Abstract
IL-4 and IL-13 are major T-helper cell (Th) 2 cytokines implicated in the pathogenesis of several lung diseases, including pulmonary fibrosis. In this study, using a novel repetitive intradermal bleomycin model in which mice develop extensive lung fibrosis and a progressive decline in lung function compared with saline-treated control mice, we investigated profibrotic functions of Th2 cytokines. To determine the role of IL-13 signaling in the pathogenesis of bleomycin-induced pulmonary fibrosis, wild-type, IL-13, and IL-4Rα-deficient mice were treated with bleomycin, and lungs were assessed for changes in lung function and pulmonary fibrosis. Histological staining and lung function measurements demonstrated that collagen deposition and lung function decline were attenuated in mice deficient in either IL-13 or IL-4Rα-driven signaling compared with wild-type mice treated with bleomycin. Furthermore, our results demonstrated that IL-13 and IL-4Rα-driven signaling are involved in excessive migration of macrophages and fibroblasts. Notably, our findings demonstrated that IL-13-driven migration involves increased phospho-focal adhesion kinase signaling and F-actin polymerization. Importantly, in vivo findings demonstrated that IL-13 augments matrix metalloproteinase (MMP)-2 and MMP9 activity that has also been shown to increase migration and invasiveness of fibroblasts in the lungs during bleomycin-induced pulmonary fibrosis. Together, our findings demonstrate a pathogenic role for Th2-cytokine signaling that includes excessive migration and protease activity involved in severe fibrotic lung disease.
Keywords: T-helper cell 2 cytokines, interleukin-13, migration, matrix metalloproteinase activity
pulmonary fibrosis is a devastating lung disease in which fibrotic lesions progressively expand because of excessive migration and accumulation of multiple mesenchymal cells involved in collagen deposition and irreversible scarring of the lungs (23, 36, 37, 44). The pathophysiology of pulmonary fibrosis is heterogeneous in part because of repetitive injury with an array of triggers, including allergens, chemicals, radiation, and environmental particles (38, 40, 43). Multiple growth factors, metalloproteinases, and profibrotic cytokines have been implicated in abnormal wound healing and excessive deposition of extracellular matrix (ECM) in the lungs. IL-4 and IL-13 are the major T-helper cell 2 (Th2) cytokines that are produced in excessive amounts and participate as key mediators of fibrotic lung remodeling (6, 19, 41, 46). Anti-IL-13 antibodies have been shown to improve lung function in a subset of patients with asthma (7). In patients with idiopathic pulmonary fibrosis (IPF), IL-13 has been shown to be elevated and inversely correlated with lung function (5, 19). Both IL-4 and IL-13 share a common cell surface receptor, IL-4Rα, as an essential signaling receptor and also downstream transcriptional regulators such as STAT6 (11, 22). Several studies have demonstrated that IL-13 plays a pathogenic role in tissue remodeling during chronic allergic diseases and also immunity against helminth infections (39, 45, 46). The IL-13Rα1 first binds to IL-13 and then recruits IL-4Rα to form a type II IL-4 receptor heterodimer. IL-4 also uses the same above receptor complex; however, the kinetics of signaling has been shown to be different between the above cytokines (25). Nevertheless, the role of IL-4 and IL-13 signaling in the pathogenesis of bleomycin-induced pulmonary fibrosis remains unclear and controversial (2, 3, 18, 20, 48). In particular, Il-13 has been shown to activate TGF-β and fizz1 activation to initiate fibrotic remodeling in the lungs (24). However, recent studies suggest bleomycin-induced fibrosis is independent of IL-13 at early stages and is largely mediated by TGF-β, IL-1β, and IL-17A to induce inflammation and fibrosis (42). These findings have resulted in a speculation that the profibrotic effects of IL-13 during bleomycin-induced injury are in part, if not completely, mediated by TGF-β, a potent regulator of extracellular matrix formation and tissue remodeling (12). In vitro stimulation with IL-13 significantly enhanced collagen production and TGF-β expression in IPF fibroblasts compared with nonfibrotic fibroblasts (32). Therefore, new studies are needed to clarify the role of Th2 cytokines in bleomycin-induced pulmonary fibrosis and to identify new therapeutic modalities for fibrotic lung diseases.
In the current study, we report a series of experiments designed to test whether the pathogenic role of IL-13 and IL-4Rα signaling is required for developing pulmonary fibrosis. Importantly, these studies provide new evidence on how IL-13-driven signaling contributes to pathological fibrotic remodeling. In particular, we demonstrate the mechanisms by which IL-13 signaling activates actin polymerization and phosphorylation of focal adhesion kinase (FAK) involved in excessive migration of fibroblasts and macrophages. Also, we show that the profibrotic effects of IL-13 are involved in pulmonary fibrosis via MMP2 and MMP9 activation. Thus, our study reveals an important role of IL-13-driven signaling in MMP activation and excessive migration of lung cells that may be involved in mesenchymal cell expansion and collagen accumulation in pulmonary fibrosis.
METHODS
Mice.
Wild-type (C57BL/6 and Balb/c mice; The Jackson Laboratories, Bar Harbor, ME), IL-4Rα knockout (IL-4Rα−/−, C57BL/6 background, inbred) and IL-13 knockout (IL-13−/−, Balb/c background, inbred) mice at 12–18 wk of age were used for all of the experiments. Mice were housed in the Cincinnati Children’s Hospital Medical Center animal facility approved by the American Association for the Accreditation of Laboratory Animal Care. All mice were maintained under aseptic conditions and received sterile food and water. Experiments were approved by the Institutional Animal Care and Use Committee.
Mouse primary lung mesenchymal cell cultures.
Primary lung mesenchymal cell cultures from C57BL/6 and IL-4Rα−/− mice were prepared as described (36). Briefly, lung tissues were collected in Iscove's Modified Dulbecco's Medium (IMDM) (Life Technologies, Waltham, MA) supplemented with 5% FBS (Life Technologies) and 1% each of penicillin and streptomycin (Life Technologies). Each lung tissue sample was finely minced and digested in 5 ml IMDM containing collagenase (2 mg/ml) and incubated at 37°C for 30 min. Digested tissues were passed through a 100-µm filter, washed two times by centrifugation at 100 g for 5 min, plated on 100-mm tissue culture plates in 10 ml IMDM containing 5% FBS, and incubated at 37°C and 5% CO2 to allow the cells to adhere and expand. Unbound cells were removed on day 3 of culture by washing cells with fresh IMDM. Adherent lung mesenchymal cells were continued in culture until confluence.
Primary peritoneal macrophage isolation.
Peritoneal macrophages were isolated as described (11). Three milliliters of solution of 4% thioglycollate medium (BD Bioscience, Sparks, MD) were injected intraperitoneally in C57BL/6 and IL-4Rα−/− mice. On day 5, mice were euthanized, and peritoneal macrophages were collected in 5 ml of PBS containing 2% fetal bovine serum (FBS). Cells were washed two times with RPMI 1640 medium (Life Technologies) containing 5% FBS and 1% each of penicillin and streptomycin and cultured overnight in 12-well plates. Nonadherent cells were removed, and the medium was replaced with fresh complete RPMI 1640 medium.
Mouse model of intradermal bleomycin-induced pulmonary fibrosis.
Wild-type or knockout mice (12–16 wk old) were administered with bleomycin (6 U/kg body wt) in 50 µl of saline solution intradermally in the center of the shaved back (within a 50-mm radius) for 5 days/wk for a total of 4 wk (29, 30, 36). Control mice were given equivalent volumes of saline. At day 28, mice were euthanized, and lung samples were collected for further analysis. Pulmonary fibrosis was evaluated by measuring lung function, histology, and Western blot analysis of collagen.
Histology and lung function measurements.
Lungs of wild-type and knockout mice were inflated and submerged in 10% neutral-buffered formalin. Formalin-fixed tissues were embedded in paraffin and cut into 5-µm-thick sections for staining with Masson trichrome as described (36, 37). All images were captured with a Leica DM2700 M bright-field microscope (Leica Microsystems, Buffalo Grove, IL). Lung fibrosis was assessed by measuring collagen levels in total lung lysates. Lung function measurements were performed using a computerized Flexi Vent system (SCIREQ, Montreal, Quebec, Canada) as described (27).
Immunofluorescence.
Immunofluorescence staining of F-actin was performed as described (11, 29, 37). Briefly, primary lung mesenchymal cells from wild-type mice were plated overnight on polylysine-coated coverslips (Sigma, St. Louis, MO). Cells were washed two times with IMDM containing 1% FBS, stimulated with IL-13 (10 ng/ml) in 1% FBS-containing media, and incubated at 37°C and 5% CO2 for 12 h. Cells were fixed with 4% paraformaldehyde for 20 min and washed three times with PBS. Fixed cells were permeabilized with 0.01% Triton X-100 (Sigma) for 5 min and washed with PBS. Cells were stained with anti-Alexa-Fluor-488-phalloidin (CST, no. 8878) in 1:20 dilution for 1 h followed by three washes with PBS. Nuclei were stained with DAPI for 5 min and mounted on microscope slides with mounting medium. Images were collected with a Nikon A1 Inverted Confocal Microscope (Nikon Instruments, Melville, NY), and data were analyzed using NIS-Element image software. Quantitative analyses were determined at five randomly selected sites from each slide as the average with four to five biological replicates for each condition.
RNA preparation and real-time PCR.
Total RNA was extracted from lung homogenates of female wild-type mice treated with saline and bleomycin by using the RNeasy Mini Kit (Qiagen Sciences, Valencia, CA) as described (11). Extracted RNA was converted to cDNA and used for real-time PCR (RT-PCR) performed with a CFX384 Touch Real-Time PCR detection system (Bio-Rad, Hercules, CA). Relative gene expression was quantified using SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA), and gene expression of IL-13 was normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT). The mouse RT-PCR primer sequences for IL-13, TGF-β, and hprt were the same as described in our previous studies (28).
Western blot analysis.
Primary lung mesenchymal cells from wild-type and knockout mice were grown in six-well plates to confluence in complete IMDM media. Cells were washed two times with IMDM containing 1% FBS and starved overnight in 1% FBS-containing media. Cells were treated with IL-13 (10 ng/ml) for 15 min, and cell lysates were prepared using radioimmunoprecipitation (RIPA) Lysis Buffer (Santa Cruz Biotechnology). The soluble fraction was separated by centrifugation at 10,000 g, and protein was estimated using the BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). Immunoblotting was performed as described (28). The soluble fractions (50 µg/lane) were separated on 4–12% SDS-PAGE under reducing conditions and transferred to nitrocellulose membranes (Millipore, Billerica, MA). The membranes were blocked by incubation for 1 h with 5% BSA at 37°C. Primary antibodies used at a 1:1,000 dilution were anti-collagen-1α, anti-phospho (p)-FAK (Tyr576/577), anti-FAK, anti-pERK, anti-ERK1, anti-cdc42, and anti-RhoA. After incubation with the corresponding primary antibodies for 1 h at 37°C, membranes were extensively washed with TBS containing 0.1% Tween 20. Appropriate secondary antibodies conjugated with peroxidase were used at a 1:10,000 dilution for 30 min at 37°C. Equal loading was confirmed using anti-GAPDH antibody (Biolegend, San Diego, CA). After the membranes were washed, the proteins bound to the secondary antibodies were visualized using ECL Prime Western Blotting Detection Reagent (Thermo Fisher Scientific).
Zymography.
Lung tissues from bleomycin-treated wild-type and IL-13−/− mice were homogenized using TissueLyser II (Qiagen) in RIPA Lysis Buffer, and the soluble fraction was separated by centrifugation at 10,000 g. Quantification of total protein was performed using the BCA kit, and 25 µg/lane of tissue lysates were loaded on 10% Gelatin Zymogram Gels (Invitrogen) (42). The gels were scanned to record the position of the colored molecular weight protein standards. According to the manufacturer’s instructions, the gels were denatured by incubation in denaturing buffer (Bio-Rad) for 30 min. Denaturation was followed by incubation in developing buffer at 37°C overnight. Gels were stained with Coomassie brilliant blue R-250 and destained in distilled water to reveal the pro and active forms of MMP2 and MMP9 within the gelatin matrix.
Migration assays.
The effect of IL-13 on the migration of primary lung mesenchymal cells and peritoneal macrophages was determined using the real-time Incucyte Live-Cell Imaging System (Essen BioScience, Ann Arbor, MI). Briefly, 50,000 cells/well were plated on fibronectin-coated (10 µg/ml) 96-well ImageLock plates (Essen Bioscience) in complete media. The confluent cell layers were scratched to generate a wound by using the Essen Wound Maker (Essen BioScience). Cells were washed two times and cultured in the presence or absence of IL-13 (10 ng/ml) in low-serum media. Wound images were continuously acquired at 2-h intervals by the IncuCyte Live-Cell imaging system for the total duration of 48 h. The data were analyzed using an integrated metric of relative cell density in the wound area (29, 37).
ELISA.
The TGF-β levels were estimated in bleomycin-treated lung lysates of wild-type and IL-13−/− mice by using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical analysis.
Data were analyzed using GraphPad Prism (version 6; GraphPad, La Jolla, CA). An unpaired Student’s t-test was used for comparisons between the two groups. One-way analysis of variance with Tukey’s multiple-comparison test was used for comparison of different experimental groups. A P value <0.05 was considered statistically significant.
RESULTS
Repetitive intradermal bleomycin induces IL-13 and severe fibrotic lung disease.
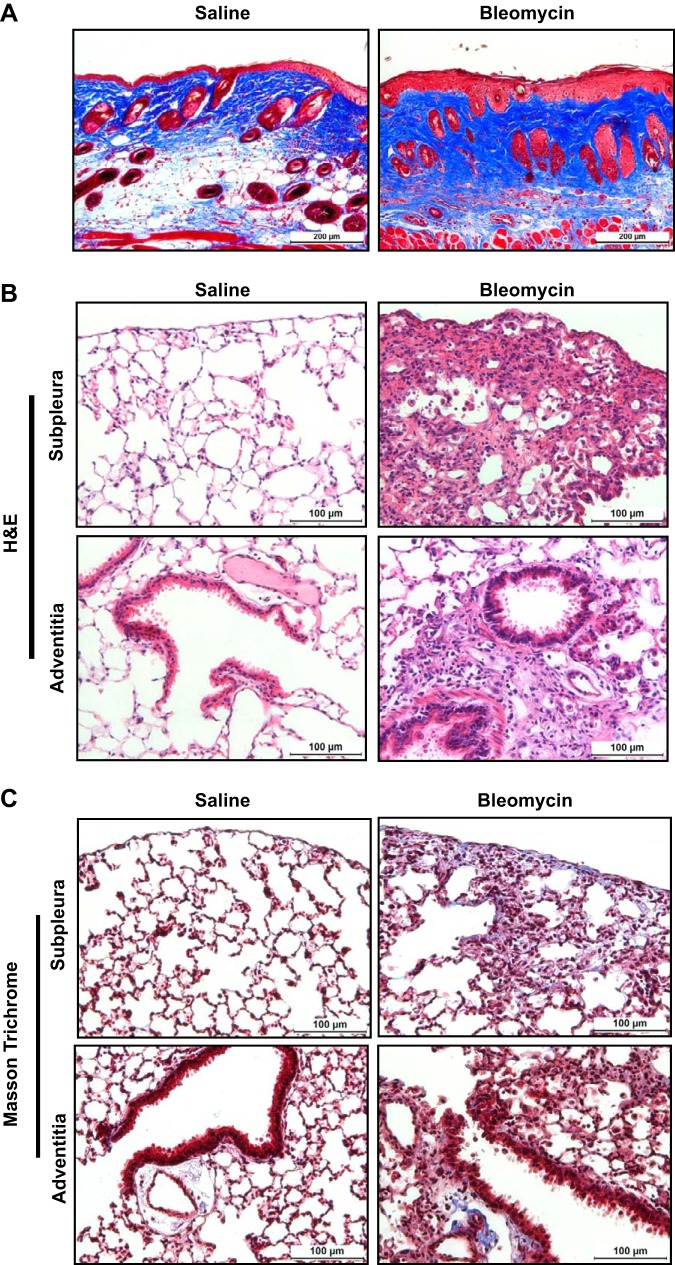
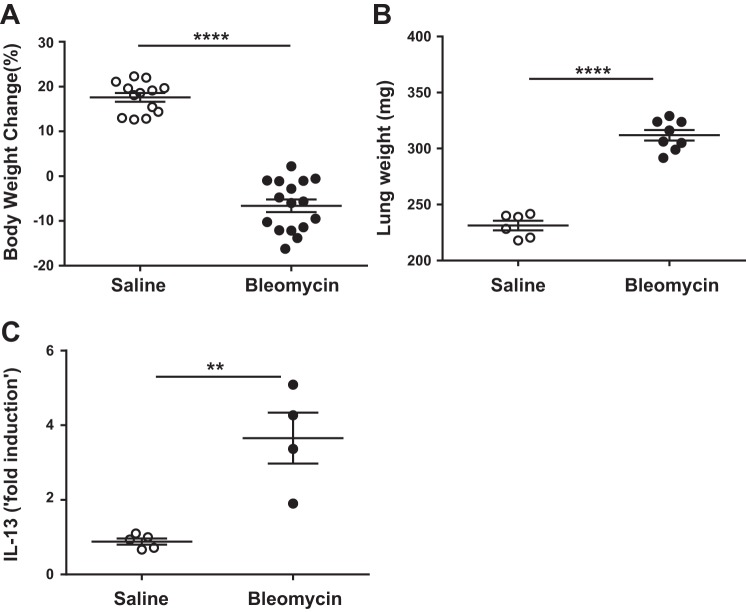
To assess whether repetitive intradermal bleomycin induces pulmonary fibrosis, bleomycin (6 U/kg body wt) was injected on the shaved back skin of mice via the intradermal route (5 days/wk) for a total of 4 wk, and the pathophysiological changes both in the lung and skin were measured. At the injection site, skin lesions showed a significant increase in collagen staining in the dermal areas with bleomycin treatment compared with saline-treated mice (Fig. 1A). Wild-type mice treated with bleomycin show a marked increase in inflammatory cell infiltration in both subpleural and adventitial lung lesions compared with saline-treated mice (Fig. 1B). Masson trichrome-stained lung sections were evaluated for collagen deposition, and increased collagen deposition in fibrotic lesions was found in bleomycin-treated mice compared with saline-treated control mice (Fig. 1C). As often seen, we observed a significant decrease in body weight in mice treated with bleomycin compared with saline treatment (Fig. 2A). Furthermore, the total lung weights were increased in bleomycin-treated mice compared with saline-treated controls (Fig. 2B), which was likely the result of the increased inflammatory response and fibrosis. These results are consistent with our recent study in which mice injected with intradermal bleomycin for 4 wk displayed a significant increase in lung inflammation and decline in lung function with a more than twofold increase in resistance and elastance compared with saline-treated control mice (36). Together, our findings suggest that repetitive intradermal bleomycin treatment induces severe fibrosis in lungs and cutaneous fibrosis at the site of skin injections. To determine whether IL-13 increases in our repetitive bleomycin injury model, we measured IL-13 transcript in lungs. Notably, IL-13 levels were significantly increased in bleomycin-treated mice compared with saline-treated control mice (Fig. 2C), suggesting a potential role of IL-13 in severe fibrotic lung disease.
Fig. 1.
Repetitive intradermal bleomycin induces inflammation and fibrosis in multiple organs. Wild-type mice were injected with saline or bleomycin (6 U/kg body wt; one time/day; 5 days/wk) via the id route for 4 wk. A: at the end of 4 wk, skin tissue of 6 mm in diameter was collected from the injected site, and 5-μm sections of paraffin-embedded skin tissues were stained with Masson’s trichrome to indicate collagen deposition (blue) in skin. All images were collected at the original magnification of ×10 (scale bars, 200 µm). B: 5-μm sections of paraffin-embedded lungs tissues were stained with hematoxylin and eosin (H&E) to indicate inflammation in the subpleural (top) and peribronchial (bottom) regions. All images were collected at an original magnification of ×40 (scale bars, 100 µm). C: 5-μm sections of paraffin-embedded lungs tissues were stained with Masson’s trichrome to indicate collagen deposition (blue) in subpleural (top) and peribronchial (bottom) regions. All images were collected at an original magnification of ×40 (scale bars, 100 µm). Data are representative of >4 independent experiments that produced similar results.
Fig. 2.
Repetitive intradermal bleomycin induces IL-13 and severe fibrotic lung disease. Wild-type mice were injected with saline and bleomycin (100 µg/day; 5 days/wk) via the id route for 4 wk. A: at the end of 4 wk, total body weights were measured at the beginning and end of bleomycin treatment, and the percentage of body weight change was calculated. B: total lung weights were measured. C: IL-13 transcript was measured using RT-PCR. Data are presented as means ± SE with 5–8 mice/group. Unpaired Student’s t-test was used to measure the significant difference between the groups, **P < 0.01 and ****P < 0.0001. All experiments were repeated >3 times with similar results.
Loss of IL-13 signaling attenuates pulmonary fibrosis.
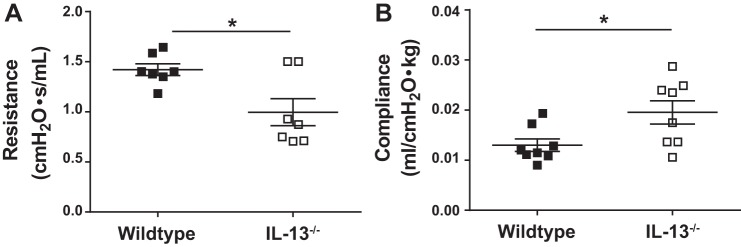
To investigate the role of IL-13 in pulmonary fibrosis, wild-type and IL-13 knockout mice were treated with bleomycin or saline for 4 wk. The lack of IL-13 resulted in decreased collagen staining in both subplural and adventitial areas of lung in IL-13-deficient mice compared with wild-type mice treated with bleomycin (Fig. 3A). Furthermore, Western blot analysis of the total lung collagen showed a significant decrease in collagen deposition in IL-13 knockout mice compared with wild-type mice treated with bleomycin (Fig. 3, B and C). Next, we measured changes in lung function by using the FlexiVent system. The lack of IL-13 was sufficient to attenuate lung function decline resulting from bleomycin-driven injury and pulmonary fibrosis. In particular, we observed a marked improvement in resistance and compliance of lungs in mice deficient for IL-13 compared with wild-type mice treated with bleomycin (Fig. 4, A and B). Thus, our new findings suggest that IL-13 functions as an important driver of pulmonary fibrosis in a chronic model of pulmonary fibrosis induced by intradermal bleomycin.
Fig. 3.
IL-13 deficiency is sufficient to attenuate pulmonary fibrosis. Wild-type and IL-13 knockout mice were treated with bleomycin via the id route for 4 wk. A: at the end of 4 wk, paraffin-embedded lung tissue sections were stained with Masson’s trichrome to indicate collagen deposition (blue) in subpleural (top) and peribronchial (bottom) regions. All images were collected at an original magnification of ×5 (scale bars, 250 µm). Dashed boxes in the ×5 images indicate the areas highlighted in the ×40 magnification (scale bars, 50 µm) and represented at the left corner of the original images. B: lung lysates were blotted with anti-collagen-1α (Col1A1), and equal loading was confirmed by GAPDH staining. C: quantification of Western blot signals using Phosphor Imager software. Data are presented as means ± SE (n = 5–6 mice/group), and unpaired Student’s t-test was used to measure the significant difference between the groups, *P < 0.05. All experiments were repeated 2 times with similar results.
Fig. 4.
IL-13 deficiency is sufficient to attenuate the lung function decline. Bleomycin (100 µg id) was given to WT and IL-13 knockout mice for 4 wk to induce pulmonary fibrosis. At the end of 4 wk, resistance (A) and compliance (B) were measured using a FlexiVent. Data are presented as means ± SE (n = 5–6 mice/group), and unpaired Student’s t-test was used to measure the significant difference between the groups, *P < 0.05. All experiments were repeated 2 times with similar results.
Loss of IL-4Rα-driven signaling attenuates pulmonary fibrosis.
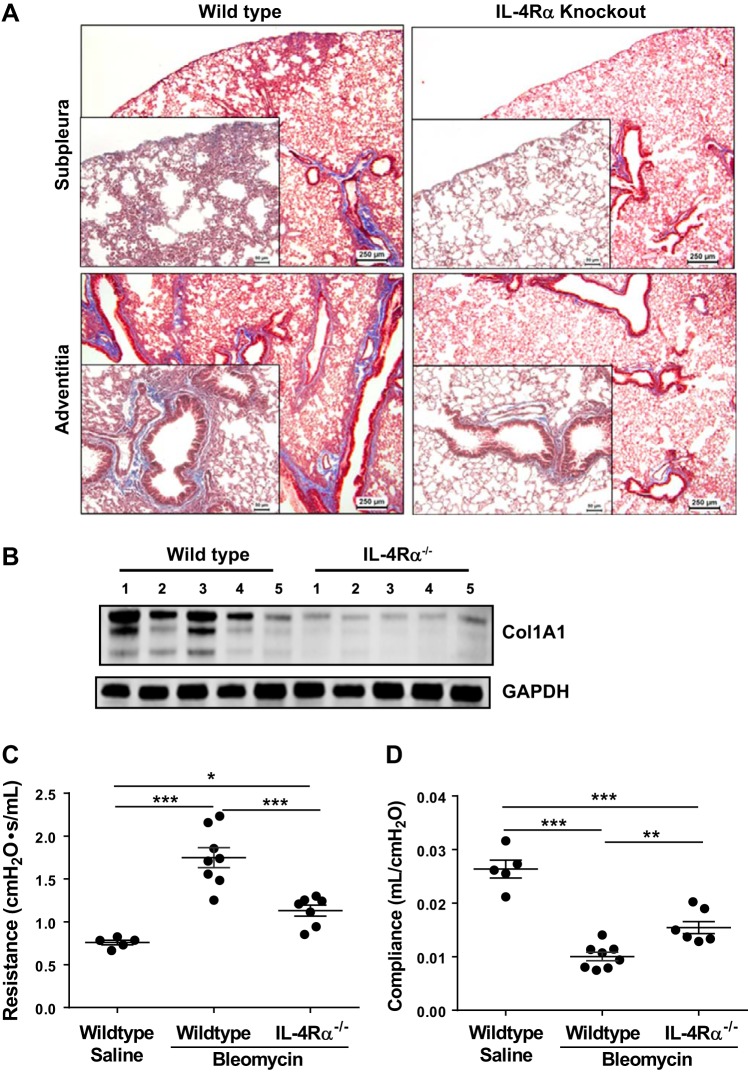
To investigate whether the loss of both IL-4 and IL-13 attenuates pulmonary fibrosis, wild-type and IL-4Rα knockout mice were treated with bleomycin for 4 wk. Masson trichrome-stained lung sections showed a significant decrease in collagen staining in both subplural and adventitial areas of lung in IL-4Rα-deficient mice compared with wild-type mice treated with bleomycin (Fig. 5A). Notably, we also observed a significant decrease in collagen levels in the lung lysates of IL-4Rα-deficient mice compared with wild-type mice treated with bleomycin (Fig. 5B). Next, we measured in vivo lung function and observed a marked improvement in resistance and compliance of lungs in mice deficient for IL-4Rα compared with wild-type mice treated with bleomycin (Fig. 5, C and D). Thus, IL-4Rα signaling is an important positive regulator in the pathogenesis of pulmonary fibrosis.
Fig. 5.
The lack of IL-4Rα-driven signaling is sufficient to attenuate pulmonary fibrosis. Wild-type and IL-4Rα knockout mice were treated with bleomycin via the id route for 4 wk. A: at the end of 4 wk, paraffin-embedded lung tissue sections were stained with Masson’s trichrome to indicate collagen deposition (blue) in subpleural (top) and peribronchial (bottom) regions. All images were collected at an original magnification of ×5 (scale bars, 250 µm). Dashed boxes in the ×5 images indicate the areas highlighted in the ×40 magnification (scale bars, 50 µm) and presented at the left corner of original images. B: lung lysates were blotted with anti-Col1A1, and equal loading was confirmed by GAPDH staining. C and D: resistance (C) and compliance (D) of the lungs were measured using a FlexiVent. Data are presented as means ± SE (n = 5–6 mice/group), and unpaired Student’s t-test was used to measure the significant difference between the groups, *P < 0.05, **P < 0.005, and ***P < 0.000.5. All experiments were repeated 2 times with similar results.
IL-13 activates macrophage and mesenchymal cell migration.
To determine whether IL-13 alters lung mesenchymal cell migration, primary lung mesenchymal cells of wild-type and IL-4Rα-deficient mice were treated with media or IL-13. Migration of mesenchymal cells was assessed by images that were taken at 2-h intervals for 48 h by using the IncuCyte live cell imaging system. Notably, the lung mesenchymal cells isolated from wild-type mice showed a significant increase in migration with IL-13 treatment compared with media alone (Fig. 6A). However, the lung mesenchymal cells of IL-4Rα knockout mice showed no significant change in their ability to migrate upon IL-13 treatment compared with media alone. Also, we observed no significant change in migration between wild-type and IL-4Rα-deficient lung mesenchymal cells treated with media alone (Fig. 6A). To investigate whether IL-13 can alter the migration of macrophages, peritoneal macrophages isolated from wild-type and IL-4Rα mice were treated with IL-13. Consistent with the findings with lung mesenchymal cells, the lack of IL-4Rα was sufficient to attenuate the migration of peritoneal macrophages by IL-13 (Fig. 6B). Thus, our new findings suggest that IL-13 plays a critical role in the migration of both lung mesenchymal cells and macrophages.
Fig. 6.

IL-13 augments mesenchymal cell and macrophage migration. Primary lung mesenchymal cells and peritoneal macrophages from WT and IL-4Rα knockout mice were prepared as described in methods. Cells were grown to confluence in complete media, and wounds were made using the 96-well Wound Maker. Cells were washed two times using 0.1% (lung mesenchymal cells) or 1% (peritoneal macrophages) serum-containing media and cultured in the presence and absence of IL-13 (10 ng/ml). Cell density in the wound area was expressed relative to the cell density outside of the wound area over time and presented as a percentage of relative wound density. A and B: graphs showing percentage of relative wound density at different time points in primary lung mesenchymal cells treated with media alone and IL-13 (10 ng/ml) (A) and peritoneal macrophages treated with IL-13 (10 ng/ml) (B). Each point represents the mean of 8 wells, with the vertical bars showing SE, **P < 0.001 and ****P < 0.00001. All experiments were repeated 2 times with similar results.
IL-13 induces F-actin polymerization in mesenchymal cell migration.
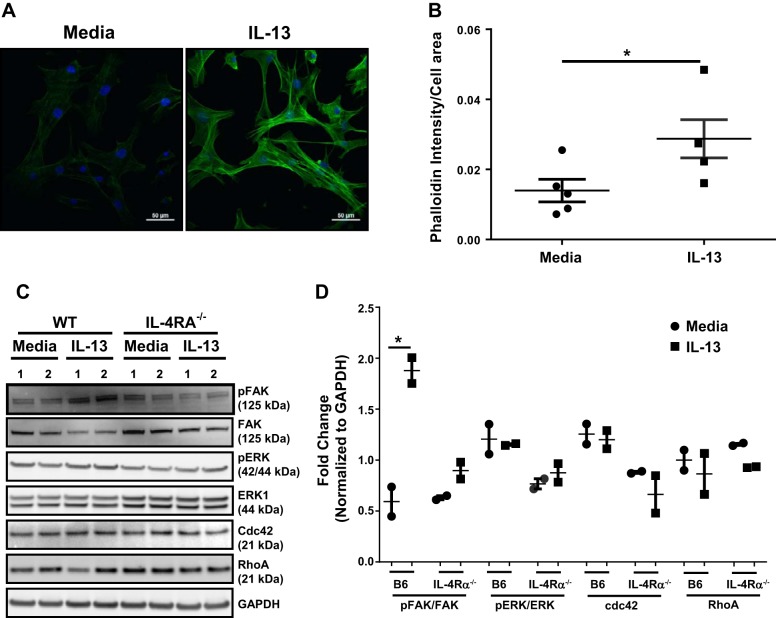
During migration, the formation of F-actin is essential to induce the formation of lamellipodia-like structures involved in cell migration (21, 47). To further understand the role of IL-13 in mesenchymal cell migration, we performed F-actin staining of primary lung mesenchymal cells treated with media or IL-13 by using anti-phalloidin Alexa-Fluor-488 and obtained immunofluorescence images by fluorescence microscopy. Notably, mesenchymal cells treated with IL-13 showed a significant increase in actin polarization compared with unstimulated cells at 12 h (Fig. 7, A and B). These new findings suggest that IL-13 functions as a critical regulator of actin polymerization in the formation of lamellipodia structures and augments mesenchymal cell migration. Multiple signaling intermediates have been shown to play an important role in the polymerization of F-actin (15, 34). To investigate the underlying signaling mechanisms by which IL-13-induces F-actin polymerization and migration, primary lung mesenchymal cells of wild-type and IL-4Rα knockout mice were treated with IL-13 for 15 min. Cell lysates were prepared to measure changes in FAK, ERK, CDC42, and Rho A and also the phosphorylation of ERK and FAK (Fig. 7, C and D). IL-13 treatment had no effect on the total protein levels of CDC42, Rho A, or phosphorylation of ERK in wild-type cells. However, treatment with IL-13 resulted in a significant increase in phosphorylation of FAK in wild-type cells. Notably, the loss of IL-13 signaling in IL-4Rα-deficient mesenchymal cells was sufficient to attenuate phosphorylation of FAK (Fig. 7, C and D). These data highlight the possibility that IL-13-driven phosphorylation of FAK signaling might play a critical role in F-actin polymerization and mesenchymal cell migration.
Fig. 7.
Mechanisms of IL-13-driven mesenchymal cell migration. A: primary lung mesenchymal cells from wild-type mice were plated on polylysine-coated coverslips overnight and stimulated with IL-13 (10 ng/ml) for 12 h in 0.1% FBS-containing media. Cells were fixed in 4% paraformaldehyde and stained with Alexa-Fluor-488-phalloidin. Images were collected using a Nikon A1 Inverted Confocal Microscope. Polymerized F-actin in cells was analyzed using NIS-Element image software. Nucleus, blue; phalloidin, green. Scale bar: 50 µm. B: quantification of phalloidin intensity/cell area using NIS-Element image software. Data are representative of 2 independent experiments and expressed as means ± SE C: primary lung mesenchymal cells from WT and IL-4Rα knockout mice were grown to confluence in complete media. Cells were starved overnight in 0.1% FBS containing media and treated with IL-13 (10 ng/ml) for 15 min. Cell lysates were prepared and blotted against phospho (p)-focal adhesion kinase (FAK), FAK, pERK, ERK1, cdc42, and RhoA antibodies. Equal loading was confirmed using GAPDH immunostaining. D: quantification of Western blot using ImageQuant LAS 4000 software (n = 2/group). Data are representative of 2 independent experiments and are expressed as means ± SE. Unpaired Student’s t-test was used to measure the significant difference, *P < 0.05 and **P < 0.01. All experiments were repeated 2 times with similar results.
IL-13 deficiency attenuates MMP2 and MMP9 activity.
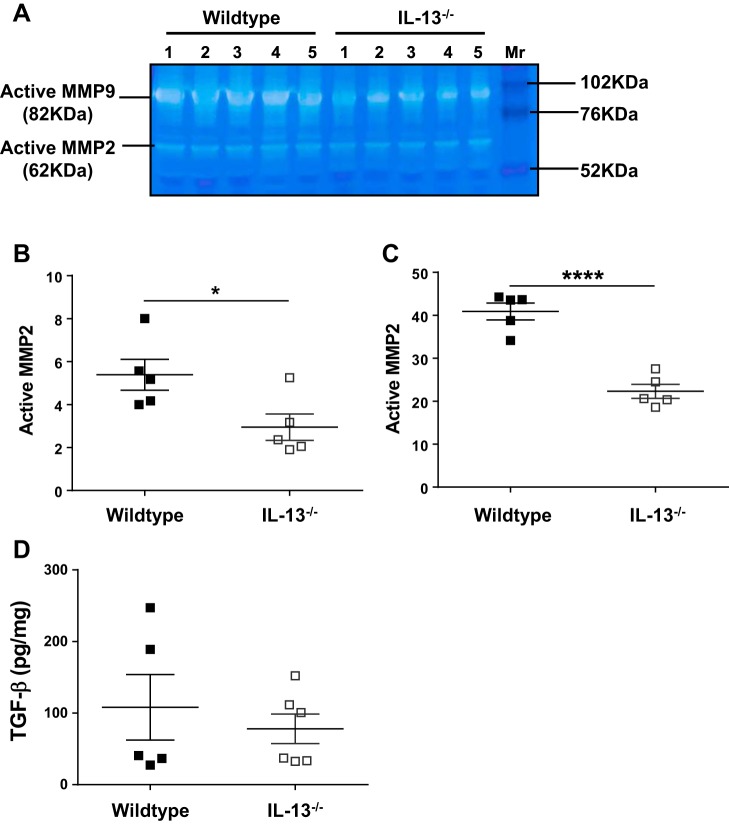
MMPs have been shown to play an important role in fibrotic remodeling in tissues (14, 28). To investigate possible mechanisms that led to the reduction in fibrosis in IL-13 knockout mice, we examined MMP activity in the lung lysates of mice treated with bleomycin. We observed a significant decrease in the levels of active MMP2 and MMP9 in lungs of mice deficient for IL-13 compared with the wild type (Fig. 8, A–C). Alterations in TGF-β levels involved in extracellular matrix synthesis may also impact pulmonary fibrosis. However, we observed no significant changes in TGF-β levels in IL-13 knockout mice compared with wild-type mice (Fig. 8D). Thus, the data suggest that IL-13 deficiency attenuated MMP2 and MMP12 activity but not TGF-β levels. Therefore, we speculate that a decrease in MMP2 and MMP9 activity may contribute to the reduction of pulmonary fibrosis observed in IL-13-deficient mice.
Fig. 8.
IL-13 deficiency attenuates matrix metalloproteinase (MMP)-2 and MMP9 activation in lungs. Wild-type and IL-13 knockout mice were treated id with bleomycin for 4 wk. A: lung lysates were homogenized, and soluble lysates (25/well) were subjected to gelatin zymography to identify active MMP2 and MMP9 on zymograms. B: quantification of active MMP2 was performed using Image-J analysis software. C: quantification of active MMP9 was performed using Image-J analysis software. D: TGF-β transcript levels were measured using RT-PCR. Data are presented as means ± SE (n = 5–6 mice/group), and unpaired Student’s t-test was used to measure the significant difference between the groups, *P < 0.05 and ****P < 0.0001. All experiments were repeated 2 times with similar results.
DISCUSSION
IL-13 is a Th2 cytokine that has been shown to play an essential role in immunity against helminth infections but promotes tissue fibrosis when produced in excess amounts during tissue injury and repair (45, 46). In the current study, we showed that IL-13 transcripts were elevated in mice repetitively treated with bleomycin via the intradermal route compared with saline-treated control mice. Also, we revealed a potential mechanism by which IL-13 mediates mesenchymal cell migration in vitro. Notably, the loss of IL-13 or IL-4Rα signaling was sufficient to attenuate bleomycin-induced pulmonary fibrosis in vivo. In particular, the decline in lung function and increase in collagen deposition were attenuated in mice deficient for IL-13 or IL-4Rα compared with wild-type mice. Our new findings have demonstrated that IL-13 plays a pathogenic role in pulmonary fibrosis induced by intradermal bleomycin administration. The role of IL-13 during bleomycin-induced fibrosis has remained controversial in part because of unknown mechanisms during bleomycin-induced injury and repair. Early profibrotic responses during bleomycin-driven injury include TNF-α, IL-17, and IL-1β (42). Later, the above responses need to be downregulated in part by counterregulatory processes mediated by Th2 cytokines. In support of this hypothesis, a recent study suggested that the loss of IL-13 had no significant effect on pulmonary fibrosis at the early stages of bleomycin-driven injury and fibrosis (42). It is possible that IL-13 might be playing an important role in severe pathological remodeling and collagen deposition at late time points during bleomycin-induced fibrosis. Furthermore, intradermal bleomycin resulted in uniform pathological changes in histology of all lung lobes compared with intratracheal treatment of bleomycin in mice. Importantly, we observed a significant increase in subpleural thickening and the loss of lung function in a mouse model of intradermal bleomycin-induced pulmonary fibrosis. In support, recent studies demonstrated that repetitive injury of lung with bleomycin was sufficient to induce more persistent and severe fibrotic lung disease compared with a single-dose administration of bleomycin (4, 9). It is possible (although not proven) that bleomycin administered via the intratracheal route induces more epithelial injury but only in the limited areas of the lung because of large airway surfaces to cover with small volumes administered. In contrast, intradermal delivery is more controlled and may cause a slow but prolonged release of bleomycin in circulation; therefore, we observed inflammation and fibrosis in all areas of the lung. These new data are consistent with our recent findings that fibrocytes augment subpleural thickening and severe fibrotic lung disease upon chronic administration of bleomycin via the intradermal route (36). The present study further demonstrated that IL-13- or IL-4Rα-driven signaling might be playing a pathogenic role during repetitive bleomycin-induced injury and pulmonary fibrosis. However, future studies are needed to determine the impact of bleomycin administered via the intradermal route compared with the intratracheal route on cellular damage, inflammation, and persistent fibrosis in the lung and other organs.
During bleomycin-induced fibrosis, both the adaptive and innate immune regulators have been shown to contribute to the pathogenesis of pulmonary fibrosis (1, 35). IL-13 is predominantly produced by hematopoietic cells but acts on both hematopoietic and nonhemaopoietic cells such as fibroblasts and macrophages during injury and repair. However, it is not clear how IL-13 regulates activation of fibroblasts and macrophages in pulmonary fibrosis. During bleomycin-induced lung injury and repair, the trafficking of mesenchymal cells and macrophages to the site of injury is mediated by several mediators and cytokines, including IL-13 (28, 42, 46). However, the mechanisms involved in the migration of fibroblasts or immune cells by IL-13 during abrupt tissue remodeling are not well studied. MMP, extracellular endopeptidases, play a pivotal role in fibrogenesis by activating multiple growth factors and also assist local infiltration of fibroblasts and macrophages in the adjacent fibrotic lesions through ECM degradation (17, 26, 28, 36). In support, previous studies showed that dysregulated action of MMPs implicated in IPF plays a central role in IPF pathogenesis (8, 28). Findings of this study suggests that IL-13 might play a critical role in regulating multiple MMPs involved in pulmonary fibrosis (28). We observed that IL-13 induces both mesenchymal cell and macrophage migration. In particular, IL-13 signals through a type II IL-4 receptor to activate mesenchymal cell migration. Furthermore, our studies demonstrate that cytoskeletal rearrangement of F-actin via phosphorylation of FAK is critical for IL-13-driven mesenchymal cell migration. Therefore, we speculate that IL-13 may function as a positive regulator of cell migration in the pathogenesis of pulmonary fibrosis. Furthermore, we observed no significant changes in TGF-β levels of IL-13-deficient mice compared with wild-type mice. This may suggest that IL-13-driven pulmonary fibrosis during intradermal bleomycin-induced fibrosis could be independent of TGF-β (13). Also, previous studies have shown that IL-13-deficient mice were protected from fluorescein isothiocyanate (20), schistosome-egg (31), and radiation-induced pulmonary fibrosis (6), suggesting a potential role of IL-13 signaling in pulmonary fibrosis. In support, recent studies have demonstrated that IL-13 and IL-13 pathway markers were upregulated in IPF (16, 33). Furthermore, in vivo administration of neutralizing antibodies against IL-13 was effective in restoring epithelial integrity and enhancing repair processes in a humanized experimental model of pulmonary fibrosis (33). However, clinical IPF often show mixed forms of inflammation, fibrosis severity, and Th2 cytokine responses (10, 33). Also, the current literature did not clearly establish unique and redundant functions between IL-4 and IL-13 in the pathogenesis of fibrotic lung disease. Therefore, these issues should be investigated further to dissect the role of Th2 cytokines in the pathogenesis of clinical IPF and also underlying mechanisms using relevant in vivo mouse models of pulmonary fibrosis.
In conclusion, the current study advances our understanding of IL-13-mediated induction of lung fibrosis in a novel mouse model of intradermal bleomycin-induced fibrosis. Our findings demonstrated a crucial role of IL-13 in mesenchymal cell and macrophage migration during wound healing, and that may depend on actin polymerization and phosphorylation of FAK. Our results validate and extend other studies that have also identified a critical role for IL-13 in the pathogenesis of pulmonary fibrosis and suggest the intradermal bleomycin model as a novel murine model to test anti-fibrotic therapeutics of the Th2 pathway.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 1R01-HL-134801 and 1R21-HL-133539 (S. K. Madala), a Department of Biotechnology Fellowship, a Government of India (R. K. Kasam) and Council of Scientific and Industrial Research Fellowship, and by the Government of India (V. Sontake).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.S., R.K.K., V.S., and S.K.M. performed experiments; B.S., R.K.K., V.S., T.A.W., and S.K.M. interpreted results of experiments; B.S. and S.K.M. prepared figures; B.S. and S.K.M. drafted manuscript; B.S., T.A.W., and S.K.M. edited and revised manuscript; B.S., R.K.K., V.S., T.A.W., and S.K.M. approved final version of manuscript; T.A.W. and S.K.M. conceived and designed research; S.K.M. analyzed data.
ACKNOWLEDGMENTS
We thank Dr. Ramakrishna Edukulla and the veterinary services at Cincinnati Children’s Hospital Medical Center for their help.
REFERENCES
- 1.Aliprantis AO, Wang J, Fathman JW, Lemaire R, Dorfman DM, Lafyatis R, Glimcher LH. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc Natl Acad Sci USA 104: 2827–2830, 2007. doi: 10.1073/pnas.0700021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, Keane MP. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 27: 419–427, 2002. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- 3.Blease K, Jakubzick C, Westwick J, Lukacs N, Kunkel SL, Hogaboam CM. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J Immunol 166: 5219–5224, 2001. doi: 10.4049/jimmunol.166.8.5219. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, Fong GH, Sakmar TP, Rafii S, Ding BS. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med 22: 154–162, 2016. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandriani S, DePianto DJ, N’Diaye EN, Abbas AR, Jackman J, Bevers J III, Ramirez-Carrozzi V, Pappu R, Kauder SE, Toy K, Ha C, Modrusan Z, Wu LC, Collard HR, Wolters PJ, Egen JG, Arron JR. Endogenously expressed IL-13Rα2 attenuates IL-13-mediated responses but does not activate signaling in human lung fibroblasts. J Immunol 193: 111–119, 2014. doi: 10.4049/jimmunol.1301761. [DOI] [PubMed] [Google Scholar]
- 6.Chung SI, Horton JA, Ramalingam TR, White AO, Chung EJ, Hudak KE, Scroggins BT, Arron JR, Wynn TA, Citrin DE. IL-13 is a therapeutic target in radiation lung injury. Sci Rep 6: 39714, 2016. doi: 10.1038/srep39714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corren J, Lemanske RF Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG. Lebrikizumab treatment in adults with asthma. N Engl J Med 365: 1088–1098, 2011. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 8.Dancer RC, Wood AM, Thickett DR. Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J 38: 1461–1467, 2011. doi: 10.1183/09031936.00024711. [DOI] [PubMed] [Google Scholar]
- 9.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 299: L442–L452, 2010. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DePianto DJ, Chandriani S, Abbas AR, Jia G, N’Diaye EN, Caplazi P, Kauder SE, Biswas S, Karnik SK, Ha C, Modrusan Z, Matthay MA, Kukreja J, Collard HR, Egen JG, Wolters PJ, Arron JR. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax 70: 48–56, 2015. doi: 10.1136/thoraxjnl-2013-204596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edukulla R, Singh B, Jegga AG, Sontake V, Dillon SR, Madala SK. Th2 Cytokines Augment IL-31/IL-31RA Interactions via STAT6-dependent IL-31RA Expression. J Biol Chem 290: 13510–13520, 2015. doi: 10.1074/jbc.M114.622126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med 12: 99–106, 2006. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 13.Fielding CA, Jones GW, McLoughlin RM, McLeod L, Hammond VJ, Uceda J, Williams AS, Lambie M, Foster TL, Liao CT, Rice CM, Greenhill CJ, Colmont CS, Hams E, Coles B, Kift-Morgan A, Newton Z, Craig KJ, Williams JD, Williams GT, Davies SJ, Humphreys IR, O’Donnell VB, Taylor PR, Jenkins BJ, Topley N, Jones SA. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity 40: 40–50, 2014. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 7: 193–203, 2014. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 7: re8, 2014. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol 18: 60–65, 1998. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 17.Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol 46: 955–975, 2007. doi: 10.1016/j.jhep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Jakubzick C, Choi ES, Joshi BH, Keane MP, Kunkel SL, Puri RK, Hogaboam CM. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J Immunol 171: 2684–2693, 2003. doi: 10.4049/jimmunol.171.5.2684. [DOI] [PubMed] [Google Scholar]
- 19.Jakubzick C, Kunkel SL, Puri RK, Hogaboam CM. Therapeutic targeting of IL-4- and IL-13-responsive cells in pulmonary fibrosis. Immunol Res 30: 339–349, 2004. doi: 10.1385/IR:30:3:339. [DOI] [PubMed] [Google Scholar]
- 20.Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, Wilke CA, Chrisman CJ, Moore BB. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol 172: 4068–4076, 2004. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]
- 21.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196, 2014. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, Keegan AD, Garcia KC. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 132: 259–272, 2008. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208: 1459–1471, 2011. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol 173: 3425–3431, 2004. doi: 10.4049/jimmunol.173.5.3425. [DOI] [PubMed] [Google Scholar]
- 25.Madala SK, Dolan MA, Sharma D, Ramalingam TR, Wilson MS, Mentink-Kane MM, Masison DC, Wynn TA. Mapping mouse IL-13 binding regions using structure modeling, molecular docking, and high-density peptide microarray analysis. Proteins 79: 282–293, 2011. doi: 10.1002/prot.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madala SK, Edukulla R, Schmidt S, Davidson C, Ikegami M, Hardie WD. Bone marrow-derived stromal cells are invasive and hyperproliferative and alter transforming growth factor-α-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 50: 777–786, 2014. doi: 10.1165/rcmb.2013-0042OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madala SK, Korfhagen TR, Schmidt S, Davidson C, Edukulla R, Ikegami M, Violette SM, Weinreb PH, Sheppard D, Hardie WD. Inhibition of the αvβ6-integrin leads to limited alteration of TGF-α-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 306: L726–L735, 2014. doi: 10.1152/ajplung.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madala SK, Pesce JT, Ramalingam TR, Wilson MS, Minnicozzi S, Cheever AW, Thompson RW, Mentink-Kane MM, Wynn TA. Matrix metalloproteinase 12-deficiency augments extracellular matrix degrading metalloproteinases and attenuates IL-13-dependent fibrosis. J Immunol 184: 3955–3963, 2010. doi: 10.4049/jimmunol.0903008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madala SK, Sontake V, Edukulla R, Davidson CR, Schmidt S, Hardie WD. Unique and redundant functions of P70S6K isoforms regulate mesenchymal cell proliferation and migration in pulmonary fibrosis. Am J Respir Cell Mol Biol 55: 792–803, 2016. doi: 10.1165/rcmb.2016-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madala SK, Thomas G, Edukulla R, Davidson C, Schmidt S, Schehr A, Hardie WD. p70 ribosomal S6 kinase regulates subpleural fibrosis following transforming growth factor-α expression in the lung. Am J Physiol Lung Cell Mol Physiol 310: L175–L186, 2016. doi: 10.1152/ajplung.00063.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mentink-Kane MM, Cheever AW, Thompson RW, Hari DM, Kabatereine NB, Vennervald BJ, Ouma JH, Mwatha JK, Jones FM, Donaldson DD, Grusby MJ, Dunne DW, Wynn TA. IL-13 receptor alpha 2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc Natl Acad Sci USA 101: 586–590, 2004. doi: 10.1073/pnas.0305064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, Beck H, Tsui P, Cochlin K, Evanoff HL, Hogaboam CM, Das AM. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol 40: 2174–2182, 2008. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Murray LA, Zhang H, Oak SR, Coelho AL, Herath A, Flaherty KR, Lee J, Bell M, Knight DA, Martinez FJ, Sleeman MA, Herzog EL, Hogaboam CM. Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model. Am J Respir Cell Mol Biol 50: 985–994, 2014. doi: 10.1165/rcmb.2013-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11: 633–643, 2010. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schrier DJ, Phan SH, McGarry BM. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am Rev Respir Dis 127: 614–617, 1983. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- 36.Sontake V, Shanmukhappa SK, DiPasquale BA, Reddy GB, Medvedovic M, Hardie WD, White ES, Madala SK. Fibrocytes regulate wilms tumor 1-positive cell accumulation in severe fibrotic lung disease. J Immunol 195: 3978–3991, 2015. doi: 10.4049/jimmunol.1500963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sontake V, Wang Y, Kasam RK, Sinner D, Reddy GB, Naren AP, McCormack FX, White ES, Jegga AG, Madala SK. Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight 2: e91454, 2017. doi: 10.1172/jci.insight.91454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 39.Urban JF Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8: 255–264, 1998. doi: 10.1016/S1074-7613(00)80477-X. [DOI] [PubMed] [Google Scholar]
- 40.White ES, Mantovani AR. Inflammation, wound repair, and fibrosis: reassessing the spectrum of tissue injury and resolution. J Pathol 229: 141–144, 2013. doi: 10.1002/path.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal 1: pe55, 2008. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 207: 535–552, 2010. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2: 103–121, 2009. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wynn TA. IL-13 effector functions. Annu Rev Immunol 21: 425–456, 2003. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 46.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 15: 271–282, 2015. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta 1773: 642–652, 2007. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang G, Volk A, Petley T, Emmell E, Giles-Komar J, Shang X, Li J, Das AM, Shealy D, Griswold DE, Li L. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine 28: 224–232, 2004. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]