This is the first study investigating the role of nitric oxide (NO) and intramuscular interstitial cells of Cajal (ICC-IM) in modulating neural release of purines in colon. We found that NO inhibited release of purines in human, monkey, and murine colons and that colons from KitW/KitW-v (W/Wv) mice, which present with partial loss of ICC-IM, demonstrated augmented neural release of purines. Interactions between nitrergic and purinergic neurotransmission may affect motility in disease conditions with ICC-IM deficiencies.
Keywords: colon, nitric oxide, purinergic, interstitial cell of Cajal, W/Wv mice
Abstract
Regulation of colonic motility depends on the integrity of enteric inhibitory neurotransmission mediated by nitric oxide (NO), purine neurotransmitters, and neuropeptides. Intramuscular interstitial cells of Cajal (ICC-IM) and platelet-derived growth factor receptor-α-positive (PDGFRα+) cells are involved in generating responses to NO and purine neurotransmitters, respectively. Previous studies have suggested a decreased nitrergic and increased purinergic neurotransmission in KitW/KitW-v (W/Wv) mice that display lesions in ICC-IM along the gastrointestinal tract. However, contributions of NO to these phenotypes have not been evaluated. We used small-chamber superfusion assays and HPLC to measure the spontaneous and electrical field stimulation (EFS)-evoked release of nicotinamide adenine dinucleotide (NAD+)/ADP-ribose, uridine adenosine tetraphosphate (Up4A), adenosine 5′-triphosphate (ATP), and metabolites from the tunica muscularis of human, monkey, and murine colons and circular muscle of monkey colon, and we tested drugs that modulate NO levels or blocked NO receptors. NO inhibited EFS-evoked release of purines in the colon via presynaptic neuromodulation. Colons from W/Wv, Nos1−/−, and Prkg1−/− mice displayed augmented neural release of purines that was likely due to altered nitrergic neuromodulation. Colons from W/Wv mice demonstrated decreased nitrergic and increased purinergic relaxations in response to nerve stimulation. W/Wv mouse colons demonstrated reduced Nos1 expression and reduced NO release. Our results suggest that enhanced purinergic neurotransmission may compensate for the loss of nitrergic neurotransmission in muscles with partial loss of ICC. The interactions between nitrergic and purinergic neurotransmission in the colon provide novel insight into the role of neurotransmitters and effector cells in the neural regulation of gastrointestinal motility.
NEW & NOTEWORTHY This is the first study investigating the role of nitric oxide (NO) and intramuscular interstitial cells of Cajal (ICC-IM) in modulating neural release of purines in colon. We found that NO inhibited release of purines in human, monkey, and murine colons and that colons from KitW/KitW-v (W/Wv) mice, which present with partial loss of ICC-IM, demonstrated augmented neural release of purines. Interactions between nitrergic and purinergic neurotransmission may affect motility in disease conditions with ICC-IM deficiencies.
nitric oxide (NO; 8, 56), purines (10, 23, 30, 46, 47), and neuropeptides [e.g., vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP); 10, 35] are the neurotransmitters released from enteric inhibitory motor neurons in the gastrointestinal (GI) tract, including the large intestine. Purines and NO are the primary neurotransmitters released by single stimuli or in response to relatively low frequency stimulation (43), whereas the inhibitory peptides require higher frequencies and/or prolonged stimulus trains for release (35). Purines and NO released from motor neurons cause stereotypical postjunctional hyperpolarization responses, consisting of fast purinergic and slow nitrergic inhibitory junction potentials (IJPs) that result in smooth muscle relaxation. No evidence has suggested the existence of discrete populations of nitrergic and purinergic motor neurons in the enteric nervous system, and therefore it is possible that NO and purines are released from the same inhibitory motor neurons (i.e., they are cotransmitters in GI muscles).
Evidence has suggested that multiple neurotransmitters contribute to enteric inhibitory purinergic neurotransmission (46). β-Nicotinamide adenine dinucleotide (β-NAD, also referred to as NAD+; 17, 30, 47), ADP-ribose (ADPR; 17), uridine adenosine tetraphosphate (Up4A; 16), and possibly adenosine 5′-triphosphate (ATP) or its immediate metabolite adenosine 5′-diphosphate (ADP; 10, 46) are released upon neural stimulation and bind to P2Y1 receptors expressed by postjunctional cells (16, 17, 22, 29, 30, 47) to activate small-conductance Ca2+-activated K+ channels (SK channels). Activation of SK channels causes membrane hyperpolarization, inhibition of Ca2+ entry via voltage-dependent Ca2+ channels, and relaxation of smooth muscle cells (SMC; 10, 16, 17, 30, 46). In contrast, NO released from enteric inhibitory nerves activates soluble guanylyl cyclase (sGC) in postjunctional cells, causing conversion of guanosine 5′-triphosphate to cyclic guanosine 5′-monophosphate (cGMP; 13, 40). The rise in cGMP is thought to activate cGMP-dependent kinase I (PRKG1; also known as cGKI; 21, 58). The targets for PRKG1 leading to the inhibitory effects in GI muscles are not fully understood.
Neurotransmitter release from motor neurons occurs from varicosities that are closely apposed to SMC, interstitial cells of Cajal (ICC), and platelet-derived growth factor receptor-α-expressing (PDGFRα+) cells, collectively called the SIP syncytium (4, 36, 55, 57). NO and purines act on different postjunctional cells within the SIP syncytium: intramuscular ICC (ICC-IM) and SMC appear to mediate nitrergic effects (4, 40, 55, 57) whereas PDGFRα+ cells express P2Y1 receptors and SK3 channels (31, 50) and appear to mediate responses to purines (3, 37, 38). The hyperpolarization responses that develop in ICC or PDGFRα+ cells in response to NO and purines conduct to SMC via gap junctions. SMC may contribute directly to the effects of neurotransmitters, particularly at higher neurotransmitter concentrations (40, 41).
Despite the relative postjunctional spatial and functional specialization of nitrergic and purinergic neurotransmission in the gut, it is possible that there are interactions between the two divisions of inhibitory neurotransmission. For example, KitW/KitW-v/J (W/Wv) mutant mice display different degrees of loss of ICC-IM in the GI tract (55, 62) and demonstrate diminished nitrergic IJPs and smooth muscle relaxation to electrical field stimulation (EFS) in the gastric fundus (9). In addition, apamin-sensitive (hence SK3/purine-mediated) IJPs are enhanced in the gastric fundus of W/Wv mice, suggesting that purinergic inhibitory neurotransmission may be increased in these mice (9). Likewise, the P2ry1 transcripts are upregulated in the W/Wv fundus compared with wild-type (WT) mouse fundus (59). Therefore enhanced postjunctional responses to purine neurotransmitters might compensate for the inhibition of nitrergic signaling in the W/Wv mice. Another possibility is that enhanced purinergic neurotransmission is mediated by augmented neural release of purines in the ICC-deficient mice to compensate for the partial loss of nitrergic inhibitory neurotransmission. However, direct quantification of purine release in the W/Wv gut has not been reported. The present study was undertaken to determine whether neural release of the purine neurotransmitters NAD+/ADPR, Up4A, and ATP is altered in colons from W/Wv mice and to investigate the role of NO in these mechanisms. We also investigated the broader role of NO in modulating purine release in colons of mice and human and nonhuman primates.
METHODS
Ethical approval.
All experimental procedures described in this study were approved by the Institutional Animal Care and Use Committee at University of Nevada. The use of human colon tissues was approved by the Human Subjects Research Committees at Renown Regional Medical Center and by the Biomedical Institutional Review Board at the University of Nevada, Reno. The method of monkey euthanasia was approved by the Charles River Laboratories Institutional Animal Care and Use Committee.
Animal and tissue preparation.
C57BL/6 mice, B6;129S4-Nos1tm1plh/J (Nos1−/−) mice, WBB6F1/J-KitW/KitW-v/J (W/Wv) mice, FVB/N-Prkg1Tn(sb-rtTA)2497B.SB2Ove/Mmjax (Prkg1+/−) mice (Jackson Laboratory, Bar Harbor, ME), and littermate WT controls when available were used in this study. Prkg1+/− mice were bred in house to generate Prkg1+/+ and Prkg1−/− mice as described previously (12). Prkg1−/− mice are smaller in size than Prkg1+/+ or Prkg1+/− littermates, die either before weaning or shortly thereafter (i.e., 4–6 wk of age), and breed inefficiently (58). Therefore the Prkg1−/− and Prkg1+/+ mice were used at age 3–6 wk whereas the other mouse strains were used at 6–12 wk of age. Mice were euthanized by sedation with isoflurane followed by cervical dislocation and exsanguination. The GI tract was removed and placed in oxygenated Krebs-bicarbonate solution (KBS) with the following composition (in mM): 118.5 NaCl, 4.2 KCl, 1.2 MgCl2, 23.8 NaHCO3, 1.2 KH2PO4, 11.0 dextrose, and 1.8 CaCl2 (pH 7.4). Colon preparations were dissected free from the remaining GI tract and opened along the mesenteric border. Tunica muscularis preparations were prepared by peeling away the mucosa and submucosa.
Human colon samples were obtained as surgical waste from male and female patients undergoing colon resections for neoplasm or diverticulitis at the Renown Medical Center (Reno, NV). Tissues from a site distant to the disease-affected area were placed in oxygenated KBS and dissected free of mucosa and submucosa.
Colon tissues of nonhuman primates [i.e., cynomolgus monkeys (Macaca fascicularis)] were used to isolate viable circular muscle preparations that contain only nerve processes and varicosities but no cell bodies (30). Colon segments of cynomolgus monkeys of both sexes were obtained from Charles River Laboratories Preclinical Services (Reno, NV). Monkeys sedated with ketamine (10 mg/kg) and 0.7 ml Beuthanasia-D (Schering-Plough Animal Health, Kenilworth, NJ) were exsanguinated. Experiments were performed in either whole tunica muscularis (WM, whole muscle) or pure circular muscles (CM) of monkey colons that were prepared by peeling away the longitudinal muscle with attached myenteric ganglia as described previously (19, 30).
Purine release.
Colonic preparations were placed in 200-µl superfusion chambers equipped with platinum electrodes and superfused with oxygenated KBS as described previously (30, 47). After equilibration, superfusate samples were collected in ice-cold Eppendorf tubes before and during EFS [480 pulses, 0.3 ms (human) or 0.5 ms (mouse and monkey), 16 Hz]. In some experiments, tissues were superfused with tetrodotoxin (TTX, 0.5 µM), Nω-nitro-l-arginine (l-NNA, 100–1,000 µM), or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10 µM) for 30–60 min before EFS or with (±)-S-nitroso-N-acetylpenicillamine (SNAP, 1–3 µM) for 5 min before EFS. Samples were acidified to pH 4.0 with citrate phosphate buffer and subjected to 1,N6-etheno derivatization at 80°C for 40 min for HPLC analysis as described previously (5, 30, 39, 47).
Nitric oxide release.
Murine colonic segments were placed into wells of a 96-well plate containing 200 µl KBS and incubated at 37°C. After equilibration, the KBS was carefully collected in ice-cold Eppendorf tubes, and fresh KBS containing 500 μM nicotine was added to the wells containing colonic segments to stimulate nicotinic acetylcholine receptors (nAChR) that are localized on enteric motor neurons. Stimulation of inhibitory motor neurons by nAChR activation has been described previously (2, 6, 19). After 5 min the KBS + nicotine solution was collected, and samples were immediately processed using a colorimetric NO assay kit according to the manufacturer’s instructions (Abcam, Cambridge, MA). Released NO was converted to nitrite, and amounts of nitrite in each sample were calculated from nitrite standards from the manufacturer processed simultaneously with each set of samples. Absorbance was measured at a wavelength of 540 nm using a Promega Glomax Multi microplate reader (Madison, WI).
Purine catabolism.
To examine the catabolism of extracellular nucleotides, colonic preparations were superfused with 1,N6-etheno-NAD (eNAD), 1,N6-etheno-Up4A (eUp4A), or 1,N6-etheno-ATP (eATP) as substrates. 1,N6-etheno nucleotides have been used previously as exogenous substrates for nucleotidases (17, 18, 61). 1,N6-etheno-derivatized and nonderivatized nucleotides share identical sites for hydrolysis (see 18); however, etheno-derivatized purines offer greater sensitivity than nonderivatized purines in detecting substrate reduction and product formation and avoid possible complications from endogenously released purines as these remain under detection limit. Three hundred microliters of the superfusion solution were collected from the beaker containing the substrate [S0, (−) tissue]. Tissues were treated with substrates (at maximum saturation concentrations of 200 nM for eNAD or 50 nM for eUp4A and eATP) for 30 s, and the contents of the chamber were collected in ice-cold Eppendorf tubes [S1, (+) tissue]. The enzymatic reactions were stopped by immersing the tubes in liquid nitrogen. HPLC with fluorescence detection (HPLC-FLD) was used to measure the reduction in 1,N6-etheno substrates and the formation of 1,N6-etheno products [i.e., eUp4A, eATP, eADP, eNAD, eADPR, eAMP, and 1,N6-ethenoadenosine (eADO)], and HPLC with diode array detection (HPLC-DAD) was used to measure the formation of uridine 5′-triphosphate (UTP), uridine 5′-diphosphate (UDP), and uridine 5′-monophosphate (UMP), as described below.
HPLC assay of 1,N6-etheno-derivatized nucleotides in tissue superfusates.
A reverse-phase gradient Agilent Technologies 1200 liquid chromatography system equipped with a fluorescence detector (Agilent Technologies, Wilmington, DE) was used to detect the 1,N6-etheno-derivatized nucleotides and nucleosides as described previously (18, 30, 47). The mobile phase consisted of 0.1 M KH2PO4 (pH 6.0) as eluent A. Eluent B consisted of 65% eluent A and 35% methanol. Gradient elution was employed according to the following linear program: time 0, 0% eluent B; 18 min, 100% eluent B. The flow rate was 1 ml/min and run time was 20 min. Column and autosampler temperatures were maintained at 25 and 4°C, respectively. The fluorescence detector was set to record 1,N6-etheno-derivatized nucleotide and nucleoside signals at an excitation wavelength of 230 nm and emission wavelength of 420 nm (5). The amount of nucleotide/nucleoside (i.e., eATP, eADP, eAMP, eADPR, eADO, and eUp4A) in each sample was calculated from calibration curves of nucleotide standards run simultaneously with each set of unknown samples. Results, normalized for sample volume and tissue weight, were expressed in femtomoles per milligram of tissue (fmol/mg). As demonstrated previously, the eADPR peak is generated by NAD+, ADPR, and very little cyclic ADPR (17, 30, 47). Detailed HPLC fraction analysis in samples collected from human and murine colons showed that NAD+ is the dominant compound in the mixture contributing ~82–93% to the mixture, whereas ADPR contributed ~6–17% of the eADPR peak. Therefore we referred to the peak at ~12 min as NAD+/ADPR throughout the study.
HPLC assay of UTP, UDP, and UMP in tissue superfusates.
The formation of UTP, UDP, and UMP from eUp4A was detected in the same samples processed for 1,N6-etheno nucleotides using a DAD connected in sequence to the FLD, with identical mobile phases, column, and run conditions. UTP, UDP, and UMP were detected at an optimum wavelength of 260 nm (16).
Preparation of etheno substrates.
Up4A and ATP were etheno derivatized as follows: 0.2 mM Up4A or ATP (dissolved in double-distilled water) was acidified to pH 4.0 with citrate phosphate buffer. Chloroacetaldehyde (1 M) was added, and substrates were heated to 80°C for 40 min to form 1,N6-etheno derivatives eUp4A and eATP (5, 17, 39). eNAD was purchased from BioLog Life Sciences (Bremen, Germany). Substrates were further diluted in the superfusion solution to final concentration needed.
Western immunoblot analysis.
Western blot experiments were performed with the Wes System (ProteinSimple, San Jose, CA), and tissue samples and reagents were prepared in accordance with the manufacturer's instructions. The protein samples (0.2 μg), primary and secondary antibodies, blocking reagent, wash buffer, and chemiluminescent substrate were loaded into assay plates and then to microcapillaries. The anti-neuronal nitric oxide synthase (nNOS)/nitric oxide synthase 1 (Nos1) rabbit antibody (sc 648; Santa Cruz Biotechnology) was used at 1:50, 1:100, and 1:200 dilutions. The anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) rabbit antibody (sc 25778; Santa Cruz Biotechnology) was used at 1:50 dilution. Data analysis was performed with Compass software (ProteinSimple). Nos1 content was normalized to the GAPDH content.
Isometric force measurements.
Strips of colon muscle (~5 × 2 mm) were cut in the long axis of the CM fibers and attached to Fort 10 (World Precision Instruments, Sarasota, FL) isometric strain gauges in organ baths filled with oxygenated KBS (47). A resting force of 6 mN was applied to each preparation. This was followed by an equilibration period of 1 h, before experiments were initiated. EFS (2 and 16 Hz, 0.1–0.5-ms pulse width, for 30 s) was applied via platinum coil electrodes positioned in parallel on both sides of the muscle strips. Signals were recorded onto a personal computer via Acknowledge software (Biopac Systems, Goleta, CA), and areas under the trace (AUT) were calculated.
Drugs.
ATP, l-NNA, and SNAP were purchased from Sigma-Aldrich (St. Louis, MO). Up4A and eNAD were purchased from BioLog Life Sciences. ODQ was purchased from R&D Systems (Minneapolis, MN). TTX was purchased from Abcam. (1R,2S,4S,5S)-4-[2-iodo-6-(methylamino)-9H-purin,-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt (MRS2500) was purchased from Tocris Bioscience (Ellisville, MO).
Statistics.
Potential differences between groups were evaluated using Student’s t-test (unpaired, paired) with Welch’s correction test where appropriate or with one-way ANOVA with Tukey’s post hoc test when multiple comparisons were appropriate. Data are presented as means ± SE. Differences were considered statistically significant when P < 0.05; n refers to number of observations. Analysis was done using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA).
RESULTS
NO inhibits the EFS-evoked overflow of NAD+/ADPR, ATP, and metabolites in human and monkey colon.
EFS stimulated release of NAD+/ADPR, ATP, and total purines (NAD+/ADPR+ATP+ADP+AMP+ADO) in human colonic muscles, and the release was increased after pretreatment with l-NNA (P < 0.05 for NAD+/ADPR, ATP, and total purines) or ODQ, an inhibitor of sGC (P < 0.01, P < 0.001, and P < 0.01 for NAD+/ADPR, ATP, and total purines, respectively; Fig. 1). The NO donor SNAP did not change the evoked release of NAD+/ADPR and ATP (P > 0.05 for both groups) but did reduce the evoked release of total purines (P < 0.05). These results suggest that endogenous NO inhibits neural release of purines in human colon likely by stimulation of sGC in target cells. EFS also evoked release of NAD+/ADPR, ATP, and total purines in monkey CM strips lacking myenteric ganglia. Release of purines was also increased by l-NNA (P < 0.0001, P < 0.01, and P < 0.001, respectively; Fig. 2, A and B). In WM preparations the EFS-stimulated release of NAD+/ADPR was also significantly increased in the presence of l-NNA (P < 0.05), but release of ATP evoked by EFS, measured simultaneously with NAD+/ADPR, was unchanged by l-NNA (P > 0.05; Fig. 2, C and D).
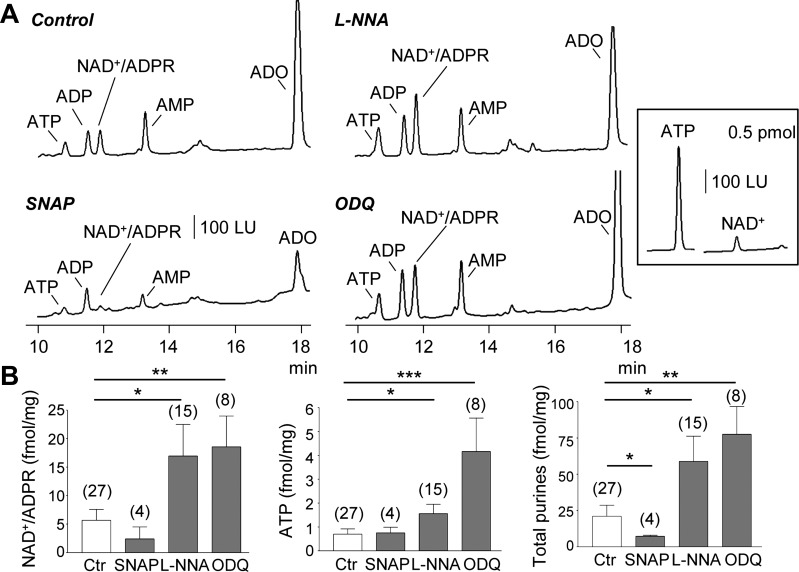
Fig. 1.
EFS-evoked release of purines upon pharmacological inhibition or stimulation of nitric oxide signaling in human colon. A: chromatograms of tissue superfusates collected during EFS of colon muscularis in the absence of drugs (control) or in the presence of l-NNA (100 μM for 60 min), ODQ (10 μM for 30 min), or SNAP (1 μM for 5 min). Inhibition of NOS with l-NNA or of soluble guanylyl cyclase with ODQ increased the EFS-evoked release of purines whereas the NO donor, SNAP, reduced the EFS-evoked release of purines. Note that the peaks of Up4A are not clearly visible in the chromatograms at the scale shown. ADO, adenosine. Inset: comparison of fluorescence of ATP and NAD+; NAD+, 0.5 pmol, generates ~10 times lower fluorescent signal than 0.5 pmol ATP. LU, luminescence units. B: averaged data (means ± SE) summarizing the EFS-evoked release of NAD+/ADPR, ATP, and total purines (i.e., ATP+ADP+NAD+/ADPR+AMP+ADO) in human colon in the absence (Ctr, control) and presence of SNAP, l-NNA, and ODQ. Release in femtomoles per milligram of tissue (fmol/mg) is calculated as evoked release minus spontaneous release. Significant difference from control release (*P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test). Number of experiments is in parentheses.
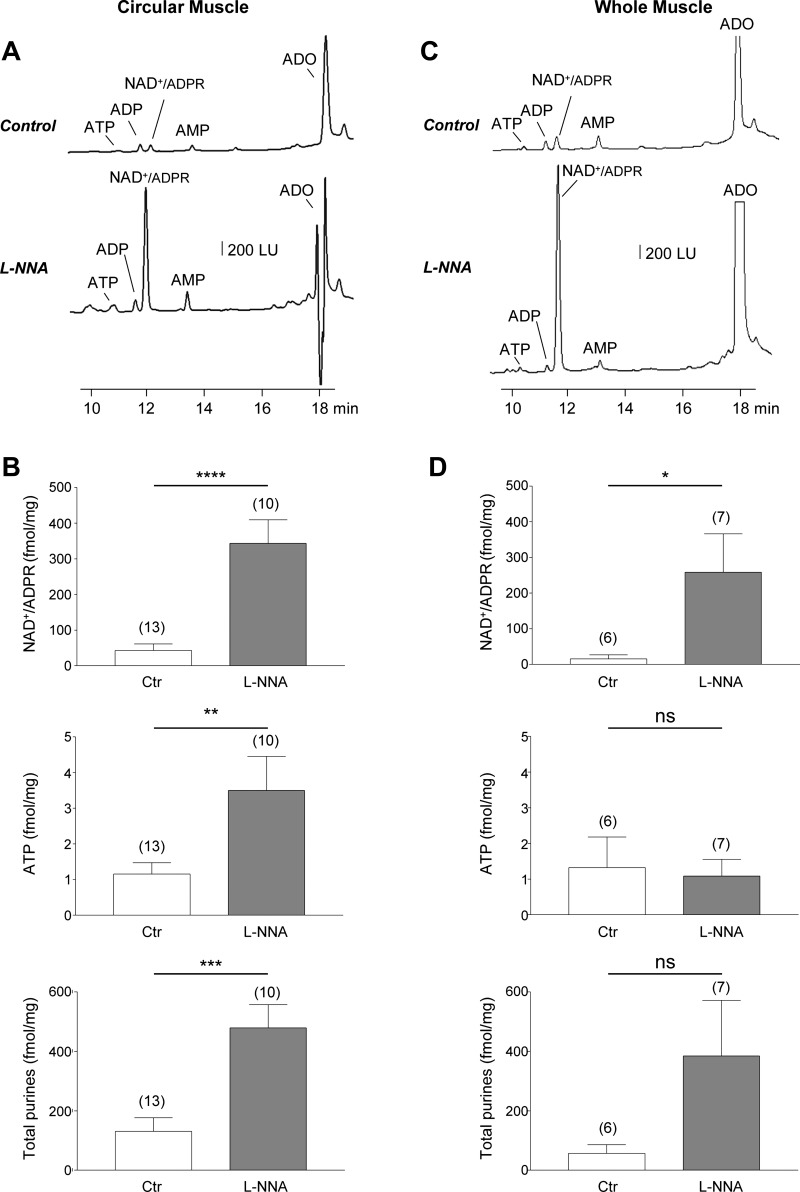
Fig. 2.
EFS-evoked release of purines after pharmacological inhibition of nitric oxide synthesis in circular muscle and whole muscle of monkey colon. A: chromatograms of tissue superfusates collected during EFS of circular muscle of colon muscularis in the absence of drugs (control) or in the presence of l-NNA (100 μM for 60 min). Inhibition of NOS with l-NNA increased the EFS-evoked release of purines. LU, luminescence units. B: averaged data (means ± SE) summarizing the EFS-evoked release of NAD+/ADPR, ATP, and total purines (i.e., ATP+ADP+NAD+/ADPR+AMP+ADO) in circular muscle of monkey colon in the absence (Ctr, control) and presence of l-NNA. C: chromatograms of tissue superfusates collected during EFS of whole tunica muscularis in the absence of drugs (control) or in the presence of l-NNA (100 μM for 60 min). D: averaged data (means ± SE) summarizing the EFS-evoked release of NAD+/ADPR, ATP, and total purines in whole muscle of monkey colon in the absence (control) and presence of l-NNA. Release in femtomoles per milligram of tissue (fmol/mg) is calculated as evoked release minus spontaneous release. Significant difference from control release (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t-test); ns, not significant (P > 0.05). Number of experiments is in parentheses.
NO inhibits the EFS-evoked overflow of NAD+/ADPR, ATP, and metabolites in colon from WT and W/Wv mice.
EFS caused release of purines in both WT and W/Wv murine colons. As reported previously with C57BL/6 mice (46), the stimulated release of NAD+/ADPR and total purines, but not of ATP, was inhibited by TTX (P < 0.01, P > 0.05, and P < 0.01 for NAD+/ADPR, ATP, and total purines, respectively). The release of purines in W/Wv mouse colons in response to EFS exceeded the release in WT (P < 0.01, P < 0.05, and P < 0.05 for NAD+/ADPR, ATP, and total purines, respectively). Release of NAD+/ADPR, ATP, and total purines evoked by EFS in colons from W/Wv mice was inhibited by TTX (P < 0.01, P < 0.05, and P < 0.05, respectively; Fig. 3).
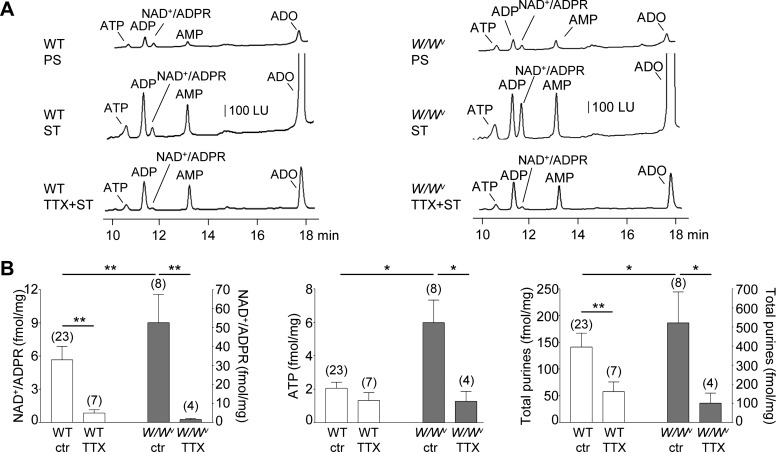
Fig. 3.
Spontaneous and evoked release of purines in wild-type (WT) and W/Wv mouse colon. A: chromatograms of tissue superfusates collected before (PS, prestimulation) and during EFS (ST, stimulation) of WT (left) and W/Wv (right) mouse colon preparations in the absence and presence of tetrodotoxin (TTX, 0.5 μM for 25 min). Small amounts of ATP, ADP, NAD+/ADPR, AMP, and ADO were present in superfusates before stimulation. EFS evoked additional release of purines. The EFS-evoked release of NAD+/ADPR, but not ATP, was reduced by TTX in the WT colon, whereas the EFS-evoked release of both NAD+/ADPR and ATP was inhibited by TTX in the W/Wv colons. LU, luminescence units. B: averaged data (means ± SE) summarizing the EFS-evoked release of NAD+/ADPR, ATP, and total purines (i.e., ATP+ADP+NAD+/ADPR+AMP+ADO) in WT and W/Wv mouse colon. Release [in femtomoles per milligram of tissue (fmol/mg)] is calculated as evoked release minus spontaneous release. Significant difference between groups is indicated with horizontal bars (*P < 0.05, **P < 0.01, Student’s t-test). Number of experiments is in parentheses.
Similar to human colon (Fig. 1), the release of NAD+/ADPR evoked by EFS in murine colon was reduced by SNAP (P < 0.01; Fig. 4). A significant reduction of stimulated release of ATP and metabolites was observed with SNAP (e.g., P < 0.05 and P < 0.01 for ATP and total purines, respectively; Fig. 4). Release of purines was also increased by ODQ in WT colons (not shown). As in human and monkey colonic muscles, the stimulated release of NAD+/ADPR in the murine colon was enhanced by l-NNA (P < 0.05; Fig. 4).
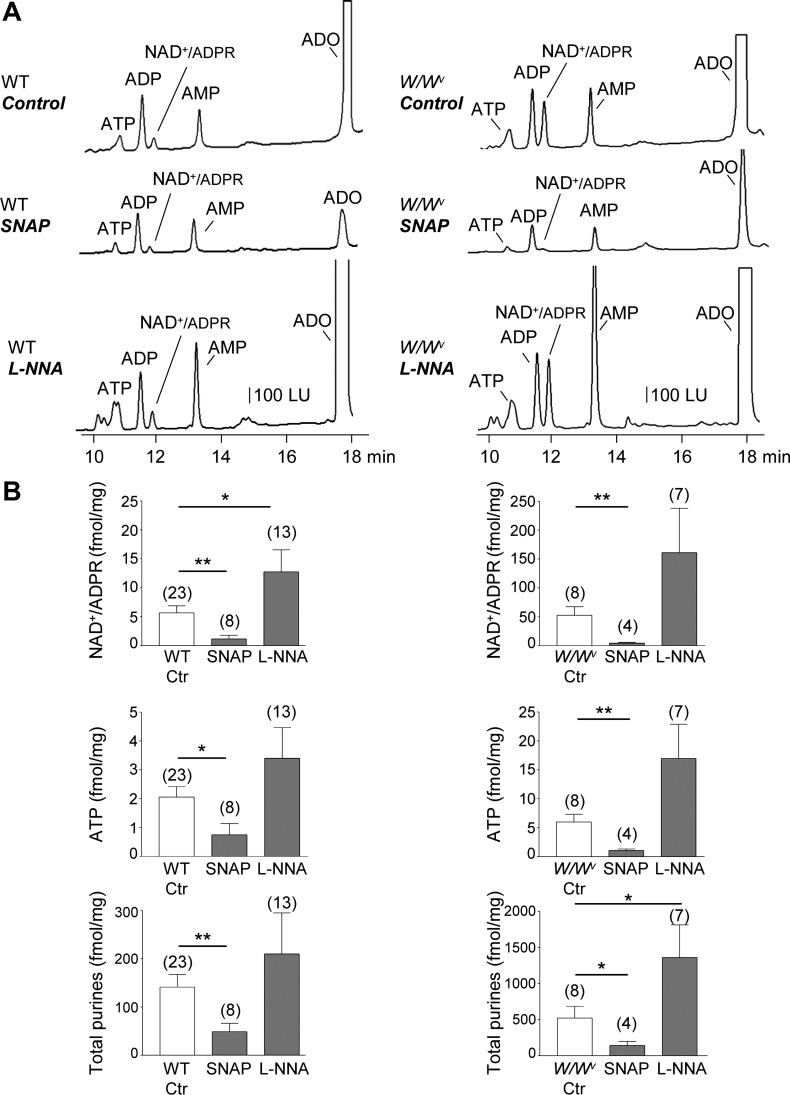
Fig. 4.
EFS-evoked release of purines upon pharmacological inhibition or stimulation of nitric oxide signaling in wild-type (WT) and W/Wv mouse colon. A: chromatograms of tissue superfusates collected during EFS of WT (left) and W/Wv (right) mouse colon in the absence of drugs (control) or in the presence of l-NNA (100 μM for 60 min) or SNAP (1 μM for 5 min). Inhibition of NO with l-NNA further increased the EFS-evoked release of purines in WT and W/Wv mice whereas the NO donor, SNAP, reduced the EFS-evoked release of purines. LU, luminescence units. B: averaged data (means ± SE) summarizing the EFS-evoked release of NAD+/ADPR, ATP, and total purines (i.e., ATP+ADP+NAD+/ADPR+AMP+ADO) in the WT and W/Wv mouse colon in the absence (Ctr, control) and presence of l-NNA and SNAP. Release in femtomoles per milligram of tissue (fmol/mg) is calculated as evoked release minus spontaneous release. Significant difference between groups is identified with horizontal bars (*P < 0.05, **P < 0.01, Student’s t-test). Number of experiments is in parentheses.
The effects of SNAP and l-NNA on purine release were more pronounced in the W/Wv colon than in WT colon (Fig. 4). Thus the P values of control vs. SNAP were P < 0.01, P < 0.01, and P < 0.05 for NAD+/ADPR, ATP, and total purines, respectively, whereas P values for control vs. l-NNA were P > 0.05, P > 0.05, and P < 0.05 for NAD+/ADPR, ATP, and total purines, respectively. As in primate colons, the results suggest that endogenous NO inhibits neural release of purines in the murine colon.
NO modulates the release of Up4A in human and murine colon.
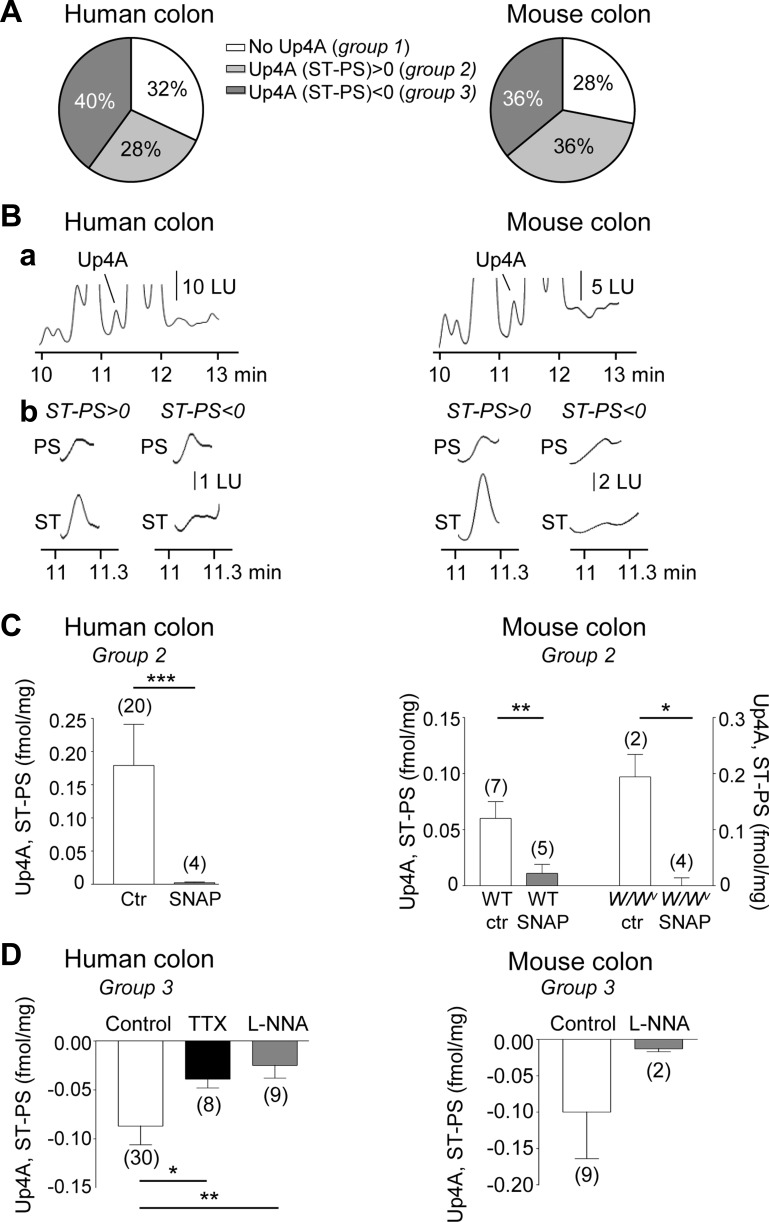
We have previously reported that in addition to NAD+/ADPR, ATP, ADP, AMP, and ADO, neural release of Up4A can be detected in ~30% of colonic muscles from humans and mice (16). In the present study we characterized further the release of Up4A at rest and during EFS and examined the effects of SNAP and l-NNA on Up4A overflow in human and murine colonic preparations. We identified three groups of preparations in human and murine colons as follows: 1) group 1, preparations in which no detectable amounts of Up4A at rest or during EFS were identified (these represent ~32 and 28% of human and murine colon preparations, respectively); 2) group 2, preparations in which EFS-evoked overflow of Up4A exceeded the resting overflow of Up4A [these represent ~28 and 36% of human and murine colon preparations, respectively (16)]; and 3) group 3, preparations in which EFS reduced the overflow of Up4A (these represent ~40 and 36% of human and murine colonic preparations, respectively; Fig. 5). In group 3 of the human colon the Up4A overflow was 0.147 ± 0.02 and 0.060 ± 0.012 fmol/mg tissue before and after EFS, respectively (n = 30, P < 0.0001), whereas in the murine colon the Up4A overflow was 0.158 ± 0.06 and 0.057 ± 0.02 fmol/mg tissue (n = 9, P > 0.05). The stimulated release of Up4A (e.g., group 2) was reduced by TTX (16), suggesting neural origin of Up4A in colon tunica muscularis (16).
Fig. 5.
Release of Up4A in human and mouse colons. A: graphs summarizing the percentage of human (left) and C57BL/6 mouse (right) colon preparations that demonstrated no EFS-evoked release of Up4A (group 1), increased EFS-evoked release of Up4A (group 2), or decreased EFS-evoked release of Up4A (group 3). B, a: segments of original chromatograms showing Up4A in tissue superfusate from human (left) and mouse (right) colon. B, b: examples of Up4A peaks in samples collected before EFS (PS, prestimulation) and during EFS (ST, stimulation) in preparations in which EFS evoked additional release of Up4A (group 2; ST-PS > 0; left) and in preparations in which EFS caused a decreased release of Up4A (group 3; ST-PS < 0; right). LU, luminescence units. C: means ± SE of ST-PS values of human (left) and mouse (right) colon preparations that demonstrated increased (group 2) EFS-evoked release. Note that the stimulated release of Up4A is reduced by SNAP in both human and mouse (WT and W/Wv) colons. Significant difference between groups is identified with horizontal bars (*P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test). D: means ± SE of ST-PS values of human (left) and mouse (right) colon preparations that demonstrated reduced (group 3) EFS-evoked release of Up4A. Note that the inhibition was reduced by either TTX (0.5 μM) or l-NNA (100 μM). Number of experiments is in parentheses.
SNAP inhibited Up4A release evoked by EFS in human (P < 0.0001) and murine colon (both from WT and W/Wv mice, P < 0.01 and P < 0.05, respectively), suggesting that similar to other purine neurotransmitters, NO inhibited the neural release of Up4A. The reduction of Up4A that was seen in response to EFS in group 3 was also diminished by TTX or l-NNA (P < 0.05 and P < 0.01 in human colon, respectively; Fig. 5), suggesting that NO released from motor neurons may have caused the decrease of Up4A during EFS in these preparations. These results suggest that release of Up4A from nerves during EFS is regulated by presynaptic inhibition by NO.
Metabolism of purines in WT and W/Wv mouse colon.
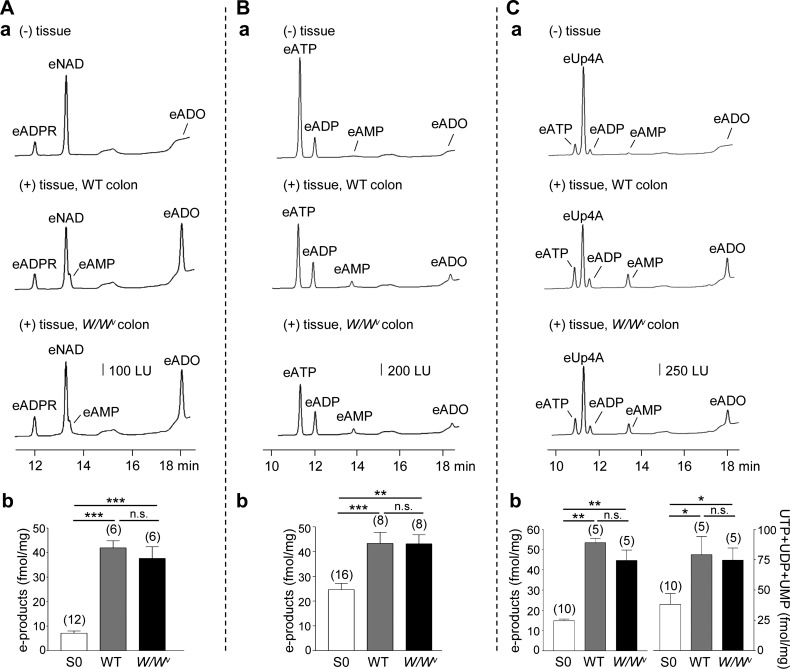
Enhanced amounts of extracellular purines could be due to either increased release of purines during neural stimulation or inhibited extracellular catabolism of purines. To determine whether the latter scenario applies to the colons isolated from W/Wv mice, we compared the degradation of eNAD, eATP, and eUp4A in WT and W/Wv mice. Superfusion of colons with eNAD (200 nM for 30 s) resulted in decreased amounts of eNAD and increased amounts of 1,N6-etheno products (e-products) eADPR+eAMP+eADO (P < 0.001 and P < 0.001 for WT and W/Wv mice, respectively). The amounts of products formed were similar in WT and W/Wv colons (P > 0.05; Fig. 6). Likewise, superfusion of colons with eATP (50 nM for 30 s) resulted in decreased eATP and increased e-products eADP+eAMP+eADO (P < 0.001 and P < 0.01 for WT and W/Wv, respectively), and there was no difference in the amounts of products formed in WT and W/Wv mice (P > 0.05; Fig. 6). Finally, superfusion of colonic muscles with eUp4A (50 nM for 30 s) resulted in reduced eUp4A and increased adenine (eATP+eADP+eAMP+eADO; P < 0.01 for WT and W/Wv mice) and pyrimidine (UTP+UDP+UMP; P < 0.05 for WT and W/Wv, respectively) products. No differences were found in the amounts of products formed from eUp4A in WT and W/Wv colons (P > 0.05; Fig. 6). These results suggest that altered purine metabolism is not likely to contribute to enhanced amounts of extracellular purines in W/Wv colons.
Fig. 6.
Degradation of eNAD, eATP, and eUp4A in wild-type (WT) and W/Wv colons. A–C, a: chromatograms of eNAD 200 nM (A, a), eATP 50 nM (B, a), and eUp4A 50 nM (C, a) in the absence of tissue [S0, (−) tissue; traces at top] and after superfusion of colonic tissues from WT mice (traces in middle) or W/Wv mice (traces at bottom) with eNAD, eATP, or eUp4A or for 30 s. Superfusion of colons from WT or W/Wv mice resulted in decreased amounts of e-substrates and increased amounts of e-products. LU, luminescence units. A, b: graphical representation of e-product (eADPR+eAMP+eADO) formation from 200 nM eNAD in (−) tissue control (S0), in WT colons, and in W/Wv colons. B, b: graphical representation of e-product (eADP+eAMP+eADO) formation from 50 nM eATP in (−) tissue control (S0), in WT colons, and in W/Wv colons. C, b: graphical representation of e-product (eATP+eADP+eAMP+eADO) formation and UTP+UDP+UMP formation from 50 nM eUp4A in (−) tissue control (S0), in WT colons, and in W/Wv colons. There was no difference in the amounts of products formed from eNAD, eATP, or eUp4A in WT and W/Wv colons (not significant). Significant differences from S0 (*P < 0.05, ***P < 0.01, ***P < 0.001, Student’s t-test); n.s., not significant, P > 0.05. Number of experiments is in parentheses.
EFS-evoked release of NAD+/ADPR, ATP, and metabolites is enhanced in colons from Nos1−/− and Prkg1−/− mice.
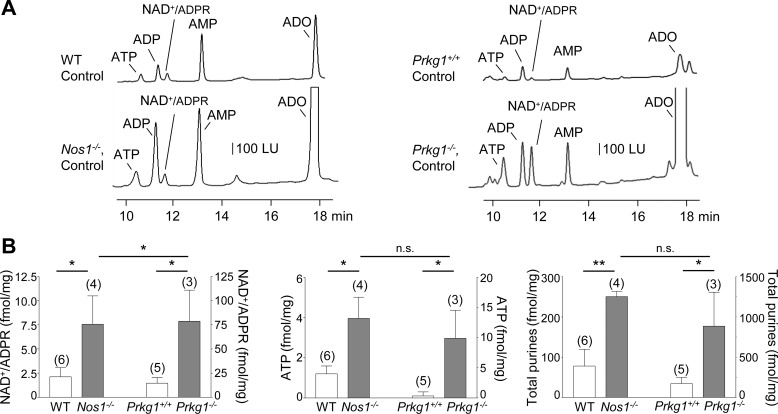
To determine further that NO modulates purine release, we evaluated the stimulated release of purines in colons from mice lacking Nos1, the gene for nNOS that is responsible for production of NO in neurons. In agreement with pharmacological studies, the evoked release of NAD+/ADPR, ATP, and total purines was increased (P < 0.05, P < 0.05, and P < 0.01, respectively) in colons from Nos1−/− mice (Fig. 7). Likewise, the stimulated release of purines was increased in colon muscles isolated from mice lacking Prkg1, the gene encoding cGKI, the downstream mediator of NO-stimulated sGC (P < 0.05 for NAD+/ADPR, ATP, and total purines, respectively; Fig. 7). These results confirm that NO likely inhibits the neural release of purines via stimulation of sGC-cGKI. No significant differences were observed between the stimulated release of ATP and total purines in colonic preparations from Nos1−/− and Prkg1−/− mice (P > 0.05) whereas the EFS-evoked release of NAD+/ADPR appeared to be higher in colon of Prkg1−/− mice than in colon of Nos1−/− mice (P < 0.05, Fig. 7B).
Fig. 7.
EFS-evoked release of purines in colons from Nos1−/− and Prkg1−/− mice. A: chromatograms of tissue superfusates collected during EFS of wild-type (WT, Nos1+/+) controls and Nos1−/− mice (left) and wild-type controls (WT, Prkg1+/+) and Prkg1−/− mice (right). The EFS-evoked release of purines was enhanced in Nos1−/− and Prkg1−/− colons. LU, luminescence units. B: averaged data (means ± SE) summarizing the EFS-evoked release of NAD+/ADPR, ATP, and total purines (i.e., ATP+ADP+NAD+/ADPR+AMP+ADO). Release in femtomoles per milligram of tissue (fmol/mg) is calculated as evoked release minus spontaneous release. Significant differences from release in WT colons (*P < 0.05, **P < 0.01, Student’s t-test); n.s., not significant, P > 0.05 between Nos1−/− and Prkg1−/− colons. Number of experiments is in parentheses.
Comparison of EFS-evoked relaxations in colons from WT and W/Wv mice.
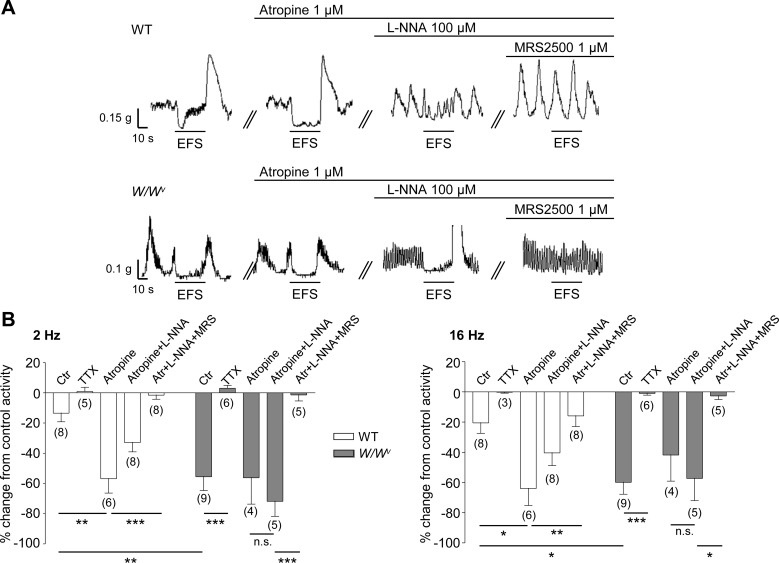
We also compared the mechanical responses of colon muscularis strips isolated from WT and W/Wv mice. As shown in Fig. 8, the dominant response to low (2 Hz) and high (16 Hz) frequency of EFS (0.1-ms pulses) for 30 s was smooth muscle relaxation. These responses were reduced by TTX in both WT and WWv colons although significance was reached in the WWv preparations only (P < 0.001 and P < 0.01 at 2 and 16 Hz, respectively). The sensitivity of EFS-evoked relaxations to TTX suggested that these responses were largely mediated by neurotransmitters released from enteric neurons. Pharmacological dissection of these responses revealed a small acetylcholine-mediated contractile component (eliminated by atropine), a nitrergic relaxation component (eliminated by adding l-NNA), and a purinergic relaxation component (eliminated by the selective P2Y1 receptor inhibitor, MRS2500). Notably, the NO-mediated relaxation was significant in WT colons, whereas the purine-mediated relaxation was dominant in the colons from W/Wv mice. These results suggest that purinergic neurotransmission (hence purine-mediated relaxation) is facilitated whereas the nitrergic neurotransmission is compromised in W/Wv mice.
Fig. 8.
Responses to EFS in WT and W/Wv isolated colonic muscle stripes. A: traces of spontaneous contractions and responses to EFS in wild-type (WT, traces at top) and W/Wv (traces at bottom) mouse colonic muscle under control conditions and in the presence of atropine (1 µM), l-NNA (100 µM), and MRS2500 (1 µM). Black lines above traces indicate when atropine, l-NNA, or MRS2500 was added to the bath. Traces showing effects of inhibitors were obtained in the same strip of WT or W/Wv colon. B: summaries of responses to EFS 2 Hz (left) and 16 Hz (right) in WT and W/Wv colon as area under the trace (AUT) in control conditions (Ctr) and in the presence of TTX (0.5 µM), atropine, atropine + l-NNA, and atropine + l-NNA + MRS2500 (Atr+l-NNA+MRS). Significant difference between groups is indicated with horizontal bars (*P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA with Tukey’s post hoc test); n.s., not significant (P > 0.05). Number of experiments is in parentheses.
W/Wv mouse colon demonstrates decreased protein levels of nNOS and reduced NO release.
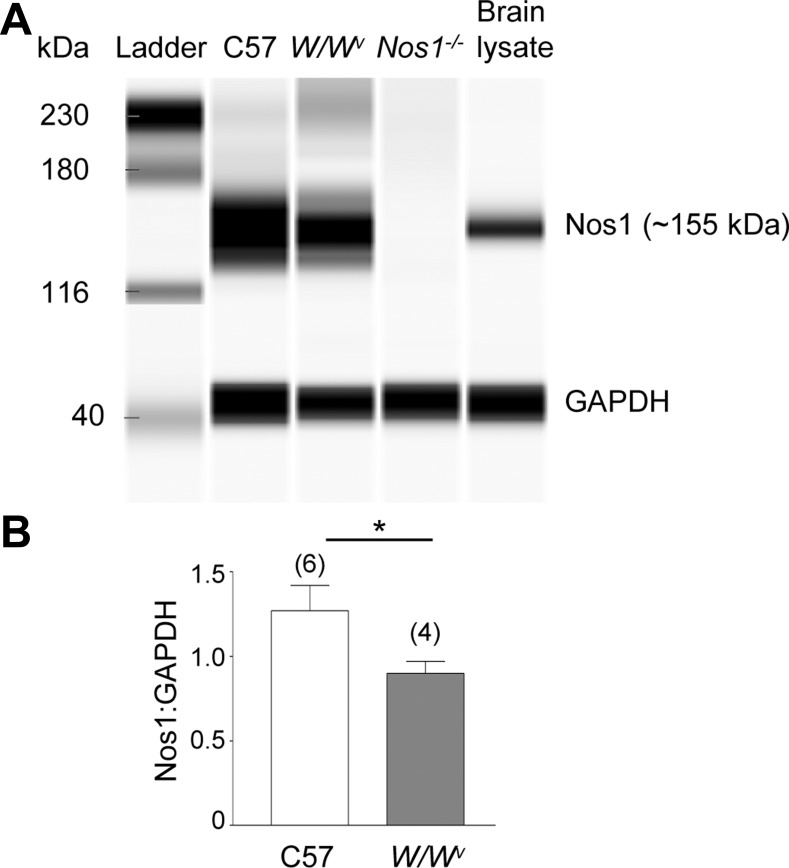
Western analysis demonstrated that nNOS protein levels were decreased in colon muscularis from W/Wv mice (Fig. 9). Stimulation of nAChR with nicotine (500 μM) evoked release of NO in colons isolated from WT mice (1.25 ± 0.49 pmol/mg, n = 3) and less from W/Wv mice (0.16 ± 0.16 pmol/mg, n = 2), P > 0.05. Solutions of nicotine (no tissue present) contained no detectable amounts of NO. Similarly, KBS collected from colonic segments before nicotinic stimulation contained no detectable amounts of NO.
Fig. 9.
Levels of Nos1 protein in colon muscularis of WT, W/Wv, and Nos1−/− mice. A: Western blot for Nos1 (upper band) and GAPDH (lower band) performed by capillary electrophoresis using the Wes System (ProteinSimple). Each lane was loaded with 0.2-μg protein. All images shown were captured at the same exposure (5 s). Note that W/Wv colon demonstrated lower Nos1 levels than WT. Nos1−/− and brain lysate samples are provided as controls. GAPDH was used to control equal loading. B: averaged data showing expression of Nos1 normalized to GAPDH in WT (C57) and W/Wv colons. Significant difference between WT and W/Wv colons (*P < 0.05, Student’s t-test). Number of experiments is in parentheses.
DISCUSSION
The present study shows that NO modulates the release of purine neurotransmitters in the large intestine and that ICC-IM affect this neuromodulation. Specifically, we demonstrated here that the neural release of purines was 1) inhibited by a NO donor, 2) increased by a NOS inhibitor or in the absence of Nos1, 3) increased in the presence of a sGC inhibitor, and 4) increased in the absence of Prkg1 in colonic muscles. These observations suggest that NO inhibits neural release of purines in the large intestine likely via activation of sGC and cGK1/PRKG1 in target cells. The second major observation in the present study was that stimulated release of purine neurotransmitters was enhanced in colons from W/Wv mice with decreased numbers of ICC-IM likely due to hampered neuromodulation by NO. We concluded, therefore, that in addition to compromised postjunctional nitrergic neurotransmission (55, 57), deficiencies in ICC-IM are accompanied with enhanced purinergic neurotransmission because of reduced inhibition of purine release by NO. This study shows the first direct evidence for NO-mediated modulation of purinergic motor transmission in the colon and suggests a role of ICC-IM in these mechanisms.
As discussed in the Introduction, changes in enteric neurotransmission of the gastric fundus of W/Wv mice have been reported extensively. However, the information about neural regulation of the colon of these mice is rather limited likely because of the fact that the colons of W/Wv mice display an incomplete loss of ICC-IM that complicates conclusions about the role of ICC in enteric motor neurotransmission (4, 55). Nevertheless, this might be a strength of this mouse model, because many human motility disorders (20), such as chronic idiopathic intestinal pseudoobstruction (33), diabetes mellitus (48), slow-transit constipation (27, 42), Hirschsprung disease (15, 24, 54), or aging-related motility disorders (25), are associated with reduced numbers of ICC but not complete loss. Therefore studies in W/Wv colon are potentially important for understanding the pathogenesis of motility disorders associated with reduced populations of ICC.
Despite the reduced number of ICC-IM in the W/Wv colon and reduced propulsive contractility in the small intestine of W/Wv mice (14), no major morphological defects were reported in enteric nerves or SMC in the colons of W/Wv mice (32). Moreover, W/Wv mice live into adulthood without apparent loss of nutritional absorption (11). Therefore it is possible that the W/Wv mice compensate for the loss of nitrergic neurotransmission by developing upregulation of other forms of enteric inhibitory neurotransmission, such as purinergic neurotransmission. The present study was designed to determine whether reduced nitrergic neurotransmission might be compensated for by enhanced release of purine neurotransmitters in colons of W/Wv mice. Since ICC-IM are major mediators of nitrergic neurotransmission and these cells are reduced in the W/Wv colons, we also investigated whether changes in the amount of NO or NO responses have effects on purine neurotransmitter release.
Pharmacological characterization of postjunctional responses has suggested that the relative role of nitrergic neurotransmission may be greater in proximal colon than in distal colon of rats (60) and CD1 mice (44) whereas purinergic neurotransmission becomes particularly important for the function of the distal colon (29, 44). Immunohistochemistry studies, based on c-Kit immunoreactivity, have reported lower densities of ICC in the CM of the distal colon than in the proximal colon of C57BL/6 mice, but the reduction was primarily due to reduced ICC in the region of the myenteric plexus, not in ICC-IM that contribute to nitrergic neurotransmission (63). Moreover, ICC-IM density, evaluated either with c-Kit antibodies or anoctamin-1 (ANO1) antibodies, showed similar reduction (~50%) in the proximal, middle, and distal colons of W/Wv mice (62). Likewise, no significant differences in density and distribution of enteric nerves have been demonstrated in different regions in the colon. Therefore the present study investigated the purine neurotransmitter release in the entire murine colon and did not distinguish between different regions of the colon.
We found that NO influenced neural release of purines in primate and mouse colon. Release of purines was enhanced when nitrergic signaling was diminished pharmacologically with l-NNA or ODQ and in mice lacking Nos1 or Prkg1 expression. Together, these data suggest that endogenous production of NO inhibits neural release of purines across species. This could be a negative-feedback mechanism to prevent “overinhibition” of colonic contractility during activation of inhibitory motor neurons. NO-mediated neuromodulation of acetylcholine release (45) or release of VIP (1) has also been suggested in previous studies. Whether purines can modulate neural release of NO in the gut remains to be determined.
Neural release of purines was augmented in colons from W/Wv mice. Drugs that affect the NO pathway had more pronounced effects in colonic muscles form W/Wv mice than in colons from WT mice. Thus the NO donor, SNAP, significantly reduced the release of purines in W/Wv mice to levels similar to those observed in WT mice. Increased levels of extracellular purines in W/Wv mouse colons were not a result of reduced purine catabolism, but were likely due to facilitated neuronal purine release because we observed similar degradation of exogenous purine neurotransmitters in WT and W/Wv colons and TTX significantly reduced the stimulated release of purines in the W/Wv colons. W/Wv mice had reduced expression of Nos1 in colonic muscles compared with WT mice, and W/Wv mouse colons appeared to have reduced levels of NO released during stimulation of inhibitory neurons with nicotine. l-NNA further augmented purine release in the W/Wv colon. We did not investigate postjunctional IJPs in response to EFS in this study, because previous reports have demonstrated a “patchy” pattern of ICC-IM loss in the W/Wv mouse colon resulting in inconsistent results of intracellular microelectrode studies in this muscle (e.g., 55). However, data from mechanical experiments demonstrated that purine-mediated relaxation responses to EFS were enhanced in W/Wv colons compared with WT colons. Thus functional data in the mouse colon support our findings from neurotransmitter release experiments and demonstrate that purinergic responses are enhanced in mice with diminished nitrergic signaling. The W/Wv colon demonstrates that apparently unaltered responses to nerve stimulation may be due to reciprocal changes in release of inhibitory neurotransmitters.
We recently reported that Up4A is released from enteric nerves and also satisfies criteria for a neurotransmitter in the human and mouse intestine (16). It should be noted that in the present study the effects of l-NNA and ODQ on the EFS-evoked release of Up4A in mouse and human colons did not always mirror the effects of these inhibitors on the release of NAD+/ADPR, ATP, and metabolites. In addition, we were not able to measure any release of Up4A in the Nos1−/− colons. These results can be accounted for by the facts that not all colonic preparations release Up4A and not all Up4A release originates from neurons [Fig. 5 and Durnin et al. (16)]. In fact, only ~30% of human and mouse colonic preparations were found to release Up4A during EFS, consistent with our previous observations in the human colon (16). Nevertheless, of the mouse colon preparations that did release Up4A (i.e., 30% of preparations), the release was significantly enhanced in the W/Wv colon and was inhibited by TTX in these mice. Thus, similar to NAD+/ADPR and ATP, the enhanced release of Up4A in W/Wv mice was of neural origin and was dependent on action potential firing. Interestingly, in ~40% of human and murine colon preparations, the release of Up4A was decreased during EFS. The EFS-evoked inhibition of Up4A release was reduced by TTX and l-NNA suggesting that neural release of NO normally inhibits the release of Up4A. We presently do not know the cell-type source of the nonneuronal Up4A in the intestine, but it may originate from the vascular endothelium (34) or another cell type. The identification of the source of Up4A in complex tissues such as the colon that are composed of multiple cell types may not be possible with current methodologies.
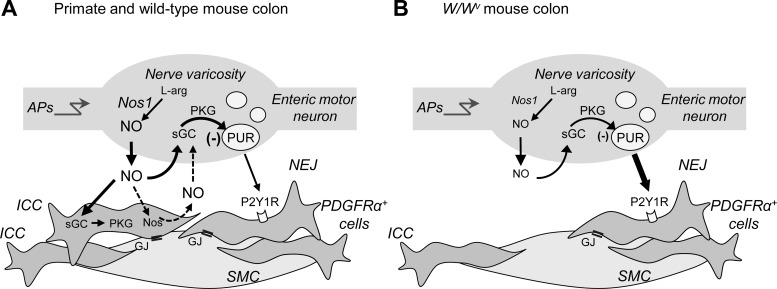
The present study shows significantly enhanced purine release in Nos1−/− mouse colons suggesting that neural NO contributes significantly to the inhibitory effects of NO on purine release. Moreover, Nos1/nNOS is the predominant NOS isoform expressed in the rat intestine, where it contributes to >95% of all NOS activity (53). The striking similarities in the release of neurogenic purines in colons isolated from Nos1−/− mice and from W/Wv mice suggested that the release of neuronal NO might be reduced in the W/Wv colon causing reduced prejunctional inhibition of purine release in these mice. In support of this hypothesis, we found that W/Wv colons have reduced levels of Nos1, in line with previous reports in the W/Wv stomach (64), and reduced neural release of NO. Therefore NO levels appear to be reduced when the primary postjunctional targets for nitrergic neurotransmission are reduced. It is also possible that part of the NO affecting purine release could come from ICC, as suggested previously (52, 65). Decreasing this source of NO in the W/Wv colons might affect purine release from enteric nerve varicosities. Both mechanisms might reduce the amount of NO available for prejunctional inhibition of purine release in colons from W/Wv mice. The proposed models are summarized in Fig. 10.
Fig. 10.
Model of NO-mediated inhibition of purine release in the colon. A: in human and wild-type mouse colon, propagation of action potentials (APs) into nerve varicosities of enteric inhibitory motor neurons stimulates release of NO that is produced from l-arginine (l-arg) via Nos1. Released NO stimulates postjunctional interstitial cells of Cajal (ICC) by binding to soluble guanylyl cyclase (sGC), which activates cGMP-dependent kinase I (PKG). Responses produced in ICC are propagated to smooth muscle cells (SMC) via gap junctions (GJ). NO also stimulates sGC (and possibly PKG) presynaptically in neurons to inhibit release of purines (PUR) from nerve varicosities (this study) and thus likely reduces purinergic responses in postjunctional PDGFRα+ cells. NO-induced release of NO from ICC (52) may also contribute to prejunctional sGC activation and inhibition of purine release (dashed arrows). Regulation of purine release by NO would limit the amounts of inhibitory neurotransmitters that are released into the neuroeffector junction (NEJ) and prevent overinhibition of colonic muscles during motor neuron activation. B: W/Wv mice have reduced colonic ICC and reduced expression of Nos1 resulting in decreased extracellular amounts of NO during AP firing and reduced sGC-mediated inhibition of purine release from nerve varicosities. Enhanced purine release and purine signaling via P2Y1 receptors (P2Y1R) on PDGFRα+ cells allows inhibitory motor responses to occur in the absence of ICC in W/Wv colons. PDGFRα+cells, platelet-derived growth factor receptor-α-positive cells.
In circular muscles (CM) of monkey colon, containing nerve fibers but not myenteric ganglia (30), l-NNA increased the release of purines, suggesting that NO can modulate the release of purines from motor neurons at the sites of neurotransmitter release. l-NNA also increased release of NAD+/ADPR (and total purines) in whole muscle (WM) preparations of monkey colon but did not affect release of ATP. This is an important difference and suggests that nerve cell bodies are not the primary site of NO neuromodulation. These observations are compatible with previous findings from studies of monkey colon showing that stimulation of nicotinic, cholinergic receptors (nAChR) or 5-hydroxytryptamine-3 receptors (5-HT3R), expressed on the cell bodies of inhibitory motor neurons, caused release of NAD+/ADPR at nerve varicosities in CM, whereas a large portion of ATP release occurred in ganglia in WM (19). Observations in monkey colon suggest that NO originating near nerve terminals, myenteric ganglia, or both can modulate release of purines stimulated by EFS at the sites of neurotransmitter release.
Enteric glial cells express inducible NOS (iNOS), which can stimulate or inhibit enteric neurons during inflammatory insults in mouse colon (7, 26). NO released from inflammatory or glial cells, however, is unlikely to modulate purine release in the W/Wv preparations as increased rather than decreased expression of iNOS in the W/Wv stomach has been reported (64). In addition, NO-mediated inhibition of purine release was dependent on activation of sGC, and cGMP-dependent protein kinase I, activated downstream of sGC, is expressed in NOS-positive myenteric nerve terminals (28) suggesting a presynaptic action of NO. We also found that purine release is enhanced in Prkg1−/− mouse colon. In fact, mice with reduced Prkg1 displayed GI dysfunction (49, 51). Our findings of augmented release of purine neurotransmitters in colon from Prkg1−/− mice suggest that excessive “compensation” by increased release of purine neurotransmitters may account for some GI motility disorders in these mice. NAD+/ADPR release, but not ATP or total purine release, appeared to be greater in colonic preparations from Prkg1−/− mice than from Nos1−/− mice. Further studies would be needed to determine the mechanisms underlying such observations. There are clearly developmental defects in the Prkg1−/− mouse line, and therefore there might be many reasons for difference in neural functions. Importantly, however, appropriate controls of age-matched WT littermates of the respective transgenic mouse strain were used for comparison of purine release in control vs. transgenic mice. The augmented release of neurogenic purines in both Prkg1−/− and Nos1−/− murine colons clearly indicates that aberrations in NO-mediated mechanisms lead to increased purine release.
In WT mouse colons, release of NAD+/ADPR, but not of ATP, was inhibited by TTX, suggesting that the primary release of ATP was not triggered by action potential firings under control conditions. This is consistent with our previous observations in mouse, human, and nonhuman primate colons where NAD+ better satisfied prejunctional and postjunctional requirements for the enteric inhibitory purine neurotransmitter than ATP (19, 30, 47). Neural release of ATP appeared to be increased in colons from W/Wv mice as a larger portion of stimulated release of ATP was eliminated by TTX in these muscles (e.g., Fig. 3). It is possible that when NO-mediated inhibition of purine release is lost in W/Wv mice, the proportion of ATP released from nerve terminals is enhanced relative to release from other cell sources and is therefore TTX sensitive. This observation also adds support to the idea that the NO-mediated inhibition likely takes place primarily at nerve terminals where the release of purine neurotransmitters occurs.
In summary, this study demonstrates that release of purines from enteric motor neurons in primate and murine colons is inhibited by NO likely by a sGC-mediated mechanism. The NO-mediated inhibition of purine release may act to prevent overinhibition of colonic muscles during increased motor neuron activation by limiting the amount of inhibitory neurotransmitter released. Such a mechanism may also contribute to the regional variation in the predominant inhibitory neurotransmitter released in distinct areas of the gut. In Nos1−/−, Prkg1−/−, and W/Wv mouse colons, inhibitory regulation of purine release by NO is diminished allowing enhancement in purinergic mechanisms. Enhanced purinergic signaling may be a safety factor to preserve inhibitory responses when nitrergic signaling is compromised prejunctionally or postjunctionally. This study reveals novel and complex regulation of enteric inhibitory motor neurotransmission in the colon and suggests a new role for interstitial cells in modulating enteric neurotransmission.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 41315.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.D. and V.N.M.-Y. conceived and designed research; L.D., A.L., S.M., and V.N.M.-Y. performed experiments; L.D., A.L., S.M., and V.N.M.-Y. analyzed data; L.D., A.L., S.M., K.C.S., K.M.S., and V.N.M.-Y. interpreted results of experiments; L.D. and V.N.M.-Y. prepared figures; L.D., K.M.S., and V.N.M.-Y. drafted manuscript; L.D., K.M.S., and V.N.M.-Y. edited and revised manuscript; L.D., A.L., S.M., K.C.S., K.M.S., and V.N.M.-Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Renown Medical Center for providing human tissues, Charles River Preclinical Services Reno for providing nonhuman primate tissues, Core C of Program Project Grant DK41315 and Nancy Horowitz for collecting primate tissue and maintaining the colonies of mice used for these studies, Drs. Brian Perrino and Caroline Cobine for technical advice on work with Wes, and Roisin McAvera for technical assistance in measuring purine release in primate colons.
A part of this study was presented as a poster at the 1st Annual Meeting of the Society for Pelvic Research, Charleston, SC, December 5–6, 2016 [Durnin L, Lees A, Manzoor S, Sasse KC, Sanders KM, Mutafova-Yambolieva VN, Loss of nitric oxide-mediated inhibition of purine neurotransmitter release in the colon in the absence of interstitial cells of Cajal (Abstract), Transl Androl Urol 5, Suppl 2: AB309, 2016].
REFERENCES
- 1.Allescher HD, Kurjak M, Huber A, Trudrung P, Schusdziarra V. Regulation of VIP release from rat enteric nerve terminals: evidence for a stimulatory effect of NO. Am J Physiol Gastrointest Liver Physiol 271: G568–G574, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Aulí M, Martínez E, Gallego D, Opazo A, Espín F, Martí-Gallostra M, Jiménez M, Clavé P. Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol 155: 1043–1055, 2008. doi: 10.1038/bjp.2008.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker SA, Hennig GW, Ward SM, Sanders KM. Temporal sequence of activation of cells involved in purinergic neurotransmission in the colon. J Physiol 593: 1945–1963, 2015. doi: 10.1113/jphysiol.2014.287599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil 20: 294–317, 2014. doi: 10.5056/jnm14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobalova J, Bobal P, Mutafova-Yambolieva VN. High-performance liquid chromatographic technique for detection of a fluorescent analogue of ADP-ribose in isolated blood vessel preparations. Anal Biochem 305: 269–276, 2002. doi: 10.1006/abio.2002.5667. [DOI] [PubMed] [Google Scholar]
- 6.Börjesson L, Nordgren S, Delbro DS. DMPP causes relaxation of rat distal colon by a purinergic and a nitrergic mechanism. Eur J Pharmacol 334: 223–231, 1997. doi: 10.1016/S0014-2999(97)01173-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345: 346–347, 1990. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- 9.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA 93: 12008–12013, 1996. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil 20, Suppl 1: 8–19, 2008. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 11.Chi MM, Powley TL. c-Kit mutant mouse behavioral phenotype: altered meal patterns and CCK sensitivity but normal daily food intake and body weight. Am J Physiol Regul Integr Comp Physiol 285: R1170–R1183, 2003. doi: 10.1152/ajpregu.00015.2003. [DOI] [PubMed] [Google Scholar]
- 12.Cobine CA, Sotherton AG, Peri LE, Sanders KM, Ward SM, Keef KD. Nitrergic neuromuscular transmission in the mouse internal anal sphincter is accomplished by multiple pathways and postjunctional effector cells. Am J Physiol Gastrointest Liver Physiol 307: G1057–G1072, 2014. doi: 10.1152/ajpgi.00331.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta 1411: 334–350, 1999. doi: 10.1016/S0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 14.Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A, Huizinga JD. Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology 114: 724–736, 1998. doi: 10.1016/S0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- 15.Do MY, Myung SJ, Park HJ, Chung JW, Kim IW, Lee SM, Yu CS, Lee HK, Lee JK, Park YS, Jang SJ, Kim HJ, Ye BD, Byeon JS, Yang SK, Kim JH. Novel classification and pathogenetic analysis of hypoganglionosis and adult-onset Hirschsprung’s disease. Dig Dis Sci 56: 1818–1827, 2011. doi: 10.1007/s10620-010-1522-9. [DOI] [PubMed] [Google Scholar]
- 16.Durnin L, Hwang SJ, Kurahashi M, Drumm BT, Ward SM, Sasse KC, Sanders KM, Mutafova-Yambolieva VN. Uridine adenosine tetraphosphate is a novel neurogenic P2Y1 receptor activator in the gut. Proc Natl Acad Sci USA 111: 15821–15826, 2014. [Erratum: Proc Natl Acad Sci USA 111: 18400, 2014.] doi: 10.1073/pnas.1409078111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with β-NAD(+). J Physiol 590: 1921–1941, 2012. doi: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durnin L, Moreland N, Lees A, Mutafova-Yambolieva VN. A commonly used ecto-ATPase inhibitor, ARL-67156, blocks degradation of ADP more than the degradation of ATP in murine colon. Neurogastroenterol Motil 28: 1370–1381, 2016. doi: 10.1111/nmo.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durnin L, Sanders KM, Mutafova-Yambolieva VN. Differential release of β-NAD(+) and ATP upon activation of enteric motor neurons in primate and murine colons. Neurogastroenterol Motil 25: e194–e204, 2013. doi: 10.1111/nmo.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil 20, Suppl 1: 54–63, 2008. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 21.Francis SH, Busch JL, Corbin JD. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563, 2010. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallego D, Hernández P, Clavé P, Jiménez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol 291: G584–G594, 2006. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- 23.Gallego D, Gil V, Aleu J, Aulí M, Clavé P, Jiménez M. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol 295: G522–G533, 2008. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- 24.Gfroerer S, Rolle U. Interstitial cells of Cajal in the normal human gut and in Hirschsprung disease. Pediatr Surg Int 29: 889–897, 2013. doi: 10.1007/s00383-013-3364-y. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, Kendrick ML, Shen KR, Cima RR, Dozois EJ, Larson DW, Ordog T, Pozo MJ, Farrugia G. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil 23: 36–44, 2011. doi: 10.1111/j.1365-2982.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green CL, Ho W, Sharkey KA, McKay DM. Dextran sodium sulfate-induced colitis reveals nicotinic modulation of ion transport via iNOS-derived NO. Am J Physiol Gastrointest Liver Physiol 287: G706–G714, 2004. doi: 10.1152/ajpgi.00076.2004. [DOI] [PubMed] [Google Scholar]
- 27.He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology 118: 14–21, 2000. doi: 10.1016/S0016-5085(00)70409-4. [DOI] [PubMed] [Google Scholar]
- 28.Huber A, Trudrung P, Storr M, Franck H, Schusdziarra V, Ruth P, Allescher HD. Protein kinase G expression in the small intestine and functional importance for smooth muscle relaxation. Am J Physiol Gastrointest Liver Physiol 275: G629–G637, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol 590: 1957–1972, 2012. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 140: 608–617.e6, 2011. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol 131: 691–702, 2009. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 32.Iino S, Horiguchi S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal in the gastrointestinal musculature of W mutant mice. Arch Histol Cytol 70: 163–173, 2007. doi: 10.1679/aohc.70.163. [DOI] [PubMed] [Google Scholar]
- 33.Isozaki K, Hirota S, Miyagawa J, Taniguchi M, Shinomura Y, Matsuzawa Y. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol 92: 332–334, 1997. [PubMed] [Google Scholar]
- 34.Jankowski V, Tölle M, Vanholder R, Schönfelder G, van der Giet M, Henning L, Schlüter H, Paul M, Zidek W, Jankowski J. Uridine adenosine tetraphosphate: a novel endothelium- derived vasoconstrictive factor. Nat Med 11: 223–227, 2005. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- 35.Keef KD, Saxton SN, McDowall RA, Kaminski RE, Duffy AM, Cobine CA. Functional role of vasoactive intestinal polypeptide in inhibitory motor innervation in the mouse internal anal sphincter. J Physiol 591: 1489–1506, 2013. doi: 10.1113/jphysiol.2012.247684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh SD, Ward SM, Sanders KM. Ionic conductances regulating the excitability of colonic smooth muscles. Neurogastroenterol Motil 24: 705–718, 2012. doi: 10.1111/j.1365-2982.2012.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurahashi M, Mutafova-Yambolieva V, Koh SD, Sanders KM. Platelet-derived growth factor receptor-α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol 307: C561–C570, 2014. doi: 10.1152/ajpcell.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol 589: 697–710, 2011. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem 137: 93–100, 1984. doi: 10.1016/0003-2697(84)90352-X. [DOI] [PubMed] [Google Scholar]
- 40.Lies B, Beck K, Keppler J, Saur D, Groneberg D, Friebe A. Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. J Physiol 593: 4589–4601, 2015. doi: 10.1113/JP270511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lies B, Gil V, Groneberg D, Seidler B, Saur D, Wischmeyer E, Jiménez M, Friebe A. Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 307: G98–G106, 2014. doi: 10.1152/ajpgi.00082.2014. [DOI] [PubMed] [Google Scholar]
- 42.Lyford GL, He CL, Soffer E, Hull TL, Strong SA, Senagore AJ, Burgart LJ, Young-Fadok T, Szurszewski JH, Farrugia G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 51: 496–501, 2002. doi: 10.1136/gut.51.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mañé N, Gil V, Martínez-Cutillas M, Clavé P, Gallego D, Jiménez M. Differential functional role of purinergic and nitrergic inhibitory cotransmitters in human colonic relaxation. Acta Physiol (Oxf) 212: 293–305, 2014. doi: 10.1111/apha.12408. [DOI] [PubMed] [Google Scholar]
- 44.Mañé N, Viais R, Martínez-Cutillas M, Gallego D, Correia-de-Sá P, Jiménez M. Inverse gradient of nitrergic and purinergic inhibitory cotransmission in the mouse colon. Acta Physiol (Oxf) 216: 120–131, 2016. doi: 10.1111/apha.12599. [DOI] [PubMed] [Google Scholar]
- 45.Mang CF, Truempler S, Erbelding D, Kilbinger H. Modulation by NO of acetylcholine release in the ileum of wild-type and NOS gene knockout mice. Am J Physiol Gastrointest Liver Physiol 283: G1132–G1138, 2002. doi: 10.1152/ajpgi.00192.2002. [DOI] [PubMed] [Google Scholar]
- 46.Mutafova-Yambolieva VN, Durnin L. The purinergic neurotransmitter revisited: a single substance or multiple players? Pharmacol Ther 144: 162–191, 2014. doi: 10.1016/j.pharmthera.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA 104: 16359–16364, 2007. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, Miyagawa J, Chen H, Miyazaki Y, Kiyohara T, Shinomura Y, Matsuzawa Y. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol 17: 666–670, 2002. doi: 10.1046/j.1440-1746.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 49.Ny L, Pfeifer A, Aszòdi A, Ahmad M, Alm P, Hedlund P, Fässler R, Andersson KE. Impaired relaxation of stomach smooth muscle in mice lacking cyclic GMP-dependent protein kinase I. Br J Pharmacol 129: 395–401, 2000. doi: 10.1038/sj.bjp.0703061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peri LE, Sanders KM, Mutafova-Yambolieva VN. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRα-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterol Motil 25: e609–e620, 2013. doi: 10.1111/nmo.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszódi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fässler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17: 3045–3051, 1998. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Publicover NG, Hammond EM, Sanders KM. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci USA 90: 2087–2091, 1993. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qu XW, Wang H, Rozenfeld RA, Huang W, Hsueh W. Type I nitric oxide synthase (NOS) is the predominant NOS in rat small intestine. Regulation by platelet-activating factor. Biochim Biophys Acta 1451: 211–217, 1999. doi: 10.1016/S0167-4889(99)00076-2. [DOI] [PubMed] [Google Scholar]
- 54.Rolle U, Piotrowska AP, Nemeth L, Puri P. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med 126: 928–933, 2002. doi: [DOI] [PubMed] [Google Scholar]
- 55.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588: 4621–4639, 2010. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol Gastrointest Liver Physiol 262: G379–G392, 1992. [DOI] [PubMed] [Google Scholar]
- 57.Sanders KM, Ward SM, Friebe A. CrossTalk proposal: interstitial cells are involved and physiologically important in neuromuscular transmission in the gut. J Physiol 594: 1507–1509, 2016. doi: 10.1113/JP271600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlossmann J, Feil R, Hofmann F. Insights into cGMP signalling derived from cGMP kinase knockout mice. Front Biosci 10: 1279–1289, 2005. doi: 10.2741/1618. [DOI] [PubMed] [Google Scholar]
- 59.Sergeant GP, Large RJ, Beckett EA, McGeough CM, Ward SM, Horowitz B. Microarray comparison of normal and W/Wv mice in the gastric fundus indicates a supersensitive phenotype. Physiol Genomics 11: 1–9, 2002. doi: 10.1152/physiolgenomics.00052.2002. [DOI] [PubMed] [Google Scholar]
- 60.Suthamnatpong N, Hata F, Kanada A, Takeuchi T, Yagasaki O. Mediators of nonadrenergic, noncholinergic inhibition in the proximal, middle and distal regions of rat colon. Br J Pharmacol 108: 348–355, 1993. doi: 10.1111/j.1476-5381.1993.tb12808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Todorov LD, Mihaylova-Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, Westfall DP. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 387: 76–79, 1997. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- 62.Wang XY, Chen JH, Li K, Zhu YF, Wright GW, Huizinga JD. Discrepancies between c-Kit positive and Ano1 positive ICC-SMP in the W/Wv and wild-type mouse colon; relationships with motor patterns and calcium transients. Neurogastroenterol Motil 26: 1298–1310, 2014. doi: 10.1111/nmo.12395. [DOI] [PubMed] [Google Scholar]
- 63.Ward SM, Gershon MD, Keef K, Bayguinov YR, Nelson C, Sanders KM. Interstitial cells of Cajal and electrical activity in ganglionic and aganglionic colons of mice. Am J Physiol Gastrointest Liver Physiol 283: G445–G456, 2002. doi: 10.1152/ajpgi.00475.2001. [DOI] [PubMed] [Google Scholar]
- 64.Winston JH, Chen J, Shi XZ, Sarna SK. Inflammation induced by mast cell deficiency rather than the loss of interstitial cells of Cajal causes smooth muscle dysfunction in W/Wv mice. Front Physiol 5: 22, 2014. doi: 10.3389/fphys.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xue C, Pollock J, Schmidt HH, Ward SM, Sanders KM. Expression of nitric oxide synthase immunoreactivity by interstitial cells of the canine proximal colon. J Auton Nerv Syst 49: 1–14, 1994. doi: 10.1016/0165-1838(94)90015-9. [DOI] [PubMed] [Google Scholar]