Serotonin (5-HT) and oxytocin reciprocally regulate the severity of intestinal inflammation and hepatotoxicity in a murine model of necrotizing enterocolitis (NEC). Selective depletion of mucosal 5-HT through genetic deletion or inhibition of tryptophan hydroxylase-1 ameliorates, while deletion of the 5-HT uptake transporter, which increases 5-HT availability, exacerbates the severity of NEC. In contrast, oxytocin reduces, while the oxytocin receptor antagonist atosiban enhances, NEC severity. Peripheral tryptophan hydroxylase inhibition may be useful in treatment of NEC.
Keywords: serotonin, oxytocin, intestine, inflammation, necrotizing enterocolitis
Abstract
Necrotizing enterocolitis (NEC), a gastrointestinal inflammatory disease of unknown etiology that may also affect the liver, causes a great deal of morbidity and mortality in premature infants. We tested the hypothesis that signaling molecules, which are endogenous to the bowel, regulate the severity of intestinal and hepatic damage in an established murine NEC model. Specifically, we postulated that mucosal serotonin (5-HT), which is proinflammatory, would exacerbate experimental NEC and that oxytocin (OT), which is present in enteric neurons and is anti-inflammatory, would oppose it. Genetic deletion of the 5-HT transporter (SERT), which increases and prolongs effects of 5-HT, was found to increase the severity of systemic manifestations, intestinal inflammation, and associated hepatotoxicity of experimental NEC. In contrast, genetic deletion of tryptophan hydroxylase 1 (TPH1), which is responsible for 5-HT biosynthesis in enterochromaffin (EC) cells of the intestinal mucosa, and TPH inhibition with LP-920540 both decrease the severity of experimental NEC in the small intestine and liver. These observations suggest that 5-HT from EC cells helps to drive the inflammatory damage to the gut and liver that occurs in the murine NEC model. Administration of OT decreased, while the OT receptor antagonist atosiban exacerbated, the intestinal inflammation of experimental NEC. Data from the current investigation are consistent with the tested hypotheses—that the enteric signaling molecules, 5-HT (positively) and OT (negatively) regulate severity of inflammation in a mouse model of NEC. Moreover, we suggest that mucosally restricted inhibition of 5-HT biosynthesis and/or administration of OT may be useful in the treatment of NEC.
NEW & NOTEWORTHY Serotonin (5-HT) and oxytocin reciprocally regulate the severity of intestinal inflammation and hepatotoxicity in a murine model of necrotizing enterocolitis (NEC). Selective depletion of mucosal 5-HT through genetic deletion or inhibition of tryptophan hydroxylase-1 ameliorates, while deletion of the 5-HT uptake transporter, which increases 5-HT availability, exacerbates the severity of NEC. In contrast, oxytocin reduces, while the oxytocin receptor antagonist atosiban enhances, NEC severity. Peripheral tryptophan hydroxylase inhibition may be useful in treatment of NEC.
necrotizing enterocolitis (NEC) is a severe intestinal inflammatory disease. It occurs in 10–15% of premature infants and causes substantial morbidity and mortality (11, 19, 64). Liver involvement, which frequently accompanies NEC, contributes significantly to NEC-associated morbidity and mortality (39, 61). The cause of NEC is not known. Serotonin (5-HT) has recently been shown to play an important role in intestinal inflammation. Deletion of the 5-HT transporter (SERT), which interferes with 5-HT inactivation, enhances intestinal inflammation (4, 33). In contrast, inhibition (58) or deletion of tryptophan hydroxylase 1 (TPH1) (23), the rate-limiting enzyme in mucosal 5-HT biosynthesis, attenuates intestinal inflammation. Although 5-HT is best known for its role as a neurotransmitter, mucosal 5-HT, which is, by far, the largest 5-HT pool in the mammalian body, is nonneuronal (21). Mucosal 5-HT is found in enterochromaffin (EC) cells and in the mast cells of rats and mice. The classical targets of the 5-HT that EC cells secrete are the mucosal processes of intrinsic primary afferent neurons that trigger peristaltic and secretory reflexes (3, 10, 13, 21, 67). The paracrine secretion of 5-HT from EC cells, however, allows 5-HT also to reach immunoeffector cells (28, 41, 55). The proinflammatory action of mucosal 5-HT could be the result of a direct effect of 5-HT on immunoeffectors or an indirect consequence of the activation of a proinflammatory neuroimmune pathway (55, 57). In either case, mucosal 5-HT release during NEC would be likely to aggravate the severity of NEC.
If mucosal 5-HT does, in fact, promote inflammation, it would seem likely that the gut would contain another molecule that is anti-inflammatory to prevent the unchecked acceleration of 5-HT-initiated inflammatory processes. Oxytocin (OT) is expressed in enteric neurons (60, 86, 87) and the OT receptor (OTR), which is developmentally regulated in the bowel, is expressed by the mucosal epithelium and enteric neurons (86). OT, moreover, is anti-inflammatory in the adult gut, and deletion of the OTR strongly enhances experimentally induced intestinal inflammation (84). In the current study, we used a well-established murine model (16, 52a, 64) to test the hypotheses that endogenous mucosal 5-HT increases the severity of both the enteric and hepatic consequences of NEC. We also employed the same model to test the ideas that exogenous and endogenous OT opposes NEC. Results suggest that selective inhibition of mucosal 5-HT biosynthesis or OT administration may be valuable forms of therapy to prevent or alleviate the inflammatory consequences of NEC when there is a high risk of its development or when NEC is suspected.
MATERIALS AND METHODS
Animals.
The Institutional Animal Care and Use Committee of Columbia University Medical Center approved all of the experiments involving animals. Wild-type (WT) C57BL/6 mice were purchased form Charles River Laboratories (Pontage, MI). Mice lacking TPH1 (TPH1KO) were derived from animals donated by Francine Côté (French National Centre for Scientific Research, Paris, France) and animals lacking SERT (SERTKO) were originally generated in the laboratory of Dennis Murphy (Clinical Science at the National Institute of Mental Health, Bethesda, MD). Heterozygous progeny were bred for all experiments, so that comparisons could be made between WT and homozygous KO littermates. All mice were on a C57BL/6 background. NEC was induced in 10- to 14-day-old mice. The protocol that we used to induce NEC has been previously shown to induce an intestinal inflammation in mice that resembles clinical NEC (30, 47, 74). This protocol includes conditions thought to be conducive to NEC in humans; it involves the combination of hyperosmolar formula feeding (Similac Advanced Infant Formula; Ross Pediatrics, Columbus, OH; Esbilac canine milk replacer 2:1; 50 μl/g), maternal separation, and induction of repeated episodes of hypoxia. During the 4-day treatment period, the experimental mice were maintained in an incubator at 37°C (a clinical neonatal isolette). The animals were fed by oral gavage five times per day, and hypoxia (5% O2-95% N2) was induced (Billups-Rothenberg, Del Mar, CA) for 10 min, 2 times per day (47). Control mice were breast fed, remained with their mothers throughout the experimental period, and were not exposed to hypoxia. In pharmacological experiments, either vehicle (methylcellulose) or the TPH inhibitor LP-920540 (100 or 200 mg/kg diluted in methylcellulose) were administered to mice once daily via oral gavage throughout the experimental period. LP-920540 selectively blocks biosynthesis of enteric mucosal 5-HT at dose levels that do not disturb 5-HT biosynthesis in neurons of either the central or enteric nervous systems (58). The weights and survival of all animals entered in each study were recorded daily.
Dissection.
Surviving mice were euthanized on postnatal day 14 (P14). The gastrointestinal (GI) tract was removed from each mouse and was examined grossly for signs of NEC, such as intestinal hemorrhage and/or ileal distention or stenosis (71). Sections, 1–2 cm in length, were taken from consistent locations of the bowel from the ileal-cecal junction to the proximal ileum. The liver was also removed. The intestinal and hepatic tissue was fixed and processed for preparation of frozen and paraffin sections. Tissue aliquots were also flash-frozen and saved for subsequent analyses of transcripts encoding proinflammatory mediators. The ileum was selected for study because it is the region of the bowel most frequently affected in human NEC and its murine models (24, 25, 72).
RT-PCR.
RNA was extracted from the ileum with TRIzol (Invitrogen, Carlsbad, CA) and treated with deoxyribonuclease I (1 U/ml). A high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA) was used to convert 1 μg of mRNA to cDNA. RT-PCR was used to quantify transcripts encoding IL-6, IL-1β, TNF-α, and IL-18 (Applied Biosystems). Expression was normalized to GAPDH (Applied Biosystems). The real-time reaction mixture contained cDNA (2 μl), primers for the cytokine/chemokine/standard (250 nmol), PCR Master Mix (12.5 μl; Applied Biosystems), and nuclease-free water (9.25 μl). A GeneAmp 7500 sequence detection system (Applied Biosystems) was used to quantify cDNA levels using a standard protocol (2 min at 50°C, 10 min at 95°C, 40 cycles of annealing at 60°C for 20 s, extension at 60°C for 1 min, and denaturation at 95°C for 15 s). TaqMan 7500 software (Applied Biosystems) was used for data analysis.
PCR microarray.
A PCR microarray, which included 84 genes, was employed to study the effects of OT on NEC. The array focused on expression of important inflammatory cytokines, chemokines, and receptors (PAMM-011z; SABiosciences; Frederick, MD). cDNA samples were mixed with master mix and loaded into the wells of 96-well PCR-array plates. Microarrays were amplified, and plates were read using a TaqMan 7500 PCR machine. The SABiosciences Web-based integrated PCR array expression analysis suite was used for analysis and data acquisition. Expression of GAPDH and β-actin were used as housekeeping genes to normalize signal intensity.
Histology and histological scoring.
Ileal and hepatic tissue were fixed in 4% formaldehyde (from paraformaldehyde) in 0.1 M phosphate buffer for 72 h and embedded in paraffin. Sections were cut at 5 μm. Sections were stained with hematoxylin and eosin, according to standard methods (57). An experienced animal pathologist who was blinded to the experimental treatment of the animals used a previously developed NEC scoring system (26, 31, 38) to grade pathological changes in intestinal architecture. The morphological changes in the intestinal epithelium were scored on a scale of 0–4 as normal, mild, moderate, or severe. The definition for each histological grade was 0, normal, no damage; 1, slight submucosal and/or lamina propria separation, without other abnormalities; 2, moderate separation of submucosa and/or lamina propria, and/or edema in submucosal and muscular layers; 3, severe separation of submucosa and/or lamina propria, and/or severe edema in submucosa and muscular layers, region villous sloughing; and 4, loss of villi with necrosis. Tissue from the liver was sectioned at 5 μm and stained with either hematoxylin and eosin for histological scoring or Sudan Black to evaluate tissue lipids. Hepatic sections were also subjected to terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis to evaluate apoptosis. An experienced animal pathologist, who was blinded to the experimental treatment of the animals used the nonalcoholic fatty liver disease activity score, as previously devised by the pathology committee of the Non-Alcoholic Steatohepatitis Clinical Research Network (45), to grade histologic changes in the liver. This scoring system evaluates the degree of steatosis, lobular inflammation, and hepatocyte ballooning. Steatosis at <5% of a low-power field was scored as 0, 5–33% was scored as 1, >33–66% was scored as 2, and >66% was scored as 3. Lobular inflammation was graded as an overall assessment of the numbers of inflammatory foci per ×20 field; none was scored as 0, <2 was scored as 1, 2–4 was scored as 2, and >4 was scored as 3. Hepatocyte ballooning was graded as 0 when none was found, 1 if few cells were visible, and 2 if many cells showed prominent ballooning. The total score was quantified as an accumulation of the combined scores of all of these groups and ranged from 0 to 8. Sudan Black staining was applied to hepatic sections to assess the quantity of fat deposition, as previously described (18). Bright-field microscopy and computer-assisted imaging (Volocity 6.0; Perkin-Elmer, Waltham, MA) were used to quantify the degree of fatty infiltration, so that experimental and control tissue could be compared statistically. Apoptosis was detected in paraffin-embedded hepatic sections by using the TUNEL procedure using the TMR red “In Situ Cell Death Detection Kit” (Roche, Nutley, NJ). Terminal transferase was omitted as a negative control, and tissue that was exposed to DNase I before the assays (10 min; Roche, Branchburg, NJ) was used as a positive control. Computer-assisted imaging was used to quantify the presence of apoptotic cells.
Statistical analysis.
Student’s t-test was used when two groups were studied, and ANOVA was employed for analysis when more than two groups were examined simultaneously. The log-rank (Mantel-Cox) test was employed to compare survival curves.
RESULTS
Intestinal and hepatic inflammation is increased in experimental NEC.
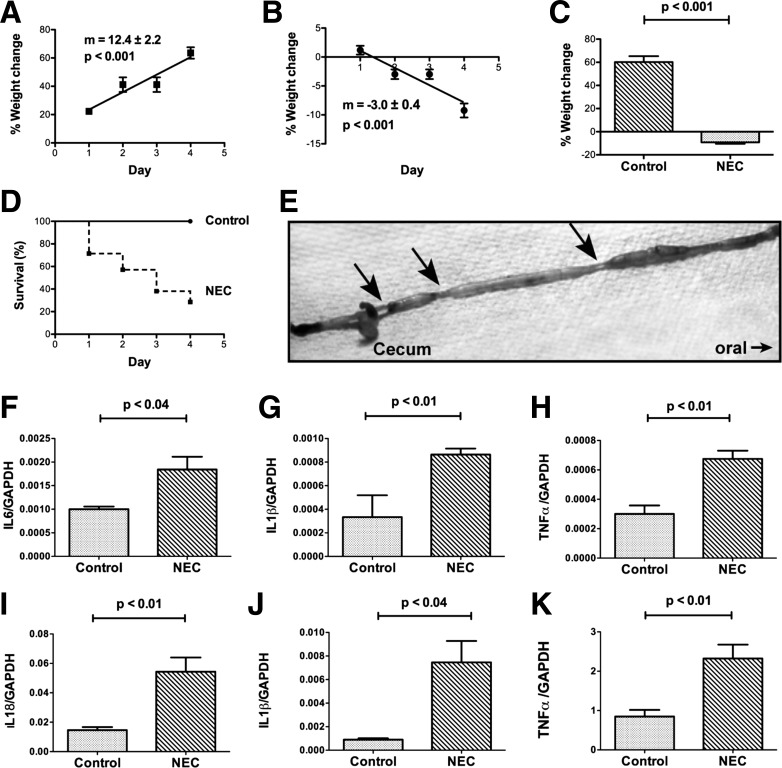
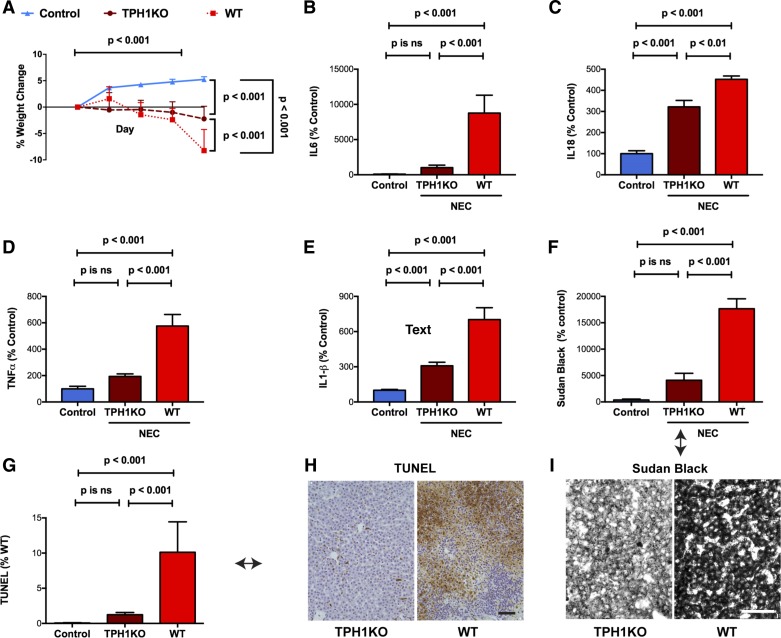
To mimic NEC experimentally, perinatal mice were removed from dams and placed in a 37° neonatal isolette, formula fed, and exposed twice daily to 10 min of hypoxia. Control mice were allowed to remain with dams in their home cages, breast-fed, and not exposed to hypoxia. The control mice gained weight significantly (P < 0.001) as a linear function of time during the 4-day treatment period (Fig. 1, A and C), while the experimental mice lost weight significantly (P < 0.001), again as a linear function of time during the same period (Fig. 1, B and C). All of the control mice survived, but only 29% of the experimental animals did so (Fig. 1D; P < 0.01). The median survival time of the experimental mice was 3 days. Multiple small intestinal strictures associated with gaseous dilation of the immediately proximal bowel were observed in each of the surviving experimental animals (Fig. 1E); these strictures/dilations, which were most common immediately proximal to the cecum and might have been functional, did not occur in the intestines of control mice. To verify that the loss of weight and poor survival of the experimental mice was associated with the development of NEC, the abundance of transcripts encoding proinflammatory cytokines in the distal 10 cm of small intestine from control and experimental animals was compared. The proinflammatory cytokines selected for study have all been shown to be elevated in human patients with NEC (32, 53, 72, 78). Significant increases were found in the abundance of transcripts encoding IL-6 (Fig. 1F), IL-1β (Fig. 1G), TNF-α (Fig. 1H), and IL-18 (Fig. 1I). In addition to increased transcription of proinflammatory cytokines in the small intestines, similarly significant increases in the abundance of transcripts encoding IL-1β (Fig. 1J) and TNF-α (Fig. 1K) were observed in the livers of the experimental mice.
Fig. 1.
Experimental necrotizing enterocolitis (NEC) leads to weight loss, mortality, and increased transcription of proinflammatory cytokines in intestine and liver. A: control mice gained weight steadily during the 4-day experimental period. The plot of the %weight change as a function of time does not differ significantly from linearity, the slope (m) of the line is positive and deviates significantly from zero (P < 0.001; n = 3). B: experimental mice lost weight steadily during the test period. The plot of the %weight change as a function of time does not differ significantly from linearity, the slope of the line is negative and deviates significantly from zero (P < 0.001; n = 15). The slope of the line in A is significantly different from that in B. C: mean %weight change of surviving control mice at the end of the 4-day test period is significantly greater than that of surviving experimental animals (P < 0.001). D: Kaplan-Meier plots of the survival of control and experimental mice during the course of the 4-day test period. Survival was 100% for control mice and 28.5% for experimental animals. The survival curves are significantly different (Mantel-Cox log-rank test; P < 0.01). E: small intestines of the mice with NEC had multiple strictures (arrows) in the small intestine. The bowel was dilated and gas-filled immediately proximal to the strictures. Strictures were most common just proximal to the cecum. Transcripts encoding proinflammatory cytokines were measured and compared in the distal small intestines of control and experimental mice (NEC). Significant increases were found in the abundance of transcripts encoding: IL-6 (F), IL-1β (G), TNF-α (H), and IL-18 (I). Transcripts encoding proinflammatory cytokines were measured and compared in the livers of NEC mice. Significant increases were found in the abundance of transcripts encoding IL-1β (J) and TNF-α (K).
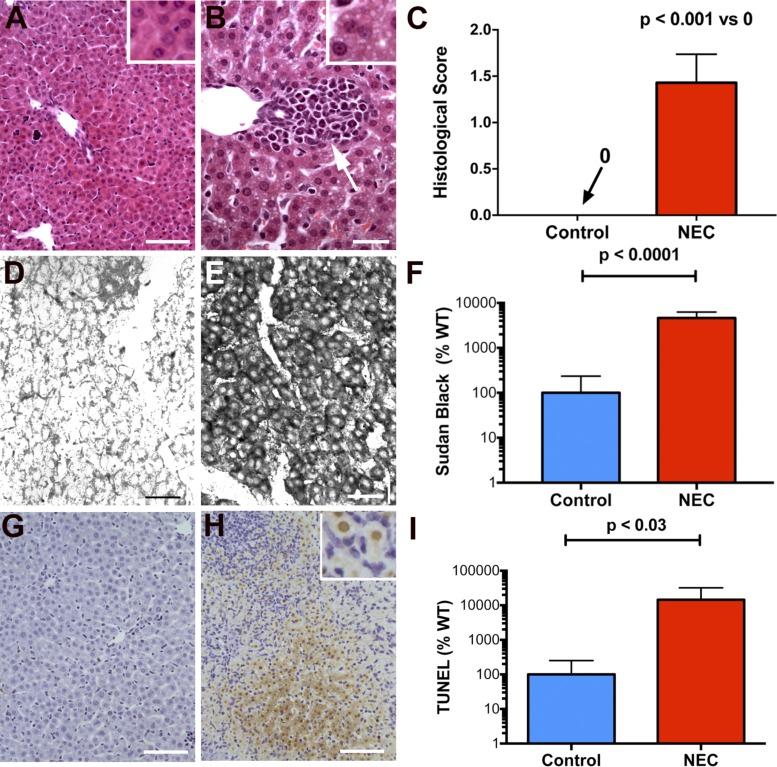
Severe inflammation was detectable in the distal ileum and proximal colon of mice subjected to the experimental NEC protocol. In contrast, inflammation was not observed in control animals. These data reproduced observations reported in earlier studies (59, 73); however, we also found that significant inflammation of the liver accompanied the intestinal inflammation that was induced in mice subjected to the NEC protocol. The livers of the mice exposed to NEC were significantly inflamed with periportal accumulations of inflammatory cells; however, no such accumulations were observed in control animals [compare Fig. 2A (control) with Fig. 2B (NEC)]. The periportal accumulations caused the scores of histological inflammation to be significantly higher in mice with NEC than in their WT control littermates (Fig. 2C). Many vacuoles were found in hepatic parenchymal cells in the livers of mice with NEC [compare inset Fig. 2A (control) with inset Fig. 2B (NEC)]; staining with Sudan Black confirmed that these vacuoles were lipid inclusions [compare Fig. 2D (control) with Fig. 2E (NEC)]. The percentage of parenchymal cell area occupied by lipid inclusions in the mice subjected to the NEC protocol was significantly greater than that in control animals (Fig. 2F). The percentage of apoptotic cells in the liver, as demonstrated by TUNEL staining [compare Fig. 2G (control) with Fig. 2H (NEC)], was also increased in the mice exposed to NEC (Fig. 2I).
Fig. 2.
Experimental NEC leads to hepatic inflammation, lipid accumulation, and apoptosis of hepatocytes. A: liver of a mouse subjected to the control protocol and stained with hematoxylin and eosin (H&E). The architecture of the liver, hepatocytes lining sinusoids, portal area, and central vein are all normal. The hepatocytes are not vacuolated (see also inset). Bar = 100 µm. B: liver of a mouse subjected to the NEC protocol and stained with H&E. An infiltrate of inflammatory cells surrounds a bile ductule in a portal area (see white arrow). Hepatocytes appear vacuolated (see also inset). Bar = 50 µm. C: histological score of inflammation is compared in sections of the livers of mice subjected to the control and NEC protocols. Significant inflammation was evident in the livers of mice exposed to NEC but not in those of control animals. D: frozen section of the liver of a mouse subjected to the control protocol and stained with Sudan Black. Very little structure is evident because the tissue lacks accumulated sudanophilic lipid. Bar = 50 µm. E: frozen section of the liver of a mouse subjected to the NEC protocol and stained with Sudan Black. Hepatocytes of the hepatic parenchyma are intensely sudanophilic, confirming that the vacuolization evident in H&E section is due to the presence of lipid droplets in the hepatocyte cytoplasm. Bar = 50 µm. F: densitometric quantitation of the Sudan Black staining of the livers of mice subjected to the control and NEC protocols. Hepatocytes of mice subjected to the NEC protocol were significantly more sudanophilic than those of control animals. Note that the scale of the ordinate is logarithmic. G: TUNEL staining of the liver of a mouse subjected to the control protocol reveals little or no apoptosis. Bar = 100 µm. H: TUNEL staining of the liver of a mouse subjected to the NEC protocol reveals an area where many hepatocytes are undergoing apoptosis. Bar = 100 µm. H, inset: nuclei of hepatocytes are filled with TUNEL reaction product. Small mononuclear cells surround the apoptotic hepatocytes. I: quantitation of the area of hepatic parenchyma containing apoptotic hepatocytes in the livers of mice subjected to the control and NEC protocols. Hepatocytes of mice subjected to the NEC protocol undergo significantly more apoptosis than those of control animals. Note that the ordinate is logarithmic.
The severity of intestinal and hepatic inflammation in experimental NEC is greater in SERTKO than in WT mice.
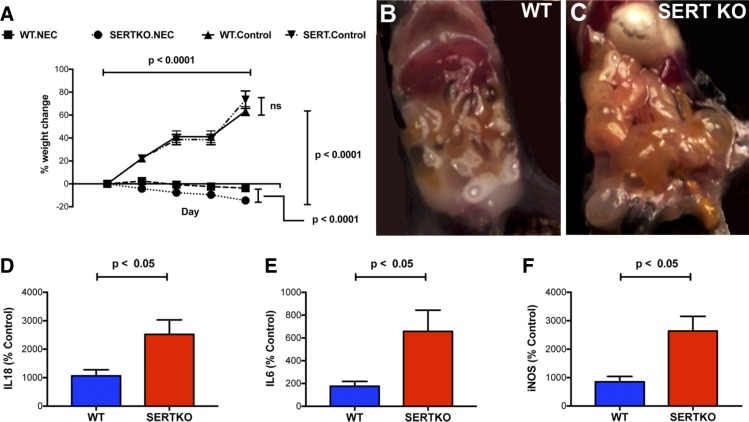
To determine the influence of SERT on the severity of inflammation in NEC, experimental NEC in SERTKO mice was compared with that in their WT littermates. There were four groups of mice: SERTKO and WT animals exposed either to the control (2 groups of 10 mice) or the NEC protocol (two groups of 30–40 mice), respectively. During the experimental period, both the WT and SERTKO mice that were exposed to the control protocol gained weight significantly (Fig. 3A). Weight gain did not differ significantly between the two control groups. In contrast, both the WT and SERTKO mice that were exposed to the NEC protocol lost weight significantly (P < 0.0001 vs. the respective controls); however, the NEC-induced loss of weight in the SERTKO animals was significantly greater than that in WT mice (P < 0.0001; Fig. 3A). Although survival was numerically worse in SERTKO than in WT mice, the difference was not statistically significant. Surviving animals were euthanized after 5 days. At this time, the intestines of NEC-exposed WT (Fig. 3B) and SERTKO mice (Fig. 3C) were grossly dilated; however, the dilation was much more severe in SERTKO than in WT animals. The abundance of transcripts encoding the proinflammatory cytokines IL-18 (Fig. 3D) and IL-6 (Fig. 3E), as well as the inflammatory marker iNOS (Fig. 3F), was significantly greater in the intestines both of WT and SERTKO mice subjected to the NEC protocol (P < 0.001), suggesting that NEC had been induced in both groups of mice. The abundance of transcripts encoding each of these mediators, however, was significantly more elevated in the NEC-exposed SERTKO animals than in the NEC-exposed WT mice (Fig. 3, D–F).
Fig. 3.
Intestinal inflammation is more severe in SERTKO than in WT mice after exposure to the NEC protocol. A: percent change in weight as a function of time is compared in WT and SERTKO mice subjected to the control and NEC protocols. Both WT (n = 10) and SERTKO (n = 10) mice gain weight during the control protocol; the difference between the two groups is not significant. In contrast, both WT (n = 36) and SERTKO (n = 40) mice lose weight during the NEC protocol; however, SERTKO mice lose significantly more weight than their WT counterparts. Photographs of the abdominal contents of a WT (B) and a SERTKO (C) mouse after exposure for 5 days to the NEC protocol. Loops of bowel are dilated with gas in both; however, the severity of the gaseous dilation is greater in the SERTKO than in the WT animal. Expression of the proinflammatory cytokines normalized to that of GAPDH, IL-18 (D) and IL-6 (E), as well as iNOS (F) are all significantly more elevated in the intestines of SERTKO than in WT mice.
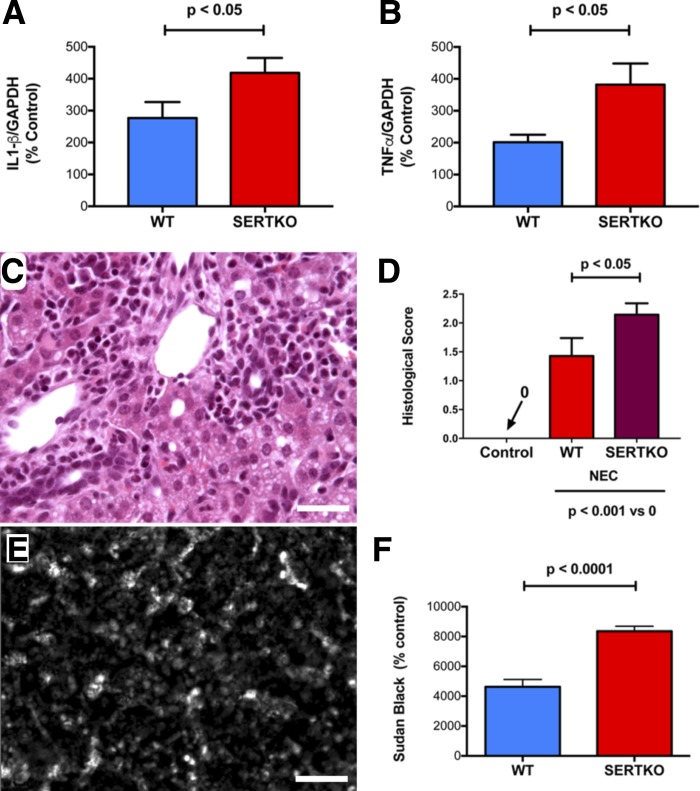
To investigate the effect of SERT deletion on the hepatic consequences of NEC, we compared parameters of inflammation and tissue damage that we had found to be abnormal in NEC-exposed mice (Fig. 2) in WT and SERTKO animals. Neither inflammation nor vacuolization of hepatocytes were detectable morphologically in the livers of SERTKO mice subjected to the control protocol. In contrast, in the livers of SERTKO mice subjected to the NEC protocol, severe inflammation was observed. The severity of the NEC-induced hepatic inflammation was significantly greater in SERTKO than in WT mice. This increase was evident in the hepatic abundance of transcripts encoding IL-1β (Fig. 4A) and TNF-α (Fig. 4B), as well as in the periportal infiltration of inflammatory cells (Fig. 4C; compare with Fig. 2, B and C), and the histologically determined inflammatory scores (Fig. 4D). The NEC-induced accumulation of lipid in hepatocytes was also greater in SERTKO than in WT mice [Fig. 4E (compare with Fig. 2, E and F)]. Apoptosis was also prominent in the livers of SERTKO mice subjected to the NEC protocol, especially in regions infiltrated with inflammatory cells; however, the distribution of apoptotic cells was highly irregular and variable between sections. The severity of NEC-induced apoptosis was, thus, not significantly greater in tissue from SERTKO than from WT mice.
Fig. 4.
Hepatic inflammation is more severe in SERTKO than in WT mice after exposure to the NEC protocol. A: transcripts encoding IL-1β are more abundant in the livers of SERTKO than WT mice. B: transcripts encoding TNF-α are more abundant in the livers of SERTKO than WT mice. C: periportal infiltration of inflammatory cells in a SERTKO mouse (compare with Fig. 2B (NEC in a WT mouse)]. Bar = 100 μm. The cytoplasm of hepatocytes appears vacuolated. D: histological score of inflammation is compared in sections of the livers of WT and SERTKO mice subjected to the control and NEC protocols. Significantly higher inflammatory scores were found in the livers of SERTKO mice exposed to the NEC protocol but not in any animals subjected to the control protocol. E: frozen section of the liver of a SERTKO mouse subjected to the NEC protocol and stained with Sudan Black. Hepatocytes of the hepatic parenchyma are intensely sudanophilic [compare with Fig. 2E (NEC in a WT mouse)]. Bar = 100 μm. F: densitometric quantitation of the Sudan Black staining of the livers of WT and SERTKO mice subjected to the NEC protocol. Hepatocytes of SERTKO mice subjected to the NEC protocol were significantly more sudanophilic than those of WT animals.
The severity of intestinal and hepatic inflammation in experimental NEC is less severe in TPH1KO than in WT mice.
The observation that NEC-induced intestinal and hepatic inflammation is enhanced in SERTKO mice is consistent with the idea that 5-HT contributes to the pathophysiology of the tissue damage in both organs during NEC. If this idea were correct, then deletion of 5-HT from the intestinal mucosa and liver would be expected to protect mice from experimental NEC. Deletion of TPH1 prevents 5-HT biosynthesis in the intestinal mucosa (14) and liver (20). The effects of the NEC protocol on the intestine and liver of WT mice and their TPH1KO littermates were, thus, compared. Although the difference in mortality between the TPH1KO mice with NEC and WT mice with NEC was not significant, WT and TPH1KO mice exposed to the NEC protocol both lost weight progressively during the experimental period (P < 0.001; Fig. 5A); however, the loss of weight that the NEC protocol induced was significantly less in TPH1KO mice than in WT animals (Fig. 5A; P < 0.001). The NEC protocol also caused the abundance of enteric transcripts encoding the proinflammatory cytokines IL-6 (Fig. 5B; P < 0.001) and IL-18 (Fig. 5C; P < 0.001) to increase in both WT and TPH1KO animals; however, in TPH1KO mice, the NEC-induced increase in the abundance of both IL-6 (Fig. 5B; P < 0.001) and IL-18 (Fig. 5C; P < 0.01) was significantly less than that in WT mice. Similarly, hepatic inflammation occurring as a result of the NEC protocol was less severe in TPH1KO than in WT mice; hepatic transcripts encoding TNF-α (P < 0.001; Fig. 5D) and IL-1β (P < 0.001; Fig. 5E) were each significantly less abundant in TPH1KO than in WT mice; moreover, both the NEC-induced lipid accumulation in hepatocytes (Fig. 5, F and I; P < 0.001) and the frequency of hepatocyte apoptosis (Fig. 5, G and H; P < 0.001) were also less in TPH1KO than WT mice.
Fig. 5.
Intestinal inflammation and hepatic toxicity are less severe in TPH1KO than in WT mice. A: percent change in weight as a function of time in mice subjected to control protocol (n = 10) is compared with both WT (n = 28) and TPH1KO (n = 28) mice subjected to the NEC protocol. Mice gained weight during the control protocol (compare with Fig. 3A (WT and SERTKO mice subjected to the control protocol). In contrast, both TPH1KO and WT mice lost weight during the NEC protocol; however, WT mice lost significantly more weight than their TPH1KO counterparts. B: transcripts encoding IL-6 are significantly more abundant in the intestines of mice subjected to the NEC than the control protocol; however, the increase in abundance of enteric IL-6 transcripts is significantly greater in WT than in TPH1KO mice. The NEC-induced increase in transcripts encoding IL-6 in the intestines of TPH1KO mice is not significantly different from control. C: transcripts encoding IL-18 are significantly more abundant in mice subjected to the NEC than the control protocol; however, the increase in the abundance of IL-18 transcripts is significantly greater in WT than in TPH1KO mice. D: transcripts encoding TNF-α are significantly more abundant in the livers of mice subjected to the NEC than the control protocol; however, the increase in abundance of TNF-α transcripts is significantly greater in WT than in TPH1KO mice. The NEC-induced increase in transcripts encoding TNF-α in the livers of TPH1KO mice is not significantly different from control. E: transcripts encoding IL-1β are significantly more abundant in the livers of mice subjected to the NEC than the control protocol; however, the increase in abundance of IL-1β transcripts is significantly greater in WT than in TPH1KO mice. F: Sudanophilia is significantly more abundant in the livers of mice subjected to the NEC than the control protocol; however, the degree of sudanophilia is significantly greater in WT than in TPH1KO mice. The NEC-induced increase in sudanophilia in the livers of TPH1KO mice is not significantly different from control. G: apoptosis, detected with the TUNEL reaction is significantly more abundant in the livers of mice subjected to the NEC than the control protocol; however, the frequency of apoptosis is significantly greater in WT than in TPH1KO mice. The NEC-induced increase in apoptosis in the livers of TPH1KO mice is not significantly different from that in the livers of control animals. H: illustrations of the TUNEL reactivity in the livers of NEC-exposed TPH1KO (left) and WT (right) mice (compare with the quantified data in G; see arrow). Note that TPH1KO protects the liver from the effects of the NEC protocol. I: frozen sections of the livers of a TPH1KO (left) and WT mouse (right) subjected to the NEC protocol and stained with Sudan Black. Hepatocytes of the hepatic parenchyma are more intensely sudanophilic in the liver of the WT than in the TPH1KO mouse (compare with the quantified data in F; see arrow). H and I: bar = 100 μm.
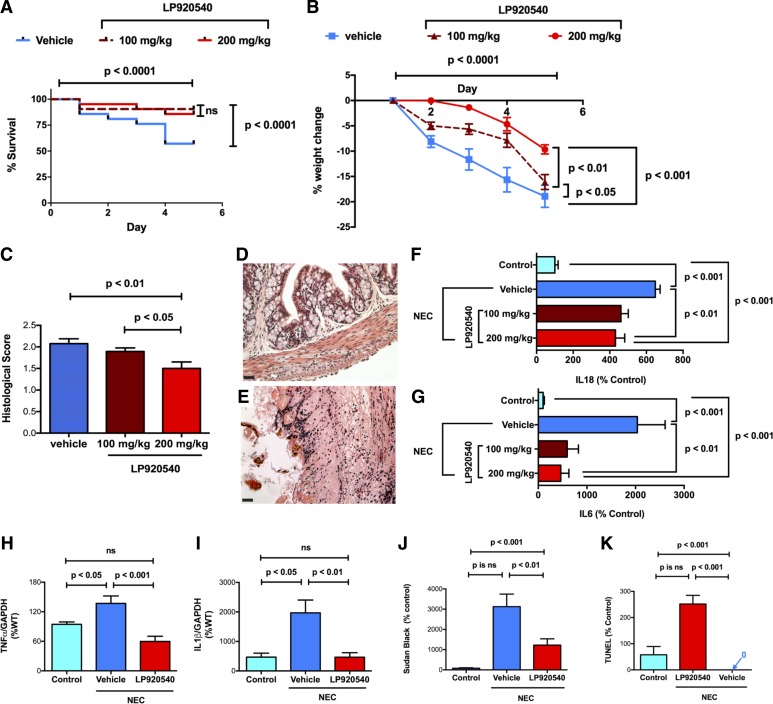
Pharmacological reduction of intestinal mucosal 5-HT opposes experimental NEC in intestine and liver.
Oral LP-920540 is a tryptophan hydroxylase inhibitor that depletes mucosal 5-HT, which is primarily contained within EC cells of the epithelium lining of the stomach, small intestine, and large intestines (58). Oral LP-920540 also depletes 5-HT from platelets, which do not synthesize 5-HT but take up 5-HT released from EC cells, as platelets circulate through the bowel. Significantly, oral LP-920540 does not affect the synthesis of the TPH2-derived 5-HT within either the enteric nervous system (ENS) or the central nervous system (CNS). Because LP-920540 depletes mucosal 5-HT selectively, we tested the hypothesis that it would mimic the ability of TPH1 deletion to protect both the intestine and the liver from NEC. Three groups of WT mice (n = 21/group) were subjected to the experimental NEC protocol. One group received only vehicle (methylcellulose, 50 μl/g), while the other two groups received oral LP-920540 diluted in vehicle, at doses of either 100 or 200 mg/kg. Both of the groups of mice that received LP-920540 were protected from the lethality associated with the NEC protocol, which was manifested in vehicle-treated animals (Fig. 6A). They also lost significantly less weight than vehicle-treated mice, although animals receiving the 200 mg/kg dose lost significantly less weight than those receiving 100 mg/kg of LP-920540 (P < 0.01) (Fig. 6B). The mice that were treated with 200 mg/kg also displayed significantly less intestinal damage than either the vehicle-treated mice or mice receiving the 100 mg/kg dose of LP-920540 (Fig. 6, C–E; Fig. 6D shows the 200 mg/kg LP-treated group, while Fig. 6E shows the vehicle-treated group). As noted earlier, the NEC protocol caused a significant increase in transcripts encoding IL-18 (Fig. 6F) and IL-6 (Fig. 6G), which was apparent when vehicle-treated mice were compared with those subjected to the control protocol. Each dose of LP-920540, however, significantly ameliorated the increases in both IL-18 (Fig. 6F) and IL-6 (Fig. 6G). Similarly, in the liver, subjection of vehicle-treated mice to the NEC protocol was associated with increases in the abundance of TNF-α (Fig. 6H) and IL-1β (Fig. 6I), as well as lipid accumulation in (Fig. 6J) and apoptosis of hepatocytes (Fig. 6K). Treatment of mice with 200 mg/kg of LP-920540 opposed the increased expression of TNF-α (Fig. 6H) and IL-1β (Fig. 6I), as well as the lipid accumulation in hepatocytes (Fig. 6J). LP-920540 also completely protected hepatocytes from the NEC-associated hepatocyte apoptosis (Fig. 6K). These observations support the idea that inflammation of the intestine, as well as the hepatotoxicity associated with subjecting mice to the NEC protocol, is dependent on the mucosal release of 5-HT.
Fig. 6.
The mucosally restricted TPH inhibitor, LP-920540, decreases the severity of the effects of the NEC protocol in the intestine and liver. A: Kaplan-Meier plots of the survival of WT mice treated with vehicle (n = 21) or LP-920540 (n = 42) after exposure to the NEC protocol. Survival was significantly better in animals treated with 100 (n = 21) or 200 mg/kg (n = 21) LP-902540 than in those treated with vehicle; the difference in survival between the two doses of LP-920540 is not significant. B: percent change in weight as a function of time is compared in WT mice treated with vehicle or LP-920540. Both vehicle- and LP-920540-treated mice lose weight during the NEC protocol; however, vehicle-treated mice lose significantly more weight than their LP-920540-treated counterparts. The 200 mg/kg dose of LP-920540 provides significantly better protection from NEC than 100 mg/kg. C: histological scores of intestinal inflammation in mice subjected to the NEC protocol are compared between vehicle- and LP-920540-treated animals. Inflammation is less severe in LP-920540-treated mice (200 mg/kg provides significantly better protection that 100 mg/kg). D and E: representative H&E-stained sections of the colons of mice subjected to the NEC protocol. Inflammation and tissue damage (vacuolization and villous blunting) are less severe in the colon of a mouse treated with 200 mg/kg LP-920540 (D) than vehicle (E). Transcripts encoding the proinflammatory cytokines IL-18 (F) and IL-6 (G) were measured and compared in the distal small intestines of vehicle- and LP-920540-treated mice undergoing the control and NEC protocols. Abundance of each transcript was greater in vehicle- than in LP-920540-treated animals undergoing the NEC protocol. H: abundance of transcripts encoding TNF-α in the livers of mice undergoing the control protocol is compared with that in vehicle- and LP-920540 (200 mg/kg)-treated mice subjected to the NEC protocol. LP-920540 provides significant protection of the liver from the effects of NEC. I: abundance of transcripts encoding IL-1β in the livers mice undergoing the control protocol is compared with that in vehicle- and LP-920540 (200 mg/kg)-treated mice subjected to the NEC protocol. LP-920540 provides significant protection of the liver from the effects of NEC. J: intensity of sudanophilia in the hepatocytes of the livers of mice undergoing the NEC protocol is significantly greater in vehicle- than in LP-920540-treated mice. K: amount of apoptosis, as measured by TUNEL assay, in the hepatocytes of the livers of mice undergoing the NEC protocol is significantly greater in vehicle- than in LP-920540-treated mice.
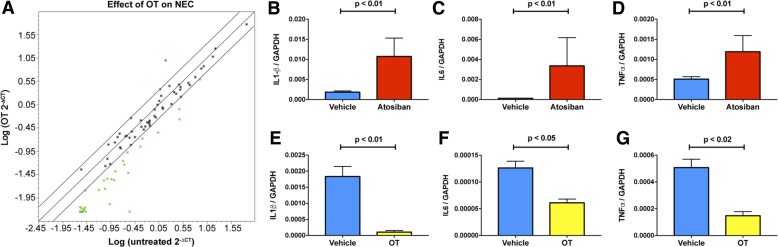
Given the proinflammatory role of endogenous mucosal 5-HT in the manifestation of experimental NEC, we determined whether the bowel might contain an endogenous signaling molecule that exerts an anti-inflammatory effect that could limit the severity of serotonergic signaling under conditions conducive to NEC. OT, which is expressed within intrinsic enteric neurons (60, 86, 87), is potentially such a molecule. The oxytocin receptor (OTR) is developmentally regulated, expressed early in fetal development, and present both on enteric neurons and on epithelial cells of the intestinal mucosa (86). OT/OTR signaling in the mouse gut has also been shown to modulate gastrointestinal inflammation, as well as the cellular stress response in GI epithelial lines and newborn villi, ENS development, motility, epithelial permeability to macromolecules, and mucosal maintenance (42–44, 84). OT/OTR signaling, moreover, is able to attenuate the excitation of enteric neurons that occurs in response to mucosal exposure to cholera toxin, which is dependent on 5-HT release from EC cells (63, 68). OT also inhibits serotonergic signaling in the brain (62). Thus, we tested the hypothesis that OT/OTR signaling can decrease the severity of the intestinal inflammation associated with the NEC protocol in mice.
Three groups of WT mice were subjected to the experimental NEC protocol. One group received only vehicle (saline; n = 48), while the others received the OT antagonist atosiban (100 mg/kg; n = 9) or OT (1.0 mg/kg; n = 9). Each of the groups undergoing the NEC protocol lost weight. Mice treated with atosiban lost significantly more weight (−19.05%) than those treated with vehicle (−14.05%; P < 0.01). To evaluate the effect of OT on intestinal inflammation in mice undergoing the NEC protocol, focused microarrays were used to enable expression of 84 inflammatory pathway genes to be studied simultaneously in five OT-treated and five untreated mice (Fig. 7A). After the NEC protocol, transcripts encoding 37/84 (44%) of such genes were at least twofold less abundant in OT-treated than in untreated animals. The downregulated transcripts encoded proinflammatory cytokines, chemokines, and their receptors. In contrast, only 2/84 (2%) transcripts, encoding a chemokine receptor (Ccr4) and a cytokine (Csf3), both of which are involved in immunosuppression, were expressed to a twofold greater extent in OT-treated than in untreated mice. Real-time PCR was used to quantify transcripts encoding the proinflammatory cytokines, IL-1β (Fig. 7, B and E), IL-6 (Fig. 7, C and F), and TNF-α (Fig. 7, D and G) to compare the effects on NEC of atosiban (Fig. 7, B–D) to those of OT and to confirm the data derived from the focused array on the effects of OT (Fig. 7, E–G). Enteric transcripts of each of the cytokines were significantly more abundant in mice treated with atosiban than in untreated mice (Fig. 7, B–D) and less abundant in animals given OT than in untreated animals (Fig. 7, E–G). These observations suggest that OT exerts an anti-inflammatory action in the bowel of mice exposed to experimental NEC; moreover, the ability of atosiban to exacerbate intestinal inflammation is consistent with the idea that endogenous enteric OT is anti-inflammatory.
Fig. 7.
Oxytocin attenuates the severity of the effects of the NEC protocol on mouse intestine. Transcripts encoding proinflammatory molecules related to inflammation are less abundant in the NEC-exposed mouse colon in OT-treated mice (n = 9) than in those receiving vehicle (n = 48). A: gene expression in a focused microarray. The abundance of transcripts in OT-treated mice is plotted logarithmically on the ordinate as a function of the abundance of the same transcripts in vehicle-treated mice plotted logarithmically on the abscissa. The central line shows the best fit of the data, and the parallel lines above and below depict twofold limits. Many genes (green dots below the lower limiting line) are expressed to a lesser extent in OT- than in vehicle-treated mice, but only two genes (red dots above the upper limiting line) are more highly expressed in OT-treated animals. Both of these gene products Ccr4 and Csf3 are anti-inflammatory or immunosuppressive. B–D: transcripts encoding IL-1β (B), IL-6 (C), and TNF-α (D) are significantly more abundant in the intestine of atosiban- (n = 9) than vehicle-treated (n = 48) mice subjected to the NEC protocol. Transcripts encoding IL-1β (E), IL-6 (F), and TNF-α (G) are significantly more abundant in the intestine of vehicle- than OT-treated mice after exposure to the NEC protocol.
DISCUSSION
NEC has been and continues to be a vexing problem for neonatal infants (15). In the United States, NEC, which carries an overall mortality of 20–40%, affects 0.3–2.4 infants/1,000 live births and occurs in 2–5% of all admissions to neonatal intensive care units (72). Despite its severity, the pathogenesis of NEC is not well understood, and the disorder is not satisfactorily treated (52). Because NEC does not occur in utero, and a bacterial dysbiosis has been associated with the condition, postnatally acquired organisms may play a role in generating NEC (2, 80). Administration of probiotics, however, has not eradicated NEC (17). The Toll-like receptor 4 (TLR4) has been implicated in the intestinal barrier failure that leads to NEC (29). One of the effects of TLR4 activation is to evoke the secretion of 5-HT from EC cells (6, 40). Although TLR4 activation leads to additional deleterious effects, such as endoplasmic reticulum stress in intestinal crypt epithelial cells (1), which probably contributes to NEC, TLR4 may also trigger 5-HT release from the mucosa, which facilitates the development of NEC.
We tested the role of 5-HT in the generation of NEC in a murine model that depends on factors shown to contribute to human NEC, including maternal deprivation, formula feeding, and transient hypoxic stress (30, 47, 52a, 74). The NEC model was reproducibly induced and was evident in the mortality, weight loss, apparent intestinal strictures, intestinal inflammation, and elevation of transcripts encoding proinflammatory cytokines in the bowel. As frequently occurs in human infants with NEC (39, 61), hepatotoxicity was also induced in mice undergoing the NEC protocol. This was manifest as lipid accumulation in hepatocytes, hepatocyte apoptosis, periportal infiltration of inflammatory cells, and elevation of transcription of proinflammatory cytokines in the liver. It should be emphasized that no animal model is a perfect recapitulation of a disorder that occurs in humans, and certainly, it cannot be claimed that this murine model of NEC is identical to human NEC in all of its clinical manifestations. Inflammation and immunity differ in humans and mice. The murine model of NEC, nevertheless, produces severe intestinal inflammation and tissue damage in a developing animal and reveals regulatory roles of intestinal molecules that would be difficult to discover in studies of the human condition. In fact, neither 5-HT nor OT has been investigated for their potential participation in patients with NEC.
Evidence that 5-HT contributes toward driving both the intestinal and hepatic manifestations of experimental NEC is shown by the exacerbation of the severity of the NEC model in both the small intestine and the colon of SERTKO mice. The inactivation of 5-HT following its release from either EC cells or neurons is SERT-dependent (5). There are no extracellular enzymes that can degrade 5-HT; therefore, the rapid removal of 5-HT from its receptors involves its SERT-mediated uptake into cells. As a result, the effects of released 5-HT are enhanced and prolonged in SERTKO mice. Enterocytes (12, 79), neurons (12, 56), and mast cells (77) express SERT in the intestine. Platelet uptake (7), which is SERT-dependent, and dissipation through the circulation probably also contribute to the inactivation of the 5-HT that EC cells secrete. Blood leaving the intestinal mucosa travels through the portal circulation to the liver; moreover, intestinal 5-HT secretion is associated with an increase in 5-HT in portal blood (27, 37). The liver, as well as the bowel, may, thus, be a target organ for 5-HT that the intestinal mucosa releases. Because deletion of mucosal SERT would impair intramucosal 5-HT inactivation, the overflow of 5-HT entering the portal circulation from the bowel would be expected to be greater in SERTKO than in WT mice. The inability of the platelets of SERTKO mice to take up 5-HT would also ensure that the 5-HT reaching the portal blood is free and not compartmentalized in cells. The effects of 5-HT would, thus, be enhanced and prolonged in both gut and liver in SERTKO animals. The increased severity of virtually all manifestations of NEC tested in both the intestine and the liver in SERTKO mice, thus, suggest that 5-HT is involved in driving NEC and is compatible with the idea that the intestine is the source of the toxic 5-HT. The expression of SERT in both mucosa and ENS, however, means that additional information is needed to establish the cellular source of the responsible 5-HT.
Two lines of evidence, both of which show that depletion of 5-HT conveys protection against experimental NEC, provide further support for the idea that 5-HT is a driver of the severity of the NEC model in gut and liver. Both also suggest that the source of the 5-HT involved is the intestinal mucosa. TPH1 is expressed in EC cells and is rate-limiting in their biosynthesis of 5-HT (22, 23). TPH1 is not expressed in the ENS, in which 5-HT biosynthesis is TPH2-dependent, and 5-HT is not depleted from enteric neurons in TPH1KO mice (22, 51). The ability of TPH1 deletion to alleviate the severity of NEC, therefore, is compatible with EC cells, rather than serotonergic neurons, being the source of the 5-HT that drives experimental NEC. Studies of the NEC model in TPH2KO mice were attempted; however, survival of these animals was so poor (0/19 TPH2 mice survived longer than 3 days when subjected to the NEC protocol; P < 0.001 vs. WT) that intestinal inflammation could not accurately be assessed. These data are consistent with previous observations that enteric neuronal 5-HT differs from mucosal 5-HT in that neuronal 5-HT is neuroprotective and anti-inflammatory (22). Hepatic TPH1-dependent biosynthesis of 5-HT in response to stress has also been reported (20), although it is minimal in comparison to that of EC cells. 5-HT has proven to be a two-faced molecule as far as the liver is concerned (48). 5-HT is beneficial in that it promotes hepatic regeneration, repair after ischemia, and protection from cholestatic injury and acetaminophen-induced hepatotoxicity (35, 49, 66, 88). 5-HT is harmful in that it mediates the oxidative stress in hepatocytes that contributes to nonalcoholic steatohepatitis (65), aggravates viral hepatitis (46), and promotes fibrosis (70). Most of the studies of hepatic effects of 5-HT, beneficial or harmful, have found that the circulation, which acquires 5-HT from the bowel, delivers it to the liver. The observations that genetic deletion of TPH1 and oral administration of LP-920540, which depletes 5-HT from the lining of the small and large intestines without affecting that of the brain or ENS (8, 58) alleviate the severity of experimental NEC in the liver, as well as the bowel is consistent with the idea that EC cells are the source of the 5-HT that drives NEC in both organs. The experiments also suggest that the hepatotoxicity seen in NEC arises because the liver is downstream from the gut. Because of its proclivity to mediate oxidative stress in hepatocytes and exacerbate nonalcoholic steatohepatitis (65), 5-HT might itself contribute to hepatotoxicity; however, given that 5-HT also has the ability to protect hepatocytes, toxic products or cytokines released from the inflamed intestine could just as well be the source of the hepatotoxicity of NEC. In either case, however, opposing the release of 5-HT from the GI mucosa and alleviating intestinal inflammation would also benefit the liver. The lack of an effect on sites of TPH2 (brain and ENS) following oral LP-920540, moreover, suggests that it could be a safe and effective therapeutic agent to alleviate the severity of NEC.
We tested the idea that OT, which is produced in enteric neurons that project widely throughout the bowel wall (86), might counteract the proinflammatory drive that occurs in the bowel in the NEC model. OTRs, moreover, are developmentally regulated and expressed, not only on neurons, but also on mucosal epithelial cells, where they are concentrated at junctional complexes (86). Exogenous OT is anti-inflammatory and can protect the adult gut from trinitrobenzene sulfonic acid- and dextran sulfate sodium-induced colitis (83, 84). Colitis was also more severe in animals lacking the OTR. In fact, OT/OTR signaling has been found to play an important role in multiple GI functions, including an inhibition of the ability of mucosal 5-HT release to activate enteric neurons, an increase in the proliferation of crypt cells, and an inhibition of mucosal permeability (83, 84). OT in colostrum, furthermore, can act protectively to alleviate the cellular stress response that occurs before first feeding in isolated newborn villi, as well as an increase in autophagy and the inflammation inhibitor, IκB, in the same tissue (44). We found that exogenous OT was able to decrease transcription of proinflammatory chemokines and cytokines during experimental NEC and also enhanced transcription of anti-inflammatory gene products such as Ccr4 and Csf3. The OTR antagonist atosiban exerted effects at baseline that were the opposite of OT, increasing the severity of the effects of the NEC protocol. These observations suggest that OT is protective against experimental NEC; moreover, the ability of atosiban to exacerbate intestinal inflammation is consistent with the idea that endogenous enteric OT is anti-inflammatory. Experiments were carried out with OTRKO mice in an attempt to validate results obtained with atosiban; however, although OTRKO mice lost significantly more weight than WT mice during the NEC protocol (16.9 ± 1.5% in OTRKO vs. 12.0 ± 0.9% in WT; P < 0.01), the mice did not survive well enough to permit cytokine expression to be validated.
Taken as a whole, the current study suggests that endogenous regulatory molecules within the bowel contribute to the occurrence and severity of experimental NEC. 5-HT, specifically from its source in EC cells, enhances the severity of the NEC protocol, while OT, which in the bowel is produced in neurons, attenuates experimental NEC severity. EC cell 5-HT appears also to promote the severity of hepatotoxicity in the NEC protocol because the liver is downstream of the gut and the target of the portal circulation. These observations are consistent with the idea that intestinal microbiota, by acting as ligands for TLR, contribute to the precipitation of experimental NEC. Bacteria may stimulate TLR4 on EC cells to trigger 5-HT release. Serotonergic enhancement of the immune response to pathogenic bacteria is probably a useful defensive mechanism. It is conceivable that this defensive mechanism is excessive and dysfunctional in the premature bowel, particularly if regulatory mechanisms are insufficiently developed to modulate inflammatory damage. OT/OTR signaling may be one such regulatory mechanism. OT opposes experimental NEC, and inhibition of its effects by the OT antagonist atosiban increases the severity of the NEC protocol. Further studies are needed to confirm whether it is the OT within the ENS and not OT from the CNS or another source that is protective against experimental NEC. OT is produced in the neonatal and postnatal hypothalamus and reaches the plasma (9, 54); however, the plasma levels of OT in an infant mouse are probably not sufficient to exert an anti-inflammatory effect in the gut (36). It is possible that breast milk, which is an important source of OT (34, 75), could supply OT or supplement the effects of enteric OT in opposing experimental NEC. Certainly, the early introduction of breast feeding decreases the incidence of NEC in premature infants (76), and formula feeding is an important component of the NEC protocol. Maternal deprivation is also an important component of this protocol (50); moreover, nurture, which heavily involves OT in the CNS (69), is beneficial as a therapeutic intervention on outcomes in neonatal intensive care units (81, 82, 85). Further work is needed, but it is possible that the benefits of nurture could be supplemented through enteric actions of OT/OTR signaling, such as the modulation of inflammatory responses.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants KO8 DK-093786 (K. Gross Margolis), NIH RO1 NINDS-15477 (M. D. Gershon), the Einhorn Family Charitable Trust, Mary Dexter Stephenson, and the Fleur Fairman Family (K. Gross Margolis, M. D. Gershon).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.G.M., M.G.W., and M.D.G. conceived and designed research; K.G.M., J.V., M.T., K.G., Z.L., A.I., K.S., V.S., N.I., and M.D.G. performed experiments; K.G.M., J.V., M.T., K.G., Z.L., A.I., K.S., V.S., N.I., and M.D.G. analyzed data; K.G.M., J.V., M.T., K.G., Z.L., A.I., K.S., V.S., N.I., and M.D.G. interpreted results of experiments; K.G.M. and M.D.G. prepared figures; K.G.M. and M.D.G. drafted manuscript; K.G.M., J.V., M.T., K.G., Z.L., A.I., K.S., V.S., N.I., M.G.W., and M.D.G. edited and revised manuscript; K.G.M., J.V., M.T., K.G., Z.L., A.I., K.S., V.S., N.I., M.G.W., and M.D.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Hackam (Johns Hopkins University School of Medicine, Baltimore, MD) for helping us to adapt the NEC protocol, Lexicon Pharmaceuticals for the donation of the LP-920540 used in this study, and Francine Côté (Université Sorbonne Paris Cité, Université Paris Descartes, INSERM, CNRS, Laboratory of Cellular and Molecular Mechanisms of Hematological Disorders and Therapeutic Implications U1163, Institut Imagine, Laboratoire d'Excellence GR-Ex, Paris, France) for donating TPH1KO mice.
Present address of M. Talavera: Dept. of Pediatrics at Nationwide Children’s Hospital, 575 Children’s Crossroad WB6233, Columbus, OH 43215.
Present address of K. Stevanovic: Dept. of Molecular Medicine at the National Institute of Environmental Health Sciences, Neurobiology Laboratory, Neurobehavioral Core, Bldg. 101, Rm. F208, 111 T.W. Alexander Dr., Research Triangle Park, NC 27709.
REFERENCES
- 1.Afrazi A, Branca MF, Sodhi CP, Good M, Yamaguchi Y, Egan CE, Lu P, Jia H, Shaffiey S, Lin J, Ma C, Vincent G, Prindle T Jr, Weyandt S, Neal MD, Ozolek JA, Wiersch J, Tschurtschenthaler M, Shiota C, Gittes GK, Billiar TR, Mollen K, Kaser A, Blumberg R, Hackam DJ. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem 289: 9584–9599, 2014. doi: 10.1074/jbc.M113.526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azcarate-Peril MA, Foster DM, Cadenas MB, Stone MR, Jacobi SK, Stauffer SH, Pease A, Gookin JL. Acute necrotizing enterocolitis of preterm piglets is characterized by dysbiosis of ileal mucosa-associated bacteria. Gut Microbes 2: 234–243, 2011. doi: 10.4161/gmic.2.4.16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand PP, Kunze WAA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101: 459–469, 2000. doi: 10.1016/S0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol 296: G685–G695, 2009. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 5.Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol 196: 263–281, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bogunovic M, Davé SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Mayer LF, Plevy SE. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol 292: G1770–G1783, 2007. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, Kilic F. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem 102: 206–215, 2007. doi: 10.1111/j.1471-4159.2007.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown PM, Drossman DA, Wood AJ, Cline GA, Frazier KS, Jackson JI, Bronner J, Freiman J, Zambrowicz B, Sands A, Gershon MD. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology 141: 507–516, 2011. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buijs RM, Velis DN, Swaab DF. Ontogeny of vasopressin and oxytocin in the fetal rat: early vasopressinergic innervation of the fetal brain. Peptides 1: 315–324, 1980. doi: 10.1016/0196-9781(80)90009-1. [DOI] [PubMed] [Google Scholar]
- 10.Bülbring E, Lin RCY, Schofield G. An investigation of the peristaltic reflex in relation to anatomical observations. Q J Exp Physiol Cogn Med Sci 43: 26–37, 1958. [DOI] [PubMed] [Google Scholar]
- 11.Caplan MS, Fanaroff A. Necrotizing: A historical perspective. Semin Perinatol 41: 2–6, 2017. doi: 10.1053/j.semperi.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Chen J-X, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol Gastrointest Liver 275: G433–G448, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Cooke HJ, Christofi F. Enteric neural regulation of mucosal secretion, 4th ed, In: Physiology of the Gastrointestinal Tract edited by Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD. New York: Elsevier, 2006, p. 737–762. doi: 10.1016/B978-012088394-3/50030-1. [DOI] [Google Scholar]
- 14.Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, Dandolo L, Hamon M, Mallet J, Vodjdani G. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA 100: 13,525–13,530, 2003. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton S, Rees CM, Hall NJ. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology 111: 423–430, 2017. doi: 10.1159/000458462. [DOI] [PubMed] [Google Scholar]
- 16.Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, Fulton WB, Niño DF, Prindle T Jr, Ozolek JA, Hackam DJ. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 126: 495–508, 2016. doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming P, Hall NJ, Eaton S. Probiotics and necrotizing enterocolitis. Pediatr Surg Int 31: 1111–1118, 2015. doi: 10.1007/s00383-015-3790-0. [DOI] [PubMed] [Google Scholar]
- 18.Frank HG, Gossrau R, Graf R. Analysis of hepatotoxicity in maternal and fetal rats after glucocorticoid administration by lipid histochemistry and thin layer chromatography. Histochemistry 91: 487–494, 1989. doi: 10.1007/BF00492521. [DOI] [PubMed] [Google Scholar]
- 19.Frost BL, Modi BP, Jaksic T, Caplan MS. New medical and surgical insights into neonatal necrotizing enterocolitis: a review. JAMA Pediatr 171: 83–88, 2017. doi: 10.1001/jamapediatrics.2016.2708. [DOI] [PubMed] [Google Scholar]
- 20.Fu J, Ma S, Li X, An S, Li T, Guo K, Lin M, Qu W, Wang S, Dong X, Han X, Fu T, Huang X, Wang T, He S. Long-term stress with hyperglucocorticoidemia-induced hepatic steatosis with VLDL overproduction is dependent on both 5-HT2 receptor and 5-HT synthesis in liver. Int J Biol Sci 12: 219–234, 2016. doi: 10.7150/ijbs.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20: 14–21, 2013. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc 123: 268–280, 2012. [PMC free article] [PubMed] [Google Scholar]
- 23.Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Côté F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137: 1649–1660, 2009. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Good M, Sodhi CP, Egan CE, Afrazi A, Jia H, Yamaguchi Y, Lu P, Branca MF, Ma C, Prindle T Jr, Mielo S, Pompa A, Hodzic Z, Ozolek JA, Hackam DJ. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol 8: 1166–1179, 2015. doi: 10.1038/mi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Good M, Sodhi CP, Ozolek JA, Buck RH, Goehring KC, Thomas DL, Vikram A, Bibby K, Morowitz MJ, Firek B, Lu P, Hackam DJ. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol 306: G1021–G1032, 2014. doi: 10.1152/ajpgi.00452.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi XH, Shah S, Ozolek JA, Hackam DJ. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 182: 636–646, 2009. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grönstad KO, Ahlund L, Dahlström A, Häggendal J, Ahlman H. A possible mechanism for the release of serotonin from the gut caused by pentagastrin. J Surg Res 44: 473–478, 1988. doi: 10.1016/0022-4804(88)90151-5. [DOI] [PubMed] [Google Scholar]
- 28.Guseva D, Holst K, Kaune B, Meier M, Keubler L, Glage S, Buettner M, Bleich A, Pabst O, Bachmann O, Ponimaskin EG. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis 20: 1516–1529, 2014. doi: 10.1097/MIB.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 29.Hackam DJ, Good M, Sodhi CP. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin Pediatr Surg 22: 76–82, 2013. doi: 10.1053/j.sempedsurg.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpern MD, Holubec H, Dominguez JA, Meza YG, Williams CS, Ruth MC, McCuskey RS, Dvorak B. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 284: G695–G702, 2003. doi: 10.1152/ajpgi.00353.2002. [DOI] [PubMed] [Google Scholar]
- 31.Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res 51: 733–739, 2002. doi: 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Halpern MD, Khailova L, Molla-Hosseini D, Arganbright K, Reynolds C, Yajima M, Hoshiba J, Dvorak B. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol 294: G20–G26, 2008. doi: 10.1152/ajpgi.00168.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haub S, Ritze Y, Bergheim I, Pabst O, Gershon MD, Bischoff SC. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil 22: 826–834, 2010. doi: 10.1111/j.1365-2982.2010.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higashida H, Yokoyama S, Munesue T, Kikuchi M, Minabe Y, Lopatina O. CD38 gene knockout juvenile mice: a model of oxytocin signal defects in autism. Biol Pharm Bull 34: 1369–1372, 2011. doi: 10.1248/bpb.34.1369. [DOI] [PubMed] [Google Scholar]
- 35.Jang JH, Rickenbacher A, Humar B, Weber A, Raptis DA, Lehmann K, Stieger B, Moritz W, Soll C, Georgiev P, Fischer D, Laczko E, Graf R, Clavien PA. Serotonin protects mouse liver from cholestatic injury by decreasing bile salt pool after bile duct ligation. Hepatology 56: 209–218, 2012. doi: 10.1002/hep.25626. [DOI] [PubMed] [Google Scholar]
- 36.Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446: 41–45, 2007. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 37.Kellum JM Jr, Jaffe BM. Release of immunoreactive serotonin following acid perfusion of the duodenum. Ann Surg 184: 633–636, 1976. doi: 10.1097/00000658-197611000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G940–G949, 2009. doi: 10.1152/ajpgi.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khailova L, Dvorak K, Arganbright KM, Williams CS, Halpern MD, Dvorak B. Changes in hepatic cell junctions structure during experimental necrotizing enterocolitis: effect of EGF treatment. Pediatr Res 66: 140–144, 2009. doi: 10.1203/PDR.0b013e3181aa3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1β- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil 21: 439–450, 2009. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JJ, Bridle BW, Ghia JE, Wang H, Syed SN, Manocha MM, Rengasamy P, Shajib MS, Wan Y, Hedlund PB, Khan WI. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol 190: 4795–4804, 2013. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- 42.Klein BY, Tamir H, Hirschberg DL, Glickstein SB, Ludwig RJ, Welch MG. Oxytocin modulates markers of the unfolded protein response in Caco2BB gut cells. Cell Stress Chaperones 19: 465–477, 2014. doi: 10.1007/s12192-013-0473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein BY, Tamir H, Hirschberg DL, Ludwig RJ, Glickstein SB, Myers MM, Welch MG. Oxytocin opposes effects of bacterial endotoxin on ER-stress signaling in Caco2BB gut cells. Biochim Biophys Acta 1860: 402–411, 2016. doi: 10.1016/j.bbagen.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Klein BY, Tamir H, Ludwig RJ, Glickstein SB, Welch MG. Colostrum oxytocin modulates cellular stress response, inflammation, and autophagy markers in newborn rat gut villi. Biochem Biophys Res Commun 487: 47–53, 2017. doi: 10.1016/j.bbrc.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 46.Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M, Cervantes-Barragan L, Ludewig B, Calzascia T, Bolinger B, Merkler D, Odermatt B, Bader M, Graf R, Clavien PA, Hegazy AN, Löhning M, Harris NL, Ohashi PS, Hengartner H, Zinkernagel RM, Lang KS. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med 14: 756–761, 2008. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 47.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 48.Lesurtel M, Clavien PA. Serotonin: a key molecule in acute and chronic liver injury! Clin Res Hepatol Gastroenterol 36: 319–322, 2012. doi: 10.1016/j.clinre.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science 312: 104–107, 2006. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Zani A, Lee C, Zani-Ruttenstock E, Zhang Z, Li X, Ip W, Gonska T, Pierro A. Endoplasmic reticulum stress is involved in the colonic epithelium damage induced by maternal separation. J Pediatr Surg 51: 1001–1004, 2016. doi: 10.1016/j.jpedsurg.2016.02.073. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Côté F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31: 8998–9009, 2011. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 368: 1271–1283, 2006. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 52a.Lu P, Sodhi CP, Jia H, Shaffley S, Good M, Branca MF, Hackam DJ. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enerocolitis: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 306: G917–G928, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maheshwari A, Schelonka RL, Dimmitt RA, Carlo WA, Munoz-Hernandez B, Das A, McDonald SA, Thorsen P, Skogstrand K, Hougaard DM, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res 76: 100–108, 2014. doi: 10.1038/pr.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchini G, Stock S. Pulsatile release of oxytocin in newborn infants. Reprod Fertil Dev 8: 163–165, 1996. doi: 10.1071/RD9960163. [DOI] [PubMed] [Google Scholar]
- 55.Margolis KG, Gershon MD. Enteric neuronal regulation of intestinal inflammation. Trends Neurosci 39: 614–624, 2016. doi: 10.1016/j.tins.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson GM, Snyder I, Veenstra-VanderWeele J, Blakely RD, Gershon MD. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest 126: 2221–2235, 2016. doi: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D’Autréaux F, Saurman V, Chalazonitis A, Gershon MD. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology 141: 588–598, 2011. doi: 10.1053/j.gastro.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, Jhaver KG, Diacou A, Gershon MD. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 63: 928–937, 2014. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mollen KP, Gribar SC, Anand RJ, Kaczorowski DJ, Kohler JW, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. Increased expression and internalization of the endotoxin coreceptor CD14 in enterocytes occur as an early event in the development of experimental necrotizing enterocolitis. J Pediatr Surg 43: 1175–1181, 2008. doi: 10.1016/j.jpedsurg.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monstein HJ, Grahn N, Truedsson M, Ohlsson B. Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study. Regul Pept 119: 39–44, 2004. doi: 10.1016/j.regpep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 61.Moss RL, Das JB, Raffensperger JG. Necrotizing enterocolitis and total parenteral nutrition-associated cholestasis. Nutrition 12: 340–343, 1996. doi: 10.1016/S0899-9007(96)80057-8. [DOI] [PubMed] [Google Scholar]
- 62.Mottolese R, Redouté J, Costes N, Le Bars D, Sirigu A. Switching brain serotonin with oxytocin. Proc Natl Acad Sci USA 111: 8637–8642, 2014. doi: 10.1073/pnas.1319810111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nilsson O, Cassuto J, Larsson P-A, Jodal M, Lidberg P, Ahlman H, Dahlström A, Lundgren O. 5-Hydroxytryptamine and cholera secretion: a histochemical and physiological study in cats. Gut 24: 542–548, 1983. doi: 10.1136/gut.24.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 13: 590–600, 2016. doi: 10.1038/nrgastro.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M, Renner EL, Clavien PA. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology 133: 608–618, 2007. doi: 10.1053/j.gastro.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 66.Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology 45: 369–376, 2007. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 67.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci 20: 3295–3309, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peregrin AT, Ahlman H, Jodal M, Lundgren O. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br J Pharmacol 127: 887–894, 1999. doi: 10.1038/sj.bjp.0702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science 345: 771–776, 2014. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, Mann DA. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol 169: 861–876, 2006. doi: 10.2353/ajpath.2006.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma A, Mittal T, Rajan R, Rathi M, Nada R, Minz RW, Joshi K, Sakhuja V, Singh S. Validation of the consensus methodology algorithm for the classification of systemic necrotizing vasculitis in Indian patients. Int J Rheum Dis 17: 408–411, 2014. doi: 10.1111/1756-185X.12219. [DOI] [PubMed] [Google Scholar]
- 72.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol 40: 27–51, 2013. doi: 10.1016/j.clp.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143: 708–718.e5, 2012. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like receptor-4 inhibits enterocyte proliferation via impaired β-catenin signaling in necrotizing enterocolitis. Gastroenterology 138: 185–196, 2010. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeda S, Kuwabara Y, Mizuno M. Concentrations and origin of oxytocin in breast milk. Endocrinol Jpn 33: 821–826, 1986. doi: 10.1507/endocrj1954.33.821. [DOI] [PubMed] [Google Scholar]
- 76.Talavera MM, Bixler G, Cozzi C, Dail J, Miller RR, McClead R Jr, Reber K. Quality improvement initiative to reduce the necrotizing enterocolitis rate in premature infants. Pediatrics 137: e20151119, 2016. doi: 10.1542/peds.2015-1119. [DOI] [PubMed] [Google Scholar]
- 77.Tamir H, Theoharides TC, Gershon MD, Askenase PW. Serotonin storage pools in basophil leukemia and mast cells: characterization of two types of serotonin binding protein and radioautographic analysis of the intracellular distribution of [3H]serotonin. J Cell Biol 93: 638–647, 1982. doi: 10.1083/jcb.93.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Upperman JS, Camerini V, Lugo B, Yotov I, Sullivan J, Rubin J, Clermont G, Zamora R, Ermentrout GB, Ford HR, Vodovotz Y. Mathematical modeling in necrotizing enterocolitis—a new look at an ongoing problem. J Pediatr Surg 42: 445–453, 2007. doi: 10.1016/j.jpedsurg.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 79.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci 16: 2352–2364, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, Shaikh N, Hoffmann JA, Linneman LA, Hamvas A, Khanna G, Rouggly-Nickless LC, Ndao IM, Shands BA, Escobedo M, Sullivan JE, Radmacher PG, Shannon WD, Tarr PI. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387: 1928–1936, 2016. doi: 10.1016/S0140-6736(16)00081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Welch MG. Calming cycle theory: the role of visceral/autonomic learning in early mother and infant/child behaviour and development. Acta Paediatr 105: 1266–1274, 2016. doi: 10.1111/apa.13547. [DOI] [PubMed] [Google Scholar]
- 82.Welch MG. Nurture in the neonatal intensive care unit. Acta Paediatr 105: 730–731, 2016. doi: 10.1111/apa.13294. [DOI] [PubMed] [Google Scholar]
- 83.Welch MG, Anwar M, Chang CY, Gross KJ, Ruggiero DA, Tamir H, Gershon MD. Combined administration of secretin and oxytocin inhibits chronic colitis and associated activation of forebrain neurons. Neurogastroenterol Motil 22: 654–e202, 2010. doi: 10.1111/j.1365-2982.2010.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Welch MG, Margolis KG, Li Z, Gershon MD. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am J Physiol Gastrointest Liver Physiol 307: G848–G862, 2014. doi: 10.1152/ajpgi.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Welch MG, Myers MM. Advances in family-based interventions in the neonatal ICU. Curr Opin Pediatr 28: 163–169, 2016. doi: 10.1097/MOP.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 86.Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J Comp Neurol 512: 256–270, 2009. doi: 10.1002/cne.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu Q, Ji R, Gao X, Fu J, Guo W, Song X, Zhao X, Burnstock G, Shi X, He C, Xiang Z. Oxytocin is expressed by both intrinsic sensory and secretomotor neurons in the enteric nervous system of guinea pig. Cell Tissue Res 344: 227–237, 2011. doi: 10.1007/s00441-011-1155-0. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, Song S, Pang Q, Zhang R, Zhou L, Liu S, Meng F, Wu Q, Liu C. Corrigendum: Serotonin deficiency exacerbates acetaminophen-induced liver toxicity in mice. Sci Rep 5: 12184, 2015. doi: 10.1038/srep12184. [DOI] [PMC free article] [PubMed] [Google Scholar]