Abstract
Advances in bioconjugation and native protein modification are appearing at a blistering pace, making it increasingly time consuming for practitioners to identify the best chemical method for modifying a specific amino acid residue in a complex setting. The purpose of this perspective is to provide an informative, graphically rich manual highlighting significant advances in the field over the past decade. This guide will help triage candidate methods for peptide alteration and will serve as a starting point for those seeking to solve long-standing challenges.
Graphical abstract
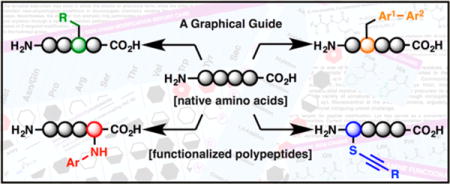
Just as some of the most pivotal advances in total synthesis can be tied to overcoming obstacles in chemoselectivity,1 advances in bioconjugation chemistry are intimately linked to the development of targeted amino acid modifications for the precise engineering of proteins. Given the sheer number of functional groups present and the general requirement for aqueous reaction media, protein modification is perhaps the ultimate expression of a chemoselective reaction. A growing appreciation for the therapeutic potential of peptides2,3 has led to numerous initiatives that necessitate an equivalent degree of chemoselectivity in diverse, late-stage peptide functionalizations. This challenge has mobilized peptide and organic chemists alike, a point illustrated by the upsurge of interdisciplinary collaborations over the past decade. Such endeavors have uniquely altered the landscape of traditional amino acid modification strategies for both peptide functionalization and protein bioconjugation, and will be the focus of this perspective.
This survey is not intended to provide a comprehensive history of bioconjugation and related chemistry;4–7 rather, it should serve as a graphically rich catalog for the practitioner interested in recently developed transformations for the discrete modification of polypeptide and protein substrates. Ligation,8 stapling,9 macrocyclization,10 and cross-linking11 strategies will not be covered, nor will methods that rely on enzymatic transformations12 or the incorporation of designer residues.13 Instead, chemical modifications specific to proteinogenic residues, select noncanonical but naturally occurring residues [e.g., dehydroalanine (Dha)], and peptide C- and N-termini will be highlighted. The format is pedagogically similar to The Portable Chemist’s Consultant,14 an e-book recently published for those engaged in chemical synthesis.
A model protein bioconjugation should be site-selective and robust and should proceed under mild conditions (physiological pH, ambient temperature and pressure, and aqueous solvent preferred). Moreover, preservation of the protein structure and conservation of activity are among the most important considerations. While shorter peptide sequences allow for some deviation, the operational simplicity epitomized by these standards is an ideal goal for the development of versatile peptide modification strategies. With these (and other) factors in mind, the aim of this user guide is to provide an etic assessment of both the advantages offered and limitations imposed by the strategies featured herein. Organized by amino acid, this schematic manual will detail key elements of each reaction, including demonstrated or implied compatibility (indicted by brackets) with common peptide functional groups, attributes unique to the method described, and challenges that may arise in reagent preparation or reaction protocol. Specific examples and general trends are highlighted in the text to guide the reader and provide essential information in a succinct manner.
Finally, this guide is supplemented with a high-level view of the field [see Table 3 (Amino Acid Side-Chain Modification Report Card) and the Supporting Information] that offers a snapshot of the available literature in the area. These resources have been compiled with an eye toward gaps in current methodology and opportunities for innovation.
Table 3.

|
See the Supporting Information for details.
ALIPHATIC SIDE CHAINS
Without an apparent functional handle, there are relatively few methods available (vide infra) for derivatization of the aliphatic residues. However, fundamental advances in the direct functionalization of C–H bonds have ushered in new opportunities for targeted modifications.15 In perhaps the most notable recent example, Yu and co-workers disclosed a palladium-catalyzed C–H arylation of N-terminal alanine (Ala) residues facilitated by coordination of the metal catalyst to the peptide backbone.16 Although currently limited to di-, tri-, and tetrapeptide substrates, this seminal report illustrates the vast potential of postassembly C(sp3)–H functionalization as an enabling tool for hydrocarbon modifications. A promising metal-mediated approach to the γ-arylation of valine (Val) and isoleucine (Ile) within dipeptide substrates has also been reported17 (Table 1).
Table 1.

|

|

|
POLAR, NONIONIZABLE SIDE CHAINS
Similar to aliphatic residues, the primary amides of asparagine (Asn) and glutamine (Gln) are all but immovable and have proven to be difficult targets for selective modification. One notable exception is the molecular recognition strategy employed by Popp and Ball, which enabled a targeted, proximity-driven modification of Asn and Gln using dirhodium metallopeptides.19 In contrast, creative reactions seeking to modify methionine (Met) are far more common. With a relatively high oxidation potential, the reversible oxidation of the thioether to the corresponding sulfoxide or sulfone is a well-defined Met reaction pathway, both synthetically and biochemically.96 Strikingly, it is the only native residue that can be alkylated under acidic conditions.97 In fact, early approaches to protein degradation for sequencing exploited these unique properties; cyanogen bromide in formic or hydrochloric acid buffer, for example, is a reliable method for Met-specific peptide bond hydrolysis that is still in use today.98 The methylthioether side chain can also be used as a homocysteine proxy, as in entry 2.21 Alkylation and subsequent demethylation provides the homologated cysteine (Cys) analogue.
In a recent joint effort, Toste, Chang, and co-workers reported the use of redox-activated chemical tagging (ReACT) for site-selective Met modification (entry 4).23 This bioconjugation strategy harnesses the previously underexplored nitrogen-transfer properties of oxaziridines to form the functionalized sulfimide. The resulting conjugates demonstrate remarkable stability when exposed to a wide pH range, disulfide reducing agents, and bioorthogonal reaction conditions. Furthermore, the ReACT approach exhibits exclusive selectivity for Met in both protein and antibody substrates.
AROMATIC SIDE CHAINS
Among the most abundant targets for new synthetic transformations, residues with aromatic side chains can be further divided into those that are readily ionized (histidine, His, and tyrosine, Tyr) and those that are not (tryptophan, Trp, and phenylalanine, Phe). It should be noted, however, that side-chain pKa is not an exclusive predictor of reactivity in this case, as indicated by the wealth of transformations specific to the indole of Trp. Importantly, the nucleophilicity of both His and Tyr can be modulated by the protonation state of the side chain. As such, the standard modes of Tyr reactivity can be effectively tuned by pH control: in acidic or neutral environments, the aromatic carbons ortho to the hydroxyl group may undergo ene-type reactions (entry 5),26,27 diazonium couplings (entry 6),28 and Mannich-type condensation reactions (entry 9),31,32 while at a pH near the pKa of phenol (∼10), alkylation or acylation of the oxygen is observed (entries 8 and 10).30,33,34 In 2009, Barbas and co-workers reported a robust aqueous ene-type reaction that enables click-like Tyr bioconjugation (entry 5).26,27 Further investigation has expanded the scope and utility of this method, allowing for the installation of diverse handles, including PEG chains and bifunctional linkers.27 In the case of Trp, modifications involving the indole C-2 position dominate the reaction landscape. A recent, metal-free organoradical conjugation strategy (entry 12)36 supplements a variety of powerful metal-based methods (entries 13–15).19,37–43 Metal-catalyzed C–H functionalization is a particularly useful strategy that enables alkynylation (entry 13)37,38 and arylation (entry 14)39–41 of the indole side chain. While not yet fully realized, emerging trends in C–H functionalization are expected to facilitate the development of robust modification protocols for Phe and His.
POLAR, IONIZABLE SIDE CHAINS
As the most readily modified of amino acids, residues with polar, ionizable side chains have been widely examined. Cys, undoubtedly the most well-studied residue, occupies a distinguished place in the bioconjugation literature.4,99 The inherently low pKa (∼8.3) and substantial nucleophilic character provide a convenient handle for site-selective derivatization. Historically, cysteine–maleimide conjugation has been the most common manner of thiol modification, but the low stability of the adducts has rendered this particular reaction outmoded.100 However, as a mild and selective modification, conjugate addition remains a popular approach for functionalization. With recent improvements in conjugate stability (entries 22–25),52–56 including the design of self-hydrolyzing maleimides,101 as well as the development of bifunctional Michael acceptors (entries 22 and 24),52,54,55 it is expected that this venerable reaction will retain prominence for years to come.
In an effort to improve the delivery of compact, high-value bioisosteres—namely, cyclobutanes, cyclopentanes, and related analogues—our lab reported the development of a variety of designer, spring-loaded reagents for strain-release functionalization.45,46 Remarkably, a number of bicyclobutane sulfones and chiral “housanes” were shown to rapidly and selectively functionalize Cys (entry 17)45,46 in the presence of several unprotected, nucleophilic peptide functional groups.
The use of transition metals in bioconjugation chemistry has gained recent traction, with the combined efforts of Pentelute, Buchwald, and co-workers at the forefront of these endeavors.47,60 In 2015, the researchers reported a palladium(II)-promoted arylation of Cys under mild conditions (entry 18).47 Of note, the palladium(II) complexes used are readily synthesized and easy to handle. In addition, the arylation reaction proved to be an effective tool in peptide stapling and antibody–drug conjugation, leading to stable bioconjugates. The site-guided palladium-mediated arylation of metal-binding proteins102 and expansion of transition metal functionalizations to the alkynylation (entry 21) and alkenylation of Cys by Messaoudi and co-workers50 demonstrate the adaptability of this approach.
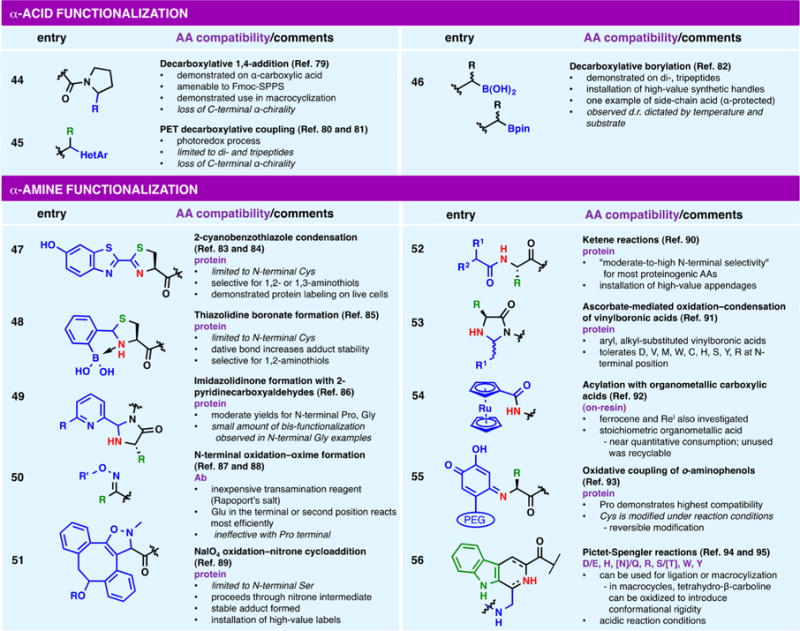
While a myriad of acid-specific reactions exists, differentiation between peptide α- and side-chain carboxylic acids (e.g., aspartic acid, Asp, and glutamic acid, Glu) has proven to be a formidable challenge in bioconjugation chemistry. Initially reported by our group in 2016,103 the use of “redox-active esters” (RAEs) as a means to activate and engage carboxylic acids in a nickel-catalyzed cross-coupling event has since been parlayed into a broad approach for the construction of carbon–carbon (entries 27, 28, and 44)58,59,79 and carbon–heteroatom (entry 46) bonds.82 The robust procedure, which repurposes activating reagents typically employed in amide bond formation (e.g., HOAt, N-hydroxyphthalimides), has been successfully applied to both resin-bound58,79 and solution-phase peptide substrates,59,79,82 allowing for the introduction of discrete cross-coupled products at either side-chain positions or α-positions.
Approaches to lysine (Lys) modification encounter similar obstacles in α- versus ε-amine differentiation, but selective acylation or alkylation can be achieved through careful pH control. Recent advances in arylation (entry 29)60 and condensation (entry 30)61 reactions have also expanded the diversity of functionalizations. The potential for reversible labeling (entries 31 and 32)62,63 of Lys is particularly intriguing, with envisioned applications toward the development of antibody–drug conjugates (ADCs). Finally, modifications to arginine (Arg) are currently limited in scope; acylation and condensation are common approaches (entries 35 and 36),66–68 with the most provocative adduct (entry 37)69 being a serendipitous byproduct formed during a copper-catalyzed azide–alkyne cycloaddition. To date, chemoselective side-chain modifications at serine (Ser) and threonine (Thr) are exceedingly rare, generally relying on proximity-driven19 or sequence-specific modifications; more broadly applicable methods represent an opportunity for invention.
NONCANONICAL AMINO ACIDS
Biosynthetically, Dha is often incorporated into peptides through the enzymatic dehydration of Ser;104 targeted chemical approaches, in contrast, typically proceed through activation–elimination of the Cys thiol105 or an analogous, and similarly facile, transformation at modified selenocysteine (Sec) residues.106 The electrophilic, unsaturated side chain is frequently utilized as an acceptor in 1,4-conjugate additions (entries 39 and 40).71–75 In a recent example, both the Davis laboratory75 and the collaborative team of Söll, Lee, and Park74 independently reported a radical-based conjugate addition (entry 40) to Dha for the site-selective introduction of diverse non-native residues into several target proteins.
In addition to its role as a Dha precursor, Sec is often purposed as a handle for native chemical ligation.107 Relatively few examples of covalent derivatization exist, primarily because of the low reduction potential and high polarizability of the selenol functional group.108 Capitalizing on these otherwise unwieldy characteristics, Pentelute and Buchwald reported a copper-promoted umpolung approach to the arylation of Sec, which takes advantage of the electrophilicity of selenyl sulfides (entry 41).76 While the reaction conditions were conclusively determined to favor Sec arylation, oxidation of unprotected Cys was unavoidable.
C-TERMINUS AND N-TERMINUS
Modification of peptide termini is an attractive approach, particularly in cases in which strict bioconjugate stoichiometry is required. The use of specialized linkers to reveal unique functionality on resin cleavage is a common approach to the alteration of C-terminal acids and has been the subject of prior reviews.109,110 Focusing instead on postassembly synthetic transformations, it is unsurprising that the aforementioned chemoselectivity challenges (see the discussion of Asp and Glu in Polar, Ionizable Side Chains) apply to α-carboxylic acids as well. Nevertheless, orthogonal protecting group strategies and judicious sequence choice have been employed to enable several decarboxylative functionalizations of α-acids, including 1,4-additions (entry 44),79 heteroarylations (entry 45),80,81 and borylations (entry 46).82
Bioconjugation reactions that target α-amines have been the subject of much investigation for use in ligation and macrocyclization; for further insight into these invaluable processes, we direct readers to the many extensive reviews available.4–10 Alternative modifications at the N-terminus111 often rely on participation from the associated side-chain functional group; reactions that engage ionizable residues (e.g., Cys) are common (entries 47 and 48),83–85 as are examples that employ aromatic (entry 56)94,95 and other nucleophilic side chains (e.g., Ser and Thr).89 The formation of heterocycles is a common approach (entries 47–49, 51, 53, and 56)83–86,89,91,94,95 as is N-terminal oxidation and further diversification (entries 50 and 51).87–89
BACKBONE MODIFICATION
As in small molecule chemistries, transition metals have enabled previously unimaginable bioconjugate transformations,112,113 an assertion particularly true in the burgeoning area of backbone modifications (Scheme 1).114–117 In 2016, White and co-workers reported an iron-catalyzed oxidative functionalization inspired by known nonribosomal peptide synthetase (NRPS) pathways.18 In an impressive display of versatility, the approach was used to synthesize 21 non-natural amino acids from four native residues, with no erosion of chirality observed. Notably, C–H oxidation of the tertiary carbon center of Leu and Val was also demonstrated.18
Scheme 1.

In another intriguing example, Ball and co-workers detailed a copper-mediated, His-directed amide N-functionalization in a rare example of selective backbone modification in the context of proteins.117 While relatively few examples of these methods currently exist, the profound effects such modifications have on peptide and protein structure and function118,119 will undoubtedly drive further innovation.
FUTURE OUTLOOK
The biological importance of modified peptides and proteins is a powerful impetus for the continued exploration of diverse amino acid-specific modifications. To this end, the operational simplicity and inherent flexibility of direct modifications of proteinogenic amino acids are undeniable. A high-level view of progress toward residue-specific peptide and protein modifications is outlined in Table 3 (Amino Acid Side-Chain Modification Report Card) and further detailed in the Supporting Information. Categorized by amino acid residue and type of chemical transformation, this graphical depiction illustrates recent and historical successes in the field, with the mild reaction conditions and exquisite chemoselectivity required for protein modifications serving as the ultimate evaluation parameters. Framed by these ideals, the amino acid landscape is marked by several remaining challenges, or rather, opportunities for invention: (1) robust aliphatic functionalization, (2) the transition from “proof of concept” (e.g., single amino acids or short, unadorned peptide sequences) to “widely applicable” (e.g., larger peptides, proteins), and (3) well-defined, site-selective modification in the presence of chemically equivalent residues. We anticipate that advances in metal-catalyzed C–H functionalization will address a number of current limitations and continue to enlist both peptide and small molecule chemists, leading to an expanding library of interdisciplinary methods. Indeed, the requisite functional group selectivity and mild reaction conditions are enviable benchmarks for all new synthetic methods. Translational approaches to peptide and protein modifications therefore have the capacity to revolutionize the capabilities of modern organic synthesis while also delivering high-value biological targets to meet urgent societal needs.
Supplementary Material
Table 2.
|
Acknowledgments
Funding
Financial support for this work was provided by the National Institutes of Health (Postdoctoral Fellowship F32GM117816 to L.R.M. and Grant GM-118176) and the National Science Foundation GRFP (J.N.D.).
ABBREVIATIONS
- PEG
polyethylene glycol
- Ab
antibody
- Ar
aryl
- Boc
tert-butyloxycarbonyl
- Alk
alkyl
- 2-pym
2-pyrimidine
- PG
protecting group
- Conj
conjugate
- HetAr
heteroaryl
- ee
enantiomeric excess
- NHS
N-hydroxysuccinimide
- HPLC
high-performance liquid chromatography
- DHA
dehydroascorbate
- PTM
post-translational modification
- Fmoc
fluorenylmethyloxycarbonyl
- SPPS
solid-phase peptide synthesis
- Bpin
boronic acid pinacol ester
- dr
diastereomeric ratio
- AA
amino acid
- HOAt
1-hydroxy-7-azabenzotriazole
- Nu
nucleophile
- LDA
lithium diisopropyl amide
- PMP
p-methoxyphenyl
- TBHP
tert-butyl hydroperoxide
- PDP
2-({(R)-2-[(R)-1-(pyridin-2-ylmethyl)-pyrrolidin-2-yl]pyrrolidin-1-yl}methyl)pyridine
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem.7b00536.
Delineation of Amino Acid Side-Chain Modification Report Card assessments, including references (PDF)
ORCID
Lara R. Malins: 0000-0002-7691-6432
Phil S. Baran: 0000-0001-9193-9053
Notes
The authors declare no competing financial interest.
References
- 1.Shenvi RA, O’Malley DP, Baran PS. Chemoselectivity: The mother of invention in total synthesis. Acc Chem Res. 2009;42:530–541. doi: 10.1021/ar800182r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 3.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discovery Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.For recent comprehensive reviews of the field, see refs4–7; Hermanson GT. Bioconjugate Techniques. 3rd. Academic Press; San Diego: 2013. [Google Scholar]
- 5.Koniev O, Wagner A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem Soc Rev. 2015;44:5495–5551. doi: 10.1039/c5cs00048c. [DOI] [PubMed] [Google Scholar]
- 6.McKay CS, Finn MG. Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chem Biol. 2014;21:1075–1101. doi: 10.1016/j.chembiol.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutureira O, Bernardes GJ. Advances in chemical protein modification. Chem Rev. 2015;115:2174–2195. doi: 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea LD, Romanelli A. Chemical ligation: Tools for biomolecule synthesis and modification. John Wiley & Sons, Inc; Hoboken, NJ: 2017. [Google Scholar]
- 9.Lau YH, de Andrade P, Wu Y, Spring DR. Peptide stapling techniques based on different macrocyclisation chemistries. Chem Soc Rev. 2015;44:91–102. doi: 10.1039/c4cs00246f. [DOI] [PubMed] [Google Scholar]
- 10.White CJ, Yudin AK. Contemporary strategies for peptide macrocyclization. Nat Chem. 2011;3:509–524. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- 11.Kodadek T, Duroux-Richard I, Bonnafous JC. Techniques: Oxidative cross-linking as an emergent tool for the analysis of receptor-mediated signalling events. Trends Pharmacol Sci. 2005;26:210–217. doi: 10.1016/j.tips.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Rashidian M, Dozier JK, Distefano MD. Enzymatic labeling of proteins: techniques and approaches. Bioconjugate Chem. 2013;24:1277–1294. doi: 10.1021/bc400102w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang K, Chin JW. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem Rev. 2014;114:4764–4806. doi: 10.1021/cr400355w. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara Y, Montero A, Baran PS. The Portable Chemist’s Consultant. Apple Publishing Group; New York: 2016. version 2.7.1. [Google Scholar]
- 15.Noisier AF, Brimble MA. C–H functionalization in the synthesis of amino acids and peptides. Chem Rev. 2014;114:8775–8806. doi: 10.1021/cr500200x. [DOI] [PubMed] [Google Scholar]
- 16.Gong W, Zhang G, Liu T, Giri R, Yu JQ. Site-selective C(sp3)–H functionalization of di-, tri-, and tetrapeptides at the N-terminus. J Am Chem Soc. 2014;136:16940–16946. doi: 10.1021/ja510233h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez N, Romero-Revilla JA, Fernandez-Ibanez MA, Carretero JC. Palladium-catalyzed N-(2-pyridyl)sulfonyl-directed C(sp3)–H γ-arylation of amino acid derivatives. Chem Sci. 2013;4:175–179. [Google Scholar]
- 18.Osberger TJ, Rogness DC, Kohrt JT, Stepan AF, White MC. Oxidative diversification of amino acids and peptides by small-molecule iron catalysis. Nature. 2016;537:214–219. doi: 10.1038/nature18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popp BV, Ball ZT. Proximity-driven metal-lopeptide catalysis: Remarkable side-chain scope enables modification of the Fos bZip domain. Chem Sci. 2011;2:690–695. [Google Scholar]
- 20.Gharakhanian EG, Deming TJ. Versatile synthesis of stable, functional polypeptides via reaction with epoxides. Biomacromolecules. 2015;16:1802–1806. doi: 10.1021/acs.biomac.5b00372. [DOI] [PubMed] [Google Scholar]
- 21.Gharakhanian EG, Deming TJ. Chemoselective synthesis of functional homocysteine residues in polypeptides and peptides. Chem Commun. 2016;52:5336–5339. doi: 10.1039/c6cc01253a. [DOI] [PubMed] [Google Scholar]
- 22.Kramer JR, Deming TJ. Reversible chemoselective tagging and functionalization of methionine containing peptides. Chem Commun. 2013;49:5144–5146. doi: 10.1039/c3cc42214c. [DOI] [PubMed] [Google Scholar]
- 23.Lin S, Yang X, Jia S, Weeks AM, Hornsby M, Lee PS, Nichiporuk RV, Iavarone AT, Wells JA, Toste FD, Chang CJ. Redox-based reagents for chemoselective methionine bioconjugation. Science. 2017;355:597–602. doi: 10.1126/science.aal3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruice TC, Schmir GL. Imidazole catalysis II. The reaction of substituted imidazoles with phenyl acetates in aqueous solution. J Am Chem Soc. 1958;80:148–156. [Google Scholar]
- 25.Liao SM, Du QS, Meng JZ, Pang ZW, Huang RB. The multiple roles of histidine in protein interactions. Chem Cent J. 2013;7:44. doi: 10.1186/1752-153X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ban H, Gavrilyuk J, Barbas CF., 3rd Tyrosine bioconjugation through aqueous ene-type reactions: a click-like reaction for tyrosine. J Am Chem Soc. 2010;132:1523–1525. doi: 10.1021/ja909062q. [DOI] [PubMed] [Google Scholar]
- 27.Ban H, Nagano M, Gavrilyuk J, Hakamata W, Inokuma T, Barbas CF., 3rd Facile and stabile linkages through tyrosine: Bioconjugation strategies with the tyrosine-click reaction. Bioconjugate Chem. 2013;24:520–532. doi: 10.1021/bc300665t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones MW, Mantovani G, Blindauer CA, Ryan SM, Wang X, Brayden DJ, Haddleton DM. Direct peptide bioconjugation/PEGylation at tyrosine with linear and branched polymeric diazonium salts. J Am Chem Soc. 2012;134:7406–7413. doi: 10.1021/ja211855q. [DOI] [PubMed] [Google Scholar]
- 29.Vilaró M, Arsequell G, Valencia G, Ballesteros A, Barluenga J. Arylation of Phe and Tyr side chains of unprotected peptides by a Suzuki–Miyaura reaction in water. Org Lett. 2008;10:3243–3245. doi: 10.1021/ol801009z. [DOI] [PubMed] [Google Scholar]
- 30.Seim KL, Obermeyer AC, Francis MB. Oxidative modification of native protein residues using cerium(IV) ammonium nitrate. J Am Chem Soc. 2011;133:16970–16976. doi: 10.1021/ja206324q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanini DW, Francis MB. Attachment of peptide building blocks to proteins through tyrosine bioconjugation. Bioconjugate Chem. 2008;19:153–157. doi: 10.1021/bc700231v. [DOI] [PubMed] [Google Scholar]
- 32.Joshi NS, Whitaker LR, Francis MB. A three-component Mannich-type reaction for selective tyrosine bioconjugation. J Am Chem Soc. 2004;126:15942–15943. doi: 10.1021/ja0439017. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Li X, Ma H. New approach for local structure analysis of the tyrosine domain in proteins by using a site-specific and polarity-sensitive fluorescent probe. ChemBioChem. 2009;10:1200–1207. doi: 10.1002/cbic.200900003. [DOI] [PubMed] [Google Scholar]
- 34.Tilley SD, Francis MB. Tyrosine-selective protein alkylation using π-allylpalladium complexes. J Am Chem Soc. 2006;128:1080–1081. doi: 10.1021/ja057106k. [DOI] [PubMed] [Google Scholar]
- 35.Malins LR, Cergol KM, Payne RJ. Chemoselective sulfenylation and peptide ligation at tryptophan. Chem Sci. 2014;5:260–266. [Google Scholar]
- 36.Seki Y, Ishiyama T, Sasaki D, Abe J, Sohma Y, Oisaki K, Kanai M. Transition metal-free tryptophan-selective bioconjugation of proteins. J Am Chem Soc. 2016;138:10798–10801. doi: 10.1021/jacs.6b06692. [DOI] [PubMed] [Google Scholar]
- 37.Ruan Z, Sauermann N, Manoni E, Ackermann L. Manganese-catalyzed C–H alkynylation: Expedient peptide synthesis and modification. Angew Chem, Int Ed. 2017;56:3172–3176. doi: 10.1002/anie.201611118. [DOI] [PubMed] [Google Scholar]
- 38.Hansen MB, Hubalek F, Skrydstrup T, Hoeg-Jensen T. Chemo- and regioselective ethynylation of tryptophan-containing peptides and proteins. Chem – Eur J. 2016;22:1572–1576. doi: 10.1002/chem.201504462. [DOI] [PubMed] [Google Scholar]
- 39.Schischko A, Ren H, Kaplaneris N, Ackermann L. Bioorthogonal diversification of peptides through selective ruthenium-(II)-catalyzed C–H activation. Angew Chem, Int Ed. 2017;56:1576–1580. doi: 10.1002/anie.201609631. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz-Rodriguez J, Albericio F, Lavilla R. Postsynthetic modification of peptides: Chemoselective C-arylation of tryptophan residues. Chem – Eur J. 2010;16:1124–1127. doi: 10.1002/chem.200902676. [DOI] [PubMed] [Google Scholar]
- 41.Reay AJ, Williams TJ, Fairlamb IJ. Unified mild reaction conditions for C2-selective Pd-catalysed tryptophan arylation, including tryptophan-containing peptides. Org Biomol Chem. 2015;13:8298–8309. doi: 10.1039/c5ob01174d. [DOI] [PubMed] [Google Scholar]
- 42.Antos JM, McFarland JM, Iavarone AT, Francis MB. Chemoselective tryptophan labeling with rhodium carbenoids at mild pH. J Am Chem Soc. 2009;131:6301–6308. doi: 10.1021/ja900094h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popp BV, Ball ZT. Structure-selective modification of aromatic side chains with dirhodium metallopeptide catalysts. J Am Chem Soc. 2010;132:6660–6662. doi: 10.1021/ja101456c. [DOI] [PubMed] [Google Scholar]
- 44.Siti W, Khan AK, de Hoog HP, Liedberg B, Nallani M. Photo-induced conjugation of tetrazoles to modified and native proteins. Org Biomol Chem. 2015;13:3202–3206. doi: 10.1039/c4ob02025a. [DOI] [PubMed] [Google Scholar]
- 45.Gianatassio R, Lopchuk JM, Wang J, Pan CM, Malins LR, Prieto L, Brandt TA, Collins MR, Gallego GM, Sach NW, Spangler JE, Zhu H, Zhu J, Baran PS. Strain-release amination. Science. 2016;351:241–246. doi: 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopchuk JM, Fjelbye K, Kawamata Y, Malins LR, Pan CM, Gianatassio R, Wang J, Prieto L, Bradow J, Brandt TA, Collins MR, Elleraas J, Ewanicki J, Farrell W, Fadeyi OO, Gallego GM, Mousseau JJ, Oliver R, Sach NW, Smith JK, Spangler JE, Zhu H, Zhu J, Baran PS. Strain-release heteroatom functionalization: Development, scope, and stereospecificity. J Am Chem Soc. 2017;139:3209–3226. doi: 10.1021/jacs.6b13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinogradova EV, Zhang C, Spokoyny AM, Pentelute BL, Buchwald SL. Organometallic palladium reagents for cysteine bioconjugation. Nature. 2015;526:687–691. doi: 10.1038/nature15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capone S, Kieltsch I, Flögel O, Lelais G, Togni A, Seebach D. Electrophilic S-trifluoromethylation of cysteine side chains in α- and β-Peptides: Isolation of trifluoro-methylated Sandostatin® (Octreotide) derivatives. Helv Chim Acta. 2008;91:2035–2056. [Google Scholar]
- 49.Lin YA, Chalker JM, Floyd N, Bernardes GJ, Davis BG. Allyl sulfides are privileged substrates in aqueous cross-metathesis: application to site-selective protein modification. J Am Chem Soc. 2008;130:9642–9643. doi: 10.1021/ja8026168. [DOI] [PubMed] [Google Scholar]
- 50.Al-Shuaeeb RA, Kolodych S, Koniev O, Delacroix S, Erb S, Nicolay S, Cintrat JC, Brion JD, Cianferani S, Alami M, Wagner A, Messaoudi S. Palladium-catalyzed chemoselective and biocompatible functionalization of cysteine-containing molecules at room temperature. Chem – Eur J. 2016;22:11365–11370. doi: 10.1002/chem.201602277. [DOI] [PubMed] [Google Scholar]
- 51.For an alternative approach to heteroaryl sulfides, see:; Toda N, Asano S, Barbas CF. Rapid, stable, chemoselective labeling of thiols with Julia–Kocienśki-like reagents: A serum-stable alternative to maleimide-based protein conjugation. Angew Chem, Int Ed. 2013;52:12592–12596. doi: 10.1002/anie.201306241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ariyasu S, Hayashi H, Xing B, Chiba S. Site-specific dual functionalization of cysteine residue in peptides and proteins with 2-azidoacrylates. Bioconjugate Chem. 2017;28:897–902. doi: 10.1021/acs.bioconjchem.7b00024. [DOI] [PubMed] [Google Scholar]
- 53.Bernardim B, Cal PM, Matos MJ, Oliveira BL, Martinez-Saez N, Albuquerque IS, Perkins E, Corzana F, Burtoloso AC, Jimenez-Oses G, Bernardes GJ. Stoichiometric and irreversible cysteine-selective protein modification using carbonylacrylic reagents. Nat Commun. 2016;7:13128. doi: 10.1038/ncomms13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolodych S, Koniev O, Baatarkhuu Z, Bonnefoy JY, Debaene F, Cianferani S, Van Dorsselaer A, Wagner A. CBTF: new amine-to-thiol coupling reagent for preparation of antibody conjugates with increased plasma stability. Bioconjugate Chem. 2015;26:197–200. doi: 10.1021/bc500610g. [DOI] [PubMed] [Google Scholar]
- 55.Koniev O, Leriche G, Nothisen M, Remy JS, Strub JM, Schaeffer-Reiss C, Van Dorsselaer A, Baati R, Wagner A. Selective irreversible chemical tagging of cysteine with 3-arylpropiolonitriles. Bioconjugate Chem. 2014;25:202–206. doi: 10.1021/bc400469d. [DOI] [PubMed] [Google Scholar]
- 56.Abbas A, Xing B, Loh TP. Allenamides as orthogonal handles for selective modification of cysteine in peptides and proteins. Angew Chem, Int Ed. 2014;53:7491–7494. doi: 10.1002/anie.201403121. [DOI] [PubMed] [Google Scholar]
- 57.Bernardes GJ, Grayson EJ, Thompson S, Chalker JM, Errey JC, El Oualid F, Claridge TD, Davis BG. From disulfide- to thioether-linked glycoproteins. Angew Chem, Int Ed. 2008;47:2244–2247. doi: 10.1002/anie.200704381. [DOI] [PubMed] [Google Scholar]
- 58.Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS. A general alkyl–alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards JT, Merchant RR, McClymont KS, Knouse KW, Qin T, Malins LR, Vokits B, Shaw SA, Bao DH, Wei FL, Zhou T, Eastgate MD, Baran PS. Decarboxylative alkenylation. Nature. 2017;545:213–218. doi: 10.1038/nature22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HG, Lautrette G, Pentelute BL, Buchwald SL. Palladium-mediated arylation of lysine in unprotected peptides. Angew Chem, Int Ed. 2017;56:3177–3181. doi: 10.1002/anie.201611202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tung CL, Wong CT, Fung EY, Li X. Traceless and chemoselective amine bioconjugation via phthalimidine formation in native protein modification. Org Lett. 2016;18:2600–2603. doi: 10.1021/acs.orglett.6b00983. [DOI] [PubMed] [Google Scholar]
- 62.Cal PMSD, Vicente JB, Pires E, Coelho AV, Veiros LSF, Cordeiro C, Gois PMP. Iminoboronates: A new strategy for reversible protein modification. J Am Chem Soc. 2012;134:10299–10305. doi: 10.1021/ja303436y. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka K, Fujii Y, Fukase K. Site-selective and nondestructive protein labeling through azaelectrocyclization-induced cascade reactions. ChemBioChem. 2008;9:2392–2397. doi: 10.1002/cbic.200800336. [DOI] [PubMed] [Google Scholar]
- 64.Chen X, Muthoosamy K, Pfisterer A, Neumann B, Weil T. Site-selective lysine modification of native proteins and peptides via kinetically controlled labeling. Bioconjugate Chem. 2012;23:500–508. doi: 10.1021/bc200556n. [DOI] [PubMed] [Google Scholar]
- 65.Diethelm S, Schafroth MA, Carreira EM. Amine-selective bioconjugation using arene diazonium salts. Org Lett. 2014;16:3908–3911. doi: 10.1021/ol5016509. [DOI] [PubMed] [Google Scholar]
- 66.Grundler V, Gademann K. Direct arginine modification in native peptides and application to chemical probe development. ACS Med Chem Lett. 2014;5:1290–1295. doi: 10.1021/ml5003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gauthier MA, Klok HA. Arginine-specific modification of proteins with polyethylene glycol. Biomacromolecules. 2011;12:482–493. doi: 10.1021/bm101272g. [DOI] [PubMed] [Google Scholar]
- 68.Gong Y, Andina D, Nahar S, Leroux JC, Gauthier MA. Releasable and traceless PEGylation of arginine-rich antimicrobial peptides. Chem Sci. 2017;8:4082–4086. doi: 10.1039/c7sc00770a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conibear AC, Farbiarz K, Mayer RL, Matveenko M, Kahlig CH, Becker CF. Arginine side-chain modification that occurs during copper-catalysed azide-alkyne click reactions resembles an advanced glycation end product. Org Biomol Chem. 2016;14:6205–6211. doi: 10.1039/c6ob00932h. [DOI] [PubMed] [Google Scholar]
- 70.Bartoccini F, Bartolucci S, Lucarini S, Piersanti G. Synthesis of boron- and silicon-containing amino acids through copper-catalysed conjugate additions to dehydroalanine derivatives. Eur J Org Chem. 2015;2015:3352–3360. [Google Scholar]
- 71.Chalker JM, Lercher L, Rose NR, Schofield CJ, Davis BG. Conversion of Cysteine into dehydroalanine enables access to synthetic histones bearing diverse post-translational modifications. Angew Chem, Int Ed. 2012;51:1835–1839. doi: 10.1002/anie.201106432. [DOI] [PubMed] [Google Scholar]
- 72.Bernardes GJL, Chalker JM, Errey JC, Davis BG. Facile conversion of cysteine and alkyl cysteines to dehydroalanine on protein surfaces: Versatile and switchable access to functionalized proteins. J Am Chem Soc. 2008;130:5052–5053. doi: 10.1021/ja800800p. [DOI] [PubMed] [Google Scholar]
- 73.Guo J, Wang J, Lee JS, Schultz PG. Site-specific incorporation of methyl- and acetyl-lysine analogues into recombinant proteins. Angew Chem, Int Ed. 2008;47:6399–6401. doi: 10.1002/anie.200802336. [DOI] [PubMed] [Google Scholar]
- 74.Yang A, Ha S, Ahn J, Kim R, Kim S, Lee Y, Kim J, Söll D, Lee HY, Park HS. A chemical biology route to site-specific authentic protein modifications. Science. 2016;354:623–626. doi: 10.1126/science.aah4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright TH, Bower BJ, Chalker JM, Bernardes GJ, Wiewiora R, Ng WL, Raj R, Faulkner S, Vallee MR, Phanumartwiwath A, Coleman OD, Thezenas ML, Khan M, Galan SR, Lercher L, Schombs MW, Gerstberger S, Palm-Espling ME, Baldwin AJ, Kessler BM, Claridge TD, Mohammed S, Davis BG. Posttranslational mutagenesis: A chemical strategy for exploring protein side-chain diversity. Science. 2016;354:1465–1. doi: 10.1126/science.aag1465. [DOI] [PubMed] [Google Scholar]
- 76.Cohen DT, Zhang C, Pentelute BL, Buchwald SL. An umpolung approach for the chemoselective arylation of selenocysteine in unprotected peptides. J Am Chem Soc. 2015;137:9784–9787. doi: 10.1021/jacs.5b05447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin YA, Boutureira O, Lercher L, Bhushan B, Paton RS, Davis BG. Rapid cross-metathesis for reversible protein modifications via chemical access to Se-allyl-selenocysteine in proteins. J Am Chem Soc. 2013;135:12156–12159. doi: 10.1021/ja403191g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedzisa L, Li X, Rader C, Roush WR. Assessment of reagents for selenocysteine conjugation and the stability of selenocysteine adducts. Org Biomol Chem. 2016;14:5141–5147. doi: 10.1039/c6ob00775a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qin T, Malins LR, Edwards JT, Merchant RR, Novak AJ, Zhong JZ, Mills RB, Yan M, Yuan C, Eastgate MD, Baran PS. Nickel-catalyzed barton decarboxylation and Giese reactions: A practical take on classic transforms. Angew Chem, Int Ed. 2017;56:260–265. doi: 10.1002/anie.201609662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng WM, Shang R, Fu Y. Photoredox/Brønsted acid co-catalysis enabling decarboxylative coupling of amino acid and peptide redox-active esters with N-heteroarenes. ACS Catal. 2017;7:907–911. [Google Scholar]
- 81.Jin Y, Jiang M, Wang H, Fu H. Installing amino acids and peptides on N-heterocycles under visible-light assistance. Sci Rep. 2016;6:20068. doi: 10.1038/srep20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li C, Wang J, Barton LM, Yu S, Tian M, Peters DS, Kumar M, Yu AW, Johnson KA, Chatterjee AK, Yan M, Baran PS. Decarboxylative borylation. Science. 2017;356:eaam7355. doi: 10.1126/science.aam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren H, Xiao F, Zhan K, Kim YP, Xie H, Xia Z, Rao J. A biocompatible condensation reaction for the labeling of terminal cysteine residues on proteins. Angew Chem, Int Ed. 2009;48:9658–9662. doi: 10.1002/anie.200903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuan Y, Liang G. A biocompatible, highly efficient click reaction and its applications. Org Biomol Chem. 2014;12:865–871. doi: 10.1039/c3ob41241e. [DOI] [PubMed] [Google Scholar]
- 85.Bandyopadhyay A, Cambray S, Gao J. Fast and selective labeling of N-terminal cysteines at neutral pH via thiazolidino boronate formation. Chem Sci. 2016;7:4589–4593. doi: 10.1039/c6sc00172f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacDonald JI, Munch HK, Moore T, Francis MB. One-step site-specific modification of native proteins with 2-pyridinecarboxyaldehydes. Nat Chem Biol. 2015;11:326–331. doi: 10.1038/nchembio.1792. [DOI] [PubMed] [Google Scholar]
- 87.Witus LS, Netirojjanakul C, Palla KS, Muehl EM, Weng CH, Iavarone AT, Francis MB. Site-specific protein transamination using N-methylpyridinium-4-carboxaldehyde. J Am Chem Soc. 2013;135:17223–17229. doi: 10.1021/ja408868a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheck RA, Dedeo MT, Iavarone AT, Francis MB. Optimization of a biomimetic transamination reaction. J Am Chem Soc. 2008;130:11762–11770. doi: 10.1021/ja802495w. [DOI] [PubMed] [Google Scholar]
- 89.Ning X, Temming RP, Dommerholt J, Guo J, Ania DB, Debets MF, Wolfert MA, Boons GJ, van Delft FL. Protein modification by strain-promoted alkyne–nitrone cycloaddition. Angew Chem, Int Ed. 2010;49:3065–3068. doi: 10.1002/anie.201000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan AOY, Ho CM, Chong HC, Leung YC, Huang JS, Wong MK, Che CM. Modification of N-terminal α-amino groups of peptides and proteins using ketenes. J Am Chem Soc. 2012;134:2589–2598. doi: 10.1021/ja208009r. [DOI] [PubMed] [Google Scholar]
- 91.Ohata J, Ball ZT. Ascorbate as a pro-oxidant: mild N-terminal modification with vinylboronic acids. Chem Commun. 2017;53:1622–1625. doi: 10.1039/c6cc09955f. [DOI] [PubMed] [Google Scholar]
- 92.Slootweg JC, Albada HB, Siegmund D, Metzler-Nolte N. Efficient reagent-saving method for the N-terminal labeling of bioactive peptides with organometallic carboxylic acids by solid-phase synthesis. Organometallics. 2016;35:3192–3196. [Google Scholar]
- 93.Obermeyer AC, Jarman JB, Francis MB. N-terminal modification of proteins with o-aminophenols. J Am Chem Soc. 2014;136:9572–9579. doi: 10.1021/ja500728c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malins LR, deGruyter JN, Robbins KJ, Scola PM, Eastgate MD, Ghadiri MR, Baran PS. Peptide macrocyclization inspired by non-ribosomal imine natural products. J Am Chem Soc. 2017;139:5233–5241. doi: 10.1021/jacs.7b01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X, Zhang L, Hall SE, Tam JP. A new ligation method for N-terminal tryptophan-containing peptides using the Pictet–Spengler reaction. Tetrahedron Lett. 2000;41:4069–4073. [Google Scholar]
- 96.Brot N, Weissbach H. The biochemistry of methionine sulfoxide residues in proteins. Trends Biochem Sci. 1982;7:137–139. [Google Scholar]
- 97.Jones JB, Hysert DW. Alkylations of the side-chain nucleophiles of cysteine, methionine, histidine, and lysine derivatives with allyl bromide, 1-bromo-2-butyne, and 2-bromoacetophenone. Can J Chem. 1971;49:3012–3019. [Google Scholar]
- 98.Yeung CWT, Carpenter FH, Busse WD. Cyanogen bromide treatment of methionine-containing compounds. Biochemistry. 1977;16:1635–1641. doi: 10.1021/bi00627a018. [DOI] [PubMed] [Google Scholar]
- 99.Chalker JM, Bernardes GJ, Lin YA, Davis BG. Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem – Asian J. 2009;4:630–640. doi: 10.1002/asia.200800427. [DOI] [PubMed] [Google Scholar]
- 100.Ross PL, Wolfe JL. Physical and chemical stability of antibody drug conjugates: Current status. J Pharm Sci. 2016;105:391–397. doi: 10.1016/j.xphs.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 101.Lyon RP, Setter JR, Bovee TD, Doronina SO, Hunter JH, Anderson ME, Balasubramanian CL, Duniho SM, Leiske CI, Li F, Senter PD. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat Biotechnol. 2014;32:1059–1062. doi: 10.1038/nbt.2968. [DOI] [PubMed] [Google Scholar]
- 102.Willwacher J, Raj R, Mohammed S, Davis BG. Selective metal-site-guided arylation of proteins. J Am Chem Soc. 2016;138:8678–8681. doi: 10.1021/jacs.6b04043. [DOI] [PubMed] [Google Scholar]
- 103.Cornella J, Edwards JT, Qin T, Kawamura S, Wang J, Pan CM, Gianatassio R, Schmidt M, Eastgate MD, Baran PS. Practical Ni-catalyzed aryl–alkyl cross-coupling of secondary redox-active esters. J Am Chem Soc. 2016;138:2174–2177. doi: 10.1021/jacs.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Repka LM, Chekan JR, Nair SK, van der Donk WA. Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem Rev. 2017;117:5457–5520. doi: 10.1021/acs.chemrev.6b00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chalker JM, Gunnoo SB, Boutureira O, Gerstberger SC, Fernandez-Gonzalez M, Bernardes GJL, Griffin L, Hailu H, Schofield CJ, Davis BG. Methods for converting cysteine to dehydroalanine on peptides and proteins. Chem Sci. 2011;2:1666–1676. [Google Scholar]
- 106.Okeley NM, Zhu Y, van der Donk WA. Facile chemoselective synthesis of dehydroalanine-containing peptides. Org Lett. 2000;2:3603–3606. doi: 10.1021/ol006485d. [DOI] [PubMed] [Google Scholar]
- 107.Malins LR, Mitchell NJ, Payne RJ. Peptide ligation chemistry at selenol amino acids. J Pept Sci. 2014;20:64–77. doi: 10.1002/psc.2581. [DOI] [PubMed] [Google Scholar]
- 108.Metanis N, Beld J, Hilvert D. IPATAI’S Chemistry of Functional Groups. John Wiley & Sons, Ltd; New York: 2009. The Chemistry of Selenocysteine. [Google Scholar]
- 109.Scott P. Linker strategies in solid-phase organic synthesis. John Wiley & Sons; West Sussex, UK: 2009. [Google Scholar]
- 110.Alsina J, Albericio F. Solid-phase synthesis of C-terminal modified peptides. Biopolymers. 2003;71:454–477. doi: 10.1002/bip.10492. [DOI] [PubMed] [Google Scholar]
- 111.For a recent overview on strategies for N-terminal protein modification, see:; Rosen CB, Francis MB. Targeting the N terminus for site-selective protein modification. Nat Chem Biol. 2017;13:697–705. doi: 10.1038/nchembio.2416. [DOI] [PubMed] [Google Scholar]
- 112.Chalker JM. Chemoselective and Bioorthogonal Ligation Reactions. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2017. Metal-Mediated Bioconjugation; pp. 231–270. [Google Scholar]
- 113.Malins LR. Transition metal-promoted arylation: An emerging strategy for protein bioconjugation. Aust J Chem. 2016;69:1360–1364. [Google Scholar]
- 114.Zhao L, Basle O, Li CJ. Site-specific C-functionalization of free-(NH) peptides and glycine derivatives via direct C-H bond functionalization. Proc Natl Acad Sci U S A. 2009;106:4106–4111. doi: 10.1073/pnas.0809052106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Datta S, Bayer A, Kazmaier U. Highly stereoselective modifications of peptides via Pd-catalyzed allylic alkylation of internal peptide amide enolates. Org Biomol Chem. 2012;10:8268–8275. doi: 10.1039/c2ob26351c. [DOI] [PubMed] [Google Scholar]
- 116.Romero-Estudillo I, Boto A. Creating diversity by site-selective peptide modification: a customizable unit affords amino acids with high optical purity. Org Lett. 2013;15:5778–5781. doi: 10.1021/ol402800a. [DOI] [PubMed] [Google Scholar]
- 117.Ohata J, Minus MB, Abernathy ME, Ball ZT. Histidine-directed arylation/alkenylation of backbone N–H bonds mediated by copper(II) J Am Chem Soc. 2016;138:7472–7475. doi: 10.1021/jacs.6b03390. [DOI] [PubMed] [Google Scholar]
- 118.Kazmaier U, Deska J. Peptide backbone modifications. Curr Org Chem. 2008;12:355–385. [Google Scholar]
- 119.Chatterjee J, Rechenmacher F, Kessler H. N-Methylation of peptides and proteins: An important element for modulating biological functions. Angew Chem, Int Ed. 2013;52:254–269. doi: 10.1002/anie.201205674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


