Abstract
G protein-coupled receptors (GPCRs) make up the largest transmembrane receptor superfamily in the human genome and are expressed in nearly all gastrointestinal cell types. Coupling of GPCRs and their respective ligands activates various phosphotransferases in the cytoplasm, and, thus, activation of GPCR signaling in intestine regulates many cellular and physiological processes. Studies in microRNAs (miRNAs) demonstrate that they represent critical epigenetic regulators of different pathophysiological responses in different organs and cell types in humans and animals. Here, we reviewed recent research on GPCR-miRNA interactions related to gastrointestinal pathophysiology, such as inflammatory bowel diseases, irritable bowel syndrome, and gastrointestinal cancers. Given that the presence of different types of cells in the gastrointestinal tract suggests the importance of cell-cell interactions in maintaining gastrointestinal homeostasis, we also discuss how GPCR-miRNA interactions regulate gene expression at the cellular level and subsequently modulate gastrointestinal pathophysiology through molecular regulatory circuits and cell-cell interactions. These studies helped identify novel molecular pathways leading to the discovery of potential biomarkers for gastrointestinal diseases.
Keywords: inflammatory bowel disease, epigenetics, inflammation, intestinal epithelial cells, neuropeptides, microribonucleic acid
receptor activation and its subsequent signaling represent an important part of cellular responses to a variety of external stimuli from the changing environment. Among different families of receptors, G protein-coupled receptors (GPCRs), which are also known as seven-transmembrane domain receptors, make up the largest transmembrane receptor superfamily in the human, mouse, Caenorhabditis elegans, and Drosophila genomes (96). Based on sequence homology analysis, the International Union of Basic and Clinical Pharmacology (IUPHAR)/The British Pharmacological Society Guide to Pharmacology has included a classification scheme that divides GPCRs as follows: class A (rhodopsin like), class B (secretin receptor family), class C (metabotropic glutamate), class D (fungal mating pheromone receptors), class E (cAMP receptors), and class F (frizzled/smoothened) (1), in which only classes A, B, C, and F are found in vertebrates. Alternatively, GPCRs are also grouped in five classes in the “GRAFS” classification system in which the five classes overlap with classes A–F in the IUPHAR classification scheme (108). In this article, the names or abbreviations of the GPCRs are used in agreement with official IUPHAR nomenclature, and the human gene symbols are written in italics.
GPCR Signal Transduction
GPCRs mediate a broad range of cellular responses to various extracellular stimuli, often in response to their ligands. GPCRs are essential to normal gastrointestinal functions, and their ligands include different types of molecules, such as peptides (e.g., neuropeptides), lipids (e.g., endocannabinoids), and proteins (e.g., chemokines) (32, 104). Structurally, a GPCR consists of an NH2-terminal extracellular end, a core made of seven transmembrane α-helices, and a COOH-terminal cytoplasmic tail. In the unbound (inactivate) state, GPCRs are associated with the membrane-bound heterotrimeric guanine nucleotide-binding proteins (G proteins) at the COOH-terminus. GPCR-associated G proteins are comprised of α-, β-, and γ-subunits in which the α-subunit binds to a GDP molecule, and the β- and γ-subunits form stable Gβγ dimers. According to the classical view of GPCR signaling activation, coupling of a ligand at the NH2-terminus mediates conformational changes in a GPCR and activates Gα subunit by exchanging the GDP for GTP, leading to the dissociation of the Gα subunit from the COOH-terminus and the Gβγ complex. The dissociated G protein subunits then interact with adenylyl cyclase or phospholipases, which are signal transducers located at the cell membrane, and generate secondary messengers, such as cAMP or phosphatidylinositol. On the other hand, activated GPCRs also transactivate various membrane-associated receptors, including epidermal growth factor receptor (EGFR), insulin-like growth factor receptor (IGFR), and transforming growth factor-α through highly regulated cross talk with receptor tyrosine kinases, matrix metalloproteinases, and integrins. Subsequently, the secondary messengers generated by the activation of GPCRs and the transactivation of other membrane-associated receptors initiate various signaling cascades by activating various downstream phosphokinases. These include mitogen-activated protein kinases (MAPKs), c-Jun NH2-terminal kinases (JNKs), phosphatidylinositol 3-kinase (PI3K), and inhibitor of κB kinases (IκB kinases) and mediate appropriate cellular responses to external stimuli (reviewed in Refs. 19 and 104).
To prevent the detrimental effect from sustained G protein-dependent signaling activation induced by extracellular ligand binding, stimulated cells achieve cellular desensitization by internalization of the ligand-bound GPCRs. In β-arrestin (β-arrs)-dependent GPCR internalization, the dissociation of G proteins from a ligand-bound GPCR is followed by phosphorylation of the intracellular region and/or COOH-terminal cytoplasmic tail of the ligand-bound GPCR by G protein-coupled receptor kinases. β-Arrs are then recruited to the COOH-terminus of GPCR and, together with adaptor protein 2 (AP2) complex, dynamin, and clathrin, initiate the process of internalization with invagination of the cell membrane. Still bound to β-arrs, the ligand-bound GPCR is believed to be inactivated and transported in a clathrin-coated vesicle to the early endosomes, where β-arrs will be dissociated from the GPCR. The GPCR will then be either transported to lysosomal vesicles for degeneration or recycled to the cell membrane for ligand binding. This β-arrs-dependent clathrin-mediated internalization mechanism appears to be common among most of the GPCRs during the desensitization process, although there are exceptions where β-arrs are not involved (reviewed in Ref. 94). However, the functional relevance of β-arrs in GPCR signal transduction is not restricted to its role in GPCR desensitization. Accumulated evidence showed that β-arrs act as functional adaptors to signaling molecules, such as c-Src, IκB-α, and other key players in MAPK-, JNK-, and PI3K-protein kinase B (AKT) signaling pathways by bringing these signaling molecules to proximity to the ligand-bound GPCRs in the intracellular vesicles. This second wave of signal transduction is dependent on β-arrs as GPCR signal transducers in early endosomes, but independent of G protein signaling activation that occurred on the cell membrane. To add to the complexity of spatial and temporal aspects of GPCR signaling, a recent study by Irannejad et al. showed that the endosome-localized β2-adrenoceptor is able to activate Gα subunits and generate cAMP after receptor internalization, in contrast to the classic G protein-dependent GPCR signaling activation model (51). Taken together, GPCR signal transduction exhibits complex spatial and temporal patterns and, thus, can mediate highly regulated and appropriate cellular responses to a broad range of external stimuli.
Activation of GPCR signaling in the intestine regulates many cellular and physiological processes, such as cell growth and proliferation (44, 63, 71), inflammation (131, 134), angiogenesis (3, 4, 50), absorption and secretion (45, 86), wound healing (44, 64), leukocyte trafficking (40), fibrogenesis (62), tumorigenesis (2, 14, 103), and intestinal motility (reviewed in Ref. 45). Importantly, GPCR signaling pathways are also targets of several existing gastrointestinal agents, such as drugs against diarrhea (opioid receptors), functional bowel disorder agents (receptors for acetylcholine, glutamate, and serotonin), gastrointestinal stimulants (dopamine and serotonin receptors), and H2 antagonists. Conventionally, studies on GPCRs and their associated protein effector signaling pathways provided significant information toward our understanding in gastrointestinal pathophysiology. However, epigenetic regulatory mechanisms of GPCR signaling evolved in recent years and represent a new interesting area to help us understand mechanisms regulating gastrointestinal pathophysiology. In this review, the interaction between GPCRs and a novel class of epigenetic regulators, microRNAs (miRNAs), will be discussed with emphasis on the gastrointestinal tract.
miRNA, an Epigenetic Regulator in GPCR Signaling
miRNAs are endogenously expressed short single-stranded RNA molecules (∼22 nucleotides) acting mainly as negative regulators in gene expression. Similar to GPCRs, miRNAs are found in C. elegans, Drosophila, mice, and humans. At the time of writing of this review, 2,588 mature human miRNAs registered in the miRbase (http://mirbase.org, Release 21, accessed February 2017) have been identified (66). Analysis from bioinformatics and proteomics estimated that 30–60% of gene expression in humans is regulated by miRNAs (33, 74, 109).
Biogenesis of miRNAs begins with the transcription of primary miRNAs (pri-miRNAs) by RNA polymerase II (11, 73) at the nucleus. pri-miRNAs are double-stranded RNA molecules in stem-loop structures, with sizes ranging from several hundred nucleotides to several kilobases. Inside the nucleus, pri-miRNAs are cleaved by a type III RNase, Drosha, to generate smaller-hairpin RNAs of ∼70 nucleotides called pre-miRNA (72). The shorter pre-miRNAs in stem-loop structures are then exported from the nucleus to the cytoplasm by exportin 5 (82, 126). This is followed by a second cleavage process in the cytoplasm. pre-miRNAs are cleaved by Dicer, another type III RNase, which releases double-stranded miRNAs from the loop regions (48). This RNA duplex immediately associates with members from the Argonaute protein family (AGO1–5 in humans) to form RNA-induced silencing complexes (RISC). The mature strand of miRNA is retained in the complex, whereas the other strand is degraded. RISC then binds to mRNA transcripts through imperfect base pairing. This results in mRNA decay by promoting deadenylation, decapping, and exonucleolytic degradation, in addition to translational repression (9). A more detailed description of miRNA biogenesis and processing is described elsewhere (8, 9, 41, 59).
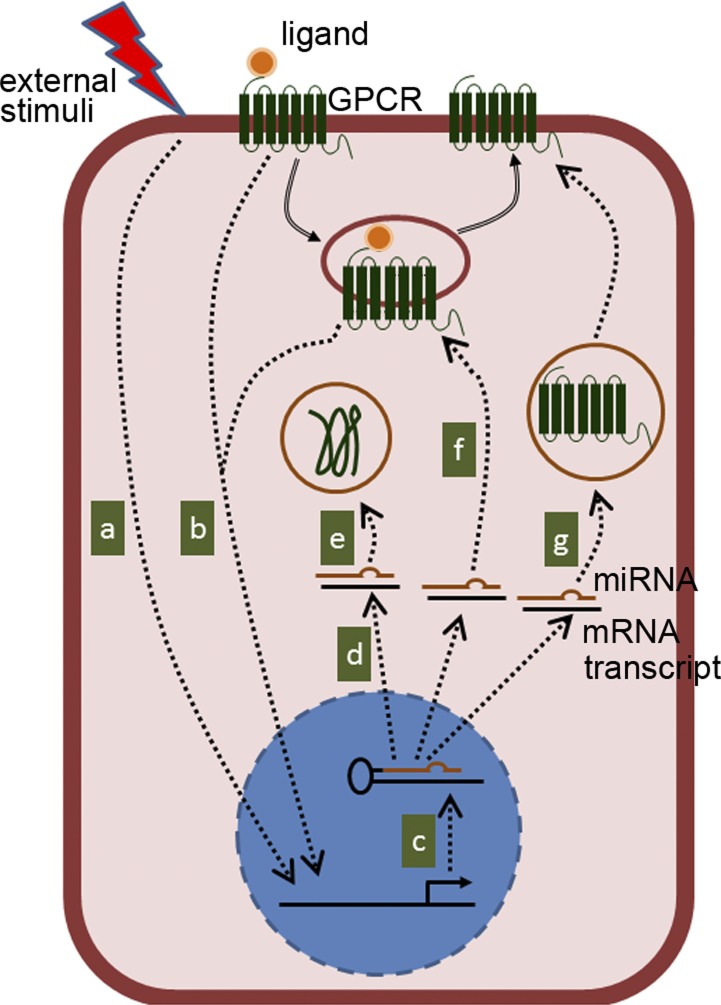
Similar to GPCRs, miRNAs regulate a large number of signaling pathways, thereby modulating the pathophysiology of several gastrointestinal disease states, such as colon cancer and inflammatory bowel disease (IBD) (52, 56, 129). The ability of miRNAs to regulate gene expression at the epigenetic level by binding to their corresponding complementary sequences in the 3′-untranslated regions (UTRs) of different target genes allows GPCR signaling pathways to regulate different targets simultaneously upon activation. GPCR expression, in some instances, is also modulated by expression of miRNAs induced by extracellular stimuli. Thus, the potential GPCR-miRNA interactions can be summarized as follows. An external stimuli or GPCR-ligand coupling activates or attenuates the transcription of miRNA in the nucleus. Once in the cytoplasm, mature miRNAs bind to their complementary mRNA sequences and reduce target protein expression either by destabilizing mRNA transcripts and/or by attenuating the translation process. Target proteins can be downstream effectors of GPCR signaling pathways, proteins that facilitate intracellular trafficking of GPCRs, or a GPCR itself. It should be noted that multiple miRNAs are transcribed upon signal activation; therefore, GPCR-miRNA interactions orchestrate a cascade of modifications in protein expression or signaling activation at the cellular level (Fig. 1). In this review, examples of different GPCR-miRNA interactions relevant to various gastrointestinal physiological conditions and their significance will be discussed.
Fig. 1.
A representative diagram of possible G protein-coupled receptor (GPCR)-microRNA (miRNA) interactions at the cellular level. miRNA transcription is initiated by either external stimuli (a) or GPCR activation (b). Primary miRNAs (pri-miRNAs) are transcribed in nucleus (c) and exported to cytoplasm as smaller-hairpin RNAs of ∼70 nucleotides (pre-miRNAs) (d). miRNAs, together with Argonaute protein family (AGO) proteins, then bind to their corresponding complementary sequence in the 3′-untranslated region (UTR) of their target mRNA transcript, in which the target mRNA transcripts will be destabilized or their translation will be inhibited. Subsequently, the expression of target proteins is reduced. The above process affects the expression of proteins that are downstream of GPCR signaling activation (e), components that facilitate GPCR signaling activation (f), or GPCR itself (g).
Inflammatory Bowel Diseases
The intestinal wall is divided into different layers comprised of different tissues and cell types. The presence of different types of cells in specialized tissues in the gastrointestinal tract strongly suggests the importance of cell-cell interactions in maintaining gastrointestinal homeostasis. Furthermore, GPCRs are expressed in nearly all gastrointestinal cell types while their associated signaling pathways are crucial in maintaining various physiological and pathophysiological functions (3, 4, 40, 44, 45, 50, 63, 86). Taken together, GPCR-miRNA interactions may affect gastrointestinal homeostasis through cell-cell communication. Studies related to NK1 receptor (TACR1), neurotensin-1 receptor (NTSR1), and their cellular and physiological roles in IBD are discussed below as examples.
IBD is a group of chronic inflammatory gastrointestinal disorders that includes Crohn’s disease and ulcerative colitis (UC), characterized by periods of persistent inflammation followed by remission. Studies have shown that miRNAs are differentially expressed in pediatric (65) and adult (98, 124, 125) colonic tissues, suggesting that miRNA expression is differentially regulated during colonic inflammation. NK1 receptor is a GPCR from the class A (rhodopsin-like) family and a high-affinity receptor for the neuropeptide substance P (SP). NK1 receptors are expressed in various gastrointestinal cell types, including colonic epithelial cells (36, 132), immune cells (116), fibroblasts (17, 62), mesenteric fat depots (37, 115), and the enteric nervous system (101). Thus, SP/NK1 receptor signaling is involved in a wide range of physiological responses, including motility, pain sensation, neurotransmission, and inflammation in the gastrointestinal tract (reviewed in Ref. 119). In cellular responses associated with inflammation, binding of SP to NK1 receptor activates the Rho family small GTPases and protein kinase C, leading to activation of kinases in MAPK, NF-κB, AKT, and JAK-STAT signaling pathways. Activation of these signaling pathways, in turn, is linked to increased interleukin-8 (IL-8) and prostaglandin E2 synthesis via a cyclooxygenase-2-dependent mechanism (37, 132). In addition, SP/NK1 receptor coupling also transactivates EGFR in murine colonic fibroblasts (17). Interestingly, part of the SP/NK1 receptor-miRNA interactions represents a regulatory circuit on NK1 receptor expression. In human astrocytoma cells, for example, prolonged exposure to SP leads to a reduction in NK1 receptor expression that coincides with an elevated expression of miR-449b and miR-500 (105). Further bioinformatics analysis suggested NK1 receptor as a direct downstream target of both miRNAs, and overexpression of miR-449b and miR-500 in human astrocytoma cells reduced NK1 receptor expression and activity (105). This study demonstrates the possibility that GPCR-miRNA interactions can directly regulate GPCR expression in a negative feedback manner. At present, the miRNAs that regulate NK1 receptor expression in human colonic epithelial cells or other gastrointestinal cell types have not been identified.
The importance of NK1 receptor signaling in IBD pathophysiology was demonstrated by the ability of NK1 receptor antagonist to attenuate mucosal healing in an experimental colitis mouse model (64). NK1 receptor expression is also increased in colonic tissues from experimental colitis models and in the colon of IBD patients (29, 36, 99). In addition, a recent study also showed that SP/NK1 receptor signaling activation induced differential expression of 29 miRNAs in human colonic epithelial cells, including miR-221–5p (29). miR-221–5p is an oncogenic miRNA associated with prostate, breast, and colorectal cancer (34, 127, 135). In UC patients, miR-221–5p expression is increased in colonic tissues, alongside with expression of SP and NK1 receptor (29). SP/NK1 receptor-induced miR-221–5p expression is NF-κB- and JNK- dependent in human colonic epithelial cells and directly targets on expression of IL-6 receptor (IL6R), which is also downregulated in the tissues from colonic biopsies taken from UC patients, supporting the notion that IL6R is a direct target of SP/NK1 receptor-miR-221–5p (29). Thus, expression levels of SP, NK1 receptor, miR-221–5p, and IL6R can be used as markers of disease or disease progression in UC patients. Recent development of intracolonic delivery of oligonucleotides in vivo is shown to be mainly targeted at the gene expression level in colonic epithelial cells (47). With the use of this mode of delivery in experimental colitis mouse models, intracolonic administration of locked nucleic acid (LNA)-antisense-miR-221–5p exacerbated colitis development, as shown by increased proinflammatory cytokine expression and higher histology scores in colonic tissues (29). Together, these results suggest that miR-221–5p is involved in the anti-inflammatory network in colonic tissues comprised of the SP/NK1 receptor-miR-221–5p-IL6R axis.
Several studies have shown that the 13-amino peptide/hormone neurotensin (NTS) is closely associated with IBD pathophysiology. NTS is found in the ileum (97) and colon (18) while its high-affinity receptor, NTSR1, is also a GPCR member of the class A (rhodopsin-like) family (121) expressed in different colonic epithelial cell lines, colonic adenocarcinoma tissues, and the enteric nervous system (2, 16, 38). In human colonic epithelial cells, NTS/NTS1 receptor coupling activates NK-κB, MAPK signaling (131, 133, 134), and AKT (2, 130), at least in part through Rho GTPases (133) and/or transactivation of EGFR1 (134) and IGFR1 (130). Activation of these signaling pathways regulates chloride secretion (102), angiogenesis (3, 4), colonic inflammation (14, 18), wound healing (13), and tumorigenesis (2, 14). Importantly, NTS and NTS1 receptor expression is increased in inflamed colonic tissues of experimental colitis models and UC patients (13, 14, 18, 61).
Recently, miRNA-associated epigenetic regulation induced by NTS/NTS1 receptor signaling was studied in human colonic epithelial cells in vitro (2). The results demonstrated that NTS/NTS1 receptor signaling activation led to a simultaneous dysregulation of 38 miRNAs in human colonic epithelial cells, including miR-210 and miR-133α (2). miR-210 has been previously associated with hypoxia, a process that plays a crucial role in regulating mucosal homeostasis during colitis development (110), through hypoxia-inducible factor-1α (HIF-1α) (20, 25, 46, 67). On the other hand, miR-133α expression is also closely related to the development of colorectal cancer (26, 55, 87, 122), of which IBD patients have a higher risk of developing. Similar to NTS and NTS1 receptor, miR-210 and miR-133α levels are increased in colonic biopsies from UC patients (3, 69), suggesting the possibility that they can be used as biomarkers for UC. Along these lines, in vitro studies in human colonic epithelial cells show that gene silencing of miR-210 and miR-133α expression attenuates NTS-induced proinflammatory cytokine production (3, 51). Importantly, in vivo intracolonic knockdown of miR-210 and miR-133α in colon tissues alleviated colitis development and reduced proinflammatory cytokine production in colon tissues taken from experimental colitis mouse models (3, 69). In summary, blocking miR-210 and miR-133α overexpression in colonic epithelial cells ameliorates NTS/NTS1 receptor-associated colonic inflammation in vivo.
Further investigation on the functional roles of individual miRNAs revealed the cellular and molecular pathways linking the GPCR-miRNA interactions observed at the cellular level to their effects in IBD pathophysiology. Similar to other GPCRs expressed on the cell membrane, membrane-bound NTS1 receptor is internalized to the cytoplasm upon NTS binding. This leads to desensitization of the NTS1 receptor to extracellular NTS (71, 92, 93) and prevents the cells from being overstimulated. On the other hand, the cells regain sensitivity toward NTS after the internalized NTS1 receptor is recycled to the cell surface and ready for ligand binding (71). Taken together, continuous NTS/NTS1 receptor signaling transduction in colonic epithelial cells largely depends on the regulation of NTS1 receptor internalization and recycling to the cell surface. In human colonic epithelial cells, NTS/NTS1 receptor-induced miR-133α expression promotes the recycling of the internalized NTS1 receptor to the cell membrane of human colonic epithelial cells (70) through its direct downstream target, aftiphilin (AFTPH) (69), a protein localized in early endosomes and the trans-golgi network (43, 70). AFTPH is originally associated with proteins involved in intracellular trafficking, such as clathrin, AP1, and AP2 complexes (15, 43, 81), and its expression is reduced upon NTS exposure and miR-133α in human colonic epithelial cells in vitro (63). Further studies suggested that reduced AFTPH expression promotes NTS1 receptor recycling and resensitization of human colonic epithelial cells to NTS stimulation (70). The above observations suggest that NTS/NTS1 receptor-miR-133α-AFTPH interactions in colonic epithelial cells regulate NTS/NTS1 receptor signaling by promoting resensitization of the cells toward NTS stimulation, which, in turn, directly affect other NTS/NTS1 receptor effector pathways, such as NTS/NTS1 receptor-miR-210 interactions.
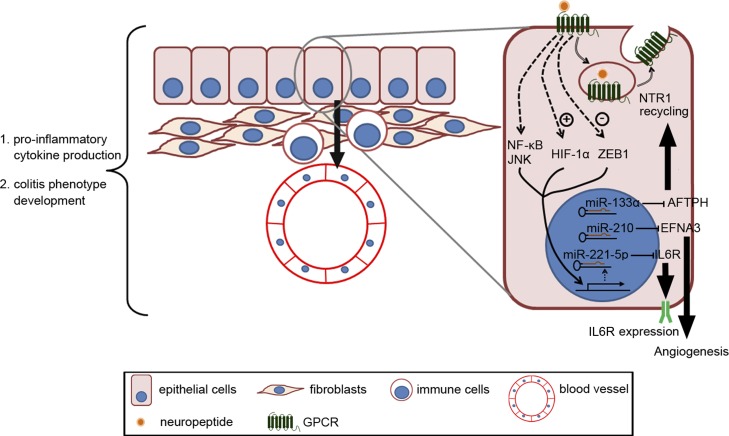
In inflamed colonic tissues of experimental colitis mouse models and IBD patients, metabolism shifts toward hypoxia, which promotes inflammation and pathogenic angiogenesis (22). A recent study shows that NTS/NTS1 receptor signaling activation promotes angiogenesis by stabilizing HIF-1α protein and increases vascular endothelial growth factor A expression in human colonic epithelial cells in vitro and in colonic tissues from experimental colitis mouse models (4). Because H1F-1α is a primary transcription regulator of miR-210 expression (25, 46, 67), NTS/NTS1 receptor signaling activation also increases miR-210 expression in human colonic epithelial cells in vitro and in inflamed colonic tissues from experimental colitis mouse models (3, 4). Moreover, NTS/NTS1 receptor-induced miR-210 expression lowers the expression of ephrin A3 (EFNA3), a direct miR-210 downstream target and an inhibitor of angiogenesis, both in vitro and in vivo (3, 30). Last, in vivo miR-210 silencing by intracolonic administration of LNA-antisense-miR-210 reduced colitis and colitis-associated angiogenesis (3). Taken together, NTS/NTS1 receptor signaling activation in colonic epithelial cells promotes angiogenesis in vitro and in colonic mucosa during colitis development, at least partially through NTS/NTS1 receptor-miR-210-EFNA3 interactions (3) (Fig. 2). Most importantly, however, these studies suggest that miR-210 represents a novel target for treatment of colonic inflammation and IBD, whereas measurements of the expression levels of miR-210 and its downstream target EFNA3 may represent potential biomarkers for UC diagnosis.
Fig. 2.
Representative diagram showing the physiological role of NK1 and neurotensin-1 (NTS1) receptor interactions with miRNAs on cellular and tissue levels. In colonic epithelial cells, substance P (SP)/NK1 receptor coupling increases miR-221–5p expression through NF-κB and c-Jun NH2-terminal kinase (JNK) activation, leading to a reduction in the expression of interleukin-6 receptor (IL6R), the downstream target of miR-221–5p. On the other hand, NTS/NTS1 receptor coupling activates hypoxia-inducible factor-1α (HIF-1α)-regulated miR-210 transcription and reduces the expression of ephrin A3 (EFNA3), a direct target of miR-210, whereas NTS/NTS1 receptor activation alleviates inhibition of miR-133α expression by zinc finger E-box-binding homeobox 1 and therefore reduces aftiphilin (AFTPH) levels. Our studies show that NTS/NTS1 receptor-associated EFNA3 reduction contributes to increased angiogenesis, whereas NTS/NTS1 receptor-associated AFTPH reduction promotes NTS1 receptor recycling in vitro. Importantly, intracolonic administration of antisense miR-221–5p exacerbates experimental colitis development, whereas intracolonic administration of antisense miR-133α and antisense miR-210 ameliorates 2,4,6-trinitrobenzenesulfonic acid-induced colonic inflammation by reducing proinflammatory cytokine production and attenuating development of colitic phenotype.
In summary, the above studies of colonic inflammation in vitro and of mouse colitis models suggest that interactions between neuropeptides, their respective GPCRs, and their downstream miRNA targets modulate cellular signaling pathways that can be self-regulatory or involved in cell-cell interactions. Thus, GPCR-miRNA interactions regulate important components of gastrointestinal mucosal homeostasis.
Irritable Bowel Syndrome
Another intestinal disorder closely related to GPCR signaling activation is irritable bowel syndrome (IBS). IBS is characterized by chronic abdominal pain or discomfort associated with changes in bowel habits, including diarrhea, constipation, or both. Thus, IBS can be further divided into IBS-D, IBS-C, and IBS-M. Although the disease mechanisms of IBS are not well understood, studies have identified a list of related risk factors, including acute psychological stress and experience of stressful life events (reviewed in Ref. 28).
Corticotropin-releasing hormone (CRH), through its receptors, CRF1 and CRF2 receptors, is an important mediator in stress-related physiological responses from the GPCR class B (secretin receptor) family. Besides CRH, other ligands of the CRF receptors include urocortin (UCN), UCN2, and UCN3. Originally identified in the central nervous system, CRF1 receptor can also be localized in myenteric and submucosal nervous plexus of the distal gut, whereas CRF2 receptors are localized in the luminal surface of crypts and myenteric neurons (reviewed in Ref. 120). Previous studies showed that chronic stress activates CRF1 receptor signaling and mediates the visceral hyperalgesia and inflammation in mesenteric adipose tissue in rats (58, 68). Although little is known about the effect of chronic stress on miRNA expression in intestine and colon, chronic water avoidance stress in rats induces visceral hyperalgesia and increases miR-17–5p expression in the dorsal horn of the spinal cord, which subsequently is found to regulate IL-6 expression and STAT3 activity (12). More importantly, reducing miR-17–5p levels in the spinal cord by intrathecal administration exacerbates stress-induced visceral hyperalgesia, supporting the notion that miR-17–5p has a potential modulatory role in stress-associated glial activity and neuroimmunomodulation (12). On the other hand, acute stress induces glucocorticoid feedback and reduces CRF1 receptor expression by increasing miR-449a levels in the rat pituitary (89). In addition, UCN2 increases miR-325–3p expression in rat pituitary cells in vitro and suppresses luteinizing hormone in rat pituitary during acute stress in vivo (88). In summary, signaling activation and expression of CRF receptors are associated with dysregulated miRNA expression in the central nervous system during acute and chronic stress and possibly mediates downstream effects in the gastrointestinal tract. Therefore, it is likely that stress regulates miRNA expression in the enteric nervous system along the gastrointestinal tract and mediates localized effects.
Opioid receptors belong to the GPCR class A (rhodopsin-like) family and are drug targets to relieve symptoms in IBS patients. Opioid receptors are expressed in the central and the enteric nervous system and classified as μ (OPRM1)-, κ (OPRK1)-, and δ (OPRD1)-receptors, with μ receptor representing a major opioid receptor in the small intestine and colon. Ligands of μ-receptors include the alkaloid morphine, which is extracted and purified from opium, and the endogenously produced ligands β-endorphin (β-endorphin, POMC), methionine-enkephalin, and leucine-encephalin ([met]- or [leu]-enkephalin, respectively, gene symbol: PENK). Along the gastrointestinal tract, μ-receptor signaling activation regulates motility, secretion, and peristalsis (reviewed in Ref. 45). Recently, studies on opioid tolerance in human neuroblastoma cells in vitro and in mouse models show that morphine treatment in vitro and in vivo increases expression of miRNAs of the let-7 family (42) and miR-103/-107 (80) and reduces μ-receptor expression by directly targeting sequences on the 3′-UTR of μ-receptor, which leads to opioid tolerance in vitro and in vivo. On the other hand, miR-134 also directly regulates μ-receptor expression in human neuroblastoma cells in vitro, whereas in a chronic inflammatory pain model miR-134 expression is reduced in the dorsal root ganglia, resulting in μ-receptor overexpression in mice (90). The above studies suggest that μ-receptor-miRNA interactions in neurons are regulated by μ-receptor signaling activation and peripheral inflammation. The role of miRNAs in the endogenous μ-opioid system expressed in the central nervous system was recently reviewed by Barbierato et al. (6). The enteric nervous system is an important component in gastrointestinal tract associated with inflammation and intestinal motility. Therefore, further studies on GPCR-miRNA interactions in the enteric nervous system will contribute to our understanding on their role in maintaining gastrointestinal homeostasis and functions.
miRNAs and Gastrointestinal Cancers
According to World Health Organization statistics, there were 774,000 and 754,000 deaths caused by colorectal cancer and gastric cancer, respectively, in 2015 (http://www.who.int/mediacentre/factsheets/fs297/en/, February 9, 2017), making these two gastrointestinal cancers the third and the fourth most common causes of cancer deaths in the world. Table 1 presents a list of GPCRs associated with gastric and colorectal cancer pathophysiology and the miRNAs involved in regulating their expression or downstream signaling pathways. These GPCRs include C-C motif chemokine receptor type 7 (CCR7) (123), C-X-C motif chemokine receptor 4 (CXCR4) (5, 27, 76, 78, 79), CCK2 receptor (cholecystokinin B receptor, CCKBR) (118), calcium-sensing receptor (CASR) (31, 117), NTS1 receptor, EP2 receptor (prostaglandin E2 receptor, PTGER2) (113), and EP4 receptor (PTGER4) (21, 113). Among these GPCRs, studies in patients and tissues from biopsy material suggest that chemokine receptors, such as CXCR4, are one of the GPCRs initiating important signaling pathways in gastrointestinal cancer pathophysiology (reviewed in Ref. 85).
Table 1.
Summary of studies related to GPCR-associated miRNAs and gastrointestinal cancers
| Pathophysiology | microRNA | Association with GPCR | Ref. No. |
|---|---|---|---|
| Gastric cancer | let-7a | Negatively correlates with CCR7 gene expression | 123 |
| miR-139 | Regulate CXCR4 levels | 5 | |
| miR-16, miR-21 | EP2- and EP4- receptors signaling regulates miR-16 and miR-21 levels | 113 | |
| Colon cancer | miR-21, miR-155 | These miRNAs are differentially regulated by NTS1 receptor signaling | 2 |
| miR-135b, miR-146b | Regulates CaS receptor | 31 | |
| miR-145, miR-21, miR-135a, miR135b | These miRNAs are differentially regulated in CaS receptor-null cells | 117 | |
| miR-101 | Regulates EP4 receptor expression | 21 | |
| miR-126 | Regulates CXCR4 expression | 76, 78, 79 | |
| miR-133b | Regulates CXCR4 expression | 27 | |
| miR-148b | Regulates CCK2 receptor expression | 118 |
miRNA, microRNA; GPCR, G protein-coupled receptor; CCR7, C-C motif chemokine receptor type 7; CXCR4, C-X-C motif chemokine receptor 4; EP2, prostaglandin E receptor 2; EP4, prostaglandin E receptor 4; NTS1, neurotensin 1 receptor; CaS, calcium-sensing receptor; CCK2, cholecystokinin B receptor. Bold type indicates GPCRs.
In gastric cancer, expression of erb-b2 receptor tyrosine kinase 2 (HER2) is associated with an increased rate of metastases in gastric cancers (23, 35). Previous studies showed that HER2/CD44 signaling activation downregulates CXCR4 expression by increasing histone deacetylation in the miR-139 promoter region (5). Importantly, two additional studies also provided evidence that miR-139 is downregulated in HER2-positive gastric carcinomas (57) and primary gastric cancer tissues from patients (39). Furthermore, similar observations were made in patients with laryngeal squamous cell carcinoma in which miR-139 also targets CXCR4 and inhibits the proliferation and metastasis of laryngeal squamous carcinoma cells (83). Results from the above studies suggest that miR-139 is a miRNA with tumor-suppressing properties; however, the functional role of miR-139 in gastric cancer in vivo needs further investigation. On the other hand, studies in colon cancer have also identified miRNAs acting as tumor suppressors by directly regulating the expression of CXCR4, such as miR-126 (76, 78, 79) and miR-133b (27). Previous studies showed that miR-126 directly targets the 3′-UTR sequence of CXCR4 in colon cancer cells (76, 78). Low expression of miR-126 is associated with poor prognosis (79) and increased migration and invasion of colon cancer cells (76). Furthermore, increased miR-126 levels in human colon cancer cells in vitro reduce cell migration and invasion by reducing CXCR4 expression (76, 78), possibly by suppressing AKT and ERK1/2 activation (78). On the other hand, expression of miR-133b and CXCR4 is inversely correlated in human colorectal cancer tissues compared with nonneoplastic adjacent tissues (27). In addition, miR-133b also directly regulates CXCR4-associated cell invasion and migration in vitro (27). These studies suggested that increased CXCR4 is associated with increased metastasis and poor prognosis in human colon cancer, possibly by promoting cell migration resulting from the downregulation of tumor suppressor miR-126 and miR-133b. In the above examples, miRNA expression induced by upstream signaling events or endogenously expressed miRNAs directly regulate CXCR4 expression in gastrointestinal cancer cells and thus affect CXCR4-mediated cellular functions along the gastrointestinal tract.
As noted in the examples of NK1 receptor (105) and CXCR4 (27, 76, 78, 79), expression of these GPCRs can be simultaneously targeted by two miRNAs in the same cell type. The recent development of high-throughput gene expression analysis and the subsequent bioinformatics analysis have enabled researchers to study the complex pattern and dynamic nature of gene expression in many tissue and cell types. Results from these studies revealed that miRNA-mRNA interactions often participate in molecular regulatory circuits. NTS1 receptor signaling pathways in human colon cancer cells are discussed below as an example.
NTS1 receptor activation in human colonic epithelial cells induced dysregulation of the expression of 38 miRNAs simultaneously (2). Among these miRNAs, NTS-induced miR-21 and miR-155 expression suppresses the expression of phosphatase and tensin homolog (PTEN) and suppressor of cytokine signaling, which represent downstream targets of miR-21 and miR-155, respectively (49, 54), in human colorectal carcinoma HCT-116 and adenocarcinoma DLD-1 cells (2). Whereas NTS/NTS1 receptor signaling has been shown to activate AKT in human colonic epithelial cells (130), PTEN is also a well-established negative regulator of AKT activity. Therefore, this study provides evidence that miR-21 is involved in NTS-induced AKT activation through suppressing PTEN expression. More importantly, this study also revealed a synergistic effect of AKT activation by NTS-induced miR-21 and miR-155 overexpression. Bioinformatics analysis suggested that miR-155 directly inhibited the expression of protein phosphatase 2 catalytic subunit-α (PPP2CA), which is another well-known negative regulator of AKT activity (100). Subsequent examination showed that NTS/NTS1 receptor-miR-21-miR-155 interactions regulate cell proliferation, migration, and invasion in vitro and in mouse cancer xenograft models (2). Last, this study also identified a positive feedback loop regulating NTS1 receptor-associated NF-κB and AKT activities by showing increased AKT activity suppresses NT-induced NF-κB activation. Importantly, this positive feedback loop is regulated by NTS-induced miR-21 and miR-155. Taken together, NTS/NTS1 receptor-miR-21-miR-155 interactions alleviate suppression on PTEN and PPP2CA expression and thus activate AKT and initiate a positive feedback loop of NTS/NTS1 receptor signaling in human colon cancer cells.
Stem Cells
Accumulated evidence from studies on various GPCRs indicate that GPCR-miRNA interactions help maintain important aspects of gastrointestinal homeostasis, including cell proliferation (84, 111), immune tolerance (24), and angiogenesis (60, 106, 136) (summarized in Table 2). Recently, intestinal stem cells became an important component of mucosal healing. Moreover, primary intestinal stem cells taken from different mouse models can now be routinely differentiated into organoids and used as tools to study intestinal epithelial cell biology. Leucine-rich repeat-containing GPCR 5 (LGR5) is an important marker of small intestinal and colonic stem cells for crypt regeneration and in vitro expansion (7, 107, 128). It is a class A (rhodopsin-like) GPCR and a part of the Wnt signaling pathway expressed in intestinal and colonic stem cells (7) and colonic adenomas (10). Previous studies showed that miR-142–3p and miR-100 directly target the 3′-UTR of LGR5 gene in colon cancer cells (111, 136) and therefore have been proposed as tumor suppressors. Importantly, both miRs are downregulated in colon cancer tissues from the patients (111, 136). Furthermore, miR-142-LGR5 interaction in colon cancer cells is associated with drug-induced apoptosis in vitro (111). However, these particular interactions have yet to be confirmed in normal intestinal and colonic stem cells in vitro and in vivo. Understanding the interactions between miRNAs and LGR5 may provide insights to the epigenetic regulation in intestinal and colonic stem cells during normal crypt regeneration and healing processes.
Table 2.
Smmary of studies related to GPCRs, their associated miRNAs, and their proposed physiological roles in gastrointestinal studies
| Function | miRNA | Association with GPCR | Ref. No. |
|---|---|---|---|
| Proliferation | miR-142–3p | Regulate CD133, ABCG2, and LGR5 | 111 |
| miR-34a | Regulate PAR2-induced CyclinD1 | 84 | |
| Monocytes/immune tolerance | miR-525–5p | Negatively regulates VPAC1 receptor expression | 24 |
| Angiogenesis | miR-802 | Regulates AT1 receptor expression | 106 |
| miR-27b, miR-218a | Regulates A2B receptor expression | 60 | |
| miR-100 | Inhibit LGR5 expression | 136 |
LGR5, leucine-rich repeat-containing G protein-coupled receptor 5; PAR2, protein-activated receptor 2; VPAC1, vasoactive intestinal peptide receptor 1; AT1, angiotensin II receptor type 1; A2B, adenosine A2b receptor. Bold type indicates GPCRs.
Clinical Implication of GPCR-miRNA Interactions.
As discussed in this review, studies with miRNAs identified novel GPCR signaling pathways as participating in several important gastrointestinal responses. Given the importance of GPCR signaling activation in gastrointestinal pathophysiology, components of GPCR signaling pathways, including miRNAs, can be potentially developed into therapeutic targets or biomarkers of drug efficacy and gastrointestinal disease modulation. From the clinical perspective, because GPCR signaling pathways in gastrointestinal tissues are highly regulated and organized in molecular regulatory circuits, measuring biomarkers developed from a combination of miRNAs and other GPCR signaling components may represent a more comprehensive view for gastrointestinal disease prognosis. In the case of human colorectal cancer, the study on NTS1 receptor-associated miRNA expression in human colon cancer cells provided evidence showing that the NTS-induced signaling pathway is activated in a molecular regulatory circuit involving increased expression of miR-155 and miR-21 and AKT activity in vitro (2). To associate these gene expression changes to human colon cancer development, expression of NTS1 receptor, miR-155, and miR-21 was examined and found upregulated significantly in human colon tumors compared with normal colon tissues. Furthermore, the expression of these two miRNAs is positively correlated to NTS1 receptor expression in human colon tumors, whereas the expression of all these genes is also correlated with tumor stages (2). Interestingly, miR-21 expression has also been shown to be increased in 5-fluorouracil-resistant human colon cancer cells and negatively correlated to patient survival (91). Taken together, combined use of NTS1 receptor, miR-155, and miR-21 expression in human colon tumors as biomarkers may serve as a useful indicator to the disease state and possibly response to treatment.
Alternatively, miRNAs could by themselves serve as drug targets, since therapies directed against the downstream mediators of GPCR signaling may provide more selective targeting of aberrant signaling cascades implicated in gastrointestinal pathophysiology. Although, in theory, delivery of antisense oligonucleotides to the appropriate tissue targets can neutralize the effects of the corresponding miRNAs, unmodified antisense oligonucleotides are prone to degradation by serum exonucleases and cellular endonucleases and have relatively poor binding affinity to tissues. Thus, antisense oligonucleotides with various chemical modifications, such as 2′-O-methyl modification of nucleotides, formation of phosphorothioate bonds by replacing nonbridging oxygen atoms in the phosphate backbone with sulfur atoms, and LNA modifications that lock the sugar structure of the nucleotide into a 3′-endo conformation, are devised to optimize the nuclease resistance of antisense oligonucleotides and their binding affinity to tissues (reviewed in Ref. 77). These modified antisense miRNAs have shown great promise in bringing therapies directed against miRNAs to clinical use. In a phase 2a study, a LNA-modified DNA phosphorothioate antisense oligonucleotide directed against miR-122 was developed to sequester mature miR-122 and inhibit its function in the context of hepatitis C virus (HCV) infection (53). The results suggested that the chemically optimized antisense miR-122 elicited a durable dose-dependent reduction in HCV RNA levels without evidence of viral resistance (53), opening up the possibilities for therapeutic use of antisense miRNA approaches in patients. Last, because various GPCRs, such as the ones mentioned in this review, are overexpressed in gastrointestinal cell types in different disease states, the recent development of surface receptor-targeted delivery using nanovectors may provide increased cellular specificity to the antisense oligonucleotide delivery. Although, at present, there are no known studies of miRNA delivery using nanovectors targeting GPCRs, results from two recent studies suggested the potential of target delivery of miRNAs using GPCRs as targets. In one study, Li et al. showed that administration of nanovectors labeled with antibodies against surface markers of pancreatic cancer cells encapsulating antisense miR-21 showed increased cellular uptake of antisense miR-21 and inhibited tumor growth in mice (75). In another study, nanovectors conjugated with octreotide, a synthetic ligand of somatostatin receptors, have successfully targeted somatostatin receptor-overexpressing tumor cells (95). Taken together, development of nanovectors conjugated with GPCR ligands or labeled with antibodies against GPCRs for delivering chemically optimized miRNAs may be one of the potential therapeutic strategies for GPCR-associated pathophysiology.
In summary, studies on GPCR-miRNA interactions may also lead to novel biomarkers or pharmaceutical interventions targeting gastrointestinal disorders. These studies suggest the possibility of using miRNAs in clinical settings, such as biomarkers or drugs that intervene with GPCR-associated miRNA functioning in disease pathology.
Conclusions
GPCR signaling plays a major role in gastrointestinal pathophysiology and is targeted by various pharmacological agents. miRNAs are a new class of negative regulators regulating gene expression. In this review, studies on miRNAs regulating GPCR expression and miRNA expression induced by GPCR signaling activation in cell types residing in gastrointestinal tracts were discussed. Although miRNAs are non-protein-coding nucleotides, results demonstrating that GPCR signaling activation induces expression of multiple miRNAs and the ability of a miRNA to target on 3′-UTRs of different genes make miRNA an important amplifier of the GPCR signaling cascade. Furthermore, GPCR-miRNA interactions occurring in a single cell type in the gastrointestinal tract can potentially regulate physiology at both cellular and tissue levels because of the close proximity of different cell types and their frequent interactions within the gastrointestinal tract. This complex network of gene regulation may explain the complexity of interactions modulating gastrointestinal homeostasis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-60729, DK-47373, CURE:DDRC P30-DK-41301(C. Pothoulakis), DK-110003 (D. Iliopoulos), T32-AM-41301 (D. Padua), fellowships from the Crohn’s and Colitis Foundation of America (I. K. M. Law), the Blinder Research Foundation for Crohn’s Disease (C. Pothoulakis), and the Eli and Edythe Broad Chair (C. Pothoulakis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.K.L. prepared figures; I.K.L. drafted manuscript; I.K.L., D.M.P., D.I., and C.P. edited and revised manuscript; C.P. approved final version of manuscript.
REFERENCES
- 1.Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Southan C, Davies JA, Collaborators C; CGTP Collaborators . The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. Br J Pharmacol 172: 5744–5869, 2015. doi: 10.1111/bph.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakirtzi K, Hatziapostolou M, Karagiannides I, Polytarchou C, Jaeger S, Iliopoulos D, Pothoulakis C. Neurotensin signaling activates microRNAs-21 and −155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology 141: 1749–1761, 2011. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakirtzi K, Law IK, Xue X, Iliopoulos D, Shah YM, Pothoulakis C. Neurotensin Promotes the Development of Colitis and Intestinal Angiogenesis via Hif-1α-miR-210 Signaling. J Immunol 196: 4311–4321, 2016. doi: 10.4049/jimmunol.1501443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakirtzi K, West G, Fiocchi C, Law IK, Iliopoulos D, Pothoulakis C. The neurotensin-HIF-1α-VEGFα axis orchestrates hypoxia, colonic inflammation, and intestinal angiogenesis. Am J Pathol 184: 3405–3414, 2014. doi: 10.1016/j.ajpath.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao W, Fu HJ, Xie QS, Wang L, Zhang R, Guo ZY, Zhao J, Meng YL, Ren XL, Wang T, Li Q, Jin BQ, Yao LB, Wang RA, Fan DM, Chen SY, Jia LT, Yang AG. HER2 interacts with CD44 to up-regulate CXCR4 via epigenetic silencing of microRNA-139 in gastric cancer cells. Gastroenterology 141: 2076–2087, 2011. doi: 10.1053/j.gastro.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Barbierato M, Zusso M, Skaper SD, Giusti P. MicroRNAs: emerging role in the endogenous μ opioid system. CNS Neurol Disord Drug Targets 14: 239–250, 2015. doi: 10.2174/1871527314666150116123932. [DOI] [PubMed] [Google Scholar]
- 7.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker L, Huang Q, Mashimo H. Immunostaining of Lgr5, an intestinal stem cell marker, in normal and premalignant human gastrointestinal tissue. Sci World J 8: 1168–1176, 2008. doi: 10.1100/tsw.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bortolin-Cavaillé ML, Dance M, Weber M, Cavaillé J. C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res 37: 3464–3473, 2009. doi: 10.1093/nar/gkp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradesi S, Karagiannides I, Bakirtzi K, Joshi SM, Koukos G, Iliopoulos D, Pothoulakis C, Mayer EA. Identification of spinal cord microRNA and gene signatures in a model of chronic stress-induced visceral hyperalgesia in rat. PLoS One 10: e0130938, 2015. doi: 10.1371/journal.pone.0130938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brun P, Mastrotto C, Beggiao E, Stefani A, Barzon L, Sturniolo GC, Palù G, Castagliuolo I. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 288: G621–G629, 2005. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 14.Bugni JM, Rabadi LA, Jubbal K, Karagiannides I, Lawson G, Pothoulakis C. The neurotensin receptor-1 promotes tumor development in a sporadic but not an inflammation-associated mouse model of colon cancer. Int J Cancer 130: 1798–1805, 2012. doi: 10.1002/ijc.26208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burman JL, Wasiak S, Ritter B, de Heuvel E, McPherson PS. Aftiphilin is a component of the clathrin machinery in neurons. FEBS Lett 579: 2177–2184, 2005. doi: 10.1016/j.febslet.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Carraway R, Leeman SE. Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem 251: 7045–7052, 1976. [PubMed] [Google Scholar]
- 17.Castagliuolo I, Morteau O, Keates AC, Valenick L, Wang CC, Zacks J, Lu B, Gerard NP, Pothoulakis C. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. Br J Pharmacol 136: 271–279, 2002. doi: 10.1038/sj.bjp.0704697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castagliuolo I, Wang CC, Valenick L, Pasha A, Nikulasson S, Carraway RE, Pothoulakis C. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. J Clin Invest 103: 843–849, 1999. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattaneo F, Guerra G, Parisi M, De Marinis M, Tafuri D, Cinelli M, Ammendola R. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int J Mol Sci 15: 19700–19728, 2014. doi: 10.3390/ijms151119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation 19: 215–223, 2012. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandramouli A, Onyeagucha BC, Mercado-Pimentel ME, Stankova L, Shahin NA, LaFleur BJ, Heimark RL, Bhattacharyya AK, Nelson MA. MicroRNA-101 (miR-101) post-transcriptionally regulates the expression of EP4 receptor in colon cancers. Cancer Biol Ther 13: 175–183, 2012. doi: 10.4161/cbt.13.3.18874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chidlow JH Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol 293: G5–G18, 2007. doi: 10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- 23.Choi Y, Ko YS, Park J, Choi Y, Kim Y, Pyo JS, Jang BG, Hwang DH, Kim WH, Lee BL. HER2-induced metastasis is mediated by AKT/JNK/EMT signaling pathway in gastric cancer. World J Gastroenterol 22: 9141–9153, 2016. doi: 10.3748/wjg.v22.i41.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cocco E, Paladini F, Macino G, Fulci V, Fiorillo MT, Sorrentino R. The expression of vasoactive intestinal peptide receptor 1 is negatively modulated by microRNA 525-5p. PLoS One 5: e12067, 2010. doi: 10.1371/journal.pone.0012067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devlin C, Greco S, Martelli F, Ivan M. miR-210: More than a silent player in hypoxia. IUBMB Life 63: 94–100, 2011. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Zhao J, Wu CW, Zhang L, Liu X, Kang W, Leung WW, Zhang N, Chan FK, Sung JJ, Ng SS, Yu J. Tumor suppressor functions of miR-133a in colorectal cancer. Mol Cancer Res 11: 1051–1060, 2013. doi: 10.1158/1541-7786.MCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 27.Duan F-T, Qian F, Fang K, Lin K-Y, Wang W-T, Chen Y-Q. miR-133b, a muscle-specific microRNA, is a novel prognostic marker that participates in the progression of human colorectal cancer via regulation of CXCR4 expression. Mol Cancer 12: 164, 2013. doi: 10.1186/1476-4598-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers 2: 16014, 2016. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang K, Sideri A, Law IK, Bakirtzi K, Polytarchou C, Iliopoulos D, Pothoulakis C. Identification of a novel substance P (SP)-neurokinin-1 receptor (NK-1R) microRNA-221–5p inflammatory network in human colonic epithelial cells. Cell Mol Gastroenterol Hepatol 1: 503–515, 2015. doi: 10.1016/j.jcmgh.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fetahu IS, Tennakoon S, Lines KE, Gröschel C, Aggarwal A, Mesteri I, Baumgartner-Parzer S, Mader RM, Thakker RV, Kállay E. miR-135b- and miR-146b-dependent silencing of calcium-sensing receptor expression in colorectal tumors. Int J Cancer 138: 137–145, 2016. doi: 10.1002/ijc.29681. [DOI] [PubMed] [Google Scholar]
- 32.Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63: 1256–1272, 2003. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 33.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galardi S, Mercatelli N, Farace MG, Ciafrè SA. NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res 39: 3892–3902, 2011. doi: 10.1093/nar/gkr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galdy S, Lamarca A, McNamara MG, Hubner RA, Cella CA, Fazio N, Valle JW. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev 36: 141–157, 2016. doi: 10.1007/s10555-016-9645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goode T, O’Connor T, Hopkins A, Moriarty D, O’Sullivan GC, Collins JK, O’Donoghue D, Baird AW, O’Connell J, Shanahan F. Neurokinin-1 receptor (NK-1R) expression is induced in human colonic epithelial cells by proinflammatory cytokines and mediates proliferation in response to substance P. J Cell Physiol 197: 30–41, 2003. doi: 10.1002/jcp.10234. [DOI] [PubMed] [Google Scholar]
- 37.Gross K, Karagiannides I, Thomou T, Koon HW, Bowe C, Kim H, Giorgadze N, Tchkonia T, Pirtskhalava T, Kirkland JL, Pothoulakis C. Substance P promotes expansion of human mesenteric preadipocytes through proliferative and antiapoptotic pathways. Am J Physiol Gastrointest Liver Physiol 296: G1012–G1019, 2009. doi: 10.1152/ajpgi.90351.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gui X, Guzman G, Dobner PR, Kadkol SS. Increased neurotensin receptor-1 expression during progression of colonic adenocarcinoma. Peptides 29: 1609–1615, 2008. doi: 10.1016/j.peptides.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, Wang Y. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol 24: 652–657, 2009. doi: 10.1111/j.1440-1746.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 40.Habtezion A, Nguyen LP, Hadeiba H, Butcher EC. Leukocyte trafficking to the small intestine and colon. Gastroenterology 150: 340–354, 2016. doi: 10.1053/j.gastro.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev 87: 3–14, 2015. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci 30: 10251–10258, 2010. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirst J, Borner GH, Harbour M, Robinson MS. The aftiphilin/p200/gamma-synergin complex. Mol Biol Cell 16: 2554–2565, 2005. doi: 10.1091/mbc.E04-12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman JM, Baritaki S, Ruiz JJ, Sideri A, Pothoulakis C. Corticotropin-releasing hormone receptor 2 signaling promotes mucosal repair responses after colitis. Am J Pathol 186: 134–144, 2016. doi: 10.1016/j.ajpath.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept 155: 11–17, 2009. doi: 10.1016/j.regpep.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X, Le QT, Giaccia AJ. MiR-210–micromanager of the hypoxia pathway. Trends Mol Med 16: 230–237, 2010. doi: 10.1016/j.molmed.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi H, Dong L, Zhang C, Zeng K, Chen J, Zhang J. miR-141 Regulates colonic leukocytic trafficking by targeting CXCL12β during murine colitis and human Crohn’s disease. Gut 63: 1247–1257, 2014. doi: 10.1136/gutjnl-2012-304213. [DOI] [PubMed] [Google Scholar]
- 48.Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293: 834–838, 2001. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 49.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 39: 493–506, 2010. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Im E, Rhee SH, Park YS, Fiocchi C, Tache Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology 138: 2457–2467, 2010. doi: 10.1053/j.gastro.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SGF, Sunahara RK, El-Samad H, Huang B, von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature 495: 534–538, 2013. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jafri MA, Zaidi SK, Ansari SA, Al-Qahtani MH, Shay JW. MicroRNAs as potential drug targets for therapeutic intervention in colorectal cancer. Expert Opin Ther Targets 19: 1705–1723, 2015. doi: 10.1517/14728222.2015.1069816. [DOI] [PubMed] [Google Scholar]
- 53.Janssen HLA, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med 368: 1685–1694, 2013. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 54.Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu H, Liu MF, Wang ED. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res 70: 3119–3127, 2010. doi: 10.1158/0008-5472.CAN-09-4250. [DOI] [PubMed] [Google Scholar]
- 55.Josse C, Bouznad N, Geurts P, Irrthum A, Huynh-Thu VA, Servais L, Hego A, Delvenne P, Bours V, Oury C. Identification of a microRNA landscape targeting the PI3K/Akt signaling pathway in inflammation-induced colorectal carcinogenesis. Am J Physiol Gastrointest Liver Physiol 306: G229–G243, 2014. doi: 10.1152/ajpgi.00484.2012. [DOI] [PubMed] [Google Scholar]
- 56.Kalla R, Ventham NT, Kennedy NA, Quintana JF, Nimmo ER, Buck AH, Satsangi J. MicroRNAs: new players in IBD. Gut 64: 504–517, 2015. doi: 10.1136/gutjnl-2014-307891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang HS, Kim J, Jang SG, Kwon SY, Park YS, Green JE, Kim HK, Ro J. MicroRNA signature for HER2-positive breast and gastric cancer. Anticancer Res 34: 3807–3810, 2014. [PubMed] [Google Scholar]
- 58.Karagiannides I, Golovatscka V, Bakirtzi K, Sideri A, Salas M, Stavrakis D, Polytarchou C, Iliopoulos D, Pothoulakis C, Bradesi S. Chronic unpredictable stress regulates visceral adipocyte-mediated glucose metabolism and inflammatory circuits in male rats. Physiol Rep 2: e00284, 2014. doi: 10.14814/phy2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139, 2009. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 60.Kolachala VL, Wang L, Obertone TS, Prasad M, Yan Y, Dalmasso G, Gewirtz AT, Merlin D, Sitaraman SV. Adenosine 2B receptor expression is post-transcriptionally regulated by microRNA. J Biol Chem 285: 18184–18190, 2010. doi: 10.1074/jbc.M109.066555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koon H-W, Kim YS, Xu H, Kumar A, Zhao D, Karagiannides I, Dobner PR, Pothoulakis C. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proc Natl Acad Sci USA 106: 8766–8771, 2009. doi: 10.1073/pnas.0903499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koon HW, Shih D, Karagiannides I, Zhao D, Fazelbhoy Z, Hing T, Xu H, Lu B, Gerard N, Pothoulakis C. Substance P modulates colitis-associated fibrosis. Am J Pathol 177: 2300–2309, 2010. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem 279: 45519–45527, 2004. doi: 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- 64.Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA 104: 2013–2018, 2007. doi: 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koukos G, Polytarchou C, Kaplan JL, Oikonomopoulos A, Ziring D, Hommes DW, Wahed R, Kokkotou E, Pothoulakis C, Winter HS, Iliopoulos D. A microRNA signature in pediatric ulcerative colitis: deregulation of the miR-4284/CXCL5 pathway in the intestinal epithelium. Inflamm Bowel Dis 21: 996–1005, 2015. doi: 10.1097/MIB.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42, Suppl D1: D68–D73, 2014. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867, 2007. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larauche M, Bradesi S, Million M, McLean P, Taché Y, Mayer EA, McRoberts JA. Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am J Physiol Gastrointest Liver Physiol 294: G1033–G1040, 2008. doi: 10.1152/ajpgi.00507.2007. [DOI] [PubMed] [Google Scholar]
- 69.Law IK, Bakirtzi K, Polytarchou C, Oikonomopoulos A, Hommes D, Iliopoulos D, Pothoulakis C. Neurotensin–regulated miR-133α is involved in proinflammatory signalling in human colonic epithelial cells and in experimental colitis. Gut 64: 1095–1104, 2015. doi: 10.1136/gutjnl-2014-307329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Law IK, Jensen D, Bunnett NW, Pothoulakis C. Neurotensin-induced miR-133α expression regulates neurotensin receptor 1 recycling through its downstream target aftiphilin. Sci Rep 6: 22195, 2016. doi: 10.1038/srep22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Law IK, Murphy JE, Bakirtzi K, Bunnett NW, Pothoulakis C. Neurotensin-induced proinflammatory signaling in human colonocytes is regulated by β-arrestins and endothelin-converting enzyme-1-dependent endocytosis and resensitization of neurotensin receptor 1. J Biol Chem 287: 15066–15075, 2012. doi: 10.1074/jbc.M111.327262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature 425: 415–419, 2003. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 73.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23: 4051–4060, 2004. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Chen Y, Li J, Zhang Z, Huang C, Lian G, Yang K, Chen S, Lin Y, Wang L, Huang K, Zeng L. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci 108: 1493–1503, 2017. doi: 10.1111/cas.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z, Li N, Wu M, Li X, Luo Z, Wang X. Expression of miR-126 suppresses migration and invasion of colon cancer cells by targeting CXCR4. Mol Cell Biochem 381: 233–242, 2013. doi: 10.1007/s11010-013-1707-6. [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov 13: 622–638, 2014. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Zhou Y, Feng X, An P, Quan X, Wang H, Ye S, Yu C, He Y, Luo H. MicroRNA-126 functions as a tumor suppressor in colorectal cancer cells by targeting CXCR4 via the AKT and ERK1/2 signaling pathways. Int J Oncol 44: 203–210, 2014. doi: 10.3892/ijo.2013.2168. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Zhou Y, Feng X, Yang P, Yang J, An P, Wang H, Ye S, Yu C, He Y, Luo H. Low expression of microRNA-126 is associated with poor prognosis in colorectal cancer. Genes Chromosomes Cancer 53: 358–365, 2014. doi: 10.1002/gcc.22146. [DOI] [PubMed] [Google Scholar]
- 80.Lu Z, Xu J, Xu M, Pasternak GW, Pan YX. Morphine regulates expression of μ-opioid receptor MOR-1A, an intron-retention carboxyl terminal splice variant of the μ-opioid receptor (OPRM1) gene via miR-103/miR-107. Mol Pharmacol 85: 368–380, 2014. doi: 10.1124/mol.113.089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lui-Roberts WW, Ferraro F, Nightingale TD, Cutler DF. Aftiphilin and gamma-synergin are required for secretagogue sensitivity of Weibel-Palade bodies in endothelial cells. Mol Biol Cell 19: 5072–5081, 2008. doi: 10.1091/mbc.E08-03-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science 303: 95–98, 2004. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 83.Luo HN, Wang ZH, Sheng Y, Zhang Q, Yan J, Hou J, Zhu K, Cheng Y, Xu YL, Zhang XH, Xu M, Ren XY. MiR-139 targets CXCR4 and inhibits the proliferation and metastasis of laryngeal squamous carcinoma cells. Med Oncol 31: 789, 2014. doi: 10.1007/s12032-013-0789-z. [DOI] [PubMed] [Google Scholar]
- 84.Ma Y, Bao-Han W, Lv X, Su Y, Zhao X, Yin Y, Zhang X, Zhou Z, MacNaughton WK, Wang H. MicroRNA-34a mediates the autocrine signaling of PAR2-activating proteinase and its role in colonic cancer cell proliferation. PLoS One 8: e72383, 2013. doi: 10.1371/journal.pone.0072383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyazaki H, Takabe K, Yeudall WA. Chemokines, chemokine receptors and the gastrointestinal system. World J Gastroenterol 19: 2847–2863, 2013. doi: 10.3748/wjg.v19.i19.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mosińska P, Zielińska M, Fichna J. Expression and physiology of opioid receptors in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 23: 3–10, 2016. doi: 10.1097/MED.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 87.Necela BM, Carr JM, Asmann YW, Thompson EA. Differential expression of microRNAs in tumors from chronically inflamed or genetic (APC(Min/+)) models of colon cancer. PLoS One 6: e18501, 2011. doi: 10.1371/journal.pone.0018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nemoto T, Mano A, Shibasaki T. Increased expression of miR-325-3p by urocortin 2 and its involvement in stress-induced suppression of LH secretion in rat pituitary. Am J Physiol Endocrinol Metab 302: E781–E787, 2012. doi: 10.1152/ajpendo.00616.2011. [DOI] [PubMed] [Google Scholar]
- 89.Nemoto T, Mano A, Shibasaki T. miR-449a contributes to glucocorticoid-induced CRF-R1 downregulation in the pituitary during stress. Mol Endocrinol 27: 1593–1602, 2013. doi: 10.1210/me.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ni J, Gao Y, Gong S, Guo S, Hisamitsu T, Jiang X. Regulation of μ-opioid type 1 receptors by microRNA134 in dorsal root ganglion neurons following peripheral inflammation. Eur J Pain 17: 313–323, 2013. doi: 10.1002/j.1532-2149.2012.00197.x. [DOI] [PubMed] [Google Scholar]
- 91.Nijhuis A, Thompson H, Adam J, Parker A, Gammon L, Lewis A, Bundy JG, Soga T, Jalaly A, Propper D, Jeffery R, Suraweera N, McDonald S, Thaha MA, Feakins R, Lowe R, Bishop CL, Silver A. Remodelling of microRNAs in colorectal cancer by hypoxia alters metabolism profiles and 5-fluorouracil resistance. Hum Mol Genet 26: 1552–1564, 2017. doi: 10.1093/hmg/ddx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem 276: 19452–19460, 2001. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 93.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275: 17201–17210, 2000. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 94.Pavlos NJ, Friedman PA. GPCR signaling and trafficking: the long and short of it. Trends Endocrinol Metab 28: 213–226, 2017. doi: 10.1016/j.tem.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peng J, Qi X, Chen Y, Ma N, Zhang Z, Xing J, Zhu X, Li Z, Wu Z. Octreotide-conjugated PAMAM for targeted delivery to somatostatin receptors over-expressed tumor cells. J Drug Target 22: 428–438, 2014. doi: 10.3109/1061186X.2013.879386. [DOI] [PubMed] [Google Scholar]
- 96.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650, 2002. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 97.Polak JM, Sullivan SN, Bloom SR, Buchan AM, Facer P, Brown MR, Pearse AG. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature 270: 183–184, 1977. doi: 10.1038/270183a0. [DOI] [PubMed] [Google Scholar]
- 98.Polytarchou C, Hommes DW, Palumbo T, Hatziapostolou M, Koutsioumpa M, Koukos G, van der Meulen-de Jong AE, Oikonomopoulos A, van Deen WK, Vorvis C, Serebrennikova OB, Birli E, Choi J, Chang L, Anton PA, Tsichlis PN, Pothoulakis C, Verspaget HW, Iliopoulos D. MicroRNA214 is associated with progression of ulcerative colitis, and inhibition reduces development of colitis and colitis-associated cancer in mice. Gastroenterology 149: 981–92.e11, 2015. doi: 10.1053/j.gastro.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Renzi D, Pellegrini B, Tonelli F, Surrenti C, Calabrò A. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol 157: 1511–1522, 2000. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Resjö S, Göransson O, Härndahl L, Zolnierowicz S, Manganiello V, Degerman E. Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cell Signal 14: 231–238, 2002. doi: 10.1016/S0898-6568(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 101.Riegler M, Castagliuolo I, So PT, Lotz M, Wang C, Wlk M, Sogukoglu T, Cosentini E, Bischof G, Hamilton G, Teleky B, Wenzl E, Matthews JB, Pothoulakis C. Effects of substance P on human colonic mucosa in vitro. Am J Physiol 276: G1473–G1483, 1999. [DOI] [PubMed] [Google Scholar]
- 102.Riegler M, Castagliuolo I, Wang C, Wlk M, Sogukoglu T, Wenzl E, Matthews JB, Pothoulakis C. Neurotensin stimulates Cl(-) secretion in human colonic mucosa In vitro: role of adenosine. Gastroenterology 119: 348–357, 2000. doi: 10.1053/gast.2000.9310. [DOI] [PubMed] [Google Scholar]
- 103.Rodriguez JA, Huerta-Yepez S, Law IK, Baay-Guzman GJ, Tirado-Rodriguez B, Hoffman JM, Iliopoulos D, Hommes DW, Verspaget HW, Chang L, Pothoulakis C, Baritaki S. Diminished expression of CRHR2 in human colon cancer promotes tumor growth and EMT via persistent IL-6/Stat3 signaling. Cell Mol Gastroenterol Hepatol 1: 610–630, 2015. doi: 10.1016/j.jcmgh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rozengurt E. Signaling pathways induced by g-protein-coupled receptors. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Elsevier, 2012, p. 75–96. [Google Scholar]
- 105.Sanchez Freire V, Burkhard FC, Kessler TM, Kuhn A, Draeger A, Monastyrskaya K. MicroRNAs may mediate the down-regulation of neurokinin-1 receptor in chronic bladder pain syndrome. Am J Pathol 176: 288–303, 2010. doi: 10.2353/ajpath.2010.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sansom SE, Nuovo GJ, Martin MM, Kotha SR, Parinandi NL, Elton TS. miR-802 regulates human angiotensin II type 1 receptor expression in intestinal epithelial C2BBe1 cells. Am J Physiol Gastrointest Liver Physiol 299: G632–G642, 2010. doi: 10.1152/ajpgi.00120.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 108.Schiöth HB, Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol 142: 94–101, 2005. doi: 10.1016/j.ygcen.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 109.Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature 455: 58–63, 2008. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 110.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 54: 17–28, 2003. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 111.Shen W-W, Zeng Z, Zhu W-X, Fu G-H. MiR-142-3p functions as a tumor suppressor by targeting CD133, ABCG2, and Lgr5 in colon cancer cells. J Mol Med (Berl) 91: 989–1000, 2013. doi: 10.1007/s00109-013-1037-x. [DOI] [PubMed] [Google Scholar]
- 113.Shin VY, Jin H, Ng EKO, Cheng ASL, Chong WWS, Wong CYP, Leung WK, Sung JJY, Chu K-M. NF-κB targets miR-16 and miR-21 in gastric cancer: involvement of prostaglandin E receptors. Carcinogenesis 32: 240–245, 2011. doi: 10.1093/carcin/bgq240. [DOI] [PubMed] [Google Scholar]
- 114.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci 36: 457–469, 2011. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sideri A, Bakirtzi K, Shih DQ, Koon HW, Fleshner P, Arsenescu R, Arsenescu V, Turner JR, Karagiannides I, Pothoulakis C. Substance P mediates pro-inflammatory cytokine release form mesenteric adipocytes in inflammatory bowel disease patients. Cell Mol Gastroenterol Hepatol 1: 420–432, 2015. doi: 10.1016/j.jcmgh.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang CC, Mykoniatis A, Pasha A, Valenick L, Sougioultzis S, Zhao D, Pothoulakis C. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci USA 100: 2957–2962, 2003. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh N, Liu G, Chakrabarty S. Isolation and characterization of calcium sensing receptor null cells: a highly malignant and drug resistant phenotype of colon cancer. Int J Cancer 132: 1996–2005, 2013. doi: 10.1002/ijc.27902. [DOI] [PubMed] [Google Scholar]
- 118.Song Y, Xu Y, Wang Z, Chen Y, Yue Z, Gao P, Xing C, Xu H. MicroRNA-148b suppresses cell growth by targeting cholecystokinin-2 receptor in colorectal cancer. Int J Cancer 131: 1042–1051, 2012. doi: 10.1002/ijc.26485. [DOI] [PubMed] [Google Scholar]
- 119.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 94: 265–301, 2014. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stengel A, Taché Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp Biol Med (Maywood) 235: 1168–1178, 2010. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron 4: 847–854, 1990. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- 122.Wang H, An H, Wang B, Liao Q, Li W, Jin X, Cui S, Zhang Y, Ding Y, Zhao L. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. Eur J Cancer 49: 3924–3935, 2013. doi: 10.1016/j.ejca.2013.07.149. [DOI] [PubMed] [Google Scholar]
- 123.Wang WN, Chen Y, Zhang YD, Hu TH. The regulatory mechanism of CCR7 gene expression and its involvement in the metastasis and progression of gastric cancer. Tumour Biol 34: 1865–1871, 2013. doi: 10.1007/s13277-013-0728-9. [DOI] [PubMed] [Google Scholar]
- 124.Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, Kwon JH. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis 16: 1729–1738, 2010. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 135: 1624–1635, 2008. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 126.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 17: 3011–3016, 2003. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan K, Xie K, Fox J, Zeng H, Gao H, Huang C, Wu M. Decreased levels of miR-224 and the passenger strand of miR-221 increase MBD2, suppressing maspin and promoting colorectal tumor growth and metastasis in mice. Gastroenterology 145: 853–864, 2013. doi: 10.1053/j.gastro.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18: 618–623, 2012. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 129.Zhang M, Du X. Noncoding RNAs in gastric cancer: research progress and prospects. World J Gastroenterol 22: 6610–6618, 2016. doi: 10.3748/wjg.v22.i29.6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao D, Bakirtzi K, Zhan Y, Zeng H, Koon HW, Pothoulakis C. Insulin-like growth factor-1 receptor transactivation modulates the inflammatory and proliferative responses of neurotensin in human colonic epithelial cells. J Biol Chem 286: 6092–6099, 2011. doi: 10.1074/jbc.M110.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao D, Keates AC, Kuhnt-Moore S, Moyer MP, Kelly CP, Pothoulakis C. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J Biol Chem 276: 44464–44471, 2001. doi: 10.1074/jbc.M104942200. [DOI] [PubMed] [Google Scholar]
- 132.Zhao D, Kuhnt-Moore S, Zeng H, Pan A, Wu JS, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves Rho family small GTPases. Biochem J 368: 665–672, 2002. doi: 10.1042/bj20020950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao D, Kuhnt-Moore S, Zeng H, Wu JS, Moyer MP, Pothoulakis C. Neurotensin stimulates IL-8 expression in human colonic epithelial cells through Rho GTPase-mediated NF-κB pathways. Am J Physiol Cell Physiol 284: C1397–C1404, 2003. doi: 10.1152/ajpcell.00328.2002. [DOI] [PubMed] [Google Scholar]
- 134.Zhao D, Zhan Y, Koon HW, Zeng H, Keates S, Moyer MP, Pothoulakis C. Metalloproteinase-dependent transforming growth factor-alpha release mediates neurotensin-stimulated MAP kinase activation in human colonic epithelial cells. J Biol Chem 279: 43547–43554, 2004. doi: 10.1074/jbc.M401453200. [DOI] [PubMed] [Google Scholar]
- 135.Zhao L, Sun Y, Hou Y, Peng Q, Wang L, Luo H, Tang X, Zeng Z, Liu M. MiRNA expression analysis of cancer-associated fibroblasts and normal fibroblasts in breast cancer. Int J Biochem Cell Biol 44: 2051–2059, 2012. doi: 10.1016/j.biocel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Zhou MK, Liu XJ, Zhao ZG, Cheng YM. MicroRNA-100 functions as a tumor suppressor by inhibiting Lgr5 expression in colon cancer cells. Mol Med Rep 11: 2947–2952, 2015. doi: 10.3892/mmr.2014.3052. [DOI] [PubMed] [Google Scholar]