Abstract
The epithelial-to-mesenchymal transition is proposed to be a key mechanism responsible for metastasis-related deaths. Similarly, cancer stem cells (CSCs) have been proposed to be a key driver of tumor metastasis. However, the link between the two events and their control mechanisms is unclear. We used a three-dimensional (3D) tumor spheroid assay and other CSC-indicating assays to investigate the role of E-cadherin in CSC regulation and its association to epithelial-to-mesenchymal transition in lung cancer cells. Ectopic overexpression and knockdown of E-cadherin were found to promote and retard, respectively, the formation of tumor spheroids in vitro but had opposite effects on tumor formation and metastasis in vivo in a xenograft mouse model. We explored the discrepancy between the in vitro and in vivo results and demonstrated, for the first time, that E-cadherin is required as a component of a major survival pathway under detachment conditions. Downregulation of E-cadherin increased the stemness of lung cancer cells but had an adverse effect on their survival, particularly on non-CSCs. Such downregulation also promoted anoikis resistance and invasiveness of lung cancer cells. These results suggest that anoikis assay could be used as an alternative method for in vitro assessment of CSCs that involves dysregulated adhesion proteins. Our data also suggest that agents that restore E-cadherin expression may be used as therapeutic agents for metastatic cancers.
Keywords: cancer stem cell, E-cadherin, lung cancer, anoikis, tumor formation, metastasis, epithelial-to-mesenchymal transition
lung cancer is a major cause of cancer-related deaths in the United States and worldwide (34). A key reason for this high mortality rate is metastasis due to the ability of lung cancer cells to overcome detachment-induced cell death, or anoikis (12, 15, 35), which is triggered by the lack of integrin-extracellular matrix (ECM) survival signaling. These cells can also invade surrounding tissues to form secondary tumors at distant sites (13, 35). Increasing evidence indicates that tumors contain a subset of cancer stem cells (CSCs) or stem-like cells that possess certain stem cell properties. These cells are highly tumorigenic and possess unlimited proliferative potential for self-renewal and differentiation into new tumor cells (14). Studies have shown that CSCs resist not only anoikis, but also chemotherapeutic agents (3, 5, 9, 19, 47), and even though normal cancer cells are killed, CSCs survive and cause tumor relapse.
Epithelial cells in solid tumors normally adhere to each other using adhesion proteins such as E-cadherin to form solid structures. E-cadherin is a transmembrane calcium-dependent adhesion molecule encoded by the CDH1 gene (8). Loss of E-cadherin has been observed in many types of cancer, including metastatic lung cancer (10, 11, 27, 33, 40). E-cadherin is generally regarded as a tumor suppressor (32). Loss of cell adhesion mediates epithelial-to-mesenchymal transition (EMT), which is required for migration and invasion of cancer cells and survival of cancer cells in the bloodstream during dissemination (7, 20, 36, 37). EMT is characterized by a downregulation of E-cadherin and an upregulation of N-cadherin and vimentin, which are less adhesive, thus allowing the cells to spread more readily (27, 42).
However, EMT alone is insufficient for metastatic cancer cells to form secondary tumors, as they later require mesenchymal-to-epithelial transition (MET) to form new solid tumors (21). Interestingly, cancer cells are often found to metastasize in the form of epithelial, rather than mesenchymal, cells. Studies have shown that metastasized cancer cells can reexpress E-cadherin protein after detachment (4, 17, 46), and an increase in E-cadherin enables cancer cells to form tumor emboli and initiate resistance to anoikis (32). Clinical data have also shown that circulating tumor cells with stem properties could circulate not only in the form of single cells, but also as aggregates of cells (32, 44).
The relationship between CSCs and EMT or MET has gained considerable attention in recent years. It is widely accepted that the presence of prominin-1 (CD133) surface protein and high aldehyde dehydrogenase (ALDHhi) activity mark lung CSCs (25). The induction of EMT by transforming growth factor-β has been shown to transform CD133-negative ALDHlow cells to CD133-positive ALDHhi cells, suggesting that EMT may contribute to the stemness of cancer cells (2). However, it has been reported that some CSCs exhibit a negative EMT phenotype (23, 24). Thus the role of EMT in CSC regulation remains controversial; specifically, the roles of E-cadherin in CSC formation, anoikis resistance, and metastasis remain obscure and are investigated in this study.
METHODS
Cell culture.
Human non-small-cell lung cancer H460 cells were obtained from American Type Culture Collection (Manassas, VA). The cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 100 U/ml penicillin-streptomycin (GIBCO, Gaithersburg, MD) in a 5% CO2 environment at 37°C.
Cell viability and anoikis assays.
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, according to the manufacturer’s protocol (Sigma Chemical, St. Louis, MO). For anoikis assay, cells were detached and dissociated into a single-cell suspension in RPMI medium containing 1% FBS and 1 mM ethylenediaminetetraacetic acid (EDTA). The suspended cells were seeded onto ultra-low-attachment well plates (Corning, Acton, MA) at a density of 1 × 104 cells/ml and analyzed for cell viability by MTT assay.
Invasion assay.
Cell invasion was assayed using a Boyden chamber with 8-μm-pore filter inserts in 24-well plates (BioCoat, BD Biosciences, Woburn, MA). Cells were added to the upper chamber of the filter at a density of 3 × 104 cells/ml. The lower chamber was filled with RPMI 1640 medium containing 10% FBS as a chemoattractant. After 48 h, noninvading cells in the upper chamber were removed with a cotton swab. The invading cells in the lower chamber were fixed and stained with Diff-Quik, according to the manufacturer’s protocol (Invitrogen, Thermo Fisher Scientific, Boston, MA).
Transfection.
Cells were transfected with pLKO.1 hygro short-hairpin (shRNA) E-cadherin or E-cadherin-GEF plasmid (Addgene, Cambridge, MA) using Nucleofector (Amaxa Biosystems, Cologne, Germany), according to the manufacturer’s protocols. The amino acid sequence for shRNA E-cadherin and E-cadherin-GEF was 5′-AAGATAGGAGTTCTCTGATGC-3′, and full-length E-cadherin was tagged with green fluorescent protein (GFP) at the COOH terminus of the cytoplasmic domain. Transfected cells were selected with 200 μg/ml hygromycin and 1 mg/ml G418, respectively.
Spheroid formation assay.
Cells were detached into a single-cell suspension in RPMI medium containing 0.25% (wt/vol) carboxymethyl cellulose (Sigma-Aldrich, St. Louis, MO) and 1% FBS at a density of 5 × 103 cells/ml using 1 mM EDTA in phosphate-buffered saline (PBS). The suspended cells were allowed to form primary spheroids for 7 days and then dissociated, seeded onto ultra-low-attachment plates, and allowed to form secondary spheroids for another 7–14 days.
Real-time quantitative PCR.
Quantitative PCR was performed using MyiQ SYBR Green supermix and quantified by MyiQ single-color real-time PCR detection software (version 2.0, Bio-Rad, Hercules, CA). PCR conditions were 50°C for 2 min followed by 95°C for 20 s and 40 cycles of amplification at 95°C for 3 s and 60°C for 30 s. E-cadherin level was calculated relative to the housekeeping gene β-actin. The following primers were used: E-cadherin [5′-GGCCAGGAAATCACATCCTA-3′ (forward) and 5′-GGCAGTGTCTCTCCAAATCC-3′ (reverse)] and α-actin [5′-GCTTCCGCTGTCCTGAGA-3′ (forward) and 5′-ATGCCAGCAGATTCCATACC-3′ (reverse)].
ALDH activity assay.
Spheroids were dissociated into single cells by 1 mM EDTA in PBS and resuspended in Aldefluor assay buffer (StemCell Technologies, Durham, NC). Each sample was made up in duplicate. An activated Aldefluor reagent (5 µl) was added to the cells at a density 1 × 105 cells/ml. Subsequently, 0.5 ml of Aldefluor-stained cells was added with or without 5 μl of diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor, as a negative control, and the cells were incubated at 37°C for 60 min. In some experiments, Aldefluor-stained cells with or without the inhibitor were blocked by 3% BSA in Aldefluor assay buffer at 4°C for 30 min and then stained with E-cadherin antibody (catalog no. 324112, BioLegend, San Diego, CA) for immunofluorescence assay. Stained cells were sorted and analyzed by fluorescence-activated cell sorting (FACSort, Becton-Dickinson, Rutherford, NJ). Gating of Aldefluor-stained cells was performed using the ALDH inhibitor DEAB. The cutoff points were used at the same level with control whether 0.1% or 0.3%.
Flow cytometry analysis.
Cells were washed with cold PBS and blocked by 3% BSA in PBS at 4°C for 30 min. Cells were later incubated on ice with CD133 (catalog no. CA1217, Cell Applications, San Diego, CA) and E-cadherin (catalog no. SC-8426, Santa Cruz Biotechnology, Santa Cruz, CA) primary antibodies for 1 h and then stained with the secondary antibodies Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 647-conjugated goat anti-mouse IgG (catalog nos. A11008 and A21235, respectively, Invitrogen, Carlsbad, CA). Fluorescence intensity was scored by flow cytometry using 488- and 635-nm excitation beams and 519- and 668-nm band-pass filters (FACSort, Becton-Dickinson). The mean fluorescence intensity was quantified using CellQuest software (Becton-Dickinson).
Immunofluorescence staining.
Secondary spheroids were collected and fixed at room temperature for 10 min with 4% paraformaldehyde and blocked by 3% BSA in PBS at 4°C on a coverslip for 30 min. Cells were then incubated with CD133 (catalog no. CA1217, Cell Applications) and E-cadherin (catalog no. SC-8426, Santa Cruz Biotechnology) primary antibodies (1:100 dilution) overnight and then stained with the secondary antibodies Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 568-conjugated goat anti-mouse IgG (catalog nos. A11008 andA11031, respectively, Invitrogen, Waltham, MA) at 1:100 dilution. The cells were treated with ProLong gold antifade reagent, and 4′,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA) was used for nuclear staining. Immunofluorescence images were acquired via confocal laser scanning microscopy (model LSM 510, Zeiss).
Western blotting.
Cells were incubated with lysis buffer (Cell Signaling Technology, Danvers, MA) containing 100 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich) and protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN) for 45 min on ice. The cell lysates were collected, and protein content was determined using the Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Hudson, NH). An equal amount of proteins from each sample (45 µg) was denatured by heating at 95°C for 5 min with Laemmli loading buffer and loaded onto a 10% SDS-polyacrylamide gel. After separation, proteins were transferred onto PVDF membranes (Bio-Rad). The membranes were blocked in 5% nonfat dry milk in TBST [25 mM Tris·HCl (pH 7.5), 125 mM NaCl, and 0.1% Tween 20] for 1 h and incubated with the appropriate primary antibodies overnight at 4°C. Membranes were washed three times with TBST for 5 min and incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature. The immune complexes were detected by chemiluminescence (Thermo Fisher Scientific, Hudson, NH) and quantified using ImageJ.
In vivo tumorigenesis and metastasis.
Six-week-old immunodeficient NOD scid gamma [strain NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG), Jackson Laboratory, Bar Harbor, ME] mice were used throughout the study. Animal care and experimental procedures were performed according to the Guidelines for Animal Experiments at West Virginia University with the approval of the Institutional Animal Care and Use Committee (IACUC no. 15-0702). Mice were anesthetized with isoflurane and subcutaneously injected with 1 × 106 cells in RPMI. For limiting-dilution assay, primary spheroids were collected and dissociated by 1 mM EDTA in PBS. The dissociated E-cadherin-overexpressing (OE-E-cad) cells were subcutaneously injected into mice at densities of 5 × 103 and 1 × 105 cells in RPMI. At the end of the experiments, the mice were euthanized, and tumors were dissected and weighed. Metastatic nodules on the surface of the liver and lungs were counted.
Statistical analysis.
Values are means ± SD from three or more independent experiments. Differences between groups were assessed by Student’s t-test using SPSS software (version 22.0). For all analysis, two-sided P = 0.05 was considered statistically significant.
RESULTS
Characteristics of tumor spheroids from human lung cancer H460 cells.
We used a 3D tumor spheroid assay to assess stem or stem-like properties of lung cancer cells. Cells were detached and suspended in an ultra-low-attachment plate and cultured under stem cell-selective conditions. For comparison, cells were grown as attached cells under normal culture conditions (2D), and the results were compared. Figure 1A shows light-microscopic images of primary and secondary tumor spheroids (3D), as well as attached cells (2D). The expression level of CSC markers, including CD133, ALDH1A1, and CD44, in these spheroid and attached cells was determined by Western blotting. Figure 1, B and C, shows a higher expression of the CSC markers in 3D than 2D cells. ALDH activity was assayed to validate the spheroid results. Figure 1, D and E, shows a higher level of ALDHhi population in 3D than 2D cells. The ALDHhi levels of primary and secondary spheroids are 15.4% and 18.6%, respectively. These data indicate that the 3D tumor spheroids contain an enriched population of cancer stem-like cells, which were used in subsequent studies.
Fig. 1.
Characteristics of tumor spheroids from human lung cancer H460 cells. A: phase-contrast images of adherent cells [2-dimensional (2D)], primary (1°) spheroids, and secondary (2°) spheroids. Scale bar = 200 µm. B and C: stem cell markers [prominin-1 (CD133), aldehyde dehydrogenase (ALDH), and CD44] were detected by Western blotting and calculated as relative protein levels. Values are means ± SD (n = 3). *P < 0.05 vs. 2D. D and E: ALDH activity was measured and analyzed. DEAB, diethylaminobenzaldehyde. Values are means ± SD (n = 3). *P < 0.05 vs. 2D.
Nonadherent conditions promote E-cadherin expression.
To test whether spheroid formation is associated with EMT under nonadherent conditions, tumor spheroids were analyzed for E-cadherin and N-cadherin by Western blotting. Upregulation of N-cadherin and downregulation of E-cadherin are generally considered markers of EMT. Figure 2, A and B, shows that E-cadherin expression was elevated in the 3D spheroids, whereas N-cadherin expression was decreased, suggesting reversed EMT. To substantiate the results, cells were analyzed for the CSC marker CD133 and E-cadherin by flow cytometry and confocal microscopy. Flow cytometry showed that both CD133 and E-cadherin were upregulated in the 3D spheroids compared with the 2D adherent cells (Fig. 2C). Similarly, confocal microscopy showed an increased expression of CD133 and E-cadherin in the spheroids (Fig. 2D), supporting their cancer stem-like cell enrichment and reversed EMT. Real-time PCR analysis of E-cadherin mRNA expression in the 3D spheroids (Fig. 2E) further substantiates this finding.
Fig. 2.
Nonadherent conditions promote E-cadherin expression. A–D: 3-dimensional (3D) spheroid formation assay. A and B: epithelial-to-mesenchymal transition (EMT) markers [E- and N-cadherin (E-cad and N-cad)] were detected by Western blotting and calculated as relative protein levels. Values are means ± SD (n = 4). *P < 0.05 vs. 2D. #P < 0.05 vs. 1° 3D. C: expression level of CD133 and E-cadherin in adherent (2D) cells and 3D spheres was determined by flow cytometry. D: secondary spheroids (3D) were captured by confocal microscopy and compared with 2D. Scale bar = 50 µm. E: relative E-cadherin mRNA level. Values are means ± SD (n = 3). *P < 0.05. vs. 2D. F and G: anoikis assay. EMT markers were detected by Western blotting and calculated as relative protein levels. Values are means ± SD (n = 3). *P < 0.05 vs. 0 h.
Resistance to anoikis, or detachment-induced apoptosis, is a key characteristic of CSCs. We analyzed E-cadherin expression in cells undergoing anoikis to investigate the potential link between EMT and CSC-like properties in lung cancer cells. Cells were suspended in an ultra-low-attachment plate and allowed to undergo apoptosis over time. The expression-time profile of E-cadherin and N-cadherin in Figure 2, F and G, shows an upregulation of the former and a downregulation of the latter during anoikis. Furthermore, detection of the EMT transcription factors zinc finger E-box-binding homeobox 1 (ZEB1), Slug, Snail, and Twist in anoikis and 3D tumor spheroid assays confirmed the results. A decrease in EMT transcription factors is shown in Fig. 3, A and B. These results suggest a potential link between E-cadherin and cancer stem-like cells and possible cancer stem-like cell regulation by E-cadherin.
Fig. 3.
EMT transcription factors. A and C: zinc finger E-box-binding homeobox 1 (ZEB1), Slug, Snail, and Twist were detected in secondary tumor spheroids and anoikis, respectively, by Western blotting. B and D: immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to control (2D and 0 h). Values are means ± SD (n = 3). *P < 0.05.
Overexpression of E-cadherin promotes 3D tumor spheroid formation in vitro but inhibits tumor formation and metastasis in vivo.
To investigate the potential role of E-cadherin in CSC regulation, lung cancer cells were stably transfected with E-cadherin or control plasmid, and their effects on tumor spheroid formation in vitro and tumor formation and metastasis in vivo were determined. Figure 4, A and B, shows the expression level of E-cadherin and related EMT markers in the cells. The stable transfected (OE-E-cad2) cells exhibited a substantial increase in E-cadherin expression and, therefore, were selected for subsequent functional studies. These cells, which were later named OE-E-cad, also exhibited reduced vimentin expression, indicating its epithelial phenotype. Since the E-cadherin plasmid used to generate the stable cells was tagged with GFP, we further analyzed GFP expression in the OE-E-cad cells by fluorescence microscopy (Fig. 4C). The results demonstrated successful transgene expression and supported E-cadherin overexpression in these cells.
Fig. 4.
Overexpression (OE) of E-cadherin increases 3D tumor spheroid formation in vitro but decreases primary tumor and metastasis in vivo. A: lung cancer H460 cells were stably transfected with E-cadherin (OE-E-cad) or control (Ctrl) plasmid, and expression of E-cadherin and the related EMT marker vimentin (Vim) was determined by Western blotting. B: immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to control. Values are means ± SD (n = 4). *P < 0.05. C: phase-contrast and fluorescence images were captured to verify E-cadherin expression in cells. D: primary spheroid images were captured by light microscopy (left), and number of spheroids formed by OE-E-cad and control cells was determined (right). Scale bar = 200 µm. Values are means ± SD (n = 3). *P < 0.05 vs. Ctrl. E–G: in vivo assay. OE-E-cad or control cells were subcutaneously injected into both flanks of mice at a density of 1 × 106 cells/side. E: representative images of tumors formed (top) and tumor weights (bottom). F and G: number of metastatic nodules in liver and lungs. Values are means ± SD (n = 6). *P < 0.05. vs. Ctrl.
Next, we assessed the ability of OE-E-cad cells to form tumor spheroids in vitro and primary and metastatic tumors in vivo compared with vector control. Figure 4D shows representative images and average numbers of tumor spheroids formed by the OE-E-cad and control cells. Interestingly, while larger and more numerous spheroids were formed by the OE-E-cad than control cells, the OE-E-cad cells formed smaller primary tumors and fewer metastatic nodules in vivo (Fig. 4, E and F). These results indicate the inconsistency of the test assays to assess CSCs and, specifically, the role of E-cadherin in cancer stem-like cell regulation, since both tumor spheroid and tumor formation and metastasis assays have been used as functional tests for CSCs. To resolve this issue, we performed additional assays, including ALDH activity and in vivo limiting-dilution assays. In the latter assay, OE-E-cad and control cells from primary spheroids were subcutaneously injected into mice at 5 × 103 and 1 × 105 cells per 100 µl and analyzed for tumor growth and metastasis. Figure 5, A and D, shows formation of substantially smaller tumors in OE-E-cad than control cells at both cell densities. Tumor and metastasis incidence data also showed a reduced capability of the OE-E-cad cells to form primary and metastatic tumors (Fig. 5C). Similarly, ALDH activity assay indicated lower ALDHhi activity in the OE-E-cad cells from tumor spheroids than in the control cells (Fig. 5B). Together, these data support the suppressive role of E-cadherin in CSC regulation, which was further substantiated by subsequent gene-knockdown experiments.
Fig. 5.
In vivo limiting-dilution assay. OE-E-cad and control cells from primary spheroids were subcutaneously injected into both flanks of mice at a density of 5 × 103 and 1 × 105 cells/side. A: representative images of tumors at different injection concentrations. B: ALDHhi activity in OE-E-cad and control cells from primary spheroids. C: tumor and metastasis incidence. D: tumor weight in OE-E-cad and control cells. Values are means ± SD (n = 3/group). *P < 0.05.
Loss of E-cadherin decreases 3D spheroid formation in vitro but increases tumor formation and metastasis in vivo.
E-cadherin-knockdown cells were generated using a shRNA strategy, and their effects on spheroid formation in vitro and tumorigenesis and metastasis in vivo were tested. In Fig. 5, A and B, Western blot analysis of E-cadherin in the knockdown cells shows their reduced expression, particularly in stable transfected (shE-cad2) cells, which were later used for functional assays and are referred to as shE-cad. In this E-cadherin-knockdown clone, vimentin expression was increased, consistent with the gene overexpression results. Real-time quantitative PCR analysis further confirmed the decreased expression of E-cadherin mRNA in the knockdown clones (Fig. 6C). The shE-cad cells were next analyzed for their ability to form tumor spheroids (Fig. 6D). Consistent with the E-cadherin overexpression results, substantially fewer spheroids were formed by shE-cad than control cells. However, shE-cad cells formed larger tumors and more metastatic nodules in vivo (Fig. 6, E–G). Together, these results support the gene overexpression study and indicate the suppressive role of E-cadherin in CSC regulation.
Fig. 6.
Loss of E-cadherin decreases 3D spheroid size in vitro but increases primary tumor weight and metastasis in vivo. H460 cells were stably transfected with short hairpin E-cadherin (shE-cad) or control (Ctrl) plasmid. A: Western blot showing expression of E-cadherin and vimentin. B: densitometric analysis of immunoblot signals. C: quantitative PCR of mRNA level of E-cadherin to confirm Western blot results. D: phase-contrast images of primary spheroids of shE-cad and control cells (left) and number of spheroids (right). Scale bar = 200 µm. E: in vivo assay. shE-cad cells were subcutaneously injected into both flanks of mice at a density of 1 × 106 cells/side. Representative images are shown (top) along with tumor weights (bottom). F and G: number of metastatic nodules in liver and lungs. Values are means ± SD (n = 3). *P < 0.05. vs. Ctrl.
E-cadherin knockdown increases ALDHhi activity but inhibits 3D spheroid formation.
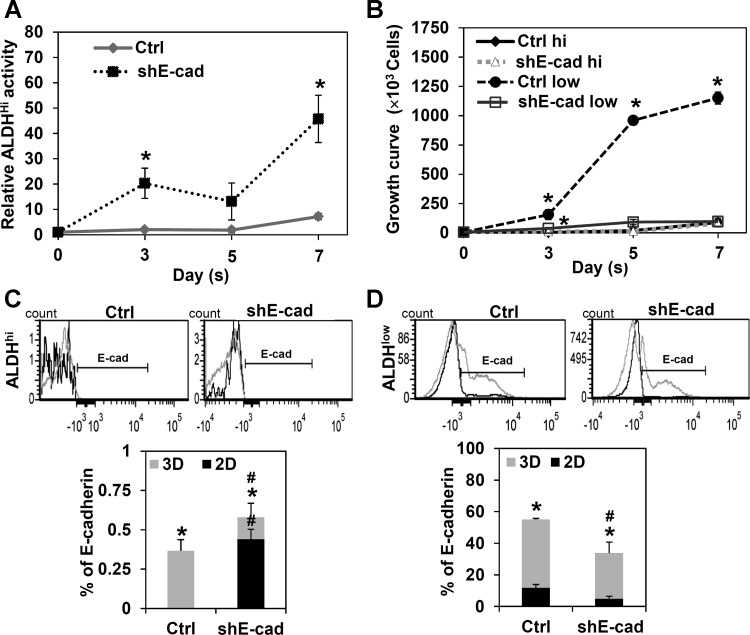
We further tested the ALDH activity of shE-cad cells during spheroid formation. ALDHhi activity was higher in shE-cad than control cells (Fig. 7A). The discrepancy in shE-cad cells between ALDHhi activity or stemness and their low tumor spheroid formation suggests that the 3D spheroid formation assay may not be indicative of CSCs under certain conditions under which E-cadherin is disrupted and that non-CSCs may play an important role in determining spheroid size. Since tumor spheroids contain both cancer stem-like cells and non-cancer stem-like cells, we hypothesized that E-cadherin is more vital to the survival and growth of non-cancer stem-like cells under detachment conditions than cancer stem-like cells. To test this hypothesis, we used Trypan blue to measure the growth rate of ALDHhi and ALDHlow cells in the shE-cad and control spheroids. Figure 7B shows that knockdown of E-cadherin had a major inhibitory effect on the growth of ALDHlow, but not ALDHhi, cells, indicating its important role in controlling the growth of non-cancer stem-like cells. This finding suggests that cell adhesion is more critical to the growth of non-cancer stem-like than cancer stem-like cells.
Fig. 7.
E-cadherin knockdown promotes ALDHhi activity and retards growth of H460 cells in 3D tumor spheroids. E-cadherin-knockdown (shE-cad) cells were suspended and analyzed for ALDH activity at days 0–7. A: relative ALDHhi activity compared with day 0. B: growth curve. C and D: E-cadherin expression at day 7 with ALDHhi or ALDHlow activity. Values are means ± SD (n = 3). *P < 0.05 vs. day 0. #P < 0.05 vs. Ctrl.
To verify the role of E-cadherin in non-cancer stem-like cells, we used flow cytometry to analyze E-cadherin expression in the ALDHlow and ALDHhi cells from shE-cad and control spheroids (3D) and attached cells (2D). Figure 7, C and D, shows much higher E-cadherin expression in the ALDHlow than ALDHhi cells in the 3D and 2D models, suggesting its importance in non-cancer stem-like cells. E-cadherin expression is also higher in 3D than 2D cells in control and shE-cad cells, suggesting that E-cadherin is required for tumor spheroid formation.
Anoikis is indicative of CSCs.
To test whether detachment-induced apoptosis, or anoikis, could be used as a functional test for lung CSCs under nonadherent conditions, we analyzed ALDH activity in control and shE-cad cells during anoikis. Figure 8A shows significantly higher ALDH activity in shE-cad than control cells: 15.5% vs. 7.4% at 12 h postdetachment and 20.1% vs. 9.4% at 24 h postdetachment. Analysis of cell viability by MTT assay showed that shE-cad cells were more resistant to detachment-induced death (Fig. 8B). Moreover, shE-cad cells were more invasive than control cells, as determined by Boyden invasion assay (Fig. 8C).
Fig. 8.
Anoikis enriches CSCs. A: ALDH activity of shE-cad cells at 12 and 24 h after cell detachment. B: viability of detached cells determined by MTT assay at 0–24 h. C: invasion of shE-cad cells was assessed using Boyden chambers with Matrigel-coated membranes. Scale bar = 200 µm. Values are means ± SD (n = 3). *P < 0.05 vs. Ctrl.
DISCUSSION
The phenotypic switch between epithelial and mesenchymal has been implicated in CSC regulation during cancer metastasis-related death (1, 6). Increasing evidence indicates that CSCs resist not only anoikis, but also chemotherapeutic agents, resulting in relapse or recurrence of malignancy (12, 15, 35). The link between CSCs and EMT or MET is not fully understood. We used multiple in vitro and in vivo approaches to study CSCs in the human lung cancer H460 cell model, which has been established for lung CSC studies (43).
Since CSCs possess a self-renewal (tumor-forming) property and a capacity to differentiate and regenerate new tumors (14), cells that can escape detachment-induced death and form tumor spheroids are generally considered CSCs. We found that E-cadherin is upregulated in the tumor spheroids, as well as in detached cells undergoing anoikis, in lung cancer H460 and H292 cells (data not shown). Protein kinase B (Akt) and MAPK 44/42 (extracellular signal-regulated kinase 1/2) activation was also found in the indicated anoikis and spheroid cells (unpublished data), suggesting activation of survival mechanisms. Detachment-induced apoptosis is characterized by loss of integrin-ECM-Akt survival signaling (13, 35). Previous studies have shown that E-cadherin-mediated cell-cell adhesion can trigger ligand-independent activation of epidermal growth factor receptor, as well as cell survival phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt and proliferation MAPK signaling pathways (30, 31), which may underlie the survival and growth of lung cancer cells under detachment conditions.
To test whether E-cadherin plays an essential role in CSC regulation, E-cadherin-knockdown (shE-cad) and OE-E-cad cells were generated. Silencing E-cadherin resulted in an increased expression of mesenchymal markers, such as vimentin, while overexpression of E-cadherin had an opposite effect. E-cadherin-expressing cells exhibited larger and more tumor spheroids, consistent with a previous report showing sphere formation with tight intracellular connection in association with E-cadherin expression (24, 41). Dissociation of spheroid cells, e.g., by calcium chelators, antibodies, or dominant-negative expression of E-cadherin, was shown to lead to massive apoptosis in various cell types, including Ewing tumor cells, oral squamous carcinoma cells, and normal enterocytes (4, 17, 46). In the present study we found that knockdown of E-cadherin inhibited tumor spheroid formation, suggesting its potential role as a positive regulator of CSCs. However, animal studies, as well as anoikis and ALDH activity assays, do not support this notion and indicate that E-cadherin is a repressor of CSCs and is more critical to the survival and growth of non-cancer stem-like cells in the tumor spheres.
Our animal studies showed that knockdown of E-cadherin promoted tumor formation and metastasis, consistent with previous findings of the suppressive effect of E-cadherin on tumor formation (11, 18). Our gene-overexpression and limiting-dilution studies in animals also support this notion. To substantiate this finding, we used 2D and 3D cell models to carry out ALDH activity assays and tested the effect of E-cadherin knockdown on the growth rate of cancer stem-like cells (ALDHhi) and non-cancer stem-like cells (ALDHlow). E-cadherin knockdown increased ALDH activity of both attached (2D) and spheroid (3D) cells, supporting the general role of E-cadherin as a negative regulator of CSCs. E-cadherin expression was upregulated in the spheroids, suggesting that it is required for spheroid formation. Previous studies showed that upregulation of E-cadherin is required for maintaining tumor emboli (26, 32, 39). Palacios and colleagues reported that E-cadherin is depleted by lysosomal degradation during EMT (29); thereby, its reexpression could be limited by such a mechanism. There is also evidence to suggest that cell-cell adhesion induces cell differentiation and proliferation through the MAPK signaling pathway (45). Lee and Ramos demonstrated that inhibition of such a pathway restrained E-cadherin expression in multicellular spheroids (22). Therefore, MAPK might serve as a positive-feedback regulator of E-cadherin.
We have demonstrated, for the first time, that E-cadherin expression is necessary for tumor spheroid formation but is more important to non-cancer stem-like than cancer stem-like cells, which are endowed with survival mechanisms under detachment conditions. Hou and colleagues reported that individual tumor cells isolated from the blood of lung cancer patients undergo apoptosis (16). Death induced by single-cell suspension was also reported to occur through focal adhesion kinase dephosphorylation and p53 activation (46). Thus, loss of E-cadherin may trigger these signaling pathways, leading to growth retardation in the tumor spheroids. We also found that E-cadherin knockdown promoted stemness (ALDHhi) of lung cancer cells to resist anoikis. It also promoted invasiveness of the cells, consistent with our in vivo metastasis data and previous reports showing the negative regulation of CSCs by E-cadherin via the Wnt/β-catenin pathway (11, 38).
In conclusion, our data indicate that E-cadherin has an essential role in the regulation of lung cancer stem-like cells under detachment conditions. Under these conditions, lung cancer cells undergo MET, or reversed EMT, as indicated by their upregulated E-cadherin expression and downregulation of mesenchymal markers, presumably to promote cell adhesion for increased survival. E-cadherin serves as a repressor of CSCs under detachment conditions. However, E-cadherin positively regulates tumor spheroids, since it has a greater effect on non-cancer stem-like cells, which comprise the major cell population in tumor spheroids, than on cancer stem-like cells. This finding suggests that the spheroid assay may not be optimal for CSC studies that involve dysregulation of adhesion proteins and that alternative methods, such as anoikis and invasion assays, may be more suitable. Finally, since CSCs are believed to be responsible for metastasis, chemoresistance, and relapse, restoration of E-cadherin expression could be a potential therapeutic strategy that may overcome metastatic and recurrent cancers.
GRANTS
This work was supported by the Doctoral Degree Chulalongkorn University 100th Year Birthday Anniversary Fund, the National Institutes of Health (Grants R01-ES-022968 and R01-EB-018857), and the International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund. Flow cytometric analysis was performed at the West Virginia University Flow Cytometry Core Facility, which is supported in part by National Institute of General Medical Sciences Grant P30-GM-103488.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.P., S.L., P.C. and X.H. performed experiments; P.P. and P.C. analyzed data; P.P. and P.C. interpreted results of experiments; P.P. and P.C. prepared figures; P.P. and P.C. drafted manuscript; Y.R. and P.C. conceived and designed research; Y.R. and P.C. edited and revised manuscript; P.P., S.L., X.H., Y.R., and P.C. approved final version of manuscript.
REFERENCES
- 1.Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 11: R46, 2009. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akunuru S, James Zhai Q, Zheng Y. Non-small cell lung cancer stem/progenitor cells are enriched in multiple distinct phenotypic subpopulations and exhibit plasticity. Cell Death Dis 3: e352, 2012. doi: 10.1038/cddis.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760, 2006. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Bergin E, Levine JS, Koh JS, Lieberthal W. Mouse proximal tubular cell-cell adhesion inhibits apoptosis by a cadherin-dependent mechanism. Am J Physiol Renal Physiol 278: F758–F768, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabrò E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA 106: 16281–16286, 2009. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell 22: 699–701, 2012. doi: 10.1016/j.ccr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Burgess DJ. Breast cancer: circulating and dynamic EMT. Nat Rev Cancer 13: 148–149, 2013. doi: 10.1038/nrc3475. [DOI] [PubMed] [Google Scholar]
- 8.Bussemakers MJG, van Bokhoven A, Völler M, Smit FP, Schalken JA. The genes for the calcium-dependent cell adhesion molecules P- and E-cadherin are tandemly arranged in the human genome. Biochem Biophys Res Commun 203: 1291–1294, 1994. doi: 10.1006/bbrc.1994.2322. [DOI] [PubMed] [Google Scholar]
- 9.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA 106: 13820–13825, 2009. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan L, Wang H, Xia X, Rao Y, Ma X, Ma D, Wu P, Chen G. Loss of E-cadherin promotes prostate cancer metastasis via upregulation of metastasis-associated gene 1 expression. Oncol Lett 4: 1225–1233, 2012. doi: 10.3892/ol.2012.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmakovskaya M, Khromova N, Rybko V, Dugina V, Kopnin B, Kopnin P. E-cadherin repression increases amount of cancer stem cells in human A549 lung adenocarcinoma and stimulates tumor growth. Cell Cycle 15: 1084–1092, 2016. doi: 10.1080/15384101.2016.1156268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124: 619–626, 1994. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guadamillas MC, Cerezo A, Del Pozo MA. Overcoming anoikis—pathways to anchorage-independent growth in cancer. J Cell Sci 124: 3189–3197, 2011. doi: 10.1242/jcs.072165. [DOI] [PubMed] [Google Scholar]
- 14.Guo W, Lasky JL 3rd, Wu H. Cancer stem cells. Pediatr Res 59: 59R–64R, 2006. doi: 10.1203/01.pdr.0000203592.04530.06. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 178: 989–996, 2011. doi: 10.1016/j.ajpath.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PH. E-cadherin cell-cell adhesion in Ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res 67: 3094–3105, 2007. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karube H, Masuda H, Ishii Y, Takayama T. E-cadherin expression is inversely proportional to tumor size in experimental liver metastases. J Surg Res 106: 173–178, 2002. doi: 10.1006/jsre.2002.6447. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Hong SH, Basse PH, Wu C, Bartlett DL, Kwon YT, Lee YJ. Cancer stem cells protect non-stem cells from anoikis: bystander effects. J Cell Biochem 117: 2289–2301, 2016. doi: 10.1002/jcb.25527. [DOI] [PubMed] [Google Scholar]
- 20.Kim YN, Koo KH, Sung JY, Yun UJ, Kim H. Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol 2012: 306879, 2012. doi: 10.1155/2012/306879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celià-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, Wei Y, Hu G, Garcia BA, Ragoussis J, Amadori D, Harris AL, Kang Y. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med 17: 1101–1108, 2011. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C, Ramos DM. Regulation of multicellular spheroids by MAPK and FYN kinase. Anticancer Res 36: 3833–3838, 2016. [PubMed] [Google Scholar]
- 23.Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, Hong S, Adams A, D’Angelo R, Ginestier C, Charafe-Jauffret E, Clouthier SG, Birnbaum D, Wong ST, Zhan M, Chang JC, Wicha MS. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports 2: 78–91, 2013. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manuel Iglesias J, Beloqui I, Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon A, Menendez JA, Dopazo J, Martin AG. Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PLoS One 8: e77281, 2013. doi: 10.1371/journal.pone.0077281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyata T, Yoshimatsu T, So T, Oyama T, Uramoto H, Osaki T, Nakanishi R, Tanaka F, Nagaya H, Gotoh A. Cancer stem cell markers in lung cancer. Personalized Medicine Universe 4: 40–45, 2015. doi: 10.1016/j.pmu.2015.03.007. [DOI] [Google Scholar]
- 26.Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S, Takeichi M, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res 53: 1696–1701, 1993. [PubMed] [Google Scholar]
- 27.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 68: 3645–3654, 2008. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 29.Palacios F, Tushir JS, Fujita Y, D’Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol 25: 389–402, 2005. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem 275: 41227–41233, 2000. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- 31.Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, Lundin E, Ottander U, Rytinki M, Liu K. Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol Endocrinol 19: 2564–2578, 2005. doi: 10.1210/me.2004-0342. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez FJ, Lewis-Tuffin LJ, Anastasiadis PZ. E-cadherin’s dark side: possible role in tumor progression. Biochim Biophys Acta 1826: 23–31, 2012. doi: 10.1016/j.bbcan.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E. Loss of E-cadherin promotes ovarian cancer metastasis via α5-integrin, which is a therapeutic target. Cancer Res 68: 2329–2339, 2008. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 67: 7–30, 2017. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 35.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett 272: 177–185, 2008. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 36.Smit MA, Peeper DS. Zeb1 is required for TrkB-induced epithelial-mesenchymal transition, anoikis resistance and metastasis. Oncogene 30: 3735–3744, 2011. doi: 10.1038/onc.2011.96. [DOI] [PubMed] [Google Scholar]
- 37.Smit MA, Geiger TR, Song J-Y, Gitelman I, Peeper DS. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol 29: 3722–3737, 2009. doi: 10.1128/MCB.01164-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su YJ, Chang YW, Lin WH, Liang CL, Lee JL. An aberrant nuclear localization of E-cadherin is a potent inhibitor of Wnt/β-catenin-elicited promotion of the cancer stem cell phenotype. Oncogenesis 4: e157, 2015. doi: 10.1038/oncsis.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251: 1451–1455, 1991. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 40.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell 22: 2423–2435, 2011. doi: 10.1091/mbc.E11-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu S, Yang Y, Dong L, Qiu W, Yang L, Wang X, Liu L. Construction and characteristics of an E-cadherin-related three-dimensional suspension growth model of ovarian cancer. Sci Rep 4: 5646, 2014. doi: 10.1038/srep05646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117: 927–939, 2004. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Yongsanguanchai N, Pongrakhananon V, Mutirangura A, Rojanasakul Y, Chanvorachote P. Nitric oxide induces cancer stem cell-like phenotypes in human lung cancer cells. Am J Physiol Cell Physiol 308: C89–C100, 2015. doi: 10.1152/ajpcell.00187.2014. [DOI] [PubMed] [Google Scholar]
- 44.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol 192: 373–382, 2011. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12: 9–18, 2002. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Lu H, Dazin P, Kapila Y. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: fibronectin and integrin-αv mediate survival signals through focal adhesion kinase. J Biol Chem 279: 48342–48349, 2004. doi: 10.1074/jbc.M407953200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Wang Z, Yu J, Shi J, Wang C, Fu W, Chen Z, Yang J. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett 322: 70–77, 2012. doi: 10.1016/j.canlet.2012.02.010. [DOI] [PubMed] [Google Scholar]