (56). Our results extend this result by demonstrating that release of eIF4E-BP1 translation inhibition at the MI spindle facilitates the production of the critical spindle protein β-tubulin, the mRNA for which is enriched at spindles (4) and is required for correct chromosome congression and spindle function. Images from previous study utilizing 4EGI inhibition indicated that an effect on β-tubulin was present on MI spindles, but that group did not quantify or note the decreased β-tubulin (56). In addition to decreased β-tubulin, the 4EGI treatment also caused an increase in the number of lagging chromosomes at the MI spindle. Although some phospho-eIF4E-BP1 is present in the ooplasm at the GV stage, the intense signals specifically associated with the spindle poles and the impact of 4EGI treatment on the spindle structure indicate local effects of inhibiting eIF4E-BP1 phosphorylation at the spindle, as opposed to nonspecific effects elsewhere in the cell.
We acknowledge that, although we did not observe a gross diminishment of pan-eIF4E-BP1 staining at the spindle pole region, a loss of eIF4E-BP1 from the specific foci of staining at the spindle poles might occur, as opposed to dephosphorylation and retention of spindle pole-associated eIF4E-BP1 or steady-state turnover and replacement without phosphorylation of newly acquired eIF4E-BP1. The first scenario, however, would be expected to result in translational activation, because the only known function for eIF4E-BP1 is to bind eIF4E and inhibit translation so that its loss would release this inhibition. The latter two scenarios would result in translational inhibition locally for mRNAs enriched at the spindle and associated with eIF4E-BP1. The reduction in tubulin staining observed with PLK1 inhibition was phenocopied by 4EGI treatment and is thus most consistent with translational inhibition, leading to deficiency of tubulin production at the spindle-forming region. This effect of PLK1 inhibition argues against the scenario of general eIF4E-BP1 loss and in favor of either of the latter two scenarios involving diminished eIF4E-BP1 phosphorylation and a resulting translation inhibition. One caveat to this interpretation would be that PLK1 inhibition might lead to sequestration of eIF4E by eIF4E-BP1 elsewhere in the cell, which could inhibit translation. Such sequestration has been reported only for nuclei (irrelevant for MI and MII cocytes) and cytoplasmic granules; we did not observe any increase in eIF4E-BP1 cytoplasmic granules (54). The negative effect of dominant negative hypophosphorylated eIF4E-BP1 mimic on oocyte spindles (22) demonstrates a negative effect of a hypophosphorylated eIF4E-BP1 on local translation at the spindle pole and spindle disruption, which is also consistent with the latter interpretations. Previous studies demonstrated that PLK1 directly phosphorylates eIF4E-BP1 in vitro and that PLK1 and phospho-eIF4E-BP1 colocalize on mitotic spindles, where they are essential for regulating spindle integrity (49). The demonstration here that PLK1 and phospho-eIF4E-BP1 also colocalize to oocyte meiotic spindles and that PLK1 inhibition results in a loss of phosphorylated eIF4E-BP1 at the oocyte spindle poles and disrupts oocyte spindle function, taken with all of the other available data, are consistent with a role for PLK1 in promoting eIF4E-BP1 phosphorylation at the spindle to support spindle formation via translateon activation. Our observations thus extend the findings from mitotic cells to the oocyte.
A recent study implied that BI2536 did not affect eIF4E-BP1 phosphorylation at the spindle (22). But the data presented in that paper do not address eIF4E-BP1 phosphorylation at the spindle. BI2536 was applied for a brief period of just 2 h starting at just 1 h after release from IBMX, a time before MI spindle formation (7 h post-release), so that direct examination of spindle-associated eIF4E-BP1 phosphorylation was not possible. Western blotting data for the effects of PLK1 inhibition did not use phosphospecific antibodies, and PLK1 inhibition was not verified. Of note, however, is that the possible persistence of phospho-eIF4E-BP1 at the level of whole oocytes reported there is consistent with our evidence for an effect of PLK1 inhibition on eIF4E-BP1 phosphorylation specifically at the spindle poles. Although PLK1 inhibition could also reduce S111 phosphorylation on cytoplasmic targets, the intense, spatially specific colocalization of eIF4E-BP1-P-S111 at the spindle poles with PLK1 is most easily explained by a localized role for PLK1 regulating eIF4E-BP1 phosphorylation and activity at the spindle poles.
PLK1 inhibition with BI2536 produced stronger effects that 4EGI treatment in some regards. For example, chromosome congression was more severely affected with BI2536 than with 4EGI. The enhanced impact of PLK1 inhibition may reflect direct effects of PLK1 on meiotic processes that are independent of eIF4E-BP1. PLK1 promotes kinetochore microtubule attachment, which likely accounts for a stronger effect of BI2536 on chromosome congression (10, 55). Despite these broader effects of PLK1, it is clear that PLK1 exerts the predominant effect on eIF4E-BP1 phosphorylation.
The effects of PLK1 inhibition observed on spindles were much more pronounced at MI than at MII, although PLK1 inhibition had a clear effect on β-tubulin expression at both stages. After PLK inhibition, the MII spindle displayed increased β-tubulin fluorescence intensity at the spindle midzone. This initially seemed inconsistent with our model of localized β-tubulin translation, but including spindle area as a variable and calculating total β-tubulin, we showed that the total amount of β-tubulin was still decreased. This change was consistent with a model of the spindle shrinking in toward the midzone, possibly as a result of the decreased localized β-tubulin translation. The dynamic nature of the MI spindle made spindle area comparison impossible, but the average β-tubulin intensity was decreased on the MI spindle after PLK1 inhibition. In addition to this, chromosome congression defects not seen at MII spindles were present at MI. This may simply reflect a greater effect of interfering with spindle formation when the inhibitors are applied before metaphase, as opposed to disrupting existing spindles in oocytes already at metaphase. Alternatively, the first and second meiotic divisions may be differentially susceptible to disruption of localized mRNA translation. Finally, PLK1 is known to regulate multiple events during meiosis and mitosis, and the prolonged treatment (7 h) may also affect MI spindle formation indirectly via targets in the ooplasm. This may also explain why the chromosome congression defects were more severe with PLK1 inhibition than with 4EGI inhibition, as PLK1 is likely affecting MI spindle formation in eIF4E-BP1-independent ways as well, such as by impairing microtubule-organizing center formation dynamics, which PLK1 regulates (8). The majority of aneuploidies detected in oocytes arise during the first meiotic division (23). Loss of sister chromatid cohesion also contributes to aneuploidy (5), but defects in spindle assembly may also contribute. Additionally, spindle assembly is driven by a Ran-GTP gradient emanating from the condensed chromosomes, but a Ran-GTP gradient-independent mechanism may also function at MI (11, 24). Because MI and MII spindle formation are regulated differently, PLK1 could differentially affect the two processes through a greater disruption of MI-specific events.
We observed no effect of mTOR or ATM inhibition on PLK1 localization at either the MI or MII stages and only a modest effect of mTOR inhibition on eIF4E-BP1-P-S64 signal exclusively on the MII stage spindle poles. mTOR regulates insulin signaling, which is dysregulated in diabetes, and diabetic mice have a variety of oocyte defects, including meiotic spindle abnormalities (57, 64). However, the hypothesis that mTOR regulates eIF4E-BP1 phosphorylation at the spindle and that this is disrupted in diabetic oocytes to negatively affect spindles is not supported by our results. The limited effects of mTOR inhibition on spindle-associated eIF4E-BP1-P-S64, lack of mTOR inhibition effect on eIF4E-BP1-P-S111, and the lack of spindle defects instead indicate that the adverse effects of maternal diabetes on meiotic spindle formation occur via an mTOR-independent mechanism or via an indirect effect of mTOR in the ooplasm (64). We note that a previous study concluded that mTOR may regulate localized translation in the oocyte (56) based on treatments with the mTOR inhibitor rapamycin. A recent study reported effects of rapamycin on eIF4E-BP1 phosphorylation, but the analysis was limited to Western blotting of whole oocytes and did not provide data for eIF4E-BP1 phosphorylation specifically at the spindle (22). It is also important to note that mTOR-mediated eIF4E-BP1 phosphorylation is resistant to rapamycin, and Torin 1 is a much more potent and specific inhibitor of this mTOR function (58), indicating that rapamycin effects on the spindle could occur through a mechanism not involving mTOR. Overall, the available data indicate that the roles of ATM and mTOR in regulating eIF4E-BP1 phosphorylation at the spindle are minor. mTOR may regulate eIF4E-BP1 phosphorylation within the ooplasm, leading to indirect spindle defects, but PLK1 is the predominant kinase regulating eIF4E-BP1 phosphorylation at the spindle poles.
The Aurora kinases (AURKs) are also important regulators of meiotic and mitotic spindle formation, and pan-AURK inhibitors result in meiotic spindle defects (50). AURKA is present at the spindle poles of meiotic MI and MII spindles (50). AURKA phosphorylates PLK1 at the start of mitosis (48). Aurora kinase B (AURKB) can be phosphorylated by PLK1 and colocalizes with PLK1 at the kinetochores of the meiotic MI spindle (6, 50) Therefore, AURKA may phosphorylate PLK1 at the MI and MII meiotic spindle poles, and PLK1 may phosphorylate AURKB at the MI kinetochores. The latter function of AURKB could explain the stronger effect of PLK1 inhibition on chromosome congression at MI that we observe. Additional upstream effects of AURKA on PLK1 would also provide functional connections between other upstream endogenous or exogenous signals and eIF4E-BP1 phosphorylation at the spindle.
CDK1 is another kinase that may work additively with PLK1 to regulate eIF4E-BP1 phosphorylation. Because global inhibition of CDK1 activity prevents germinal vesicle breakdown and causes oocyte activation at MII (21, 41, 46), the role of CDK1 in eIF4E-BP1 phosphorylation at the spindle cannot be tested directly through chemical inhibition or genetic ablation, although a role for CDK1 can be inferred from our data, as past studies of eIF4E-BP1 phosphorylation in cultured cells revealed a complex hierarchy wherein the T36/45 sites are priming phosphorylation sites and necessary for the subsequent S64 and T69 phosphorylations (14). The presence eIF4E-BP1-S64 (which CDK1 phosphorylates) on the MI and MII spindles and the lack of mTOR inhibitor effects on eIF4E-BP1 phosphorylation suggest that CDK1 likely works with PLK1 to regulate eIF4E-BP1 phosphorylation at the meiotic spindle (18). This interaction is further supported by the known autoamplification loop between PLK1 and maturation-promoting factor (a complex of cyclin B and CDK1) during meiosis (39). It remains unknown whether CDK1 is upstream or downstream or works in concert with PLK1. If CDK1 activation is downstream of PLK1, then PLK1 is likely regulating CDK1 activity solely at the spindle structure, as CDK1 is carefully regulated throughout the rest of the cytoplasm to regulate meiotic progression and PLK1 is localized only at high levels to the spindle structure (10).
The importance of the eIF4E-BP1-S111 phosphorylation site to eIF4E binding has remained unclear, with different results emerging from different experimental systems (17, 65). S111 could be an alternative priming site to promote phosphorylation at other sites, or it could directly affect eIF4E-BP1 binding to eIF4E (17, 65). Regardless of which of these functions is fulfilled by S111 phosphorylation, our data indicate that PLK1-mediated phosphorylation at this site may be essential for correct spindle formation and chromosome congression.
Our results highlight the specialized nature and complexity of the meiotic spindle structure in oocytes. First and second meiotic divisions in the oocyte differ in key respects, and both meiotic divisions differ from mitotic divisions. Similarly to a mitotic spindle, the first meiotic spindle forms in the center of the oocytes, but then the meiotic spindle moves to the cell cortex via an actin-dependent mechanism, an event that does not occur during mitosis. The MII spindle does not form in the oocyte center but instead forms in close proximity to the location of first polar body extrusion (31). The MI spindle does not arrest at the metaphase, whereas the MII spindle arrests at the metaphase until a sperm penetrates the oocyte (34), a potential explanation for why aneuploidies primarily result for incorrect chromosome segregation at MI, as there is not sufficient time to ensure correct homologous chromosome alignment (5). In addition, the first meiotic spindle requires the alignment and segregation of homologous chromosomes, whereas the second meiotic spindle is more similar to a mitotic spindle in that it is separating sister chromatids. With these key differences in mind, it is perhaps understandable that mammalian oocytes are predisposed to aneuploidy and sensitive to a range of factors such as maternal age, hormonal stimulation, nutrition, diabetes, obesity, disease, and environmental toxins. Additional future studies to dissect further the roles of PLK1 and eIF4E-BP1 in spindle dynamics may offer novel approaches to mitigate these negative effects on oocyte quality and fertility.
GRANTS
This work was supported in part by the Eunice Kennedy Shiver National Institute of Child Health and Human Development of the National Institutes of Health under the award no. T32-HD-087166, by MSU AgBioResearch, and by Michigan State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.S. and K.E.L. conceived and designed research; A.L.S. performed experiments; A.L.S. analyzed data; A.L.S. and K.E.L. interpreted results of experiments; A.L.S. prepared figures; A.L.S. and K.E.L. drafted manuscript; A.L.S. and K.E.L. edited and revised manuscript; A.L.S. and K.E.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Melinda Frame at the Michigan State University Center for Advanced Microscopy for exceptional confocal and image assistance on this project.
REFERENCES
- 1.Acosta-Jaquez HA, Keller JA, Foster KG, Ekim B, Soliman GA, Feener EP, Ballif BA, Fingar DC. Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol 29: 4308–4324, 2009. doi: 10.1128/MCB.01665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari D, Zheng W, Shen Y, Gorre N, Ning Y, Halet G, Kaldis P, Liu K. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum Mol Genet 21: 2476–2484, 2012. doi: 10.1093/hmg/dds061. [DOI] [PubMed] [Google Scholar]
- 3.Baran V, Solc P, Kovarikova V, Rehak P, Sutovsky P. Polo-like kinase 1 is essential for the first mitotic division in the mouse embryo. Mol Reprod Dev 80: 522–534, 2013. doi: 10.1002/mrd.22188. [DOI] [PubMed] [Google Scholar]
- 4.Blower MD, Feric E, Weis K, Heald R. Genome-wide analysis demonstrates conserved localization of messenger RNAs to mitotic microtubules. J Cell Biol 179: 1365–1373, 2007. doi: 10.1083/jcb.200705163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 20: 1522–1528, 2010. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu Y, Yao PY, Wang W, Wang D, Wang Z, Zhang L, Huang Y, Ke Y, Ding X, Yao X. Aurora B kinase activation requires survivin priming phosphorylation by PLK1. J Mol Cell Biol 3: 260–267, 2011. doi: 10.1093/jmcb/mjq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark BF, Grunberg-Manago M, Gupta NK, Hershey JWB, Hinnebusch AG, Jackson RJ, Maitra U, Mathews MB, Merrick WC, Rhoads RE, Sonenberg N, Spremulli LL, Trachsel H, Voorma HO. Prokaryotic and eukaryotic translation factors. Ad Hoc Nomenclature Subcommittee Report. Biochimie 78: 1119–1122, 1996. doi: 10.1016/S0300-9084(97)86738-7. [DOI] [PubMed] [Google Scholar]
- 8.Clift D, Schuh M. A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nat Commun 6: 7217, 2015. doi: 10.1038/ncomms8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davydenko O, Schultz RM, Lampson MA. Increased CDK1 activity determines the timing of kinetochore-microtubule attachments in meiosis I. J Cell Biol 202: 221–229, 2013. doi: 10.1083/jcb.201303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J, Cao Y, Wang Q, Zhang N, Liu X, Chen D, Liu X, Xu Q, Ma W. Unique subcellular distribution of phosphorylated Plk1 (Ser137 and Thr210) in mouse oocytes during meiotic division and pPlk1(Ser137) involvement in spindle formation and REC8 cleavage. Cell Cycle 14: 3566–3579, 2015. doi: 10.1080/15384101.2015.1100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumont J, Petri S, Pellegrin F, Terret ME, Bohnsack MT, Rassinier P, Georget V, Kalab P, Gruss OJ, Verlhac MH. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol 176: 295–305, 2007. doi: 10.1083/jcb.200605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellederova Z, Kovarova H, Melo-Sterza F, Livingstone M, Tomek W, Kubelka M. Suppression of translation during in vitro maturation of pig oocytes despite enhanced formation of cap-binding protein complex eIF4F and 4E-BP1 hyperphosphorylation. Mol Reprod Dev 73: 68–76, 2006. doi: 10.1002/mrd.20368. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca BD, Alain T, Finestone LK, Huang BP, Rolfe M, Jiang T, Yao Z, Hernandez G, Bennett CF, Proud CG. Pharmacological and genetic evaluation of proposed roles of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK), extracellular signal-regulated kinase (ERK), and p90(RSK) in the control of mTORC1 protein signaling by phorbol esters. J Biol Chem 286: 27111–27122, 2011. doi: 10.1074/jbc.M111.260794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 13: 1422–1437, 1999. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 15: 2852–2864, 2001. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gladding CM, Fan J, Zhang LY, Wang L, Xu J, Li EH, Lombroso PJ, Raymond LA. Alterations in STriatal-Enriched protein tyrosine Phosphatase expression, activation, and downstream signaling in early and late stages of the YAC128 Huntington’s disease mouse model. J Neurochem 130: 145–159, 2014. doi: 10.1111/jnc.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heesom KJ, Avison MB, Diggle TA, Denton RM. Insulin-stimulated kinase from rat fat cells that phosphorylates initiation factor 4E-binding protein 1 on the rapamycin-insensitive site (serine-111). Biochem J 336: 39–48, 1998. doi: 10.1042/bj3360039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heesom KJ, Gampel A, Mellor H, Denton RM. Cell cycle-dependent phosphorylation of the translational repressor eIF-4E binding protein-1 (4E-BP1). Curr Biol 11: 1374–1379, 2001. doi: 10.1016/S0960-9822(01)00422-5. [DOI] [PubMed] [Google Scholar]
- 19.Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev 41: 232–238, 1995. doi: 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- 20.Jang CY, Coppinger JA, Seki A, Yates JR III, Fang G. Plk1 and Aurora A regulate the depolymerase activity and the cellular localization of Kif2a. J Cell Sci 122: 1334–1341, 2009. doi: 10.1242/jcs.044321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang WI, Lin ZL, Lee SH, Namgoong S, Kim NH. A specific inhibitor of CDK1, RO-3306, reversibly arrests meiosis during in vitro maturation of porcine oocytes. Anim Reprod Sci 144: 102–108, 2014. doi: 10.1016/j.anireprosci.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Jansova D, Koncicka M, Tetkova A, Cerna R, Malik R, Del Llano E, Kubelka M, Susor A. Regulation of 4E-BP1 activity in the mammalian oocyte. Cell Cycle 16: 927–939, 2017. doi: 10.1080/15384101.2017.1295178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum Reprod Update 14: 143–158, 2008. doi: 10.1093/humupd/dmm043. [DOI] [PubMed] [Google Scholar]
- 24.Kaláb P, Solc P, Motlík J. The role of RanGTP gradient in vertebrate oocyte maturation. Results Probl Cell Differ 53: 235–267, 2011. doi: 10.1007/978-3-642-19065-0_12. [DOI] [PubMed] [Google Scholar]
- 25.Kogasaka Y, Hoshino Y, Hiradate Y, Tanemura K, Sato E. Distribution and association of mTOR with its cofactors, raptor and rictor, in cumulus cells and oocytes during meiotic maturation in mice. Mol Reprod Dev 80: 334–348, 2013. doi: 10.1002/mrd.22166. [DOI] [PubMed] [Google Scholar]
- 26.Latham KE, Garrels JI, Chang C, Solter D. Quantitative analysis of protein synthesis in mouse embryos. I. Extensive reprogramming at the one- and two-cell stages. Development 112: 921–932, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Lawitts JA, Biggers JD. Culture of preimplantation embryos. Methods Enzymol 225: 153–164, 1993. doi: 10.1016/0076-6879(93)25012-Q. [DOI] [PubMed] [Google Scholar]
- 28.Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci USA 95: 9301–9306, 1998. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lénárt P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol 17: 304–315, 2007. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 30.Lin ZL, Kim NH. Role of ataxia-telangiectasia mutated (ATM) in porcine oocyte in vitro maturation. Cell Biol Int 39: 710–720, 2015. doi: 10.1002/cbin.10439. [DOI] [PubMed] [Google Scholar]
- 31.Longo FJ, Chen DY. Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol 107: 382–394, 1985. doi: 10.1016/0012-1606(85)90320-3. [DOI] [PubMed] [Google Scholar]
- 32.López-Mateo I, Villaronga MA, Llanos S, Belandia B. The transcription factor CREBZF is a novel positive regulator of p53. Cell Cycle 11: 3887–3895, 2012. doi: 10.4161/cc.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma D, Yu H, Lin D, Sun Y, Liu L, Liu Y, Dai B, Chen W, Cao J. S6K1 is involved in polyploidization through its phosphorylation at Thr421/Ser424. J Cell Physiol 219: 31–44, 2009. doi: 10.1002/jcp.21647. [DOI] [PubMed] [Google Scholar]
- 34.Madgwick S, Jones KT. How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div 2: 4, 2007. doi: 10.1186/1747-1028-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol Cell 3: 707–716, 1999. doi: 10.1016/S1097-2765(01)80003-4. [DOI] [PubMed] [Google Scholar]
- 36.Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177: 129–145, 1971. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 37.Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A, Gross JD, Degterev A, Yuan J, Chorev M, Halperin JA, Wagner G. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 128: 257–267, 2007. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 38.Mu XF, Jin XL, Farnham MM, Li Y, O’Neill C. DNA damage-sensing kinases mediate the mouse 2-cell embryo’s response to genotoxic stress. Biol Reprod 85: 524–535, 2011. doi: 10.1095/biolreprod.110.089334. [DOI] [PubMed] [Google Scholar]
- 39.Pahlavan G, Polanski Z, Kalab P, Golsteyn R, Nigg EA, Maro B. Characterization of polo-like kinase 1 during meiotic maturation of the mouse oocyte. Dev Biol 220: 392–400, 2000. doi: 10.1006/dbio.2000.9656. [DOI] [PubMed] [Google Scholar]
- 40.Pause A, Belsham GJ, Gingras AC, Donzé O, Lin TA, Lawrence JC Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371: 762–767, 1994. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 41.Phillips KP, Petrunewich MA, Collins JL, Booth RA, Liu XJ, Baltz JM. Inhibition of MEK or cdc2 kinase parthenogenetically activates mouse eggs and yields the same phenotypes as Mos(−/−) parthenogenotes. Dev Biol 247: 210–223, 2002. doi: 10.1006/dbio.2002.0680. [DOI] [PubMed] [Google Scholar]
- 42.Pomerantz Y, Elbaz J, Ben-Eliezer I, Reizel Y, David Y, Galiani D, Nevo N, Navon A, Dekel N. From ubiquitin-proteasomal degradation to CDK1 inactivation: requirements for the first polar body extrusion in mouse oocytes. FASEB J 26: 4495–4505, 2012. doi: 10.1096/fj.12-209866. [DOI] [PubMed] [Google Scholar]
- 43.Potireddy S, Amarnath D, Latham KE. Transcription, accumulation, storage, recruitment, and degradation of maternal mRNA in mammalian oocytes. In: Biology and Pathology of the Oocyte: Role in Fertility, Medicine and Nuclear Reprogramming (2nd ed.). Cambridge, UK: Cambridge University Press, 2013, p. 154–163. [Google Scholar]
- 44.Potireddy S, Midic U, Liang CG, Obradovic Z, Latham KE. Positive and negative cis-regulatory elements directing postfertilization maternal mRNA translational control in mouse embryos. Am J Physiol Cell Physiol 299: C818–C827, 2010. doi: 10.1152/ajpcell.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romasko EJ, Amarnath D, Midic U, Latham KE. Association of maternal mRNA and phosphorylated EIF4EBP1 variants with the spindle in mouse oocytes: localized translational control supporting female meiosis in mammals. Genetics 195: 349–358, 2013. doi: 10.1534/genetics.113.154005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saskova A, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. Aurora kinase A controls meiosis I progression in mouse oocytes. Cell Cycle 7: 2368–2376, 2008. doi: 10.4161/cc.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science 320: 1655–1658, 2008. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang ZF, Yu L, Li B, Tu WZ, Wang Y, Liu XD, Guan H, Huang B, Rang WQ, Zhou PK. 4E-BP1 participates in maintaining spindle integrity and genomic stability via interacting with PLK1. Cell Cycle 11: 3463–3471, 2012. doi: 10.4161/cc.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shuda K, Schindler K, Ma J, Schultz RM, Donovan PJ. Aurora kinase B modulates chromosome alignment in mouse oocytes. Mol Reprod Dev 76: 1094–1105, 2009. doi: 10.1002/mrd.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shuda M, Velásquez C, Cheng E, Cordek DG, Kwun HJ, Chang Y, Moore PS. CDK1 substitutes for mTOR kinase to activate mitotic cap-dependent protein translation. Proc Natl Acad Sci USA 112: 5875–5882, 2015. doi: 10.1073/pnas.1505787112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Si H, Verma SC, Lampson MA, Cai Q, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded LANA can interact with the nuclear mitotic apparatus protein to regulate genome maintenance and segregation. J Virol 82: 6734–6746, 2008. doi: 10.1128/JVI.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solc P, Kitajima TS, Yoshida S, Brzakova A, Kaido M, Baran V, Mayer A, Samalova P, Motlik J, Ellenberg J. Multiple requirements of PLK1 during mouse oocyte maturation. PLoS One 10: e0116783, 2015. doi: 10.1371/journal.pone.0116783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sukarieh R, Sonenberg N, Pelletier J. The eIF4E-binding proteins are modifiers of cytoplasmic eIF4E relocalization during the heat shock response. Am J Physiol Cell Physiol 296: C1207–C1217, 2009. doi: 10.1152/ajpcell.00511.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sumara I, Giménez-Abián JF, Gerlich D, Hirota T, Kraft C, de la Torre C, Ellenberg J, Peters JM. Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr Biol 14: 1712–1722, 2004. doi: 10.1016/j.cub.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 56.Susor A, Jansova D, Cerna R, Danylevska A, Anger M, Toralova T, Malik R, Supolikova J, Cook MS, Oh JS, Kubelka M. Temporal and spatial regulation of translation in the mammalian oocyte via the mTOR-eIF4F pathway. Nat Commun 6: 6078, 2015. doi: 10.1038/ncomms7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takano A, Usui I, Haruta T, Kawahara J, Uno T, Iwata M, Kobayashi M. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol 21: 5050–5062, 2001. doi: 10.1128/MCB.21.15.5050-5062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032, 2009. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Till A, Saito R, Merkurjev D, Liu JJ, Syed GH, Kolnik M, Siddiqui A, Glas M, Scheffler B, Ideker T, Subramani S. Evolutionary trends and functional anatomy of the human expanded autophagy network. Autophagy 11: 1652–1667, 2015. doi: 10.1080/15548627.2015.1059558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tong C, Fan HY, Lian L, Li SW, Chen DY, Schatten H, Sun QY. Polo-like kinase-1 is a pivotal regulator of microtubule assembly during mouse oocyte meiotic maturation, fertilization, and early embryonic mitosis. Biol Reprod 67: 546–554, 2002. doi: 10.1095/biolreprod67.2.546. [DOI] [PubMed] [Google Scholar]
- 61.Tyrode MV. The mode of action of some purgative salts. Arch Int Pharmacodyn Ther 20: 205–223, 1910. [Google Scholar]
- 62.van Vugt MA, Medema RH. Getting in and out of mitosis with Polo-like kinase-1. Oncogene 24: 2844–2859, 2005. doi: 10.1038/sj.onc.1208617. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, Luo Y, Lin Z, Lee IW, Kwon J, Cui XS, Kim NH. Effect of ATM and HDAC inhibition on etoposide-induced DNA damage in porcine early preimplantation embryos. PLoS One 10: e0142561, 2015. doi: 10.1371/journal.pone.0142561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol 23: 1603–1612, 2009. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Li W, Parra JL, Beugnet A, Proud CG. The C terminus of initiation factor 4E-binding protein 1 contains multiple regulatory features that influence its function and phosphorylation. Mol Cell Biol 23: 1546–1557, 2003. doi: 10.1128/MCB.23.5.1546-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev 10: 2411–2422, 1996. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto A, Mizushima N, Tsukamoto S. Fertilization-induced autophagy in mouse embryos is independent of mTORC1. Biol Reprod 91: 7, 2014. doi: 10.1095/biolreprod.113.115816. [DOI] [PubMed] [Google Scholar]
- 68.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol 2: 893–898, 2000. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 69.Yuan J, Li M, Wei L, Yin S, Xiong B, Li S, Sheng-Li L, Schatten H, Sun QY. Astrin regulates meiotic spindle organization, spindle pole tethering and cell cycle progression in mouse oocytes. Cell Cycle 8: 3384–3395, 2009. doi: 10.4161/cc.8.20.9885. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Y, King ML. Sending RNAs into the future: RNA localization and germ cell fate. IUBMB Life 56: 19–27, 2004. doi: 10.1080/15216540310001658886. [DOI] [PubMed] [Google Scholar]