Abstract
Tissue matrix remodeling and fibrosis leading to loss of pulmonary arterial and right ventricular compliance are important features of both experimental and clinical pulmonary hypertension (PH). We have previously reported that transglutaminase 2 (TG2) is involved in PH development while others have shown it to be a cross-linking enzyme that participates in remodeling of extracellular matrix in fibrotic diseases in general. In the present studies, we used a mouse model of experimental PH (Sugen 5416 and hypoxia; SuHypoxia) and cultured primary human cardiac and pulmonary artery adventitial fibroblasts to evaluate the relationship of TG2 to the processes of fibrosis, protein cross-linking, extracellular matrix collagen accumulation, and fibroblast-to-myofibroblast transformation. We report here that TG2 expression and activity as measured by serotonylated fibronectin and protein cross-linking activity along with fibrogenic markers are significantly elevated in lungs and right ventricles of SuHypoxic mice with PH. Similarly, TG2 expression and activity, protein cross-linking activity, and fibrogenic markers are significantly increased in cultured cardiac and pulmonary artery adventitial fibroblasts in response to hypoxia exposure. Pharmacological inhibition of TG2 activity with ERW1041E significantly reduced hypoxia-induced cross-linking activity and synthesis of collagen 1 and α-smooth muscle actin in both the in vivo and in vitro studies. TG2 short interfering RNA had a similar effect in vitro. Our results suggest that TG2 plays an important role in hypoxia-induced pulmonary and right ventricular tissue matrix remodeling in the development of PH.
Keywords: pulmonary hypertension, transglutaminase 2, tissue fibrosis, pulmonary remodeling, right ventricular remodeling
primary manifestations of pulmonary hypertension (PH) include decreased compliance (increased stiffness) of both the pulmonary vasculature (40, 43) and right ventricle (RV) of the heart (1, 32). The decreased compliance of the pulmonary artery (PA) is an important predictor of mortality in heart failure with diastolic dysfunction in patients with PH (2). Although other mechanisms are possible, the most likely contributor to the tissue stiffness is the presence of tissue matrix changes that include the development of fibrosis (32, 42). We and others have previously identified the development of fibrosis in RVs of animals with experimental PH (30, 34, 44, 47). In experimental rodent models of PH the vascular and ventricular tissue stiffness has been attributed to deposition of collagen and development of fibrosis (32, 37, 43). Fibroblast activation to myofibroblast transdifferentiation, which exhibits de novo expression of α-smooth muscle actin (α-SMA), is implicated in the tissue matrix remodeling and stiffness (16). Protein cross-linking also participates in the tissue matrix changes, which include posttranslational modification of extracellular matrix proteins (16). Smooth muscle proliferative processes have received considerable attention as contributors to vascular remodeling in PH, but little is known about how fibrogenesis and protein cross-linking occur in the tissue matrix remodeling of this disease.
We have previously reported marked elevations of both transglutaminase 2 (TG2) expression and activity in lungs of mice with chronic hypoxia-induced (46) and Sugen 5416/hypoxia (SuHypoxia)-induced PH (9) and in rats with chronic hypoxia-induced and monocrotaline-induced PH (46). TG2 is a multifunctional enzyme that covalently modifies proteins, and the resulting posttranslationally modified products are largely resistant to proteolysis, resulting in extracellular matrix stability and tissue rigidity (10, 23). TG2 has been shown to mediate incorporation of serotonin (serotonylation) into vascular proteins, such as fibronectin (Fn), and to participate in vascular cell function (28, 29). Since TG2 is also known to stimulate fibrosis (38, 41) and cross-linking between glutamine and lysine residues of proteins facilitating the formation of ε-(γ-glutamyl)-lysine isopeptide bonds (23), we undertook the present study to determine whether TG2 might be responsible for the development of tissue fibrosis and protein cross-linking in experimental PH. As a surrogate marker for tissue fibrosis we used type 1 collagen (Col1), which is known to participate in PH pathology (12, 21). Col1 is among the matrix proteins that may be synthesized (41) and cross-linked (11) by TG2. Development of tissue fibrosis in various diseases such as idiopathic pulmonary fibrosis (25), interstitial kidney disease (13), and dermatologic disorders (26) has been attributed to elevation of TG2 activity. Because of the importance of tissue stiffness in PH we sought to determine whether a relationship exists between elevated TG2 and tissue fibrosis and matrix protein cross-linking in experimental PH. For in vivo studies, we used mice exposed to SuHypoxia and for in vitro studies, we used human RV cardiac and PA adventitial fibroblasts exposed to hypoxia, which we have found to regulate TG2 also in the absence of Sugen 5416 (29, 46). The mice from which we obtained tissues for these studies were the same ones for which we previously demonstrated physiologic changes of increased pulmonary arterial pressure and RV hypertrophy with exposure to SuHypoxia (9). Additionally, we have reported that the elevated pressures were reduced by treatment with the TG2 inhibitor, ERW1041E, in these animals (9). The results of our present studies collectively support a role for TG2 in the development of tissue matrix changes in PH.
MATERIALS AND METHODS
Animal model.
C57BL/6 adult male mice 6−8 wk of age (Charles River Laboratories, Wilmington, MA) were used for the study in accordance with the Tufts University Institutional Animal Care and Use Committee-approved protocol. RV systolic pressure measurements were recorded and followed by exsanguination while mice were anesthetized as reported (9). Lung and cardiac tissue obtained from mice placed in normobaric hypoxic [inspired O2 fraction () 10.5%] chambers (Biospherix, Parish, NY) for 3 wk and administered 200-μl subcutaneous weekly injections of 20 mg/kg Sugen 5416 (Tocris Bioscience, Minneapolis, MN), as previously reported (9), were used. Similar mice exposed to vehicle plus normoxia (21% O2) for 3 wk were used as controls.
Reagents.
2-[(3-Bromo-4,5-dihydro-isoxazol-5-ylmethyl)-carbamoyl]-pyrrolidine-1-carboxylic acid quinolin-3-ylmethyl ester (ERW1041E) is an active site-directed, small molecule and selective inhibitor of TG2 activity (45). ERW1041E was dissolved in 20% dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) in water and used as previously described in in vivo (9) and in vitro (29) experiments.
Cell culture.
Deidentified human primary cardiac fibroblasts were obtained from discarded hearts of both male and female recipient patients, ages 33–67 yr, receiving cardiac transplants at Tufts Medical Center (14). Two were of Caucasian descent, and one was of Hispanic descent. All patients had nonischemic dilated cardiomyopathy of unknown etiology. Human PA adventitial fibroblasts were purchased from a commercial supplier (cat. no. 3120; ScienCell Research Laboratories, Carlsbad, CA). Both sets of fibroblasts were cultured in fibroblast growth medium (Lonza, Portsmouth, NH) supplemented with growth factors (Lonza), fetal bovine serum (FBS; Sigma), and antibiotics (Sigma). Cell passages 2–5 were used, and cellular purity was assessed by morphological appearance under Axio light microscopy (Carl Zeiss) and by immunofluorescence staining for α-SMA (cat. no. sc-32251; Santa Cruz Biotechnology, Dallas, TX).
Stable transfection of fibroblasts.
Fibroblasts were grown to 70% confluence and transfected with 20 nM final concentration of TG2 stealth short interfering RNA (siRNA; cat. no. 1299001; Thermo Fisher Scientific, Waltham, MA) and control siRNA (Thermo Fisher Scientific) using Lipofectamine 2000 reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, fibroblasts were transfected with siRNA for 6 h in growth media without antibiotics and then incubated overnight in reduced serum (0.2% FBS) medium containing antibiotics. Transfected cells were then exposed to normoxia (95% room air and 5% CO2) or hypoxia (3% O2, 5% CO2, and balance N2) in tightly sealed humidified modular incubator chambers (Billups-Rothenberg, Del Mar, CA) at 37°C.
Cell treatment.
Fibroblasts were grown to ~70% confluence and then incubated overnight with reduced serum in media containing antibiotics. Cells were next pretreated for 1 h with vehicle (DMSO) or TG2 inhibitor (ERW1041E) and then exposed to normoxia or hypoxia in tightly sealed humidified modular incubator chambers at 37°C.
RNA isolation and quantitative real-time polymerase chain reaction analysis.
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer’s instructions. In brief, 1 μg of total RNA was reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). The quantitative real-time polymerase chain reaction (PCR) analyses were performed using the SYBR Green Master Mix (Thermo Fisher Scientific) method on an ABI Prism 7900 Sequence Detection System (Thermo Fisher Scientific). Samples were quantified in triplicate using mouse and human specific primer sets (IDT Technologies, Coralville, IA; Table 1). The cycle threshold (Ct) values were normalized using 18S ribosomal RNA as an internal standard and analyzed by the ΔΔCt method for relative quantification as previously described (29).
Table 1.
Primers for quantitative PCR
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| Mouse | ||
| TG2 | GGAGATCCAGGGTGACAAGA | CAGAGAAAGGCTCCAGGTTG |
| TGF-β1 | CACCGGAGAGCCCTGGATA | TGCCGCACACAGCAGTTC |
| Col1 | AAGGGTCCCTCTGGAGAACC | TCTAGAGCCAGGGAGACCCA |
| Fn | ATGTGGACCCCTCCTGATAGT | GCCCAGTGATTTCAGCAAAGG |
| α-SMA | GGCATCCACGAAACCACCTA | CACGAGTAACAAATCAAAGC |
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| Human | ||
| TG2 | ATGAGAAATACCGTGACTGCCTTAC | CAGCTTGCGTTTCTGCTTGG |
| TGF-β1 | CGAGCCTGAGGCCGACTAC | TCGGAGCTCTGATGTGTTGAA |
| Col1 | GTCGAGGGCCAAGACGAAG | CAGATCACGTCATCGCACAAC |
| Fn | AAACCAATTCTTGGAGCAGG | CCATAAAGGGCAACCAAGAG |
| α-SMA | CAAAGCCGGCCTTACAGAG | AGCCCAGCCAAGCACTG |
| 18S | CAAAGCCGGCCTTACAGAG | AGCCCAGCCAAGCACTG |
Protein extraction and immunoblot analysis.
Tissues were homogenized and fibroblasts were lysed and processed for SDS-PAGE as previously reported (29). Immunoblotting was performed using antibodies against target protein TG2 (cat. no. sc-20621; Santa Cruz Biotechnology), serotonin (cat. no. S5545; Sigma), Col1 (cat. no. ab34710; Abcam, Cambridge, MA), fibronectin (Fn; cat. no. sc-9068; Santa Cruz Biotechnology), α-SMA (cat. no. sc-32251; Santa Cruz Biotechnology), and β-actin (cat. no. 4970; Cell Signaling Technology, Danvers, MA). The protein bands were detected using horseradish peroxidase-tagged secondary antibodies (Santa Cruz Biotechnology). Nε-(γ-glutamyl)-lysine (cat. no. ab424; Abcam) protein bands were detected using goat anti-mouse IgM µ-chain horseradish peroxidase-tagged secondary antibody (cat. no. ab97230; Abcam). Enhanced chemiluminescent Western blotting substrate (Thermo Fisher Scientific) was used to visualize the protein bands. Densitometry analysis was performed using Un-Scan-It gel analysis software (Silk Scientific, Orem, UT; 29).
Statistics.
Statistical analyses were performed using Student’s t-test for comparisons between 2 groups and Kruskal-Wallis one-way ANOVA for comparisons between >2 groups using SigmaPlot 12.5 software (Systat Software, San Jose, CA). In figure legends, n refers to sample number. P < 0.05 was considered statistically significant.
RESULTS
SuHypoxia upregulates fibrogenic markers in mouse lung and RV tissues.
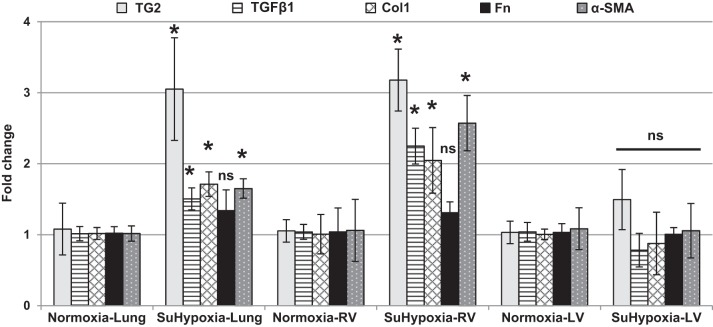
We have previously reported that in addition to producing PH, exposure of mice to SuHypoxia for 3 wk elevates TG2 expression and activity in lungs of the exposed mice compared with normoxic controls (9). In addition to these findings we report that quantitative PCR analysis of lung and cardiac tissues from the same mice exposed to SuHypoxia in our previous study (9) shows significant upregulation of TG2 and key fibrogenic markers that include transforming growth factor-β1 (TGF-β1), Col1, and α-SMA, but not fibronectin (Fn), mRNA in lungs and RVs (Fig. 1), indicating enhanced tissue fibrosis compared with room air-exposed controls. In contrast, TG2 and the tested fibrogenic markers were not significantly affected by SuHypoxia in left ventricular (LV) tissues (Fig. 1). Additionally, our studies showed that TG2 activity (as discussed below) was also found to be significantly elevated in SuHypoxic RVs similar to that found in lungs (9).
Fig. 1.
Fibrogenic marker and TG2 mRNA expression is upregulated in SuHypoxia-exposed mouse lung and RV tissues. Quantitative PCR analysis demonstrates the effect of normoxia (21% O2) and SuHypoxia (10.5% O2) on transglutaminase 2 (TG2), transforming growth factor-β1 (TGF-β1), type 1 collagen (Col1), fibronectin (Fn), and α-smooth muscle actin (α-SMA) levels in mouse lung, right ventricular (RV), and left ventricular (LV) tissues. Bar graphs demonstrate average fold change in mRNA expression normalized to mouse 18S ribosomal RNA by ΔΔCt method performed in triplicates. Data are presented as means ± SD. Statistical analysis was performed by t-test. P < 0.05 was considered statistically significant; ns, not significant; n = 4–6/group. *Significantly different compared with vehicle+normoxia control.
Pharmacological inhibition of TG2 activity attenuates the SuHypoxia-induced TG2 cross-linking activity and fibrogenic phenotype in mouse lung tissue.
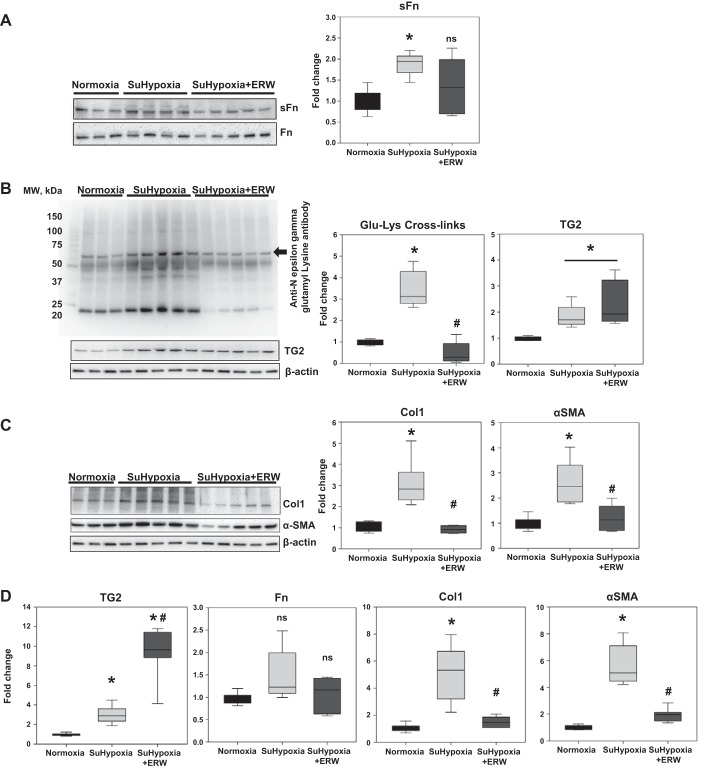
To test the hypothesis that TG2 activity has functional relevance to the fibrogenic phenotype in SuHypoxic lung tissue, we used SuHypoxia-exposed mice treated with or without the TG2 inhibitor, ERW1041E, as previously described (9). We found that ERW1041E reduced but did not significantly block SuHypoxia-induced serotonylated fibronectin (sFn/Fn) levels (Fig. 2A; see discussion); however, the TG2 cross-linking product, ε-(γ-glutamyl)-lysine isopeptide bonds (Glu-Lys cross-links), was significantly reduced (Fig. 2B). TG2 protein expression was induced in response to SuHypoxia but was unaffected by ERW1041E treatment (Fig. 2B). Since Col1 constitutes a significant portion of tissue fibrosis in PH (12, 21), it was used as a surrogate marker for evaluating reduction of fibrosis by ERW1041E. Inhibition of TG2 activity did significantly reduce the SuHypoxia-induced protein (Fig. 2C) and mRNA (Fig. 2D) expressions of Col1 and α-SMA. Treatment with ERW1041E significantly stimulated TG2 (Fig. 2D) but did not affect Fn (Fig. 2D) mRNA expression in lung tissue.
Fig. 2.
Pharmacological inhibition of TG2 activity blocks SuHypoxia-induced serotonylation of fibronectin, TG2 cross-linking activity, and protein and mRNA expression of Col1 and α-SMA in mouse lung tissue. Representative Western blot analysis of lung protein extracts from mice exposed to normoxia (21% O2), SuHypoxia (10.5% O2), and SuHypoxia+ERW1041E (ERW) showing serotonylated fibronectin (sFn; 220 kDa) and total fibronectin (Fn; 220 kDa) levels (A); transglutaminase 2 (TG2) reaction product [60 kDa; Nε-(γ-glutamyl)-lysine isopeptide bonds; Glu-Lys cross-links; denoted by arrow], TG2 (78 kDa), and β-actin (45 kDa) levels (B); and (C) type 1 collagen (Col1; 130 kDa), α-smooth muscle actin (α-SMA; 42 kDa), and β-actin levels. Each lane corresponds to protein extracts from an individual mouse. MW, molecular weight. Box plots demonstrate fold change in protein expression normalized to total Fn or β-actin assessed by densitometry analysis. D: quantitative PCR analysis demonstrates the effect of normoxia, SuHypoxia, and SuHypoxia+ERW1041E on TG2, Fn, Col1, and α-SMA levels in mouse lung tissues. Box plots demonstrate average fold change in mRNA expression normalized to 18S ribosomal RNA by ΔΔCt method performed in triplicates. Data are presented as 25th to 75th percentiles (boxes), medians (lines), and 5th and 95th percentiles (whiskers). Statistical analysis was performed by one-way ANOVA (Tukey post hoc test). P < 0.05 was considered statistically significant; ns, not significant; n = 6/group. *Significantly different compared with vehicle+normoxia control. #Significantly different compared with vehicle+SuHypoxia.
Pharmacological inhibition of TG2 activity attenuates the SuHypoxia-induced TG2 cross-linking activity and fibrogenic phenotype in mouse RV tissue.
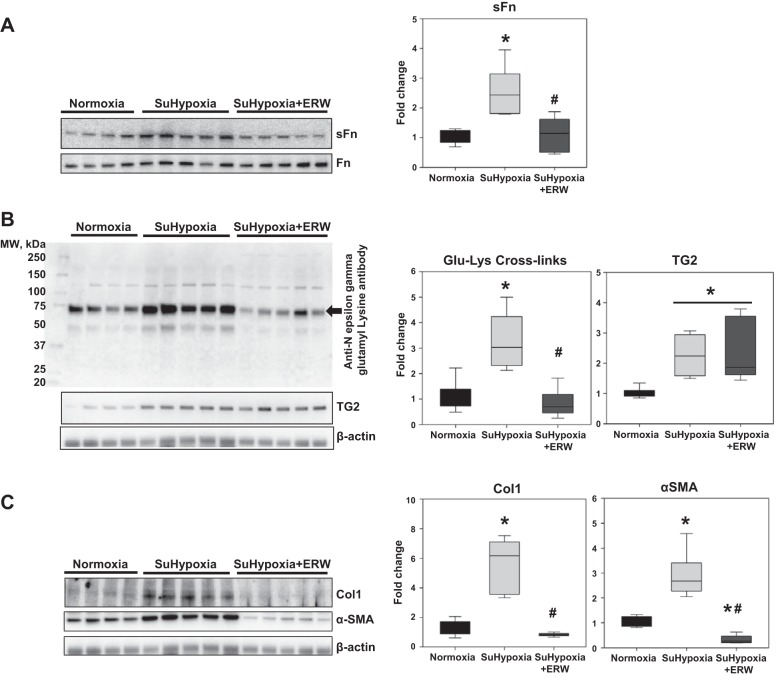
We previously reported that pharmacological inhibition of TG2 activity with ERW1041E significantly reduced RV systolic pressure in an intact mouse model of SuHypoxia (9). Given these findings, we assessed the effect of ERW1041E on TG2-mediated cross-linking activity and tissue fibrogenesis in SuHypoxia-exposed mouse RVs. Analysis of TG2 function and activity revealed significant reduction of SuHypoxia-induced sFn levels (Fig. 3A) and TG2-mediated isopeptide bonds (Glu-Lys cross-links; Fig. 3B) in RVs of mice treated with ERW1041E. Consistent with the findings in mouse lung tissue, we also found that ERW1041E did not affect SuHypoxia-induced TG2 expression (Fig. 3B), but blocked the SuHypoxia-induced expression of Col1 and α-SMA proteins in mouse RVs (Fig. 3C). Similar to that in the lung, the reduction of Col1 by ERW1041E in the RV is indicative of a block of cardiac tissue fibrosis.
Fig. 3.
Pharmacological inhibition of TG2 activity blocks SuHypoxia-induced serotonylation of fibronectin, TG2 cross-linking activity, and protein expression of Col1 and α-SMA in mouse RV tissue. Representative Western blot analysis of RV protein extracts from mice exposed to normoxia (21% O2), SuHypoxia (10.5% O2), and SuHypoxia+ERW1041E (ERW) showing serotonylation of fibronectin (sFn; 220 kDa) and total fibronectin (Fn; 220 kDa) levels (A); transglutaminase 2 (TG2) reaction product [60 kDa; Nε-(γ-glutamyl)-lysine isopeptide bonds; Glu-Lys cross-links; denoted by arrow], TG2 (78 kDa) and β-actin (45 kDa) levels (B); and type 1 collagen (Col1; 130 kDa), α-smooth muscle actin (α-SMA; 42 kDa), and β-actin levels (C). Each lane corresponds to protein extracts from an individual mouse. Box plots demonstrate fold change in protein expression normalized to total Fn or β-actin assessed by densitometry analysis. Data are presented as 25th to 75th percentiles (boxes), medians (lines), and 5th and 95th percentiles (whiskers). Statistical analysis was performed by one-way ANOVA (Tukey post hoc test). P < 0.05 was considered statistically significant; n = 6/group. *Significantly different compared with vehicle+normoxia control. #Significantly different compared with vehicle+SuHypoxia.
TG2 expression and fibrogenic phenotype are regulated by hypoxia in isolated human RV cardiac fibroblasts.
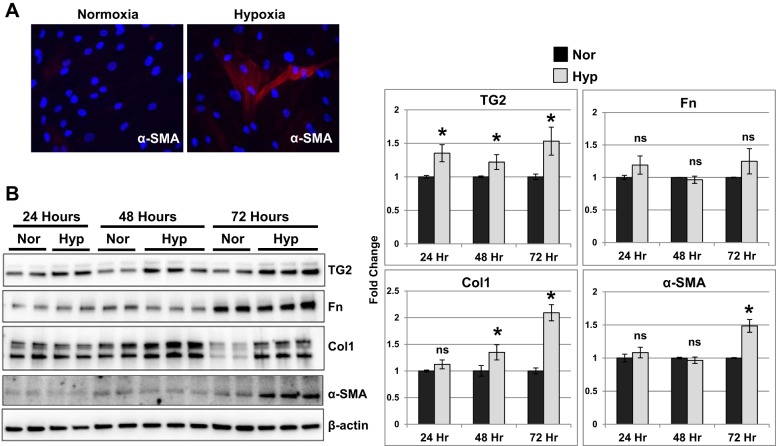
Next, using RV fibroblasts obtained from discarded hearts of transplanted subjects (non-PH patients), we performed in vitro studies to determine the sequence of events leading to enhanced expression of fibrogenic markers and TG2 as observed in the in vivo experiments. We selected fibroblasts for the studies since they are well known to be a primary regulator of tissue matrix (15). Fibroblast-to-myofibroblast transdifferentiation marker, α-SMA, was elevated in response to 24-h hypoxia exposure in cardiac fibroblasts (Fig. 4A).
Fig. 4.
Hypoxia exposure stimulated TG2, Col1, and myofibroblast transdifferentiation marker, α-SMA, expression in human RV cardiac fibroblasts. A: representative immunostained images for α-smooth muscle actin (α-SMA; red) and nucleus [4′,6-diamidino-2-phenylindole (DAPI); blue] in RV cardiac fibroblasts exposed to normoxia (21% O2) and hypoxia (3% O2) for 24 h. B: Western blots of protein extracts from RV cardiac fibroblasts exposed to normoxia (Nor) and hypoxia (Hyp) for 24, 48, and 72 h showing transglutaminase 2 (TG2; 78 kDa), fibronectin (Fn; 220 kDa), type 1 collagen (Col1; 147 kDa), α-smooth muscle actin (α-SMA; 42 kDa), and β-actin (45 kDa) levels. Representative blots of three independent experiments performed in duplicates/triplicates. Bar graphs demonstrate fold change in protein expression assessed by normalizing to β-actin levels at each time point by densitometry analysis. Data are presented as means ± SD. Statistical analysis was performed by t-test. P < 0.05 was considered statistically significant; ns, not significant; n = 6–9/group. *Significantly different compared with normoxia control.
We observed that TG2 expression levels were significantly elevated in response to hypoxia at 24-, 48-, and 72-h time points (Fig. 4B), whereas Fn levels did not significantly differ between normoxic and hypoxic conditions tested at these time points (Fig. 4B). Additionally, we observed that the fibrogenic marker, Col1, expression was significantly increased at 48 and 72 h of hypoxia, whereas α-SMA expression was significantly increased at 72 h of hypoxia exposure in cardiac fibroblasts (Fig. 4B).
TG2 activity regulates hypoxia-induced sFn, matrix Col1 accumulation, and fibroblast-to-myofibroblast transformation in RV cardiac fibroblasts.
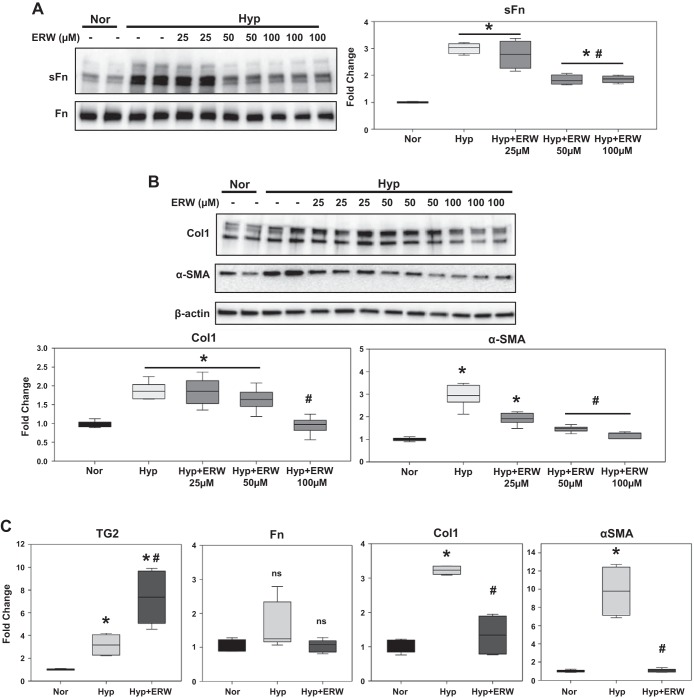
We previously demonstrated that the small-molecule inhibitor of TG2, ERW1041E, effectively blocked TG2 activity in in vitro studies using PA smooth muscle cells (29). To determine the influence of TG2 activity on hypoxia-mediated fibrogenesis, we assessed the effects of ERW1041E in cultured RV cardiac fibroblasts. A concentration-response effect showed that ERW1041E (50 and 100 µM doses) pretreatment significantly inhibited the hypoxia-induced expression of sFn (Fig. 5A) and α-SMA (Fig. 5B) proteins. In addition, significant inhibition of hypoxia-induced Col1 protein expression (Fig. 5B) was obtained with ERW1041E (100 µM dose).
Fig. 5.
Hypoxia-induced fibrogenesis is mediated by TG2 activity in human RV cardiac fibroblasts. Western blots of protein extracts from RV cardiac fibroblasts pretreated with vehicle control (DMSO) and increasing concentrations (25–100 µM) of TG2 inhibitor, ERW1041E (ERW), followed by normoxia (Nor; 21% O2) and hypoxia (Hyp; 3% O2) exposure for 72 h showing serotonylated fibronectin (sFn; 220 kDa) and total Fn (220 kDa) levels (A) and type 1 collagen (Col1; 147 kDa), α-smooth muscle actin (α-SMA; 42 kDa), and β-actin (45 kDa) levels (B). Representative of three independent experiments performed in duplicates/triplicates. Box plots demonstrate fold change in protein expression normalized to total Fn or β-actin assessed by densitometry analysis. C: quantitative PCR analysis demonstrates the effect of pretreatment with vehicle control (DMSO) and ERW (100 µM), followed by normoxia and hypoxia exposure for 72 h, on TG2, Fn, Col1, and α-SMA mRNA levels in RV cardiac fibroblasts. Box plots demonstrate average fold change in mRNA expression normalized to 18S ribosomal RNA by ΔΔCt method performed in triplicates. Data are presented as 25th to 75th percentiles (boxes), medians (lines), and 5th and 95th percentiles (whiskers). Statistical analysis was performed by one-way ANOVA (Tukey post hoc test). P < 0.05 was considered statistically significant; ns, not significant; n = 6–9/group. *Significantly different compared with vehicle+normoxia control. #Significantly different compared with vehicle+hypoxia control.
To further assess the effect of pharmacological inhibition of TG2 activity with higher concentration of ERW1041E (100 µM dose), we compared the mRNA expression of fibrosis-associated markers in RV cardiac fibroblasts. Similar to the in vivo mouse lung tissue findings (Fig. 2D), there was a marked increase in TG2 mRNA levels in response to ERW1041E treatment (Fig. 5C). In addition, we found no significant effect of both hypoxia and ERW1041E on Fn mRNA levels (Fig. 5C). Similar to in vivo findings, pretreatment with ERW1041E significantly reduced the hypoxia-induced mRNA expression of Col1 and α-SMA (Fig. 5C).
TG2 expression regulates hypoxia-induced sFn, matrix Col1 accumulation, and fibroblast-to-myofibroblast transformation in RV cardiac fibroblasts.
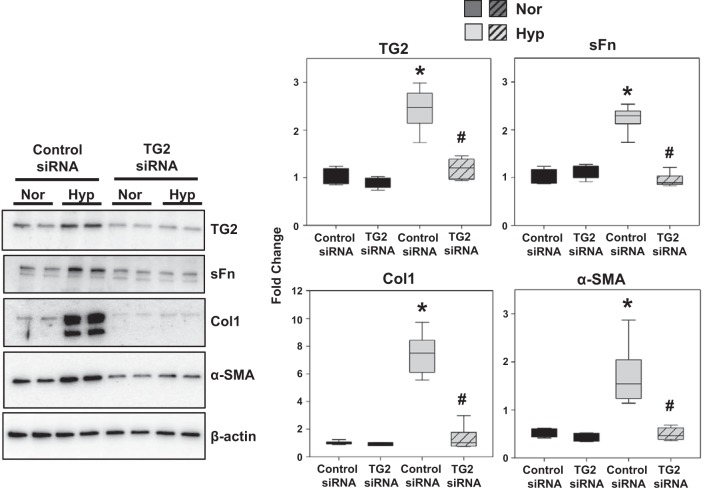
To confirm the influence of hypoxia-induced TG2 expression, we tested the effect of TG2 knockdown (Fig. 6) with siRNA transfection. RV cardiac fibroblasts transfected with control siRNA and TG2 siRNA were exposed to normoxia and hypoxia for comparing TG2 activity and the fibrogenic and contractile phenotype. In addition to our findings with pharmacological inhibition of TG2 activity, TG2 knockdown significantly abrogated hypoxia-induced expression of sFn, Col1, and α-SMA proteins (Fig. 6).
Fig. 6.
Hypoxia-induced fibrogenesis is mediated by TG2 expression in human RV cardiac fibroblasts. Western blots of protein extracts from RV cardiac fibroblasts transfected with control siRNA and TG2 siRNA and exposed to normoxia (Nor; 21% O2) or hypoxia (Hyp; 3% O2) exposure for 72 h showing transglutaminase 2 (TG2; 78 kDa), serotonylated fibronectin (sFn; 220 kDa), type 1 collagen (Col1; 147 kDa), α-smooth muscle actin (α-SMA; 42 kDa), and β-actin (45 kDa) levels. Representative blots of three independent experiments performed in duplicates. Box plots demonstrate fold change in protein expression normalized to total β-actin assessed by densitometry analysis. Data are presented as 25th to 75th percentiles (boxes), medians (lines), and 5th and 95th percentiles (whiskers). Statistical analysis was performed by one-way ANOVA (Tukey post hoc test). P < 0.05 was considered statistically significant; n = 6/group. *Significantly different compared with control siRNA+normoxia. #Significantly different compared with control siRNA+hypoxia.
Inhibition of TG2 activity reduces hypoxia-induced sFn and synthesis of Col1 and α-SMA in PA adventitial fibroblasts.
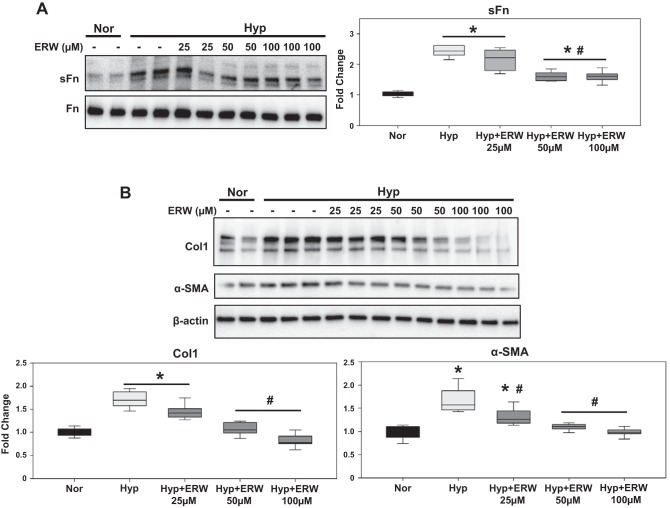
Next, to ascertain the relevance of TG2 activity in PA adventitial fibrosis, we tested human PA adventitial fibroblasts obtained commercially. Similar to the findings in RV cardiac fibroblasts, the concentration-response effect showed that pharmacological inhibition of TG2 activity with ERW1041E (50 and 100 µM doses) pretreatment significantly blocked the hypoxia-induced expression of sFn (Fig. 7A), Col1 (Fig. 7B), and α-SMA (Fig. 7B) proteins in PA adventitial fibroblasts.
Fig. 7.
Hypoxia-induced fibrogenesis is mediated by TG2 activity in human PA adventitial fibroblasts. Western blots of protein extracts from PA adventitial fibroblasts pretreated with vehicle control (DMSO) and increasing concentrations (25–100 µM) of TG2 inhibitor, ERW1041E (ERW), followed by normoxia (Nor; 21% O2) and hypoxia (Hyp; 3% O2) exposure for 72 h showing serotonylated fibronectin (sFn; 220 kDa) and total Fn (220 kDa) levels (A) and type 1 collagen (Col1; 147 kDa), α-smooth muscle actin (α-SMA; 42 kDa), and β-actin (45 kDa) levels (B). Representative blots of three independent experiments performed in duplicates/triplicates. Box plot demonstrating fold change in protein expression normalized to total Fn or β-actin assessed by densitometry analysis. Data are presented as 25th to 75th percentiles (boxes), medians (lines), and 5th and 95th percentiles (whiskers). Statistical analysis was performed by one-way ANOVA (Tukey post hoc test). P < 0.05 was considered statistically significant; n = 6–9/group. *Significantly different compared with vehicle+normoxia control. #Significantly different compared with vehicle+hypoxia.
DISCUSSION
It is now generally accepted that clinical features of PH of all categories include decreased compliance of the pulmonary vasculature (43) and RV of the heart (33). The pathobiology by which this occurs is subject to some debate, but it is likely that these conditions include remodeling of tissue matrix that in most cases is associated with tissue fibrosis. Tissue stiffness has been illustrated in various experimental models of PH (21, 44); however, the precise biologic/biochemical process by which the tissue stiffness occurs remains uncertain. One mechanism for production of tissue stiffness may involve enhanced lysyl oxidase (LOX) activity initiated by the LOX gene, which creates cross-linking of amine and lysine residues of proteins (7). Another proposed mechanism includes yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) and micro-RNA-130/301 (6, 22). Yet another possible mechanism that has remained previously unexplored in PH and discussed in this article is through activity of the multifunctional enzyme TG2, which is also known to cross-link proteins, including collagen and fibronectin, through glutamine and lysine residues (23, 28).
We have previously demonstrated marked elevations of TG2 expression and activity in lungs of experimental rodent models of PH (9, 46). Additionally, we have found TG2 to be elevated in sera of patients with pulmonary arterial hypertension (46). In the present communication we have utilized the SuHypoxia-exposed mouse model at a time when we have identified both elevated RV pressure and TG2 expression and activity (3-wk exposure; 9) to explore the question of whether the enhanced TG2 expression and activity we observed may be related to the fibrotic tissue remodeling (and perhaps stiffness) associated with the hypoxic exposure and the mechanism by which this may occur. Although gender relations favoring females have been reported in PH (5), we selected males for the studies because RV stiffness has been previously reported in male mice exposed to SuHypoxia (44) and we have previously reported elevation of TG2 in lung tissues from male mice exposed to SuHypoxia (9). In relation to known gender differences in serotonin-dependent models of PH (8), further understanding of the sex differences in TG2-mediated pathology may be useful in the development of effective therapeutic strategies. Our initial mRNA analyses of the effects of SuHypoxia showed that both mouse lung and RV tissue had a marked increase in TG2 expression and fibrogenesis (Fig. 1), whereas similar analyses of mouse LV tissue showed no effect (Fig. 1), indicating the differential cardiac remodeling that is expected in this model.
Our previous report showed significant block by ERW1041E in TG2 activity as measured by TG2 substrate, 5-biotinylamidopentylamine, incorporation in mouse lung tissue sections (9). Although our present studies demonstrate a trend toward reduced sFn levels, we did not observe a significant block in SuHypoxia-induced sFn in mouse lungs of mice treated with ERW1041E (Fig. 2A). Interestingly, we did find significant reduction of sFn levels in RVs of SuHypoxic mice treated with ERW1041E (Fig. 3). This discrepancy could be related to biological tissue heterogeneity or in vivo kinetics of ERW1041E in blocking sFn. In addition, TG2 belongs to a family of nine transglutaminases (10), and further studies are needed to test whether other members compensate the TG2 functional activity in ERW1041E-treated tissues. However, our in vitro studies showed that TG2 inhibition significantly blocked hypoxia-induced sFn in both RV cardiac (Fig. 5A) and PA adventitial (Fig. 7A) fibroblasts. Furthermore, we observed that TG2 mRNA expression was markedly increased in ERW1041E-treated mouse lung tissues (Fig. 2D), suggesting a possible positive feedback mechanism of TG2 gene transcription. Because of the limited availability of mouse RV tissue from SuHypoxic mice, we were unable to perform a similar comparative analysis of mRNA expression in the RVs. On this note, pharmacological inhibition of TG2 activity showed marked elevation of TG2 mRNA expression in hypoxia-exposed RV cardiac fibroblasts (Fig. 5C), suggesting a possible similar response to ERW1041E in the RV.
We then assessed whether fibrogenesis and cross-linking activity stimulated in mouse lung and RV by exposure to SuHypoxia are dependent on enhancement of TG2 activity. We found that production of the fibrogenic marker Col1, the myofibroblast differentiation marker α-SMA, and the cross-linking product glutamyl-lysine isopeptide bonds requires TG2 activity, indicating the participation of TG2 in both lung and RV remodeling. We also observed that control human RV cardiac and PA adventitial fibroblasts in culture demonstrated similar fibrogenic responses to hypoxia exposure and further showed that TG2 inhibition blocked the hypoxia-induced fibrogenic phenotype in both cell types. Given the ubiquitous expression of TG2 in several other cell types (35) including endothelial cells, smooth muscle cells, cardiomyocytes, immune cells, etc., future studies may unravel novel TG2-mediated pathways in PH. Our previous studies reported that TG2-mediated serotonylation and cross-linking activity are implicated in PA smooth muscle cell signaling (27) and function (29).
Telci et al. (41) have shown that stimulation of TG2 expression results in TGF-β1 induction and matrix protein synthesis via nitric oxide (NO)-dependent nuclear factor-κB activation in Swiss 3T3 fibroblasts. Relevant to these findings, Steppan et al. (39) reported that reduced NO-mediated decrease in nitrosylation and increase in cross-linking activity of TG2 mediate aortic stiffness in an aging rat model. Although these NO-dependent mechanisms remain to be tested, we report from the results of our studies that the enhanced TG2 expression and activity are closely associated with fibroblast differentiation, increased Col1 synthesis, and protein cross-linking in experimental PH. This may provide a mechanism through which matrix stiffness occurs in PH, and TG2 may be a target for treatment of pulmonary vascular and cardiac dysfunction of PH. Further studies related to determining tissue and cell physical properties may provide insights into showing TG2-mediated stiffness-dependent changes in the PH experimental models.
The actions of TG2 in tissue remodeling of PH may extend beyond those of alteration of tissue matrix. Recent evidence indicates that TG2-mediated cross-linked extracellular matrix protein collagen has been shown to induce nuclear translocation and transcriptionally activate YAP/TAZ in stromal fibroblasts promoting cancer cell proliferation (19). We have already demonstrated that TG2 participates in PA smooth muscle cell proliferation (29), and others have shown that inflammatory processes that occur in PH (31, 36) also may regulate TG2 expression (24). Furthermore, PH has been identified more recently to demonstrate an alteration in cellular metabolism with an upregulation of glycolytic metabolism (the so-called glycolytic shift; 3, 4, 20). With indications that there may be a close relationship between TG2 and glycolysis in tumor cell biology (17, 18), further exploration of the associations between glycolytic and mitochondrial metabolism, inflammation, stiffness-dependent cell-matrix signaling and mechanotransduction, and TG2 may be thought-provoking next steps in studies of PH.
In conclusion, taken together, we demonstrate in the present study that under experimental hypoxic conditions, TG2 expression and activity are involved in a number of profibrogenic mechanisms, which include matrix protein cross-linking, Col1 deposition, and fibroblast activation. Therefore disrupting TG2-mediated fibrogenesis may provide a viable strategy to counter both the vascular and cardiac ventricular matrix changes that are known to occur in PH.
GRANTS
This work was supported by National Institutes of Health Grants RO1-HL-107713 (B. L. Fanburg) and R01-DK-063158 (C. Khosla) and by the Gilead Sciences Research Scholars Program in Pulmonary Arterial Hypertension (K. C. Penumatsa).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.C.P., D.T., I.R.P., and B.L.F. conceived and designed research; K.C.P., R.R.W., and M.K. performed experiments; K.C.P. and M.K. analyzed data; K.C.P., D.T., I.R.P., N.K.K., C.K., N.S.H., and B.L.F. interpreted results of experiments; K.C.P. and M.K. prepared figures; K.C.P., D.T., and B.L.F. drafted manuscript; K.C.P., D.T., R.R.W., I.R.P., N.K.K., C.K., N.S.H., and B.L.F. edited and revised manuscript; K.C.P., D.T., R.R.W., M.K., I.R.P., N.K.K., C.K., N.S.H., and B.L.F. approved final version of manuscript.
REFERENCES
- 1.Alaa M, Abdellatif M, Tavares-Silva M, Oliveira-Pinto J, Lopes L, Leite S, Leite-Moreira AF, Lourenço AP. Right ventricular end-diastolic stiffness heralds right ventricular failure in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 311: H1004–H1013, 2016. doi: 10.1152/ajpheart.00202.2016. [DOI] [PubMed] [Google Scholar]
- 2.Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 3: 467–474, 2015. doi: 10.1016/j.jchf.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer SL, Fang YH, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ 3: 144–152, 2013. doi: 10.4103/2045-8932.109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assad TR, Hemnes AR. Metabolic dysfunction in pulmonary arterial hypertension. Curr Hypertens Rep 17: 20, 2015. doi: 10.1007/s11906-014-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin ED, Lahm T, West J, Tofovic SP, Johansen AK, Maclean MR, Alzoubi A, Oka M. Gender, sex hormones and pulmonary hypertension. Pulm Circ 3: 294–314, 2013. doi: 10.4103/2045-8932.114756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang YY, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Black SM, Fratz S, Fineman JR, Vargas SO, Haley KJ, Waxman AB, Chau BN, Fredenburgh LE, Chan SY. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Reports 13: 1016–1032, 2015. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 73: 1721–1732, 2013. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dempsie Y, MacLean MR. The influence of gender on the development of pulmonary arterial hypertension. Exp Physiol 98: 1257–1261, 2013. doi: 10.1113/expphysiol.2012.069120. [DOI] [PubMed] [Google Scholar]
- 9.DiRaimondo TR, Klöck C, Warburton R, Herrera Z, Penumatsa K, Toksoz D, Hill N, Khosla C, Fanburg B. Elevated transglutaminase 2 activity is associated with hypoxia-induced experimental pulmonary hypertension in mice. ACS Chem Biol 9: 266–275, 2014. doi: 10.1021/cb4006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GV, Mehta K. Transglutaminase regulation of cell function. Physiol Rev 94: 383–417, 2014. doi: 10.1152/physrev.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortunati D, Chau DY, Wang Z, Collighan RJ, Griffin M. Cross-linking of collagen I by tissue transglutaminase provides a promising biomaterial for promoting bone healing. Amino Acids 46: 1751–1761, 2014. doi: 10.1007/s00726-014-1732-0. [DOI] [PubMed] [Google Scholar]
- 12.Golob MJ, Wang Z, Prostrollo AJ, Hacker TA, Chesler NC. Limiting collagen turnover via collagenase-resistance attenuates right ventricular dysfunction and fibrosis in pulmonary arterial hypertension. Physiol Rep 4: e12815, 2016. doi: 10.14814/phy2.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson TS, Fisher M, Haylor JL, Hau Z, Skill NJ, Jones R, Saint R, Coutts I, Vickers ME, El Nahas AM, Griffin M. Transglutaminase inhibition reduces fibrosis and preserves function in experimental chronic kidney disease. J Am Soc Nephrol 18: 3078–3088, 2007. doi: 10.1681/ASN.2006070690. [DOI] [PubMed] [Google Scholar]
- 14.Kapur NK, Qiao X, Paruchuri V, Mackey EE, Daly GH, Ughreja K, Morine KJ, Levine J, Aronovitz MJ, Hill NS, Jaffe IZ, Letarte M, Karas RH. Reducing endoglin activity limits calcineurin and TRPC-6 expression and improves survival in a mouse model of right ventricular pressure overload. J Am Heart Assoc 3: e000965, 2014. [Erratum. J Am Heart Assoc 3(4): e000419, 2014.] doi: 10.1161/JAHA.114.000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5: 123, 2014. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 229: 298–309, 2013. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku BM, Lee CH, Lee SH, Kim SY. Increased expression of transglutaminase 2 drives glycolytic metabolism in renal carcinoma cells. Amino Acids 46: 1527–1536, 2014. doi: 10.1007/s00726-014-1714-2. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Donti TR, Agnihotri N, Mehta K. Transglutaminase 2 reprogramming of glucose metabolism in mammary epithelial cells via activation of inflammatory signaling pathways. Int J Cancer 134: 2798–2807, 2014. doi: 10.1002/ijc.28623. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Condello S, Yakubov B, Emerson R, Caperell-Grant A, Hitomi K, Xie J, Matei D. Tissue transglutaminase mediated tumor-stroma interaction promotes pancreatic cancer progression. Clin Cancer Res 21: 4482–4493, 2015. doi: 10.1158/1078-0432.CCR-15-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Riddle S, Zhang H, D’Alessandro A, Flockton A, Serkova NJ, Hansen KC, Moldvan R, McKeon BA, Frid M, Kumar S, Li H, Liu H, Caánovas A, Medrano JF, Thomas MG, Iloska D, Plecitá-Hlavatá L, Ježek P, Pullamsetti S, Fini MA, El Kasmi KC, Zhang Q, Stenmark KR. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation, 134: 1105–1121, 2016. doi: 10.1161/CIRCULATIONAHA.116.023171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AM, Suárez Velandia MM, Vitali S, Colas RA, Norris PC, Marinković A, Liu X, Ma J, Rose CD, Lee SJ, Comhair SA, Erzurum SC, McDonald JD, Serhan CN, Walsh SR, Tschumperlin DJ, Fredenburgh LE. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 1: e86987, 2016. doi: 10.1172/jci.insight.86987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4: 140–156, 2003. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 24.Luo R, Liu C, Elliott SE, Wang W, Parchim N, Iriyama T, Daugherty PS, Tao L, Eltzschig HK, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Transglutaminase is a critical link between inflammation and hypertension. J Am Heart Assoc 5: e003730, 2016. doi: 10.1161/JAHA.116.003730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen KC, Epa AP, Kulkarni AA, Kottmann RM, McCarthy CE, Johnson GV, Thatcher TH, Phipps RP, Sime PJ. Inhibition of transglutaminase 2, a novel target for pulmonary fibrosis, by two small electrophilic molecules. Am J Respir Cell Mol Biol 50: 737–747, 2014. doi: 10.1165/rcmb.2013-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsons AC, Yosipovitch G, Sheehan DJ, Sangüeza OP, Greenberg CS, Sane DC. Transglutaminases: the missing link in nephrogenic systemic fibrosis. Am J Dermatopathol 29: 433–436, 2007. doi: 10.1097/DAD.0b013e318156e43f. [DOI] [PubMed] [Google Scholar]
- 27.Penumatsa K, Abualkhair S, Wei L, Warburton R, Preston I, Hill NS, Watts SW, Fanburg BL, Toksoz D. Tissue transglutaminase promotes serotonin-induced AKT signaling and mitogenesis in pulmonary vascular smooth muscle cells. Cell Signal 26: 2818–2825, 2014. doi: 10.1016/j.cellsig.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penumatsa KC, Fanburg BL. Transglutaminase 2-mediated serotonylation in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306: L309–L315, 2014. doi: 10.1152/ajplung.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penumatsa KC, Toksoz D, Warburton RR, Hilmer AJ, Liu T, Khosla C, Comhair SA, Fanburg BL. Role of hypoxia-induced transglutaminase 2 in pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 307: L576–L585, 2014. doi: 10.1152/ajplung.00162.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preston IR, Sagliani KD, Warburton RR, Hill NS, Fanburg BL, Jaffe IZ. Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 304: L678–L688, 2013. doi: 10.1152/ajplung.00300.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rain S, Andersen S, Najafi A, Gammelgaard Schultz J, da Silva Gonçalves Bós D, Handoko ML, Bogaard HJ, Vonk-Noordegraaf A, Andersen A, van der Velden J, Ottenheijm CA, de Man FS. Right ventricular myocardial stiffness in experimental pulmonary arterial hypertension: relative contribution of fibrosis and myofibril stiffness. Circ Heart Fail 9: e002636, 2016. doi: 10.1161/CIRCHEARTFAILURE.115.002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT, Dorfmüller P, Guignabert C, Humbert M, Macdonald P, Dos Remedios C, Postmus PE, Saripalli C, Hidalgo CG, Granzier HL, Vonk-Noordegraaf A, van der Velden J, de Man FS. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 128: 2016–2025, 2013. doi: 10.1161/CIRCULATIONAHA.113.001873. [DOI] [PubMed] [Google Scholar]
- 34.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 115: 176–188, 2014. doi: 10.1161/CIRCRESAHA.113.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sane DC, Kontos JL, Greenberg CS. Roles of transglutaminases in cardiac and vascular diseases. Front Biosci 12: 2530–2545, 2007. doi: 10.2741/2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 10: 6, 2009. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreier D, Hacker T, Song G, Chesler N. The role of collagen synthesis in ventricular and vascular adaptation to hypoxic pulmonary hypertension. J Biomech Eng 135: 021018, 2013. doi: 10.1115/1.4023480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small K, Feng JF, Lorenz J, Donnelly ET, Yu A, Im MJ, Dorn GW II, Liggett SB. Cardiac specific overexpression of transglutaminase II (G(h)) results in a unique hypertrophy phenotype independent of phospholipase C activation. J Biol Chem 274: 21291–21296, 1999. doi: 10.1074/jbc.274.30.21291. [DOI] [PubMed] [Google Scholar]
- 39.Steppan J, Sikka G, Jandu S, Barodka V, Halushka MK, Flavahan NA, Belkin AM, Nyhan D, Butlin M, Avolio A, Berkowitz DE, Santhanam L. Exercise, vascular stiffness, and tissue transglutaminase. J Am Heart Assoc 3: e000599, 2014. doi: 10.1161/JAHA.113.000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan W, Madhavan K, Hunter KS, Park D, Stenmark KR. Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm Circ 4: 560–580, 2014. doi: 10.1086/677370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telci D, Collighan RJ, Basaga H, Griffin M. Increased TG2 expression can result in induction of transforming growth factor beta1, causing increased synthesis and deposition of matrix proteins, which can be regulated by nitric oxide. J Biol Chem 284: 29547–29558, 2009. doi: 10.1074/jbc.M109.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc 13: 276–284, 2016. doi: 10.1513/AnnalsATS.201509-599FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 1: 212–223, 2011. doi: 10.4103/2045-8932.83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Schreier DA, Hacker TA, Chesler NC. Progressive right ventricular functional and structural changes in a mouse model of pulmonary arterial hypertension. Physiol Rep 1: e00184, 2013. doi: 10.1002/phy2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watts RE, Siegel M, Khosla C. Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles. J Med Chem 49: 7493–7501, 2006. doi: 10.1021/jm060839a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei L, Warburton RR, Preston IR, Roberts KE, Comhair SA, Erzurum SC, Hill NS, Fanburg BL. Serotonylated fibronectin is elevated in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L1273–L1279, 2012. doi: 10.1152/ajplung.00082.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zungu-Edmondson M, Shults NV, Wong CM, Suzuki YJ. Modulators of right ventricular apoptosis and contractility in a rat model of pulmonary hypertension. Cardiovasc Res 110: 30–39, 2016. doi: 10.1093/cvr/cvw014. [DOI] [PMC free article] [PubMed] [Google Scholar]