Abstract
IL-6 and leukemia inhibitory factor (LIF), members of the IL-6 family of cytokines, play recognized paradoxical roles in skeletal muscle mass regulation, being associated with both growth and atrophy. Overload or muscle contractions can induce a transient increase in muscle IL-6 and LIF expression, which has a regulatory role in muscle hypertrophy. However, the cellular mechanisms involved in this regulation have not been completely identified. The induction of mammalian target of rapamycin complex 1 (mTORC1)-dependent myofiber protein synthesis is an established regulator of muscle hypertrophy, but the involvement of the IL-6 family of cytokines in this process is poorly understood. Therefore, we investigated the acute effects of IL-6 and LIF administration on mTORC1 signaling and protein synthesis in C2C12 myotubes. The role of glycoprotein 130 (gp130) receptor and downstream signaling pathways, including phosphoinositide 3-kinase (PI3K)-Akt-mTORC1 and signal transducer and activator of transcription 3 (STAT3)-suppressor of cytokine signaling 3 (SOCS3), was investigated by administration of specific siRNA or pharmaceutical inhibitors. Acute administration of IL-6 and LIF induced protein synthesis, which was accompanied by STAT3 activation, Akt-mTORC1 activation, and increased SOCS3 expression. This induction of protein synthesis was blocked by both gp130 siRNA knockdown and Akt inhibition. Interestingly, STAT3 inhibition or Akt downstream mTORC1 signaling inhibition did not fully block the IL-6 or LIF induction of protein synthesis. SOCS3 siRNA knockdown increased basal protein synthesis and extended the duration of the protein synthesis induction by IL-6 and LIF. These results demonstrate that either IL-6 or LIF can activate gp130-Akt signaling axis, which induces protein synthesis via mTORC1-independent mechanisms in cultured myotubes. However, IL-6- or LIF-induced SOCS3 negatively regulates the activation of myotube protein synthesis.
Keywords: IL-6, LIF, gp130, Akt, mTORC1, STAT3, muscle protein synthesis
skeletal muscle mass maintenance plays a well-documented role in health and quality of life. To this end, our knowledge of processes governing skeletal muscle mass, which involves the balance between protein synthesis and degradation, has been rapidly expanding (16). However, the multifactorial regulation of muscle proteostasis by systemic changes related to nutritional status, physical activity level, inflammation, and underlying disease has created significant gaps in our understanding of muscle protein synthesis regulation. Identified effectors of these systemic conditions that regulate skeletal muscle protein proteostasis include mechanical stimuli, cytokines, growth factors, and hormones (16, 56).
The interleukin-6 (IL-6) family of cytokines regulates many physiological and pathological processes, including immune system regulation, inflammation, wound healing, and cell survival (22). Skeletal muscle serves as both a biological target and a source of the IL-6 family of cytokines. Two members of the IL-6 family, IL-6 and leukemia inhibitory factor (LIF), play recognized roles in both skeletal muscle hypertrophy and atrophy (26, 45). Plasma elevation and muscle production of IL-6 and LIF occur under physiological conditions, involving repeated contractions during exercise and overload or muscle injury (10, 18). This response is thought to initiate important autocrine and paracrine signaling that contributes to muscle hypertrophy by controlling myocyte proliferation and differentiation (10, 21, 61). Notably, the systematic release of IL-6 in response to exercise is in a temporal manner, increasing rapidly and remaining elevated for several hours (42, 58). Unlike the beneficial effects of IL-6 and LIF under these transient physiological conditions, long-term pathological elevation of circulating IL-6 and LIF is tightly linked with muscle wasting (12, 44, 59, 65, 66). Currently, the mechanisms underlying this complex dichotomy warrant further investigation, which will advance our understanding of muscle mass regulation (45).
IL-6 and LIF can induce intracellular signaling pathways via type I cytokine receptors. These receptors contain a ligand-binding α-receptor subunit and two signal transducing β-receptor subunits, which contain a cytoplasmic signaling transducing domain (11, 22). Glycoprotein 130 (gp130), a transmembrane protein, functions as the β-receptor subunit for the IL-6 cytokine family. The ligand-receptor protein complex activates constitutively bound Janus family kinase 2 (JAK2), which phosphorylates tyrosine residues in the cytoplasmic domains of gp130 (11, 22). gp130 can then phosphorylate and activate signal transducer and activator of transcription 3 (STAT3), leading to STAT3 dimerization and nuclear translocation to induce target gene transcription. Notably, activated STAT3 induces suppressor of cytokine signaling 3 (SOCS3) expression, which can suppress gp130-dependent signaling through direct interaction with the kinase domain of JAK2 (22, 57). This negative feedback inhibition serves to suppress gp130-dependent signaling pathways under physiological conditions (57). Phosphorylated gp130 also leads to Ras-Raf-ERK1/2 signaling activation through the phosphorylation of Src homology domain-containing protein tyrosine phosphatase 2 (SHP-2) and subsequent interaction of growth-factor receptor bound protein 2 (Grb2) (22). The IL-6 family of cytokines can also induce the phosphoinositide 3-kinase (PI3K)/Akt signaling axis (22), but the molecular events mediating this regulation are not well understood, Interestingly, gp130 induction of PI3K-Akt signaling has been demonstrated a cell-type specific regulation; gp130 activation has increased Akt phosphorylation in myeloma cells (13, 23, 60), cardiac myocytes (17, 46), and basal carcinoma cells (32) but not in HepG2 hepatoma cells (36). Despite mounting evidence for cytokine-induced gp130 signaling in other cell types, the specific regulatory role of this signaling pathway in skeletal muscle fibers remains to be determined.
The induction of protein synthesis plays an established role in skeletal muscle hypertrophy (16). The mammalian target of rapamycin (mTOR), which functions in the multiprotein complex mTORC1, is thought to sit at the nexus of skeletal muscle protein synthesis and muscle mass regulation (6). Activated mTORC1 can induce muscle protein synthesis through the phosphorylation of the p70 ribosomal S6 kinase (p70S6K) and eukaryotic initiation factor 4E binding protein (4E-BP1). This signaling increases ribosomal biogenesis and cap-dependent protein translation initiation (37). Many muscle hypertrophic stimuli, such as growth factors like insulin-like growth factor 1 (IGF-1) and mechanical overload, can stimulate muscle mTORC1 and protein synthesis through PI3K-Akt (6, 29) and ERK1/2 signaling (43), respectively. Both Akt and ERK1/2 can phosphorylate tuberous sclerosis 1/2 (TSC1/2), which further relieves TSC1/2 suppression on Ras homolog enriched in brain (Rheb), allowing Rheb to stimulate mTORC1 (6, 28, 30, 37, 43). Muscle IL-6 and LIF also have a role in muscle overload-induced muscle hypertrophy (58, 61). However, their regulation in mTOR signaling and protein synthesis during overload-induced growth has not been established.
Intracellular signaling of the IL-6 family of cytokines initiated by the gp130 has the potential to induce mTORC1 upstream regulators that increase protein synthesis. The gp130 induction of PI3K-Akt and ERK1/2 signaling plays a role in IL-6 and LIF induction of cardiomyocyte protein synthesis and cardiac hypertrophy (17, 47). However, the specific interaction between gp130 and the PI3K/Akt-mTORC1 or ERK1/2-mTORC1 signaling axis within muscle cells requires further investigation. The purpose of this study was to investigate if acute administration of either IL-6 or LIF can regulate myotube protein synthesis through gp130-dependent signaling pathways. We hypothesized that acute IL-6 or LIF administration will induce mTOR signaling and protein synthesis in cultured myotubes through the activation of gp130-Akt-mTOR signaling axis. The role of gp130 and its potential downstream effectors, including PI3K-Akt-mTOR and STAT3 signaling pathways, were investigated with siRNA knockdown or the administration of their specific pharmaceutical inhibitors.
METHODS
Cell culture.
C2C12 myoblasts (American Type Culture Collection, Manassas, VA) were cultured in DMEM supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin. To induce C2C12 myoblast differentiation, C2C12 myoblasts were incubated in differentiation medium (DMEM supplemented with 2% horse serum, 50 U/ml penicillin, and 50 μg/ml streptomycin) for 4 days after they reached ∼95% confluence. Myotubes were differentiated for 8 days in some experiments to further reduce the residual myoblasts in cell culture.
Myobloast proliferation inhibition.
To exlude the proliferating myoblasts in myotubes culture, we pretreated myotubes with the cell proliferation inhibitor Ara-C before the cytokine administration. Two-day differentiated myotubes were incubated in differentiation medium containing 50 μΜ cell cycle inhibitor Ara-C (Sigma-Aldrich, St. Louis, MO) for 24 h. This dose of Ara-C has been demonstrated to block C2C12 myoblasts proliferation (data not shown). Myotubes were allowed to recovery in differentiation medium for another 24 h after Ara-C treatment. In another experiment, myotubes were allowed to differentiate for 8 days to reduce the residual myoblasts.
Cytokine treatment.
Differentiated C2C12 myotubes were treated with different doses (5, 10, 20, or 100 ng/ml) of IL-6 (Sigma-Aldrich), TNF-α (Sigma-Aldrich), or LIF (Thermo Scientific, Waltham, MA) for different time periods (30 min and 1, 2, or 4 h). These dosages of IL-6 and LIF has been demonstrated to activate mTORC1 signaling and protein synthesis in cardiac myocytes or induce cardiac myocytes hypertrophy (17, 24, 47, 55). To exclude the potential effects of cytokines in serum, myotubes were maintained in serum-free condition during cytokine treatment.
RNA interference.
Three days after the onset of differentiation, C2C12 myotubes were transfected with scramble siRNA (GE Dharmacon, Lafayette, CO) or siRNA targeting to gp130 or SOCS3 (Santa Cruz Biotechnology, Santa Cruz, CA) using Dharmafect 3 transfection reagent (GE Dharmacon) according to the manufacturer’s instructions. Briefly, siRNA and transfection reagent were separately diluted in serum-free and antibiotics-free DMEM and incubated at room temperature for 5 min. The diluted transfection reagent was then added to the siRNA mixture and allowed to complex with siRNA for 20 min. siRNA-transfection reagent complexes were then added to the antibiotic-free differentiation medium, and myotubes were incubated for 24 h in the medium containing the transfection mixture. The final concentration of siRNA was set at 100 nM.
Intracellular signaling inhibition.
To inhibit gp130-dependent STAT3, ERK1/2, and PI3K/Akt signaling pathways and the Akt downstream target mTOR signaling pathway, the STAT3-specific inhibitor LLL12 (1 μM final concentration and 0.3 μl DMSO stock solution per 1 ml culture medium; BioVision, Milpitas, CA), the PI3K inhibitor-specific wortmannin (2 μM final concentration and 1 μl DMSO stock solution per 1 ml culture medium; Cell Signaling Technology), or the mTORC1 inhibitor rapamycin (20 nM final concentration and 1 μl DMSO stock solution per 1 ml culture medium; Cell Signaling Technology) was added to cell culture medium 1 h before the IL-6 or LIF administration. For each inhibitor, an equal volume of DMSO was added into cell culture medium as vehicle controls.
Western blotting.
Western blot analysis was performed as previously described (19). Briefly, cells were washed by ice-cold PBS and then scraped into ice-cold RIPA buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM NaF, 1 mM NaVO4, 1 mM β-glycerophosphate, and 1% protease inhibitor cocktail; Sigma-Aldrich). Cell lysates were homogenized on ice and centrifuged (16,000 g for 15 min at 4°C), and supernatants were collected. Protein concentrations were determined by the Bradford reagent (Bio-Rad) following the manufacturer’s instructions. Protein were fractionated in SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes. After the membranes were blocked, primary antibodies were incubated overnight at 4°C in 2% Tris-buffered saline-Tween 20 milk. Anti-rabbit or anti-mouse IgG-conjugated secondary antibodies were incubated with the membranes at 1:2,000 dilutions for 2 h in 2% Tris-buffered saline-Tween 20 milk. The blotting condition for each protein is shown in Table 1. Enhanced chemiluminescence (Advansta, Menlo Park, CA) developed by autoradiography was used to visualize the antibody-antigen interactions. Blots were analyzed by measuring the integrated optical density of each band with ImageJ software (National Institutes of Health, Bethesda, MD).
Table 1.
Conditions of Western blotting experiments
| Primary Antibody |
Secondary Antibody |
||||
|---|---|---|---|---|---|
| Protein | Protein Loaded, μg | Company/Catalog no. | Dilution | Company/Catalog no. | Dilution |
| p-Akt | 40 | Cell Signaling no. 9725 | 1:1,000 | ||
| Akt | 10 | Cell Signaling no. 9272 | 1:2,000 | ||
| p-STAT3 | 20 | Cell Signaling no. 9145 | 1:2,000 | ||
| STAT3 | 10 | Cell Signaling no. 4904 | 1:2,000 | ||
| p-ERK1/2 | 20 | Cell Signaling no. 9101 | 1:2,000 | ||
| ERK1/2 | 10 | Cell Signaling no. 9102 | 1:5,000 | ||
| p-p70S6K | 20 | Cell Signaling no. 9205 | 1:2000 | ||
| p-4E-BP1 | 10 | Cell Signaling no. 2855 | 1:5,000 | Cell Signaling no. 7074 | 1:2,000 |
| 4E-BP1 | 10 | Cell Signaling no. 9452 | 1:5,000 | ||
| p-NF-κB | 60 | Cell Signaling no. 3033 | 1:1,000 | ||
| NF-κB | 20 | Cell Signaling no. 8242 | 1:2,000 | ||
| GAPDH | 10 | Cell Signaling no. 2118 | 1:10,000 | ||
| gp130 | 20 | Santa Cruz Biotechnology no. sc-656 | 1:2,000 | ||
| Myogenin | 20 | Santa Cruz Biotechnology no. sc-576 | 1:2,000 | ||
| p70S6K | 10 | Santa Cruz Biotechnology no. sc-230 | 1:2,000 | ||
| Cyclin D | 20 | Santa Cruz Biotechnology no. sc-8396 | 1:2,000 | ||
| Puromycin | 10 | Millipore MABE343 | 1:2,000 | Cell Signaling no. 7076 | 1:2,000 |
For each protein to be probed, the amount of protein loaded to gel and primary and secondary antibody source and dilution are specified. 4E-BP1, eukaryotic initiation factor 4E binding protein; gp130, glycoprotein 130; p70S6K, p70 ribosomal S6 kinase; STAT3, signal transducer and activator of transcription 3; SOCS, suppressor of cytokine signaling 3.
Protein synthesis measurement.
The myotube protein synthesis was determined by puromycin incorporation as previously described (20). In brief, puromycin (EMD Chemicals, San Diego, CA) was added into cell culture media (1 μM final concentration) 30 min before protein collection. The amount of puromycin incorporated into newly synthesized protein was determined by Western blotting. The protein samples from myotubes without puromycin labeling were used as negative control.
RNA isolation, cDNA synthesis, and real-time PCR.
RNA isolation was performed using TRIzol Reagent (Thermo Scientific) according to the manufacturer’s instructions. cDNA synthesis and real-time PCR were performed as previously described, using reagents from Applied Biosystems (Foster City, CA). Quantitative real-time PCR analysis was carried out in 20-ul reactions consisting of 2× SYBR Green PCR buffer (AmpliTaq Gold DNA Polymerase, Buffer, dNTP mix, AmpErase UNG, MgCl2), 0.1 μl of cDNA, RNase-free water, and 60 nM of each primer. The sequences of SOCS3 primers set were 5′-TGCAGGAGAGCTGATTCTAC-3′ (forward) and 5′-TGACGCTCAACGTGAAGAAG-3′ (reverse). The sequences of GAPDH primers were published elsewhere (50). Data were analyzed by ABI software using the cycle threshold, which is the cycle number at which the fluorescence emission is midway between detection and saturation of the reaction. The SOCS3 data of each sample were normalized by its GAPDH level. All treatment in this study had no significant effects on myotube GAPDH expression (data not shown).
Data presentation and statistical analysis.
Each experiment group contained three technical replicates. All experiments were conducted at least twice to verify the observations. The quantified result of each observation (phosphorylation level, protein level, or mRNA expression) was normalized by the mean value of control group, and the mean value of three technical replicates in each group was considered as a biological observation. The two biological observations in each group were analyzed by Student's t-test, one-way ANOVA, or two-way ANOVA (indicated in the figure legends). Post hoc analyses were performed with Student-Newman-Keuls method if necessary. All values are means ± SE. P < 0.05 was considered significant.
RESULTS
Regulation of myotubes protein synthesis and mTORC1 signaling by different doses of cytokines.
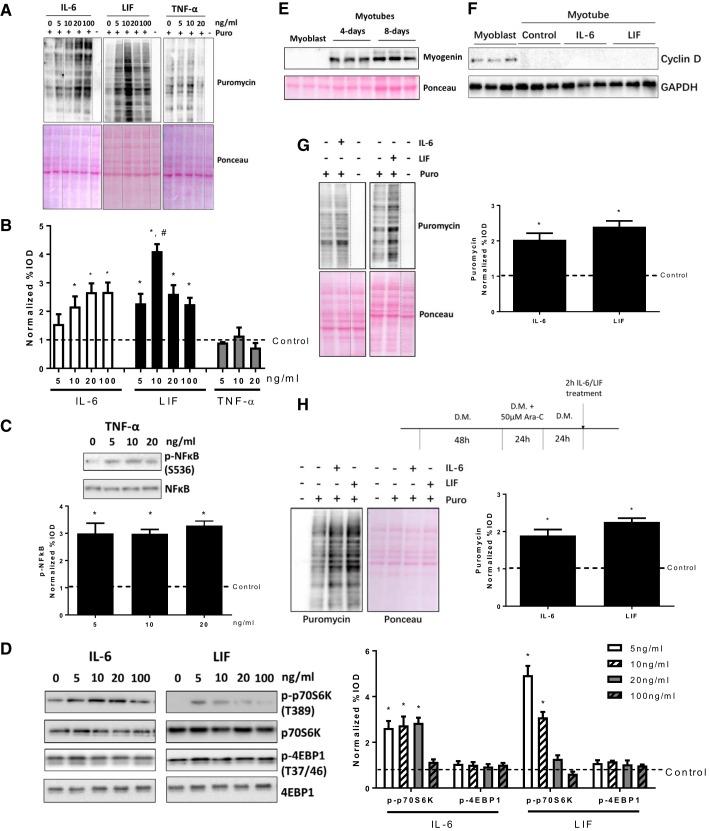
To determine the effect of IL-6 and LIF administration on myotube protein synthesis and mTORC1 signaling, myotubes were treated with different doses (5, 10, 20, and 100 ng/ml) of IL-6 or LIF for 2 h. IL-6 increased myotube protein synthesis, which was determined by puromycin incorporation, at the doses of 10, 20, and 100 ng/ml (Fig. 1A and B). Myotube protein synthesis was induced by all doses of LIF (Fig. 1, A and B), and the 10 ng/ml LIF dose demonstrated the maximal protein synthesis induction (Fig. 1, A and B). To determine whether myotube protein synthesis is specifically upregulated by IL-6 family of cytokines, we treated with myotubes with different doses of TNF-α, which is not an IL-6 family cytokine member. Neither TNF-α doses (5, 10, or 20 ng/ml) demonstrated any changes in myotube protein synthesis (Fig. 1, A and B). The TNF-α action was validated by the increased NF-κB phosphorylation (Fig. 1C). We also measured the p70S6K and 4E-BP1 phosphorylation, two downstream targets of mTORC1. IL-6 (5, 10, and 20 ng/ml) and LIF (5 and 10 ng/ml) doses significantly increased p70S6K phosphorylation (Fig. 1D), while high doses of IL-6 or LIF elicited no change (100 ng/ml IL-6 and 20 ng/ml LIF) or even decreased (100 ng/ml LIF) p70S6K phosphorylation (Fig. 1D). However, 4E-BP1 phosphorylation was not affected by any doses of IL-6 or LIF (Fig. 1D). These results demonstrate that the IL-6-related cytokines IL-6 and LIF can induce myotube protein synthesis and mTOR signaling and that this response is dose sensitive.
Fig. 1.
Myotube protein synthesis and mammalian target of rapamycin complex 1 (mTORC1) signaling regulation by short-term exposure of different cytokines. C2C12 myotubes were treated with different doses (5, 10, 20, and 100 ng/ml) of IL-6, leukemia inhibitory factor (LIF), and TNF-α for 2 h. A: the protein synthesis was determined by puromycin incorporation. Dash lines represent different positions on the same gel. B: quantified results of puromycin incorporation in A. IOD, integrated optical density. *P < 0.05 vs. control group; #P < 0.05 vs. all other groups; n = 2; one-way ANOVA. C: the phosphorylation of NF-κB in myotubes treated with different doses of TNF-α was measured by Western blotting. *P < 0.05 vs. control group; n = 2; one-way ANOVA. D: the phosphorylation of p70S6K and eukaryotic initiation factor 4E binding protein (4E-BP1) in myotubes treated with different doses of IL-6 or LIF was measured by Western blotting. *P < 0.05 vs. control group; n = 2; one-way ANOVA. E: expression of myogenin in proliferating myoblasts in 4- and 8-day differentiated myotubes. F: expression of myogenin in proliferating myoblasts and 4-day differentiated myotubes with/without 2-h IL-6 (20 ng/ml) or LIF (10 ng/ml) administration. G: 8-day differentiated myotubes were treated with IL-6 (20 ng/ml) or LIF (10 ng/ml) for 2 h, and the puromycin incorporation was measured by Western blotting. *P < 0.05 vs. control group; n = 2; Student’s t-test. H: C2C12 myotubes were treated with Ara-C for 24 h during differentiation (3rd day of differentiation), followed with 2-h administration of IL-6 (20 ng/ml) or LIF (10 ng/ml) for 2 h. The puromycin incorporation was measured by Western blotting. *P < 0.05 vs. control group; n = 2; Student’s t-test.
The IL-6 family of cytokines can control the satellite cells expansion and myogenic differentiation process by regulating cyclin D1 expression (25, 58, 64). Interestingly, activation of mTOR signaling is also necessary for satellite cell proliferating and the myogenesis process (71). To exclude the possibility that IL-6 or LIF was regulating protein synthesis only in the residual myoblasts in C2C12 myotube culture, we measured the expression of the cell cycle regulator cyclin D1 and the molecular marker of myogenesis myogenin in proliferating myoblasts and 4-day differentiated myotubes. The differentiated myotubes showed high myogenin expression (Fig. 1E), while the cyclin D1 protein was below the detection level (Fig. 1F). Furthermore, unlike the IL-6 and LIF induction of cyclin D1 expression in proliferating C2C12 myoblasts (25, 64), neither IL-6 or LIF induced cyclin D1 expression in differentiated myotubes (Fig. 1F). We also allowed myotubes to differentiate for 8 days or pretreated myotubes with Ara-C to exclude the residual proliferating myoblasts in myotube culture. We found IL-6 and LIF significantly increased protein synthesis in both 8-day differentiated myotubes (Fig. 1G) and Ara-C pretreated myotubes (Fig. 1H), although both long-term differentiated myotubes and Ara-C-pretreated myotubes showed less induction of protein synthesis by IL-6 and LIF compared with 4-day differentiated myotubes (for IL-6, ~2.9 fold in 4-day differentiated myotubes vs. ~2-fold in 4-day differentiated myotubes and ~1.7-fold in Ara-C-treated myotubes; for LIF, ~4.4-fold in 4-day differentiated myotubes vs. ~2.4-fold in 4-day differentiated myotubes and ~2.3-fold in Ara-C-treated myotubes). These results demonstrate that the protein synthesis both IL-6 and LIF can upregulate protein synthesis in highly differentiated myotubes.
gp130 knockdown attenuated myotube protein synthesis induction by IL-6 or LIF.
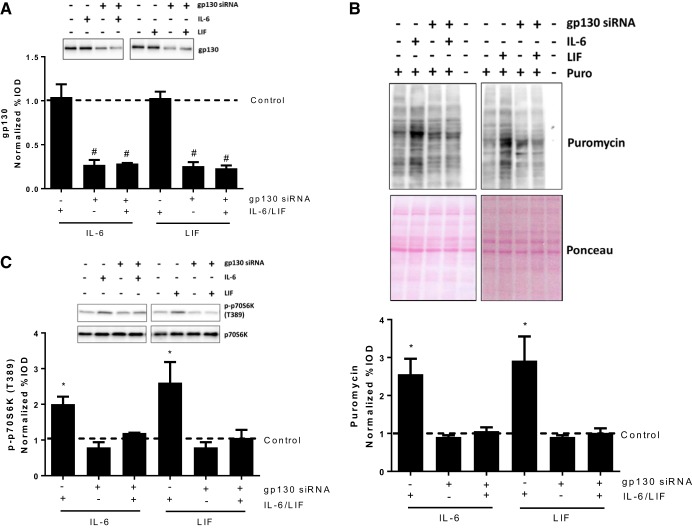
The IL-6 family of cytokines regulate intracellular signal transducers through the interaction with their functional receptor complex containing specific cytokine receptors and the gp130 transmembrane protein. To determine whether gp130 was necessary for the IL-6 and LIF induction of myotube protein synthesis, we transfected myotubes with gp130 siRNA. gp130 siRNA transfections significantly decreased myotube gp130 protein expression, while IL-6 or LIF administration did not change the gp130 protein level in either control or gp130 siRNA-transfected myotubes (Fig. 2A). Both IL-6 and LIF significantly increased protein synthesis in control siRNA-transfected myotubes but not in gp130 siRNA-transfected myotubes (Fig. 2B). Consistent with the changes in protein synthesis, IL-6 or LIF was unable to increase p70S6K phosphorylation in gp130 siRNA-transfected myotubes (Fig. 2C). These results demonstrate gp130 is necessary for IL-6 or LIF regulation of myotube mTOR signaling and protein synthesis.
Fig. 2.
Role of glycoprotein 130 (gp130) in IL-6/LIF regulation of myotube protein synthesis and mTORC1 signaling. C2C12 myotubes were transfected with 100 nM control or gp130 siRNA for 24 h, which was followed with 2-h exposure of IL-6 (20 ng/ml) or LIF (10 ng/ml). A: gp130 protein level in control or gp130 siRNA-transfected myotubes treated with/without IL-6/LIF. #Main effect of gp130 siRNA; n = 2; two-way ANOVA. B: protein synthesis in control or gp130 siRNA-transfected myotubes treated with/without IL-6/LIF was determined by puromycin incorporation. *P < 0.05 vs. all other groups; n = 2; two-way ANOVA. C: phosphorylation of p70S6K in control or gp130 siRNA-transfected myotubes treated with/without IL-6/LIF was measured by Western blotting. *P < 0.05 vs. all other groups; n = 2; two-way ANOVA.
Time course regulation of IL-6 and LIF on myotube gp130-dependent signaling pathways.
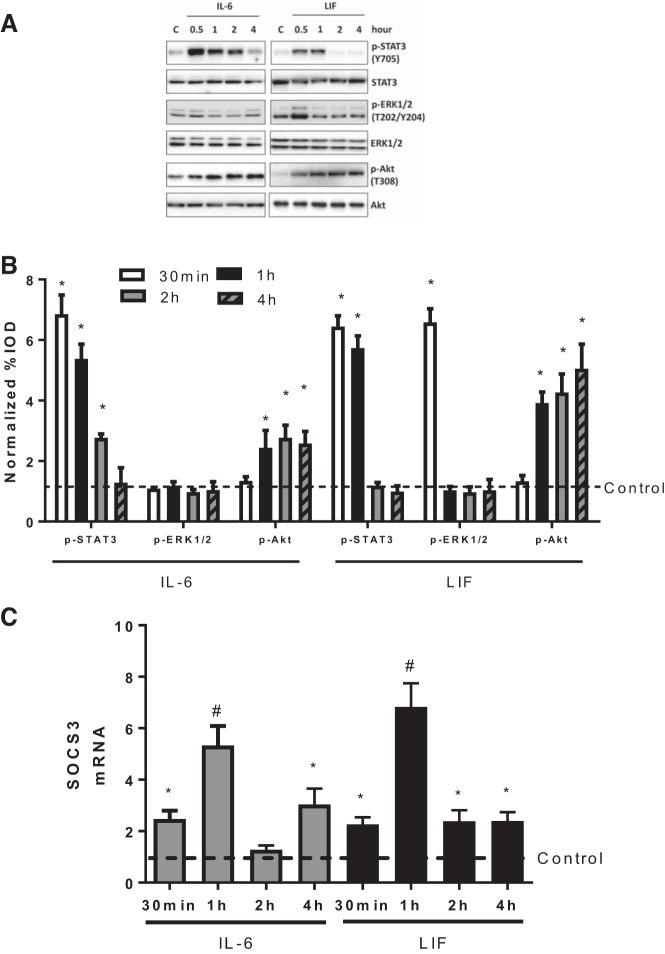
gp130 signaling induction results in the activation of STAT3, ERK1/2, and PI3K/Akt signaling pathways. STAT3 signaling can induce SOCS3 expression, which under physiological conditions creates negative feedback to prevent long-term gp130 signaling induction. Therefore, we next investigated the time course of IL-6 and LIF administration on gp130-dependent signaling pathways. Myotubes were treated with IL-6 (20 ng/ml) or LIF (10 ng/ml) for different time periods (30 min and 1, 2, and 4 h). The IL-6 and LIF doses used were selected based on the maximal protein synthesis induction (Fig. 1, A and B). The phosphorylation of STAT3 increased as early as 30 min after addition of either IL-6 or LIF (Fig. 3A) and remained elevated after 2 h of IL-6 exposure and 1 h of LIF exposure (Fig. 3A). However, STAT3 phosphorylation returned to the basal level after 4-h IL-6 exposures or 2-h LIF exposure (Fig. 3A). LIF administration for 30 min elevated ERK1/2 phosphorylation, but elevated ERK1/2 phosphorylation was not maintained after LIF exposure for 1 h or longer (Fig. 3A). Interestingly, ERK1/2 phosphorylation was not increased by IL-6 (Fig. 3A). Akt phosphorylation was induced as early as 1 h after either IL-6 or LIF exposure and remained elevated at 4 h (Fig. 3A). The short duration of increased STAT3 phosphorylation suggested a negative feedback involving gp130 signaling. Therefore, we examined IL-6 and LIF regulation of SOCS3 expression. IL-6 or LIF exposure increased SOCS3 mRNA expression as early as 30 min (Fig. 3B). One-hour IL-6 or LIF administration demonstrated the maximum induction of SOCS3 expression, while longer IL-6 or LIF exposure showed less (4-h IL-6 exposure and 2- and 4-h LIF exposure) or no (2-h IL-6 exposure) induction of SOCS3 expression (Fig. 3B). Similar with previous reports (70), we did not detect a change in SOCS3 protein after LIF or IL-6 treatment. However, the data were extremely variable and may reflect limitations in measurement due to the fast SOCS3 turnover in cultured myotubes (data not shown). These results demonstrate that IL-6 and LIF can induce an acute induction of gp130 signaling, which includes SOCS3 expression.
Fig. 3.
Regulation of IL-6 or LIF on myotube gp130-dependent signaling pathways. C2C12 myotubes were treated with IL-6 (20 ng/ml) or LIF (10 ng/ml) for different time periods (30 min and 1, 2, or 4 h). A: the phosphorylation of signal transducer and activator of transcription 3 (STAT3), ERK1/2, and Akt was measured by Western blotting. *P < 0.05 vs. control group; n = 2; one-way ANOVA. B: the suppressor of cytokine signaling 3 (SOCS3) mRNA expression in myotubes treated with IL-6 or LIF for different time periods was determined by RT-PCR. *P < 0.05 vs. control group; #P < 0.05 vs. all other groups; n = 2; one-way ANOVA.
Role of PI3K-Akt-mTOR signaling in IL-6/LIF regulation of myotube protein synthesis.
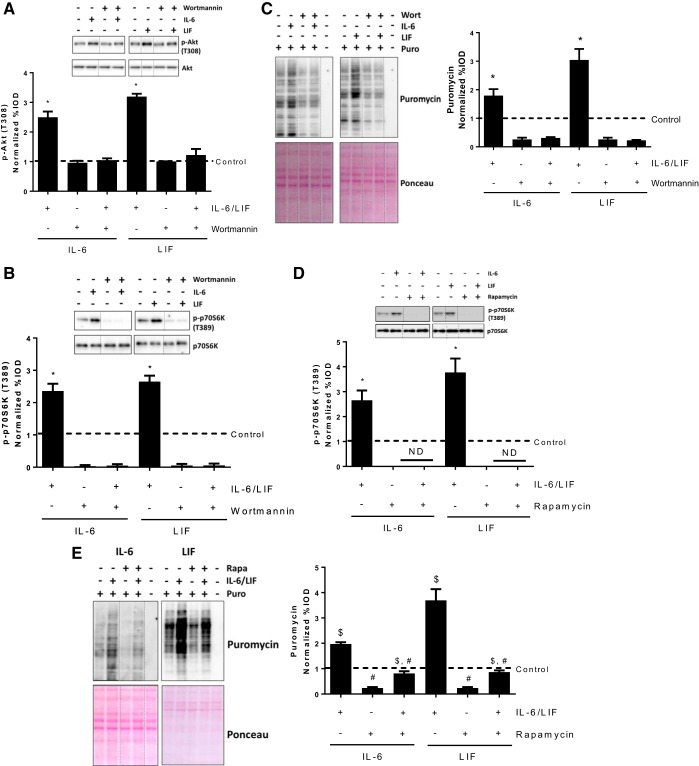
Since IL-6 and LIF were both able to induce Akt phosphorylation, we used wortmannin to inhibit PI3K/Akt signaling and examined if PI3K/Akt signaling is necessary for IL-6 or LIF induction of myotube protein synthesis. Myotubes were pretreated with wortmannin (1 μM) for 2 h before the administration of IL-6 or LIF. As expected, IL-6 or LIF did not increase Akt phosphorylation in wortmannin-pretreated myotubes (Fig. 4A). Furthermore, the wortmannin decreased basal phosphorylation of p70S6K and prevented IL-6 or LIF induction of p70S6K phosphorylation (Fig. 4B). Both IL-6 and LIF increased protein synthesis in control (DMSO) condition (Fig. 4C). Wortmannin decreased basal protein synthesis and blocked protein synthesis induction by IL-6 or LIF (Fig. 4C). These results demonstrate that PI3K/Akt signaling is necessary for myotube mTORC1 signaling and protein synthesis induction by either IL-6 or LIF.
Fig. 4.
Role of Akt-mTORC1 signaling in short-term IL-6/LIF regulation of myotube protein synthesis. C2C12 myotubes were pre-treated with the phosphoinositide 3-kinase (PI3K)/Akt signaling inhibitor, wortmannin (1 μM) or mTORC1 inhibitor, rapamycin (10 nM) for 1 h, which was followed with a 2-h exposure of IL-6 (20 ng/ml) or LIF (10 ng/ml). The equal volume of DMSO was added into cell culture medium of control group. A and B: phosphorylation of Akt (A) and p70S6K (B) in myotubes treated with/without IL-6/LIF or wortmannin was measured by Western blotting. Dash lines represent different positions on the same gel. *P < 0.05 vs. all other groups; n = 2; two-way ANOVA. C: the protein synthesis in myotubes treated with/without IL-6/LIF or wortmannin was determined by puromycin incorporation. *P < 0.05 vs. all other groups; n = 2; two-way ANOVA. D: phosphorylation of p70S6K in myotubes treated with/without IL-6/LIF or rapamycin was measured by Western blotting. Dash lines represent different positions on the same gel. *P < 0.05 vs. all other groups; n = 2; two-way ANOVA. E: the protein synthesis in myotubes treated with/without IL-6/LIF or rapamycin was determined by puromycin incorporation. #Main effect of rapamycin; $main effect of IL-6/LIF; n = 2; two-way ANOVA.
It has been well established that Akt signaling induction can induce muscle protein synthesis though the activation of mTORC1. To examine the role of mTORC1 in myotube protein synthesis induction by IL-6 and LIF, we pretreated myotubes with the mTORC1-specific inhibitor rapamycin before IL-6 or LIF administration. As expected, rapamycin decreased basal p70S6K phosphorylation, and IL-6 or LIF did not increase p70S6K phosphorylation in rapamycin-pretreated myotubes (20 nM for 2 h, Fig. 4D). Although rapamycin decreased basal myotube protein synthesis, both IL-6 and LIF still significantly increased protein synthesis in the presence of rapamycin (Fig. 4E). These results demonstrate that IL-6 and LIF can induce myotube protein synthesis though mTORC1-independent mechanisms.
Role of STAT3 in IL-6/LIF regulation of myotube protein synthesis.
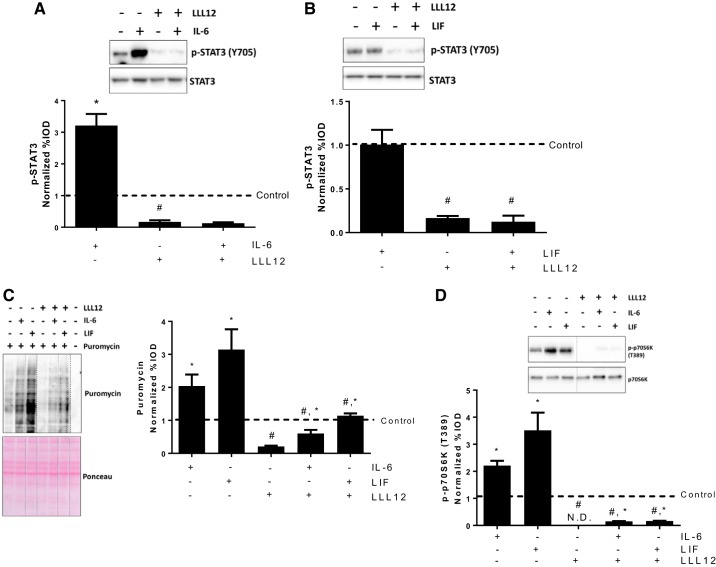
STAT3 is a classical downstream effector of IL-6/gp130 signaling, and we have observed a short-term induction of STAT3 phosphorylation by either IL-6 or LIF (Fig. 3A). To examine the role of STAT3 signaling in IL-6 and LIF regulation of myotube protein synthesis, we pretreated myotubes with the STAT3-specific inhibitor LLL12 to block the STAT3 signaling. LLL12 decreased basal STAT3 phosphorylation and blocked the IL-6 induction of STAT3 phosphorylation at 2 h (Fig. 5A). LIF administration alone for 2 h did not change the STAT3 phosphorylation in control or LLL12-treated myotubes (Fig. 5B), which we have shown occurs at 1 h or earlier (Fig. 3A). While LLL12 decreased basal myotube protein synthesis, both IL-6 and LIF significantly increased protein synthesis in the presence of LLL12 (Fig. 5C). LLL12 also decreased basal myotube p70S6K phosphorylation, but p70S6K phosphorylation was still induced by IL-6 or LIF in LLL12-treated myotubes (Fig. 5D). These results suggest that STAT3 signaling is necessary for maintaining basal myotube protein synthesis but is not involved in IL-6 or LIF induction of myotube mTORC1 signaling and protein synthesis.
Fig. 5.
Role of STAT3 signaling in IL-6/LIF regulation of myotube protein synthesis. C2C12 myotubes were pretreated with the STAT3 signaling inhibitor LLL12 (1 μM) for 1 h, which was followed with a 2-h exposure of IL-6 (20 ng/ml) or LIF (10 ng/ml). A: phosphorylation of STAT3 in myotubes treated with/without LLL12 or IL-6 for 2 h. *P < 0.05 vs. all other groups; #P < 0.05 vs. control group; n = 2; two-way ANOVA. B: phosphorylation of STAT3 in myotubes treated with/without LLL12 or LIF for 2 h. #main effect of LLL12; n = 2; two-way ANOVA. C: protein synthesis in myotubes treated with/without LLL12, IL-6, or LIF was determined by puromycin incorporation. Dash lines represent different positions on the same gel. #Main effect of LLL12; $main effect of IL-6/LIF, n = 2; two-way ANOVA. D: phosphorylation of p70S6K in myotubes treated with/without LLL12, IL-6 or LIF. Dash lines represent different positions on the same gel. #Main effect of LLL12; $main effect of IL-6/LIF; n = 2; two-way ANOVA.
Role of SOCS3 in the regulation of myotube protein synthesis.
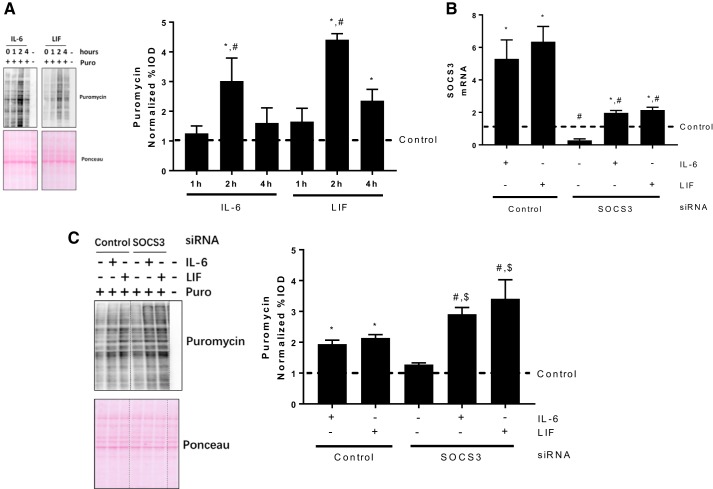
We have demonstrated that gp130 is necessary for either IL-6 or LIF induction of myotube protein synthesis (Fig. 2B). Both IL-6 and LIF caused a transient induction of STAT3 phosphorylation, which was associated with increased SOCS3 expression. We measured myotube protein synthesis after different durations of IL-6 or LIF exposure. Myotube protein synthesis induction peaked after 2-h IL-6 or LIF exposure (Fig. 6A). Interestingly, longer IL-6 or LIF exposure (4 h) showed no (for IL-6) or less (for LIF) protein synthesis induction (Fig. 6A). These results demonstrate that IL-6 and LIF only result in short-term induction of myotube protein synthesis, which coincides with the increased SOCS3 expression.
Fig. 6.
Role of SOCS3 in IL-6 and LIF time course regulation of myotube protein synthesis. A: protein synthesis in myotubes treated with IL-6 (20 ng/ml) or LIF (10 ng/ml) for different time periods (1, 2, and 4 h) was determined by puromycin incorporation. *P < 0.05 vs. control group; #P < 0.05 vs. all other groups; n = 2; one-way ANOVA. B: C2C12 myotubes were transfected with control or SOCS3 siRNA for 24 h, which was followed with 1 h IL-6 (20 ng/ml) or LIF (10 ng/ml) administration. The SOCS3 mRNA expression was measured by RT-PCR. *Main effect of IL-6/LIF; #main effect of SOCS3 siRNA; n = 2; two-way ANOVA. C: the control or SOCS3 siRNA-transfected myotubes were treated with IL-6 (20 ng/ml) or LIF (10 ng/ml) for 4 h, the puromycin incorporation was measured by Western blotting. *P < 0.05 vs. control group; #P < 0.05 vs. SOCS3 siRNA group; $P < 0.05 vs. control siRNA with IL-6/LIF group; n = 2; two-way ANOVA.
To determine the role of SOCS3 in myotube protein synthesis regulation, we inhibited SOCS3 expression by siRNA transfection before IL-6 and LIF administration. SOCS3 siRNA transfection decreased the basal SOCS3 mRNA level and attenuated the IL-6/LIF induction of SOCS3 expression (Fig. 6B). However, SOCS3 siRNA did not completely block the IL-6 and LIF induction of SOCS3 expression (Fig. 6B). We examined IL-6 or LIF administration for 4 h on protein synthesis in control or SOCS3 siRNA-transfected myotubes. IL-6 and LIF administration for 4 h resulted in a slight induction of protein synthesis (Fig. 6C). Either IL-6 or LIF treatment further increased protein synthesis in SOCS3 siRNA-transfected myotubes (Fig. 6C). These results demonstrate that SOCS3 serves as a suppressor of myotube protein synthesis induction by either IL-6 or LIF.
DISCUSSION
The IL-6 family of cytokines appears to have paradoxical roles in skeletal muscle mass regulation. Under physiological conditions, IL-6 family of cytokines produced in skeletal muscle, including IL-6 and LIF, is an important regulator of skeletal muscle hypertrophy. However, the regulation of skeletal muscle protein synthesis by the IL-6 family of cytokines warrants further investigation. In this study, we report the important and novel finding that either IL-6 or LIF can transiently induce protein synthesis in differentiated myotubes. This induction requires gp130 and downstream PI3K-Akt signaling. Interestingly, mTORC1 signaling inhibition did not block protein synthesis induction by IL-6 or LIF. While STAT3 signaling was necessary for maintaining basal protein synthesis, we report that STAT3 was not required for the induction of protein synthesis by either IL-6 or LIF. The transient induction of protein synthesis by either IL-6 or LIF was associated with increased SOCS3 expression. SOCS3 knockdown increased basal and cytokine-induced protein synthesis, suggesting that SOCS3 is a feedback inhibitor of protein synthesis. Overall, these results demonstrate that either IL-6 or LIF can induce a transient elevation of protein synthesis in cultured myotubes, which requires the activation of gp130-Akt signaling but is independent of STAT3 signaling.
Mechanical stimuli from overload or resistance exercise results in increased muscle production of IL-6 and LIF, which can regulate the muscle hypertrophic process though autocrine/paracrine manner (10, 58, 61). Our previous work suggested that gp130 signaling may play a role in mechanical stretch regulation of myotube protein synthesis under a Lewis lung carinoma-induced atrophic environment (19). IL-6 and LIF may facilitate muscle protein synthesis indirectly though different mechanisms. For example, IL-6 and LIF can sensitize the insulin/IGF-1 action in skeletal muscle (27, 68, 69), and IL-6 and LIF also induce muscle glucose uptake (1, 9), which is an inducer of muscle protein synthesis (33). Our results demonstrate that either IL-6 or LIF can induce protein synthesis in cultured myotubes, which provides another potential mechanism underlying the mechanical stimuli regulation of muscle protein synthesis and the IL-6 family of cytokines regulation of muscle hypertrophy. Interestingly, the overload induction of p70S6K phosphorylation was not attenuated in IL-6 deficient mice, which suggests that the direct mTORC1 signaling and protein synthesis induction by IL-6 are not the only mechanisms (21). Indeed, multiple biochemical events have been identified as mediators of protein synthesis induction by mechanical stimulus. Further work is needed to determine whether regulation of protein synthesis by IL-6 or LIF contributes to the mechanical stimuli-induced muscle hypertrophy.
The binding of the IL-6 family of cytokines on the receptor complex containing its specific receptor and gp130 causes the phosphorylation of gp130 intracellular tyrosine residues and STAT3 by the Janus kinases JAK1 and JAK2 (22). This active receptor complex also leads to the activation of the Ras-Raf-ERK/MAPK pathway and the PI3K/Akt pathway (22, 48). The PI3K-Akt is the most well-defined hypertrophic signaling pathway, which leads to the anabolic shift of muscle protein turnover regulation (6, 51). The insulin/IGF-1induced PI3K-Akt signaling activation leads to the phosphorylation of TSC2, which relieves suppression of the TSC1/2 protein complex on Rheb, allowing Rheb to stimulate mTORC1 and subsequent protein synthesis (6, 29). Although the mechanism is still poorly understood, gp130 signaling can activate PI3K-Akt signaling in skeletal muscles, which is a key mechanism of muscle glucose uptake induced by the IL-6 family of cytokines (9, 54, 62). Consistent with previous findings (54, 68), we report that both IL-6 and LIF administration increased Akt phosphorylation. Most importantly, inhibition of Akt signaling blocked protein synthesis induction by IL-6 or LIF. These findings demonstrate that induction of PI3K/Akt signaling is also necessary for IL-6/LIF regulation of protein synthesis in skeletal muscle cells, which is similar to its central role in cardiac myocyte protein synthesis and hypertrophy (17, 46). Our results also highlight the importance of PI3K/Akt signaling in myotube protein synthesis regulation by the IL-6 family of cytokines.
mTORC1 signaling plays a central role in regulating skeletal muscle protein synthesis and is essential for muscle hypertrophy response to growth factors (6). mTORC1 signaling activation induces muscle protein synthesis through the phosphorylation and activation of p70S6K and 4E-BP1 (67). This event regulates ribosomal biogenesis (35, 67) and cap-dependent protein translation initiation (49). Akt is a classical upstream stimulator of mTORC1, and previous work has demonstrated that the gp130-Akt-mTORC1 signaling axis is critical for IL-6 or LIF induction of cardiac myocytes protein synthesis and cardiac hypertrophy (17, 46). Here, we propose that this signaling axis exists in myotubes, because IL-6 or LIF transiently increased p70S6K phosphorylation, which was blocked by either gp130 knockdown or Akt signaling inhibition. Inhibition of mTORC1 by rapamycin blocked IL-6/LIF induction of p70S6K phosphorylation and decreased protein synthesis in both control and IL-6/LIF-treated myotubes, which further supports the central role of mTORC1 signaling in protein synthesis regulation. Surprisingly, mTORC1 signaling inhibition did not completely block the protein synthesis induction by IL-6 or LIF. Notably, PI3K-Akt signaling inhibition by wortmannin fully blocked the IL-6/LIF induction of protein synthesis, suggesting that IL-6/LIF-induced mTORC1-independent protein synthesis upregulation is still dependent on PI3K/Akt signaling. Previously, studies have proposed that glucose and branched chain amino acid supplementation can stimulate muscle protein synthesis in the absence of mTORC1 signaling activation (2, 33) and that rapamycin only partially inhibited leucine-induced muscle protein synthesis (3). A potential mechanism by which leucine stimulates protein synthesis independent of mTORC1 activity is through direct phosphorylation of eukaryotic initiation factor 4G (7, 14). Interestingly, both IL-6 and LIF can stimulate muscle glucose uptake through the activation of PI3K/Akt signaling (1, 9), and glucose can stimulate muscle protein synthesis, possibly through mTORC1-independent mechanisms (33, 53). Combined together, these reports suggest that increased glucose uptake is a potential mTORC1-independent protein synthesis stimulator induced by IL-6-related cytokines. However, whether the increase in glucose translocation is the mTORC1-independent mechanism of IL-6/LIF-induced protein synthesis still needs to be investigated.
STAT3 is a well-defined downstream target of IL-6-gp130 signaling (40). Once phosphorylated, STAT3 proteins dimerize and translocate into the nucleus, where they promote the transcription of downstream genes that are responsible for a variety of cellular functions, including proliferation, migration, and the prevention of apoptosis (31, 58). STAT3 plays a critical role during development, and its deletion leads to early embryonic lethality (63). In skeletal muscles, STAT3 activation is also required for hypertrophy under the action of IL-6 (58) by regulating the transcription of myogenesis genes (64). However, to our knowledge, there is no evidence demonstrating direct STAT3 regulation of either mTORC1 signaling or protein synthesis. We report that STAT3 inhibition resulted in a strong suppression of mTORC1 signaling and protein synthesis in both control and cytokine-treated myotubes, which suggests a role for STAT3 in basal protein synthesis regulation in myotubes. However, STAT3 inhibition did not block the myotube mTORC1 signaling and protein synthesis induction by either IL-6 or LIF. Previous studies in nonmuscle cells suggest that STAT3 is indirectly involved in anabolic signaling pathways. In primary cultured renal proximal tubule cells, STAT3 plays a permissive role in IL-6 induction of PI3K/Akt signaling (38). In cancer cells, STAT3 signaling induction is critical for increased glucose uptake and aerobic glycolysis (15), both of which have been demonstrated as positive regulators of mTORC1 signaling (33, 53). Interestingly, IL-6/STAT3 signaling activation also plays a role in insulin resistance by activating mTOR signaling in hepatocarcinoma cells (34). However, the potential cross talk between STAT3 signaling and protein synthesis in skeletal muscle needs to be further defined.
SOCS3, whose expression is induced by STAT3 activation, can inhibit STAT3 signaling by binding the phosphotyrosines of JAK2 and thus physically block the binding of STAT3 to JAK2 (22). Several previous studies suggested a potential catabolic role for SOCS3 in skeletal muscle mass regulation (4, 8, 39, 41). Long-term IL-6 overexpression in vivo suppresses muscle protein synthesis suppression and induces wasting (17, 41), which are associated with muscle SOCS3 expression (41). Increased SOCS3 expression is also observed in wasting skeletal muscles during cancer cachexia and sarcopenia (4, 8, 39). SOCS3 may play an indirect role in muscle protein synthesis regulation by desensitizing the IGF-1-PI3K-Akt signaling pathways. For example, SOCS3 can target and degrade insulin receptor substrate-1 in inflammation-induced insulin resistance, possibly via the elongin BC ubiquitin-ligase (52). SOCS3 is also associated with muscle atrophy and growth impairment by disrupting the anabolic effects of growth hormones in IL-6-overexpressing mice (41). Consistent with previous reports (25, 59), we observed that STAT3 phosphorylation returned to baseline after a transient increase after either IL-6 or LIF exposure. Interestingly, we found similar time course regulation of IL-6 and LIF on myotube protein synthesis: peaking after 2 h of IL-6 or LIF exposure and decreasing after longer cytokine exposure. The time course regulation of STAT3 signaling and protein synthesis is associated with increased SOCS3 mRNA expression. Interestingly, we and others (70) did not find induction of SOCS3 protein level by IL-6 or LIF, which suggests a complicated posttranscriptional regulation of SOCS3. In this study, we reported that SOCS3 siRNA knockdown resulted in a longer induction of IL-6 and LIF on myotube protein synthesis. These results suggest that increased SOCS3 mRNA expression is a potential negative-feedback mechanism of IL-6/LIF-induced myotube protein synthesis. These results also strengthen our understanding of the catabolic role of SOCS3 in muscle mass regulation. However, it is still unknown whether SOCS3 can inhibit myotube protein synthesis through inhibiting gp130 signaling or insulin receptor substrate-1/Akt signaling. More research is needed to determine the potential interplay of SOCS3 with anabolic signaling pathways in skeletal muscles.
IL-6 and LIF can also regulate satellite proliferation and differentiation, which are key mechanisms for their regulatory role during muscle hypertrophy (58, 61). IL-6 can induce the expression of cell cycle regulators such as cyclin D1 and c-myc, which further contributes to satellite cell proliferation (5). Unlike the high expression of cyclin D1 in myoblasts, which can be further induced by IL-6 (25), 4-day differentiated myotubes showed no expression of cyclin D1, and IL-6 or LIF did not induce Cyclin D1 expression. Most importantly, we also found similar protein synthesis induction by IL-6 or LIF in long-term differentiated or Ara-C-treated myotubes. The long-term differentiated and Ara-C-pretreated myotubes showed less protein synthesis induction by IL-6 or LIF compared with the 4-day differentiated myotubes, which suggests a potential protein synthesis upregulation by IL-6 and LIF in residual myoblasts in 4-day differentiated myotubes. However, our results demonstrate that IL-6 and LIF upregulation of protein synthesis happens in differentiated myotubes.
In summary, we examined the acute regulation of IL-6 and LIF on protein synthesis in cultured myotubes. Both IL-6 and LIF can activate myotube mTORC1 signaling and protein synthesis in a time- and dose-sensitive manner. The gp130 receptor and PI3K/Akt signaling pathway was critical for this induction. IL-6 and LIF both induced the gp130-Akt signaling axis through activated mTORC1 signaling, but mTORC1-independent regulation was apparent. STAT3 signaling played a regulatory role in maintaining basal myotube protein synthesis, but STAT3 signaling was not required for the induction by IL-6 and LIF of protein synthesis. Finally, either IL-6 or LIF induced SOCS3 mRNA expression, and knockdown of SOCS3 expression enhanced the cytokine induction of protein synthesis. Our study provides new insights into the involvement of the IL-6 family of cytokines in protein synthesis regulation. Further work is necessary to determine the potential mTORC1-independent mechanisms underlying IL-6/LIF induction of protein synthesis and the role of IL-6 or LIF in muscle protein turnover regulation during regeneration and muscle hypertrophy.
GRANTS
This work was supported by National Cancer Institute Grant R01 CA121249A501 (J. A. Carson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.G., J.L.D., H.-J.K., W.E.C., N.F., and J.A.C. conceived and designed research; S.G. performed experiments; S.G. analyzed data; S.G., J.L.D., H.-J.K., W.E.C., N.F., and J.A.C. interpreted results of experiments; S.G. prepared figures; S.G. and J.A.C. drafted manuscript; S.G., J.L.D., H.-J.K., W.E.C., N.F., and J.A.C. edited and revised manuscript; S.G., J.L.D., H.-J.K., W.E.C., N.F., and J.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gaye Christmus for editorial review of the manuscript and Dennis Fix, Brandon Vanderveen, and Justin Hardee for help in the experiments.
REFERENCES
- 1.Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006. doi: 10.1210/me.2005-0490. [DOI] [PubMed] [Google Scholar]
- 2.Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, Jefferson LS. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes 51: 928–936, 2002. doi: 10.2337/diabetes.51.4.928. [DOI] [PubMed] [Google Scholar]
- 3.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 294: R393–R401, 2008. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 5.Begue G, Douillard A, Galbes O, Rossano B, Vernus B, Candau R, Py G. Early activation of rat skeletal muscle IL-6/STAT1/STAT3 dependent gene expression in resistance exercise linked to hypertrophy. PLoS One 8: e57141, 2013. doi: 10.1371/journal.pone.0057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 7.Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr 134: 1704–1710, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, Zimmers TA. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 6: e22538, 2011. doi: 10.1371/journal.pone.0022538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt N, O’Neill HM, Kleinert M, Schjerling P, Vernet E, Steinberg GR, Richter EA, Jørgensen SB. Leukemia inhibitory factor increases glucose uptake in mouse skeletal muscle. Am J Physiol Endocrinol Metab 309: E142–E153, 2015. doi: 10.1152/ajpendo.00313.2014. [DOI] [PubMed] [Google Scholar]
- 10.Broholm C, Laye MJ, Brandt C, Vadalasetty R, Pilegaard H, Pedersen BK, Scheele C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol (1985) 111: 251–259, 2011. doi: 10.1152/japplphysiol.01399.2010. [DOI] [PubMed] [Google Scholar]
- 11.Carbia-Nagashima A, Arzt E. Intracellular proteins and mechanisms involved in the control of gp130/JAK/STAT cytokine signaling. IUBMB Life 56: 83–88, 2004. doi: 10.1080/15216540410001668064. [DOI] [PubMed] [Google Scholar]
- 12.Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 38: 168–176, 2010. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML. Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J Biol Chem 274: 23013–23019, 1999. doi: 10.1074/jbc.274.33.23013. [DOI] [PubMed] [Google Scholar]
- 14.Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 135: 376–382, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D’Angeli L, Bartoli A, Gough DJ, Turkson J, Levy DE, Watson CJ, Wieckowski MR, Provero P, Pinton P, Poli V. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany NY) 2: 823–842, 2010. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 49: 59–68, 2014. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahmi A, Smart N, Punn A, Jabr R, Marber M, Heads R. p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell Signal 25: 898–909, 2013. doi: 10.1016/j.cellsig.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16: 1335–1347, 2002. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 19.Gao S, Carson JA. Lewis lung carcinoma regulation of mechanical stretch-induced protein synthesis in cultured myotubes. Am J Physiol Cell Physiol 310: C66–C79, 2016. doi: 10.1152/ajpcell.00052.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25: 1028–1039, 2011. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerci A, Lahoute C, Hébrard S, Collard L, Graindorge D, Favier M, Cagnard N, Batonnet-Pichon S, Précigout G, Garcia L, Tuil D, Daegelen D, Sotiropoulos A. Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 15: 25–37, 2012. doi: 10.1016/j.cmet.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374: 1–20, 2003. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene 20: 5991–6000, 2001. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka E, Kawashima S, Takahashi T, Rikitake Y, Hirase T, Yokoyama M. PI 3-kinase-Akt-p70 S6 kinase in hypertrophic responses to leukemia inhibitory factor in cardiac myocytes. Kobe J Med Sci 49: 25–37, 2003. [PubMed] [Google Scholar]
- 25.Hoene M, Runge H, Häring HU, Schleicher ED, Weigert C. Interleukin-6 promotes myogenic differentiation of mouse skeletal muscle cells: role of the STAT3 pathway. Am J Physiol Cell Physiol 304: C128–C136, 2013. doi: 10.1152/ajpcell.00025.2012. [DOI] [PubMed] [Google Scholar]
- 26.Hunt LC, White J. The role of leukemia inhibitory factor receptor signaling in skeletal muscle growth, injury and disease. Adv Exp Med Biol 900: 45–59, 2016. doi: 10.1007/978-3-319-27511-6_3. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda SI, Tamura Y, Kakehi S, Sanada H, Kawamori R, Watada H. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem Biophys Res Commun 473: 947–952, 2016. doi: 10.1016/j.bbrc.2016.03.159. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17: 1829–1834, 2003. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657, 2002. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 30.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 31.Ivanova AV, Ivanov SV, Zhang X, Ivanov VN, Timofeeva OA, Lerman MI. STRA13 interacts with STAT3 and modulates transcription of STAT3-dependent targets. J Mol Biol 340: 641–653, 2004. doi: 10.1016/j.jmb.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Jee SH, Chiu HC, Tsai TF, Tsai WL, Liao YH, Chu CY, Kuo ML. The phosphotidyl inositol 3-kinase/Akt signal pathway is involved in interleukin-6-mediated Mcl-1 upregulation and anti-apoptosis activity in basal cell carcinoma cells. J Invest Dermatol 119: 1121–1127, 2002. doi: 10.1046/j.1523-1747.2002.19503.x. [DOI] [PubMed] [Google Scholar]
- 33.Jeyapalan AS, Orellana RA, Suryawan A, O’Connor PM, Nguyen HV, Escobar J, Frank JW, Davis TA. Glucose stimulates protein synthesis in skeletal muscle of neonatal pigs through an AMPK- and mTOR-independent process. Am J Physiol Endocrinol Metab 293: E595–E603, 2007. doi: 10.1152/ajpendo.00121.2007. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Kim JE, Liu HY, Cao W, Chen J. Regulation of interleukin-6-induced hepatic insulin resistance by mammalian target of rapamycin through the STAT3-SOCS3 pathway. J Biol Chem 283: 708–715, 2008. doi: 10.1074/jbc.M708568200. [DOI] [PubMed] [Google Scholar]
- 35.Kimball SR, Jefferson LS. Molecular mechanisms through which amino acids mediate signaling through the mammalian target of rapamycin. Curr Opin Clin Nutr Metab Care 7: 39–44, 2004. doi: 10.1097/00075197-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Kortylewski M, Feld F, Krüger KD, Bahrenberg G, Roth RA, Joost HG, Heinrich PC, Behrmann I, Barthel A. Akt modulates STAT3-mediated gene expression through a FKHR (FOXO1a)-dependent mechanism. J Biol Chem 278: 5242–5249, 2003. doi: 10.1074/jbc.M205403200. [DOI] [PubMed] [Google Scholar]
- 37.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 122: 3589–3594, 2009. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YJ, Heo JS, Suh HN, Lee MY, Han HJ. Interleukin-6 stimulates alpha-MG uptake in renal proximal tubule cells: involvement of STAT3, PI3K/Akt, MAPKs, and NF-κB. Am J Physiol Renal Physiol 293: F1036–F1046, 2007. doi: 10.1152/ajprenal.00034.2007. [DOI] [PubMed] [Google Scholar]
- 39.Léger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res 11: 163–175, 2008. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- 40.Levy DE, Lee CK. What does Stat3 do? J Clin Invest 109: 1143–1148, 2002. doi: 10.1172/JCI0215650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieskovska J, Guo D, Derman E. Growth impairment in IL-6-overexpressing transgenic mice is associated with induction of SOCS3 mRNA. Growth Horm IGF Res 13: 26–35, 2003. [DOI] [PubMed] [Google Scholar]
- 42.McKay BR, De Lisio M, Johnston AP, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One 4: e6027, 2009. doi: 10.1371/journal.pone.0006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol 589: 1831–1846, 2011. doi: 10.1113/jphysiol.2011.205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori M, Yamaguchi K, Honda S, Nagasaki K, Ueda M, Abe O, Abe K. Cancer cachexia syndrome developed in nude mice bearing melanoma cells producing leukemia-inhibitory factor. Cancer Res 51: 6656–6659, 1991. [PubMed] [Google Scholar]
- 45.Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J 280: 4131–4148, 2013. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, Kishimoto T, Yamauchi-Takihara K. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction. Circulation 103: 555–561, 2001. doi: 10.1161/01.CIR.103.4.555. [DOI] [PubMed] [Google Scholar]
- 47.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem 273: 9703–9710, 1998. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol 536: 329–337, 2001. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ptushkina M, von der Haar T, Vasilescu S, Frank R, Birkenhäger R, McCarthy JE. Cooperative modulation by eIF4G of eIF4E-binding to the mRNA 5′ cap in yeast involves a site partially shared by p20. EMBO J 17: 4798–4808, 1998. doi: 10.1093/emboj/17.16.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130's role in Lewis lung carcinoma-induced cachexia. FASEB J 28: 998–1009, 2014. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009–1013, 2001. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 52.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277: 42394–42398, 2002. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 53.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 59: 2426–2434, 2010. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saini A, Faulkner SH, Moir H, Warwick P, King JA, Nimmo MA. Interleukin-6 in combination with the interleukin-6 receptor stimulates glucose uptake in resting human skeletal muscle independently of insulin action. Diabetes Obes Metab 16: 931–936, 2014. doi: 10.1111/dom.12299. [DOI] [PubMed] [Google Scholar]
- 55.Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, Takahashi T, Makino S, Kato T, Ogawa S. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem 275: 29717–29723, 2000. doi: 10.1074/jbc.M003128200. [DOI] [PubMed] [Google Scholar]
- 56.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280: 4294–4314, 2013. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 57.Schmitz J, Weissenbach M, Haan S, Heinrich PC, Schaper F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J Biol Chem 275: 12848–12856, 2000. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- 58.Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Seto DN, Kandarian SC, Jackman RW. A key role for leukemia inhibitory factor in C26 cancer cachexia. J Biol Chem 290: 19976–19986, 2015. doi: 10.1074/jbc.M115.638411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Y, Hsu JH, Hu L, Gera J, Lichtenstein A. Signal pathways involved in activation of p70S6K and phosphorylation of 4E-BP1 following exposure of multiple myeloma tumor cells to interleukin-6. J Biol Chem 277: 15712–15720, 2002. doi: 10.1074/jbc.M200043200. [DOI] [PubMed] [Google Scholar]
- 61.Spangenburg EE, Booth FW. Leukemia inhibitory factor restores the hypertrophic response to increased loading in the LIF(−/−) mouse. Cytokine 34: 125–130, 2006. doi: 10.1016/j.cyto.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Steinberg GR, Watt MJ, Ernst M, Birnbaum MJ, Kemp BE, Jørgensen SB. Ciliary neurotrophic factor stimulates muscle glucose uptake by a PI3-kinase-dependent pathway that is impaired with obesity. Diabetes 58: 829–839, 2009. doi: 10.2337/db08-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA 94: 3801–3804, 1997. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L, Sacco A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med 20: 1182–1186, 2014. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2: 862–871, 2002. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 66.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 89: 381–410, 2009. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21: 362–369, 2006. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 68.Weigert C, Hennige AM, Brodbeck K, Häring HU, Schleicher ED. Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. Am J Physiol Endocrinol Metab 289: E251–E257, 2005. doi: 10.1152/ajpendo.00448.2004. [DOI] [PubMed] [Google Scholar]
- 69.Weigert C, Hennige AM, Lehmann R, Brodbeck K, Baumgartner F, Schaüble M, Häring HU, Schleicher ED. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J Biol Chem 281: 7060–7067, 2006. doi: 10.1074/jbc.M509782200. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol 20: 604–612, 2009. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang P, Liang X, Shan T, Jiang Q, Deng C, Zheng R, Kuang S. mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration. Biochem Biophys Res Commun 463: 102–108, 2015. doi: 10.1016/j.bbrc.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]