Ovariectomized guinea pig cardiomyocytes have higher frequencies of Ca2+ waves, and isoprenaline-challenged cells display more early afterdepolarizations, delayed afterdepolarizations, and extra beats compared with sham myocytes. These alterations to Ca2+ regulation were not observed in myocytes from ovariectomized guinea pigs supplemented with 17β-estradiol, suggesting that ovarian hormone deficiency modifies cardiac Ca2+ regulation, potentially creating proarrhythmic substrates.
Keywords: ovariectomy, calcium regulation, arrhythmia, 17β-estradiol
Abstract
This study addressed the hypothesis that long-term deficiency of ovarian hormones after ovariectomy (OVx) alters cellular Ca2+-handling mechanisms in the heart, resulting in the formation of a proarrhythmic substrate. It also tested whether estrogen supplementation to OVx animals reverses any alterations to cardiac Ca2+ handling and rescues proarrhythmic behavior. OVx or sham operations were performed on female guinea pigs using appropriate anesthetic and analgesic regimes. Pellets containing 17β-estradiol (1 mg, 60-day release) were placed subcutaneously in selected OVx animals (OVx + E). Cardiac myocytes were enzymatically isolated, and electrophysiological measurements were conducted with a switch-clamp system. In fluo-4-loaded cells, Ca2+ transients were 20% larger, and fractional sarcoplasmic reticulum (SR) Ca2+ release was 7% greater in the OVx group compared with the sham group. Peak L-type Ca2+ current was 16% larger in OVx myocytes with channel inactivation shifting to more positive membrane potentials, creating a larger “window” current. SR Ca2+ stores were 22% greater in the OVx group, and these cells showed a higher frequency of Ca2+ sparks and waves and shorter wave-free intervals. OVx myocytes showed higher frequencies of early afterdepolarizations, and a greater percentage of these cells showed delayed afterdepolarizations after exposure to isoprenaline compared with sham myocytes. The altered Ca2+ regulation occurring in the OVx group was not observed in the OVx + E group. These findings suggest that long-term deprivation of ovarian hormones in guinea pigs lead to changes in myocyte Ca2+-handling mechanisms that are considered proarrhythmogenic. 17β-Estradiol replacement prevented these adverse effects.
NEW & NOTEWORTHY Ovariectomized guinea pig cardiomyocytes have higher frequencies of Ca2+ waves, and isoprenaline-challenged cells display more early afterdepolarizations, delayed afterdepolarizations, and extra beats compared with sham myocytes. These alterations to Ca2+ regulation were not observed in myocytes from ovariectomized guinea pigs supplemented with 17β-estradiol, suggesting that ovarian hormone deficiency modifies cardiac Ca2+ regulation, potentially creating proarrhythmic substrates.
although the risk of cardiovascular disease (CVD) is lower in premenopausal women than in age-matched men, with CVD developing ~10 yr later in women compared with men (56), the risk increases noticeably in women after menopause (39, 45). Furthermore, in animal models, long-term deficiency of estrogen was associated with cardiac contractile dysfunction (36, 71), reduced myofilament force, and increased Ca2+ sensitivity (37). Clinical observations show that although premenopausal women have lower incidence of sudden cardiac death compared with men, which could be related to reduced risk of malignant ventricular arrhythmias and delayed onset of coronary artery disease, postmenopausal women seem to lose this protection (19, 29, 40, 41, 46). Postmenopausal women are more vulnerable to arrhythmia-related sudden cardiac death compared with premenopausal women (54, 64). Notably, premenopausal women with ventricular premature beats that mostly originate from the right ventricular outflow tract show decreased premature beat frequency around ovulation when serum estradiol is at its peak (14).
Although these clinical findings suggest that ovarian hormones may provide some cardiovascular protection and reduce the incidence of proarrhythmic events (2, 32, 34, 65), large studies examining hormone replacement therapy (HRT) showed no cardiovascular benefit (47). These conflicting results led to the testing of a hypothesis, which suggests that the timing of HRT is important and that women receiving such therapy early after menopause benefit from reduced risk of serious cardiovascular events (49). However, the association between serum estrogen levels and arrhythmias appears complex. For example, in acquired long-QT syndrome, drug-induced QT prolongation is more pronounced, and the risk for polymorphic ventricular tachycardia is greater when estradiol serum levels peak during the estrous cycle, suggesting a proarrhythmic role for estradiol (35). Therefore, further investigation is required to clarify the effect of ovarian hormones on cardiac function and elucidate underlying mechanisms.
Although there have been some studies on excitation-contraction (EC) coupling and Ca2+ regulation in cardiomyocytes after ovariectomy (OVx) in rats and mice (8, 17, 26), the underlying mechanisms altered by estrogens remain ambiguous and, given the species used, may not be translatable to humans. More benefit may be achieved by developing and studying an animal whose cardiac EC coupling and Ca2+ regulation are more closely akin to humans.
Furthermore, ineffective Ca2+ regulation is potentially arrhythmogenic, so a deficiency of estrogen could provide a proarrhythmic substrate. Indeed, an increase in susceptibility to epinephrine-induced arrhythmias (for example, premature ventricular contractions) was noted in OVx rats compared with gonad-intact animals (59). A clinical investigation also revealed that postmenopausal women who have idiopathic outflow tract ventricular arrhythmia had lower serum estradiol levels than control postmenopausal women, and the occurrence of ventricular arrhythmia events was lower in women with estrogen replacement (23).
The aims of the present study were to assess whether long-term deprivation of ovarian estrogen in the guinea pig alters intracellular Ca2+ regulation in ventricular myocytes and leads to more arrhythmogenic events. We aimed to identify which Ca2+ regulatory mechanisms are affected and whether estrogen supplementation to OVx guinea pigs reverses any alterations to cardiac Ca2+ handling and rescues proarrhythmic behavior. Our rationale for using the guinea pig in these experiments is that in many relevant physiological aspects it displays similar features to humans. The relative amounts of transsarcolemmal Ca2+ influx and sarcoplasmic reticulum (SR) Ca2+ release in their cardiac myocytes are almost identical to that of humans in contrast to the corresponding fluxes in rats or mice, which are far removed from the human situation because of their very “high gain” EC coupling system (3). Most ionic currents shaping the ventricular action potential of the guinea pig are similar to humans and very dissimilar to rats or mice. In terms of sex hormone balance and steroidogenesis, again the guinea pig is similar to humans (33, 58).
METHODS
All animal experiments were carried out with the approval of the Home Office, United Kingdom, and in accordance with the United Kingdom Home Office Guide on the Operation of the Animals (Scientific Procedures) Act 1986 under assurance number A5634-01.
Animal Preparation
Female adult Dunkin-Hartley guinea pigs (400–500 g) underwent either OVx through a bilateral flank approach or a sham operation that left the gonads intact. Animals were anesthetized with 2–4% isoflurane (Isoflo, Abbott)-95% O2 mix. Atropine sulfate (0.05 mg/kg sc; atrocare, Animalcare) was administered to anaesthetized animals to reduce saliva secretions followed by prophylactic antibiotic [enrofloxacin (5 mg/kg sc), Baytril, Bayer] and analgesic [carprofen (4.4 mg/kg sc), Rimadyl, Pfizer, and buprenorphine (0.05 mg/kg sc), Vetergesic, Alstoe]. Local anesthetic was injected [Bupivacaine (2 mg/kg sc), Macaine Polyamp, AstraZeneca] around the incision site. Carprofen (Rimadyl, 15 mg in 300 ml water) was given orally for 2 days postoperatively.
To establish a long-term absence of ovarian estrogen in OVx-operated animals, the three animal groups (sham, OVx, and OVx + E) were used 150 days postsurgery. Pellets (60-day release) containing 1 mg 17β-estradiol (Innovative Research of America) were implanted subcutaneously in the back region of selected OVx animals under isoflurane inhalation anesthesia 90 days post-OVx surgery. Throughout the study, animals were housed at 21 ± 1°C in a controlled lighting environment (12:12-h light-dark cycles). After surgery, guinea pigs were fed ad libitum with water and a casein-based, soy-free diet to minimize the uptake of phytoestrogens.
Echocardiography
M-mode echocardiography was performed using a HP Sonos 5500 echo machine on unanesthetized guinea pigs to avoid anesthetic effects on heart function. Left ventricular (LV) internal diameters at diastole (LVIDd) and systole (LVIDs) were measured to calculate fractional shortening (FS) as follows: FS = (LVIDd − LVIDs)/LVIDd.
Serum 17β-Estradiol
Blood samples were collected in serum separator tubes (Becton Dickinson) and centrifuged at 3,000 revolutions/min for 15 min at 4°C. The serum supernatant was transferred to low-binding protein Eppendorf tubes and stored at −80°C until assayed. Levels of serum 17β-estradiol were measured by quantitative sandwich ELISA according to the manufacturer's instructions (MyBioSource).
Myocyte Isolation
LV myocytes were isolated from sham, OVx, or OVx + E guinea pig hearts as previously described (31) but using the Liberase TL enzyme (Liberase, Roche). Hearts were retrogradely perfused at 37°C with Krebs-Henseleit (KH) solution for 1–2 min. The perfusate was switched to low-Ca2+ solution for 4–5 min before Liberase TL enzyme (5 mg in 50 ml enzyme solution) was added for 6–10 min. The hearts were then removed from the cannula that supplied the retrograde perfusion, and the LVs were chopped into 2- to 4-mm tissues. Tissues were transferred to a small jar containing the same enzyme solution, shaken, and oxygenated. The supernatant was filtered every 3 min until the digestion was complete. The flow through was centrifuged at low speed (400 revolutions/min) for 1 min. The resulting cell pellet was resuspended in fresh low-Ca2+ solution in the absence of Liberase TL enzyme. Finally, myocytes were stored in DMEM (GIBCO-BRL, Life Technologies) at 20–22°C and were used within 6−8 h.
Composition of Solutions
Tyrode solution.
Normal Tyrode (NT) solution was composed of (in mM) 137.00 NaCl, 5.40 KCl, 10.00 glucose, 10.00 HEPES, 1.00 MgCl2, and 2.00 CaCl2 (pH adjusted to 7.4 with 1 M NaOH).
KH solution.
KH solution was composed of (in mM) 119.00 NaCl, 4.70 KCl, 0.94 MgSO4, 1.00 CaCl2, 1.20 KH2PO4, 25.00 NaHCO3, and 11.50 glucose (pH adjusted to 7.4 with 1 M HCl).
Low-Ca2+ solution.
Low-Ca2+ solution was composed of (in mM) 120.00 NaCl, 5.00 KCl, 5.00 MgSO4, 5.00 Na pyruvate, 20.00 glucose, 20.00 taurine, 10.00 HEPES, and 5.00 nitrilotriacetic acid (NTA) with 35.00 µM CaCl2 (estimated final free Ca2+ concentration: 12–15 µM) (pH adjusted to 6.96 with 1 M NaOH).
Enzyme solution.
Enzyme solution was composed of (in mM) 120.00 NaCl, 5.00 KCl, 5.00 MgSO4, 5.00 Na pyruvate, 20.00 glucose, 20.00 taurine, and 10.00 HEPES with 200.00 µM CaCl2 (pH adjusted to 7.4 with 1 M NaOH).
0 Na+-0 Ca2+ solution.
0 Na+-0 Ca2+ solution was composed of (in mM) 140.00 LiCl, 6.00 KOH, 10.00 glucose, 10.00 HEPES, 1.00 MgCl2, and 1.00 EGTA (pH adjusted to 7.4 with 1 M LiOH).
Intracellular Ca2+ Monitoring
Ca2+ dye loading.
Cells were loaded with the Ca2+-sensitive fluorescent dye fluo-4 AM (Invitrogen, Life Technologies). Cells were incubated with 10 µM fluo-4 AM at room temperature on a rocking platform for 25 min. Thereafter, the cell suspension was centrifuged at low speed (400 revolutions/min) for 1 min before the supernatant was replaced with fresh DMEM. Cells were further incubated at room temperature for at least 30 min to allow for deesterification of the intracellular Ca2+ indicator.
Ca2+ transient.
Cells were superfused at 37°C with NT solution. Ca2+ transients were assessed during steady-state external field stimulation at 0.5 Hz as previously described (52, 55). For Ca2+ transient decay rate determination, a standard single-exponential equation was fitted to the decay trace of a steady-state Ca2+ transient to obtain a time constant from which a rate constant for the Ca2+ transient decay was calculated (13). After the SR Ca2+ content loading protocol (1-Hz stimulation for 20 s), cells were rapidly superfused with 10 mM caffeine in NT or in 0 Na+-0 Ca2+ solution to induce a transient. The decay phase of the caffeine-induced Ca2+ transients were used to determine the rate constants of SERCA and the Na+/Ca2+ exchanger (NCX) as previously described (6). Fractional release (x/y) was calculated using the steady-state Ca2+ transient amplitude (x) and the size of the caffeine-evoked Ca2+ transient in 0 Na+-0 Ca2+ solution (y) (1). One caveat of these approaches is that they are oversimplified. When one extrusion system is inhibited, the relative roles of the other systems may change, so more accurate estimations of their relative roles require consideration of cytosolic Ca2+ buffering, which was not measured here.
Ca2+ sparks and waves.
Briefly, Ca2+ sparks and waves were acquired using a line scanning confocal microscope consisting of an inverted microscope with a Bio-Rad Radiance 2000 confocal attachment. Ca2+ sparks and waves were recorded during a 30-s quiescence period after the SR Ca2+ loading protocol (2-Hz stimulation for 20 s). When isoprenaline (ISO) was added to the superfusate in these types of experiments, it was used at a concentration of 0.5 μM. The SparkMaster plugin for ImageJ (National Institutes of Health) was used to analyze Ca2+ sparks from the line-scan images (42). The detection criterion for Ca2+ sparks was set at 4.2 times the standard deviation above the mean background value, instead of 3.8, to reduce false identification of Ca2+ sparks resulting from background noise. Spark mass was calculated using the following formula: spark mass = amplitude × 1.206 × full width at half-maximal amplitude (FWHM)3 (22). Ca2+ waves were analyzed using an ImageJ macro (52). Wave-free survival analysis was performed using Kaplan-Meier estimates.
Electrophysiological Measurements
Action potential duration (APD), NCX inward current, and L-type Ca2+ channel current (ICa,L) were recorded using a switch-clamping system (Axoclamp 2B amplifier, Molecular Devices) with high-resistance microelectrodes to minimize cell dialysis and preserve the intracellular milieu. Sharp microelectrodes with resistances of 20–40 MΩ were filled with a solution containing 2 M KCl, 5 mM HEPES, and 100 μM EGTA (pH adjusted to 7.20 with KOH). In current-clamp mode, action potentials were elicited with 3-ms current pulses (typically 1.5–2.5 nA) applied at a rate of 0.5 Hz. APDs were expressed as the time to 10% and 90% repolarization (APD10 and APD90, respectively). ICa,L was recorded in discontinuous single electrode voltage-clamp mode (gain: 0.5–1.5 nA/mV and switching rate: 4–6 kHz) in the presence and absence of 200 µM cadmium. ICa,L was evoked from a holding potential of −40 mV by 200-ms steps from −45 to +55 mV in 5-mV increments. Steady-state inactivation of ICa,L was assessed with double-pulse protocols in which conditioning pulses of 200-ms duration and ranging from −45 to +55 mV were followed by a 5-ms step to −40 mV and a 200-ms step to +5 mV. ICa,L was determined by subtracting the trace in the presence of cadmium from the original trace at each voltage. SR Ca2+ content was assessed by integrating the inward NCX current induced by the rapid application of 10 mM caffeine when cells were voltage clamped at −80 mV, as previously described (15).
To elicit early and delayed afterdepolarizations (EADs and DADs, respectively), cells were exposed to 50 nM ISO and paced at various frequencies (0.5, 1.0, and 2.0 Hz) for 30 s. The presence of EADs, appearing as transient repression of the repolarization process and/or reversal of the membrane potential during repolarization, was confirmed by the reversal of the negative first derivative (57) of the membrane potential toward less negative and/or positive values, respectively. DADs were counted if their amplitudes were ≥5 mV.
mRNA Expression of Cav1.2 and Cav β2
Total RNA from cells was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. Primers were obtained from Invitrogen and made using a desalted purification method. Real-time RT-PCR was performed using the one-step QuantiTect SYBR Green PCR kit (Qiagen). Primers were designed using National Center for Biotechnology Information (NCBI)/Primer-Blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Target gene sequences were sourced from NCBI/Gene (https://www.ncbi.nlm.nih.gov/gene/). The primer sequences were as follows: Cav1.2 (encoded by CACNA1C), sense 5′-ACACTGCTGTGGACCTTCATC-3′ and antisense 5′-TGCATACCAATCACGGCATA-3′; Cav β2 (encoded by CACNB2), sense 5′-CCAAGCCCAGTCAAACTAGAAAAC-3′ and antisense 5′-CAAACTGGACGATGAATTTCCTCC-3′; and β-actin (encoded by ACTB), sense 5′-ACTTTGCTGCGTTACACCCT-3′ and antisense 5′-AATCAAAGTCCTCGGCCACA-3′.
Cycle threshold (Ct) values for target mRNA were normalized to the housekeeping gene coding β-actin (ACTB), and the relative gene expression was calculated using the method (27).
Acquisition Systems and Statistical Analysis
The electrophysiological and fluorescence data were recorded and analyzed using Clampex (version 10) acquisition and analysis software (Molecular Devices). The Ca2+ spark morphological parameters of amplitude and mass were log transformed, resulting in normally distributed populations for statistical analysis. Statistical differences between means were calculated using Student’s t-test, two-sample proportion test, and one-way ANOVA with a Fisher post hoc test. Values were expressed as means ± SD; where appropriate, n is the number of cells from the total number of hearts (n = cells/hearts). Statistical significance is denoted as *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
Physical Characteristics of Experimental Animals
The serum estrogen level decreased significantly in OVx guinea pigs and increased when estrogen was replaced (OVx + E treatment group; Table 1). Average body weight significantly increased by 10% in the OVx group, but the ratio of heart weight to body weight remained unchanged compared with the sham group (Table 1). Both uterine and heart weights normalized to body weight increased significantly in the OVx + E group (Table 1).
Table 1.
Physical characteristics of the experimental animals
| Number of Animals/Group | Body Weight, g | Uterine Weight, g | Heart Weight, g | Uterine Weight/Body Weight, % | Heart Weight/Body Weight, % | Serum E2, pg/ml | |
|---|---|---|---|---|---|---|---|
| Sham | 9 | 784.00 ± 85.00 | 1.52 ± 0.47 | 2.38 ± 0.08 | 0.19 ± 0.05 | 0.31 ± 0.04 | 7.80 ± 4.37 |
| OVx | 10 | 870.00 ± 67.00* | 0.37 ± 0.08† | 2.66 ± 0.23† | 0.04 ± 0.01† | 0.31 ± 0.02 | 4.19 ± 1.93* |
| OVx + E | 10 | 711.00 ± 57.00* | 3.89 ± 0.60† | 2.48 ± 0.13 | 0.55 ± 0.10† | 0.35 ± 0.04* | 24.22 ± 3.83† |
Values are expressed as means ± SD. Sham, sham operation; OVx, ovariectomy; OVx + E, OVx with 17β-estradiol treatment.
P < 0.05 and
P < 0.001 represent significant differences compared with the sham group (by one-way ANOVA and Fisher’s test).
In Vivo M-Mode Echocardiography
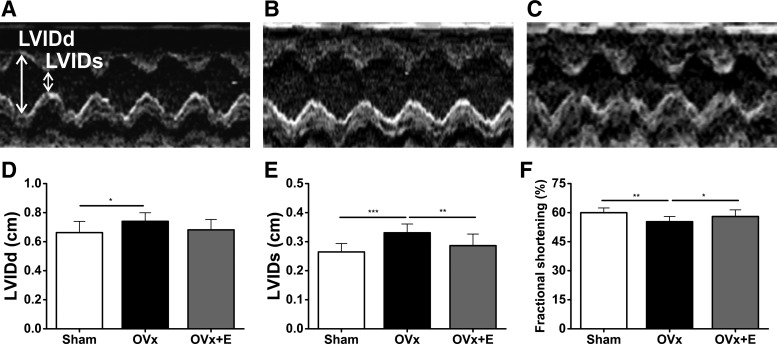
FS, assessed by measuring LVIDd and LVIDs, decreased in the OVx group but remained unchanged in the OVx + E group compared with the sham group (Fig. 1). The OVx group showed larger LVIDs and LVIDd than those in the sham group (Fig. 1).
Fig. 1.
A–C: representative in vivo M-mode echocardiograms measuring left ventricular internal diameters at diastole and systole (LVIDd and LVIDs, respectively) from sham-operated (Sham; A), ovariectomized (OVx; B), and OVx + 17β-estradiol (OVx + E; C) guinea pigs. D–F: mean data of LVIDd (D), LVIDs (E), and fractional shortening (FS; F). Sham: n = 9, OVx: n = 10, and OVx + E: n = 10. *P < 0.05; **P < 0.01; ***P < 0.001.
Ca2+ Transient Amplitudes and SR Ca2+ Fractional Release
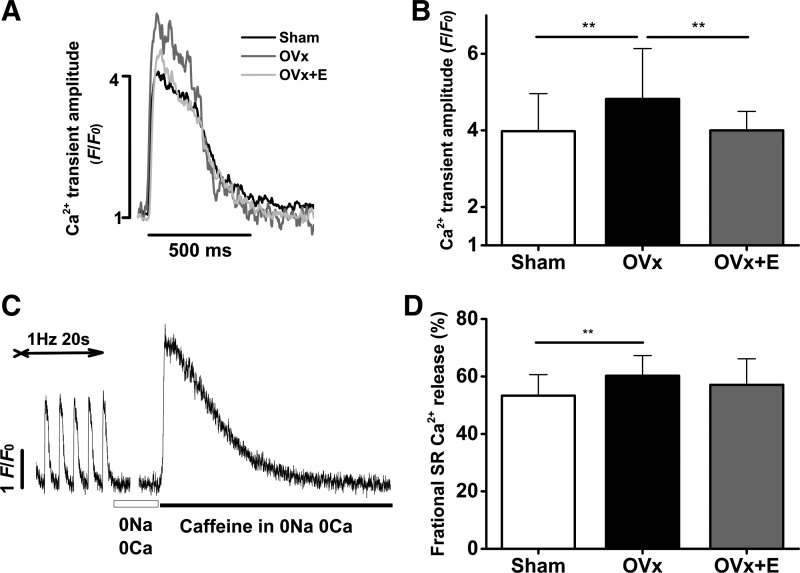
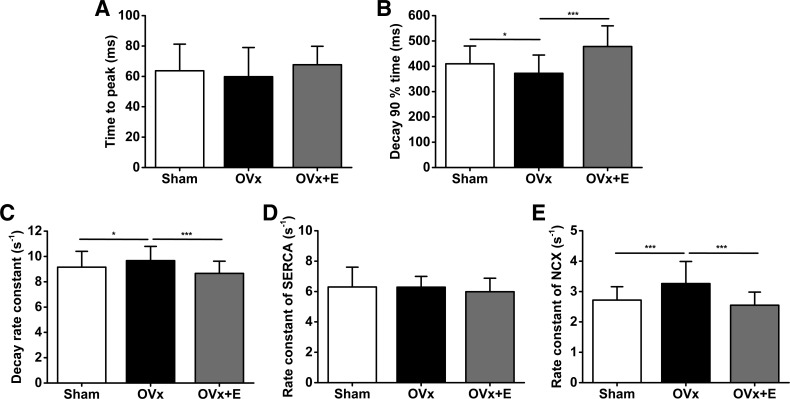
Cells were field stimulated at 0.5 Hz to achieve steady states. Ca2+ transient amplitudes, measured as F/F0, were 20% larger in the OVx group than in the sham group (Fig. 2, A and B). The OVx group had greater SR Ca2+ fractional release compared with the sham group (Fig. 2, C and D). Myocytes isolated from the OVx + E group had similar Ca2+ transient amplitudes and SR Ca2+ fractional release compared with myocytes isolated from the sham group. The time to peak of the Ca2+ transients remained unchanged between sham, OVx, and OVx + E groups (Fig. 3A). Ca2+ transients measured from the OVx group had shorter decay times and faster decay rate constants compared with sham and OVx + E groups, respectively (Fig. 3C). Cells from the sham and OVx + E groups had similar decay rate constants (Fig. 3C). The rate constant representative of SERCA function was not different between sham, OVx, and OVx + E cells (Fig. 3D). The rate constant indicative of NCX function was 20% and 27% faster in OVx myocytes compared with sham and OVx + E myocytes, respectively (Fig. 3E).
Fig. 2.
Effect of long-term absence of estrogen on Ca2+ handling. A: representative traces comparing Ca2+ transients between sham-operated (Sham), ovariectomized (OVx), and OVx + 17β-estradiol (OVx + E) Fluo-4-loaded myocytes. B: myocytes from OVx animals elicited a larger Ca2+ transient compared with Sham and OVx + E animals. Sham: n = 44/4, OVx: n = 55/4, and OVx + E: n = 40/3. **P < 0.01. C: experimental protocol used to measure fractional sarcoplasmic reticulum (SR) Ca2+ release in myocytes (see methods). D: fractional SR Ca2+ release was larger in OVx myocytes compared with Sham myocytes. Sham: n = 34/3, OVx: n = 48/4, and OVx + E: n = 40/3. **P < 0.01.
Fig. 3.
Ca2+ transient parameters. Mean time to peak differences are shown in A and time to 90% decay variation is shown in B for sham-operated (Sham), ovariectomized (OVx), and OVx + 17β-estradiol (OVx + E) groups. C shows mean differences in decay rate constants for the transients and D and E show variations in rate constants for SERCA and the Na+/Ca2+ exchanger (NCX), respectively. Sham: n = 56/3, OVx: n = 38/3, and OVx + E: n = 33/3. *P < 0.05; **P < 0.01; ***P < 0.001.
SR Ca2+ Content
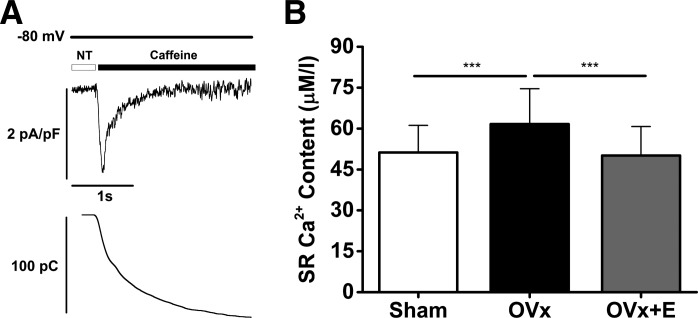
SR Ca2+ content, obtained by integrating the caffeine-evoked inward NCX current (Fig. 4A), was on average 20% greater in the OVx group compared with the sham group (Fig. 4B). SR Ca2+ content was unaltered between cells isolated from sham and OVx + E groups.
Fig. 4.
A: typical traces of caffeine-induced Na+/Ca2+ exchanger inward current (top) and the integrated current used to measure sarcoplasmic reticulum (SR) Ca2+ content (bottom). B: SR Ca2+ content was significantly greater in ovariectomized (OVx) myocytes compared with sham-operated (Sham) and OVx + 17β-estradiol replacement (OVx + E) myocytes (Sham: 51 ± 10 µM/l accessible cell volume, OVx: 62 ± 13 µM/l accessible cell volume, and OVx + E: 50 ± 11 µM/l accessible cell volume). Sham: n = 33/3, OVx: n = 34/3, and OVx + E: n = 33/5. ***P < 0.001.
Ca2+ Sparks and Waves
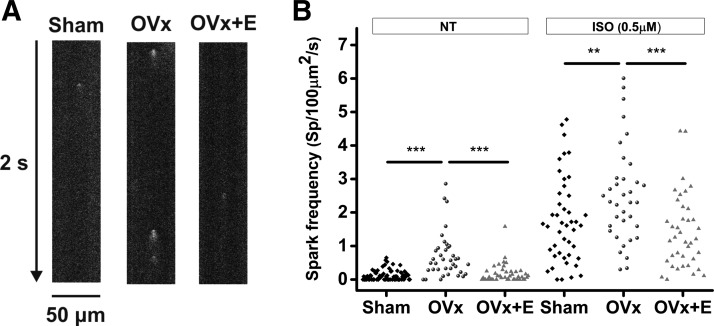
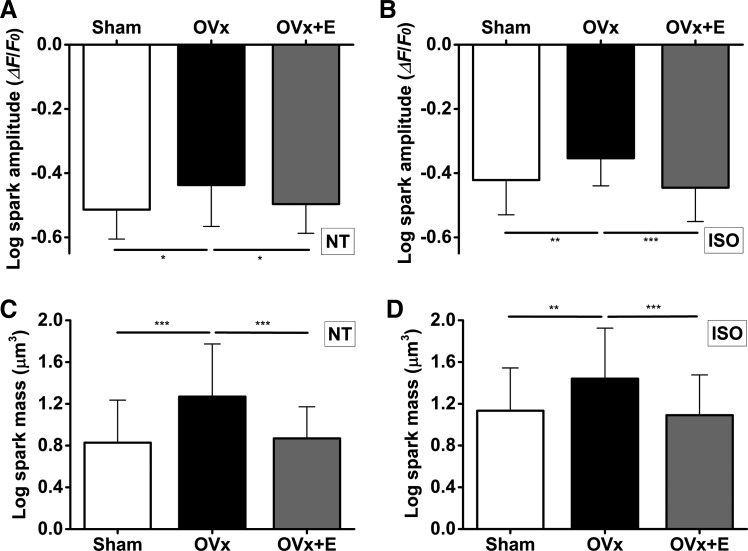
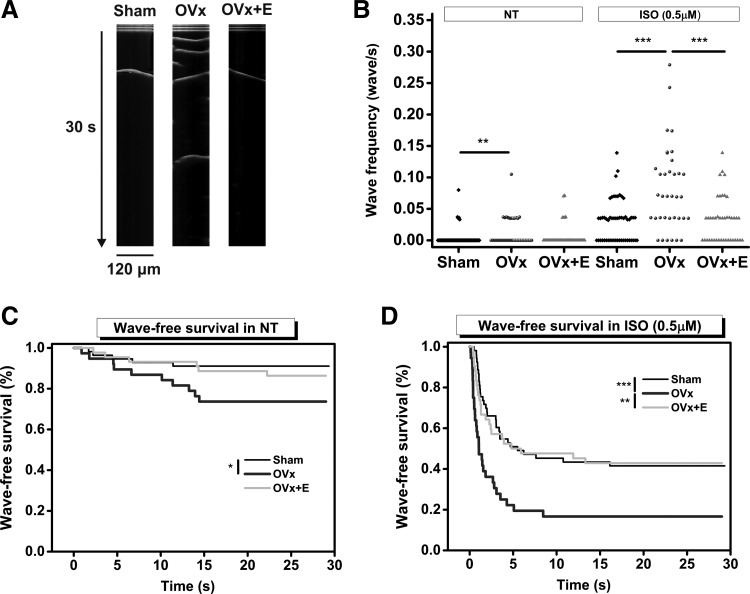
Because SR Ca2+ content was greater in the OVx group, we examined Ca2+ spark frequencies to assess whether there were any changes in spontaneous Ca2+ release. In addition, we assessed whether these cells had an increased tendency to produce Ca2+ waves when provoked by an ISO challenge. Spark frequencies were higher in the OVx group compared with the sham group (Fig. 5B). In the presence of 0.5 µM ISO, myocytes showed more sparks, and the frequencies at which they occurred remained higher in cells isolated from the OVx group (Fig. 5B). Spark amplitude and mass were also larger in OVx myocytes with or without ISO (Fig. 6). In conjunction with Ca2+ sparks, wave frequencies were higher in the OVx group compared with the sham group (Fig. 7B). In the presence of 0.5 µM ISO, myocytes showed more waves, and the frequencies at which they occurred remained higher in the OVx group (Fig. 7B). Spark and wave frequencies significantly decreased in myocytes from animals supplemented with estrogen compared with OVx animals, and wave-free survival times were longer in sham and OVx + E animals in the absence or presence of ISO compared with OVx animals (Fig. 7, C and D).
Fig. 5.
A: detection of Ca2+ sparks in sham-operated (Sham), ovariectomized (OVx), and OVx + 17β-estradiol (OVx + E) Fluo-4-loaded myocytes using line scanning confocal microscopy. B: mean spark frequency was higher in OVx myocytes compared with Sham and OVx + E myocytes in the presence and absence of isoprenaline (ISO). Sham: n = 55/5, OVx: n = 36/3, and OVx + E: n = 40/4. **P < 0.01; ***P < 0.001.
Fig. 6.
Effect of long-term absence of estrogen on Ca2+ spark amplitude (A) and mass (C). Spark amplitudes were larger in the ovariectomized (OVx) group than in the sham-operated (Sham) and OVx + 17β-estradiol replaced (OVx + E) group in the absence (A) and presence (B) of isoprenaline (ISO). OVx myocytes had larger spark masses compared with Sham and OVx + E myocytes in the absence (C) and presence (D) of ISO. Sham: n = 55/5, OVx: n = 36/3, and OVx + E: n = 40/4. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 7.
Measuring Ca2+ wave frequency and wave-free survival in sham-operated (Sham), ovariectomized (OVx), and OVx + 17β-estradiol (OVx + E) Fluo-4-loaded myocytes. A: typical traces of Ca2+ waves recorded in Sham, OVx, and OVx + E myocytes. B: wave frequency was higher in OVx compared with Sham and OVx + E both in the absence (NT) and in the presence of isoprenaline (ISO; 0.5 mM). Sham: n = 61/5, OVx: n = 38/3; and OVx + E: n = 40/4. **P < 0.01; ***P < 0.001. C and D: wave-free survival times in the absence (C) and presence of ISO (D). Wave-free survival time was longer in Sham compared with OVx myocytes in NT and after ISO challenge (*P < 0.05 and ***P < 0.001, respectively, log rank test). The wave-free survival was significantly longer in the OVx + E group compared with the OVx group only in the presence of ISO (**P < 0.01, log rank test).
L-type Ca2+ Current Amplitude and Gating
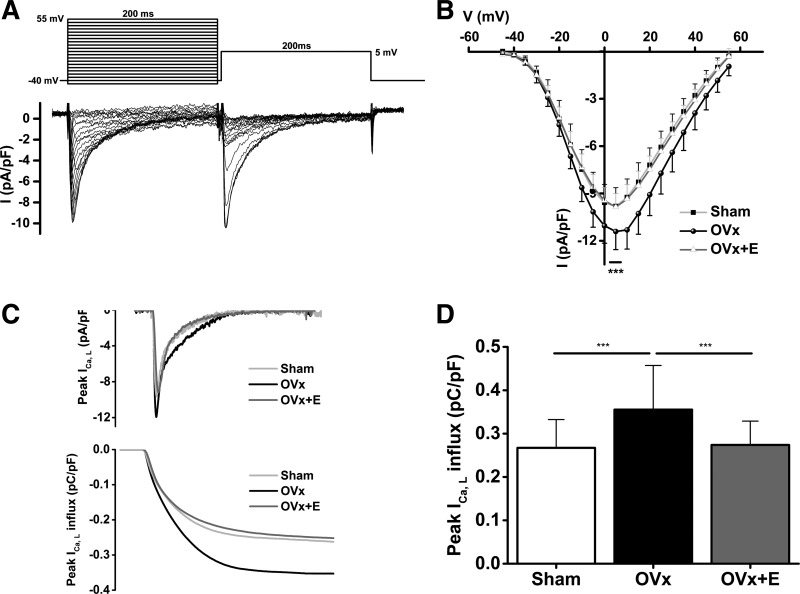
This assessment of Ca2+ regulation indicated that there were changes in intracellular Ca2+ handling after long-term absence of estrogen. It was therefore necessary to measure ICa,L to assess whether changes in Ca2+ influx occurred after OVx. Peak ICa,L was 16% larger in the OVx group than in the sham and OVx + E groups (Fig. 8B), and the amount of Ca2+ influx calculated during the 200-ms activating clamp step was 33% larger in OVx myocytes compared with sham and OVx + E myocytes (Fig. 8D).
Fig. 8.
A: the voltage-clamp protocol and the corresponding traces of L-type Ca2+ current (ICa,L; cadmium-sensitive currents) elicited by voltage steps. B: current-voltage (I-V) relationships determined by the typical protocol shown in A for sham-operated (Sham), ovariectomized (OVx), and OVx + 17β-estradiol (OVx + E) myocytes. Peak ICa,L was larger in OVx myocytes compared with Sham and OVx+E myocytes (***P < 0.001). Sham: n = 40/5, OVx: n = 44/5, and OVx + E: n = 37/7. C: representative traces of peak ICa,L (top) and calculated Ca2+ influx (bottom) in Sham, OVx, and OVx + E myocytes. D: mean data from the experiments shown in C. Ca2+ influx was larger in OVx myocytes compared with Sham and OVx + E myocytes (***P < 0.001).
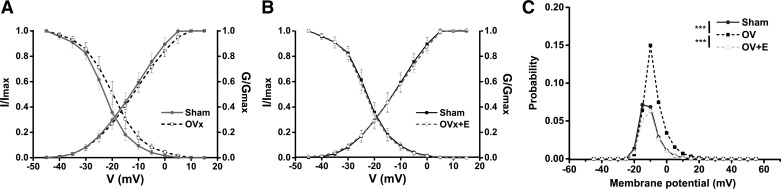
Voltage-dependent activation and steady-state inactivation curves were fitted with the following Boltzmann equation: y = 1/(1 + exp[(x − V0.5)/K]) (Fig. 9). The steady-state inactivation of ICa,L shifted to more positive potentials in OVx myocytes (V1/2,inactivation shift: 3.0 mV, P < 0.001). Channel gating, determined by the ICa,L inactivation shift, was unaltered in OVx + E myocytes compared with sham myocytes (Fig. 9, A and B).
Fig. 9.
Activation and inactivation plots of the L-type Ca2+ channel from sham-operated (Sham), ovariectomized (OVx), and OVx + 17β-estradiol (OVx + E) myocytes. The activation curve was plotted using the test voltage and the corresponding normalized conductance (G/Gmax). Conductance (G) was calculated using the following equation: G = I/(V − Vrev), where V was the test voltage, I was the peak current at the test voltage, and Vrev was the reversal potential obtained by extrapolating the tail part of the I-V curve to its intersection with the voltage axis. Steady-state inactivation was obtained by normalizing the individual currents to the maximal current (I/Imax) and plotting against the test voltage. A and B: L-type Ca2+ channel gating shifted to more positive membrane potential (Em) in OVx myocytes compared with Sham myocytes (V1/2 inactivation shift: 3.0 mV, P < 0.001). Channel gating was unaltered in OVx + E compared with Sham myocytes. Sham: n = 40/5, OVx: n = 46/6, and OVx + E, n = 37/7. C: the probability of having the channel opening within the “window” range of voltages in Sham myocytes peaked at −15 mV with a probability value of 0.07. This value increased to 0.15 at −10 mV in OVx cells. In OVx + E cells, the probability value was 0.06 at −10 mV.
The probability of Ca2+ channels being open within the “window” range of voltages was calculated using the following equation: (1/{1+exp[(V0.5, activation − V)/Kactivation]}− 1/{1 + exp[(V − V0.5,inactivation)/Kinactivation]}), as previously described (24). In sham myocytes, the probability of Ca2+ channels being open was 0.07 at −15 mV, whereas in OVx myocytes, this reached 0.15 at −10 mV and subsequently decreased in OVx + E myocytes to 0.06 at −10 mV (Fig. 9C).
L-Type Ca2+ Channel α1c- and β2-Subunit Relative mRNA Expression
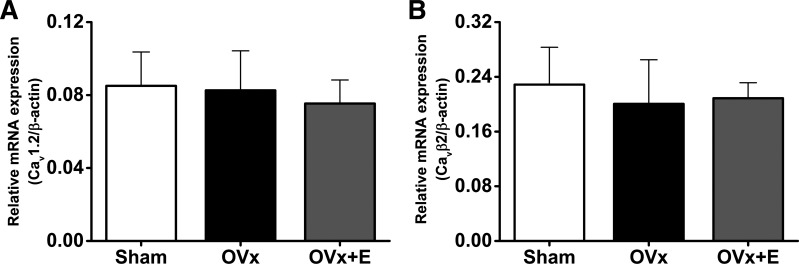
Quantitative RT-PCR was performed to measure relative mRNA expression levels to determine whether the augmented OVx ICa,L was a consequence of altered gene expression of α1c- or β2-subunits. The relative mRNA expression of these subunits was unaltered between sham, OVx, and OVx + E groups (Fig. 10).
Fig. 10.
Relative amounts of Cav1.2 and Cavβ2 mRNA after ovariectomy (OVx). A: relative Cav1.2 mRNA expression (normalized to β-actin mRNA) was unaltered between sham-operated (Sham), OVx, and OVx + 17β-estradiol replacement (OVx + E) myocytes. Sham: n = 7, OVx: n = 7, and OVx + E: n = 6. B: relative Cav β2 mRNA expression (normalized to β-actin mRNA) did not change after OVx and OVx + E compared with Sham treatment. Sham: n = 7, OVx: n = 7, and OVx + E: n = 6.
EADs and DADs Induced by ISO
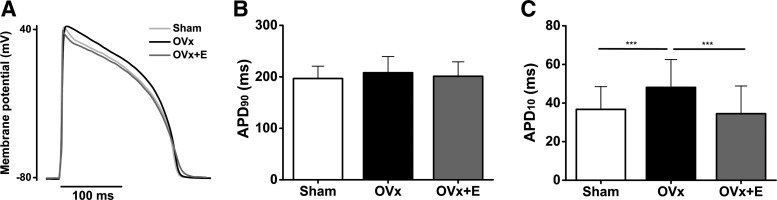
The right shift in Ca2+ current inactivation, increasing the fraction of Ca2+ current that fails to inactivate within the “window” voltage range, may favor the generation of arrhythmic events after OVx. To assess proarrhythmic susceptibility after long-term deprivation of estrogen, the frequency of EADs or DADs after an ISO challenge was determined. First, we measured action potentials in cells isolated from each group (Fig. 11A). APD90 remained unchanged between sham, OVx, and OVx + E groups (Fig. 11B); however, APD10 was 1.3 times longer in cells isolated from the OVx group compared with the sham and OVx + E groups (Fig. 11C). This may indicate more inward current flow early in the action potential supporting the Ca2+ current measurements.
Fig. 11.
Assessing the long-term absence of estrogen on action potential duration (APD). A: representative recordings of action potentials from ventricular myocytes. B: mean data show that APD at 90% repolarization (APD90) remained unchanged between sham-operated (Sham), ovariectomized (OVx), and OVx + 17β-estradiol (OVx + E). C: mean data indicate that APD at 10% repolarization (APD10) was longer in OVx compared with Sham and OVx + E, but no differences in APD10 were observed between Sham and OVx + E (***P < 0.001). Sham: n = 7, OVx: n = 7, and OVx + E: n = 6.
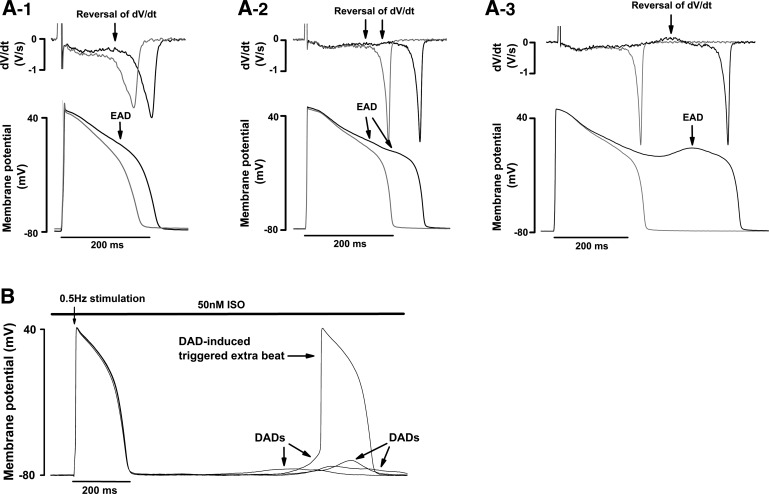
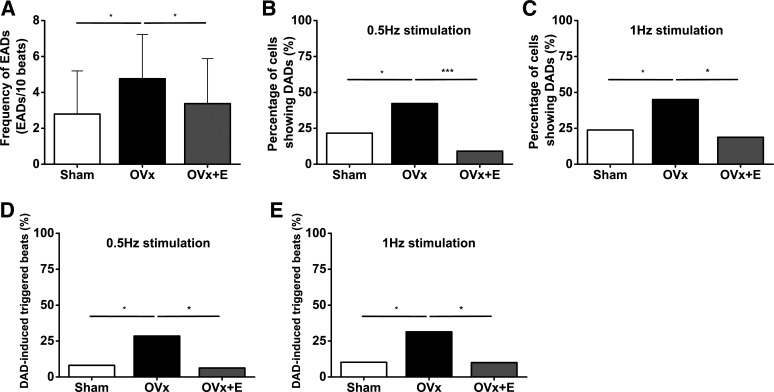
EADs or DADs were elicited by pacing cells at 0.5, 1.0, or 2.0 Hz for 30 s in the presence of 50 nM ISO (Fig. 12). Myocytes from the OVx group showed a higher frequency of EADs compared with the sham group (Fig. 13A), and, in addition, a higher percentage of OVx cells showed DADs when stimulated at 0.5 and 1.0 Hz compared with sham and OVx + E cells (Fig. 13, B and C). OVx myocytes also had a higher percentage of cells showing DAD-induced triggered extra beats during 0.5- and 1.0-Hz stimulation compared with sham and OVx + E myocytes (Fig. 13, D and E).
Fig. 12.
Identification of early (EAD) and delayed afterdepolarizations (DAD) after isoprenaline (ISO) challenges. A: typical traces of EAD formation in action potentials with short (1), normal (2), and long duration (3) and the corresponding first derivative (dV/dt) traces from ovariectomized (OVx) myocytes. Action potentials were recorded during 2-Hz stimulation in 50 nM ISO. B: typical DADs that form in one OVx myocyte during 0.5-Hz stimulation in 50 nM ISO. The recording illustrates a DAD-induced (triggered) extrasystole.
Fig. 13.
Quantification of early (EADs) and delayed afterdepolarizations (DADs) after isoprenaline (ISO) challenges. A: ovariectomized (OVx) myocytes had a higher mean frequency of EADs per 10 beats compared with sham-operated (Sham) and OVx + 17β-estradiol replaced (OVx + E) myocytes after 50 nM ISO challenges at 2-Hz stimulation. Sham: 2.8 ± 2.4, OVx; 4.7 ± 2.5, and OVx + E: 3.4 ± 2.5; Sham: n = 39/5, OVx: n = 30/4, and OVx + E: 30/5. P < 0.05. B and C: percentages of cells with DADs in the Sham, OVx, and OVx + E groups after treatment with 50 nM ISO during 0.5-Hz stimulation (B; Sham: n = 37/5, OVx: n = 45/4, and OVx + E: 33/5; *P < 0.05 and ***P < 0.001) and during 1.0-Hz stimulation (C; Sham: n = 42/5, OVx: n = 40/4, and OVx + E: 32/5; *P < 0.05). D and E: percentages of cells in each group that exhibit DAD-induced extrasystoles during 0.5-Hz stimulation (D) and 1.0-Hz stimulation (E). *P < 0.05.
DISCUSSION
Observations
This study addressed the hypothesis that long-term deficiency of ovarian hormones alters intracellular Ca2+ regulation in a way that may predispose cardiac myocytes to more potentially arrhythmogenic events. Alongside testing this central hypothesis, we assessed whether 17β-estradiol supplementation reversed altered intracellular Ca2+ handling, thus preventing proarrhythmic behavior. After ISO challenge, OVx myocytes had higher frequencies of EADs, and a greater percentage of them showed DADs and DAD-induced triggered extra beats. Significantly fewer proarrhythmic events were observed in myocytes isolated from OVx guinea pigs with 17β-estradiol supplementation. These observations suggest that long-term deprivation of ovarian estrogen can modify Ca2+ regulation in ventricular myocytes in ways that may promote proarrhythmic behavior.
Ca2+ Transient and SR Ca2+ Load
OVx myocytes had greater SR Ca2+ content and larger Ca2+ transient amplitudes, supporting a previous report (17). We also found that OVx myocytes had larger peak ICa,L and corresponding Ca2+ influx. We suggest that the greater SR Ca2+ content may result from the loading effect of the larger Ca2+ influx. Not only is the size of SR Ca2+ release proportional to the size of the Ca2+ current (4), but also increased Ca2+ influx loads the SR with more Ca2+ (16) that augments fractional SR Ca2+ release (50). The Ca2+ transient decay rate constant was significantly greater in the OVx group compared with the sham and OVx + E groups. Although the rate constant of SERCA function remained unchanged between sham, OVx, and OVx + E groups, the rate constant of NCX function significantly increased in OVx hearts compared with sham and OVx + E groups, leading to a faster Ca2+ transient decay in the OVx group. The enhanced NCX forward mode may help to facilitate Ca2+ efflux from the cell in response to increased Ca2+ influx after OVx and could potentially prolong the action potential and increase the likelihood of EAD formation (57). Upregulated NCX function was also reported in an OVx rat model and was suggested to be associated with NCX phosphorylation by upregulation of PKA (26).
Ca2+ Sparks and Waves
Our data show significant increases in the frequency of Ca2+ sparks and waves after OVx. Spark amplitudes and mass were also larger in OVx myocytes. All these indexes are indicative of an enhanced SR Ca2+ load (48), which is proposed to alter ryanodine receptor Ca2+ sensitivity at luminal regulatory sites (21) or directly at the store-sensing gate in the receptor (10) and increase single channel current amplitude that governs cluster behavior (20). These changes increase the probability of Ca2+ wave formation and propagation that are considered to be proarrhythmic (62). These will induce sufficient Ca2+-sensitive inward current, particularly forward-mode NCX current, to cause depolarization of the cardiac cell membrane, producing spontaneously triggered beats under certain conditions (51). Therefore, OVx myocytes may have an increased tendency to develop arrhythmias, which could explain the finding that OVx increased the susceptibility to ischemia-induced arrhythmias (29) and the increased incidence of arrhythmias in postmenopausal women (40).
In addition, we also investigated the cell responses to β-adrenergic stimulation (ISO challenge). Cells from sham, OVx, and OVx + E groups all produced significantly more sparks and waves when treated with ISO. OVx myocytes again showed higher frequencies of sparks and waves compared with sham myocytes. In contrast, OVx + E myocytes had comparable responses to ISO challenge as sham myocytes. Therefore, OVx myocytes were more sensitive to adrenergic stimulation, which elicited significantly more SR Ca2 release events. 17β-Estradiol supplementation suppressed these potentially adverse effects.
Ca2+ Current
ICa,L was measured to determine whether it could be responsible for the increases in cellular Ca2+ content. We found that peak ICa,L and the corresponding Ca2+ influx were larger in OVx myocytes. Furthermore, we observed that current inactivation shifted to more positive voltages, and the probability of Ca2+ channels being open within the “window” voltage range was greater in OVx myocytes. Both parameters may favor the generation of proarrhythmic events (5, 70). Evidence suggests that the activity of L-type Ca2+ channels increased after OVx, and these changes are reversed by estrogen supplementation (25, 38, 60). The changes appear to take place without alterations to the relative amounts of gene expression for L-type Ca2+ channel subunits α1c and β2. This was a similar finding to the levels of mRNA coding for L-type Ca2+ channel α-subunits in smooth muscle cells of coronary arteries after estrogen withdrawal (60). It is tempting to suggest that the increased ICa,L may result from a change in phosphorylation status of the channel. Enhanced basal activity of PKA and increased basal activity of the L-type Ca2+ channel have been shown in rat OVx myocytes, and the increased basal activity of L-type Ca2+ channel was reversed by a PKA inhibitor (25). In addition, CaMKIIδ and phosphorylated CaMKII were both upregulated in cardiomyocytes from an OVx rat model and were restored to normal levels after estrogen replacement (30).
EADs and DADs
A key finding in our study was the observation that OVx myocytes had higher frequencies of EADs, and a greater percentage of these cells showed DADs and DAD-induced triggered extra beats during ISO challenge. Significantly fewer proarrhythmic events were observed in myocytes isolated from OVx guinea pigs with 17β-estradiol supplementation. To the best of our knowledge, this is the first ISO challenge experiment to induce EADs/DADs in cardiomyocytes from OVx animals. EADs are likely to occur when a proportion of Ca2+ channels reactivate or fail to inactivate, especially in the case of increased window current (68). Late-phase EADs may share the same underlying mechanisms as DADs, which are SR Ca2+ overload and spontaneous Ca2+ release (63). Adrenergic stimulation-induced EADs are regarded as the underlying mechanism of torsade de pointes ventricular tachycardia and sudden death in patients with idiopathic long-QT syndrome (43). Our observations may help explain an in vivo whole heart study showing an increase in susceptibility to adrenergic stimulation-induced arrhythmias in OVx rats compared with gonad-intact animals (59).
Comparing Ca2+ Handling in OVx Animal Models
Table 2 shows the changes in cardiac myocyte Ca2+ handling in OVx animal models. We have reported an increased peak ICa,L in OVx that is in good agreement with effects seen in rat and mouse OVx models (18, 25). The amount of mRNA coding for the L-type Ca2+ channel was unaltered after OVx, a finding similar to Fares et al. in the rat (18), but it is uncertain whether L-type Ca2+ channel protein expression changes (11, 18). We suggest the increased ICa,L may result from the change in phosphorylation status of the channel. Further research using PKA/CaMKII inhibitors is required to assess the role of L-type Ca2+ channel phosphorylation in estrogen-mediated responses.
Table 2.
Changes in cardiomyocyte Ca2+ handling in ovariectomized animal models
| OVx Animal | Guinea Pig (This Study) | Rat | Mouse |
|---|---|---|---|
| Peak L-type Ca2+ current | ↑ | A↑ (25) | ↑ (18) and ↔ (17) |
| L-type Ca2+ channel | mRNA↔ | WB↑ (11) | WB↓ (18) |
| mRNA↔ (11) | |||
| PKA | E↑ (26) | ||
| A↑ (25) | |||
| CaMKIIδ and phosphorylated CaMKII | WB↑ (30) | ||
| Sarcoplasmic reticulum Ca2+ content | ↑ | ↑ (17, 18) | |
| Ca2+ transient amplitude | ↑ | ↑ (12, 25, 26) and ↓ (9) | ↑ (17, 18) and ↓ (61) |
| Ca2+ transient time to peak | ↔ | ↓ (18) | |
| Ca2+ transient rate constant | ↑ | ↑ (12, 26) | ↑ (17, 18) and ↓ (61) |
| Fractional sarcoplasmic reticulum Ca2+ release | ↑ | ↔ (17, 18) | |
| SERCA | R↔ | A↔ (26) and ↓ (8) | WB↔ (18) and ↓ (61) |
| WB↔ (11, 26) and ↓ (7, 8) | |||
| mRNA↔ (11) and ↓ (8) | |||
| PLB↔ (7, 8, 11) | |||
| Na+/Ca2+ exchanger | R↑ | A↑ (26) | WB↔ (18) |
| RC↑ (26) | |||
| WB↔ (26) and ↓ (11) | |||
| mRNA↔ (11) | |||
| Ryanodine receptor 2 | A↑ (26) | ||
| WB↔ (11) | |||
| Spark frequency | ↑ | ↑ (17, 18) | |
| Spark amplitude | ↑ | ↑ (17) | |
| Spark mass | ↑ | ↑ (18) and ↔ (17) | |
| Wave frequency | ↑ | ||
| Isoprenaline-induced early afterdepolarizations and delayed afterdepolarizations | ↑ |
↑, increase compared with the sham group; ↔, no difference compared with the sham group; ↓, decrease compared with the sham group; A, activity; WB, Western blot; E, expression of fluorescence intensity; PLB, phospholamban; R, rate constant; RC, relaxation contribution.
Most studies of the effects of OVx have reported increased Ca2+ transient amplitudes (12, 17, 18, 25, 26), although discrepancies exist (9, 61). OVx in the guinea pig speeds the decay of the Ca2+ transient, and our results suggest that this is due to faster Ca2+ removal via NCX. Although mRNA and protein expression of NCX are unaltered in most studies (18, 26), there is one report (26) of increased activity of NCX resulting from PKA phosphorylation. The rate constant representing SERCA function remained unaltered after OVx, implying unchanged Ca2+ uptake into the SR, but the expression and regulation of SERCA seem to vary among studies (7, 8, 11, 18, 26, 61).
Here, we report increased spark frequency, amplitude, and mass in the OVx group, which supports similar findings in the mouse, an animal whose cardiac EC coupling is more reliant on SR Ca2+ release than the guinea pig (17, 18).
All these effects after OVx could be reversed with 17β-estradiol supplementation, confirming that it is the withdrawal of estrogen that mediates the changes in Ca2+ regulation.
In this study, we used an ISO challenge to assess whether OVx creates a proarrhythmic substrate. OVx myocytes had more sparks and waves after the ISO challenges compared with sham and OVx + E cells. Furthermore, these cells had a higher frequency of EADs and a higher percentage of DADs and DAD-induced triggered extra beats. To further explore the proarrhythmic mechanism, gating of the L-type Ca2+ channel was investigated and was found to shift toward a more positive membrane potential with a larger window current, allowing a higher probability of reactivation of L-type Ca2+ channels. These novel findings may provide new insights for the mechanisms underlying postmenopausal arrhythmia.
Myofilament Ca2+ Sensitivity
Our data also showed a decrease in in vivo FS and increases in LVIDd and LVIDs in the OVx group compared with the ovary-intact sham group, observations that are difficult to reconcile with increased Ca2+ current and SR Ca2+ content. A possible explanation is that there are changes to myofilament Ca2+ sensitivity in OVx hearts. Ca2+ desensitization and/or decreased ATPase activity have been observed in in vitro studies of troponin T mutant proteins (28, 44, 53, 69) and accompany many dilated cardiomyopathy phenotypes (69) that show decreases in in vivo FS yet increases to the Ca2+ transient, as found here. A deficiency of female sex hormones alters the cardiac contractile machinery (36, 46, 66), but the few studies that have been done in rats present an ambiguous picture of the effects of OVx (37, 67).
In conclusion, OVx had detrimental effects on Ca2+ regulation in guinea pig cardiac myocytes. The dysregulation of Ca2+ handling contributes to a higher frequency of spontaneous or triggered SR Ca2+ release events. With β-adrenergic stimulation, the probability of formation of EAD and DAD and DAD-induced triggered extrasystoles was higher in the OVx group. These findings suggest that long-term absence of ovarian hormones leads to adverse changes in cardiac Ca2+-handling mechanisms that may cause the formation of a more proarrhythmic substrate. 17β-Estradiol replacement prevented these changes.
GRANTS
This research was funded by British Heart Foundation Grant S/P 16/2/32004 and the Tri-Service General Hospital, National Defense Medical Center, Taiwan, Republic of China.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.-Y.Y., J.M.F., A.J.F., and A.A.-L. performed experiments; H.-Y.Y., J.M.F., A.J.F., A.A.-L., and K.T.M. analyzed data; H.-Y.Y., J.M.F., A.J.F., A.A.-L., and K.T.M. interpreted results of experiments; H.-Y.Y. prepared figures; H.-Y.Y. and K.T.M. drafted manuscript; H.-Y.Y., J.M.F., and K.T.M. edited and revised manuscript; H.-Y.Y., J.M.F., A.J.F., A.A.-L., and K.T.M. approved final version of manuscript; K.T.M. conceived and designed research.
ACKNOWLEDGMENTS
We thank Dr. Markus Sikkel for kindly providing self-coded ImageJ plugins for the analysis of Ca2+ sparks and waves. We thank Dr. Matthew Turley and Dr. Claire Poulet for advice on primer design and real-time PCR using SYBR green.
REFERENCES
- 1.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol 268: C1313–C1319, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Bell JR, Bernasochi GB, Varma U, Raaijmakers AJ, Delbridge LM. Sex and sex hormones in cardiac stress−mechanistic insights. J Steroid Biochem Mol Biol 137: 124–135, 2013. doi: 10.1016/j.jsbmb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Bers D. Excitation-Contraction Coupling and Cardiac Contractile Force, 2nd ed Boston, MA: Klewer, 2001. doi: 10.1007/978-94-010-0658-3 [DOI] [Google Scholar]
- 4.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM, Morotti S. Ca2+ current facilitation is CaMKII-dependent and has arrhythmogenic consequences. Front Pharmacol 5: 144, 2014. doi: 10.3389/fphar.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bode EF, Briston SJ, Overend CL, O’Neill SC, Trafford AW, Eisner DA. Changes of SERCA activity have only modest effects on sarcoplasmic reticulum Ca2+ content in rat ventricular myocytes. J Physiol 589: 4723–4729, 2011. doi: 10.1113/jphysiol.2011.211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bupha-Intr T, Laosiripisan J, Wattanapermpool J. Moderate intensity of regular exercise improves cardiac SR Ca2+ uptake activity in ovariectomized rats. J Appl Physiol 107: 1105–1112, 2009. doi: 10.1152/japplphysiol.00407.2009. [DOI] [PubMed] [Google Scholar]
- 8.Bupha-Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 291: H1101–H1108, 2006. doi: 10.1152/ajpheart.00660.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bupha-Intr T, Wattanapermpool J, Peña JR, Wolska BM, Solaro RJ. Myofilament response to Ca2+ and Na+/H+ exchanger activity in sex hormone-related protection of cardiac myocytes from deactivation in hypercapnic acidosis. Am J Physiol Regul Integr Comp Physiol 292: R837–R843, 2007. doi: 10.1152/ajpregu.00376.2006. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, Tian X, Jones PP, O’Mara ML, Liu Y, Mi T, Zhang L, Bolstad J, Semeniuk L, Cheng H, Zhang J, Chen J, Tieleman DP, Gillis AM, Duff HJ, Fill M, Song LS, Chen SR. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med 20: 184–192, 2014. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu SH, Goldspink P, Kowalski J, Beck J, Schwertz DW. Effect of estrogen on calcium-handling proteins, beta-adrenergic receptors, and function in rat heart. Life Sci 79: 1257–1267, 2006. doi: 10.1016/j.lfs.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Curl CL, Wendt IR, Canny BJ, Kotsanas G. Effects of ovariectomy and 17 beta-oestradiol replacement on [Ca2+]i in female rat cardiac myocytes. Clin Exp Pharmacol Physiol 30: 489–494, 2003. doi: 10.1046/j.1440-1681.2003.03864.x. [DOI] [PubMed] [Google Scholar]
- 13.Dibb KM, Eisner DA, Trafford AW. Regulation of systolic [Ca2+]i and cellular Ca2+ flux balance in rat ventricular myocytes by SR Ca2+, L-type Ca2+ current and diastolic [Ca2+]i. J Physiol 585: 579–592, 2007. doi: 10.1113/jphysiol.2007.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dogan M, Yiginer O, Uz O, Kucuk U, Degirmencioglu G, Isilak Z, Uzun M, Davulcu E. The effects of female sex hormones on ventricular premature beats and repolarization parameters in physiological menstrual cycle. Pacing Clin Electrophysiol 39: 418–426, 2016. doi: 10.1111/pace.12821. [DOI] [PubMed] [Google Scholar]
- 15.Egdell RM, De Souza AI, Macleod KT. Relative importance of SR load and cytoplasmic calcium concentration in the genesis of aftercontractions in cardiac myocytes. Cardiovasc Res 47: 769–777, 2000. doi: 10.1016/S0008-6363(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 16.Eisner D, Bode E, Venetucci L, Trafford A. Calcium flux balance in the heart. J Mol Cell Cardiol 58: 110–117, 2013. doi: 10.1016/j.yjmcc.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Fares E, Parks RJ, Macdonald JK, Egar JM, Howlett SE. Ovariectomy enhances SR Ca2+ release and increases Ca2+ spark amplitudes in isolated ventricular myocytes. J Mol Cell Cardiol 52: 32–42, 2012. doi: 10.1016/j.yjmcc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Fares E, Pyle WG, Ray G, Rose RA, Denovan-Wright EM, Chen RP, Howlett SE. The impact of ovariectomy on calcium homeostasis and myofilament calcium sensitivity in the aging mouse heart. PLoS One 8: e74719, 2013. doi: 10.1371/journal.pone.0074719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillis AM. Atrial fibrillation and ventricular arrhythmias: sex differences in electrophysiology, epidemiology, clinical presentation, and clinical outcomes. Circulation 135: 593–608, 2017. doi: 10.1161/CIRCULATIONAHA.116.025312. [DOI] [PubMed] [Google Scholar]
- 20.Guo T, Gillespie D, Fill M. Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle. Circ Res 111: 28–36, 2012. doi: 10.1161/CIRCRESAHA.112.265652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Györke I, Györke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J 75: 2801–2810, 1998. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingworth S, Peet J, Chandler WK, Baylor SM. Calcium sparks in intact skeletal muscle fibers of the frog. J Gen Physiol 118: 653–678, 2001. doi: 10.1085/jgp.118.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Wang J, Xu C, He B, Lu Z, Jiang H. Effect of oestrogen replacement therapy on idiopathic outflow tract ventricular arrhythmias in postmenopausal women. Arch Cardiovasc Dis 104: 84–88, 2011. doi: 10.1016/j.acvd.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Priori SG, Napolitano C, O’Leary ME, Chahine M. Y1767C, a novel SCN5A mutation, induces a persistent Na+ current and potentiates ranolazine inhibition of Nav1.5 channels. Am J Physiol Heart Circ Physiol 300: H288–H299, 2011. doi: 10.1152/ajpheart.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kam KW, Kravtsov GM, Liu J, Wong TM. Increased PKA activity and its influence on isoprenaline-stimulated L-type Ca2+ channels in the heart from ovariectomized rats. Br J Pharmacol 144: 972–981, 2005. doi: 10.1038/sj.bjp.0706123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kravtsov GM, Kam KW, Liu J, Wu S, Wong TM. Altered Ca2+ handling by ryanodine receptor and Na+-Ca2+ exchange in the heart from ovariectomized rats: role of protein kinase A. Am J Physiol Cell Physiol 292: C1625–C1635, 2007. doi: 10.1152/ajpcell.00368.2006. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Lu QW, Morimoto S, Harada K, Du CK, Takahashi-Yanaga F, Miwa Y, Sasaguri T, Ohtsuki I. Cardiac troponin T mutation R141W found in dilated cardiomyopathy stabilizes the troponin T-tropomyosin interaction and causes a Ca2+ desensitization. J Mol Cell Cardiol 35: 1421–1427, 2003. doi: 10.1016/j.yjmcc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Lujan HL, Dicarlo SE. Sex differences to myocardial ischemia and beta-adrenergic receptor blockade in conscious rats. Am J Physiol Heart Circ Physiol 294: H1523–H1529, 2008. doi: 10.1152/ajpheart.01241.2007. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Cheng WT, Wu S, Wong TM. Oestrogen confers cardioprotection by suppressing Ca2+/calmodulin-dependent protein kinase II. Br J Pharmacol 157: 705–715, 2009. doi: 10.1111/j.1476-5381.2009.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLeod KT, Harding SE. Effects of phorbol ester on contraction, intracellular pH and intracellular Ca2+ in isolated mammalian ventricular myocytes. J Physiol 444: 481–498, 1991. doi: 10.1113/jphysiol.1991.sp018889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 297: R525–R545, 2009. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- 34.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 109: 687–696, 2011. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odening KE, Koren G. How do sex hormones modify arrhythmogenesis in long QT syndrome? Sex hormone effects on arrhythmogenic substrate and triggered activity. Heart Rhythm 11: 2107–2115, 2014. doi: 10.1016/j.hrthm.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paigel AS, Ribeiro RF Jr, Fernandes AA, Targueta GP, Vassallo DV, Stefanon I. Myocardial contractility is preserved early but reduced late after ovariectomy in young female rats. Reprod Biol Endocrinol 9: 54, 2011. doi: 10.1186/1477-7827-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandit S, Woranush W, Wattanapermpool J, Bupha-Intr T. Significant role of female sex hormones in cardiac myofilament activation in angiotensin II-mediated hypertensive rats. J Physiol Sci 64: 269–277, 2014. doi: 10.1007/s12576-014-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson E, Ma L, Szabo B, Robinson CP, Thadani U. Ovariectomy and estrogen-induced alterations in myocardial contractility in female rabbits: role of the L-type calcium channel. J Pharmacol Exp Ther 284: 586–591, 1998. [PubMed] [Google Scholar]
- 39.Pérez-López FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci 17: 511–531, 2010. doi: 10.1177/1933719110367829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters RW, Gold MR. The influence of gender on arrhythmias. Cardiol Rev 12: 97–105, 2004. doi: 10.1097/01.crd.0000096416.94304.bd. [DOI] [PubMed] [Google Scholar]
- 41.Petrie MC, Dawson NF, Murdoch DR, Davie AP, McMurray JJV. Failure of women’s hearts. Circulation 99: 2334–2341, 1999. doi: 10.1161/01.CIR.99.17.2334. [DOI] [PubMed] [Google Scholar]
- 42.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol 293: C1073–C1081, 2007. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 43.Priori SG, Napolitano C, Schwartz PJ. Electrophysiologic mechanisms involved in the development of torsades de pointes. Cardiovasc Drugs Ther 5: 203–212, 1991. doi: 10.1007/BF03029822. [DOI] [PubMed] [Google Scholar]
- 44.Ramratnam M, Salama G, Sharma RK, Wang DW, Smith SH, Banerjee SK, Huang XN, Gifford LM, Pruce ML, Gabris BE, Saba S, Shroff SG, Ahmad F. Gene-targeted mice with the human troponin T R141W mutation develop dilated cardiomyopathy with calcium desensitization. PLoS One 11: e0167681, 2016. doi: 10.1371/journal.pone.0167681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regitz-Zagrosek V, Oertelt-Prigione S, Seeland U, Hetzer R. Sex and gender differences in myocardial hypertrophy and heart failure. Circ J 74: 1265–1273, 2010. doi: 10.1253/circj.CJ-10-0196. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro RF Jr, Pavan BM, Potratz FF, Fiorim J, Simoes MR, Dias FM, Lima FL, Fernandes AA, Vassallo DV, Stefanon I. Myocardial contractile dysfunction induced by ovariectomy requires AT1 receptor activation in female rats. Cell Physiol Biochem 30: 1–12, 2012. doi: 10.1159/000339041. [DOI] [PubMed] [Google Scholar]
- 47.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 48.Satoh H, Blatter LA, Bers DM. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol Heart Circ Physiol 272: H657–H668, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, Køber L, Jensen JE. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 345: e6409, 2012. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 50.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys J 78: 334–343, 2000. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiferaw Y, Aistrup GL, Wasserstrom JA. Intracellular Ca2+ waves, afterdepolarizations, and triggered arrhythmias. Cardiovasc Res 95: 265–268, 2012. doi: 10.1093/cvr/cvs155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sikkel MB, Collins TP, Rowlands C, Shah M, O’Gara P, Williams AJ, Harding SE, Lyon AR, MacLeod KT. Flecainide reduces Ca2+ spark and wave frequency via inhibition of the sarcolemmal sodium current. Cardiovasc Res 98: 286–296, 2013. doi: 10.1093/cvr/cvt012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sommese RF, Nag S, Sutton S, Miller SM, Spudich JA, Ruppel KM. Effects of troponin T cardiomyopathy mutations on the calcium sensitivity of the regulated thin filament and the actomyosin cross-bridge kinetics of human β-cardiac myosin. PLoS One 8: e83403, 2013. doi: 10.1371/journal.pone.0083403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sourander L, Rajala T, Räihä I, Mäkinen J, Erkkola R, Helenius H. Cardiovascular and cancer morbidity and mortality and sudden cardiac death in postmenopausal women on oestrogen replacement therapy (ERT). Lancet 352: 1965–1969, 1998. doi: 10.1016/S0140-6736(98)05066-1. [DOI] [PubMed] [Google Scholar]
- 55.Stagg MA, Malik AH, MacLeod KT, Terracciano CM. The effects of overexpression of the Na+/Ca2+ exchanger on calcium regulation in hypertrophied mouse cardiac myocytes. Cell Calcium 36: 111–118, 2004. doi: 10.1016/j.ceca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Stock EO, Redberg R. Cardiovascular disease in women. Curr Probl Cardiol 37: 450–526, 2012. doi: 10.1016/j.cpcardiol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Szabo B, Sweidan R, Rajagopalan CV, Lazzara R. Role of Na+:Ca2+ exchange current in Cs+-induced early afterdepolarizations in Purkinje fibers. J Cardiovasc Electrophysiol 5: 933–944, 1994. doi: 10.1111/j.1540-8167.1994.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 58.Taggart MJ, Hume R, Lartey J, Johnson M, Tong WC, Macleod KT. Cardiac remodelling during pregnancy: whither the guinea pig? Cardiovasc Res 104: 226–227, 2014. doi: 10.1093/cvr/cvu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teplitz L, Igić R, Berbaum ML, Schwertz DW. Sex differences in susceptibility to epinephrine-induced arrhythmias. J Cardiovasc Pharmacol 46: 548–555, 2005. doi: 10.1097/01.fjc.0000179435.26373.81. [DOI] [PubMed] [Google Scholar]
- 60.Tharp DL, Ivey JR, Shaw RL, Bowles DK. Ovariectomy increases L-type Ca2+ channel activity in porcine coronary smooth muscle. Menopause 21: 661–668, 2014. doi: 10.1097/GME.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 61.Turdi S, Huff AF, Pang J, He EY, Chen X, Wang S, Chen Y, Zhang Y, Ren J. 17-β Estradiol attenuates ovariectomy-induced changes in cardiomyocyte contractile function via activation of AMP-activated protein kinase. Toxicol Lett 232: 253–262, 2015. doi: 10.1016/j.toxlet.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venetucci LA, Trafford AW, O’Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res 77: 285–292, 2008. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 63.Volders PGA, Kulcśar A, Vos MA, Sipido KR, Wellens HJJ, Lazzara R, Szabo B. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc Res 34: 348–359, 1997. doi: 10.1016/S0008-6363(96)00270-2. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Wang Q, Zhao Y, Gong D, Wang D, Li C, Zhao H. Protective effects of estrogen against reperfusion arrhythmias following severe myocardial ischemia in rats. Circ J 74: 634–643, 2010. doi: 10.1253/circj.CJ-09-0223. [DOI] [PubMed] [Google Scholar]
- 65.Wang YC, Xiao XL, Li N, Yang D, Xing Y, Huo R, Liu MY, Zhang YQ, Dong DL. Oestrogen inhibits BMP4-induced BMP4 expression in cardiomyocytes: a potential mechanism of oestrogen-mediated protection against cardiac hypertrophy. Br J Pharmacol 172: 5586–5595, 2015. doi: 10.1111/bph.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wattanapermpool J. Increase in calcium responsiveness of cardiac myofilament activation in ovariectomized rats. Life Sci 63: 955–964, 1998. doi: 10.1016/S0024-3205(98)00353-1. [DOI] [PubMed] [Google Scholar]
- 67.Wattanapermpool J, Reiser PJ. Differential effects of ovariectomy on calcium activation of cardiac and soleus myofilaments. Am J Physiol 277: H467–H473, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 7: 1891–1899, 2010. doi: 10.1016/j.hrthm.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J Mol Cell Cardiol 48: 882–892, 2010. doi: 10.1016/j.yjmcc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X, Ai X, Nakayama H, Chen B, Harris DM, Tang M, Xie Y, Szeto C, Li Y, Li Y, Zhang H, Eckhart AD, Koch WJ, Molkentin JD, Chen X. Persistent increases in Ca2+ influx through Cav1.2 shortens action potential and causes Ca2+ overload-induced afterdepolarizations and arrhythmias. Basic Res Cardiol 111: 4, 2016. doi: 10.1007/s00395-015-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol 306: H628–H640, 2014. doi: 10.1152/ajpheart.00859.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]