Abstract
Transient muscle paralysis engendered by a single injection of botulinum toxin A (BTxA) rapidly induces profound focal bone resorption within the medullary cavity of adjacent bones. While initially conceived as a model of mechanical disuse, osteoclastic resorption in this model is disproportionately severe compared with the modest gait defect that is created. Preliminary studies of bone marrow following muscle paralysis suggested acute upregulation of inflammatory cytokines, including TNF-α and IL-1. We therefore hypothesized that BTxA-induced muscle paralysis would rapidly alter the inflammatory microenvironment and the osteoclastic potential of bone marrow. We tested this hypothesis by defining the time course of inflammatory cell infiltration, osteoinflammatory cytokine expression, and alteration in osteoclastogenic potential in the tibia bone marrow following transient muscle paralysis of the calf muscles. Our findings identified inflammatory cell infiltration within 24 h of muscle paralysis. By 72 h, osteoclast fusion and pro-osteoclastic inflammatory gene expression were upregulated in tibia bone marrow. These alterations coincided with bone marrow becoming permissive to the formation of osteoclasts of greater size and greater nuclei numbers. Taken together, our data are consistent with the thesis that transient calf muscle paralysis induces acute inflammation within the marrow of the adjacent tibia and that these alterations are temporally consistent with a role in mediating muscle paralysis-induced bone resorption.
Keywords: bone, inflammation, muscle paralysis, osteoclast, osteoclast fusion
transient muscle paralysis induced by a single injection of botulinum toxin A (BTxA) rapidly induces focal and profound bone resorption within the medullary cavity of adjacent bones. While initially conceived as a model of bone loss due to reduced gait-induced loading, osteoclastic resorption in this model is disproportionately severe compared with the modest gait defect that is created (19, 32, 49). Instead, the severe bone loss following BTxA-induced muscle paralysis is comparable in rapidity and magnitude to that induced by sciatic neurectomy and spinal cord injury (31, 34). Subsequent studies demonstrated that acute trabecular bone loss following muscle paralysis arises via profound receptor activator of NF-κB ligand (RANKL)-mediated osteoclastogenesis, but the initial signaling cascade responsible for the rapid activation of this bone catabolic pathway is not known (2).
Preliminary studies to identify candidate signaling pathways responsible for activating RANKL-mediated osteoclastogenesis indicated that the inflammatory cytokines TNF-α and IL-1 were upregulated before the onset of bone resorption and altered bone marrow gene expression previously observed at 7 and 14 days postparalysis (33, 53). As these cytokines have been shown to regulate osteoclast activity (1, 12, 27, 56), we speculated that inflammatory alterations within the marrow in response to muscle paralysis create a focal microenvironment that is permissive to osteoclastogenesis.
The linkage between inflammation, immune function, and bone catabolism is strongly supported by the literature. Predisposition to bone loss and osteoporotic fracture is observed in a number of chronic inflammatory diseases with immune components such as rheumatoid arthritis, inflammatory bowel disease, chronic obstructive pulmonary disease, and multiple sclerosis (8, 15, 17, 41). Inflammatory pathways have also been implicated in postmenopausal bone loss (40). When focal inflammatory disorders occur adjacent to bone surfaces (e.g., periodontitis or adjuvant arthritis), bone resorption is especially destructive (3, 29). One mechanism for this resorption is that RANKL can be highly expressed on activated CD4+ T cells and other inflammatory regulating cells (24, 25, 48), leading to enhanced osteoclast formation/recruitment and bone destruction. As well, inflammatory cytokines such as TNF-α, IL-1, and IL-6 have been found to promote osteoclastogenesis (3, 20) and are implicated in postmenopausal bone loss (40). Lastly, both genetic and pharmacologic inhibition of the immune response through T-cell depletion and/or anti-inflammatory treatment reduces bone loss in models of ovariectomy and adjuvant arthritis (9, 40, 42).
In addition to clear evidence of immune system involvement in chronic inflammatory bone loss, the rapidity and magnitude of bone resorption induced by muscle paralysis implies that immune-inflammatory pathways may play a critical role in paralysis-induced bone loss. In this context, an advantage of exploring this phenomenon in the BTxA bone loss model is the brief, contained period of de novo osteoclast activity [6 to 13 days (5)] induced by muscle paralysis. We therefore hypothesized that BTxA-induced muscle paralysis would rapidly alter the inflammatory microenvironment and the osteoclastic potential of bone marrow. We tested this hypothesis by defining the time course of inflammatory cell infiltration, osteoinflammatory cytokine expression, and alteration in osteoclastogenic potential in the tibia bone marrow following transient muscle paralysis of the adjacent calf muscle group.
METHODS
This study comprised three complementary experiments. All mice were 16-wk-old female C57Bl/6 obtained from Jackson Laboratories. Each experiment utilized a single injection of botulinum toxin A (BTxA; 2 U/100 g body wt; Allergan, Irvine, CA) into the right calf muscle group to induce transient muscle paralysis on day 0 (39). Mice receiving BTxA treatment were allowed free cage activity for the remainder of each experiment. Calf paralysis was confirmed 24 h postinjection by visual examination of reduced toe extension and ankle plantarflexion in the affected limb. We chose to use age-matched naïve mice as controls in our studies for two primary reasons. First, while we have observed minimal alterations in RANKL protein levels, osteoclast numbers and bone volume alterations in control limbs following BTxA-induced muscle paralysis (2, 50), we had no data confirming the absence of inflammatory gene expression alterations in contralateral limbs. Second, we had previously used naïve control mice to define the onset of osteoclastic resorption following transient muscle paralysis (4, 5). All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee, University of Washington.
Flow cytometric analysis of T cells.
Mice were euthanized 24 or 48 h following BTxA injection (n = 4/group). Marrow within the proximal metaphysis of the right tibia (~5 mm section distal to the growth plate) was flushed with RPMI and pelleted by centrifugation. Red blood cells in the cell pellet were lysed by incubation in ACK lysing buffer (BioWhittaker) for 2 min. Cells were resuspended in RPMI and aliquoted into FACS tubes (5 × 106 cells/sample). FACS samples were centrifuged and resuspended in surface antibody dilution (1:200 ratio of surface antibody to FACS buffer consisting of HBSS with 2% FBS) for 20 min at 4°C. Finally, samples were washed twice and resuspended in FACS buffer for flow cytometry analysis. Marrow from naïve mice (n = 5) was identically collected and processed as the baseline control. Marrow cell populations were stained for T-cell surface markers [T-cell receptor-β (TCRβ), CD4, and CD8 antibodies; Biolegend). Individual sample data were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar). Prior to analysis, one mouse from the BTxA group was removed owing to a failed physical examination for muscle paralysis.
Quantitative RT-PCR of osteoinflammatory and osteoclast fusion genes.
Mice in this study were euthanized on day 1, 3, or 7 post-BTxA injection (n = 6/group). Whole marrow was flushed from the right tibia with RPMI. For quantitative RT-PCR, total RNA was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol and cDNA was synthesized using Super Script III reverse transcriptase (Invitrogen/Life Technologies). Control marrow from naïve mice was prepared identically (n = 6). We identified a candidate panel of inflammatory and osteoclastogenic genes from the literature and based on our preliminary data (TNF, CD9, CD44, CD47, CD81, IL-1a, IL-1b, IL-4, IL-11, DC-STAMP, OC-STAMP). We analyzed gene expression with quantitative real-time PCR using SYBR green and the Applied Biosystems 7900 HT sequence detection system. Gene expression levels were quantified using the 2−ΔΔCt method relative to the inflammatory specific housekeeping gene HPRT (45). Primers used for this analysis are shown in Table 1.
Table 1.
Primer sequences for RT-PCR analysis
| Target | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| HPRT | AGTGTTGGATACAGGCCAGAC | CGTGATTCAAATCCCTGAAGT |
| TNF | AGCCCCCAGTCTGTATCCTT | CTCCCTTTGCAGAACTCAGG |
| CD9 | GCCGCCGTCTGGGGCTATAC | TGCCACAGCAGTCCAACGCC |
| CD44 | TCCAGGGGGAGTTCCCGCAC | CCGCGATGCAGACGGCAAGA |
| CD47 | AAGCGCGATGCCATGGTGGG | AGTGTTGAAGGCCGTGCGGTT |
| CD81 | TGGGGACGTTCTTCACCTGCCTT | GGGGGTGAGTATGTTGCCGCC |
| IL-1a | GCAACGGGAAGATTCTGAAG | TGACAAACTTCTGCCTGACG |
| IL-1b | GCCCATCCTCTGTGACTCAT | AGGCCACAGGTATTTTGTCG |
| IL-4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT |
| IL-11 | CTGTGGGGACATGAACTGTG | CGTCAGCTGGGAATTTGTCT |
| DC-STAMP | GCCGTTCTGTCGTGTGGCCT | GCGGAGTGGCAAGGCCGTAAA |
| OC-STAMP | ACCTCCGGTGGAGAGCGTGT | ACAGTCGTGGGGCGTGAAGC |
Primary osteoclast culture.
Tissue culture medium and supplements were purchased from Invitrogen (Life Technologies). Media were supplemented with heat-inactivated FBS (HyClone, Thermo Scientific). Marrow was collected from mouse tibiae at 1, 3, and 7 days post-BTxA injection (n = 2/group). Marrow was flushed in culture medium (α-MEM with 10% FBS) and red blood cells were lysed as described above in Flow cytometric analysis of T cells. Cells were seeded in duplicate at 1.5 × 104/cm2 in chamber well slides (Thermo Scientific), supplemented with 50 ng/ml M-CSF (Peprotech) and 10 ng/ml RANKL (Biolegend) and incubated at 37°C and 5% CO2. Cell media were refreshed on day 3 of culture, and cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) on day 8 of culture using the Leukocyte (TRAP) kit and protocol (Sigma-Aldrich). The experiment was replicated (total combined group size of n = 4/group), with data normalized within each experiment to identically treated marrow from treatment-naïve mice (n = 2/experiment). For each group, normal osteoclasts (i.e., TRAP-positive cells with 3+ nuclei) and giant osteoclasts (i.e., a subset of normal osteoclasts with 20+ nuclei) were quantified on a per well basis using bright-field microscopy (Olympus BH2 microscope, ×10 and ×40 magnification). The cutoff for a giant osteoclast (20+ nuclei) was based on previous studies (22, 51) to distinguish an increase in cell size between experimental groups. Once this difference was identified, a secondary analysis was performed to quantify the cell size distribution within these identified samples to confirm that our original analysis was robust to our choice of what was considered a giant osteoclast. For this, we assessed osteoclast area in naïve and 3 days post-BTxA cultures via automated calculation of osteoclast TRAP+ cell area in two 10 mm2 subsamples of the culture area (composite of eighty ×20 light microscopy images, Zeiss) using custom ImageJ software (Fiji). Specifically, the software identified individual cell perimeters through image thresholding, binarized the images, and filled in the cell area for individual cell area calculations. The average size of a three-nuclei osteoclast was quantified by imaging a series of three-nuclei TRAP+ cells (n = 20 cells across all groups).
Statistical analysis.
Because of nonhomogeneity of variance, flow cytometry data were analyzed using Kruskal-Wallis rank-sum tests with Dunn’s post hoc analysis for pairwise comparisons. Differential gene regulation in the quantitative RT-PCR study was determined using planned comparison one-way multivariate ANOVAs (i.e., gene expression on all days compared with naïve mice) with Dunnett’s two-way post hoc for multiple comparisons to controls (i.e., naïve). Multiple one-way ANOVAs with Tukey post hoc analysis were used to determine significant differences in the primary osteoclast culture. To compare osteoclast size in cultures, a Kolmogorov-Smirnov (KS) test was first used to compare the distribution in cell areas. Next, normalized population distributions (i.e., percentage of cell population at a given area) were determined and fit with separate power law curves using maximum likelihood estimation (MLE) and likelihood ratio testing (LRT) as described previously (46). Finally, we assessed whether the data sets could be fit by a single power law model via MLE and LRT. Statistical significance was determined to be P < 0.05.
RESULTS
CD4+ T cells are acutely upregulated following muscle paralysis.
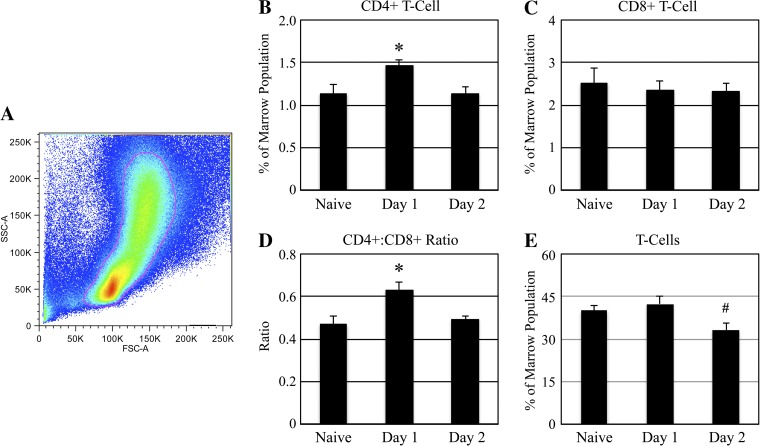
Flow cytometry revealed rapid transient alterations in tibia marrow cell populations in the proximal tibia metaphysis following muscle paralysis (Fig. 1). The percentage of CD4+ T cells (means ± SE) within the bone marrow was elevated 25% within 24 h of BTxA injection (1.47 ± 0.07%) vs. naïve (1.14 ± 0.10%, P < 0.04; Fig. 1B) but returned to baseline levels within 48 h after BTxA injection (1.14 ± 0.07%, P = 0.75 vs. naive; Fig. 1B). CD8+ populations were not significantly altered at any time point (2.36 ± 0.21% vs. 2.32 ± 0.20% vs 2.52 ± 0.35 in day 1, day 2, and naïve, respectively, P > 0.9; Fig. 1C). Thus, the ratio of CD4+/CD8+ T cells within the marrow was increased 34% on day 1 (0.63 ± 0.04) vs. naïve (0.47 ± 0.04, P < 0.03; Fig. 1D), but not on day 2 (0.49 ± 0.01, P = 0.82 vs. naive; Fig. 1C). The overall T cell population size in naïve marrow (40.17 ± 1.78%) was not significantly altered at 24 h (42.40 ± 2.91%, P = 0.36 vs. naive; Fig. 1E) or 48 h (33.20 ± 2.24%, P = 0.10 vs. naive; Fig. 1E).
Fig. 1.
Acute CD4+ T-cell upregulation in bone marrow following muscle paralysis. Bone marrow was extracted from the proximal metaphysis of the right tibia of naïve mice and mice that had received an intramuscular (IM) injection of botulinum toxin A (BTxA) either 24 or 48 h previously (2 U/100 g body wt in right calf muscle group). Samples were lysed of red blood cells and stained with T-cell receptor-β (TCRβ), CD4, and CD8 antibodies, and FACS analysis was performed. Prior to determination of T-cell populations within bone marrow, cellular debris was excluded from analysis using traditional forward side-scatter gating (A). Percentages of CD4+ (B) and CD8+ (C) T cells are shown, and the ratio of CD4+:CD8+ cells was determined (D). Additionally, total percentages of T cells in the marrow were graphed (E). Values represent means ± SE (n = 4–5 per group), *P < 0.05 vs. naïve and day 2, #P < 0.05 vs. day 1.
Osteoinflammatory genes are rapidly altered following muscle paralysis.
Temporal gene expression patterns within the tibia marrow following muscle paralysis were consistent with transient inflammatory signaling. While none of the panel of nine genes was significantly altered at 1 day following muscle paralysis (vs. naïve controls), three genes showed statistically significant regulation by day 3. Two genes were upregulated (TNF: 299%, IL-4: 243%; each P < 0.02, Table 2), while one was downregulated (CD47: −88%; P < 0.03, Table 2). Only IL-4 was significantly upregulated on day 7 (250%, P < 0.02, Table 2). Additionally, three other genes (CD9, IL-1a, and IL-1b; Table 2) were detectable in naïve and day 1 samples but were undetectable on days 3 and 7. IL-6 gene expression was also assayed, but the levels were undetectable in most samples and thus are not reported.
Table 2.
Relative mRNA expression in whole tibia following calf paralysis
| Days After Botox Injection |
||||
|---|---|---|---|---|
| Naïve | Day 1 | Day 3 | Day 7 | |
| TNF | 1.15 ± 0.30 | 1.87 ± 0.80 | 4.59 ± 0.80 ↑ | 2.81 ± 0.79 |
| CD9 | 1.47 ± 0.48 | 1.23 ± 0.45 | Undetectable | Undetectable |
| CD44 | 1.41 ± 0.43 | 1.04 ± 0.38 | 0.19 ± 0.11 | 0.95 ± 0.57 |
| CD47 | 0.99 ± 0.23 | 0.91 ± 0.28 | 0.12 ± 0.06 ↓ | 0.48 ± 0.25 |
| CD81 | 1.44 ± 0.54 | 0.98 ± 0.25 | 0.98 ± 0.15 | 1.10 ± 0.31 |
| IL-1a | 0.05 ± 0.01 | 0.05 ± 0.02 | Undetectable | Undetectable |
| IL-1b | 0.12 ± 0.04 | 0.13 ± 0.04 | Undetectable | Undetectable |
| IL-4 | 0.60 ± 0.13 | 0.72 ± 0.16 | 2.06 ± 0.38 ↑ | 2.10 ± 0.50 ↑ |
| IL-11 | 0.17 ± 0.04 | 0.16 ± 0.05 | 0.26 ± 0.07 | 0.21 ± 0.08 |
Values are means ± SE. Significant increase (↑) or decrease (↓) vs. Naïve (all P < 0.05). Undetectable = unquantifiable expression in at least half of the samples.
The osteoclastic potential of bone marrow is altered by muscle paralysis.
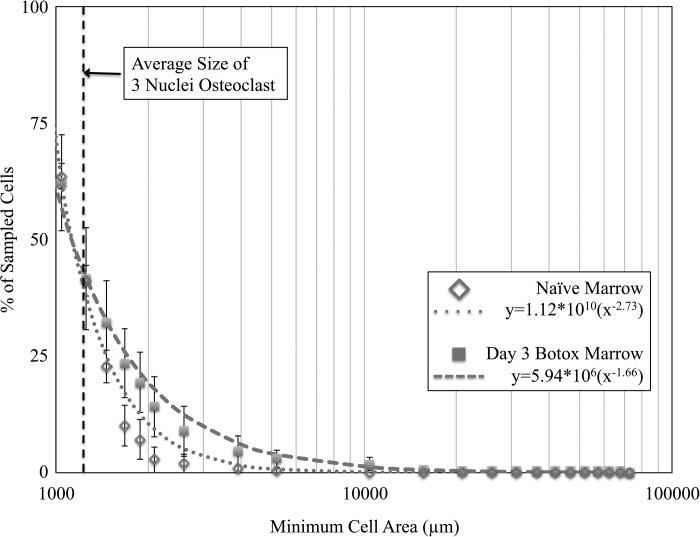
The culture conditions were permissive to the formation of osteoclasts with a small subset of giant osteoclasts (>20 nuclei; Fig. 2). Marrow from naïve mice generated 96.8 ± 26.7 (means ± SE) normal osteoclasts/well, including 2.5 ± 1.0 giant osteoclasts/well. Transient muscle paralysis did not significantly alter the number of normal osteoclasts formed in marrow culture at any time point (Fig. 3A). However, the capacity to form giant osteoclasts was acutely upregulated 3 days following muscle paralysis and was not significantly different from naïve mice by day 7. Marrow harvested 3 days after BTxA injection produced 268% more giant osteoclasts than naïve control marrow (P = 0.008) and 352% more giant osteoclasts than day 1 marrow (P = 0.003; Fig. 3B). Secondary analyses indicated that this observation was robust to the number of nuclei chosen to represent a giant osteoclast. A KS test indicated that the distribution of osteoclast size arising from day 3 BTxA marrow was significantly different from that of naïve bone marrow (P < 0.001). Additionally, we observed that two separate power law models (a*xb) were required to describe the in vivo data (P = 0.87, formulas in Fig. 4). Given that the overall osteoclast numbers were not different in naïve and day 3 BTxA cultures (see above), this analysis indicates that the percentages of cells (and thus total cell number) in the day 3 BTxA culture were higher than in the naïve cultures regardless of the threshold chosen to classify a giant osteoclast.
Fig. 2.
Marrow culture conditions were permissive to formation of osteoclasts. Whole bone marrow was collected from the right tibia of experimental mice. Samples were lysed of red blood cells, seeded in culture medium supplemented with M-CSF (50 ng/ml) and RANKL (10 ng/ml), incubated at 37°C, and stained for tartrate-resistant acid phosphatase (TRAP) on day 8 of culture. Representative image of bone marrow harvested 3 days after transient muscle paralysis is shown. Giant osteoclasts (blue arrows) and smaller osteoclasts (green arrows) can be seen.
Fig. 3.
Transient muscle paralysis augmented osteoclastic potential of tibia marrow through enhanced osteoclast fusion, but not increased osteoclast number. Whole bone marrow was collected from the right tibia of naïve mice and from mice that had received an IM injection of BTxA injection in the right calf 1, 3 and 7 days previously. Cells were cultured for 8 days and stained for TRAP. Normal osteoclasts (TRAP-positive cells with 3+ nuclei) and giant osteoclasts (a subset of normal osteoclasts with 20+ nuclei) were quantified in each group on a per well basis using bright-field microscopy (×10 and ×40 magnification). Fold changes of normal osteoclasts (A) and giant osteoclasts (B) are shown at day 1, 3, and 7 postinjection normalized to the naïve controls. Values represent means ± SE (n = 4/group), **P < 0.01 vs. naïve and day 1.
Fig. 4.
Size of osteoclasts generated from marrow harvested 3 days after muscle paralysis was consistently larger than that of naïve marrow cultures. An automated quantification of osteoclast area was performed on two subsamples of the naïve and day 3 cultures (10 mm2 per subsample using a composite of 80 light microscopy ×20 images) using custom software as described in methods. A Kolmogorov-Smirnov (KS) analysis indicated that the e area distributions from naïve and day 3 BTxA marrow were significantly different (P < 0.001). The figure represents the distribution of cell areas in each culture with separate power law models (dashed and dotted lines) fit to simulate the in vitro data. Values represent means ± SE.
Osteoclast fusion genes are upregulated 3 days following muscle paralysis.
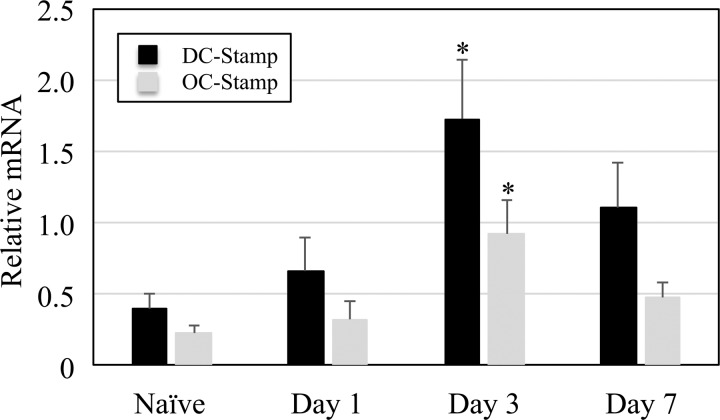
The elevated osteoclast size and number of nuclei generated by marrow following muscle paralysis might be explained by enhanced cell fusion. We therefore reassessed cDNA from the gene expression experiment and quantified alterations in the osteoclast fusion genes (DC-STAMP and OC-STAMP). Both DC-STAMP (243% increase) and OC-STAMP (218% increase) were significantly upregulated in bone marrow removed 3 days postparalysis as compared with naïve bone marrow (both P < 0.02, Fig. 5). Consistent with the in vitro experiments, osteoclast fusion gene expression was not significantly altered on day 1 or day 7 following muscle paralysis compared with naïve samples.
Fig. 5.
Osteoclast fusion gene expression following muscle paralysis. Whole bone marrow was flushed from the right tibia of naïve mice and mice that had received an IM injection of BTxA in the right calf 1, 3, and 7 days previously. After preparation of total RNA and synthesis of cDNA, quantitative RT-PCR was performed using DC-STAMP and OC-STAMP gene-specific primers. Gene expression levels were quantified relative to the housekeeping gene HPRT. Values represent means ± SE (n = 6 per group), *P < 0.05 vs. naïve.
DISCUSSION
We used flow cytometry, quantitative RT-PCR, and primary osteoclast culture to explore the presence of inflammatory signaling within bone marrow following calf paralysis. We observed inflammatory cell infiltration within 24 h and proinflammatory gene expression within 72 h of muscle paralysis. Surprisingly, alterations in the osteoclastic potential of bone marrow extracted following muscle paralysis were not associated with increased numbers of osteoclasts but instead manifested as an increase in osteoclast size and number of nuclei per osteoclast. The observation of enhanced numbers of giant osteoclasts was supported by simultaneous upregulation of the osteoclast fusion genes DC- and OC-STAMP. In combination, these data are consistent with muscle paralysis acutely precipitating a localized inflammatory response within bone marrow that is permissive to generating osteoclasts of greater size and nuclei number.
These observations must be considered in the context of the limitations of our approach. The impetus for this study was to assess our thesis that inflammatory signaling within bone marrow precedes or is coincident with the onset of bone resorption induced by muscle paralysis. To that end, we quantified inflammatory cell influx (FACS) and altered osteoinflammatory signaling (RT-PCR) within bone marrow. While recognizing that these outcomes are indirect and do not define a causal relationship between bone marrow inflammation and subsequent bone resorption, we believe this limitation is mitigated, in part, by the cadence of events underlying bone resorption following muscle paralysis. Specifically, we observed alterations in inflammatory cell populations (day 1) upstream of alterations in osteoinflammatory gene expression (day 3). The timing in which marrow became permissive to formation of giant osteoclasts (day 3), coupled with the time needed to form functioning osteoclasts from precursor populations [≈3–5 days (13, 47)] is congruent with the onset of diaphyseal endocortical resorption following paralysis (which almost entirely occurs between days 6 and 13 postparalysis) (5).
To further support our gene expression data, we performed a follow-on ELISA-based study to quantify protein level alterations in TNF-α and IL-4 (as both genes showed significant upregulation following muscle paralysis). Utilizing the same time points as the gene expression experiment, we did not observe elevated expression in either protein following muscle paralysis. There are many potential explanations for the lack of direct correlation between mRNA and protein levels, and the inflammatory literature illustrates this challenge even in a simple cell culture environment (e.g., see Ref. 44). One clear challenge in our system is that the temporal dynamics of protein expression and stability (e.g., half-life on the scale of minutes in an experiment of many days) is likely to be exceedingly nonlinear. Though we believe that the cumulated data across our studies suggest otherwise, it is possible that the inflammatory genes upregulation does not manifest in alteration in protein level expression. Regardless, these data support the need to further clarify the role of inflammatory mediation and suggest that either transgenic (6, 40) or pharmaceutical (9, 42) strategies would most effectively explore a mechanistic relation between inflammation and muscle paralysis-induced bone loss.
Using a primary culture system to explore whether bone marrow had altered osteoclastic potential following transient muscle paralysis, we observed greater numbers of giant osteoclasts (>20 nuclei). This finding ostensibly contradicts our previous in vivo observation of increased osteoclast numbers in the proximal tibia following BTxA-induced paralysis of the calf (2). However, as histologic quantification of osteoclast numbers in adjacent cross sections relies upon an assumption of equivalent cell size, a significant increase in osteoclast size (as observed in vitro) would likely manifest as an increased osteoclast number in stereological assessments. Additionally, we did not determine the resorptive potential of the giant osteoclasts observed in cell culture. The literature suggests that osteoclast size does not always correlate with increased bone resorption. For example, giant osteoclasts observed following long-term bisphosphonate therapy are generally not thought to resorb bone at all but rather are an artifact caused by prolonged apoptosis (22, 51). In contrast, however, giant osteoclasts associated with inflammatory pathologies such as Paget’s are generally thought to be capable of resorbing large volumes of bone mineral (16, 21, 29, 38). If so, it is reasonable to speculate that a relatively few giant osteoclasts could account for a sizeable proportion of bone resorption observed following muscle paralysis. As confirmation of the in vivo presence of giant osteoclasts via histology may prove technically challenging, given our observations of enhanced cell fusion, it is possible that pharmaceutical strategies to transiently inhibit cell fusion could be implemented to explore this question in vivo (18).
Despite these limitations, our results are consistent with the upregulation of inflammatory cells and inflammatory genes before the initiation of bone resorption, and our in vivo RT-PCR data are also consistent with this altered marrow environment facilitating the formation of giant osteoclasts observed in vitro. Following muscle paralysis, several genes implicated in osteoclast fusion and giant osteoclast formation were upregulated, including the essential protein for osteoclast fusion, DC-STAMP (35, 43, 54), and a second osteoclast fusion-related protein, OC-STAMP (36). Inhibition studies have indicated that these genes are essential for normal osteoclast activity, while overexpression studies having resulted in elevated osteoclast formation/bone resorption (11, 28, 52, 55). IL-4 has been shown to promote the formation of multinucleated giant cells from macrophage precursors through the overexpression of E-cadherin (37). Other recent reports identified that upregulation of TNF in the absence of CD44 results in the generation of highly resorbing giant osteoclasts (21). While we did not detect a statistical decrease in CD44 gene expression on day 3 (P = 0.10), the 87% decrease in comparison to naïve bone marrow and elevated TNF in our data are also consistent with this thesis.
Ultimately, understanding how muscle paralysis of the calf induces inflammatory alterations in tibia bone marrow may reveal unexpected targets to mitigate the catabolic effects of neuromuscular dysfunction, regardless of initiating pathology. One possible explanation is that muscle paralysis induces neurogenic inflammation, which is characterized by the local release of inflammatory mediators in response to trauma or noxious stimuli (10, 23, 30). Classically, antidromic depolarization causes sensory neurons to rapidly release neuropeptides [primarily SP and CGRP (10, 14)] that act on a variety of cell populations to rapidly orchestrate a focal inflammatory response (7). While direct evidence of neuropeptide release following muscle paralysis has not yet been reported, even brief periods of weightlessness have been associated with afferent sensory nerve depolarization (26).
Taken together, our data suggest that transient calf muscle paralysis induces acute inflammation within the marrow of the adjacent tibia that is temporally consistent with an active role in mediating bone resorption induced by this neuromuscular dysfunction. Our data additionally suggest that the inflammatory cascade swiftly triggered by transient muscle paralysis predisposes bone marrow to the formation of giant osteoclasts, which may account for the rapid and severe destruction of bone observed in this model. The identification of an acute inflammatory cascade in bone marrow leading to the formation of giant osteoclasts has potential to reveal novel therapeutic strategies for mitigating paralysis-induced bone loss following neuromuscular trauma.
GRANTS
This work was supported, in part, by National Institute on Aging Grant F31AG037287 (to B. J Ausk), National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR060304 and AR064735 (to T. S. Gross), the Sigvard T. Hansen, Jr Endowed Chair (to T. S. Gross), and the Zimmer FX Biology Professorship (to S. D. Bain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.J.A., L.E.W., K.S.S., R.Y.K., E.M.G., and T.S.G. conceived and designed research; B.J.A., L.E.W., and K.S.S. performed experiments; B.J.A., L.E.W., S.D.B., and S.S. analyzed data; B.J.A., L.E.W., K.S.S., S.D.B., S.S., E.M.G., and T.S.G. interpreted results of experiments; B.J.A. and S.S. prepared figures; B.J.A. and T.S.G. drafted manuscript; B.J.A., L.E.W., S.D.B., S.S., E.M.G., and T.S.G. edited and revised manuscript; B.J.A., L.E.W., K.S.S., R.Y.K., S.D.B., S.S., E.M.G., and T.S.G. approved final version of manuscript.
REFERENCES
- 1.Adamopoulos IE, Bowman EP. Immune regulation of bone loss by Th17 cells. Arthritis Res Ther 10: 225, 2008. doi: 10.1186/ar2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliprantis AO, Stolina M, Kostenuik PJ, Poliachik SL, Warner SE, Bain SD, Gross TS. Transient muscle paralysis degrades bone via rapid osteoclastogenesis. FASEB J 26: 1110–1118, 2012. doi: 10.1096/fj.11-196642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol 160: 403–409, 1998. [PubMed] [Google Scholar]
- 4.Ausk BJ, Huber P, Poliachik SL, Bain SD, Srinivasan S, Gross TS. Cortical bone resorption following muscle paralysis is spatially heterogeneous. Bone 50: 14–22, 2012. doi: 10.1016/j.bone.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausk BJ, Huber P, Srinivasan S, Bain SD, Kwon RY, McNamara EA, Poliachik SL, Sybrowsky CL, Gross TS. Metaphyseal and diaphyseal bone loss in the tibia following transient muscle paralysis are spatiotemporally distinct resorption events. Bone 57: 413–422, 2013. doi: 10.1016/j.bone.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker PJ, Howe L, Garneau J, Roopenian DC. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol Med Microbiol 34: 45–50, 2002. doi: 10.1111/j.1574-695X.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 7.Biosse-Duplan M, Baroukh B, Dy M, de Vernejoul MC, Saffar JL. Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am J Pathol 174: 1426–1434, 2009. doi: 10.2353/ajpath.2009.080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckland-Wright JC, Walker SR. Incidence and size of erosions in the wrist and hand of rheumatoid patients: a quantitative microfocal radiographic study. Ann Rheum Dis 46: 463–467, 1987. doi: 10.1136/ard.46.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-alpha and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res 22: 724–729, 2007. doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- 10.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15: 1063–1067, 2012. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu YH, Mensah KA, Schwarz EM, Ju Y, Takahata M, Feng C, McMahon LA, Hicks DG, Panepento B, Keng PC, Ritchlin CT. Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP). J Bone Miner Res 27: 79–92, 2012. doi: 10.1002/jbmr.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colburn NT, Zaal KJ, Wang F, Tuan RS. A role for gamma/delta T cells in a mouse model of fracture healing. Arthritis Rheum 60: 1694–1703, 2009. doi: 10.1002/art.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collin-Osdoby P, Yu X, Zheng H, Osdoby P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol Med 80: 153–166, 2003. doi: 10.1385/1-59259-366-6:153. [DOI] [PubMed] [Google Scholar]
- 14.Corrigan F, Vink R, Turner RJ. Inflammation in acute CNS injury: a focus on the role of substance P. Br J Pharmacol 173: 703–715, 2016. doi: 10.1111/bph.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosman F, Nieves J, Komar L, Ferrer G, Herbert J, Formica C, Shen V, Lindsay R. Fracture history and bone loss in patients with MS. Neurology 51: 1161–1165, 1998. doi: 10.1212/WNL.51.4.1161. [DOI] [PubMed] [Google Scholar]
- 16.da Costa CE, Annels NE, Faaij CM, Forsyth RG, Hogendoorn PC, Egeler RM. Presence of osteoclast-like multinucleated giant cells in the bone and nonostotic lesions of Langerhans cell histiocytosis. J Exp Med 201: 687–693, 2005. doi: 10.1084/jem.20041785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimai HP, Domej W, Leb G, Lau KH. Bone loss in patients with untreated chronic obstructive pulmonary disease is mediated by an increase in bone resorption associated with hypercapnia. J Bone Miner Res 16: 2132–2141, 2001. doi: 10.1359/jbmr.2001.16.11.2132. [DOI] [PubMed] [Google Scholar]
- 18.Fan X, Biskobing DM, Bain S, Rubin J. Ketoconazole and phorbol myristate acetate regulate osteoclast precursor fusion in primary murine marrow culture. J Bone Miner Res 11: 1274–1280, 1996. doi: 10.1002/jbmr.5650110912. [DOI] [PubMed] [Google Scholar]
- 19.Gross TS, Poliachik SL, Prasad J, Bain SD. The effect of muscle dysfunction on bone mass and morphology. J Musculoskelet Neuronal Interact 10: 25–34, 2010. [PubMed] [Google Scholar]
- 20.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 47: 1635–1640, 2008. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 21.Hayer S, Steiner G, Görtz B, Reiter E, Tohidast-Akrad M, Amling M, Hoffmann O, Redlich K, Zwerina J, Skriner K, Hilberg F, Wagner EF, Smolen JS, Schett G. CD44 is a determinant of inflammatory bone loss. J Exp Med 201: 903–914, 2005. doi: 10.1084/jem.20040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain N, Weinstein RS. Giant osteoclasts after long-term bisphosphonate therapy: diagnostic challenges. Nat Rev Rheumatol 5: 341–346, 2009. doi: 10.1038/nrrheum.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jancsó N, Jancsó-Gábor A, Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother 31: 138–151, 1967. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josien R, Wong BR, Li HL, Steinman RM, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol 162: 2562–2568, 1999. [PubMed] [Google Scholar]
- 25.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 169: 987–998, 2006. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawano F, Nomura T, Ishihara A, Nonaka I, Ohira Y. Afferent input-associated reduction of muscle activity in microgravity environment. Neuroscience 114: 1133–1138, 2002. doi: 10.1016/S0306-4522(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 27.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304–309, 1999. doi: 10.1038/35005552. [DOI] [PubMed] [Google Scholar]
- 28.Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med 200: 941–946, 2004. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuratani T, Nagata K, Kukita T, Hotokebuchi T, Nakasima A, Iijima T. Induction of abundant osteoclast-like multinucleated giant cells in adjuvant arthritic rats with accompanying disordered high bone turnover. Histol Histopathol 13: 751–759, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Levine JD, Dardick SJ, Basbaum AI, Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci 5: 1380–1386, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu D, Zhao CQ, Li H, Jiang SD, Jiang LS, Dai LY. Effects of spinal cord injury and hindlimb immobilization on sublesional and supralesional bones in young growing rats. Bone 43: 119–125, 2008. doi: 10.1016/j.bone.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Manske SL, Boyd SK, Zernicke RF. Vertical ground reaction forces diminish in mice after botulinum toxin injection. J Biomech 44: 637–643, 2011. doi: 10.1016/j.jbiomech.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Marchand-Libouban H, Le Drévo MA, Chappard D. Disuse induced by botulinum toxin affects the bone marrow expression profile of bone genes leading to a rapid bone loss. J Musculoskelet Neuronal Interact 13: 27–36, 2013. [PubMed] [Google Scholar]
- 34.Marenzana M, De Souza RL, Chenu C. Blockade of beta-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone 41: 206–215, 2007. doi: 10.1016/j.bone.2007.04.184. [DOI] [PubMed] [Google Scholar]
- 35.Mensah KA, Ritchlin CT, Schwarz EM. RANKL induces heterogeneous DC-STAMP(lo) and DC-STAMP(hi) osteoclast precursors of which the DC-STAMP(lo) precursors are the master fusogens. J Cell Physiol 223: 76–83, 2010. doi: 10.1002/jcp.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto H, Suzuki T, Miyauchi Y, Iwasaki R, Kobayashi T, Sato Y, Miyamoto K, Hoshi H, Hashimoto K, Yoshida S, Hao W, Mori T, Kanagawa H, Katsuyama E, Fujie A, Morioka H, Matsumoto M, Chiba K, Takeya M, Toyama Y, Miyamoto T. Osteoclast stimulatory transmembrane protein and dendritic cell–specific transmembrane protein cooperatively modulate cell-cell fusion to form osteoclasts and foreign body giant cells. J Bone Miner Res 27: 1289–1297, 2012. doi: 10.1002/jbmr.1575. [DOI] [PubMed] [Google Scholar]
- 37.Moreno JL, Mikhailenko I, Tondravi MM, Keegan AD. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: contribution of E-cadherin. J Leukoc Biol 82: 1542–1553, 2007. doi: 10.1189/jlb.0107058. [DOI] [PubMed] [Google Scholar]
- 38.Muzylak M, Price JS, Horton MA. Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcif Tissue Int 79: 301–309, 2006. doi: 10.1007/s00223-006-0082-7. [DOI] [PubMed] [Google Scholar]
- 39.Poliachik SL, Bain SD, Threet D, Huber P, Gross TS. Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone 46: 18–23, 2010. doi: 10.1016/j.bone.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98: 13960–13965, 2001. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roux C, Abitbol V, Chaussade S, Kolta S, Guillemant S, Dougados M, Amor B, Couturier D. Bone loss in patients with inflammatory bowel disease: a prospective study. Osteoporos Int 5: 156–160, 1995. doi: 10.1007/BF02106094. [DOI] [PubMed] [Google Scholar]
- 42.Saidenberg-Kermanac’h N, Corrado A, Lemeiter D, deVernejoul MC, Boissier MC, Cohen-Solal ME. TNF-alpha antibodies and osteoprotegerin decrease systemic bone loss associated with inflammation through distinct mechanisms in collagen-induced arthritis. Bone 35: 1200–1207, 2004. doi: 10.1016/j.bone.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Sanecka A, Ansems M, Prosser AC, Danielski K, Warner K, den Brok MH, Jansen BJ, Eleveld-Trancikova D, Adema GJ. DC-STAMP knock-down deregulates cytokine production and T-cell stimulatory capacity of LPS-matured dendritic cells. BMC Immunol 12: 57, 2011. doi: 10.1186/1471-2172-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shebl FM, Pinto LA, García-Piñeres A, Lempicki R, Williams M, Harro C, Hildesheim A. Comparison of mRNA and protein measures of cytokines following vaccination with human papillomavirus-16 L1 virus-like particles. Cancer Epidemiol Biomarkers Prev 19: 978–981, 2010. doi: 10.1158/1055-9965.EPI-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silver N, Cotroneo E, Proctor G, Osailan S, Paterson KL, Carpenter GH. Selection of housekeeping genes for gene expression studies in the adult rat submandibular gland under normal, inflamed, atrophic and regenerative states. BMC Mol Biol 9: 64, 2008. doi: 10.1186/1471-2199-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivasan S, Threet D, Worton LE, Ausk BJ, Bain SD, Gardiner EM, Kwon RY, Gross TS. Distinct cyclosporin a doses are required to enhance bone formation induced by cyclic and rest-inserted loading in the senescent skeleton. PLoS One 9: e84868, 2014. doi: 10.1371/journal.pone.0084868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tinkler SM, Williams DM, Johnson NW. Osteoclast formation in response to intraperitoneal injection of 1 alpha-hydroxycholecalciferol in mice. J Anat 133: 91–97, 1981. [PMC free article] [PubMed] [Google Scholar]
- 48.Tunyogi-Csapo M, Kis-Toth K, Radacs M, Farkas B, Jacobs JJ, Finnegan A, Mikecz K, Glant TT. Cytokine-controlled RANKL and osteoprotegerin expression by human and mouse synovial fibroblasts: fibroblast-mediated pathologic bone resorption. Arthritis Rheum 58: 2397–2408, 2008. doi: 10.1002/art.23653. [DOI] [PubMed] [Google Scholar]
- 49.Warden SJ, Galley MR, Richard JS, George LA, Dirks RC, Guildenbecher EA, Judd AM, Robling AG, Fuchs RK. Reduced gravitational loading does not account for the skeletal effect of botulinum toxin-induced muscle inhibition suggesting a direct effect of muscle on bone. Bone 54: 98–105, 2013. doi: 10.1016/j.bone.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warner SE, Sanford DA, Becker BA, Bain SD, Srinivasan S, Gross TS. Botox induced muscle paralysis rapidly degrades bone. Bone 38: 257–264, 2006. doi: 10.1016/j.bone.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med 360: 53–62, 2009. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wisitrasameewong W, Kajiya M, Movila A, Rittling S, Ishii T, Suzuki M, Matsuda S, Mazda Y, Torruella MR, Azuma MM, Egashira K, Freire MO, Sasaki H, Wang CY, Han X, Taubman MA, Kawai T. DC-STAMP is an osteoclast fusogen engaged in periodontal bone resorption. J Dent Res 96: 685–693, 2017. doi: 10.1177/0022034517690490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Worton LE, Gardiner EM, Bain SD, Gross TS. Transient muscle paralysis increases the osteoclastogenic differentiation potential of marrow. Long Beach, CA: Orthopaedic Research Society, Jan. 13-15, 2011, Poster No. 2317. Available at https://www.ors.org/Transactions/57/2317.pdf. [Google Scholar]
- 54.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202: 345–351, 2005. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang M, Birnbaum MJ, MacKay CA, Mason-Savas A, Thompson B, Odgren PR. Osteoclast stimulatory transmembrane protein (OC-STAMP), a novel protein induced by RANKL that promotes osteoclast differentiation. J Cell Physiol 215: 497–505, 2008. doi: 10.1002/jcp.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem 283: 11535–11540, 2008. doi: 10.1074/jbc.M607999200. [DOI] [PubMed] [Google Scholar]