Abstract
We are interested in understanding mechanisms that govern the protective role of exercise against lipid-induced insulin resistance, a key driver of type 2 diabetes. In this context, cell culture models provide a level of abstraction that aid in our understanding of cellular physiology. Here we describe the development of an in vitro myotube contraction system that provides this protective effect, and which we have harnessed to investigate lipid-induced insulin resistance. C2C12 myocytes were differentiated into contractile myotubes. A custom manufactured platinum electrode system and pulse stimulator, with polarity switching, provided an electrical pulse stimulus (EPS) (1 Hz, 6-ms pulse width, 1.5 V/mm, 16 h). Contractility was assessed by optical flow flied spot noise mapping and inhibited by application of ammonium acetate. Following EPS, myotubes were challenged with 0.5 mM palmitate for 4 h. Cells were then treated with or without insulin for glucose uptake (30 min), secondary insulin signaling activation (10 min), and phosphoinositide 3-kinase-α (PI3Kα) activity (5 min). Prolonged EPS increased non-insulin-stimulated glucose uptake (83%, P = 0.002), Akt (Thr308) phosphorylation (P = 0.005), and insulin receptor substrate-1 (IRS-1)-associated PI3Kα activity (P = 0.048). Palmitate reduced insulin-specific action on glucose uptake (−49%, P < 0.001) and inhibited insulin-stimulated Akt phosphorylation (P = 0.049) and whole cell PI3Kα activity (P = 0.009). The inhibitory effects of palmitate were completely absent with EPS pretreatment at the levels of glucose uptake, insulin responsiveness, Akt phosphorylation, and whole cell PI3Kα activity. This model suggests that muscle contraction alone is a sufficient stimulus to protect against lipid-induced insulin resistance as evidenced by changes in the proximal canonical insulin-signaling pathway.
Keywords: exercise, insulin resistance, diabetes, muscle, lipid
cell culture-based models are widely used to study cellular physiology. Such models are not beholden to the methodological limitations of human and animal studies, allowing for higher throughput, while also eliminating the need for tissue procurement. In vitro models have been applied to every facet of physiological research, but their relevance hinges upon their reproducibility of observed phenomena demonstrated in vivo and ex vivo. Several skeletal muscle contraction models have been published to date, with the goal of improving our understanding of exercise physiology. These models have relied on electrical pulse stimulation (EPS) of differentiated skeletal myotubes, and have faithfully reproduced exercise-induced hormone secretion (36, 38, 42, 45), substrate utilization and metabolic signaling (15, 41), protein synthesis (6, 17), and gene expression (7, 9). Despite their extensive application, many exercise-related phenomena still need cellular models to understand their beneficial roles with regard to metabolism.
We (24) and others (46) have shown that physical exercise in adults can protect against lipid-induced insulin resistance, a known driver of type 2 diabetes (12). This protective phenomenon has also been demonstrated in a rat model of exercise (51) but is yet to be established in an in vitro cellular model system. At the cellular level, insulin resistance manifests as a reduction in insulin-stimulated glucose uptake (11). Our earlier work indicated that the inflammatory cytokine tumor necrosis factor-α (TNF-α) reduces insulin-stimulated glucose uptake and inhibits insulin signaling in C2C12 skeletal myotubes (13), a murine cell line widely used to study muscle physiology (56). Subsequent experiments have shown that the TNF-α effect is mediated through saturated lipids (54), such as palmitate (16:0), which inhibit insulin signaling in vitro through phosphoinositide-3-kinase (PI3K), Akt/PKB, and glucose uptake (16, 23, 49). Given the methodological limitations of clinical and animal studies, in depth investigation is needed to better understand the protective role of exercise on elements of the canonical insulin signaling pathway.
Our interest lies in determining the mechanisms that impart the beneficial effects of exercise, especially in protection against insulin resistance. Although exercise is a systemic event resulting in various metabolic and cardiovascular adaptations (32), the primary component of skeletal muscle contraction could underlie the beneficial effects. Thus, we hypothesized that contraction in isolation is a sufficient stimulus to protect muscle against lipid-induced insulin resistance by exploring the protective phenomenon along the canonical insulin signaling pathway. We compare data from this in vitro muscle contraction model with clinical observations, to contextualize the systemic and cell specific-responses to exercise.
EXPERIMENTAL PROCEDURES
Materials.
All cell culture media (Dulbecco’s modified Eagle’s medium, phosphate-buffered saline, and penicillin-streptomycin) were obtained from the Cleveland Clinic Lerner Research Institute Media Preparations Core (Cleveland, OH). Fetal bovine serum (FBS) and horse serum were obtained from Life Technologies (Carlsbad, CA). Palmitate, sodium pyruvate, d-mannitol, d-(+)-glucose, 2-deoxy-d-glucose, gelatin (from porcine skin), HEPES, potassium chloride, calcium chloride, magnesium chloride, magnesium sulfate, and PMSF were purchased from Sigma Aldrich (St. Louis, MO). Dimethyl sulfoxide, glycine, sodium chloride, Tween 20, Tris base, and ScintiSafe Econo2 scintillation cocktail were purchased from Fischer Scientific (Pittsburgh, PA). Bovine serum albumin and PhosSTOP Easy Pack were purchased from Roche (Indianapolis, IN). Sodium dodecyl sulfate was purchased from Pierce (Rockford, IL). Cell extraction buffer was purchased from Life Technologies.
Cell culture.
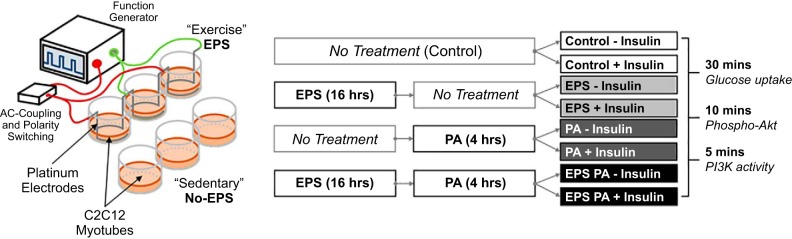
C2C12 myoblasts were purchased from the American Type Culture Collection (Manassas, VA) and proliferated in high glucose (25 mM) DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (100 U/ml) placed in a water-jacketed incubator set at 37°C and 5% CO2. Prior to 80% confluence, cells at passages 4 through 7 were plated onto gelatin-coated 12-well plates. Gelatin coating was achieved by incubating wells with 0.1% gelatin (wt/vol in ddH2O) for 2 h at 37°C followed by PBS washing. Upon confluence, media were switched to differentiation media composed of DMEM high glucose supplemented with 2% horse serum and 1% penicillin-streptomycin. Differentiation media were replenished daily by removing 75% of cell media followed by the addition of fresh media. Cells were deemed ready for stimulation after the C2C12 myoblasts fully differentiated into contractile myotubes (4 to 5 days). Cells were then treated as shown in Fig. 1B according to the techniques described below.
Fig. 1.
A: schematic of electrical pulse stimulus (EPS) system for inducing C2C12 myotube contraction. B: experimental design for 4 different conditions [control, EPS, palmitate (PA), and EPS+PA] stimulated with or without insulin for 5, 10, or 30 min for the indicated experimental outcomes.
Electrical pulse stimulation.
Fully differentiated C2C12 myotubes were induced to contract via electrical pulse stimulation (EPS), as represented schematically in Fig. 1A. An electric field was generated by submerged platinum (99.95%) wire electrodes to promote membrane depolarization and action potential propagation as an artificial excitation-contraction-coupling stimulus. Electrical current was provided at 1 Hz, 6-ms pulse width (0.6% duty cycle), and 1.5 V/mm with a Grass S88 Dual Output Square Pulse Stimulator (Warwick, RI) set at stimulus isolation unit mode. An AC-coupling capacitor (250 μF) was included to remove the direct current component to attenuate reduction/oxidation at the electrodes. A Double Pole Double Throw (DPDT) mechanical relay switched the electrical field polarity at 0.5 Hz to negate electrophoretic effects. Differentiation medium was replaced before the 16 h EPS bout for both stimulated and nonstimulated control wells to ensure that nutrient deprivation did not blunt the responses.
Contractility.
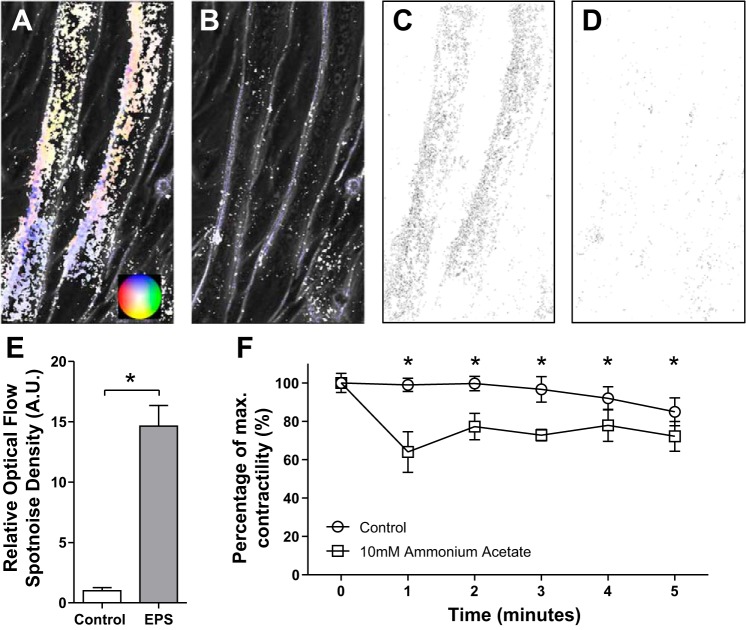
Relative contractility of C2C12 myotubes was quantified using a modified adaptation of optical flow measurements with the FlowJ plugin in ImageJ (National Institutes of Health, Bethesda, MD). FlowJ is generally used to detect movement in video for object recognition, producing an optical flow field (a vector map) that represents individual pixel movement (1). Video recordings of EPS (1 Hz, 60-ms pulse width, 3.3 V/mm) contracting myotubes were captured with an Olympus IX81 Inverted Microscope at 10 frames per second. The optical flow field was calculated using the Lucas and Kanade algorithm (all parameters set to 1). Shown in Fig. 2 are representative optical flow fields for two adjacent myotubes over the course of a single contraction (Fig. 2A), or at rest (Fig. 2B). The direction and magnitude of each pixel vector is indicated by the color wheel insert in Fig. 2A. Spot noise maps were then created for each optical flow field, combining both magnitude and direction into a single pixel color value (Fig. 2, C–D). A mean histogram density of spot noise flow field maps for each myotube was calculated to quantify contractility (53). Since hyperammonemia impairs skeletal muscle contractile function in vivo and modifies contractile proteins in cell culture in vitro (37), the impact of 10 mM ammonium acetate on contractile function in C2C12 myotubes was quantified and compared with vehicle-treated control cells. Decreases in contractility are represented by decreases in mean histogram density relative to that of control contractions before the addition of a final concentration of 10 mM ammonium acetate (Sigma Aldrich). Videos were taken every 60 s for 5 s to quantify the average contractility for each myotube (2–4) over five contractions. Relative contractility may be represented as a relative (to control) mean of histogram density or as a percentage of maximal density (at baseline).
Fig. 2.
Quantification of reductions in in vitro C2C12 myotube contractility with ammonium acetate. A and B: directional two-dimensional optical flow maps of pixel movement from a single contraction over 10 frames (A) and with no contraction control (B). Direction and intensity of pixel movement is represented by the color wheel insert (A). C and D: spot noise maps depicting total pixel movement for each point over 10 frames with a single contraction (C) and no contraction control (D). E: average spot noise histogram density for each myotube (n = 4) relative to no contraction control. AU, arbitrary units. F: average spot noise histogram density (n = 10) of control myotubes (○) or 10 mM ammonium acetate-incubated myotubes (□). *P < 0.01.
Palmitic acid challenge.
Palmitate was dissolved in ethanol (200 Proof, Pharmaco-Aaper, Brookfield, CT) at a concentration of 100 mM. On the day of the challenge, the palmitic acid stock was conjugated with Free Fatty Acid-free BSA (10% wt/vol in ddH2O) via incubation at 60°C for 10 min with constant mild agitation. The palmitate:BSA molar ratio during conjugation was 10:3, providing BSA binding sites for long-chain free fatty acids in excess (48). The conjugated solution was diluted to a final concentration of palmitic acid at 0.5 mM and BSA at 1% with Krebs-Ringer-HEPES (KRH) buffer (20 mM HEPES, 136 mM sodium chloride, 4.7 mM potassium chloride, 1.25 mM magnesium sulfate, 1.25 mM calcium chloride) supplemented 32 mM d-mannitol, and 8 mM d-(+)-glucose. EPS was stopped, electrodes were removed, and media were aspirated before the addition of the palmitic acid-containing media. Control wells received the same media (including BSA at 1% wt/vol) without palmitic acid. Cells were incubated for a total challenge time of 4 h. Once this treatment step was complete, media were carefully aspirated before the addition of insulin or non-insulin control media before final analysis.
Glucose uptake.
Insulin responsiveness was measured by an insulin-stimulated glucose uptake assay adapted from Gao et al. (21), whose methods ensured robust insulin-stimulated glucose uptake. After EPS and palmitic acid incubation, including control conditions, cells were stimulated with 1.0 µM insulin (Novolin R Regular Human Insulin - Recombinant DNA Origin, Novo Nordisk, Plainsboro, NJ) in KRH supplemented with 2 mM sodium pyruvate, 32 mM d-mannitol, and 0.1% BSA for 30 min. Mannitol served as an osmolyte, while sodium pyruvate served as a metabolic substrate during glucose-free incubation. Insulin stimulation media were then aspirated and cells were incubated for 10 min in KRH supplemented with 1 μCi/ml [3H]-2-deoxy-d-glucose (Perkin Elmer, Waltham, MA) and 2 mM cold 2-deoxy-d-glucose. Uptake of radiolabeled glucose was terminated by aspiration and rapid washing three times with ice-cold PBS. Cells were then lysed with 0.1% SDS in ddH2O on a plate shaker for 30 min. Lysate was used for both BCA protein quantification as an internal control and for scintillation counting following the addition of ScintiSafe scintillation cocktail. Glucose uptake rate was calculated as a percentage increase over control uptake (without EPS, palmitic acid, or insulin treatment). Results from three independent experiments, each in triplicate, were normalized to control non-insulin and insulin-stimulated glucose before compiling data sets.
Lipid kinase assay.
Phosphoinositide-3-kinase-α (PI3Kα) activity was determined as previously described (39, 40). Briefly, following EPS and palmitic acid challenge, cells were incubated with or without 1.0 µM insulin for 5 min. Insulin stimulation was stopped by aspiration and cells were rapidly washed once with ice-cold PBS. Washed cells were placed on dry ice for immediate freezing. Plates were scraped and experimental triplicates combined for a minimum of 300 µg, of which 150 µg was used for immunoprecipitation with insulin receptor substrate-1 (IRS-1; Cell Signaling Technologies, Danvers, MA) or pan-PI3Kα-P110 (Santa Cruz, Dallas, TX) antibodies. The same antibodies were also used for Western blotting. Lysates were then incubated with phosphatidylinositol (PI) (Sigma Aldrich) and radiolabeled ATP [γ-32P] (Perkin Elmer) with continuous agitation. Samples were then blotted onto thin layer chromatography plates for separation. Subsequent radiographs were quantified by fixed area density calculations using ImageJ.
Western blotting.
Following treatment with or without EPS and palmitate, myotubes were incubated with or without 1.0 µM insulin for 10 min. Cells were then washed twice with ice-cold PBS before the addition of a cell extraction cocktail (5% protease inhibitor, 5% PhosSTOP, 1% sodium orthovanadate, 0.35% PMSF, in cell extraction buffer). Plates were then stored at −80°C until ready for scraping and cell lysate preparation. Collected crude cell lysates were spun down at 10,000 g for 10 min to pellet any insoluble matter. Supernatant was collected and quantified for total protein content (Pierce BCA Protein Assay Kit, Thermo Scientific). Protein (25 µg) lysate for each condition was prepared with Laemmli buffer containing β-mercaptoethanol, heated to 95°C for 30 min, and loaded onto a precast 4–12% Tris-glycine gel (Novex WedgeWell, Life Technologies). Gels were run until completion at fixed voltage (125 V) before setup for transfer onto PVDF membranes (Bio-Rad, Hercules, CA) for 90 min at fixed voltage (60 V, Criterion Blotter, Bio-Rad). Membranes were blocked with 5% BSA in PBS-T (PBS-0.15% Tween 20) for at least 30 min before overnight incubation of primary antibodies (all at 1:1,000 in blocking buffer): Glucose transporter 4 (Glut4; no. G4048, Sigma Aldrich), phospho-Akt-Thr308 (9275S, CST), total Akt (9272S, CST), total IRS-1 (2390S, CST), PI3Kα-P110 (sc-7189, Santa Cruz), and HSC 70 (sc-7298, Santa Cruz) as an internal loading control. Mouse and rat ECL secondary antibodies tagged to horseradish peroxidase (GE Healthcare, Chicago, IL) were used (2 h secondary incubation), and chemiluminescent detection was performed on a LAS 500 imager (GE Healthcare). Blots were quantified using ImageJ.
Statistical analysis.
All analyses were performed in GraphPad Prism 5 (La Jolla, CA). Data were tested for normality using the Kolmogorov-Smirnov test. For normally distributed data, a Student’s t-test was used to determine statistically significant differences between treatments. For non-normally distributed data a Mann-Whitney test was used instead. A Δ% between mean insulin and non-insulin-stimulated glucose uptake was calculated for the four treatment combinations [control, EPS, palmitate (PA), EPS+PA] to represent insulin-specific action on glucose uptake (see Fig. 3B). Standard deviations were determined from the variance of the difference of the means as calculated using the Central Limit Theorem. Data from independent experiments were normalized to the internal (non-insulin stimulated) control within each experiment. The accepted P-value for significance was set at less than 0.05. All data are presented as means and standard deviations, with replicate numbers indicated in figure legends.
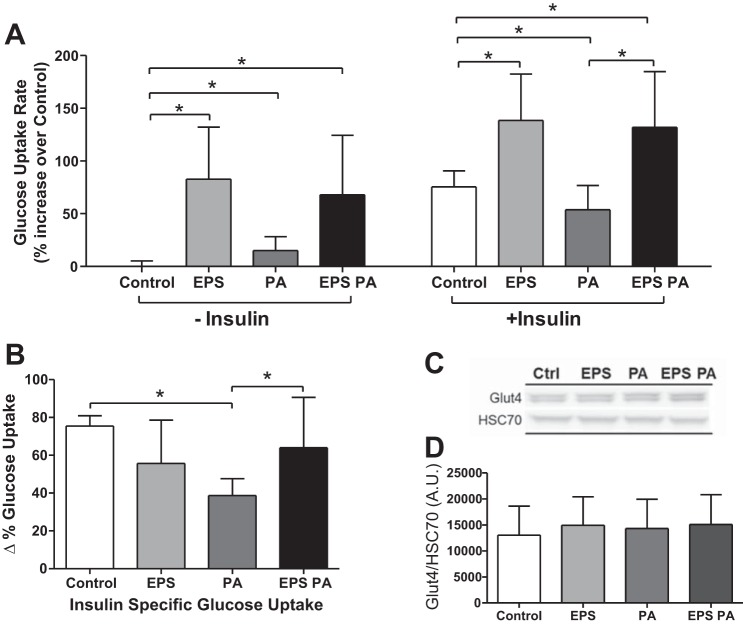
Fig. 3.
Glucose uptake rate and glucose transporter expression. A: relative glucose uptake rate of control (Ctrl), EPS, palmitate (PA), and combined stimulus (EPS+PA), under non-insulin and insulin-stimulated conditions (n = 3). B: Δ% change in glucose uptake rate between non-insulin and insulin-stimulated conditions (shown in A), representing insulin-specific action on the background of non-insulin-mediated changes in glucose uptake. C and D: representative Western blots for Glut4 (C) and quantification (n = 12) of total protein expression (both bands; D). *P < 0.05.
RESULTS
Contractility.
A reliable model of exercise will most obviously produce contracting muscle cells, but more importantly respond to contraction stimuli in a predictable manner. Supplemental Video S1 shows that EPS induced contractions are directly dependent on stimulus frequency, with higher frequencies (>4 Hz) almost inducing a tetanic state. (Supplemental Material for this article is available at the Journal website.) Optical flow field spot noise mapping (Fig. 2, A–D) calculations show that EPS readily increases myotube contraction (Fig. 2E). The addition of 10 mM ammonium acetate instantly inhibited contraction, despite continued EPS (Supplemental Video S2). Within 1 min, contractility was reduced significantly (99.0 ± 3.5% vs. 64.0 ± 10.6%, P = 0.008) (Fig. 2F). Mean contractility of ammonium acetate-treated myotubes remained significantly lower than control contractions throughout the 5-min observation period.
Glucose uptake.
Radiolabeled glucose uptake by C2C12 myotubes is presented here as a percentage increase over control without insulin stimulation (Fig. 3A). In the absence of insulin, EPS significantly (P = 0.002 vs. control) increased the glucose uptake rate by 83%. Palmitate treatment alone acutely increased glucose uptake (15%, P = 0.01), to a much lower extent than EPS. EPS treatment followed by palmitate challenge and EPS alone had similar glucose uptake rates in the absence of insulin (P = 0.58) and was still significantly greater than non-insulin-stimulated control uptake rate (68%, P = 0.007). Insulin stimulation, as expected, resulted in an increase in glucose uptake (75%, P < 0.001), with an effect size similar to that of EPS alone (P = 0.70) (Fig. 3A). Prior EPS stimulation, followed by a 4 h “rest” period, produced a higher insulin stimulated glucose uptake rate than insulin stimulation alone (138%, P = 0.003). Prior palmitate treatment reduced the insulin-stimulated glucose uptake rate (54%, P = 0.03, Fig. 3A), with an effective reduction in insulin-specific action on glucose uptake of 49% (75% vs. 39%, P < 0.001, Fig. 3B). EPS was able to rescue the inhibitory effect of palmitate on insulin-stimulated glucose uptake and insulin responsiveness (Fig. 3, A and B). Total Glut4 protein expression remained unchanged (Fig. 3, C and D).
Canonical insulin signaling pathway.
A key downstream signaling mediator of insulin-stimulated glucose uptake is the serine and threonine kinase Akt/PKB. This kinase is phosphorylated at the threonine-308 residue in response to insulin stimulation (3). Insulin stimulation of muscle cells results in phosphorylation of Akt due to recruitment of phosphoinositide-dependent kinase-1 (PDK1) and Akt to the plasma membrane through generation of phosphatidylinositol(3,4,5)-trisphosphate (PIP3) due to activation of PI3Kα. PI3Kα is recruited to activated insulin receptors through insulin receptor substrates, wherein PI3Kα converts phosphatidylinositol 4,5-bisphosphate (PIP2) to PIP3, leading to subsequent recruitment of downstream molecules (3).
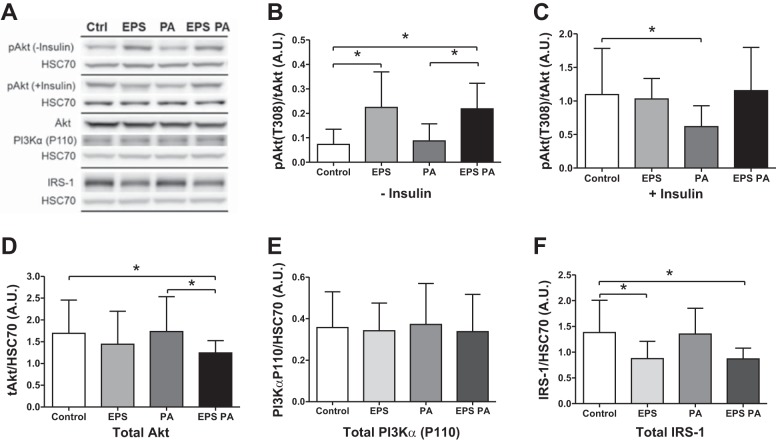
EPS increased Akt phosphorylation in the absence of insulin (P = 0.005), which was unaffected by palmitate (Fig. 4B). As expected, insulin stimulation increased Akt phosphorylation by ten-fold (Fig. 4C). Prior EPS stimulation did not affect this insulin-stimulated increase in phosphorylation. As with the glucose uptake rate, palmitate significantly reduced insulin-stimulated phosphorylation of Akt to only fivefold of non-insulin-stimulated levels (P = 0.048). This inhibitory effect was fully rescued by prior EPS stimulation (Fig. 4C). Total protein expression of Akt was decreased by EPS stimulation, with a significant reduction in total expression in the combination of EPS and palmitate treatment (P = 0.013, Fig. 4D). PI3Kα catalytic subunit expression was unaltered by either EPS or palmitate treatment (Fig. 4E). IRS-1 protein expression, however, was significantly reduced by ~50% following EPS treatment in the presence (P = 0.037) or absence (P = 0.024) of the palmitate challenge (Fig. 4F).
Fig. 4.
Protein expression and phosphorylation for Akt (n = 11), phosphoinositide 3-kinase-α (PI3Kα; n = 9), and IRS-1 (n = 9). A: representative Western blots. B–D: non-insulin (B) and insulin-stimulated (C) phosphorylated Akt (Thr308) expression corrected to total Akt protein (D; both corrected to HSC 70 expression). E and F: total expression of PI3Kα (E) and IRS-1 (F). *P < 0.05.
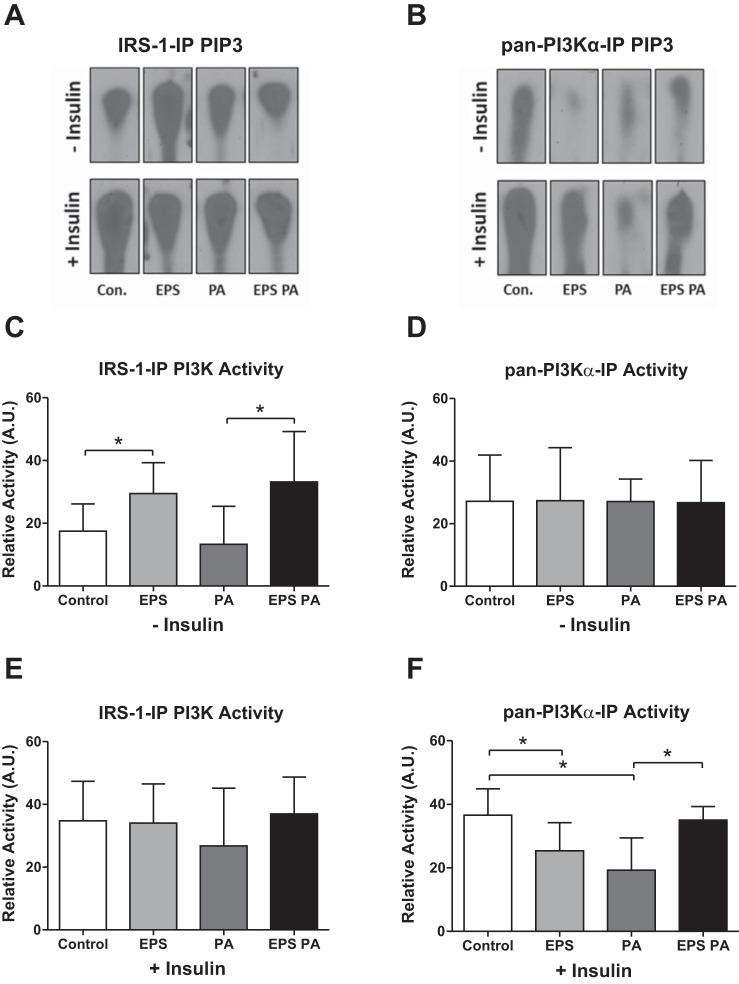
We measured the activity of IRS-1-associated PI3K and PI3Kα to determine the specificity of PI3Kα activation in all treatment conditions (see Fig. 1B). IRS-1-associated PI3K activity was increased in response to EPS treatment (P = 0.048, Fig. 5C), similar to non-insulin-stimulated Akt phosphorylation (Fig. 4B), while insulin-stimulated IRS-1-associated PI3K activity was not altered by EPS (Fig. 5E). However, IRS-1-associated PI3K activity showed a slight but nonsignificant reduction with palmitate challenge (Fig. 5E). Interestingly, PI3Kα activity was not altered with either EPS or palmitate treatments in the absence of insulin stimulation (Fig. 5D). Significantly, EPS protected against palmitate-induced reductions in PI3Kα activity, despite the reduction in enzyme activity (Fig. 5F). These data match the insulin responsiveness data shown in Fig. 3B.
Fig. 5.
PI3K catalytic activity. A and B: representative radiographs arranged to match bar graph layouts. PIP3, phosphatidylinositol(3,4,5)-trisphosphate. C and D: non-insulin-stimulated relative PI3K activity for IRS-1-IP (C; n = 6) and pan-PI3Kα-IP (D; n = 6) as quantified from PIP3 radiographs. E and F: insulin-stimulated relative PI3K activity for IRS-1-IP (E; n = 5) and pan-PI3Kα-IP (F; n = 6). *P < 0.05.
DISCUSSION
The objective of this study was to develop an in vitro contraction model that allows for elucidation of the protective effects of exercise against lipid-induced insulin resistance. By taking a reductionist approach, we were able to abstract the effects of contraction on muscle insulin signaling, independent of the systemic effects of exercise. We demonstrate that the protective effect of exercise on peripheral insulin sensitivity may be explained by muscle contraction alone. The protection is evident at the proximal level of the insulin-signaling pathway, which is inhibited under insulin-resistant states in type 2 diabetes (5).
Much of the difficulty in developing in vitro models lies in the optimization of cell culture methods. We reviewed the literature of successfully implemented C2C12 contraction models (2, 4, 14, 18–20, 34, 35, 41, 43, 50, 55, 57) and tested a variety of approaches. Precoating plates with gelatin, growth until confluence, and daily partial changes in media resulted in full differentiation of C2C12 myocytes into contractile myotubes within 5 days. These specific technical details were absent from previously reported methods. Visually confirmed contraction was immediately evident upon EPS, without the need for an acclimation period to align sarcomeres, as reported by others (18–20, 41). To confirm and quantify myotube contractility, we used a novel application of a video analysis algorithm (Fig. 2, A–E) (53). With this technique, we are able to confirm the inhibitory effect of ammonium acetate on muscle contraction, further demonstrating the ability of our model to replicate in vivo contractile dysfunction in response to a specific intervention (Fig. 2F) (37). Traditionally, contractility measures are made via cell shortening microscopy, force transducers, or other engineered devices (22). This application of the model with optical flow field mapping provides an accurate and higher throughput approach to study the effects of various stimuli on contractility in vitro, which can be applied to simple bright-field microscopy videos.
We used a total of 16 h of EPS in this model. An exercise bout of this length is not common in humans or rodents. However, this duration produced a persistent effect on potentiating glucose uptake (Fig. 3A) and interleukin-6 secretion (data not shown), and is consistent with effects previously observed after physical training (25). IRS-1 protein expression data give an indication as to what type of exercise this model mimics. Chibalin et al. (10) have shown that 6 h of daily swimming in rats reduces muscle expression of IRS-1 only after 5 days, suggesting that our model is equivalent to several consecutive days of aerobic training (Fig. 4F). IRS-1-associated PI3K activity as well as Akt phosphorylation were also elevated following the 5 days of training in rats, consistent with our observations (Fig. 4B and Fig. 5C). However, in contrast to their study, marked reduction in total Akt protein expression was observed following EPS (Fig. 4D), suggesting a negative-feedback downregulation as described by Chibalin et al. for the reduced IRS-1 expression (10). Despite protecting against insulin resistance, EPS reduced expression of key elements in the canonical insulin-signaling pathway, indicating a role for alternative noncanonical signaling pathways. Previous studies have suggested that the insulin receptor substrate-2 (IRS-2) may mediate the alternative pathways in downstream signals (8, 10, 28, 29). Furthermore, insulin-stimulated PI3Kα activity in our model (Fig. 5F) supports the idea that mechanisms independent of the insulin receptor substrate may play a role in the protective effect of contraction against palmitate-induced insulin resistance.
Shorter, 20 min, palmitate incubations of L6 and C2C12 myotubes increase glucose uptake in an Akt/PKB- and ERK1/2-dependent manner (44). Here we show that this acute effect of palmitate persists for up to 4 h (Fig. 3A). Palmitate does not appear to alter EPS-induced increases in glucose uptake, Akt phosphorylation, or PI3K activity in the absence of insulin. In the presence of insulin, the inhibitory effects of palmitate are seen in our glucose uptake and Akt phosphorylation data, consistent with many other observations (16, 49, 51, 52). We also show that EPS drives an increase in Akt phosphorylation (Fig. 4B). Camera et al. (8) reported that both endurance and resistance exercise can increase muscle Akt phosphorylation in the absence of insulin stimulation for up to 60 min after exercise. Our data here suggest that increased Akt phosphorylation is likely driven by increased IRS-1-associated PI3K activity under non-insulin-stimulated conditions (Fig. 5C), which persists in vitro for up to 4 h (Fig. 4A). The effect of the combination of EPS and insulin stimuli on glucose uptake was not fully additive (Fig. 3, A and B), which may be due to a saturation in uptake rate with the dual stimuli. Alternatively, this finding supports the notion of shared pools of recruitable glucose transporters (12).
Even one week of daily exercise results in improved peripheral insulin sensitivity in humans (33). We have also previously shown that individuals engaged in regular exercise training have greater insulin-stimulated activation of IRS-1-associated PI3K activity (31), which is diminished in muscle of type 2 diabetes patients (30). This in vitro model does not display a characteristic increase in insulin responsiveness at the levels of glucose uptake (Fig. 3B) or IRS-1-associated PI3K activity (Fig. 5E) (26). Gao et al. (21) have demonstrated that a serum factor is necessary to produce contraction-induced increases in muscle insulin sensitivity. Although a low concentration of horse serum is present during the EPS bout in our model, subsequent treatments were performed in serum-free medium to avoid the confounding effects of additional fatty acids in serum. As a result, the necessary insulin-sensitizing factor would not be present during palmitate challenge or insulin stimulation. Furthermore, the horse serum and host serum (mouse in this model) may be different in regard to its role in modulating insulin signaling. This observation, like other aforementioned inconsistencies, suggests different systemic versus cell-specific effects of exercise that can only be identified through abstraction.
In this report, we have only used the model to examine the proximal insulin-signaling pathway. Our clinical findings suggested that increased whole body lipid kinetics and skeletal muscle free fatty acid oxidation play an important role in the reduction in lipid-induced insulin resistance after exercise training (47). Schenk and Horowitz observed increased triglyceride synthesis, reduced ceramides, and reduced proinflammatory signaling in human skeletal muscle when protected from lipid-induced insulin resistance by a single exercise bout (27, 46). Thrush et al. (51) also observed the same increases in lipid kinetics and fatty acid oxidation in their rat exercise model. Through ex vivo palmitate incubations of excised muscle following treadmill running in rats, Thrush et al. also showed that in response to an insulin stimulus, the protective effect was evident at the levels of skeletal muscle glucose uptake and Akt phosphorylation (51). Within the proximal insulin-signaling pathway, these in vivo findings are consistent with what we have observed here in vitro. Future applications of this model are ideally suited to investigate lipid metabolism, as radioisotopic tracer methods, toxic inhibitors, and siRNA are readily applied in cell culture. However, as with any model, there are limitations to in vitro interpretations as they relate to the systemic effects of exercise.
We conclude that contraction alone may protect muscle from lipid-induced insulin resistance, evident at the levels of glucose uptake and proximal insulin signaling. Our data support the presence of a noncanonical PI3Kα-activating pathway that could potentially be independent of insulin receptor substrate, mediating the protective role of exercise. The robustness and validity of this “exercise in a petri dish” system has been thoroughly tested here and may be used as a high-throughput tool to investigate exercise metabolism.
GRANTS
This study was funded by investigator initiated grants from the Metabolic Translational Research Center (MTRC 13-1563, Cleveland Clinic, Cleveland, OH) and the National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R21 AR067477).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.N., A.M., C.E.F., S.D., S.V.N.P., and J.P.K. conceived and designed research; S.N., A.M., C.E.F., and E.M. performed experiments; S.N. and A.M. analyzed data; S.N., A.M., C.E.F., S.V.N.P., and J.P.K. interpreted results of experiments; S.N. prepared figures; S.N. drafted manuscript; S.N., A.M., C.E.F., S.D., S.V.N.P., and J.P.K. edited and revised manuscript; S.N. and J.P.K. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Abràmoff MD, Niessen WJ, Viergever MA. Objective quantification of the motion of soft tissues in the orbit. IEEE Trans Med Imaging 19: 986–995, 2000. doi: 10.1109/42.887614. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama Y, Furukawa Y, Morishima K. Controllable bio-microactuator powered by muscle cells. Conf Proc IEEE Eng Med Biol Soc Suppl: 6565–6568, 2006. doi: 10.1109/IEMBS.2006.260890. [DOI] [PubMed] [Google Scholar]
- 3.Andjelković M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem 272: 31515–31524, 1997. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 4.Asano T, Ishizua T, Yawo H. Optically controlled contraction of photosensitive skeletal muscle cells. Biotechnol Bioeng 109: 199–204, 2012. doi: 10.1002/bit.23285. [DOI] [PubMed] [Google Scholar]
- 5.Björnholm M, Kawano Y, Lehtihet M, Zierath JR. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes 46: 524–527, 1997. doi: 10.2337/diab.46.3.524. [DOI] [PubMed] [Google Scholar]
- 6.Brevet A, Pinto E, Peacock J, Stockdale FE. Myosin synthesis increased by electrical stimulation of skeletal muscle cell cultures. Science 193: 1152–1154, 1976. doi: 10.1126/science.959833. [DOI] [PubMed] [Google Scholar]
- 7.Burch N, Arnold AS, Item F, Summermatter S, Brochmann Santana Santos G, Christe M, Boutellier U, Toigo M, Handschin C. Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS One 5: e10970, 2010. doi: 10.1371/journal.pone.0010970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42: 1843–1852, 2010. doi: 10.1249/MSS.0b013e3181d964e4. [DOI] [PubMed] [Google Scholar]
- 9.Chahine KG, Baracchini E, Goldman D. Coupling muscle electrical activity to gene expression via a cAMP-dependent second messenger system. J Biol Chem 268: 2893–2898, 1993. [PubMed] [Google Scholar]
- 10.Chibalin AV, Yu M, Ryder JW, Song XM, Galuska D, Krook A, Wallberg-Henriksson H, Zierath JR. Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: differential effects on insulin-receptor substrates 1 and 2. Proc Natl Acad Sci USA 97: 38–43, 2000. doi: 10.1073/pnas.97.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciaraldi TP, Abrams L, Nikoulina S, Mudaliar S, Henry RR. Glucose transport in cultured human skeletal muscle cells. Regulation by insulin and glucose in nondiabetic and non-insulin-dependent diabetes mellitus subjects. J Clin Invest 96: 2820–2827, 1995. doi: 10.1172/JCI118352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, Suppl 2: S157–S163, 2009. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol Endocrinol Metab 276: E849–E855, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Dennis RG, Kosnik PE II. Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim 36: 327–335, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 15.Evers-van Gogh IJ, Alex S, Stienstra R, Brenkman AB, Kersten S, Kalkhoven E. Electric pulse stimulation of myotubes as an in vitro exercise model: cell-mediated and non-cell-mediated effects. Sci Rep 5: 10944, 2015. doi: 10.1038/srep10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng XT, Wang TZ, Leng J, Chen Y, Liu JB, Liu Y, Wang WJ. Palmitate contributes to insulin resistance through downregulation of the Src-mediated phosphorylation of Akt in C2C12 myotubes. Biosci Biotechnol Biochem 76: 1356–1361, 2012. doi: 10.1271/bbb.120107. [DOI] [PubMed] [Google Scholar]
- 17.Freud-Silverberg M, Shainberg A. Electric stimulation regulates the level of Ca-channels in chick muscle culture. Neurosci Lett 151: 104–106, 1993. doi: 10.1016/0304-3940(93)90057-R. [DOI] [PubMed] [Google Scholar]
- 18.Fujita H, Endo A, Shimizu K, Nagamori E. Evaluation of serum-free differentiation conditions for C2C12 myoblast cells assessed as to active tension generation capability. Biotechnol Bioeng 107: 894–901, 2010. doi: 10.1002/bit.22865. [DOI] [PubMed] [Google Scholar]
- 19.Fujita H, Nedachi T, Kanzaki M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res 313: 1853–1865, 2007. doi: 10.1016/j.yexcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Fujita H, Shimizu K, Nagamori E. Novel method for measuring active tension generation by C2C12 myotube using UV-crosslinked collagen film. Biotechnol Bioeng 106: 482–489, 2010. doi: 10.1002/bit.22705. [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Gulve EA, Holloszy JO. Contraction-induced increase in muscle insulin sensitivity: requirement for a serum factor. Am J Physiol Endocrinol Metab 266: E186–E192, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Grosberg A, Nesmith AP, Goss JA, Brigham MD, McCain ML, Parker KK. Muscle on a chip: in vitro contractility assays for smooth and striated muscle. J Pharmacol Toxicol Methods 65: 126–135, 2012. doi: 10.1016/j.vascn.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haghani K, Pashaei S, Vakili S, Taheripak G, Bakhtiyari S. TNF-α knockdown alleviates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Biochem Biophys Res Commun 460: 977–982, 2015. doi: 10.1016/j.bbrc.2015.03.137. [DOI] [PubMed] [Google Scholar]
- 24.Haus JM, Solomon TP, Marchetti CM, Edmison JM, González F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab 95: 323–327, 2010. doi: 10.1210/jc.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol 546: 299–305, 2003. doi: 10.1113/jphysiol.2002.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol (1985) 99: 338–343, 2005. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- 27.Horowitz JF. Exercise-induced alterations in muscle lipid metabolism improve insulin sensitivity. Exerc Sport Sci Rev 35: 192–196, 2007. doi: 10.1097/jes.0b013e318156e084. [DOI] [PubMed] [Google Scholar]
- 28.Howlett KF, Sakamoto K, Hirshman MF, Aschenbach WG, Dow M, White MF, Goodyear LJ. Insulin signaling after exercise in insulin receptor substrate-2-deficient mice. Diabetes 51: 479–483, 2002. doi: 10.2337/diabetes.51.2.479. [DOI] [PubMed] [Google Scholar]
- 29.Howlett KF, Sakamoto K, Yu H, Goodyear LJ, Hargreaves M. Insulin-stimulated insulin receptor substrate-2-associated phosphatidylinositol 3-kinase activity is enhanced in human skeletal muscle after exercise. Metabolism 55: 1046–1052, 2006. doi: 10.1016/j.metabol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest 104: 733–741, 1999. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirwan JP, del Aguila LF, Hernandez JM, Williamson DL, O’Gorman DJ, Lewis R, Krishnan RK. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J Appl Physiol (1985) 88: 797–803, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med 84, Suppl 1: S15–S21, 2017. doi: 10.3949/ccjm.84.s1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 297: E151–E156, 2009. doi: 10.1152/ajpendo.00210.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manabe Y, Miyatake S, Takagi M, Nakamura M, Okeda A, Nakano T, Hirshman MF, Goodyear LJ, Fujii NL. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS One 7: e52592, 2012. doi: 10.1371/journal.pone.0052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marotta M, Bragós R, Gómez-Foix AM. Design and performance of an electrical stimulator for long-term contraction of cultured muscle cells. Biotechniques 36: 68–73, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Matthews VB, Aström MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, Akerström T, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Bruunsgaard H, Pedersen BK, Febbraio MA. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 52: 1409–1418, 2009. [Erratum: 53: 854, 2015.] doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 37.McDaniel J, Davuluri G, Hill EA, Moyer M, Runkana A, Prayson R, van Lunteren E, Dasarathy S. Hyperammonemia results in reduced muscle function independent of muscle mass. Am J Physiol Gastrointest Liver Physiol 310: G163–G170, 2016. doi: 10.1152/ajpgi.00322.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyatake S, Bilan PJ, Pillon NJ, Klip A. Contracting C2C12 myotubes release CCL2 in an NF-κB-dependent manner to induce monocyte chemoattraction. Am J Physiol Endocrinol Metab 310: E160–E170, 2016. doi: 10.1152/ajpendo.00325.2015. [DOI] [PubMed] [Google Scholar]
- 39.Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem 276: 18953–18959, 2001. doi: 10.1074/jbc.M102376200. [DOI] [PubMed] [Google Scholar]
- 40.Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA. Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex. J Cell Biol 158: 563–575, 2002. doi: 10.1083/jcb.200202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab 295: E1191–E1204, 2008. doi: 10.1152/ajpendo.90280.2008. [DOI] [PubMed] [Google Scholar]
- 42.Nedachi T, Hatakeyama H, Kono T, Sato M, Kanzaki M. Characterization of contraction-inducible CXC chemokines and their roles in C2C12 myocytes. Am J Physiol Endocrinol Metab 297: E866–E878, 2009. doi: 10.1152/ajpendo.00104.2009. [DOI] [PubMed] [Google Scholar]
- 43.Pan H, Xu X, Hao X, Chen Y. Changes of myogenic reactive oxygen species and interleukin-6 in contracting skeletal muscle cells. Oxid Med Cell Longev 2012: 145418, 2012. doi: 10.1155/2012/145418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pu J, Peng G, Li L, Na H, Liu Y, Liu P. Palmitic acid acutely stimulates glucose uptake via activation of Akt and ERK1/2 in skeletal muscle cells. J Lipid Res 52: 1319–1327, 2011. doi: 10.1194/jlr.M011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, Beckers J, Hrabé de Angelis M, Häring HU, Weigert C. Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol 305: C877–C886, 2013. doi: 10.1152/ajpcell.00043.2013. [DOI] [PubMed] [Google Scholar]
- 46.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon TP, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab 297: E552–E559, 2009. doi: 10.1152/ajpendo.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spector AA, John K, Fletcher JE. Binding of long-chain fatty acids to bovine serum albumin. J Lipid Res 10: 56–67, 1969. [PubMed] [Google Scholar]
- 49.Storz P, Döppler H, Wernig A, Pfizenmaier K, Müller G. Cross-talk mechanisms in the development of insulin resistance of skeletal muscle cells palmitate rather than tumour necrosis factor inhibits insulin-dependent protein kinase B (PKB)/Akt stimulation and glucose uptake. Eur J Biochem 266: 17–25, 1999. doi: 10.1046/j.1432-1327.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 50.Thelen MH, Simonides WS, van Hardeveld C. Electrical stimulation of C2C12 myotubes induces contractions and represses thyroid-hormone-dependent transcription of the fast-type sarcoplasmic-reticulum Ca2+-ATPase gene. Biochem J 321: 845–848, 1997. doi: 10.1042/bj3210845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thrush AB, Harasim E, Chabowski A, Gulli R, Stefanyk L, Dyck DJ. A single prior bout of exercise protects against palmitate-induced insulin resistance despite an increase in total ceramide content. Am J Physiol Regul Integr Comp Physiol 300: R1200–R1208, 2011. doi: 10.1152/ajpregu.00091.2010. [DOI] [PubMed] [Google Scholar]
- 52.Thrush AB, Heigenhauser GJ, Mullen KL, Wright DC, Dyck DJ. Palmitate acutely induces insulin resistance in isolated muscle from obese but not lean humans. Am J Physiol Regul Integr Comp Physiol 294: R1205–R1212, 2008. doi: 10.1152/ajpregu.00909.2007. [DOI] [PubMed] [Google Scholar]
- 53.Van Wijk JJ. Spot noise texture synthesis for data visualization. In: Proceedings of the 18th annual conference on computer graphics and interactive techniques. New York: Assoc. for Computing Machinery, 1991, p. 309- 318. doi: 10.1145/122718.122751. [DOI] [Google Scholar]
- 54.Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T, Gurley C, Simpson P, McGehee RE Jr, Kern PA, Peterson CA. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 296: E1300–E1310, 2009. doi: 10.1152/ajpendo.90885.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitham M, Chan MH, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Brüning JC, Wunderlich FT, Lancaster GI, Febbraio MA. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem 287: 10771–10779, 2012. doi: 10.1074/jbc.M111.310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270: 725–727, 1977. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 57.Yamasaki K, Hayashi H, Nishiyama K, Kobayashi H, Uto S, Kondo H, Hashimoto S, Fujisato T. Control of myotube contraction using electrical pulse stimulation for bio-actuator. J Artif Organs 12: 131–137, 2009. doi: 10.1007/s10047-009-0457-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.