Abstract
Arterial medial calcification (AMC) is thought to share some outward similarities to skeletal mineralization and has been associated with the transdifferentiation of vascular smooth muscle cells (VSMCs) to an osteoblast-like phenotype. ATP and UTP have previously been shown to inhibit bone mineralization. This investigation compared the effects of extracellular nucleotides on calcification in VSMCs with those seen in osteoblasts. ATP, UTP and the ubiquitous mineralization inhibitor, pyrophosphate (PPi), dose dependently inhibited VSMC calcification by ≤85%. Culture of VSMCs in calcifying conditions was associated with an increase in apoptosis; treatment with ATP, UTP, and PPi reduced apoptosis to levels seen in non-calcifying cells. Extracellular nucleotides had no effect on osteoblast viability. Basal alkaline phosphatase (TNAP) activity was over 100-fold higher in osteoblasts than VSMCs. ATP and UTP reduced osteoblast TNAP activity (≤50%) but stimulated VSMC TNAP activity (≤88%). The effects of extracellular nucleotides on VSMC calcification, cell viability and TNAP activity were unchanged by deletion or inhibition of the P2Y2 receptor. Conversely, the actions of ATP/UTP on bone mineralization and TNAP activity were attenuated in osteoblasts lacking the P2Y2 receptor. Ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) hydrolyses ATP and UTP to produce PPi. In both VSMCs and osteoblasts, deletion of NPP1 blunted the inhibitory effects of extracellular nucleotides suggesting involvement of P2 receptor independent pathways. Our results show that although the overall functional effect of extracellular nucleotides on AMC and bone mineralization is similar there are clear differences in the cellular mechanisms mediating these actions.
1 INTRODUCTION
Vascular calcification is a common consequence of ageing, atherosclerosis, diabetes and chronic kidney disease. It is the pathological deposition of calcium phosphate mineral, usually as hydroxyapatite, in the medial and/or intimal layer of the arteries and heart valves. Arterial medial calcification (AMC) refers to the calcification that occurs within the tunica media of blood vessels and is characterized by increased vessel stiffness and reduced blood flow (Young, Adams, Anderson, Boulton, & Cavanagh, 1993). Traditionally, AMC was thought to be a passive process caused by high serum levels of phosphate and calcium. However, it is now accepted that it is a complex cell-mediated process that shares some similarities with the process of physiological bone formation. Specifically, AMC is thought to involve the loss of calcification inhibitors (e.g., pyrophosphate (PPi), osteopontin, fetuin A), gain of calcification inducers (e.g., alkaline phosphatase (TNAP)) and increased apoptosis (Proudfoot et al., 2000; Zhu, Mackenzie, Farquharson, & Macrae, 2012). While many cell types can contribute towards the development of AMC, vascular smooth muscle cells (VSMCs) are thought to be the major cell type involved (Narisawa et al., 2007; Zhu, Mackenzie, Millan, Farquharson, & Macrae, 2011). Within a calcifying environment (high phosphate), VSMCs can undergo a phenotypic transdifferentiation to take on characteristics usually associated with bone-forming osteoblasts (Shroff & Shanahan, 2007; Zhu et al., 2011).
ATP has long been recognized for its role in intracellular energy metabolism; however, it is also an important extracellular signalling molecule. ATP and related compounds (UTP, ADP, UDP) act via purinergic P2 receptors to regulate cell proliferation, differentiation, survival, and function in many tissues (Burnstock, 2007). The P2 receptor family is made up of seven P2X ion channels (P2 × 1–7) and eight P2Y G-protein coupled receptors (P2Y1,2,4,6,11–14) (Abbracchio & Burnstock, 1994; Burnstock & Kennedy, 1985).
Purinergic signalling plays a number of roles in the cardiovascular system (see review (Burnstock & Ralevic, 2014)). VSMCs express multiple P2 receptor subtypes (Wang et al., 2002) and, in recent years, several investigations have examined the role of purinergic signalling in the different forms of vascular calcification (Fish et al., 2013). However, they provide conflicting evidence as to whether extracellular nucleotides are harmful or protective. P2Y2 receptor mediated signalling has been shown to promote the survival of aortic valve interstitial cells and protect against aortic valve calcification (Cote et al., 2012) and arterial intimal calcification (Qian et al., 2017). Additionally, a biochemical study showed that ATP inhibited calcium phosphate deposition in rat VSMCs (Villa-Bellosta & Sorribas, 2013). In contrast, activation of P2Y receptors by Up4A (a non-selective P2R agonist) has been reported to enhance VSMC calcification (Schuchardt et al., 2012).
Once released extracellular nucleotides are rapidly broken down by ecto-nucleotidases to limit their actions to cells within close proximity of the release site. PPi, a ubiquitous and potent inhibitor of calcification (Fleisch & Bisaz, 1962), is generated when nucleotide triphosphates are hydrolyzed by ecto-nucleotide pyrophosphatase/phosphodiesterases (NPPs). VSMCs express many ecto-nucleotidases, including NPP1, and can release ATP in a controlled manner (Prosdocimo, Douglas, Romani, O’Neill, & Dubyak, 2009; Prosdocimo, Wyler, Romani, O’Neill, & Dubyak, 2010; Villa-Bellosta, Wang, Millan, Dubyak, & O’Neill, 2011). Accumulating evidence now suggests that the hydrolysis of locally released ATP by NPP1 is the major source of extracellular PPi (Orriss, Arnett, & Russell, 2016; Prosdocimo et al., 2009). This PPi can then act locally to regulate the level of calcification. Consistent with this, NPP1 knockout mice (Enpp1−/−) display extensive ectopic calcification in a variety of soft tissues including the aorta, kidney, cartilage, ear pinna, and whisker vibrissae (Hajjawi et al., 2014; Johnson et al., 2003; Mackenzie et al., 2012). Furthermore, mutations in the gene encoding NPP1 lead to the recessive condition Generalised Arterial Calcification of Infancy (GACI) which is characterized by extensive vascular calcification (Rutsch et al., 2001).
The regulation of bone formation by extracellular nucleotides has been widely studied often with conflicting results (see reviews Burnstock, Arnett, & Orriss, 2013; Gartland et al., 2012; Noronha-Matos & Correia-de-Sa, 2016; Orriss, 2015). Activation of several P2 receptors (e.g., P2Y2, P2X1, P2X7) has been shown to both inhibit and promote bone mineralization (Hoebertz, Mahendran, Burnstock, & Arnett, 2002; Noronha-Matos et al., 2014; Orriss, Key, et al., 2012; Orriss et al., 2007; Panupinthu et al., 2007; Xing et al., 2014). We have previously reported that ATP and UTP selectively inhibit the mineralization of the organic matrix and TNAP expression and activity (Orriss et al., 2007). These actions are due to both P2 receptor mediated signalling and also direct hydrolysis by NPP1 to produce PPi (Orriss et al., 2007; Orriss, Key, et al., 2012; Orriss, Key, Hajjawi, & Arnett, 2013). Recently, we also demonstrated that activation of the P2Y2 receptor exerts some of its effects on bone mineralization indirectly by promoting the release of ATP from osteoblasts (Orriss et al., 2017). In contrast to the potent actions of ATP and UTP, ADP and UDP do not influence bone mineralization (Orriss et al., 2007).
The aim of this study was to investigate the effects of extracellular nucleotides on AMC and, given the apparent similarities to bone mineralization, to compare any functional effects to those seen in osteoblasts. Established in vitro mouse models of AMC and bone formation were used to determine the cellular mechanisms mediating these actions. Since the inhibitory effects of ATP and UTP on bone mineralization involve both P2 receptor dependent and independent signalling we examined both pathways in parallel.
2 METHODS
2.1 Reagents
All tissue culture reagents were purchased from Life Technologies (Paisley, UK); unless mentioned, all chemicals were purchased from Sigma Aldrich (Poole, UK). The selective P2Y2 receptor antagonist, ARC118935XX, was obtained from Tocris Bioscience (Bristol, UK).
2.2 Animals
Mice lacking the P2Y2 receptor gene (P2Y2R−/−) were obtained from Jackson Laboratories (Bar Harbor, ME). The generation and characterization of P2Y2R−/− mice, which are on a C57BL/6J background, has been previously described (Homolya, Watt, Lazarowski, Koller, & Boucher, 1999). Animals were bred from homozygote (P2Y2R−/−) and parental strain wildtype (P2Y2R+/+) breeding pairs. The generation and characterization of mice lacking NPP1 (Enpp1−/−), which are on a 129 Sv/TerJ genetic background, has previously been described (Sali, Favaloro, Terkeltaub, & Goding, 1999). Animals were bred from heterozygote (Enpp1+/−) breeding pairs due to the inability of homozygotes to breed. All mice were housed under standard conditions with free access to food and water. All procedures complied with the UK animals (Scientific Procedures) Act 1986 and were reviewed and approved by the Royal Veterinary College Research Ethics Committee.
2.3 Vascular smooth muscle cell (VSMC) calcification assay
Primary VSMCs were isolated from aortas of P2Y2R−/− or Enpp1−/− mice and their corresponding wildtypes (P2Y2R+/+ or Enpp1+/+). After removal of the adventitia, the aorta was opened to expose the endothelial layer under a dissection microscope. Tissues from six to eight animals were pooled and incubated with trypsin (0.25% w/v) for 10 min to remove any remaining adventitia and endothelium. Tissues were incubated overnight in alpha Minimum Essential Medium, supplemented with 10% foetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin (complete mixture abbreviated to αMEM) before being digested with 425 U/ml collagenase type II (Worthington Biomedical Corporation, Lakewood, NJ) for 5 hr. Isolated VSMCs were expanded in T25 tissue culture flasks in a humidified atmosphere of 5% CO2-95% air at 37°C until confluent. Following seeding into 24-well plates at a density of 2.5 × 104 cells/well, VSMCs were cultured in control (αMEM only) or calcifying medium (αMEM + 2 mM sodium phosphate) for up to 14 days, with half medium changes every 3 days. Cells were treated with 1–100 μM extracellular nucleotides (ATP, ADP, UTP, UDP) or pyrophosphate (PPi) for the duration of the culture; fresh nucleotide was added at each medium change. The selective P2Y2 receptor antagonist, ARC118935XX, was used to confirm results obtained with P2Y2R−/− cells.
2.4 Aortic ring calcification assay
Aortas were isolated from P2Y2R+/+ or P2Y2R−/− mice and the adventitia layer removed. The vessels were cut into 2–3 mm rings and cultured overnight in serum free αMEM. After 24 hr, the rings were transferred to calcification medium (αMEM plus 2.5 mM phosphate and 2.7 mM calcium chloride). Aortic rings were cultured for a further 9 days with half medium changes every 3 days.
2.5 Determination of VSMC and aortic ring calcification
Calcifying VSMCs or aortic rings were washed twice with phosphate buffered saline (PBS) and incubated with 0.6M HCl at room temperature for 24 hr. Calcium content was measured colorimetrically by stable interaction with o-cresolphthalein using a commercially available kit (Sigma-Adrich, Poole, UK) and corrected for total protein concentration using the Bradford assay. Calcium deposition was visualised by alizarin red staining of VSMC cell layers as previously described (Taylor, Shah, & Orriss, 2014).
2.6 Osteoblast bone formation assay
Osteoblasts were isolated from the calvariae of 3–5 day old mice by trypsin/collagenase digestion as previously described (Orriss, Hajjawi, Huesa, MacRae, & Arnett, 2014; Orriss, Taylor, & Arnett, 2012; Taylor et al., 2014). Cells were obtained from P2Y2R−/−, P2Y2R+/+, Enpp1−/− or Enpp1+/+ animals. Following isolation, cells were resuspended in αMEM and cultured for 2–4 days in a humidified atmosphere of 5% CO2-95% air at 37°C in 75 cm2 flasks until fully confluent. Cells were sub-cultured into 6-well trays in αMEM supplemented with 2 mM β-glycerophosphate and 50 μg/ml ascorbic acid, with half medium changes every 3 days. Cells were treated with 1–100 μM ATP, UTP or PPi for the duration of the culture; fresh nucleotide was added at each medium change.
To assess bone formation, experiments were terminated by fixing the cells in 2.5% glutaraldehyde for 5 min. Cell culture plates were imaged at 800 dpi using a flat-bed scanner (Epson Perfection 4990 Photo, Epson Ltd UK, Hemel Hempstead, UK) and the total area of bone nodules formed was quantified by image analysis, as described previously (Orriss, Taylor, & Arnett, 2012). Cell layers were stained with alizarin red for microscopy.
2.7 Analysis of P2 receptor gene expression by real time PCR
VSMCs were cultured in control or calcification medium for 14 days. Osteoblasts were cultured until the onset of mineralization (14 days). RNA was extracted using RNeasy total RNA (Qiagen Ltd, Crawley, UK), according to the manufacturer’s instructions. RNA was quantified and reverse transcribed as previously described (Mackenzie, Staines, Zhu, Genever, & Macrae, 2014). Levels of mRNA expression were measured using the SYBR green detection method (Roche, East Sussex, UK) as previously reported (Staines, Zhu, Farquharson, & MacRae, 2014). Data are presented as (1) the fold change in expression in calcifying VSMCs relative to control cells and (2) the fold change in expression in calcifying VSMCs compared to mineralizing osteoblasts. Primer sequences are shown in Table 1.
Table 1.
Primer sequences for real time PCR
| Gene | Primer sequence (5′-3′) | |
|---|---|---|
| P2X1 | S | tgt acg ggg aga aga acc tg |
| AS | tcc caa aca cct tga aga gg | |
| P2X2 | S | cgt ctt cat cgt gca gaa aa |
| AS | cac ttt gtg ttc cga cat gg | |
| P2X3 | S | tac caa gtc ggt ggt tgt ga |
| AS | cca ccc cac aaa gta gga ga | |
| P2X4 | S | gca ccc tcc acc atc tct aa |
| AS | aaa cct ctt gcc aga agc aa | |
| P2X5 | S | ggg ctt tct tct gtg acc tg |
| AS | gtt ggc ctc aac ctc aac at | |
| P2X6 | S | agc cat ggc ata aaa act gg |
| AS | gtg aag ttc ttg gcc tga gc | |
| P2X7 | S | ggc act gga gga aaa ttt ga |
| AS | tga gca agt caa tgc aca ca | |
| P2Y1 | S | agg aaa gct tcc agg agg ag |
| AS | cgt gtc tcc att ctg ctt ga | |
| P2Y2 | S | gtc agc agt gac gac tca aga c |
| AS | tca gag gat atc agc ccc ttt a | |
| P2Y4 | S | agg aag cag cag aac acc at |
| AS | caa gga gtc tgc act ggt ca | |
| P2Y6 | S | ttc cat ctt gca tga gac aga |
| AS | gct tga aat cct cac ggt aga | |
| P2Y13 | S | ggc cac tag atg tca cct ttt c |
| AS | gat ggt ggg gtg gta act aga a | |
2.8 Cell viability assay
VSMCs and osteoblasts were cultured for 14 days in medium supplemented with ATP, UTP, ADP, UDP or PPi (1–100 μM); fresh nucleotide was added at each medium change. Cell number and viability was determined using the CytoTox 96® colorimetric cytotoxicity assay (Promega, Southampton, UK), as described previously (Orriss, Key, et al., 2012). Cell supernatants were collected to determine medium LDH levels (cell viability). To establish total cellular LDH levels, cells were lysed with 1% Triton X-100 in water (lysis buffer, 15 μl/ml of medium) for 1 hr. The LDH content of the supernatants and cell lysates were measured colorimetrically (490 nm) as per manufacturer’s instructions. A standard curve for determination of cell numbers was constructed using cells seeded at 102 to 106/well. Cell viability (shown as percentage of dead cells) was calculated by expressing medium LDH as a percentage of the total cellular LDH.
2.9 Quantification of apoptosis by flow cytometry
VSMCs plated in 24-well trays were cultured in control or calcification medium (± treatment) for 7 days. Apoptosis was assessed via flow cytometry using an annexin V antibody conjugated to fluorescein (Life Technologies, Paisley, UK), as per manufacturer’s instructions. Briefly, cells were detached using trypsin (0.25%) and resultant pellet washed in ice cold PBS. This suspension was centrifuged and resuspended in 1X annexin-binding buffer (Life Technologies, Paisley, UK). A sample of this suspension was incubated with the annexin V antibody for 15 min, after which, analyzed using a BD FACSCanto II Flow Cytometer (Becton, Dickinson and Company, Oxford, UK). Data was processed to calculate percentage apoptosis using Flowing Software (version 2.5.1) (Turku University, Finland).
2.10 Determination of TNAP activity
VSMCs and osteoblasts VSMCs were cultured with 1–100 μM ATP, UTP or PPi for 14 days; fresh nucleotide was added at each medium change. TNAP activity was measured in P2Y2R−/−, Enpp1−/− or wildtype (P2Y2R+/+ / Enpp1+/+) cell lysates using a colorimetric assay (Anaspec, CA), as previously described (Orriss, Key, et al., 2012). TNAP activity was normalized to cell protein using the Bradford assay.
2.11 Histology
Histological analysis was performed on aortas obtained from 24-week old male P2Y2R+/+ or P2Y2R−/−mice. Tissues were fixed in 10% neutral buffered formalin and embedded in paraffin wax blocks. Serial sections were cut every 5 μm and mounted onto slides. Before staining, the samples were de-paraffinised using xylene, then rehydrated through a series of decreasing ethanol solutions and finally water. Slides were stained with haematoxylin and eosin (H&E) to examine cell morphology and alizarin red to visualize vascular calcification.
2.12 Statistical analysis
Data were analyzed using GraphPad Prism 6 software (San Diego, CA). Statistical comparisons were made using one-way or two-way analysis of variance (ANOVA) with a post-hoc Bonferroni correction for multiple comparisons. Results are expressed as means ± SEM for six replicates and are representative of experiments performed at least three times using cells or tissues obtained from different animals.
3 RESULTS
3.1 P2 receptor expression is increased in calcifying VSMCs
Analysis of mRNA expression revealed that mouse VSMCs express multiple P2 receptor subtypes including all the P2X receptors and the majority of the P2Y receptors (Figure 1a). Expression of the P2X1, P2X2, P2X4, P2X5, P2X6, and P2Y2 receptors was increased up to threefold in calcifying VSMCs compared to control VSMCs (Figure 1a).
Figure 1.
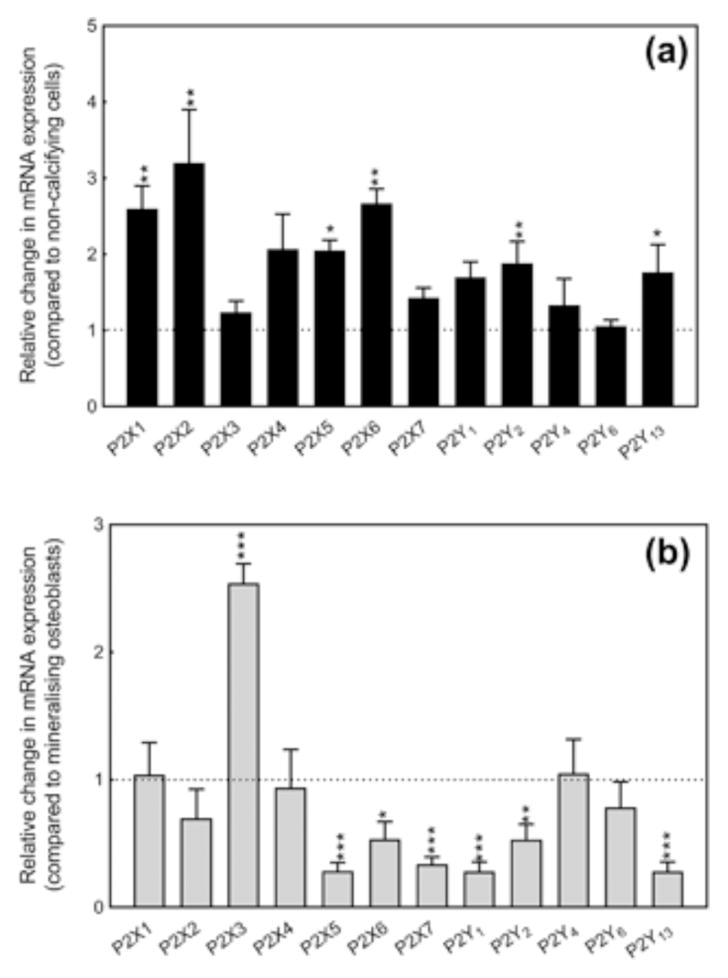
Expression of P2 receptors by calcifying VSMCs. (a) VSMCs express mRNA for multiple P2 receptor subtypes; expression of the P2X1, P2X2, P2X4, P2X5, P2X6 and P2Y2 receptors is increased up to threefold in calcifying cells compared to control cells. (b) Compared to mineralizing osteoblasts, calcifying VSMCs show increased P2X3 receptor (2.5 fold) expression but reduced levels of the P2X5, P2X6, P2X7, P2Y1, P2Y2 and P2Y13 receptors. Values are mean ± SEM (n = 4), *p < 0.05, **p< 0.01, ***p< 0.001
The relative level of P2 receptor mRNA expression between calcifying VSMCs and mineralizing osteoblasts was also investigated (Figure 1b). Expression of P2X3 receptor mRNA was increased 2.5 fold in calcifying VSMCs. In contrast, levels of the P2X5, P2X, P2X7, P2Y1, P2Y2, and P2Y13 receptors were reduced in calcifying VSMCs compared to mineralizing osteoblasts (Figure 1b). All other P2 receptors was displayed a similar level of expression
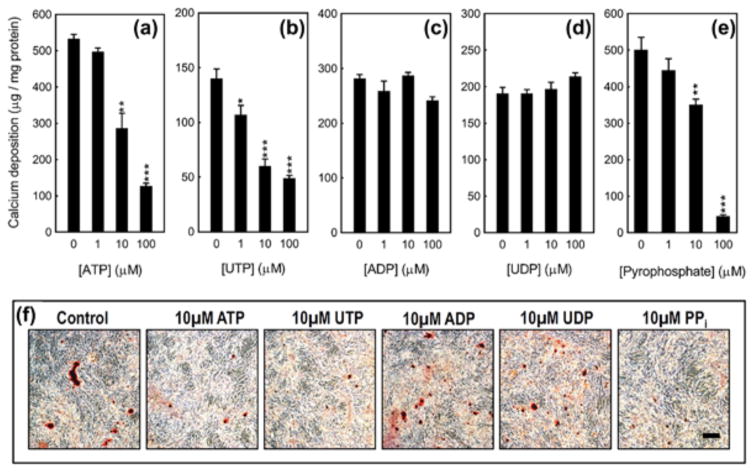
3.2 ATP, UTP and PPi inhibit VSMC calcification
ATP (≥10 μM) and UTP (≥1 μM) dose dependently inhibited VSMC calcification by up to 80% (Figures 2a, 2b, and 2f). UTP was more potent, exerting inhibitory actions from 1 μM. ADP and UDP had no effect at any of the concentrations tested (Figures 2c, 2d, and 2f). The ubiquitous mineralization inhibitor, PPi (≥10 μM), reduced VSMC calcification up to 85% (Figures 2e and 2f). Representative images in Figure 2f show the inhibitory actions of 10 μM ATP, UTP or PPi on calcification in mouse VSMC cultures.
Figure 2.
The functional effects of extracellular nucleotides on VSMC calcification. (a) ATP and (b) UTP dose dependently (≥1 μM) inhibit VSMC calcification by up to 80%. (c) ADP and (d) UDP have no effect on VSMC calcification. (e) PPi (≥10 μM) reduces VSMC calcification by up to 85%. Values are mean ± SEM (n = 6), *p < 0.05, **p< 0.01, ***p < 0.001. (f) Representative light microscopy images of alizarin red stained VSMC cultures treated with extracellular nucleotides. Scale bar = 50 μm
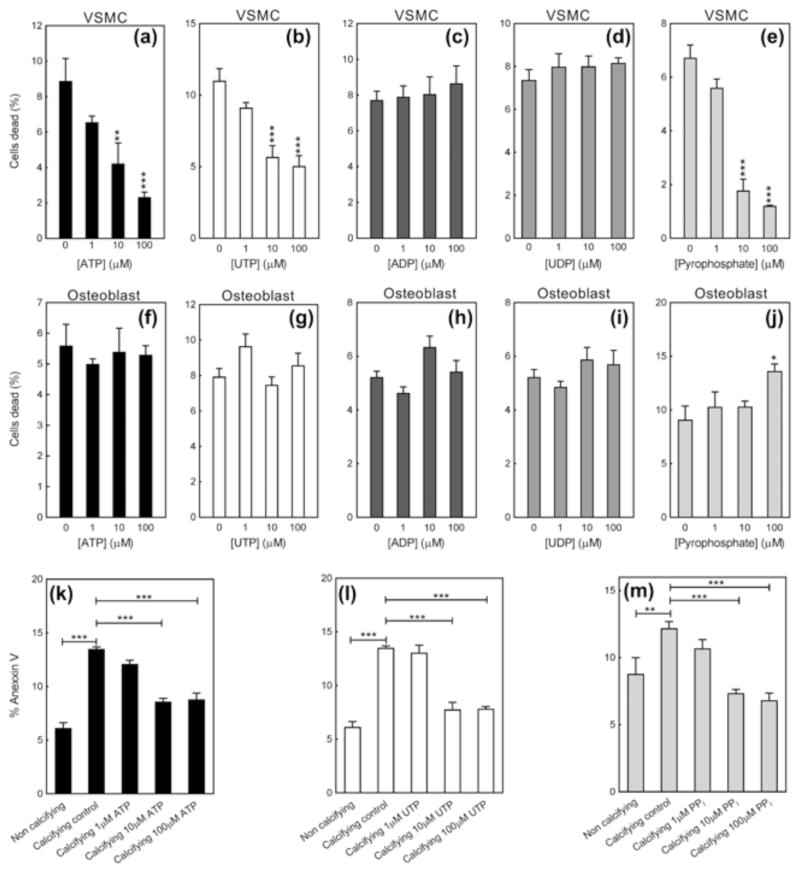
3.3 ATP, UTP and PPi increase VSMC viability and decrease apoptosis
In calcifying VSMCs, treatment with ATP, UTP and PPi (≥10 μM) decreased the percentage of dead cells present by up to 75%, 60%, and 80%, respectively; ADP and UDP had no effect (Figure 3a–e). ATP, UTP, ADP, and UDP had no effect on osteoblast cell viability (Figure 3f–i). PPi at the highest dose (100 μM) caused a 45% increase in the proportion of dead cells (Figure 3j).
Figure 3.
ATP, UTP, and PPi reduce VSMC cell death but have no effect on osteoblast survival. (a) ATP and (b) UTP dose dependently decrease the proportion of dead cells in calcifying VSMC cultures by up to 75%; (c) ADP and (d) UDP have no effect. (e) PPi reduced the level of dead cells by up to 80%. (f–i) ATP, UTP, ADP and UDP do not influence osteoblast viability. (j) PPi at the highest dose tested caused a small increase in the percentage of dead cells. (k–m) Culture of VSMCs in calcifying conditions was associated by an increased level of apoptosis (up to 2.3 fold). ATP, UTP and PPi (≥10 μM) reduced the amount of apoptosis to levels seen in non-calcifying cells. Values are mean ± SEM (n = 6), *p < 0.05, **p < 0.01, ***p < 0.001
Apoptosis was increased up to 2.3-fold in calcifying VSMCs compared to control VSMCs (Figure 3k–m). Treatment with ATP, UTP and PPi (≥10 μM) decreased the level of apoptosis in calcifying cells to the level seen in control VSMCs (Figure 3k–m).
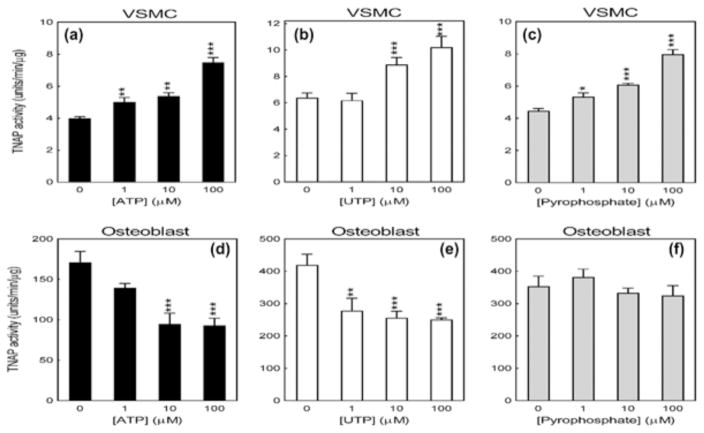
3.4 The effect of ATP, UTP and PPi on TNAP activity in calcifying VSMCs and osteoblasts
ATP and UTP (≥1 μM) increased VSMC TNAP activity by up to 88% and 50%, respectively (Figures 4a and 4b). PPi, (≥1 μM), which is a substrate for TNAP, dose dependently stimulated enzyme activity by up to 80% (Figure 4c). In contrast, ATP and UTP (≥1 μM) inhibited osteoblast TNAP activity by up to 50% (Figures 4d and e). PPi had no effect on TNAP activity in osteoblasts (Figure 3f). It should also be noted that the basal TNAP activity of mineralizing osteoblasts was at least 100-fold higher than that of a calcifying VSMCs (Figure 4).
Figure 4.
Opposing effects of extracellular nucleotides on TNAP activity in VSMCs and osteoblasts. (a) ATP, (b) UTP, and (c) PPi (≥1 μM) increased VSMC TNAP activity by up to 88%. (d) ATP and (e) UTP reduced osteoblast TNAP activity by up to 50%. (f) No effect of PPi on osteoblast TNAP activity. Note that the basal levels of TNAP activity are approximately 100-fold higher in mineralizing osteoblasts than calcifying VSMCs. Values are mean ± SEM (n = 6), *p < 0.05, **p <0.01, *** p< 0.001
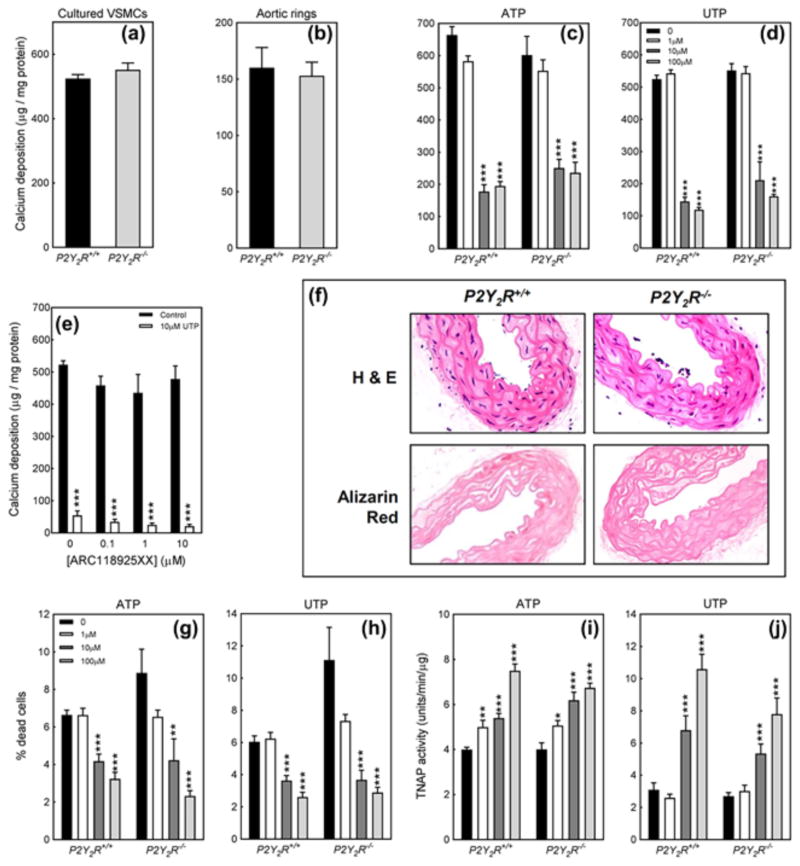
3.5 The P2Y2 receptor does not mediate the effects of ATP & UTP on VSMC calcification
Deletion of the P2Y2 receptor had no effect on the basal level of calcification in VSMC or aortic ring cultures (Figures 5a and 5b). The inhibitory effects of ATP and UTP on calcification were unchanged in P2Y2R−/− VSMCs (Figures 5c and 5d). The selective P2Y2 receptor antagonist, ARC118935XX, also failed to attenuate the inhibitory effects of UTP (Figure 5e). Isolated aortas from 24-week old P2Y2R+/+ and P2Y2R−/− mice showed no obvious differences in structure or cell morphology (Figure 5f). Alizarin red staining revealed no spontaneous calcium deposition within any of the arterial layers (Figure 5f). Representative images shown are from the descending aorta. The effects of ATP and UTP on VSMC cell viability and TNAP activity were also unaffected by the deletion of the P2Y2 receptor (Figure 5g–j).
Figure 5.
Lack of involvement of the P2Y2 receptor in regulating AMC. (a) Cultured VSMCs and (b) aortic rings from P2Y2R−/− animals displayed no differences in the basal level of calcification. The inhibitory effects of (c) ATP and (d) UTP were unchanged in P2Y2R−/− VSMCs. (e) The selective P2Y2 receptor antagonist, ARC118925XX, did not block the inhibitory effects of UTP on VSMC calcification. (f) Histological analysis of isolated aortas from 24-week old P2Y2R−/− mice showed no difference in cell morphology or the presence of AMC. Scale bar = 100 μm. The protective effects of (g) ATP and (h) UTP on cell viability were unaffected by deletion of the P2Y2 receptor. The stimulatory actions of (i) ATP and (j) UTP on TNAP activity were unchanged in P2Y2R−/− VSMCs. Values are mean ± SEM (n = 6), *p < 0.05, **p < 0.01, ***p < 0.001
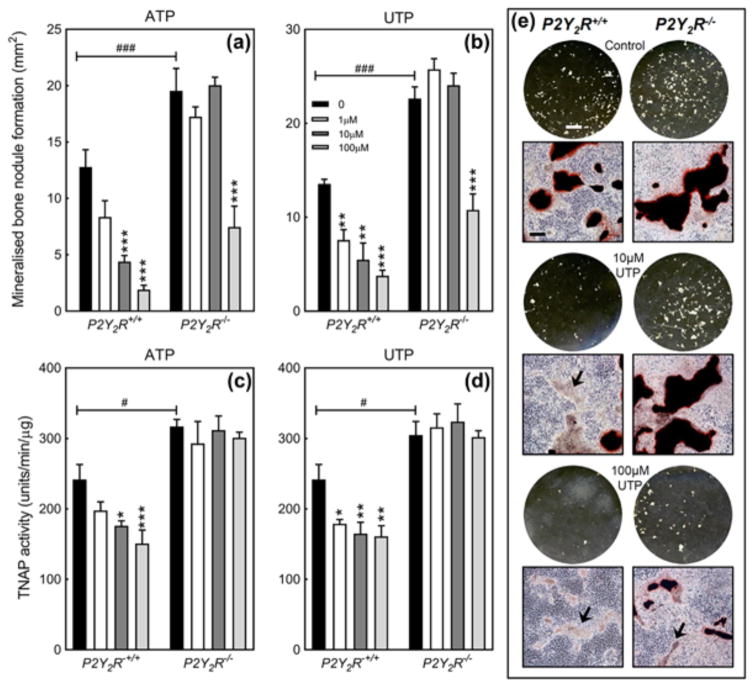
3.6 Reduced inhibitory effects of ATP and UTP on bone mineralization in P2Y2R−/− osteoblasts
ATP (≥10 μM) and UTP (≥1 μM) dose dependently reduced bone mineralization in P2Y2R+/+ osteoblasts by up to 80% (Figures 6a, 6b, and 6e). The overall level of mineralized bone nodule formation was up to 60% higher in P2Y2R−/− cells compared to P2Y2R+/+. In P2Y2R−/− osteoblasts, the effects of ATP and UTP were only observed at 100 μM making them 10-fold and 100-fold less potent at inhibiting bone mineralization, respectively (Figures 6a, 6b, and 6e). Representative whole well scans (unstained) and phase contrast microscopy images (alizarin red stained) of osteoblast cell layers show the reduced inhibitory effects of UTP in P2Y2R−/− osteoblasts. They also illustrate the increased level of bone formation in P2Y2R−/− cells (Figure 6e).
Figure 6.
The role of the P2Y2 receptor in the inhibitory effects of ATP and UTP on bone mineralization. The inhibitory effects of (a) ATP and (b) UTP were 10-fold and 100-fold less potent, respectively, in P2Y2R−/− osteoblasts; mineralized bone nodule formation was 60% higher in P2Y2R−/− osteoblasts. The inhibitory actions of (c) ATP and (d) UTP on TNAP activity were lost in P2Y2R−/− osteoblasts. Basal TNAP activity was increased up to 40% in P2Y2R−/− cells. Values are mean ± SEM (n = 6), *p < 0.05, **p < 0.01, ***p < 0.001. Comparison of P2Y2R+/+ to P2Y2R−/−: #p < 0.05, ##p < 0.01, ###= p < 0.001. (E) Representative whole well scans (unstained) and phase contrast microscopy images (alizarin red stained) showing the effects of UTP on bone mineralization in P2Y2R−/− and P2Y2R+/+ osteoblasts. Areas of unmineralized matrix are highlighted by the arrows. Scale bars: whole well scans = 0.5 cm, microscopy images = 200 μm
3.7 The inhibitory effects of ATP and UTP on TNAP activity are lost in P2Y2R−/− osteoblasts
In P2Y2R+/+osteoblasts ATP (≥10 μM) and UTP (≥1 μM) reduce TNAP activity by up to 40%. P2Y2R−/− osteoblasts display basal TNAP activity levels that are up to 30% higher than wildtype cells. The inhibitory effects of ATP and UTP on TNAP activity were lost in P2Y2R−/− osteoblasts (Figures 6c and 6d).
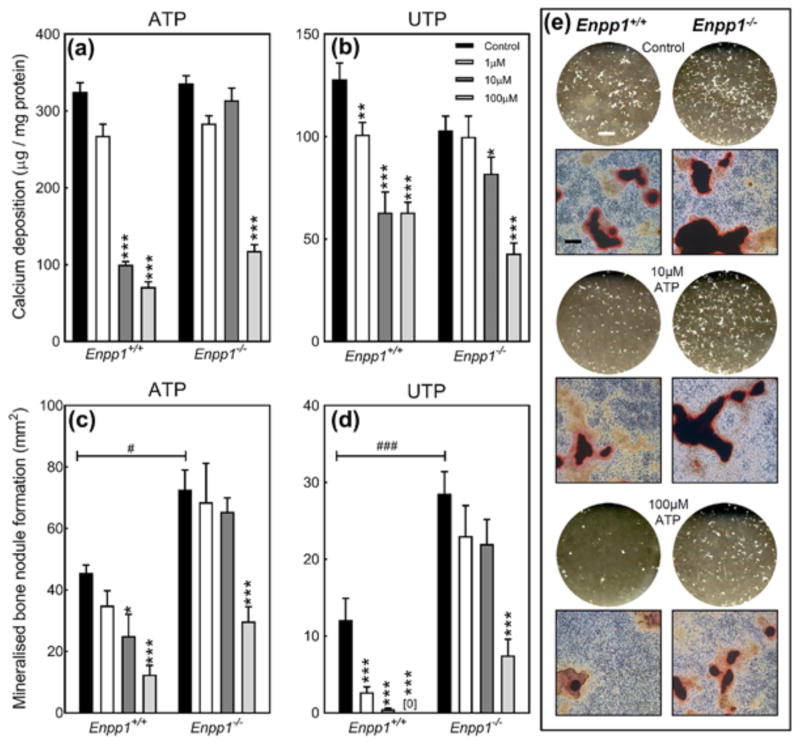
3.8 The inhibitory effects of ATP and UTP on VSMC calcification and bone mineralization are reduced in Enpp1−/− mice
Since PPi mimics many of the actions of ATP and UTP we investigated whether the effects of these extracellular nucleotides involved P2 receptor independent mechanisms. In Enpp1+/+ cells, ATP and UTP inhibit VSMC calcification from concentrations of 10 μM and 1 μM, respectively. In Enpp1−/− VSMCs, ATP and UTP are 10-fold less potent only exerting inhibitory effects at 100 and 10 μM, respectively (Figures 7a and 7b).
Figure 7.
The actions of NPP1 contribute towards the inhibitory effects of ATP and UTP on VSMC calcification and bone mineralization. The inhibitory effects of (a) ATP and (b) UTP on calcification were 10-fold less potent in Enpp1−/− VSMCs. In Enpp1−/− osteoblasts (c) ATP and (d) UTP were 10-fold and 100-fold less potent, respectively, at blocking bone mineralization. Values are mean ± SEM (n = 6), *p < 0.05, **p < 0.01, ***p < 0.001. Comparison of Enpp1+/+ to Enpp1−/−: #p< 0.05, ###p < 0.001. (e) Representative whole well scans (unstained) and phase contrast microscopy images (alizarin red stained) showing the effects of ATP on bone mineralization in Enpp1+/+ and Enpp1−/− osteoblasts. Scale bars: whole well scans = 0.5 cm, microscopy images = 200 μm
Consistent with earlier studies, Enpp1−/− osteoblasts displayed increased levels of bone mineralization compared to Enpp1+/+ cells (Anderson et al., 2005; Orriss, Key, Hajjawi, Millan, & Arnett, 2015). The inhibitory effects of ATP and UTP on bone mineralization were 10 and 100-fold less potent, respectively, in Enpp1−/− osteoblasts (Figures 7c and 7d). Representative whole well scans (unstained) and phase contrast microscopy images (alizarin red stained) of osteoblast cell layers show the reduced inhibitory effects of ATP in Enpp1−/− osteoblasts (Figure 7e).
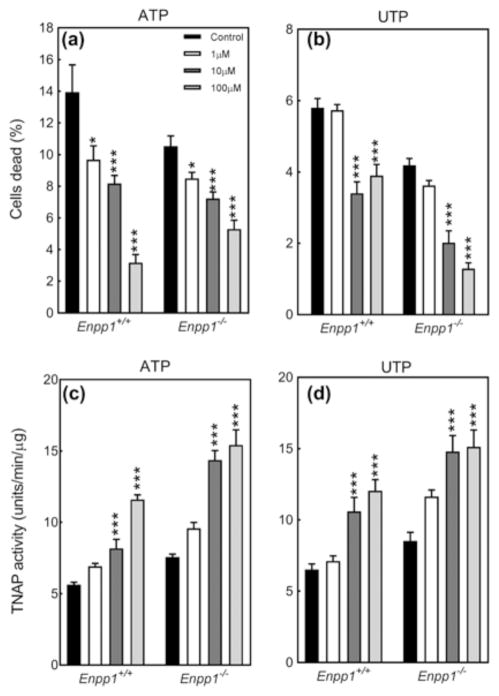
3.9 NPP1 deletion does not blunt the effects of ATP and UTP on cell viability and TNAP activity
The protective effects of ATP and UTP against loss of cell viability were unchanged in cultures of Enpp1−/− VSMCs (Figures 8a and 8b). Deletion of NPP1 also had no effect on the ATP/UTP-mediated increase in VSMC TNAP activity (Figures 8c and 8d).
Figure 8.
The effect of ATP and UTP on VSMC survival and TNAP activity does not involve NPP1. The protective effects of (a) ATP and (b) UTP on cell viability were unchanged in Enpp1−/− VSMCs. The stimulatory actions of (c) ATP and (d) UTP on TNAP activity were the same in Enpp1+/+ and Enpp1−/− cells. Values are mean ± SEM (n = 6), *p < 0.05, ***p < 0.001
4 DISCUSSION
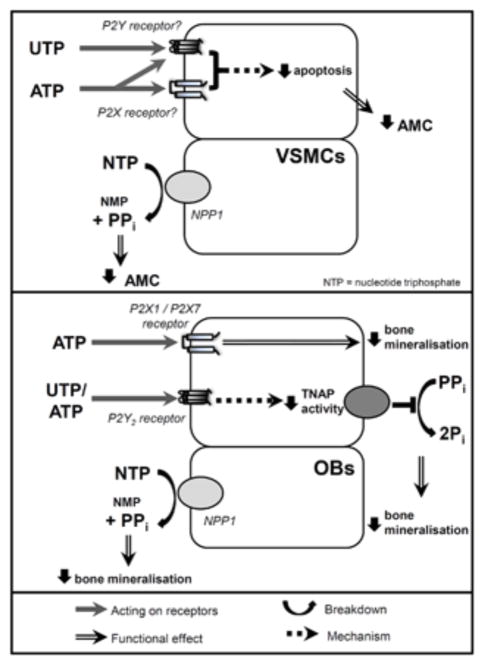
This study compared the effects of extracellular nucleotides on AMC and bone mineralization. We found that, while the functional effect was the same, the cellular mechanisms mediating the observed inhibitory actions of ATP and UTP differed. In VSMCs, ATP and UTP prevent calcification, at least in part, by reducing VSMC apoptosis. In contrast, ATP and UTP have no effect on osteoblast survival and instead block bone mineralization via the inhibition of TNAP. Furthermore, the P2Y2 receptor does not appear to mediate the actions of ATP and UTP on AMC but is involved in the effects on bone mineralization. However, NPP1-mediated generation of PPi contributes to the inhibitory effects observed in both VSMCs and osteoblasts (see summary Figure 9).
Figure 9.
Summary of the differing cellular mechanisms by which ATP and UTP exert their effects on AMC and bone mineralization. ATP and UTP inhibit both AMC and bone mineralization but there are some differences in the mechanisms mediating these actions. In VSMCs (top), ATP and UTP act via P2 receptors to decrease apoptosis and, as a consequence, calcification. In osteoblasts (OBs, bottom panel), ATP and UTP act via the P2Y2 receptor to inhibit alkaline phosphatase (TNAP) activity. However, for both cell types hydrolysis of ATP and UTP to produce PPi by NPP1 also contributes to the inhibitory effects observed
This study demonstrated, like others, that VSMCs express multiple P2X and P2Y receptor subtypes (Lewis & Evans, 2000; Wang et al., 2002). Interestingly, the mRNA expression of several P2 receptors was upregulated in calcifying VSMCs compared to control cells. Since the expression of P2 receptors by osteoblasts is differentiation-dependent (Dixon, Bowler, Walsh, & Gallagher, 1997; Orriss, Knight, Ranasinghe, Burnstock, & Arnett, 2006; Noronha-Matos et al., 2012), this could reflect the changes in the VSMC phenotype which occur during the development of AMC.
Consistent with earlier work that described protective effects of extracellular nucleotides on aortic valve calcification (Cote et al., 2012) and VSMC calcification (Villa-Bellosta & Sorribas, 2013), we also found that both ATP and UTP were inhibitory. Whereas, ADP and UDP were without effect. UTP, which was more potent than ATP, only activates the P2Y2 and P2Y4 receptor subtypes. Given the involvement of the P2Y2 receptor in inhibiting bone mineralization (Figure 6) (Hoebertz et al., 2002; Orriss et al., 2007), aortic valve calcification and intimal calcification (Cote et al., 2012; Qian et al., 2017), the P2Y2 receptor appeared to be a strong candidate for mediating the effects in VSMCs. However, the effects of ATP and UTP on VSMC calcification were not lost in P2Y2R−/− cells or blocked by a selective P2Y2 receptor antagonist. Furthermore, P2Y2R−/− VSMCs and aortic rings displayed no differences in the basal level of calcification in vitro and isolated aortas from 24-week old animals also showed no signs of AMC. It should be noted that aged animals were not studied here and so it is possible that the condition develops in older P2Y2R−/− mice. Taken together, these data suggest that ATP and UTP are not acting via the P2Y2 receptor to regulate AMC.
Our findings contrast to previous work which has implicated the P2Y2 receptor in the effects of nucleotides on valve calcification (Cote et al., 2012) and arterial intimal calcification (Qian et al., 2017). There are a number of potential explanations for these observed differences. First, the lack of involvement of the P2Y2 receptor in aortic VSMCs could represent variation in the cellular mechanisms that underpin these different forms of pathological vascular calcification. Second, it could be due to differences in the experimental models used since each investigation has utilised distinct in vitro and in vivo methods to study the different forms of vascular calcification. Finally, we observed that P2Y2 receptor mRNA expression was significantly lower in calcifying VSMCs than mineralizing osteoblasts. This suggests that P2Y2 receptor-mediated signalling could be of lesser importance in the regulation of calcification processes in aortic VSMCs.
Extracellular nucleotides are rapidly hydrolysed by ecto-nucleotidases, meaning that the parent molecule is only present in the culture medium for a very short period of time. Particularly important is the breakdown of ATP and UTP by NPP1 to produce the key local inhibitor of mineralization, PPi. Since the effects of PPi on VSMC calcification mimicked those of ATP and UTP, we used Enpp1−/− cells to determine the involvement of P2 receptor-independent mechanisms. The effects of ATP and UTP were blunted in Enpp1−/− VSMCs and osteoblasts suggesting that NPP1-mediated hydrolysis to produce PPi does contribute to the observed inhibitory actions in both cell types. This is consistent with earlier work that examined the role of ATP-derived PPi in VSMC calcification (Prosdocimo et al., 2009, 2010) and bone formation (Orriss et al., 2013, 2007). However, since the effects were not completely attenuated it suggests that other mechanisms are also involved. In osteoblasts, this is likely to involve activation of the P2 receptors implicated in the regulation of bone mineralization by ATP and/or UTP, such as the P2Y2, P2X1, and P2X7 receptors (Hoebertz et al., 2002; Orriss et al., 2007; Orriss, Key, et al., 2012). In VSMCs, the other mechanisms leading the functional effects are less clear. Although unlikely to be mediated by the P2Y2 receptor, involvement of other P2 receptor subtypes (e.g., the P2Y4 receptor) is probable, and presents an area for future study. Furthermore, since VSMCs have been shown to also express functional NPP3 (Prosdocimo et al., 2009), a role for other ecto-nucleotidases cannot be discounted.
Increased VSMC apoptosis has been implicated in the development of vascular calcification (Proudfoot et al., 2000). In this study we found that, at the concentrations which inhibited calcification, ATP, UTP and PPi prevented the increase in apoptosis usually seen in calcifying VSMC cultures. The effect of extracellular nucleotides on cell viability was unchanged in Enpp1−/− VSMCs. This suggests that, although PPi itself appears to exert protective actions, ATP and UTP are working directly to promote VSMC survival in calcifying conditions. Apoptosis is thought to promote calcification because the VSMC-derived apoptotic bodies can act as a nucleation site for hydroxyapatite crystal formation (Proudfoot et al., 2000). Thus, the inhibitory effects of extracellular nucleotides (and PPi) on AMC could be mediated, at least in part, by their ability to reduce apoptosis. The overall result being that there are less apoptotic bodies on which calcification can be initiated. In contrast, extracellular nucleotides have no effect on osteoblast viability and so the inhibitory effects on bone mineralization are clearly not mediated via alterations in apoptosis.
These differential effects of extracellular nucleotides on cell survival may also illustrate the differences between physiological bone mineralization and pathological AMC. Bone mineralization is not associated with increased levels of osteoblast apoptosis and instead matrix vesicles are the primary nucleation site for hydroxyapatite formation (Anderson, Cecil, & Sajdera, 1975). The initiation of AMC appears more heterogeneous with multiple factors acting as nucleation sites for calcification including apoptotic bodies and mineralization-competent vesicles (Kapustin & Shanahan, 2012; Proudfoot et al., 2000).
TNAP is a crucial enzyme in bone mineralization, with deficiencies leading to hypophosphatasia, a condition characterized by hypomineralization and rickets (Caswell, Whyte, & Russell, 1991). Increased TNAP expression and activity has also been associated with AMC (Narisawa et al., 2007; Sheen et al., 2015). We have previously shown that ATP and UTP reduce TNAP expression and activity in osteoblasts (Orriss et al., 2007). Here, we demonstrate that these inhibitory actions involve P2Y2 receptor activation since the effects on TNAP activity were lost in P2Y2R−/− osteoblasts. In contrast, at concentrations where calcification is inhibited by up to 80%, ATP and UTP stimulated VSMC TNAP activity. The reasons for this counterintuitive increase are unclear but since PPi, and to a lesser extent ATP and UTP, are hydrolyzed by TNAP the higher substrate levels could lead to alterations in enzyme kinetics. It is important to note that, even when stimulated, the TNAP activity of calcifying VSMCs was at least 100-fold lower than that of mineralizing osteoblasts in vitro. Taken together, our observations suggest that while reduced TNAP activity contributes to the inhibition of bone mineralization by ATP and UTP, alterations in TNAP activity are unlikely to mediate the functional effects of nucleotides on VSMC calcification. Instead the effects on VSMC apoptosis appear to predominate. Furthermore, although TNAP is essential for bone mineralization its role in AMC appears more complex.
It should be noted that, while cell culture conditions were very similar, they were not identical because the use of different phosphate sources was required; VSMCs and osteoblasts were grown in 2 mM sodium phosphate or 2 mM β-glycerophosphate, respectively. VSMCs did not calcify in 2 mM β-glycerophosphate probably because they lack sufficient TNAP to generate the free phosphate needed for calcification. While it is unlikely that this small variation in culture conditions caused the different cellular responses to extracellular nucleotides, contributory effects cannot be fully discounted.
It is widely accepted that the development of AMC shares similarities to physiological bone formation. While the role of NPP1 was similar in both VSMCs and osteoblasts, this study demonstrated that there are also clear differences in the cellular mechanisms mediating the inhibitory effects of extracellular nucleotides. This suggests that some of the underlying processes leading to AMC and bone mineralization are not the same. It is these differences which need to be fully understood in order to identify a drug target or therapeutic agent that can prevent or regress AMC without exerting negative actions on the skeleton.
Acknowledgments
The authors are grateful for funding from the British Heart Foundation (grant number: PG/15/13/31296) and Arthritis Research UK (grant number: 19205).
Footnotes
CONFLICT OF INTEREST
The authors have no disclosures or other conflicts of interest to report.
References
- Abbracchio MP, Burnstock G. Purinoceptors: Are there families of P2X and P2Y purinoceptors? Pharmacology & Therapeutics. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Cecil R, Sajdera SW. Calcification of rachitic rat cartilage in vitro by extracellular matrix vesicles. The American Journal of Pathology. 1975;79(2):237–254. [PMC free article] [PubMed] [Google Scholar]
- Anderson HC, Harmey D, Camacho NP, Garimella R, Sipe JB, Tague S, … Millan JL. Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia-related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double-deficient mice. The American Journal of Pathology. 2005;166(6):1711–1720. doi: 10.1016/S0002-9440(10)62481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiology Reviews. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Arnett TR, Orriss IR. Purinergic signalling in the musculoskeletal system. Purinergic Signal. 2013;9(4):541–572. doi: 10.1007/s11302-013-9381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? General Pharmacology. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacological Reviews. 2014;66(1):102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- Caswell AM, Whyte MP, Russell RG. Hypophosphatasia and the extracellular metabolism of inorganic pyrophosphate: Clinical and laboratory aspects. Critical Reviews in Clinical Laboratory Sciences. 1991;28(3):175–232. doi: 10.3109/10408369109106862. [DOI] [PubMed] [Google Scholar]
- Cote N, El HD, Pepin A, Guauque-Olarte S, Ducharme V, Bouchard-Cannon P, … Mathieu P. ATP acts as a survival signal and prevents the mineralization of aortic valve. Journal of Molecular and Cellular Cardiology. 2012;52(5):1191–1202. doi: 10.1016/j.yjmcc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Dixon CJ, Bowler WB, Walsh CA, Gallagher JA. Effects of extracellular nucleotides on single cells and populations of human osteoblasts: Contribution of cell heterogeneity to relative potencies. British Journal of Pharmacology. 1997;120(5):777–780. doi: 10.1038/sj.bjp.0700961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish RS, Klootwijk E, Tam FW, Kleta R, Wheeler DC, Unwin RJ, Norman J. ATP and arterial calcification. European Journal of Clinical Investigation. 2013;43(4):405–412. doi: 10.1111/eci.12055. [DOI] [PubMed] [Google Scholar]
- Fleisch H, Bisaz S. Mechanism of calcification: Inhibitory role of pyrophosphate. Nature. 1962;195:911. doi: 10.1038/195911a0. [DOI] [PubMed] [Google Scholar]
- Gartland A, Orriss IR, Rumney RM, Bond AP, Arnett T, Gallagher JA. Purinergic signalling in osteoblasts. Frontiers in Bioscience. 2012;17:16–29. doi: 10.2741/3912. [DOI] [PubMed] [Google Scholar]
- Hajjawi MO, MacRae VE, Huesa C, Boyde A, Millan JL, Arnett TR, Orriss IR. Mineralization of collagen rich soft tissues and osteocyte lacunae in Enpp1 −/− mice. Bone. 2014;69C:139–147. doi: 10.1016/j.bone.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebertz A, Mahendran S, Burnstock G, Arnett TR. ATP and UTP at low concentrations strongly inhibit bone formation by osteoblasts: A novel role for the P2Y2 receptor in bone remodeling. Journal of Cellular Biochemistry. 2002;86(3):413–419. doi: 10.1002/jcb.10236. [DOI] [PubMed] [Google Scholar]
- Homolya L, Watt WC, Lazarowski ER, Koller BH, Boucher RC. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y(2) receptor (−/−) mice. Journal of Biological Chemistry. 1999;274(37):26454–26460. doi: 10.1074/jbc.274.37.26454. [DOI] [PubMed] [Google Scholar]
- Johnson K, Goding J, Van Etten D, Sali A, Hu SI, Farley D, … Terkeltaub R. Linked deficiencies in ext racellu lar PP(i) and osteopontin mediate pathologic calcification associated with defective PC-1 and ANK expression. Journal of Bone and Mineral Research. 2003;18(6):994–1004. doi: 10.1359/jbmr.2003.18.6.994. [DOI] [PubMed] [Google Scholar]
- Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell-derived matrix vesicles. Trends in Cardiovascular Medicine. 2012;22(5):133–137. doi: 10.1016/j.tcm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Evans RJ. Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels. British Journal of Pharmacology. 2000;131(8):1659–1666. doi: 10.1038/sj.bjp.0703744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie NC, Staines KA, Zhu D, Genever P, Macrae VE. MiRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochemistry and Function. 2014;32(2):209–216. doi: 10.1002/cbf.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie NC, Zhu D, Milne EM, van ‘t HR, Martin A, Darryl QL, … Macrae VE. Altered bone development and an increase in FGF-23 expression in Enpp1(−/−) mice. PLoS ONE. 2012;7(2):32177. doi: 10.1371/journal.pone.0032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa S, Harmey D, Yadav MC, O’Neill WC, Hoylaerts MF, Millan JL. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. Journal of Bone and Mineral Research. 2007;22(11):1700–1710. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- Noronha-Matos JB, Coimbra J, Sa-e-Sousa A, Rocha R, Marinhas J, Freitas R, … Correia-de-Sa P. P2 × 7-induced zeiosis promotes osteogenic differentiation and mineralization of postmenopausal bone marrow-derived mesenchymal stem cells. FASEB Journal. 2014;28(12):5208–5222. doi: 10.1096/fj.14-257923. [DOI] [PubMed] [Google Scholar]
- Noronha-Matos JB, Correia-de-Sa P. Mesenchymal Stem Cells Ageing: Targeting the “Purinome” to promote osteogenic differentiation and bone repair. Journal of Cellular Physiology. 2016;231(9):1852–1861. doi: 10.1002/jcp.25303. [DOI] [PubMed] [Google Scholar]
- Noronha-Matos JB, Costa MA, Magalhaes-Cardoso MT, Ferreirinha F, Pelletier J, … Correia-de-Sa P. Role of ecto-NTPDases on UDP-sensitive P2Y(6) receptor activation during osteogenic differentiation of primary bone marrow stromal cells from postmenopausal women. Journal of Cellular Physiology. 2012;227(6):2694–2709. doi: 10.1002/jcp.23014. [DOI] [PubMed] [Google Scholar]
- Orriss IR. The role of purinergic signalling in the musculoskeletal system. Autonomic Neuroscience. 2015;191:124–134. doi: 10.1016/j.autneu.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Arnett TR, Russell RG. Pyrophosphate: A key inhibitor of mineralization. Current Opinion in Pharmacology. 2016;28:57–68. doi: 10.1016/j.coph.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Gueneri D, Hajjawi MO, Shaw K, Patel JJ, Arnett TR. Activation of the P2Y2 receptor regulates bone cell function by enhancing ATP release. Journal of Endocrinology. 2017;233:341–356. doi: 10.1530/JOE-17-0042. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Hajjawi MO, Huesa C, MacRae VE, Arnett TR. Optimisation of the differing conditions required for bone formation in vitro by primary osteoblasts from mice and rats. International Journal of Molecular Medicine. 2014;34(5):1201–1208. doi: 10.3892/ijmm.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orriss IR, Key ML, Brandao-Burch A, Patel JJ, Burnstock G, Arnett TR. The regulation of osteoblast function and bone mineralization by extracellular nucleotides: The role of P2X receptors. Bone. 2012;51(3):389–400. doi: 10.1016/j.bone.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Key ML, Hajjawi MO, Arnett TR. Extracellular ATP released by osteoblasts is a key local inhibitor of bone mineralization. PLoS ONE. 2013;8(7):69057. doi: 10.1371/journal.pone.0069057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orriss IR, Key ML, Hajjawi MO, Millan JL, Arnett TR. Acidosis Is a key regulatior of osteoblast ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) expression and activity. Journal of Cellular Physiology. 2015;230:3049–3056. doi: 10.1002/jcp.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orriss IR, Knight GE, Ranasinghe S, Burnstock G, Arnett TR. Osteoblast responses to nucleotides increase during differentiation. Bone. 2006;39(2):300–309. doi: 10.1016/j.bone.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Taylor SE, Arnett TR. Rat osteoblast cultures. Methods in Molecular Biology. 2012;816:31–41. doi: 10.1007/978-1-61779-415-5_3. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Utting JC, Brandao-Burch A, Colston K, Grubb BR, Burnstock G, Arnett TR. Extracellular nucleotides block bone mineralization in vitro: Evidence for dual inhibitory mechanisms involving both P2Y2 receptors and pyrophosphate. Endocrinology. 2007;148(9):4208–4216. doi: 10.1210/en.2007-0066. [DOI] [PubMed] [Google Scholar]
- Panupinthu N, Zhao L, Possmayer F, Ke HZ, Sims SM, Dixon SJ. P2×7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. The Journal of Biological Chemistry. 2007;282(5):3403–3412. doi: 10.1074/jbc.M605620200. [DOI] [PubMed] [Google Scholar]
- Prosdocimo DA, Douglas DC, Romani AM, O’Neill WC, Dubyak GR. Autocrine ATP release coupled to extracellular pyrophosphate accumulation in vascular smooth muscle cells. American Journal of Physiology Cell Physiology. 2009;296(4):C828–C839. doi: 10.1152/ajpcell.00619.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosdocimo DA, Wyler SC, Romani AM, O’Neill WC, Dubyak GR. Regulation of vascular smooth muscle cell calcification by extracellular pyrophosphate homeostasis: Synergistic modulation by cyclic AMP and hyperphosphatemia. American Journal of Physiology Cell Physiology. 2010;298(3):C702–C713. doi: 10.1152/ajpcell.00419.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circulation Research. 2000;87(11):1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- Qian S, Regan JN, Shelton MT, Hoggatt A, Mohammad KS, Herring PB, Seye CI. The P2Y2 nucleotide receptor is an inhibitor of vascular calcification. Atherosclerosis. 2017;257:38–46. doi: 10.1016/j.atherosclerosis.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, … Terkeltaub R. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. The American Journal of Pathology. 2001;158(2):543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Favaloro JM, Terkeltaub R, Goding JW. Germline deletion of the nucleoside triphosphate pyrophosphohydrolase (NTPPPH) plasma cell membrane glycoprotein (PC-1) produces abnormal calcification of the periarticular tissues. In: Vanduffe L, Lemmens R, editors. Ecto-ATPases and related ectonucleotides. Mastricht, the Netherlands: Shaker Publishing BV; 1999. pp. 267–282. [Google Scholar]
- Schuchardt M, Tolle M, Prufer J, Prufer N, Huang T, Jankowski V, … van der Giet M. Uridine adenosine tetraphosphate activation of the purinergic receptor P2Y enhances in vitro vascular calcification. Kidney International. 2012;81(3):256–265. doi: 10.1038/ki.2011.326. [DOI] [PubMed] [Google Scholar]
- Sheen CR, Kuss P, Narisawa S, Yadav MC, Nigro J, Wang W, … Millan JL. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. Journal of Bone and Mineral Research. 2015;30(5):824–836. doi: 10.1002/jbmr.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff RC, Shanahan CM. The vascular biology of calcification. Seminars in Dialysis. 2007;20(2):103–109. doi: 10.1111/j.1525-139X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- Staines KA, Zhu D, Farquharson C, MacRae VE. Identification of novel regulators of osteoblast matrix mineralization by time series transcriptional profiling. Journal of Bone and Mineral Metabolism. 2014;32(3):240–251. doi: 10.1007/s00774-013-0493-2. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Shah M, Orriss IR. Generation of rodent and human osteoblasts. BoneKEy Reports. 2014;3:585. doi: 10.1038/bonekey.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Bellosta R, Sorribas V. Prevention of vascular calcification by polyphosphates and nucleotides- role of ATP. Circulation Journal. 2013;77(8):2145–2151. doi: 10.1253/circj.cj-13-0016. [DOI] [PubMed] [Google Scholar]
- Villa-Bellosta R, Wang X, Millan JL, Dubyak GR, O’Neill WC. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. American Journal of Physiology Heart and Circulatory Physiology. 2011;301(1):H61–H68. doi: 10.1152/ajpheart.01020.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, … Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. Journal of Cardiovascular Pharmacology. 2002;40(6):841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Xing Y, Gu Y, Bresnahan JJ, Paul EM, Donahue HJ, You J. The roles of P2Y2 purinergic receptors in osteoblasts and mechano-transduction. PLoS ONE. 2014;9(9):108417. doi: 10.1371/journal.pone.0108417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Adams JE, Anderson GF, Boulton AJ, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia. 1993;36(7):615–621. doi: 10.1007/BF00404070. [DOI] [PubMed] [Google Scholar]
- Zhu D, Mackenzie NC, Farquharson C, Macrae VE. Mechanisms and clinical consequences of vascular calcification. Frontiers in Endocrinology (Lausanne) 2012;3:95. doi: 10.3389/fendo.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Mackenzie NC, Millan JL, Farquharson C, Macrae VE. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE. 2011;6(5):19595. doi: 10.1371/journal.pone.0019595. [DOI] [PMC free article] [PubMed] [Google Scholar]