Solutes of increasing size crossed cultured endothelium through intercellular junctions, through tricellular junctions, or transcellularly. Cells aligned to minimize the shear stress acting across their long axis. Paracellular transport correlated with the level of this minimized shear, but transcellular transport was reduced uniformly by flow regardless of the shear profile.
Keywords: endothelial permeability, wall shear stress, transverse wall shear stress, vesicles, tricellular junction
Abstract
Transport of macromolecules across vascular endothelium and its modification by fluid mechanical forces are important for normal tissue function and in the development of atherosclerosis. However, the routes by which macromolecules cross endothelium, the hemodynamic stresses that maintain endothelial physiology or trigger disease, and the dependence of transendothelial transport on hemodynamic stresses are controversial. We visualized pathways for macromolecule transport and determined the effect on these pathways of different types of flow. Endothelial monolayers were cultured under static conditions or on an orbital shaker producing different flow profiles in different parts of the wells. Fluorescent tracers that bound to the substrate after crossing the endothelium were used to identify transport pathways. Maps of tracer distribution were compared with numerical simulations of flow to determine effects of different shear stress metrics on permeability. Albumin-sized tracers dominantly crossed the cultured endothelium via junctions between neighboring cells, high-density lipoprotein-sized tracers crossed at tricellular junctions, and low-density lipoprotein-sized tracers crossed through cells. Cells aligned close to the angle that minimized shear stresses across their long axis. The rate of paracellular transport under flow correlated with the magnitude of these minimized transverse stresses, whereas transport across cells was uniformly reduced by all types of flow. These results contradict the long-standing two-pore theory of solute transport across microvessel walls and the consensus view that endothelial cells align with the mean shear vector. They suggest that endothelial cells minimize transverse shear, supporting its postulated proatherogenic role. Preliminary data show that similar tracer techniques are practicable in vivo.
NEW & NOTEWORTHY Solutes of increasing size crossed cultured endothelium through intercellular junctions, through tricellular junctions, or transcellularly. Cells aligned to minimize the shear stress acting across their long axis. Paracellular transport correlated with the level of this minimized shear, but transcellular transport was reduced uniformly by flow regardless of the shear profile.
transport of macromolecules across the vascular endothelium and its modification by mechanical forces are important for the normal functioning of tissue. They may also be important in the development of atherosclerosis; the disease has a patchy distribution within the arterial system (2, 7, 17, 25, 33) that has been attributed to variation in the hemodynamic stresses imposed on the endothelium (7, 17, 25, 26, 33) and to variation in the transendothelial transport of macromolecules such as low- and high-density lipoproteins (LDL and HDL, respectively) (2, 7, 17, 49, 57). However, the routes by which macromolecules cross the endothelium (32, 39, 44, 46, 56), the hemodynamic stresses that maintain normal endothelial function or trigger disease (7, 17, 25, 26, 33, 36), and the dependence of transendothelial transport on hemodynamic stresses (21, 50, 55) have been matters of controversy over several decades.
In the present study, the method of Dubrovskyi et al. (13) was modified to investigate how particles of different charge and size cross cultured aortic endothelium. In the original method, fluorescein isothiocyanate-labeled avidin (FITC-avidin; 66–69 kDa) is added to the medium above an endothelial monolayer cultured on biotinylated collagen. When the tracer crosses the endothelium, it binds to the biotin and thus becomes immobilized; its distribution is imaged en face by fluorescence microscopy and compared with the morphology of the overlying cells to determine the structure through which it traveled. [Recently, Richter et al. (43) used the reverse method of applying biotinylated tracers to epithelial cells grown on an avidin-labeled substrate.] Here, FITC-labeled deglycosylated avidin (“NeutrAvidin”) was also used, to obtain a tracer with a more physiological isoelectric point, and bigger fluorescent labels attached to avidin gave larger tracers: labelling NeutrAvidin with R-phycoerythrin (RPE), a fluorescent phycobilliprotein, produced a tracer with a fivefold larger mass, within the range for HDL, while a streptavidin-labelled nanoparticle (Qdot 800, diameter approximately 20 nm) provided a tracer having the size of LDL. Additionally, the same principle was used to obtain preliminary results in vivo; an intravital tracer that is immobilized on crossing the endothelium was created by conjugating a fluorescent dye to an antibody raised against components of the basement membrane.
To investigate the relation between permeability and hemodynamic stress, the avidin-based tracers were used in experiments where endothelium cultured in multiwell plates was exposed to flow using an orbital shaker (37, 38, 55); fluorescence from immobilized tracer, mapped with a scanning fluorimetric plate reader, was compared with local values of shear stress metrics derived from numerical simulations of the flow.
METHODS
Chemicals were obtained from Sigma-Aldrich unless otherwise stated.
Isolation and culture of endothelial cells.
Porcine aortic endothelial cells were isolated from the descending thoracic aorta of Landrace cross pigs aged 4–6 mo as previously described by Bogle et al. (4) and cultured at 37°C in a humidified incubator under 95% air-5% CO2. The medium was DMEM containing 10% (vol/vol) FBS, 5 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B, 50 μg/ml gentamycin, 90 μg/ml heparin, and 5 μg/ml endothelial cell growth supplement from bovine neural tissue. Medium was replaced every 2–3 days. When confluent, cells were passaged by brief exposure to trypsin-EDTA.
Endothelial cell purity was assessed from the internalization of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (Dil)-labeled acetylated LDL (Molecular Probes), which was added to confluent monolayers at a final concentration of 10 μg/ml and incubated at 37°C for 12 h. The wells were then washed in PBS and fixed in 4% paraformaldehyde for 15 min; nuclei were stained with DRAQ5 (1:1,000, BioStatus), and the cells were washed in PBS again before being imaged using a laser scanning confocal microscope (Leica SP5) with a ×10, 0.40-numerical aperture (NA) objective. Dil-acetylated-LDL was excited at 514 nm and detected at 539–593 nm; equivalent wavelengths for DRAQ5 were 633 and 675–725 nm. The fraction of cells taking up Dil-acetylated LDL, determined with a manual counting program developed in MATLAB (The MathWorks), was 99.7% [±0.1% (SE), n = 5 isolations].
Application of flow in vitro.
Porcine aortic endothelial cells were seeded at 104 cells/cm2 in 12-well plates that had been coated with biotinylated gelatin as previously described by Dubrovskyi et al. (13) and cultured until confluent (~72 h). The plates (medium height of 2 mm when stationary) were then placed on the platform of an orbital shaker (POS-300, Grant Instruments) in the incubator for 7 days. The platform was horizontal, and its orbit, also in the horizontal plane, was circular with a diameter of 10 mm and a rotation rate of 150 rpm. Static controls were included for each experiment: 12-well plates containing cells from the same aorta and of the same passage as those on the orbital shaker were placed in the incubator for the same period.
Application of tracer in vitro.
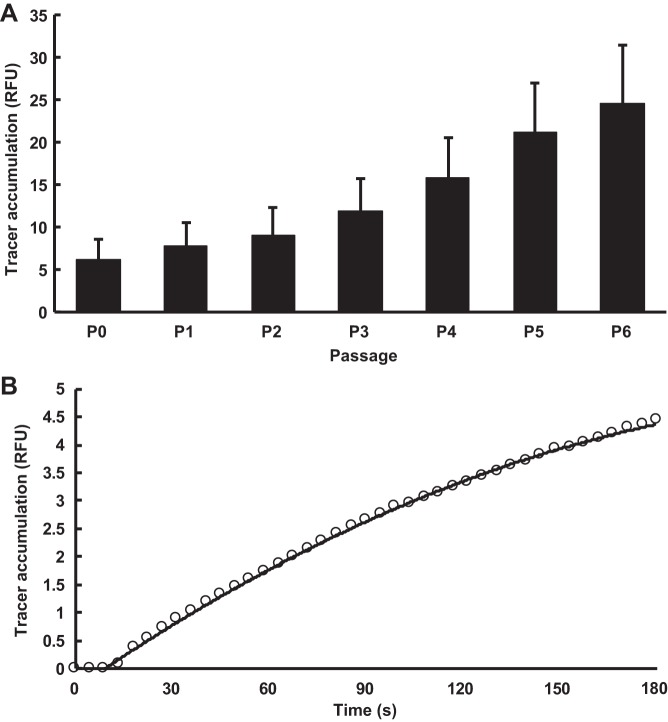
Preliminary measurements showed that the permeability of monolayers to FITC-avidin increased with passage number (Fig. 1A). Although passage 0 cells gave monolayer permeabilities closest to those seen in vivo, it is not practicable to use them for large numbers of experiments. Permeability increased by 46% between passages 0 and 2 but changed more rapidly thereafter, increasing 2.7-fold between passages 2 and 6 (Fig. 1A). Cells were therefore used at passage 2.
Fig. 1.
A: effect of passage number (P) on 3-min subendothelial accumulation of the 66- to 69-kDa FITC-avidin tracer [expressed in relative fluorescence units (RFU)], measured by a scanning fluorimetric plate reader. Values are means + SD; n = 10 isolations. B: subendothelial accumulation of FITC-avidin as a function of time. Tracer fluorescence was measured by confocal microscopy of a living monolayer. Tracer was added to the well at time ≈ 9 s. A second-order polynomial was fitted to the data.
Cells were starved of serum by changing to culture medium that contained 1% (wt/vol) BSA instead. One percent is sufficient to obtain the permeability-reducing effect of albumin (10); withdrawal of serum did not have a significant effect on the permeability of the monolayer to FITC-NeutrAvidin (P = 0.27, n = 3 isolations). After 24 h, DMEM containing FITC-avidin, FITC-NeutrAvidin, and/or RPE-NeutrAvidin (Life Technologies; Table 1), each at 0.38 μM, was applied to the wells, and the plate was returned to the incubator, on the orbital shaker for cells exposed to flow. Alternatively, a 0.076 μM solution of nanocrystals coated in streptavidin (Qdot 800-streptavidin; Life Technologies; Table 1) was used. The Qdot 800-streptavidin had been purified of free streptavidin by repetitive ultrafiltration with a centrifugal filter (100-kDa cutoff, Amicon) and centrifuged to remove aggregates.
Table 1.
Relative molecular mass and isoelectric point of the proteins used to make tracers
| Protein | Relative Molecular Mass, kDa | Isoelectric Point, pH |
|---|---|---|
| Avidin | 66−69 | 10.5 |
| NeutrAvidin | 60 | 6.5 |
| Streptavidin | 53 | 6.8−7.5 |
| R-phycoerythrin | 250 | 4.8 [Sun et al. (48)] |
Tracer solutions were removed after 3 min, and the wells were rinsed with PBS (4 × 2 min), fixed with 4% paraformaldehyde for 10 min, and stained with DRAQ5 as above to reveal nuclei. Preliminary results showed that the average rate at which FITC-avidin accumulated over 3 min was only 20% lower than the average rate at which it accumulated over the first minute (Fig. 1B). Hence, even for the smallest tracer, there was insignificant saturation of subendothelial avidin binding sites or self-quenching of fluorescence at this duration of exposure. (Saturation of binding sites or self-quenching would limit any further increase in detected tracer, even if tracer were still crossing the monolayer.) For this reason, accumulation over 3 min is assumed to indicate monolayer permeability.
Visualization of transport pathways in vitro.
To visualize the distribution of tracer under the cells, wells were imaged using the confocal microscope. FITC, RPE, DRAQ5, and Qdot 800 were excited at 488, 565, 633, and 405 nm and detected at 515–525, 580–590, 690–700, and 760–800 nm, respectively. Maximum intensity projections were computed from z-stacks that had 1,024 × 1,024 pixels, an average height of 62 µm, and a slice thickness of 2.4 µm. Images were generally acquired using 12-well plates and either a ×10, 0.40-NA objective (Figs. 1 and 8) or a ×20, 0.75-NA objective (Figs. 3 and 4). To obtain the higher-resolution images shown in Fig. 2, some static experiments were conducted using 96-well plates, which have thinner bases than 12-well plates and thus permit the use of a ×40, 1.25-NA objective; all culture conditions and imaging settings were otherwise kept consistent.
Fig. 8.
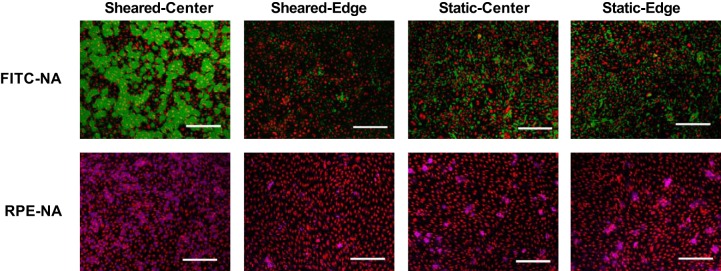
Images of sheared and nonsheared endothelial cells at the center and toward the edge of a well after the application of 60-kDa FITC-NeutrAvidin (FITC-NA; green) or 310-kDa R-phycoerythrin-NeutrAvidin (RPE-NA; purple). DRAQ5 nuclear staining is shown in red. Bar = 200 μm. n = 3 isolations.
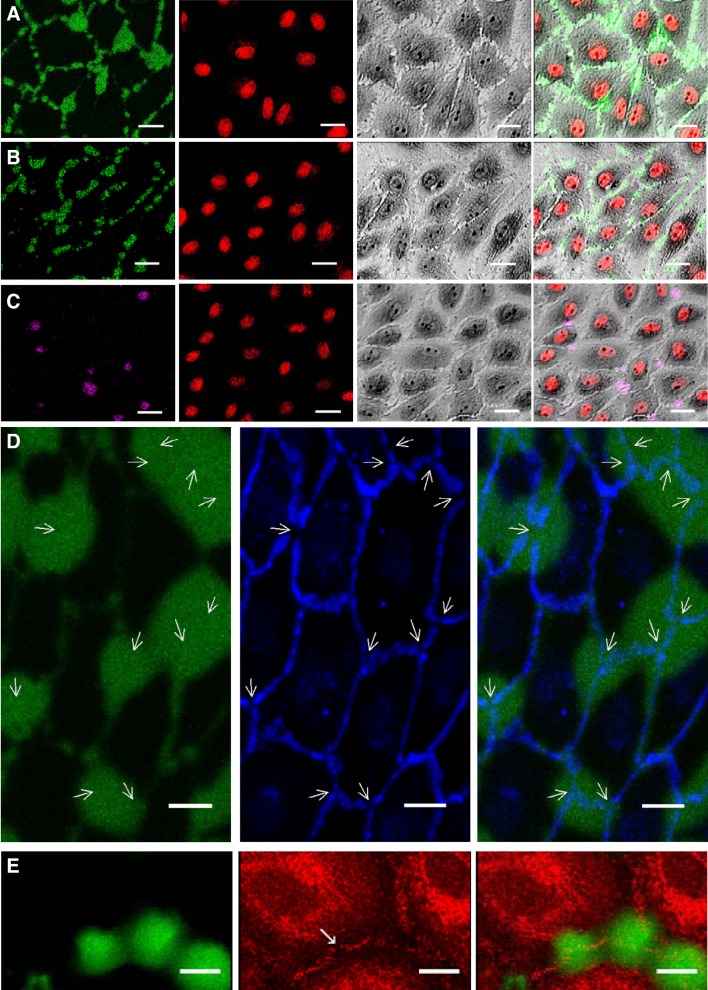
Fig. 3.
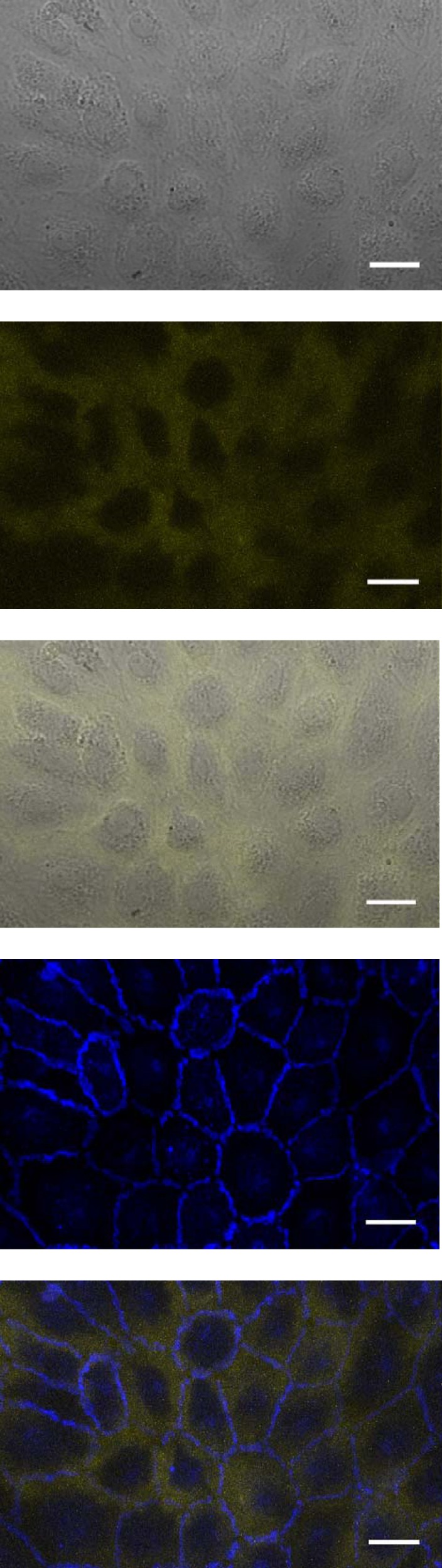
Confocal images from in vitro transport experiments showing, from top to bottom, a grayscale phase contrast image of the cells, Qdot 800-streptavidin (yellow, ~20-nm radius), an overlay of these two images, anti-vascular-endothelial (VE)-cadherin (blue), and an overlay of the Qdot and VE-cadherin images. The overlays demonstrate tracer localization under the cells, except in nuclear regions, and no enhancement of tracer concentration under intercellular junctions. Bar = 20 μm. n = 3 isolations.
Fig. 4.
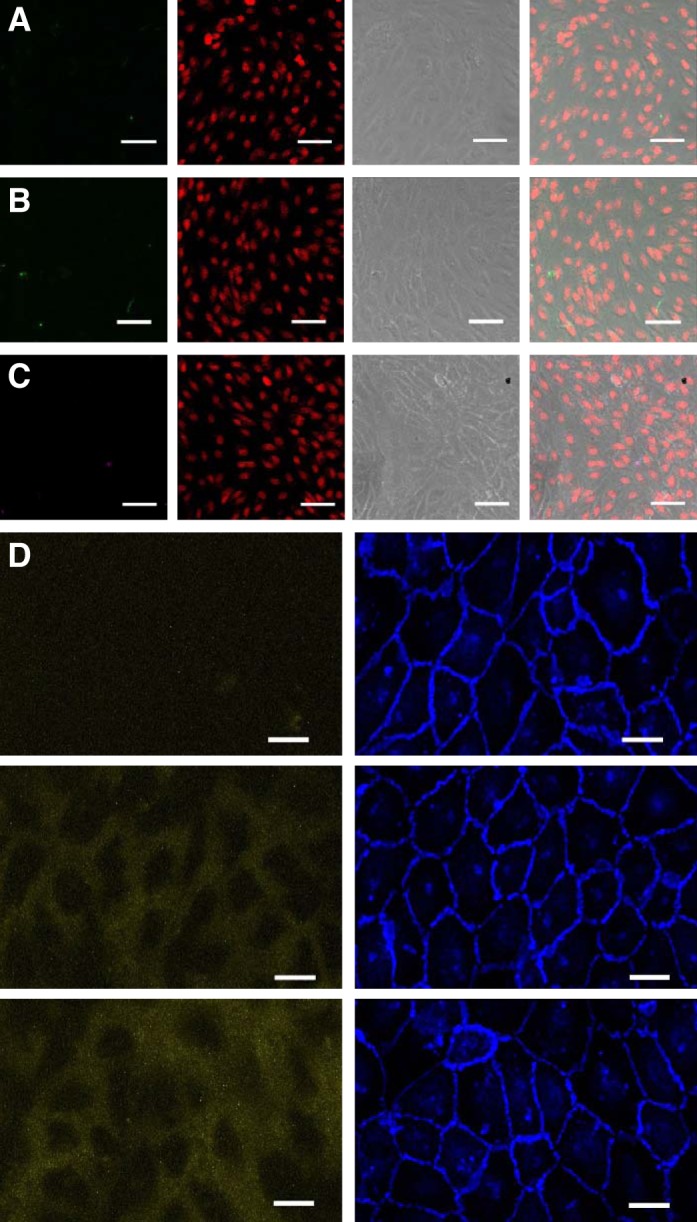
Confocal images from control transport experiments in vitro showing 66- to 69-kDa FITC-avidin (green; A), 60-kDa FITC-NeutrAvidin (green; B), or 310-kDa R-phycoerythrin-NeutrAvidin (purple; C) together with DRAQ5-stained nuclei (red) and a grayscale phase-contrast image. Overlaying the images in A−C demonstrated the lack of tracer visible when the gelatin substrate was not biotinylated. Bar = 50 µm. D: confocal images showing Qdot 800-streptavidin (yellow, ~20-nm radius) and anti-vascular-endothelial cadherin (blue). The row at the top demonstrates the lack of tracer visible when the gelatin substrate was not biotinylated. The row in the middle shows 3-min uptake with biotinylated gelatin, as in Fig. 3, and the row at the bottom shows that extending the uptake time from 3 to 15 min still did not result in the lines and spots seen for the smaller tracers. Bar = 20 μm. n = 3 isolations.
Fig. 2.
Confocal images from in vitro transport experiments showing 66- to 69-kDa FITC-avidin (green; A), 60-kDa FITC-NeutrAvidin (green; B), or 310-kDa R-phycoerythrin-NeutrAvidin (purple; C) together with DRAQ5-stained nuclei (red) and a grayscale phase-contrast image. Overlaying the images in each subpanel demonstrated tracer localization under bicellular and tricellular junctions. Bar = 20 µm. D: confocal images showing FITC-avidin (green) and vascular-endothelial (VE)-cadherin immunostaining (blue). The overlay shows that spots of high tracer uptake colocalized with breaks in the pericellular VE-cadherin staining (arrows). Bar = 10 µm. E: confocal images showing FITC-avidin (green) and zonula occludens-1 immunostaining (red). The overlay shows a spot of high tracer uptake colocalized with a break in the pericellular zonula occuldens-1 staining (indicated with an arrow in the red channel). Bar = 10 μm. n = 3 isolations.
Vascular endothelial (VE)-cadherin immunostaining was used to visualize the distribution of tracer in relation to cell borders. Cells were fixed with 4% paraformaldehyde for 5 min and permeabilized with 0.1% Triton X-100. After being blocked with a 1% BSA solution for 2 h, cells were incubated overnight at 4°C with a 1:200 dilution of anti-VE-cadherin (Santa Cruz Biotechnology) and then with a 1:300 dilution of Alexa Fluor 568-labeled donkey anti-goat IgG (Life Technologies) for 1 h before being imaged by confocal microscopy (excitation: 561 nm; emission: 580–610 nm). The same methods were used for staining zonula occludens (ZO)-1 except that cells were blocked with 5% BSA, stained with anti-ZO-1 (Santa Cruz Biotechnology) and Alexa Fluor 546-labeled goat anti-rabbit IgG (Life Technologies), and imaged with excitation and emission wavelengths of 561 and 575–595 nm, respectively.
Quantification of in vitro transport.
The distribution of tracer bound to the biotinylated gelatin was mapped using the well scan function of an automated plate reader (SpectraMax M5) to produce a 19 × 19-pixel image. The radius of each well was 11 mm, but the plate reader could only measure intensities at distances up to 9 mm from the center. (Maps and graphs derived from the numerical simulations of flow were therefore truncated to this radius.)
Quantification of cell and nuclear orientation in vitro.
Cell borders and nuclei were stained with anti-VE-cadherin and DRAQ5, respectively, and imaged with the confocal microscope using excitation and emission wavelengths for DRAQ5 of 633 and 680–720 nm and the wavelengths for VE-cadherin immunostaining described above. A tile scan of z-stacks was obtained from the center to the edge of the well, each stack having 1,024 × 1,024-pixel resolution in the x-y plane and an average of 30 slices covering a total depth of 40 µm in the z direction.
Images were processed using code written in MATLAB. Intensity, area, and perimeter thresholding were used to differentiate nuclei from the background. Ellipses were fitted to the nuclei, and the centroid of each ellipse was calculated. A vector connecting the centroid with the farthest boundary point of the same ellipse was obtained. This vector gave the orientation of the nucleus in the x-y plane. A vector connecting the center point of the well to each nuclear centroid was produced. This vector acted as the reference axis from which the orientation of the nucleus was determined in the polar system. Data were plotted as histograms, and modal orientations were obtained by fitting a seventh-order polynomial.
To obtain the orientation of the cells, intensity thresholding was applied to differentiate the anti-VE-cadherin staining from background fluorescence. Morphological processing functions were used to establish clear borders of the cells: lines were reduced to single pixel thickness by thinning, residual nonboundary pixels (i.e., those with all 4 connected neighbors equal to 1) were removed, and breaks were filled by dilation. Grayscales were then inverted, area thresholding was applied, and ellipses were fitted. Vectors and orientations of the cells were defined using the coordinate system described for nuclei.
Computation of shear stress applied in vitro.
The movement of fluid above the cultured monolayer was computed using STAR-CCM+ software (version 9, CD-adapco). The culture well was discretized using a structured cylindrical mesh of diameter 22.1 mm and height 10 mm with 291,600 grid elements. The base was assumed to be flat, meaning that variation in the height of the cells themselves was neglected. A noninertial frame of reference was used that moved with the well center. Values of 0.78 × 10−3 Pa·s and 1,003 kg/m3 were used for the dynamic viscosity and density of medium containing 10% serum at 37°C, respectively. The corresponding values for saturated air at 37°C were 18.688 10−6 Pa·s and 1.1115 kg/m3. The gas-liquid interface was tracked using the volume-of-fluid method; the Courant-Friedrichs-Lewy (CFL) number was set to 0.5. A convergence study was performed to confirm that the results were mesh independent, and the simulation was run for 10 orbits to ensure that a periodic state had been achieved. The shear stress (frictional force per unit area) experienced by the cells was determined from the velocity gradient at the well base. This stress was termed “wall shear stress” (WSS), by analogy with the stress acting on blood vessel walls.
Four established WSS metrics were calculated. Time-average WSS (TAWSS) is the average of the magnitudes of the instantaneous WSS vectors (, whereas the magnitude of the mean WSS (MagMeanWSS) is the magnitude of the average of the vectors, as follows:
where t is time and T is the duration of the cycle. Note that = 0 because of the no-slip boundary condition and continuity equation. For flow of constant speed oriented in the positive x direction for half of the cycle and the negative x direction for the other half of the cycle, TAWSS would be nonzero, whereas MagMeanWSS would be zero.
The oscillatory shear index (OSI) (19) compares MagMeanWSS with TAWSS, whereas transWSS is the time average of WSS components perpendicular to the mean direction, as follows:
where is the unit vector perpendicular to the well base. For flow of constant speed oriented in the positive x direction for half of the cycle and the negative x direction for the other half of the cycle, the OSI would be 0.5 since MagMeanWSS = 0. TransWSS would be indeterminate in this artificial case because there is no mean flow direction; however, if the flow were slightly greater in the positive than the negative x direction, allowing a mean to be defined, then transWSS would be zero since there are no components perpendicular to the mean direction.
A new metric, the minimum transverse WSS (transWSSmin), a generalization of transWSS, was defined as follows:
This metric is associated with the angle (ϕ) that minimizes the time average of WSS components perpendicular to it.
Visualization of transport pathways in vivo.
Antibodies were raised to a component of the endothelial basement membrane, labeled with a fluorescent dye, and administered to rabbits intravenously. The antibodies were raised in sheep by standard methods; the immunogen was the NC1 domain of collagen type IV, prepared from rabbit lung as previously described by Hudson et al. (20). Antiserum was labeled with Lissamine rhodamine using the methods we previously used to label albumin (8), and antibodies were then extracted with an affinity column in which the immunogen was linked to beads with cyanogen bromide. The specificity of unlabeled antibody was tested by using it to stain histological sections of formalin-fixed, paraffin-embedded rabbit aorta. Binding was examined by digital-imaging fluorescence microscopy after application of rhodamine-labeled rabbit anti-sheep IgG, using a conventional epifluorescence microscope (Zeiss Axioplan), ×40 oil immersion lens, and a cooled charge-coupled device (CCD) camera (Axiom Viper AX2).
In vivo experiments were conducted according to our previous protocols (47). All procedures complied with the Animals (Scientific Procedures) Act 1986 and were approved by the Local Ethical Review Panel of the University of Reading. Briefly, tracer was administered via the marginal ear vein to conscious, unrestrained male New Zealand White rabbits (HSDIF strain, Harlan, n = 6) that had been individually housed at 18 ± 2°C under a 12:12-h light-dark cycle and fed a normal laboratory diet (9603 TRB, Harlan Teklad, Bicester, UK) ad libitum. The tracer was allowed to circulate for various times before the animals were administered heparin (2,000 USP units iv, Sigma) and, after a further 2 min, an overdose of pentobarbital (~200 mg/kg Euthatal, Rhône Mérieux) by the same route. After a ventral midline laparotomy and thoracotomy, the aorta was cannulated, flushed by retrograde perfusion with saline, and fixed at physiological pressure with formalin. The thoracic segment was excised, stained with acridine orange to reveal nuclei, and examined en face by confocal microscopy (TCS SP2, Leica) using the 568-nm line of a krypton-argon mixed-gas laser for excitation. Stacks of optical slices were acquired in the z direction, each slice being approximately parallel to the endothelial surface. A maximum intensity projection was obtained from each stack.
Statistics.
Differences in permeability were assessed by Student’s unpaired t-test using P < 0.05 as the criterion of significance. The uniformity of cell orientations was assessed by the χ2-test.
RESULTS
Transport pathways in static culture.
FITC-avidin bound to the biotinylated gelatin in lines and spots (Fig. 2A). Comparison with the pattern of nuclear staining and with phase-contrast images showed that the lines, which were sometimes punctate, correspond to bicellular junctions (i.e., junctions between two neighboring cells). The spots occurred chiefly at the intersections of the lines, corresponding to locations where three or more cells came together, here termed “tricellular junctions” for simplicity: 65.5 ± 5.7% (mean ± SE, n = 6 isolations) of tricellular junctions had spots, and only 6.3 ± 4.1% of spots were not at tricellular junctions. Immunostaining showed that the spots tended to colocalize with breaks in pericellular VE-cadherin expression (Fig. 2D), and colocalization with breaks in ZO-1 staining was also occasionally observed (Fig. 2E).
The lines for FITC-NeutrAvidin were often thicker and less continuous than those for FITC-avidin; however, they were still located at the cell borders. Spots also occurred under tricellular junctions, but in the majority of isolations they were less pronounced or less frequent than those for FITC-avidin (Fig. 2B).
RPE-NeutrAvidin bound only in spots: no lines were visible. Comparison with phase-contrast images showed that the spots were located under tricellular junctions (Fig. 2C) or, less frequently, under large gaps at cell borders.
Dual-tracer experiments showed that 99.1 ± 1.2% (n = 6 isolations) of RPE-NeutrAvidin binding sites coincided with spots of FITC-avidin and 96.1 ± 2.0% coincided with spots of FITC-NeutrAvidin.
Qdot 800-streptavidin bound under the cells (Fig. 3). Comparison with VE-cadherin staining showed that the tracer concentration was not elevated under bicellular or tricellular junctions. Comparison with phase-contrast images showed that fluorescence was less intense in nuclear regions, suggesting that the nucleus provided a barrier to the transcellular transport of the tracer. This was the case even when the nuclei were close to an intercellular junction, demonstrating that the tracer had not entered through such junctions and diffused laterally before binding.
For all tracers, little fluorescence was seen in control experiments where the gelatin was not biotinylated (Fig. 4), demonstrating that the patterns shown in Figs. 2–4 represent tracer bound to the substrate and not tracer within cells, attached to them, or free underneath them.
Shear stress patterns in orbiting wells.
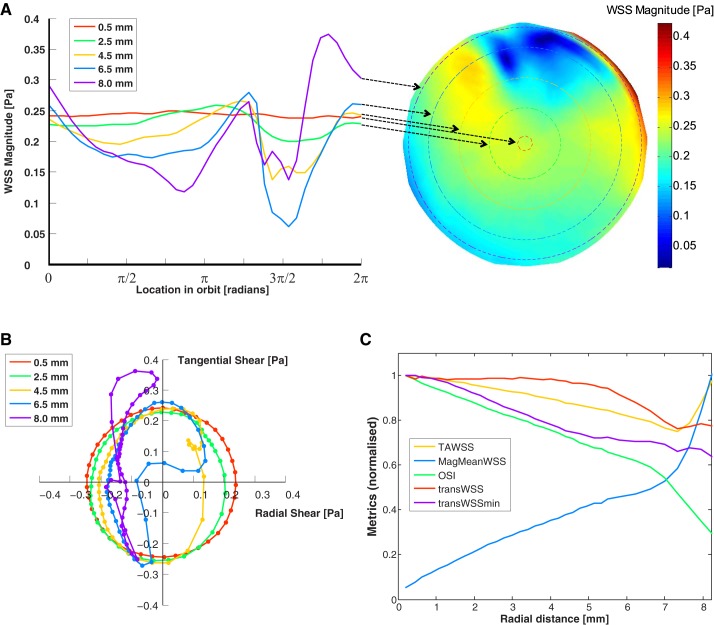
The liquid surface and underlying velocity field were characterized by a wave that traveled around the well with the period of rotation of the orbital shaker. There was a region of rapid change in surface elevation, termed a “front,” that was approximately aligned with the radial direction. The magnitude of WSS vectors across the base of the well at a single instant is shown in Fig. 5A. This spatial pattern remained constant but rotated around the center of the well in synchrony with the wave. Hence, at a given distance from the center, all cells experienced the same temporal pattern of WSS magnitude, albeit with a different phase. The temporal pattern of WSS magnitude changed continuously upon moving from the center to the edge of the computational domain (Fig. 5A). At the center, the magnitude was approximately constant over time, but a double-peaked oscillation with increasing maximum was observed farther away from the center.
Fig. 5.
A, right: map of the instantaneous wall shear stress (WSS) magnitude acting on the base of the well. The radius of the map has been truncated so that only the area of the well covered by the scanning plate reader is shown. Left: WSS magnitude for different radii throughout one cycle (2π rad). The arrows indicate the radial position for each curve. B: polar plot of the magnitude and direction of instantaneous WSS vectors during one cycle, with different colors representing different radii. Each point of the curve represents the tip of the WSS vector at a single instant. The symbols are spaced at 10-ms intervals. A uniformly rotating vector would appear as a circle centered on the origin. A vector that oscillated between forward and backward uniaxial flow would appear as a line passing through the origin. C: WSS metrics (defined in text) as a function of distance from the center of the well. Each metric has been normalized by its maximum value: time-average WSS (TAWSS), 0.24 Pa; magnitude of the mean WSS (MagMeanWSS), 0.17 Pa; oscillatory shear index (OSI), 0.48; time average of WSS components perpendicular to the mean direction (transWSS), 0.16 Pa; and minimum transverse WSS (transWSSmin), 0.15 Pa. Small ripples in the lines are artifacts caused by the finite size of mesh elements.
All WSS vectors pointed broadly to the wave front at each instant. Consequently, the directionality of the WSS vector also changed between the center and the edge of the well (Fig. 5B). There was no preferred direction at the center of the well; the vector rotated through 360°, spending equal time in each direction and, as noted above, having equal magnitude at each time. With increasing distance from the well center, the radial components of the instantaneous vectors, particularly components in the positive direction (i.e., pointing away from the center), became smaller in magnitude; vectors with negative radial components occupied a larger fraction of the cycle than those with positive radial components. At the largest radius examined, the radial component was always negative and approximately constant throughout much of the cycle.
The five computed WSS metrics varied in magnitude with increasing distance from the center of the well (Fig. 5C). TAWSS decreased, whereas MagMeanWSS increased. OSI, transWSS, and transWSSmin decreased, like TAWSS, but their curves had different shapes.
Mean transport in static culture.
In the following sections, subendothelial accumulation of tracer, assessed using the fluorimetric plate reader, is expressed in arbitrary but self-consistent relative fluorescence units (RFU). A preliminary experiment was conducted to determine monolayer permeability in absolute mass transport coefficients. A 2-mm-deep, 0.025 μg/ml solution of FITC-avidin was placed in wells (n = 6) that had been coated with biotin-labeled gelatin but were free of cells. Spectrophotometry of the medium after 2 days showed that all the tracer had bound. The wells were then rinsed thoroughly (no tracer being detected in the washes), and fluorescence from the tracer bound to gelatin was determined with the plate reader: a mean value of 16.8 RFU was obtained, whereas 7 RFU was obtained for cell monolayers (n = 3 isolations) incubated as usual for 3 min with 25 μg/ml of tracer in 2-mm-deep medium. An absolute permeability could be calculated for the monolayers, assuming that the tracer was well mixed and that tracer transport across the endothelium during the 3-min uptake period did not materially affect the tracer concentration in the medium, as follows:
The quantity of bound tracer was obtained from the fluorescence measured in the endothelial transport experiment, using the fluorescence measured in the calibration experiment and the knowledge that in the latter each square centimeter of substrate will have bound 0.005 μg of tracer (i.e., all the tracer in the solution above the substrate).
The permeability value corresponding to 7 RFU was 4.6 × 10−7 cm/s. This can be used to convert other RFU values obtained for FITC-avidin transport. For example, the RFU values obtained for passage 0 and passage 6 cells shown in Fig. 1 are equivalent to ∼4 × 10−7 and 16 × 10−7 cm/s, respectively.
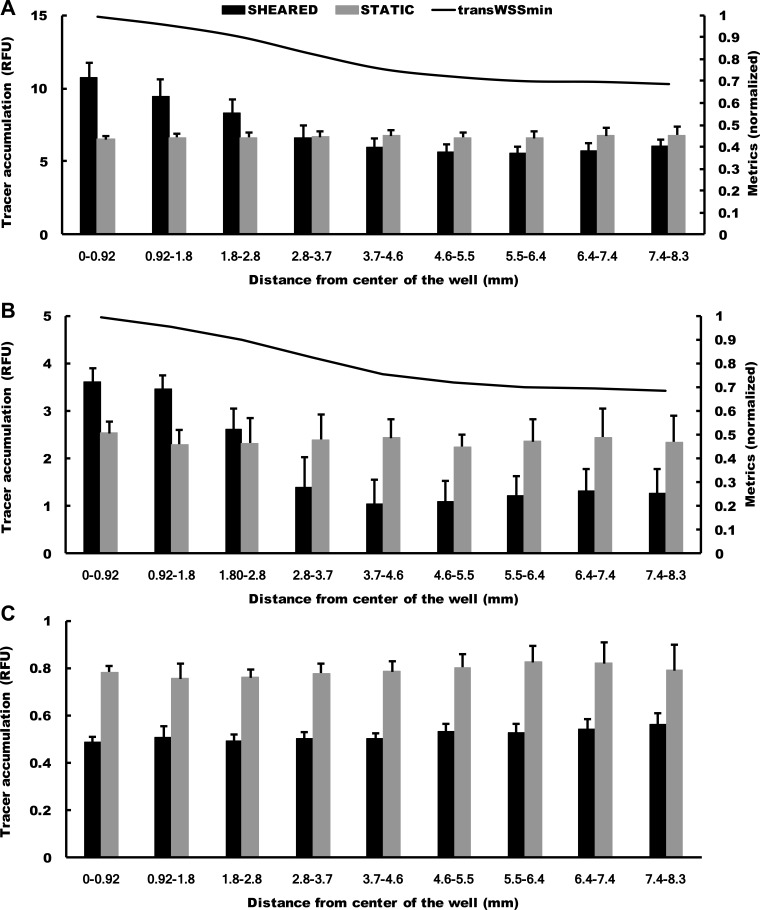
Effects of chronic shear stress on transport across cultured endothelium.
The quantitative maps produced by the plate reader did not reveal any systematic large-scale inhomogeneities in monolayer permeability to FITC-NeutrAvidin, RPE-NeutrAvidin, or Qdot 800-streptavidin across wells cultured under static conditions (Fig. 6, A, C, and E). Monolayers exposed to FITC-NeutrAvidin and RPE-NeutrAvidin under shear showed increased permeability at the center of the well and decreased permeability toward the edge (Fig. 6, B and D). Overall, the application of shear decreased uptake along a radius by 5% for FITC-NeutrAvidin (from 6.75 ± 0.034 to 6.41 ± 0.080 RFU, means ± SE, n = 3 isolations, P = 0.039) and by 36% for RPE-NeutrAvidin (from 2.36 ± 0.23 to 1.52 ± 0.017 RFU, P < 0.001). Monolayers exposed to Qdot 800-streptavidin under shear showed a nearly uniform decrease in permeability across the well (Fig. 6F).
Fig. 6.
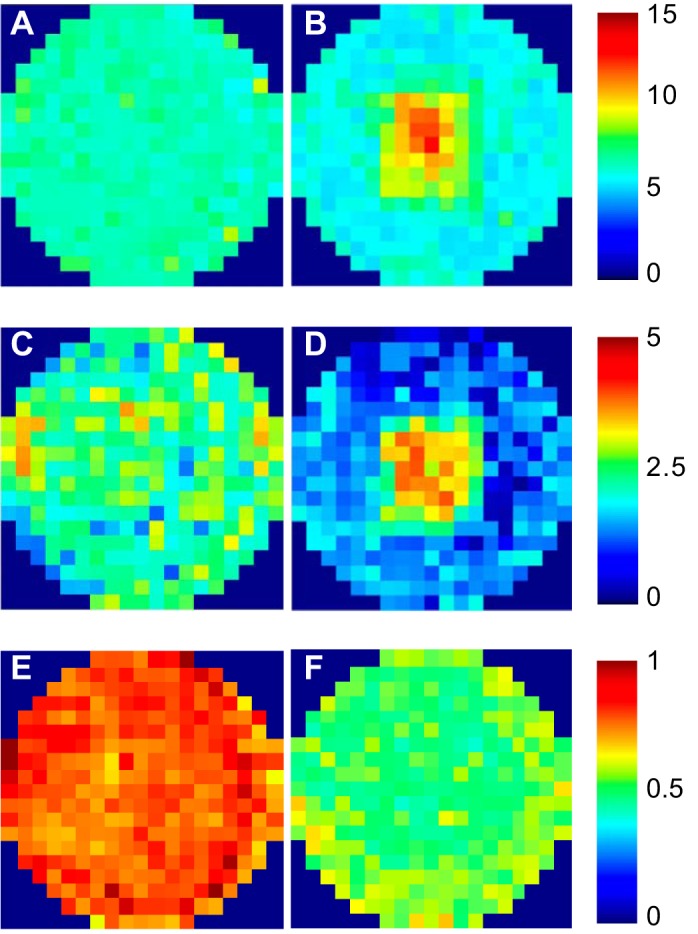
Average maps of cultured monolayer permeability to 60-kDa FITC-NeutrAvidin under static conditions (A) and shear (B), 310-kDa R-phycoerythrin-NeutrAvidin under static conditions (C) and shear (D; n = 6 isolations in each case), and ~20-nm Qdot 800-streptavidin under static conditions (E) and shear (F; n = 3 isolations). Intensity values are in arbitrary units.
Histograms of permeability with and without shear at different radial locations showed that the fractional increase in permeability induced by shear at the center of the well was smaller for RPE-NeutrAvidin than for FITC-NeutrAvidin (Fig. 7, A and B). They also revealed that the effect fell faster with radial distance for RPE-NeutrAvidin and that the eventual reduction in permeability was larger. The effect of shear was significant at all radii except 3–4 mm (P = 0.74) for FITC-NeutrAvidin and 2–3 mm (P = 0.058) for RPE-NeutrAvidin. The permeability for Qdot 800-streptavidin was significantly decreased by shear at all radii examined (all P < 0.0001), but there was a slight rise in permeability from the center of the well toward the edge (Fig. 7C) that is also visible in Fig. 6F.
Fig. 7.
Histogram showing means + SD of tracer accumulation under cultured monolayers, with and without shear, at different radial distances from the center of a well for 60-kDa FITC-NeutrAvidin (A), 310-kDa R-phycoerythrin-NeutrAvidin (B), and ~20-nm Qdot 800-streptavidin (C). n = 6 isolations for FITC-NeutrAvidin and R-phycoerythrin-NeutrAvidin and n = 3 isolations for Qdot 800-streptavidin. In A and B, a plot of minimum transverse WSS (transWSSmin) has also been included (see discussion).
In agreement with the permeability maps, higher-resolution confocal images also showed that the monolayer was more permeable to FITC-NeutrAvidin and RPE-NeutrAvidin at the center of the well than toward its edge under shear but not under static conditions (Fig. 8).
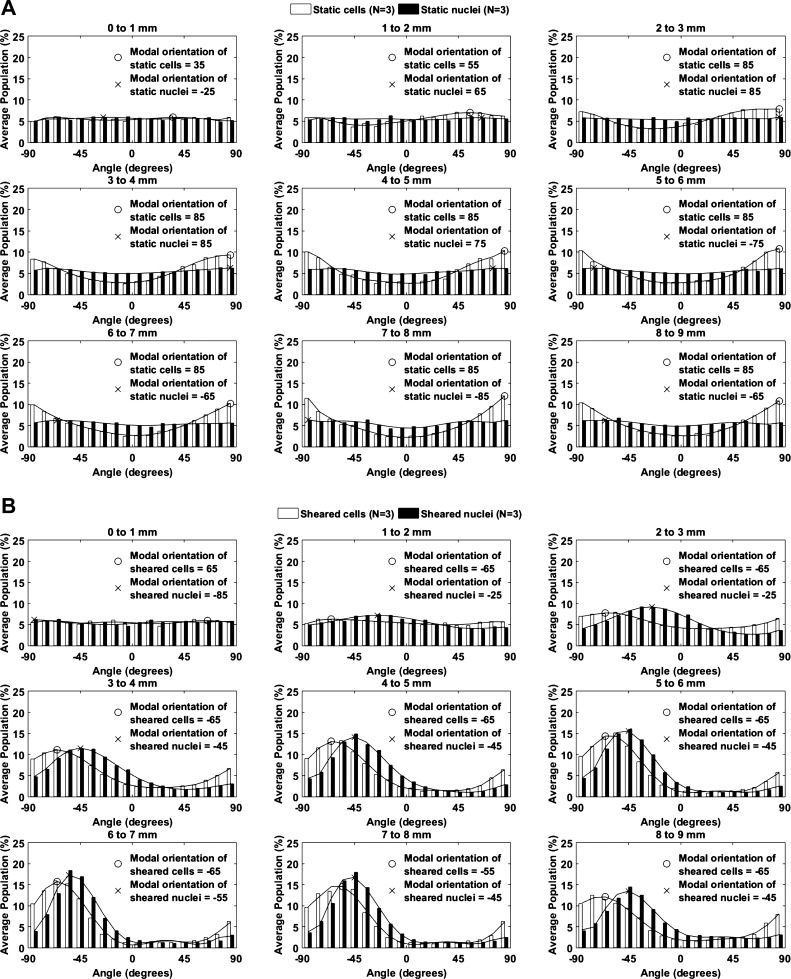
Cell and nuclear alignment and orientation in vitro.
The orientation of cultured endothelial cells and their nuclei at different radial distances from the center of the well is shown in Fig. 9. Static conditions (Fig. 9A) are considered first. Near the center of the well, all orientations were approximately equally represented. With increasing distance from the center, nuclei remained randomly oriented, but there was a tendency for the cells to become aligned in the circumferential direction (i.e., ±90°), presumably reflecting a confining influence of the edge of the well (where cells align circumferentially) that propagated toward the center. When shear was applied for 1 wk (Fig. 9B), cells and nuclei remained randomly oriented near the center of the well. At distances ≥3 mm from the center, different angles predominated for cells (typically −65°) and nuclei (−45°).
Fig. 9.
Orientations of endothelial cells and their nuclei at different distances from the center of the well under static conditions (A) and after exposure to shear for 1 wk (B). Orientation was defined as the angular deviation of the long axis of the nucleus from a radial line to the center of the well, with positive numbers indicating a clockwise rotation of the end of the nucleus nearest the center of the well. Lines are best-fit seventh-order polynomials, and the modal orientation for each line is shown (n = 3 isolations).
Transport pathways in vivo.
Immunofluorescence staining with the antibody raised against the NC1 domain of collagen type IV was used to test its specificity to the endothelial basement membrane in rabbit aorta. Figure 10A shows a thin line of staining immediately under the endothelium in a section through the aorta. The line was discontinuous, being seen under some regions of the endothelium (such as the region illustrated) but not others. There was little fluorescence from the aortic media, suggesting that the antibody did not recognize the basement membrane of smooth muscle cells or other matrix components. Equivalent findings have previously been obtained for sections of bovine aorta stained with an antibody raised against a synthetic fragment from the NC1 domain of the α3-chain of collagen type IV (40). The pattern is consistent with specific staining of the endothelial basement membrane.
Fig. 10.
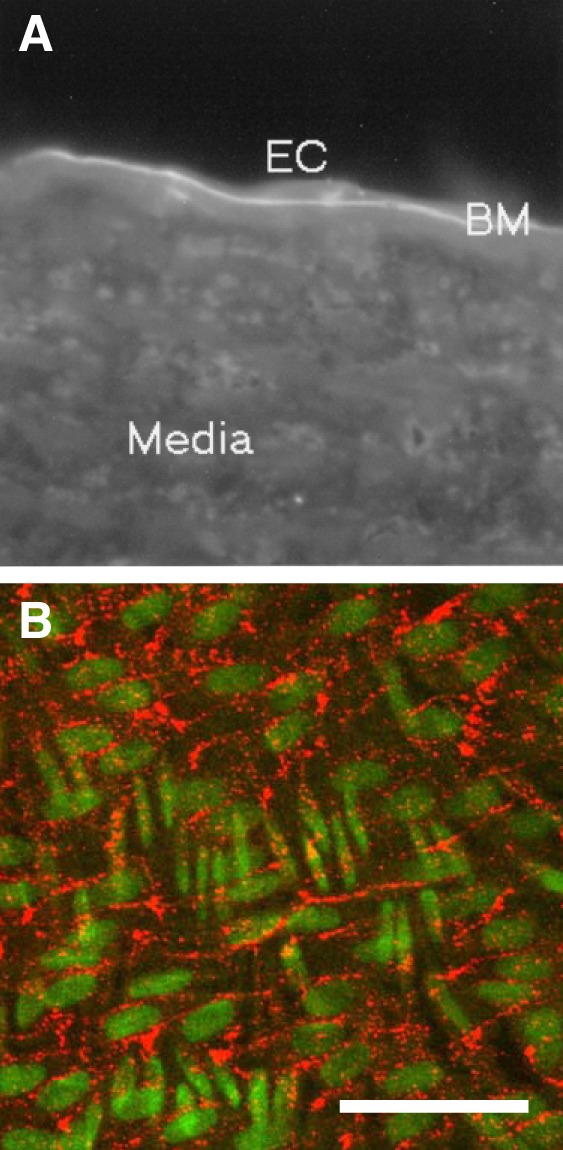
A: binding of an antibody raised against the NC1 domain of collagen type IV to a section of the rabbit aorta, examined by digital-imaging fluorescence microscopy after application of rhodamine-labeled rabbit anti-sheep IgG. The pattern is consistent with specific binding to the endothelial basement membrane (BM). EC, endothelial cell. B: maximum intensity projection of an en face confocal image stack of the descending thoracic aorta of a rabbit administered the same antibody, labeled with rhodamine, intravenously. Nuclei (green) of endothelial cells are oriented approximately left–right, whereas the more fusiform nuclei of underlying smooth muscle cells run approximately top–bottom. The localization of antibody (red) is consistent with transendothelial transport through bicellular and tricellular junctions. Bar = 100 μm. n = 6 rabbits.
Figure 10B shows a maximum intensity projection of a confocal image stack of the aorta of a rabbit that had been administered the rhodamine-labeled anti-NC1 antibody intravenously. The aorta was fixed in situ, excised, and imaged from the endothelial surface. The counterstained nuclei of endothelial and underlying smooth muscle cells can clearly be distinguished from each other by their orientation. Fluorescence from the antibody was distributed in lines and lines of spots that formed a tessellated pattern. The lines did not intersect endothelial nuclei, and each “tile” contained such a nucleus. The tiles were elongated in broadly the same direction as the nuclei themselves. Larger spots were seen at the intersections of many of the lines. Overall, the pattern bore a strong resemblance to the pattern of uptake for FITC-avidin and FITC-NeutrAvidin in vitro (Fig. 2, A and B). There was no bright staining under the body of the endothelial cells (although fainter staining cannot be ruled out), and there were no obvious lines oriented in the direction of the smooth muscle cell nuclei, as would be the case if tracer had penetrated into the media. The pattern is consistent with transendothelial transport through bicellular and tricellular junctions and immobilization of the tracer before the media is reached.
DISCUSSION
The classical “two-pore theory” of transendothelial transport was proposed 60 yr ago by Grotte, who found that the lymph-to-plasma concentration ratio for circulating dextran dropped sharply with increasing dextran size up to a radius of 4–5 nm but was not strongly affected by further increases (18). A similar discontinuity is seen in direct measurements of the permeability of capillary endothelium to lipid-insoluble molecules of various sizes (42). There is considerable evidence that the conceptual small pore, allowing passage of molecules of radius up to ~4 nm, represents gaps in tight junctions between adjacent endothelial cells, overlain by glycocalyx (11). Grotte’s large pores, allowing the transport of macromolecules with radius >4 nm, would have to be substantially wider to explain the insensitivity of permeability to solute radius and would have to be rare, otherwise they would also dominate the transport of the smaller solutes. There is no consensus concerning their physical nature; it is debated whether such transport is active or passive, whether it is receptor dependent or independent, and whether it occurs through or between cells (32, 39, 44, 46, 56).
Plasma proteins of the size of albumin (Stokes-Einstein radius: 3.6 nm) should be transported to a significant extent through both small and large pores. In the present study, as in that of Dubrovskyi et al. (13), FITC-avidin (similar in mass to albumin) was transported through bicellular junctions, the site of the putative small pore, in cultured endothelium. The lines were sometimes discontinuous, consistent with structural inhomogeneity. Furthermore, not all bicellular junctions were involved; in any one image, tracer was seen under some junctions but not others, suggesting that the permeability of this pathway can be regulated. Consistent with this view, Dubrovskyi et al. (13) demonstrated that transport through the junctions was increased by thrombin, an agent known to raise permeability and to modify the structure of intercellular junctions (40). A novel observation of the present study was the frequent occurrence of spots of high FITC-avidin uptake at, or close to, tricellular junctions.
The same general trends were observed when FITC-NeutrAvidin rather than FITC-avidin was used. However, the lines of high uptake tended to be broader and less continuous, and uptake at tricellular junctions was somewhat less pronounced, although exceptions were observed. Weaker interactions of the less highly charged FITC-NeutrAvidin with the negatively charged glycocalyx, or altered hydrated radius, may modify its transendothelial passage compared with that of FITC-avidin (29, 51).
RPE alone is expected to have a Stokes-Einstein radius of ~5.5 nm (31), so RPE-NeutrAvidin should not pass through small pores to a significant extent. It crossed the endothelium primarily at tricellular junctions and not through bicellular junctions, implying that the tricellular junctions serve as a pathway for larger macromolecules in cultured endothelium, whereas the bicellular junctions have a cutoff somewhere between the size of FITC-avidin and RPE-NeutrAvidin. Not all tricellular junctions were involved, again consistent with some form of regulation in which the fraction of open junctions is under physiological control, leading to alterations of permeability to molecules using this pathway. For example, 18 ± 0.8% of tricellular junctions were associated with FITC-avidin spots under static conditions, but toward the edge of the well this decreased to 6.4 ± 0.4% (mean ± SE, n = 3 isolations, P < 0.001) when shear was applied.
We are not aware of any previous studies demonstrating the tricellular pathway for macromolecule transport across vascular endothelium, although the possibility has been postulated on theoretical grounds (49). Krug et al. (24) recently demonstrated that tricellular junctions are important in transport across cultured epithelium, but only for solutes of relative molecular mass up to 20 kDa; 70-kDa dextran (similar in mass to avidin) was used as an impermeant marker, clearly indicating different barrier properties. In endothelium, tricellular junctions are preferred sites for leukocyte transmigration (6). The tricellular junction has a number of features that make it a convincing candidate for a large pore. First, these junctions occupy an even smaller fraction of the endothelial surface area than bicellular junctions. Second, electron microscopy and immunofluorescence studies suggest that tight and adherens junctions are particularly discontinuous at tricellular borders (3, 5, 52). Third, the junctions would allow convective transport and hence can account in a simple fashion for effects of pressure gradients on macromolecule transport.
Qdot 800-streptavidin, which has a radius of ~20 nm, was not seen under bicellular or tricellular junctions but underneath the body of the cells, consistent with the existence of a third, even larger, pore. The absence of elevated concentrations under junctions was confirmed at longer durations of exposure to tracer (Fig. 4D), and absence of tracer under nuclei was observed even when the nucleus lay close to a junction, reinforcing the view that paracellular transport was not involved. The diffuse distribution of the tracer and the negligible concentration under nuclei suggest transport via some vesicular process, such as transcytosis, transient vesicular fusion with transfer of contents, or the fusion of vesicles to form patent transcellular tubes (9, 14, 39, 45). Transport of the smaller tracers may also have occurred via this pathway (fluorescence from such transport would have been hard to detect because of the much brighter emission near junctions), but it represented, at most, a minor component of their overall accumulation.
Tarbell and colleagues have proposed the existence of three pores for macromolecule transport across cultured endothelium (23, 30), but the proposed pores were bicellular junctions, vesicles, and leaky junctions around dying or dividing cells. On the basis of a mathematical analysis of transport data, they deduced that >90% of LDL transport and >40% of albumin transport occurs through the leaky junctions. Our data, obtained by direct visualization, do not suggest a substantial role for leaky junctions, at least under nonconvective conditions. We did observe cell-to-cell variation in permeability, but this was too prevalent to be explained by dying and dividing cells.
It is conceivable that endothelial transport pathways identified in culture may not pertain in vivo, and this applies particularly to the paracellular routes since permeability to albumin-based tracers appears to be substantially higher in culture than in intact animals (1). Bicellular and tricellular junctions might be more leaky in vitro than in vivo, perhaps because the endothelial glycocalyx is less well developed in culture; the distribution of the glycocalyx in regions where two or more cells come into contact should be a particular focus of further evaluation. The avidin-biotin method cannot be applied in vivo, but it should be possible to exploit the same general principle by developing tracers that become immobilized in the subendothelium. In preliminary experiments to demonstrate the feasibility of this approach, we raised antibodies to a component of the endothelial basement membrane, labeled them with a fluorescent dye, and administered them to rabbits intravenously. Immunostaining of histological sections with the antibody gave a pattern of fluorescence that was consistent with specific labeling of the endothelial basement membrane. Maximum projections of en face confocal image stacks of rabbit aortas exposed to the tracer in vivo showed a tracer distribution that strongly resembled the one seen for FITC-avidin and FITC-NeutrAvidin in vitro, suggesting transendothelial tracer transport through bicellular and tricellular junctions.
These methods are less developed than the ones we used in vitro; further validation is required, for example, by using labeled nonspecific antibodies to check for passive sieving of the tracer [although a previous study (21) has suggested that fluorescent microparticles as large as 30 nm (3 times the size of IgG) can pass through endothelial basement membrane]. The images appear to confirm that the same general approach to determining transport routes can be employed in vivo, and they also suggest that our in vitro findings, at least for smaller macromolecular tracers, are physiologically relevant. Preparation of antigen-binding fragments (Fab) and F(ab′)2 and selective purification of IgG and IgM would allow characterization of transport routes for a homologous tracer series with molecular weights of ~50, 110, 150, and 970 kDa.
To investigate effects of flow on transendothelial transport, we swirled the multiwell culture plates on an orbital shaker. Traditional methods for shearing endothelial cells such as parallel-plate flow chambers and cone-and-plate viscometers allow the imposition of steady, oscillatory, and pulsatile flows along one axis. Rotating the substrate can additionally give the multidirectional flow that recent work suggests is important in disrupting endothelial homeostasis (53) and triggering disease (34, 35), but current implementations allow only occasional changes of the axis along which the WSS is imposed (54). The swirling-well method provides both uniaxial and multidirectional flows at physiological frequencies in conjunction with mean WSS values and amplitudes of oscillation that are appropriate for the arterial cell type. It additionally permits high throughput and chronic exposure. Although the flows in this system are hard to control, they can be characterized (37, 38, 55). Combining the swirling-well method with the avidin-biotin technique of Dubrovskyi et al. (13), detection with a scanning plate reader, and numerical simulation of the flow enables effects of multidirectional flows to be studied conveniently in vitro.
For FITC-NeutrAvidin and RPE-NeutrAvidin, chronic application of flow increased permeability in the center of the well and decreased it toward the edge. Although both tracers were affected in a qualitatively similar way, there were quantitative differences: the increase in the center was smaller and the decrease at the edge was larger with the larger tracer (increase >60 vs. <50% and decrease <10 vs. >50%, respectively). This suggests that bicellular and tricellular junctions may respond to flow in subtly different ways.
The instantaneous WSS vectors were used to calculate four established WSS metrics: one pair (the TAWSS and MagMeanWSS) represent the average magnitude of the WSS over each cycle, while a second pair (the OSI and transWSS) characterize the directionality of the flow. The radial gradient of permeability to FITC-NeutrAvidin and RPE-NeutrAvidin in orbiting wells was in the same general direction as the gradients for TAWSS, the OSI, and transWSS and in the opposite direction to the gradient for the MagMeanWSS. However, none of the correlations were convincing: the plot of radial variation in permeability had a markedly different shape from the plots for these shear metrics.
We therefore examined the possibility that a novel WSS metric might correlate better with the pattern of paracellular permeability under flow. TransWSS averages over the cycle those components of instantaneous WSS vectors that act perpendicularly to the mean WSS vector. Endothelial cells are thought to elongate and align in the direction of the mean WSS vector (12, 15, 16, 27, 28), putatively to minimize the overall shear stress that they experience (58), and transWSS is therefore thought to quantify shear acting across the long axis of the cell. However, cells might not align with the mean WSS vector under complex multidirectional flow. We speculated that they align so as to minimize the components of shear across their long axis, rather than to minimize total WSS. Transverse flows upregulate proinflammatory signals in endothelium (53), and the cells may have evolved a mechanism for reducing this. To test this possibility, we computed a novel metric, the transWSSmin, which is the time-average WSS component that would occur across the long axis of the cell if the cell aligned so as to minimize it. The radial distribution of this new metric shows a convincing correspondence with the pattern of permeability to FITC-NeutrAvidin and RPE-NeutrAvidin under shear (Fig. 7): correlation coefficients (r = 0.97 for FITC-NeutrAvidin and r = 0.94 for RPE-NeutrAvidin) were higher than for the other metrics (all <0.9).
This hypothesis can be further tested because it predicts a different orientation of the endothelial cells from the one that would result if the cells aligned with the mean WSS vector. Figure 11 shows the orientation that would be expected at different distances from the center of the well if the cells aligned with the mean WSS vector, as commonly assumed, or if they aligned so as to minimize WSS components across their long axis, as proposed here. The observed modal orientations of cells and their nuclei, also shown in Fig. 11, follow the trend computed for minimizing transverse WSS. Taken together, these data suggest that transWSSmin is a critical metric in defining endothelial alignment and paracellular permeability, and probably other properties as well, at least within the range of shear metrics employed in the present study.
Fig. 11.
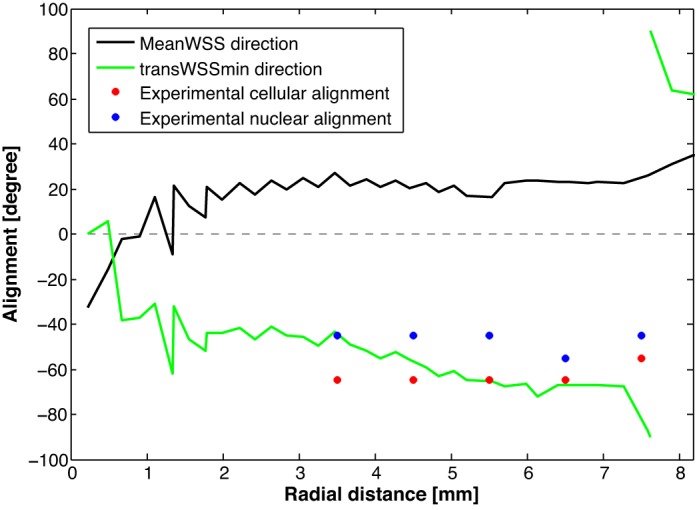
The orientations of cells at different radial locations that would occur if the cells aligned with the mean wall shear stress (WSS) or aligned to obtain minimum transverse WSS (transWSSmin) and the observed modal orientations of cells and their nuclei. These alignments are defined as for Fig. 9; −90 and +90° are equivalent since the two ends of the nuclei are indistinguishable. Data from the three locations nearest the center of the well were excluded since χ2-tests showed random cellular orientations in all three replicates, and the modal values are therefore determined by noise in these cases. (Directions defined by the shear metrics also become increasingly noisy toward the center.)
Surprisingly, the application of the highly nonuniform shear field resulted in a nearly uniform reduction in the transport of Qdot 800-streptavidin. The uniform reduction may mean that many kinds of flow reduce vesicular transport. An alternative explanation is that flow causes the release of a soluble mediator from at least some of the cells that is then well mixed in the swirling medium and reduces transcellular transport in all the cells to the same extent.
In conclusion, our data are consistent with the presence of three different pathways for the transendothelial transport of macromolecules across cultured endothelium and with different effects of flow on the paracellular and transcellular pathways. Furthermore, we have shown that endothelial cells align close to the direction that minimizes WSS across their long axis and presented evidence that this minimized shear component, termed “transWSSmin,” plays a critical role in determining paracellular permeability. Our results are consistent with the release from sheared endothelium of a soluble mediator that reduces transport via the transcellular route. Additional work is required to determine whether these phenomena also occur under different values of the shear metrics and with cells grown on different substrates and to further develop the proof-of-concept in vivo methods.
GRANTS
This work was supported by a Department of Bioengineering studentship (to M. Ghim), a Talentia Fellowship from the Andalucian Regional Ministry of Economy, Innovation, Science and Employment (to P. Alpresa), a studentship from the Imperial College British Heart Foundation Centre of Research Excellence (to S. G. Gray), and a British Heart Foundation program grant (to P. D. Weinberg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.G., P.A., S.J.S., M.v.R., and P.D.W. conceived and designed research; M.G., P.A., S.Y., S.T.B., and S.G.G. performed experiments; M.G., P.A., S.Y., S.T.B., and S.G.G. analyzed data; M.G., P.A., S.Y., S.J.S., M.v.R., and P.D.W. interpreted results of experiments; M.G. and P.A. prepared figures; M.G., P.A., S.Y., S.G.G., and P.D.W. drafted manuscript; M.G., P.A., and P.D.W. edited and revised manuscript; M.G., P.A., S.Y., S.T.B., S.G.G., S.J.S., M.v.R., and P.D.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sally E. Barnes, Dr. Andrew R. Bond, and Dr. Mike K. Shaw for assistance with generating antibodies and using the antibodies in vivo.
REFERENCES
- 1.Albelda SM, Sampson PM, Haselton FR, McNiff JM, Mueller SN, Williams SK, Fishman AP, Levine EM. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol 64: 308–322, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Anitschkow NN. Experimental arteriosclerosis in animals. In: Arteriosclerosis: a Survey of the Problem, edited by Cowdry EV. New York: MacMillan, 1933, p. 271–322. [Google Scholar]
- 3.Barry PA, Petroll WM, Andrews PM, Cavanagh HD, Jester JV. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Invest Ophthalmol Vis Sci 36: 1115–1124, 1995. [PubMed] [Google Scholar]
- 4.Bogle RG, Baydoun AR, Pearson JD, Mann GE. Regulation of l-arginine transport and nitric oxide release in superfused porcine aortic endothelial cells. J Physiol 490: 229–241, 1996. doi: 10.1113/jphysiol.1996.sp021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev 83: 309–336, 2003. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 6.Burns AR, Walker DC, Brown ES, Thurmon LT, Bowden RA, Keese CR, Simon SI, Entman ML, Smith CW. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol 159: 2893–2903, 1997. [PubMed] [Google Scholar]
- 7.Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci 177: 109–159, 1971. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- 8.Clarke LA, Zahra Mohri, Weinberg PD. High throughput en face mapping of arterial permeability using tile scanning confocal microscopy. Atherosclerosis 224: 417–425, 2012. doi: 10.1016/j.atherosclerosis.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Clough G, Michel CC. The role of vesicles in the transport of ferritin through frog endothelium. J Physiol 315: 127–142, 1981. doi: 10.1113/jphysiol.1981.sp013737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry FE. Determinants of capillary permeability: a review of mechanisms based on single capillary studies in the frog. Circ Res 59: 367–380, 1986. doi: 10.1161/01.RES.59.4.367. [DOI] [PubMed] [Google Scholar]
- 11.Curry FE, Michel CC. A fiber matrix model of capillary permeability. Microvasc Res 20: 96–99, 1980. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- 12.Dewey CF Jr, Bussolari SR, Gimbrone MA Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103: 177–185, 1981. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 13.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 93: 254–263, 2013. doi: 10.1038/labinvest.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem 49: 419–432, 2001. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 15.Eskin SG, Ives CL, McIntire LV, Navarro LT. Response of cultured endothelial cells to steady flow. Microvasc Res 28: 87–94, 1984. doi: 10.1016/0026-2862(84)90031-1. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res 30: 23–33, 1972. doi: 10.1161/01.RES.30.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Fry DL. Certain chemorheologic considerations regarding the blood vascular interface with particular reference to coronary artery disease. Circulation 40, 5S4: 38–57, 1969. doi: 10.1161/01.CIR.40.5S4.IV-38. [DOI] [Google Scholar]
- 18.Grotte G. Passage of dextran molecules across the blood-lymph barrier. Acta Chir Scand Suppl 211: 1–84, 1956. [PubMed] [Google Scholar]
- 19.He X, Ku DN. Pulsatile flow in the human left coronary artery bifurcation: average conditions. J Biomech Eng 118: 74–82, 1996. doi: 10.1115/1.2795948. [DOI] [PubMed] [Google Scholar]
- 20.Hudson BG, Gunwar S, Chung AE, Burgeson RE. Matrix components (types IV and VII collagen, entactin, and laminin) found in basement membranes. In: Extracellular Matrix: a Practical Approach, edited by Haralson MA and Hassell JR. Oxford, UK: IRL, 1995, chapt. 4, p. 99–129. [Google Scholar]
- 21.Jiang Y, Wen K, Zhou X, Schwegler-Berry D, Castranova V, He P. Three-dimensional localization and quantification of PAF-induced gap formation in intact venular microvessels. Am J Physiol Heart Circ Physiol 295: H898–H906, 2008. doi: 10.1152/ajpheart.00309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo H, Dull RO, Hollis TM, Tarbell JM. Endothelial albumin permeability is shear dependent, time dependent, and reversible. Am J Physiol Heart Circ Physiol 260: H1992–H1996, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Kang H, Cancel LM, Tarbell JM. Effect of shear stress on water and LDL transport through cultured endothelial cell monolayers. Atherosclerosis 233: 682–690, 2014. doi: 10.1016/j.atherosclerosis.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell 20: 3713–3724, 2009. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5: 293–302, 1985. doi: 10.1161/01.ATV.5.3.293. [DOI] [PubMed] [Google Scholar]
- 26.Lei M, Kleinstreuer C, Truskey GA. Numerical investigation and prediction of atherogenic sites in branching arteries. J Biomech Eng 117: 350–357, 1995. doi: 10.1115/1.2794191. [DOI] [PubMed] [Google Scholar]
- 27.Levesque MJ, Liepsch D, Moravec S, Nerem RM. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis 6: 220–229, 1986. doi: 10.1161/01.ATV.6.2.220. [DOI] [PubMed] [Google Scholar]
- 28.Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng 107: 341–347, 1985. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 29.Levick JR, Michel CC. The effect of bovine albumin on the permeability of frog mesenteric capillaries. Q J Exp Physiol Cogn Med Sci 58: 87–97, 1973. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Quintero SV, Ji XY, Antonetti DA, Tarbell JM. A three-pore model describes transport properties of bovine retinal endothelial cells in normal and elevated glucose. Invest Ophthalmol Vis Sci 52: 1171–1180, 2011. doi: 10.1167/iovs.10-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacColl R, Eisele LE, Williams EC, Bowser SS. The discovery of a novel R-phycoerythrin from an antarctic red alga. J Biol Chem 271: 17157–17160, 1996. doi: 10.1074/jbc.271.29.17157. [DOI] [PubMed] [Google Scholar]
- 32.Michel CC, Nanjee MN, Olszewski WL, Miller NE. LDL and HDL transfer rates across peripheral microvascular endothelium agree with those predicted for passive ultrafiltration in humans. J Lipid Res 56: 122–128, 2015. doi: 10.1194/jlr.M055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JRA, Schwartz CJ. Arterial Disease. Oxford, UK: Blackwell, 1965. [Google Scholar]
- 34.Mohamied Y, Rowland EM, Bailey EL, Sherwin SJ, Schwartz MA, Weinberg PD. Change of direction in the biomechanics of atherosclerosis. Ann Biomed Eng 43: 16–25, 2015. doi: 10.1007/s10439-014-1095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peiffer V, Sherwin SJ, Weinberg PD. Computation in the rabbit aorta of a new metric - the transverse wall shear stress - to quantify the multidirectional character of disturbed blood flow. J Biomech 46: 2651–2658, 2013. doi: 10.1016/j.jbiomech.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peiffer V, Sherwin SJ, Weinberg PD. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res 99: 242–250, 2013. doi: 10.1093/cvr/cvt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potter CM, Lundberg MH, Harrington LS, Warboys CM, Warner TD, Berson RE, Moshkov AV, Gorelik J, Weinberg PD, Mitchell JA. Role of shear stress in endothelial cell morphology and expression of cyclooxygenase isoforms. Arterioscler Thromb Vasc Biol 31: 384–391, 2011. doi: 10.1161/ATVBAHA.110.214031. [DOI] [PubMed] [Google Scholar]
- 38.Potter CM, Schobesberger S, Lundberg MH, Weinberg PD, Mitchell JA, Gorelik J. Shape and compliance of endothelial cells after shear stress in vitro or from different aortic regions: scanning ion conductance microscopy study. PLoS One 7: e31228, 2012. doi: 10.1371/journal.pone.0031228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Predescu D, Palade GE. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am J Physiol Heart Circ Physiol 265: H725–H733, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol 16: 488–496, 1996. doi: 10.1161/01.ATV.16.3.488. [DOI] [PubMed] [Google Scholar]
- 41.Reddy GK, Gunwar S, Kalluri R, Hudson BG, Noelken ME. Structure and composition of type IV collagen of bovine aorta. Biochim Biophys Acta 1157: 241–251, 1993. doi: 10.1016/0304-4165(93)90106-I. [DOI] [PubMed] [Google Scholar]
- 42.Renkin EM, Curry FE. Transport of water and solutes across capillary endothelium. In: Membrane Transport in Biology, edited by Giebisch G, Tosteson DC, and Ussing HH. Berlin: Springer-Verlag, 1978, vol. IV, p. 1–45. [Google Scholar]
- 43.Richter JF, Schmauder R, Krug SM, Gebert A, Schumann M. A novel method for imaging sites of paracellular passage of macromolecules in epithelial sheets. J Control Release 229: 70–79, 2016. doi: 10.1016/j.jconrel.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Rippe B, Rosengren BI, Carlsson O, Venturoli D. Transendothelial transport: the vesicle controversy. J Vasc Res 39: 375–390, 2002. doi: 10.1159/000064521. [DOI] [PubMed] [Google Scholar]
- 45.Schneeberger EE. Proteins and vesicular transport in capillary endothelium. Fed Proc 42: 2419–2424, 1983. [PubMed] [Google Scholar]
- 46.Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am J Physiol Heart Circ Physiol 262: H246–H254, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Sebkhi A, Weinberg PD. Effect of age on the pattern of short-term albumin uptake by the rabbit aortic wall near intercostal branch ostia. Arterioscler Thromb Vasc Biol 16: 317–327, 1996. doi: 10.1161/01.ATV.16.2.317. [DOI] [PubMed] [Google Scholar]
- 48.Sun L, Wang S, Gong X, Zhao M, Fu X, Wang L. Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expr Purif 64: 146–154, 2009. doi: 10.1016/j.pep.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Tarbell JM. Mass transport in arteries and the localization of atherosclerosis. Annu Rev Biomed Eng 5: 79–118, 2003. doi: 10.1146/annurev.bioeng.5.040202.121529. [DOI] [PubMed] [Google Scholar]
- 50.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 87: 320–330, 2010. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner MR, Clough G, Michel CC. The effects of cationised ferritin and native ferritin upon the filtration coefficient of single frog capillaries. Evidence that proteins in the endothelial cell coat influence permeability. Microvasc Res 25: 205–222, 1983. doi: 10.1016/0026-2862(83)90016-X. [DOI] [PubMed] [Google Scholar]
- 52.Walker DC, MacKenzie A, Hosford S. The structure of the tricellular region of endothelial tight junctions of pulmonary capillaries analyzed by freeze-fracture. Microvasc Res 48: 259–281, 1994. doi: 10.1006/mvre.1994.1054. [DOI] [PubMed] [Google Scholar]
- 53.Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arterioscler Thromb Vasc Biol 33: 2130–2136, 2013. doi: 10.1161/ATVBAHA.113.301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C, Lu H, Schwartz MA. A novel in vitro flow system for changing flow direction on endothelial cells. J Biomech 45: 1212–1218, 2012. doi: 10.1016/j.jbiomech.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warboys CM, Eric Berson R, Mann GE, Pearson JD, Weinberg PD. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am J Physiol Heart Circ Physiol 298: H1850–H1856, 2010. doi: 10.1152/ajpheart.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinbaum S, Tzeghai G, Ganatos P, Pfeffer R, Chien S. Effect of cell turnover and leaky junctions on arterial macromolecular transport. Am J Physiol Heart Circ Physiol 248: H945–H960, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Weinberg PD. Rate-limiting steps in the development of atherosclerosis: the response-to-influx theory. J Vasc Res 41: 1–17, 2004. doi: 10.1159/000076124. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi T, Yamamoto Y, Liu H. Computational mechanical model studies on the spontaneous emergent morphogenesis of the cultured endothelial cells. J Biomech 33: 115–126, 2000. doi: 10.1016/S0021-9290(99)00159-1. [DOI] [PubMed] [Google Scholar]