Aprepitant increases fasting, postprandial, and accommodation gastric volumes. Aprepitant increases volume to fullness and maximum tolerated volume during a nutrient drink test. NK1 receptors are involved in the control of gastric volume and in determining postprandial satiation and symptoms.
Keywords: gastric motor functions, satiation, NK1 receptor antagonist
Abstract
Aprepitant, an NK1 receptor antagonist, is approved for the treatment of chemotherapy-induced or postoperative emesis by blocking NK1 receptors in the brain stem vomiting center. The effects of NK1 receptors on gastric functions and postprandial symptoms in humans are unclear; a single, crossover study did not show a significant effect of aprepitant on gastrointestinal transit. Our aim was to compare, in a randomized, double-blind, placebo-controlled, parallel-group study (12 healthy volunteers per group), the effects of aprepitant vs. placebo on gastric emptying of solids (by scintigraphy) with a 320-kcal meal, gastric volumes (GVs; fasting and accommodation by single photon emission-computed tomography ), satiation [maximum tolerated volume (MTV)], and symptoms after a dyspeptogenic meal of Ensure. Aprepitant (125 mg on day 1, followed by 80 mg on days 2–5) or placebo, one tablet daily, was administered for 5 consecutive days. Statistical analysis was by unpaired rank sum test, adjusted for sex difference and body mass index. To assess treatment effects on symptoms, we incorporated MTV in the model. Aprepitant increased fasting, postprandial, and accommodation GV and tended to increase volume to fullness and MTV by ~200 kcal. However, aprepitant increased aggregate symptoms, nausea, and pain scores after ingestion the MTV of Ensure. There was no significant effect of aprepitant on gastric half-emptying time of solids. We conclude that NK1 receptors are involved in the control of GV and in determining postprandial satiation and symptoms. Further studies of the pharmacodynamics and therapeutic role of NK1 receptor antagonists in patients with gastroparesis and dyspepsia are warranted.
NEW & NOTEWORTHY Aprepitant increases fasting, postprandial, and accommodation gastric volumes. Aprepitant increases volume to fullness and maximum tolerated volume during a nutrient drink test. NK1 receptors are involved in the control of gastric volume and in determining postprandial satiation and symptoms.
tachykinins, such as substance P, comprise a series of structurally related peptides that derive from alternate processing of three Tac genes and are expressed throughout the nervous and immune systems. Tachykinins interact with three neurokinin G protein-coupled receptors and participate in important physiological processes in the nervous, immune, gastrointestinal, respiratory, urogenital, and dermal systems, including inflammation, nociception, smooth muscle contractility, epithelial secretion, and proliferation.
When released from intrinsic enteric or extrinsic primary afferent neurons, tachykinins have the potential to influence both nerves and muscles by way of interactions with three different types of tachykinin receptors, termed NK1, NK2, and NK3 receptors. Most prominent among the effects of tachykinins is their excitatory action on mammalian gastrointestinal motor activity by direct activation of the muscle through NK1 and/or NK2 receptors or by stimulation of excitatory enteric motor pathways through NK3 and/or NK1 receptors. In addition, tachykinins can inhibit motor activity by stimulating either inhibitory neuronal pathways or interrupting excitatory relays (14). Therefore, the effects of modulating NK1 receptors on regional gastrointestinal motor function cannot be accurately predicted.
In fact, there are conflicting results regarding the effects of substance P (and other tachykinins) on gastric emptying in rats (11, 15, 21), with potential excitation of antral contractions (via NK1 receptors, with costimulation with acetyl choline), stimulation of NK1 receptors on interstitial cells of Cajal, and stimulation of pyloric contractility (15).
Neurokinin receptor antagonists are selective, are potent, and have shown efficacy in models of gastrointestinal motor or inflammatory diseases. In clinical trials, there has been a singular success: NK1 receptor antagonists to treat nausea and vomiting (21), an effect that is mediated through brainstem effects in the least shrew (10–12, 19).
Based on a single, crossover study of 12 healthy volunteers in the literature (16), a 125-mg capsule of aprepitant or placebo on day 1, followed by an 80-mg capsule of aprepitant or placebo on days 2–5 did not change gastric retention at 15 min, gastric half-emptying time (T1/2), gastric mean transit time, time to small intestinal transit of 10%, small intestinal mean transit time, or colonic geometric center after 24, 48, and 72 h. To further characterize the effects of NK1 receptors on gastric motor functions and symptoms in humans, our aim was to compare, in healthy volunteers, the effects of aprepitant vs. placebo on gastric emptying, gastric volumes (GVs; fasting and accommodation), and satiation and symptoms after a dyspeptogenic meal.
METHODS
Study Design
We conducted a randomized, double-blind, placebo-controlled, parallel-group study to assess the effects of the NK1 antagonist aprepitant on gastric motor functions and satiation in healthy human volunteers. Participants were randomized to either aprepitant, 125 mg, on day 1, followed by 80 mg on days 2–5, or to placebo on five consecutive dates, with studies of quantitative traits being conducted on the last three days of treatment. This study was approved by Mayo Clinic Institutional Review Board and was conducted in the Mayo Clinic Clinical Research and Trials Unit. Informed consent was obtained, and patients expressed willingness to comply with the study procedures.
Participants
Healthy adult participants, 18–65 yr of age with body mass index (BMI) of 18–35 kg/m2, were recruited from the local community. For females of childbearing age, a hormonal (i.e., oral, implantable, or injectable) and single-barrier method or a double-barrier method of birth control was required throughout the study. Participants had to avoid medications that altered gastrointestinal transit and analgesic drugs for 48 h before the baseline period and throughout the study. Stable doses of thyroid replacement, estrogen replacement, low-dose aspirin for cardioprotection, and birth control medications were permitted.
We excluded patients with a recent history of surgery (within 60 days of screening), acute or chronic illness or history of illness which could interfere with interpretation of study data, acute gastrointestinal illness within 48 h of initiation of the baseline period, history of excessive alcohol use or substance abuse, and participation in an investigational study within the 30 days before dosing in the present study.
In total, we enrolled 24 participants from an initial 27 who were recruited for the study. Of the 27 recruited, 2 failed screening and 1 withdrew consent. Of the 24 participants, 12 (8 females and 4 males) were randomized to the aprepitant arm and 12 (9 females and 3 males) to the placebo arm. The trial flow is summarized in Fig. 1.
Fig. 1.
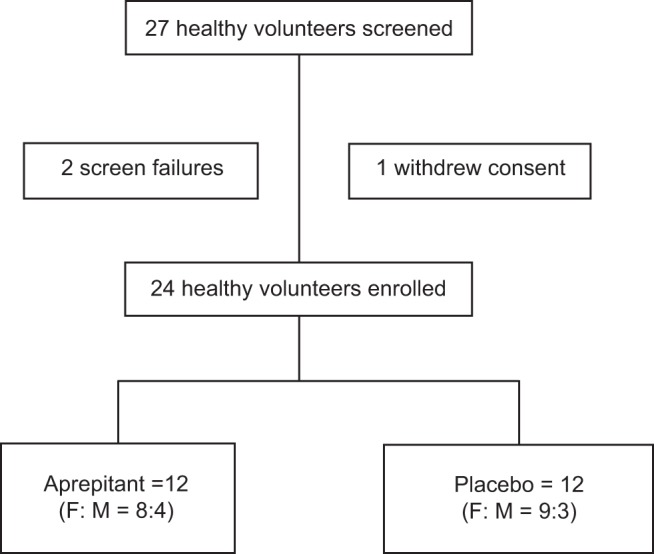
CONSORT flow diagram showing participant disposition. F:M, female:male.
Study Drug: Aprepitant
Aprepitant was administered for 5 days at approximately the same time each day; given the reported pharmacokinetics, in particular, Tmax after oral administration is 3–4 h; bioavailability is 60–65% (80- to 125-mg dose range); and elimination half-life is 9–13 h. The medications were supplied as a tablet from the Mayo Clinic Research Pharmacy, where matching placebo was also prepared. All study drugs were kept in a secure, limited-access storage area at the clinical site at a temperature between 2 and 8°C.
Experimental Protocol and Measurement of Quantitative Traits
Subjects received either aprepitant or placebo on all 5 days. On the last 3 days of the treatment, subjects were required to fast for a total of 8 h before gastrointestinal testing.
On day 3, gastric emptying of solids [based on a well-validated method (7) using an egg-based, 320 kcal, 30% fat meal] was measured using scintigraphy. Subjects were given the study medication on a fasting stomach 1 h before ingestion of the radiolabeled meal, and scans were taken every 15 min for the first hour and every 30 min for 2–4 h.
On day 4, volume to fullness (VTF) and maximum tolerated volume (MTV) were assessed by drinking Ensure (Abbott Laboratories, Lake Bluff, IL) nutrient drink (1 kcal/ml, 11% fat, 73% carbohydrate, and 16% protein) at a constant rate of 30 ml/min (8). Briefly, starting at the time of first ingestion of Ensure, participants recorded fullness sensations using a numerical scale from 0 to 5, with 0 being no symptoms, 3 corresponding to fullness sensation after a typical meal (VTF), and 5 corresponding to the MTV (maximum or unbearable fullness/satiation). Nutrient intake was stopped when subjects reached the score of 5, which constituted a “dyspeptogenic” stimulus. Postprandial symptoms of fullness, nausea, bloating, and pain were measured 30 min after the meal using 100-mm horizontal visual analog scales, with the words “none” and “worst ever” anchored at each end. Subjects were dosed on a fasting stomach 1 h before commencement of the drink test.
On day 5, gastric accommodation by single photon emission-computed tomography (SPECT) was assessed. Subjects were required to fast for 8 h before the test and were given the study medication 1 h before testing. 99mTc-pertechnetate was then administered intravenously. After a 10- to 15-min wait period, a fasting scan was acquired using a dual-head SPECT gamma camera. At the completion of the fasting scan, the subject consumed 300 ml of Ensure; a postprandial scan was then obtained. The method for quantifying GVs during fasting and postprandially and the test performance characteristics have been reported elsewhere (4, 6).
Statistical Methods
End points.
The primary end points of this study were fasting and accommodation GVs by SPECT, VTF (ml) of Ensure, and gastric half-emptying time (T1/) of solids. The secondary end points were postprandial GV, MTV during satiation test, and aggregate and individual (nausea, bloating, fullness, and pain) symptom scores 30 min after reaching the MTV on satiation test.
Statistical power.
The selected sample size was calculated based on previous experience with clinically relevant effect sizes of around 25% in the two primary end points, and the results of the primary end points were based on data previously collected using the same methods in in the same Mayo Clinic laboratory: for gastric emptying of solids T1/2 122 ± 29.8 (SD) min; for gastric accommodation 507 ± 100 mL (6, 7). We calculated that a sample size of 12 patients in each of the 2 treatment groups would provide 80% power at α = 0.05 to demonstrate differences for aprepitant compared with placebo of 23.6% for gastric accommodation volume and 29.2% for gastric emptying T1/2.
Statistical analysis.
Ranked dependent quantitative traits and symptoms ANOVA models were used to compare effects of placebo and aprepitant. Each model predicting quantitative traits also included the predictors of sex and BMI, while each model predicting symptoms also included sex, BMI, and MTV. The α-level was set at 0.05 for statistical significance in accordance with recommendations on comparing two therapies for several end points in a single trial (17). Adjustments for sex difference and BMI are necessary because of prior evidence, based on 214 females and 105 males, that sex difference is significantly associated with gastric emptying T1/2 (7), and a subset of patients with increased BMI have faster gastric emptying (2).
RESULTS
Participant Characteristics
Twenty-seven participants volunteered for the study; two failed screening and one withdrew voluntarily before receiving any study medication (Fig. 1). The remaining 24 volunteers were randomized (12 per group) to aprepitant or placebo; 17 participants were females and 7 were males. Table 1 summarizes the patient demographics by treatment group.
Table 1.
Participant baseline characteristics and effects of aprepitant and placebo on the primary end points
| Data Median (IQR) |
|||
|---|---|---|---|
| Aprepitant | Placebo | P Value | |
| n | 12 | 12 | |
| Demographics | |||
| Sex (F:M) | 8:4 | 9:3 | 0.653 |
| Age, yr | 34.5 (30.5, 47.0) | 34.5 (27.0, 47.5) | 0.840 |
| BMI, kg/m2 | 26.6 (25.3, 28.8) | 23.8 (23.0, 28.7) | 0.273 |
| Gastric functions | |||
| Gastric emptying solids T1/2, min | 95.4 (85.7, 114.0) | 99.8 (85.6, 120.0) | 0.453 |
| Fasting GV, ml | 191.8 (158.7, 216.4) | 174.1 (136.6, 187.9) | 0.037 |
| Postprandial GV, ml | 675.5 (577.8, 746.9) | 618.4 (590.6, 648.6) | 0.004 |
| Accommodation GV, ml | 476.1 (395.4, 531.3) | 457.2 (429.1, 502.5) | 0.047 |
| Satiation (Ensure) drink test | |||
| VTF, ml (= kcal) | 630.0 (420.0, 750.0) | 420.0 (345.0, 555.0) | 0.248 |
| MTV, ml (= kcal) | 1007.0 (886.8, 1066.5) | 808.0 (711.0, 1046.5) | 0.099 |
| Aggregate score (/400) | 179.5 (164.5, 231.0) | 119.5 (98.5, 169.5) | 0.020 |
| Nausea score (/100) | 36.0 (19.0, 48.5) | 4.0 (1.0, 8.5) | 0.023 |
Shown are the primary end points of gastric half-emptying time (T1/2) of solids and gastric volume (GV), assessed incorporating sex difference and body mass index (BMI) in the analysis, as well as the secondary end points of fasting and postprandial GVs and measurements related to satiation. There was no significant group difference in gastric emptying of solids, while the higher accommodation volume with aprepitant was significant. IQR, interquartile range; VTF, volume to fullness; MTV, maximum tolerated volume.
Effects of Aprepitant on Fasting, Postprandial, and Gastric Accommodation Volumes
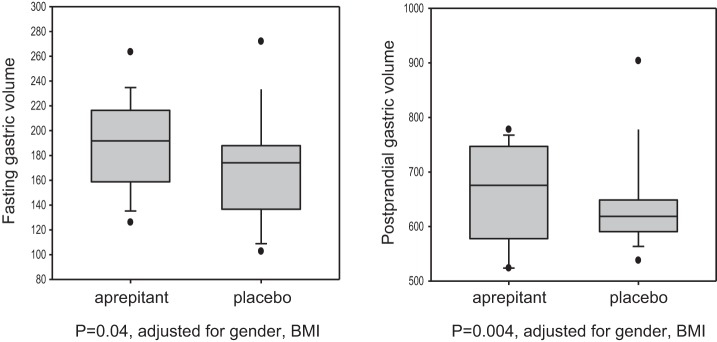
When adjusted for sex difference and BMI, aprepitant increased fasting, postprandial (Fig. 2), and gastric accommodation volumes (Table 1). There were no significant correlations between measured fasting or postprandial GVs and either VTF or MTV (data not shown).
Fig. 2.
Effects of aprepitant and placebo on fasting and postprandial gastric volumes. Data show median, interquartile range (IQR), 5–95 percentile range, and outliers. Note that there is marked increase in these volumes in the aprepitant group as compared with the placebo group. BMI, body mass index.
Effect of Aprepitant on Gastric Emptying of Solids
Gastric emptying of solids was not significantly different between the aprepitant and placebo arms of the study, even after being adjusted for sex difference and BMI (Table 1).
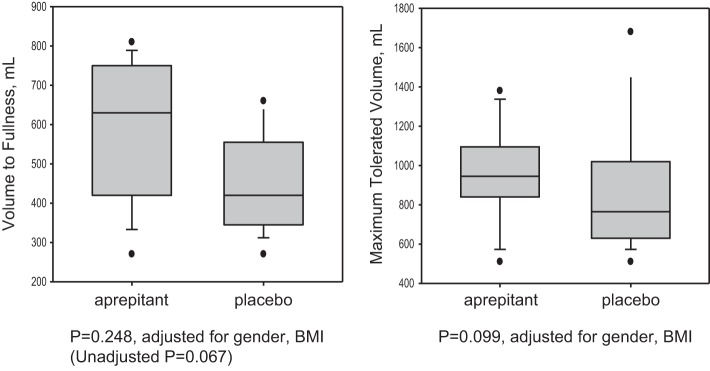
Effect of Aprepitant on Satiation as Measured by VTF and MTV
Aprepitant demonstrated a tendency to increase the MTV (P = 0.099) compared with placebo by ~200 kcal (Table 1, Fig. 3). Although a similar numerical difference in VTF of ~200 ml was noted between the two treatments, this was not statistically significant (P = 0.248).
Fig. 3.
Effects of aprepitant and placebo on volume to fullness and maximal tolerated volume. This was assessed on day 4 after patients were given an Ensure nutrient drink (characteristics as listed in the article) at a rate of 30 ml/min. Data shown are median, IQR, 5–95 percentile range, and outliers.
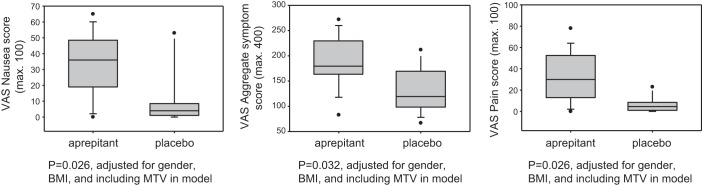
Effect of Aprepitant on Symptoms 30 Min After Reaching MTV
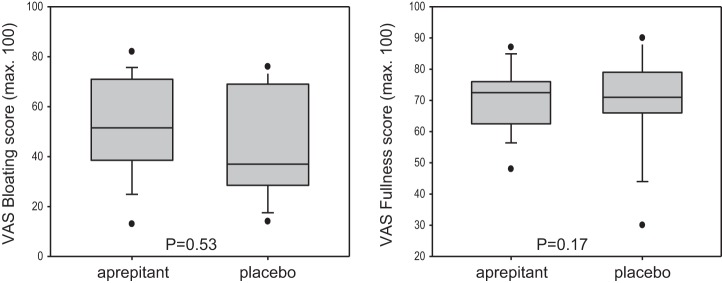
In the aprepitant group, symptom scores for nausea, pain and aggregate symptoms were increased compared with placebo (Fig. 4). These differences were present with and without inclusion of MTV in the statistical model, suggesting that the increased symptom scores are not accounted for by the 25% higher calorie and volume ingested in the group that received aprepitant. In contrast, there were no significant differences in bloating and fullness scores (Fig. 5).
Fig. 4.
Aggregate symptom scores and nausea and pain scores for aprepitant and placebo groups. Data shown are median, IQR, 5–95 percentile range, and outliers. Note the increase in overall symptoms in the aprepitant group, even when the maximum tolerated volume is included. Postprandial symptoms of nausea and pain were measured 30 min after the meal using 100-mm horizontal visual analog scales (VAS), with the words “none” and “worst ever” anchored at each end.
Fig. 5.
Postprandial symptoms of fullness and bloating were measured 30 min after the meal using 100-mm horizontal VAS, with the words “none” and “worst ever” anchored at each end. Data shown are median, IQR, 5–95 percentile range, and outliers. No significant difference was noted in these scores in response to aprepitant and placebo.
DISCUSSION
This randomized, double-blind, placebo-controlled, parallel-group study demonstrated the effects of the NK1 receptor antagonist aprepitant on gastric emptying, satiation, and gastric accommodation volumes in healthy volunteers. The study showed that aprepitant increased gastric fasting volume, postprandial volume, accommodation volume and caloric intake but not the gastric emptying of solids. It also increased upper gastrointestinal symptoms in response to a dyspeptogenic meal. The increase in gastric accommodation was demonstrated by the ability of participants in the aprepitant group to tolerate an additional 200 kcal compared with the placebo group. The increase in symptoms that were more pronounced in the aprepitant group compared with the placebo group was not altered even when we accounted for the increase in maximal tolerated volume in the aprepitant group. There was no significant association of symptoms observed during the dyspeptogenic meal with gastric emptying or gastric accommodation measured independently in the same participants exposed to the same treatments during each test.
A recent review summarizes the important effects of NK receptor modulation on the neuromuscular apparatus in the gastrointestinal tract (21). Although tachykinins are found in intestinal immune and enterochromaffin cells, the major sources in the gut are enteric neurons, followed by nerve fibers from dorsal root and vagal ganglia. Tachykinin-containing fibers surround enteric ganglia, ramify through muscle, form a perivascular mesh around submucosal arteries, and supply the mucosa. These fibers are in close proximity to cells expressing NK receptors. The NK1 receptor is expressed by enteric neurons, interstitial cells of Cajal, epithelial cells, and lymphocytes and macrophages of the lamina propria. The NK2 receptor is expressed by myocytes, neuronal varicosities, and epithelial cells. The NK3 receptor is mostly neuronal. The locations of tachykinins and NK receptors are consistent with the regulation of neuro-neuronal transmission, motility, secretion, inflammation, and pain.
Aprepitant, an NK1 receptor antagonist, was approved more than a decade ago for the prevention of chemotherapy-induced nausea and vomiting. NK1 receptor antagonists are thought to act centrally, inhibiting emesis by blocking binding of substance P at the NK1 receptor in the brain stem emetic center (22). Aprepitant is effective for the relief of nausea and vomiting in the prophylaxis of highly emetogenic chemotherapy (1), as well as postoperative nausea and vomiting (20).
The importance of the current study on the potential role of NK1 receptors in the functions of the stomach and postprandial symptoms is emphasized by recent preliminary observations from the APRON Trial conducted in over 100 patients with gastroparesis and related disorders, which showed that aprepitant reduced symptoms in such patients (18). There are also anecdotal reports of the beneficial long-term effects of this NK1 receptor antagonist in patients with significant gastroparesis (9, 13). While our observation of increased GV and calorie volume tolerated could be beneficial in patients with gastroparesis, including enhancement of nutritional intake without retardation of gastric emptying, the increase in symptoms of dyspepsia such as pain and nausea and aggregate symptoms could potentially be deleterious. We controlled for the increased MTV and still observed the increase in symptoms after the dyspeptogenic meal of almost 1,000 kcal (average). There is evidence in the literature that dyspepsia may be induced almost twice as frequently by aprepitant (8.4%) compared with placebo (4.9%) in patients with breast cancer receiving highly emetogenic chemotherapy (25).
It has been demonstrated that patients with upper gastrointestinal symptoms may have either accelerated or delayed gastric emptying, or reduced gastric accommodation, or combinations of these alterations in functions (5, 23, 24), and these constitute potential targets for treatment. NK1 receptor antagonists have several potential pharmacological effects that could reverse the abnormal functions or conceivably aggravate other pathophysiological mechanisms. For example, NK1 antagonists may reduce gastric emptying by inhibiting antral motility or may accelerate gastric emptying by inhibition of pyloric tone. NK1 antagonists could enhance GVs during fasting or postprandially, and they could act centrally or peripherally to reduce afferent activity arising in the proximal gut.
Before our comprehensive study, there was only one documentation of the effects of NK1 antagonists on gastric accommodation or compliance (3). Thus, in healthy volunteers, NK1 receptors did not appear to be involved in the control of gastric compliance, accommodation or sensitivity to distention (3). The difference in the results reported by Ang et al. (3) and our current studies may reflect differences in methods used, specifically 5 days of treatment (day 1 with aprepitant 125 mg, and days 2–5 with aprepitant 80 mg) in our study in contrast to the 1 day of treatment with 80 or 125 mg aprepitant in the study by Ang et al. (3). This difference is relevant given the pharmacokinetics of aprepitant, the Tmax of ~4 h, and the likelihood that at least 3 days of administration are required to achieve steady-state blood levels. Another difference is the use of SPECT imaging that measured entire GV in our experiments and the possibility that the gastric barostat balloon measured predominantly fundic compliance and accommodation.
Given the paucity of available treatments for patients with decreased gastric accommodation and gastroparesis, our study shows some promise that aprepitant might be used to enhance gastric accommodation and, in turn, improve appetite in this subgroup of patients. While the APRON Trial (18) showed improvement in overall symptoms in patients with nausea and vomiting, the primary end point of improvement in nausea was not achieved; this was consistent with the results in our study in healthy volunteers. However, it is unclear whether long-term improvement in symptoms can be explained by the ability of the aprepitant group to tolerate more overall GVs (fasting, postprandial, and accommodation volumes), as opposed to any effects on gastric emptying or sensation.
The limitations of our study are the relatively small sample size conducted only in healthy volunteers, and therefore, we are unable to comment on the potential effects of NK1 modulation on the same functions in patients with gastroparesis or dyspepsia. However, the study used validated methods and end points in humans using the highest doses approved for use in humans, and the study had sufficient power to detect clinically relevant changes in quantitative traits. Indeed, for the volume and satiation symptoms, significant effects were observed, suggesting that our study could indeed assess the effects of the NK1 antagonist in humans. Further studies are required to assess the pharmacodynamic effects of aprepitant in patients with gastroparesis and decreased gastric accommodation.
GRANTS
This study was supported Morrie K. Abramson Research Fund at Mayo Clinic. M. Camilleri receives research support from National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-67071-R56; the study was made possible by CCaTS Grant UL1-TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C. conceived and designed research; D.J., I.A.B., D.D.B., H.H., I.O., D.R., and M.R. performed experiments; D.J., W.S.H., and M.C. interpreted results of experiments; D.J. and M.C. prepared figures; D.J. and M.C. drafted manuscript; D.J., I.A.B., D.D.B., H.H., I.O., W.S.H., and M.C. edited and revised manuscript; D.J., I.A.B., D.D.B., H.H., I.O., D.R., W.S.H., and M.C. approved final version of manuscript; I.A.B., D.D.B., D.R., M.R., and W.S.H. analyzed data.
ACKNOWLEDGMENTS
We thank Cindy Stanislav for excellent secretarial assistance.
REFERENCES
- 1.Abdel-Rahman O. Neurokinin-1 inhibitors in the prevention of nausea and vomiting from highly emetogenic chemotherapy: a network meta-analysis. Ther Adv Med Oncol 8: 396–406, 2016. doi: 10.1177/1758834016654902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta A, Camilleri M, Shin A, Vazquez-Roque M, Iturrino J, Burton D, O’Neill J, Eckert D, Zinsmeister AR. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology 148: 537–546, 2015. doi: 10.1053/j.gastro.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang D, Pauwels A, Akyuz F, Vos R, Tack J. Influence of a neurokinin-1 receptor antagonist (aprepitant) on gastric sensorimotor function in healthy volunteers. Neurogastroenterol Motil 25: e830–e838, 2013. doi: 10.1111/nmo.12210. [DOI] [PubMed] [Google Scholar]
- 4.Bouras EP, Delgado-Aros S, Camilleri M, Castillo EJ, Burton DD, Thomforde GM, Chial HJ. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut 51: 781–786, 2002. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol 1: 264–272, 2003. doi: 10.1016/S1542-3565(03)00130-7. [DOI] [PubMed] [Google Scholar]
- 6.Breen M, Camilleri M, Burton D, Zinsmeister AR. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterol Motil 23: 308–315, 2011. doi: 10.1111/j.1365-2982.2010.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong ID, Zinsmeister AR. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 24: 1076–e562, 2012. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chial HJ, Camilleri C, Delgado-Aros S, Burton D, Thomforde G, Ferber I, Camilleri M. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol Motil 14: 249–253, 2002. doi: 10.1046/j.1365-2982.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 9.Chong K, Dhatariya K. A case of severe, refractory diabetic gastroparesis managed by prolonged use of aprepitant. Nat Rev Endocrinol 5: 285–288, 2009. doi: 10.1038/nrendo.2009.50. [DOI] [PubMed] [Google Scholar]
- 10.Darmani NA, Chebolu S, Amos B, Alkam T. Synergistic antiemetic interactions between serotonergic 5-HT3 and tachykininergic NK1-receptor antagonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav 99: 573–579, 2011. doi: 10.1016/j.pbb.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Darmani NA, Crim JL, Janoyan JJ, Abad J, Ramirez J. A re-evaluation of the neurotransmitter basis of chemotherapy-induced immediate and delayed vomiting: evidence from the least shrew. Brain Res 1248: 40–58, 2009. doi: 10.1016/j.brainres.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 12.Darmani NA, Dey D, Chebolu S, Amos B, Kandpal R, Alkam T. Cisplatin causes over-expression of tachykinin NK(1) receptors and increases ERK1/2- and PKA- phosphorylation during peak immediate- and delayed-phase emesis in the least shrew (Cryptotis parva) brainstem. Eur J Pharmacol 698: 161–169, 2013. doi: 10.1016/j.ejphar.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Fahler J, Wall GC, Leman BI. Gastroparesis-associated refractory nausea treated with aprepitant. Ann Pharmacother 46: e38, 2012. doi: 10.1345/aph.1R484. [DOI] [PubMed] [Google Scholar]
- 14.Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther 73: 173–217, 1997. doi: 10.1016/S0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Micci MA, Murthy KS, Pasricha PJ. Substance P is essential for maintaining gut muscle contractility: a novel role for coneurotransmission revealed by botulinum toxin. Am J Physiol Gastrointest Liver Physiol 306: G839–G848, 2014. doi: 10.1152/ajpgi.00436.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madsen JL, Fuglsang S. A randomized, placebo-controlled, crossover, double-blind trial of the NK1 receptor antagonist aprepitant on gastrointestinal motor function in healthy humans. Aliment Pharmacol Ther 27: 609–615, 2008. doi: 10.1111/j.1365-2036.2008.03618.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien PC, Shampo MA. Statistical considerations for performing multiple tests in a single experiment. 5. Comparing two therapies with respect to several endpoints. Mayo Clin Proc 63: 1140–1143, 1988. doi: 10.1016/S0025-6196(12)65511-6. [DOI] [PubMed] [Google Scholar]
- 18.Pasricha PJ, Yates K, Sarosiek I, McCallum R, Clarke J, Nguyen L, Dhalla S, Stein E, Koch K, Abell T, Hasler W, Snape W, Lee L, Grover M, Miriel L, Van Natta M, Farrugia G, Tonascia J, Hamilton F, Parkman H. Aprepitant for symptoms of gastroparesis and related disorders: the APRON randomized clinical trial. Am J Gastroenterol 111: S480, 2016.27685301 [Google Scholar]
- 19.Rojas C, Raje M, Tsukamoto T, Slusher BS. Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol 722: 26–37, 2014. doi: 10.1016/j.ejphar.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Singh PM, Borle A, Rewari V, Makkar JK, Trikha A, Sinha AC, Goudra B. Aprepitant for postoperative nausea and vomiting: a systematic review and meta-analysis. Postgrad Med J 92: 87–98, 2016. doi: 10.1136/postgradmedj-2015-133515. [DOI] [PubMed] [Google Scholar]
- 21.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 94: 265–301, 2014. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tattersall FD, Rycroft W, Francis B, Pearce D, Merchant K, MacLeod AM, Ladduwahetty T, Keown L, Swain C, Baker R, Cascieri M, Ber E, Metzger J, MacIntyre DE, Hill RG, Hargreaves RJ. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology 35: 1121–1129, 1996. doi: 10.1016/S0028-3908(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 23.Vakil NB, Howden CW, Moayyedi P, Tack J. White paper AGA: functional dyspepsia. Clin Gastroenterol Hepatol 15: 1191–1194, 2017. doi: 10.1016/j.cgh.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Vanheel H, Carbone F, Valvekens L, Simren M, Tornblom H, Vanuytsel T, Van Oudenhove L, Tack J. Pathophysiological abnormalities in functional dyspepsia subgroups according to the Rome III criteria. Am J Gastroenterol 112: 132–140, 2017. doi: 10.1038/ajg.2016.499. [DOI] [PubMed] [Google Scholar]
- 25.Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, Raftopoulos H, Grunberg SM, Gabriel M, Rodgers A, Bohidar N, Klinger G, Hustad CM, Horgan KJ, Skobieranda F. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23: 2822–2830, 2005. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]