Abstract
Along with amide bond formation, Suzuki cross-coupling, and reductive amination, the Buchwald–Hartwig–Ullmann-type amination of aryl halides stands as one of the most employed reactions in modern medicinal chemistry. The work herein demonstrates the potential of utilizing electrochemistry to provide a complementary avenue to access such critical bonds using an inexpensive nickel catalyst under mild reaction conditions. Of note is the scalability, functional-group tolerance, rapid rate, and the ability to employ a variety of aryl donors (Ar–Cl, Ar–Br, Ar–I, Ar–OTf), amine types (primary and secondary), and even alternative X–H donors (alcohols and amides).
Keywords: amination, arylation, cross-coupling, electrochemistry, nickel
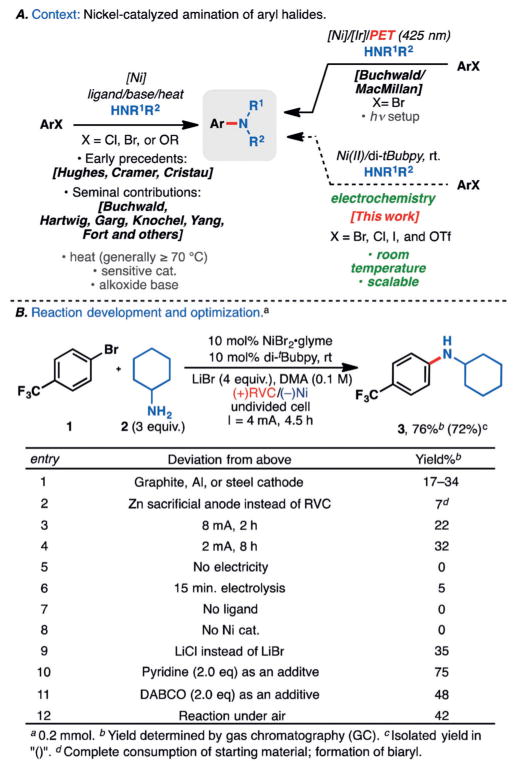
Despite its short history of merely several decades, palladium-catalyzed amination of aryl halides has emerged rapidly as one of the most widely utilized reactions in modern organic chemistry.[1] In fact, a recent study ranks the venerable Buchwald–Hartwig amination amongst one of the 20 most frequently used reactions in medicinal chemistry in 2014.[2] Similarly, the copper-catalyzed Ullmann coupling has also been a regular tool in medicinal chemists’ armamentorium.[3] Unequivocally, the formation of aryl C–N bonds is of paramount importance in drug discovery. The first example of nickel-mediated C–N coupling dates back to the 1950s using NiCl2 at 200 °C.[4,5] Subsequent efforts by the groups of Cramer[6a] and Cristau[6b] broadened and established the scope of similar reactions. Nevertheless, the harsh reaction conditions still precluded broad adoption (Figure 1A). It was not until 1997 when Buchwald’s seminal efforts spawned strong interests in utilizing nickel(0)/ligand complexes to catalyze the cross-coupling reactions between aryl halides and amines.[7] Over the years, efficient protocols have been developed by the groups of Buchwald,[8a] Hartwig,[8b] Garg,[8c] Knochel,[8d] Yang,[8e] Fort,[8f] and others.[8g–j] Nickel catalysts are inexpensive and exhibit high reactivities toward less reactive electrophiles such as aryl chlorides, thus offering an alternative approach to palladium and copper catalysis. Nevertheless, these efforts are plagued by several drawbacks, including the use of air-sensitive nickel(0) catalysts, the need for high temperatures, and the necessity of alkoxide bases. While elegant nickel(II) pre-catalysts and in situ methods of catalyst generation have been devised to address the first problem,[8] the other issues remain largely unresolved. In 2016, Buchwald, MacMillan, and co-workers reported a photochemically assisted C–N cross-coupling by nickel catalysis wherein the photoinduced electron transfer between an iridium sensitizer and nickel catalysts allows readily available nickel(II) salts to serve as catalysts under milder reaction conditions.[9]
Figure 1.
A) Background and historical context of nickel-based aryl amination methods. B) Invention and optimization of nickel-catalyzed amination. DABCO =1,4-diazobicyclo[2.2.2]octane, DMA =N,N′-dimethylacetamide, RVC =reticulated vitreous carbon, di-tBubpy =4,4′-di-tert-butyl-2,2′-bipyridine.
These studies spanning two decades point to two critical challenges to achieve nickel-catalyzed amination of aryl halides, namely the generation of a reactive low-valent nickel catalyst and the C–N bond-forming process by reductive elimination. The former often entails the reduction of a nickel(II) species[10] while the latter may be promoted by the intermediacy of a high-valent nickel species accessible by oxidation[11,12]—the ability to access nickel complexes of various oxidation states, in the same pot, is thus crucial. Electrochemistry represents the most direct and controllable means of redox manipulation as each electrochemical process seamlessly combines concurrent anodic oxidations with cathodic reductions.[13] As such, it was surmized that various oxidation states of nickel complexes could coexist in harmony under electrolytic conditions. This realization, coupled with the innate scalability, sustainability, and tunability of electrochemistry,[14] prompted the investigation of electrochemically promoted cross-coupling reactions under nickel catalysis.[15]
Herein an electrochemical method to achieve the cross-coupling between aryl halides and alkyl amines at room temperature and in the absence of an external base is presented. The scope of this electrolytic protocol encompasses aryl bromides, chlorides, triflates, and iodides. Additionally, alcohols and amides can also serve as nucleophiles.
Figure 1B provides the optimal reaction conditions alongside an abbreviated picture of reaction optimization on the coupling of the aryl bromide 1 with cyclohexylamine (2). The use of expensive electrode materials was avoided at the outset of the study and the highest yield was obtained with an RVC anode and a nickel foam cathode. Coupling products were still observed with alternative cathode materials such as graphite, aluminum, and stainless steel, albeit in diminished yields (entry 1, Figure 1B). The use of a zinc sacrificial anode exerted deleterious effects on the reaction (entry 2) as homocoupling of the aryl halide ensued instead. This result has mechanistic significance as most nickel-catalyzed electrochemical coupling of aryl halides utilize sacrificial anodes to prevent the competitive oxidation of low-valent nickel catalysts and to avoid the need for a divided cell.[15] Thus, the intermediacy of high-valent nickel species appears to be essential.[16,17] As mentioned above, such concurrent oxidation/reduction cycles are ideally suited for electrochemistry. A current of 4 mA was optimal on 0.2 mmol scale, and adjusting the current (while maintaining the total amount of electron passage constant) lowered the yields of coupling products (entries 3 and 4). Unsurprisingly, no coupling products were observed in the absence of an electric current (entry 5). Reducing the duration of electrolysis (the overall reaction time is still 4.5 h), too, had detrimental effects (entry 6), thus electricity does not merely initiate a chain reaction. The inexpensive combination of NiBr2·glyme and a bipyridyl ligand provides the most effective catalyst system for the transformation—on substrate 1, the omission of either component led to no product formation (entries 7 and 8). The choice of electrolyte has a significant impact on the reaction outcome as well and the use of LiCl instead of LiBr led to a significant drop in yield (entry 9). The addition of external bases is unnecessary. In fact, the presence of pyridine had little influence on the yield (entry 10), whereas the use of DABCO had deleterious effects (entry 11). Although optimal results are obtained when the reaction was set up under a protective atmosphere of argon, rigorous deoxygenation is unnecessary. The coupling product was afforded in 42% yield even under air (entry 12). Overall, C–N coupling is accomplished under galvanostatic (constant current) conditions in a simple undivided cell at room temperature, generally within five hours.
With the optimized reaction conditions in hand, the scope of the reaction was probed next. Medicinally privileged cyclic secondary amines such as pyrrolidine, piperazine derivatives, morpholine, and piperidine have all proven to be viable substrates, thus affording 4–7 in good yields (Scheme 1). Amines substituted with hydroxy, pyridinyl, cyano, and ester substituents (8–11) have also been successfully coupled with 1, thus underscoring the functional-group compatibility of the reaction. Cyclic amines bearing an additional α-substituent (e.g., 2-methylpyrrolidine and 2-methylpiperidine) can be used in the reaction as well, as is evidenced through the formation of 12 and 13. Notably, the attempted coupling of 2-methyl-piperidine (to furnish 13) under PET was unsuccessful. Additionally, cross-couplings using acyclic secondary amines have been demonstrated. Although dibutylamine is not a competent coupling partner under PET, it was found to react by this electrolytic system to afford 14 in 69% yield. Aside from cyclohexylamine (3), other primary amines can also be coupled with aryl bromides and these include ethylene-glycol-derived 2-(2-aminoethoxy)ethanol. It is of note that the ability to rapidly incorporate poly-PEG motifs into small-molecule pharmaceuticals has recently gained importance because of an exploding interest in PROTACs, where a poly-PEG chain often links a target binding motif with an E3 ligase recognition motif.[19]
Scheme 1.
Scope of the electrochemically enabled nickel-catalyzed amination of aryl bromides. [a] Reaction conditions: aryl bromide (1.0 equiv), amine (3.0 equiv), NiBr2·glyme (10 mol%), di-tBubpy (10 mol%), DMA (0.1 M), LiBr (4 equiv), RVC anode, Ni cathode, constant current (I = 4 mA for 0.2 mmol scale), RT. [b] Based on results reported in Ref. [9 a]. [c] Based on specified references for each substrate (Ref. [18]). [d] I =8 mA for 0.2 mmol scale. [e] Using NiBr2·glyme (5 mol%), di-tBubpy (5 mol%) [f] I = 2 mA for 0.2 mmol scale. Boc = tert-butoxycarbonyl.
This reaction also showcased a broad scope with respect to the aryl bromide electrophiles (Scheme 1). Various electron-withdrawing functional groups were tolerated, such as amide (18), ester (19), nitrile (20, 25), and sulfonamide (21). Couplings with heteroaryl bromides derived from pyridine, pyrimidine, and quinoline have also been successful (24–27). In addition, unsubstituted bromobenzene can be employed as a coupling partner (see 23).
Across a number of substrates, coupling products were afforded in comparable yields to those obtained under photochemical, palladium-catalyzed, copper-catalyzed, and nickel-catalyzed (thermal) conditions. The amount of the catalyst/ligand can be reduced to 5 mol%, and 12 of the substrates were furnished in similar yield under these conditions. In fact, 4 and 19 were afforded in higher yields with lower catalyst loading. The use of electrochemistry enables C–N coupling at ambient conditions. Furthermore, 5 could even be synthesized at 0°C (55%, 4.5 h) with this technology.
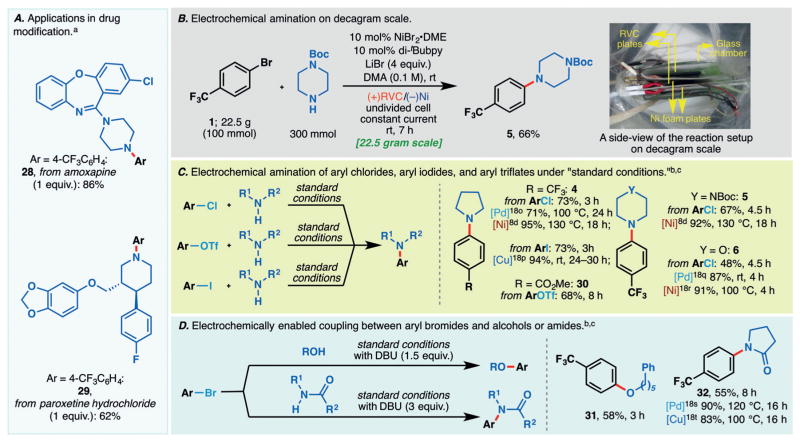
Electrochemical amination can facilitate many applications in organic synthesis. For instance, this reaction may be utilized to derivatize amine motifs in bioactive molecules. Electrochemcial N-arylation has been achieved on amoxapine and paroxetine, affording 28 and 29, respectively (Scheme 2A). In each case, the amine was used as the limiting reagent (3–5 equiv of aryl bromide; 2 equiv of DBU was added), thus highlighting the applicability of this method to complex and possibly precious amine starting materials. It is also noteworthy that the aryl chloride motif in amoxapine was left unscathed after the coupling.
Scheme 2.
Applications and extensions of the electrochemically enabled amination reaction to achieve drug modifications (A), decagram scale C–N coupling (B), amination of aryl chlorides/triflates/iodides (C), and cross-coupling using alcohol and amide as nucleophiles (D). [a] Reaction conditions: aryl bromide (3.0–5.0 equiv), amine (1.0 equiv), NiBr2·glyme (10 mol%), di-tBubpy (10 mol%), DBU (2.0 equiv), DMA (0.08 M), LiBr (4.8 equiv), RVC anode, Ni cathode, constant current (I =4 mA for 0.167 mmol scale), RT (see the Supporting Information for experimental details). [b] Experimental procedures adapted from the standard conditions with modifications indicated. For details, see the Supporting Information. [c] Comparisons based on specified references for each substrate (Ref. [18] and [8d]). DBU =1,8-diaza-bicyclo[5.4.0]undec-7-ene.
The scalability of this reaction has also been demonstrated through the cross-coupling of 1 and N-Boc-piperazine on a 23 gram scale (Scheme 2B). The aniline product 5 was afforded in 66% yield. Moreover, this large-scale electrolysis was complete within 7 hours, thus attesting to the high reaction rate and practicality.
Aside from aryl bromides, other aryl (pseudo)halides can also serve as coupling partners under electrochemical conditions. For example, cross-coupling of aryl chlorides have been successfully achieved under room temperature to afford 4–6 in good yields with no changes to the standard reaction conditions in Scheme 2C. Additionally, the coupling using aryl triflates and aryl iodides is also possible under the standard reaction conditions, as shown by the preparations of 30 and 4, respectively.
This versatile system may be adapted for the coupling between aryl halides and other nucleophilic species (Scheme 2D). For example, the coupling of an aryl bromide with a primary alcohol has been demonstrated, and 31 was afforded in a moderate yield with the addition of a base (DBU). Inclusion of an external base also allowed pyrrolidinone to serve as the nucleophile in this electrochemically facilitated cross-coupling such that 32 was furnished in 55% yield.
From a practical vantage point, this robust reaction does not require rigorous deoxygenation procedures (a simple air/ argon exchange usually suffices; see the Supporting Information for details). Admittedly, as with other electrochemical reactions under constant current conditions, fluctuations in applied potential as a result of varying cell resistance stemming from variabilities in setups may undermine the yield of the coupling and this can be circumvented through the use of additional electrolyte. As shown in Figure 1, the choice of electrode materials also exerted a substantial impact on the reaction. Nevertheless, preliminary results indicate that readily available graphite plates and stainless steel rods can serve as anode and cathode materials. With regards to substrate scope, the current conditions are not compatible with anilines. Under the present system, the use of 3 equivalents of amine is optimal when the aryl halide is the limiting reagent (for the synthesis of 3, the use 1.5 equiv of amine led to 41% GC yield instead of 76%; see the Supporting Information).
To summarize, nickel-catalyzed coupling between aryl (pseudo)halides and aliphatic amines has been enabled at room temperature, in the absence of an external base, through a simple and inexpensive experimental setup (constant current, undivided cell) using electrochemistry. The scalability and practicality of this protocol, which utilizes inexpensive catalysts and electrode materials, is not surprising as this is often the case in electrochemistry.[14a] The utility of reaction has already been field-tested in both process (Asymchem) and medicinal chemistry (Pfizer) settings.
This work represents a rare example where anodic and cathodic processes are reconciled to synergistically generate reactive catalyst species in different oxidation states, thus a sacrificial electrode is not involved.[20] From a holistic standpoint, this study is reminiscent of the electrochemically assisted Heck reaction using simple unmodified electrodes as reported by Tian and Moeller[21], a pioneering contribution which has largely been overlooked by the community. The work of Little and co-workers work on electroreductive coupling provides another instructive example where electro-chemistry opens up new dimensions in nickel catalysis.[22] Taken together, electrochemical strategies to facilitate challenging cross-coupling reactions in a simple and sustainable fashion represent an exciting area whose full potential is yet to be realized.[23]
Supplementary Material
Acknowledgments
Financial support for this work was provided by NIH (grant number GM-118176), Pfizer, China Scholarship Council (CSC) (postdoctoral fellowship to C.L.), Hewitt Foundation (postdoctoral fellowship for Y.K.), JSPS (postdoctoral fellowship to H.N.), and GlaxoSmithKline/University of Strathclyde (travel grant to J.C.V.). We are grateful to D.-H. Huang and L. Pasternack (The Scripps Research Institute) for assistance with nuclear magnetic resonance spectroscopy. We acknowledge Dr. Evan Horn (Celgene) for helpful discussions during the early stages of this work.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.201707906.
Contributor Information
Dr. Chao Li, The Scripps Research Institute (TSRI), North Torrey Pines Road, La Jolla, CA 92037 (USA).
Dr. Yu Kawamata, The Scripps Research Institute (TSRI), North Torrey Pines Road, La Jolla, CA 92037 (USA).
Dr. Hugh Nakamura, The Scripps Research Institute (TSRI), North Torrey Pines Road, La Jolla, CA 92037 (USA)
Julien C. Vantourout, The Scripps Research Institute (TSRI), North Torrey Pines Road, La Jolla, CA 92037 (USA)
Dr. Zhiqing Liu, Asymchem Laboratories (Tianjin) Co., Ltd., TEDA, Tianjin, 300457 (P. R. China)
Qinglong Hou, Asymchem Laboratories (Tianjin) Co., Ltd., TEDA, Tianjin, 300457 (P. R. China).
Dr. Denghui Bao, Asymchem Laboratories (Tianjin) Co., Ltd., TEDA, Tianjin, 300457 (P. R. China)
Dr. Jeremy T. Starr, Discovery Sciences, Medicine Design, Pfizer Global Research and Development, Groton, CT 06340 (USA)
Dr. Jinshan Chen, Discovery Sciences, Medicine Design, Pfizer Global Research and Development, Groton, CT 06340 (USA)
Ming Yan, The Scripps Research Institute (TSRI), North Torrey Pines Road, La Jolla, CA 92037 (USA).
Prof. Dr. Phil S. Baran, The Scripps Research Institute (TSRI), North Torrey Pines Road, La Jolla, CA 92037 (USA)
References
- 1.Ruiz-Castillo P, Buchwald SL. Chem Rev. 2016;116:12564. doi: 10.1021/acs.chemrev.6b00512.Hartwig JF. Angew Chem Int Ed. 1998;37:2046. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L.Angew Chem. 1998;110:2154.For another recent summary, see: Ishihara Y, Montero A, Baran PS. The Portable Chemist’s Consultant, version 2.7.1. Apple Publishing Group; New York: 2016.
- 2.Analysis based on representative publications in J Med Chem Brown DG, Boström J. J Med Chem. 2016;59:4443. doi: 10.1021/acs.jmedchem.5b01409.
- 3.For a review on the Ullmann coupling, see: Sambiagio C, Marsden SP, Blacker AJ, McGowan PC. Chem Soc Rev. 2014;43:3525. doi: 10.1039/c3cs60289c.
- 4.Hughes EC, Veatch F, Elersich V. Ind Eng Chem. 1950;42:787. [Google Scholar]
- 5.For a review on nickel-catalyzed amination of aryl halides, see: Mar%n M, Rama RJ, Nicasio MC. Chem Rec. 2016;16:1819. doi: 10.1002/tcr.201500305.
- 6.a) Cramer R, Coulson DR. J Org Chem. 1975;40:2267. [Google Scholar]; b) Cristau HJ, Desmurs JR. Ind Chem Libr. 1995;7:240. [Google Scholar]
- 7.Wolfe JP, Buchwald SL. J Am Chem Soc. 1997;119:6054. [Google Scholar]
- 8.For other selected examples of aryl C–N couplings using nickel catalysis, see: Park NH, Teverovskiy G, Buchwald SL. Org Lett. 2014;16:220. doi: 10.1021/ol403209k.Ge S, Green RA, Hartwig JF. J Am Chem Soc. 2014;136:1617. doi: 10.1021/ja411911s.Fine Nathel NF, Kim J, Hie L, Jiang X, Garg NK. ACS Catal. 2014;4:3289. doi: 10.1021/cs501045v.Manolikakes G, Gavryushin A, Knochel P. J Org Chem. 2008;73:1429. doi: 10.1021/jo702219f.Chen C, Yang LM. J Org Chem. 2007;72:6324. doi: 10.1021/jo0709448.Brenner E, Fort Y. Tetrahedron Lett. 1998;39:5359.Matsubara K, Ueno K, Koga Y, Hara K. J Org Chem. 2007;72:5069. doi: 10.1021/jo070313d.Shimasaki T, Tobisu M, Chatani N. Angew Chem Int Ed. 2010;49:2929. doi: 10.1002/anie.200907287.Angew Chem. 2010;122:2991.Iglesias MJ, Prieto A, Nicasio MC. Adv Synth Catal. 2010;352:1949.Lavoie CM, MacQueen PM, Rotta-Loria NL, Sawatzky RS, Borzenko A, Chisholm AJ, Hargreaves BKV, McDonald R, Ferguson MJ, Stradiotto M. Nat Commun. 2016;7:11073. doi: 10.1038/ncomms11073.
- 9.Corcoran EB, Pirnot MT, Lin S, Dreher SD, DiRocco DA, Davies IW, Buchwald SL, MacMillan DWC. Science. 2016;353:279. doi: 10.1126/science.aag0209.the PET facilitated amination can also be achieved using semiconductor quantum dots: Caputo JA, Frenette LC, Zhao N, Sowers KL, Krauss TD, Weix DJ. J Am Chem Soc. 2017;139:4250. doi: 10.1021/jacs.6b13379.
- 10.a) Tsou TT, Kochi JK. J Am Chem Soc. 1979;101:6319. [Google Scholar]; b) Morrell DG, Kochi JK. J Am Chem Soc. 1975;97:7262. [Google Scholar]
- 11.a) Koo K, Hillhouse GL. Organometallics. 1995;14:4421. [Google Scholar]; b) Koo K, Hillhouse GL. Organometallics. 1996;15:2669. [Google Scholar]; c) Mindiola DJ, Hillhouse GL. J Am Chem Soc. 2001;123:4623. doi: 10.1021/ja010358a. [DOI] [PubMed] [Google Scholar]; d) Lin BL, Clough CR, Hillhouse GL. J Am Chem Soc. 2002;124:2890. doi: 10.1021/ja017652n. [DOI] [PubMed] [Google Scholar]
- 12.For a review on reductive eliminations in C-Heteroatom coupling reactions, see: Hartwig JF. Acc Chem Res. 1998;31:852.
- 13.Hammerich O, Speiser B, editors. Organic Electrochemistry. 5. CRC; Boca Raton, FL: 2015. Revised and Expanded. [Google Scholar]
- 14.For selected reviews on electrochemistry, see: Horn EJ, Rosen BR, Baran PS. ACS Cent Sci. 2016;2:302. doi: 10.1021/acscentsci.6b00091.Moeller KD. Tetrahedron. 2000;56:9527.Sperry JB, Wright DL. Chem Soc Rev. 2006;35:605. doi: 10.1039/b512308a.Yoshida JI, Kataoka K, Horcajada R, Nagaki A. Chem Rev. 2008;108:2265. doi: 10.1021/cr0680843.Francke R, Little RD. Chem Soc Rev. 2014;43:2492. doi: 10.1039/c3cs60464k.
- 15.For seminal reports on the use of nickel complexes in the electrochemical coupling of ArX, see: Mori M, Hashimoto Y, Ban Y. Tetrahedron Lett. 1980;21:631.Rollin Y, Troupel M, Tuck DG, Perichon J. J Organomet Chem. 1986;303:131.Schiavon G, Bontempelli G, Corain B. J Chem Soc Dalton Trans. 1981;0:1074.Yamamoto T, Saito N. Mol Chem Phys. 1996;197:165.For selected reviews on metal-catalyzed electrochemical cross-couplings, see: Nédélec JY, Périchon J, Troupel M. Top Curr Chem. 1997;185:141.Duñach E, Franco D, Olivero S. Eur J Org Chem. 2003:1605.
- 16.Nakamura and co-workers reported an example of oxidatively induced reductive elimination in nickel-catalyzed N-arylation of aryl Grignard reagents with a chemical oxidant: Ilies L, Matsubara T, Nakamura E. Org Lett. 2012;14:5570. doi: 10.1021/ol302688u.
- 17.During the electrochemical reaction between 1 and 2, the potential at the cathode was measured to be about −1.5 V vs. Ag/AgCl, and which corresponds to the reduction of nickel(II) bipyridine complexes. The anodic potential was about +1 V vs. Ag/AgCl.
- 18.Yield, reaction temperature, and reaction times based on the reaction of the same starting materials (for substrates with multiple reports, data from the latest one was chosen): Keenan M, Chaplin JH, Alexander PW, Abbott MJ, Best WM, Khong A, Botero A, Perez C, Cornwell S, Thompson RA, White KL, Shackleford DM, Koltun M, Chiu FCK, Morizzi J, Ryan E, Campbell M, von Geldern TW, Scandale I, Chatelain E, Charman SA. J Med Chem. 2013;56:10158. doi: 10.1021/jm401610c.Winkler A, Brandhorst K, Freytag M, Jones PG, Tamm M. Organometallics. 2016;35:1160.Conesa Lerma I, Cawley MJ, Cloke FGN, Arentsen K, Scott JS, Pearson SE, Hayler J, Caddick S. J Organomet Chem. 2005;690:5841.Ding X, Huang M, Yi Z, Du D, Zhu X, Wan Y. J Org Chem. 2017;82:5416. doi: 10.1021/acs.joc.7b00290.Masui M, Adachi M, Mikamiyama H, Matsumura A, Tsuno M. 2009067784 A. JP. 2009Bozzoli A, Branch CL, Marshall H, Nash DJ. 2006002956. WO. 2006Hagopian LE, Campbell AN, Golen JA, Rheingold AL, Nataro C. J Organomet Chem. 2006;691:4890.Wolfe JP, Buchwald SL. J Org Chem. 2000;65:1144. doi: 10.1021/jo9916986.Singh C, Rathod J, Jha V, Panossian A, Kumar P, Leroux FR. Eur J Org Chem. 2015:6515.Tao C-Z, Liu W-W, Sun J-Y, Cao Z-L, Li H, Zhang Y-F. Synthesis. 2010:1280.Kamp-mann SS, Skelton BW, Wild DA, Koutsantonis GA, Stewart SG. Eur J Org Chem. 2015:5995.Yang JC, Niu D, Karsten BP, Lima F, Buchwald SL. Angew Chem Int Ed. 2016;55:2531. doi: 10.1002/anie.201509922.Angew Chem. 2016;128:2577.Xu HJ, Zheng FY, Liang YF, Cai ZY, Feng YS, Che DQ. Tetrahedron Lett. 2010;51:669.Maiti D, Fors BP, Henderson JL, Nakamura Y, Buchwald SL. Chem Sci. 2011;2:57. doi: 10.1039/C0SC00330A.Fang W, Jiang J, Xu Y, Zhou J, Tu T. Tetrahedron. 2013;69:673.Wang Y, Ling J, Zhang Y, Zhang A, Yao Q. Eur J Org Chem. 2015:4153.Wheaton CA, Bow J-PJ, Stradiotto M. Organometallics. 2013;32:6148.Magano J, Monfette S. ACS Catal. 2015;5:3120.Shakespeare WC. Tetrahedron Lett. 1999;40:2035.Jiang L. Molecules. 2014;19:13448. doi: 10.3390/molecules190913448.
- 19.a) Gilbert AM, Noe MC. Med Chem Rev. 2016;51:347. [Google Scholar]; b) Raina K, Crews CM. Curr Opin Chem Biol. 2017;39:46. doi: 10.1016/j.cbpa.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lai AC, Crews CM. Nat Rev Drug Discovery. 2017;16:101. doi: 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Gadd MS, Testa A, Lucas X, Chan KH, Chen W, Lamont DJ, Zengerle M, Ciulli A. Nat Chem Biol. 2017;13:514. doi: 10.1038/nchembio.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arene amination can also be achieved in an anodic processes. See: Morofuji T, Shimizu A, Yoshida JI. J Am Chem Soc. 2013;135:5000. doi: 10.1021/ja402083e.Waldvogel SR, Möhle S. Angew Chem Int Ed. 2015;54:6398. doi: 10.1002/anie.201502638.Angew Chem. 2015;127:6496.Herold S, Möhle S, Zirbes M, Richter F, Nefzger H, Waldvogel SR. Eur J Org Chem. 2016:1274.Möhle S, Herold S, Richter F, Nefzger H, Waldvogel SR. ChemElectroChem. 2017 ( https://doi.org/10.1002/celc.201700476)Wesenberg LJ, Herold S, Shimizu A, Yoshida J-i, Waldvogel SR. Chem Eur J. 2017 doi: 10.1002/chem.201701979. DOI: ( https://doi.org/10.1002/chem.201701979)Morofuji T, Shimizu A, Yoshida JI. Chem Eur J. 2015;21:3211. doi: 10.1002/chem.201406398.Morofuji T, Shimizu A, Yoshida JI. J Am Chem Soc. 2014;136:4496. doi: 10.1021/ja501093m.Morofuji T, Shimizu A, Yoshida JI. J Am Chem Soc. 2015;137:9816. doi: 10.1021/jacs.5b06526.
- 21.Tian J, Moeller KD. Org Lett. 2005;7:5381. doi: 10.1021/ol0519487. [DOI] [PubMed] [Google Scholar]
- 22.Miranda JA, Wade CJ, Little RD. J Org Chem. 2005;70:8017. doi: 10.1021/jo051148+. [DOI] [PubMed] [Google Scholar]
- 23.For examples of electrochemical cross-coupling reactions, see Ref. [15]; also see: Elsler B, Schollmeyer D, Dyballa KM, Francke R, Waldvogel S. Angew Chem Int Ed. 2014;53:5210. doi: 10.1002/anie.201400627.Angew Chem. 2014;126:5311.Morofuji T, Shimizu A, Yoshida JI. Angew Chem Int Ed. 2012;51:7259. doi: 10.1002/anie.201202788.Angew Chem. 2012;124:7371.Yang QL, Li YQ, Ma C, Fang P, Zhang XJ, Mei TS. J Am Chem Soc. 2017;139:3293. doi: 10.1021/jacs.7b01232.Perkins RJ, Pedro DJ, Hansen EC. Org Lett. 2017;19:3755. doi: 10.1021/acs.orglett.7b01598.Sengmany S, Vitu-Thiebaud A, Le Gall E, Condon S, Léonel E, Thobie-Gautier C, Pipelier M, Lebreton J, Dubreuil D. J Org Chem. 2013;78:370. doi: 10.1021/jo3022428.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.