Abstract
Significance: Excessive scarring is major clinical and financial burden in the United States. Improved therapies are necessary to reduce scarring, especially in patients affected by hypertrophic and keloid scars.
Recent Advances: Advances in our understanding of mechanical forces in the wound environment enable us to target mechanical forces to minimize scar formation. Fetal wounds experience much lower resting stress when compared with adult wounds, and they heal without scars. Therapies that modulate mechanical forces in the wound environment are able to reduce scar size.
Critical Issues: Increased mechanical stresses in the wound environment induce hypertrophic scarring via activation of mechanotransduction pathways. Mechanical stimulation modulates integrin, Wingless-type, protein kinase B, and focal adhesion kinase, resulting in cell proliferation and, ultimately, fibrosis. Therefore, the development of therapies that reduce mechanical forces in the wound environment would decrease the risk of developing excessive scars.
Future Directions: The development of novel mechanotherapies is necessary to minimize scar formation and advance adult wound healing toward the scarless ideal. Mechanotransduction pathways are potential targets to reduce excessive scar formation, and thus, continued studies on therapies that utilize mechanical offloading and mechanomodulation are needed.
Keywords: : mechanotransduction, wound healing, scar, therapy

Geoffrey C. Gurtner, MD
Scope and Significance
Scarring of the skin after cutaneous injury is a source of major morbidity to patients and is a financial burden to the healthcare system. Recent improvements in our understanding of the role of mechanical forces in wound healing and repair open up the possibility of targeting mechanotransduction pathways to reduce scar formation. This review will discuss the role of mechanical forces in wound healing and scarless wound repair and provides an update on therapies that offload mechanical tension in the wound environment to encourage a healing response closer to the “scarless ideal.”
Translational Relevance
Since the original observation that fetal wounds heal without scars in utero,1 a major goal of skin and wound healing research has been to identify the changes that cause neonatal and adult skin to assume a scarring phenotype. Human skin is particularly sensitive and responsive to mechanical forces in the environment and converts mechanical cues to biochemical signals that promote scar formation.2–5 It may be possible to specifically target these signaling pathways to return adult wound healing to the scarless state of fetal skin.
Clinical Relevance
The clinical and financial burden resulting from excessive scarring is tremendous. Severe burns result in more than 40,000 hospitalizations and nearly 4,000 deaths per year in the United States, and much of the care required is related to the ensuing burn scar.6 Wounds that heal with excessive scar tissue result in poor functional and aesthetic outcomes through the formation of hypertrophic scar and keloid scar. The economic impact is greater when the costs of disability and revision surgeries due to dysfunctional tissue and disfiguring scars are included.7
Discussion of Findings and Relevant Literature
Overview of uncomplicated wound healing
The classic stages in adult wound repair have been well described in the literature.8 There are three distinct, sequential phases of repair leading to the formation of a fibrotic scar: (1) inflammation, (2) new tissue formation, and (3) remodeling. Inflammation occurs immediately after tissue injury. During this phase, hemostasis is achieved via the platelet plug and fibrin matrix, bacterial products are degraded via complement activation and the recruitment of neutrophils, and monocytes localize to the wound and differentiate into macrophages.8–10 Neutrophils immediately diapedese to the wound to kill microbes, whereas macrophages arrive later to phagocytose debris and produce cytokines. The mechanisms by which these immune mediators induce scar formation are not yet fully understood.11,12 The second phase, new tissue formation, occurs through the proliferation and migration of various cell types (e.g., keratinocytes, endothelial cells, fibroblasts, myofibroblasts). Granulation tissue, consisting of connective tissue and a dense network of new blood vessels, forms from 2 to 10 days after tissue injury. Of particular interest are the actions of fibroblasts and myofibroblasts, because they interact with and produce extracellular matrix (ECM; i.e., collagen) that comprises a substantial component of the mature scar.13
Two to 3 weeks after tissue injury, in the final remodeling phase, many cells undergo apoptosis or migrate from the wound and leave behind type I and type III collagen and other ECM proteins that they previously produced. Fibroblasts, macrophages, and endothelial cells secrete metalloproteinases that remodel the ECM. In the early wound and immature scar, the ratio of type I to type III collagen in the acellular matrix is ∼2:1 (33% type III collagen). As the scar matures, the composition of the acellular matrix transitions to contain more type I collagen, changing the ratio of type I to type III collagen to ∼4:1, the ratio typically found in normal skin.14–16 This process strengthens the repaired tissue over the course of 6–12 months.17 The remodeling phase lasts for a year or more.8 The strength of previously wounded skin is at most 75–80% that of unwounded skin.14 All of the aforementioned stages of wound healing are influenced by mechanical forces, as will be described in later sections.
Scarless wound healing
The existence of scarless healing in the fetus was first observed in 1971,1 but the transition from scarless to scar-forming wound healing was first demonstrated in fetal lambs in 1990.18 This transition was further demonstrated in fetal rhesus monkeys in 1993.19 In 75-day gestation (term = 165 days) fetal monkeys, full-thickness lip wounds healed completely with normal tissue architecture and epidermal appendages (hair follicles and sebaceous glands) and without scars (scarless and regenerative repair). In the 85–100 day gestation group, healed wounds displayed normal collagen patterning, but lacked epidermal appendages (scarless, but not regenerative repair). By 107 days of gestation, the wounds healed with a thin scar and with no epidermal appendages.19 Differences in growth factor distribution among fetal, neonate, and adult mouse lip wounds are associated with this transition. Though platelet-derived growth factor was observed in all three populations, transforming growth factor β (TGFβ) and basic fibroblast growth factor were absent in scarless fetal wounds and were present in scarring neonatal and adult wounds.20 Furthermore, trophic factors such as TGFβ are sufficient to induce fibrosis in fetal animal models. Implants containing TGFβ placed subcutaneously induced adult-like fibrosis and collagen deposition in fetal rabbit wounds.21 Similarly, a fibrotic and angiogenic response was induced 48–72 h after TGFβ was injected directly into the skin of newborn mice.22 Thus, inflammation can induce scarring in otherwise scarless fetal wound healing. There are many more documented differences between adult and scarless wound healing.23,24 Of note, the phenomenon of fetal scarless repair is organ specific: Fetuses that heal cutaneous wounds without scars will form scar tissue in the stomach, intestines, and diaphragm.25,26 The timing of the transition from scarless to scarring cutaneous healing may be related to the development of acute inflammation and the increasingly complex architecture of fetal skin.27 Remarkably, there are adult mammals that are able to heal scarlessly and regeneratively. In two species of African spiny mouse (Acomys kempi and Acomys percivali), adults are able to fully regenerate epidermal-derived structures in response to large excisional wounding.28 Future studies that characterize the wound environment and mechanisms of skin regeneration in African spiny mice will be critical for our understanding of scar mechanisms and therapeutic interventions for fetal scarless and regenerative repair.
In addition, fetal mammalian skin contains thin collagen fibers that exhibit low levels of resting stress, whereas adult skin contains thick collagen bundles that exhibit high levels of resting stress. This suggests a relationship between mechanical tension and scar formation.24 Studies addressing the mechanics of embryonic wound healing in vitro have given rise to a model known as “purse-string” healing in which a circular cable of connected actin filaments encircling the epidermal wound margin gradually contracts and closes the wound.29,30 This has been further studied and computationally modeled in the chick embryo. As a result, three different phases in early chick embryo healing were proposed: (1) contraction of a thick actin cable of cells in the first ∼30 s to close the wound area by >50%, (2) formation and contraction of a thin actin cable at the wound edge to close the wound almost completely over several minutes, and (3) “zipping” of wound edges via filopodia.31 In adult healing, fibroblasts convert to myofibroblasts to form contractile granulation tissue and keratinocytes migrate from the edges of the wound via lamellipodia to re-epithelize the wound bed.32 Given that fetal wounds experience much lower resting stress and have a different mechanism of wound contraction, mechanical forces in the wound environment likely play a key role in scarless fetal wound healing.
Mechanotransduction in the wound environment
Early observations in anatomy and surgery have hinted at the importance of mechanical tension on wound-healing outcomes.33 For example, Langer lines in human skin correspond to the bands of tension naturally occurring in skin due to collagen fibril and fibroblast interactions. Incisions made parallel to these lines experience reduced tension and tend to heal with less scarring than those placed perpendicular to them.33–35 Increased scar formation has also been noted when wounds are in locations subjected to increased mechanical force, such as wounds along the sternum and across joints.35,36 Conversely, reduction of mechanical tension through tension shielding has been shown to reduce scarring.2,5
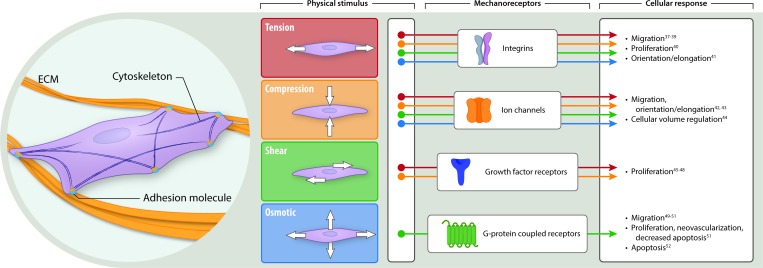
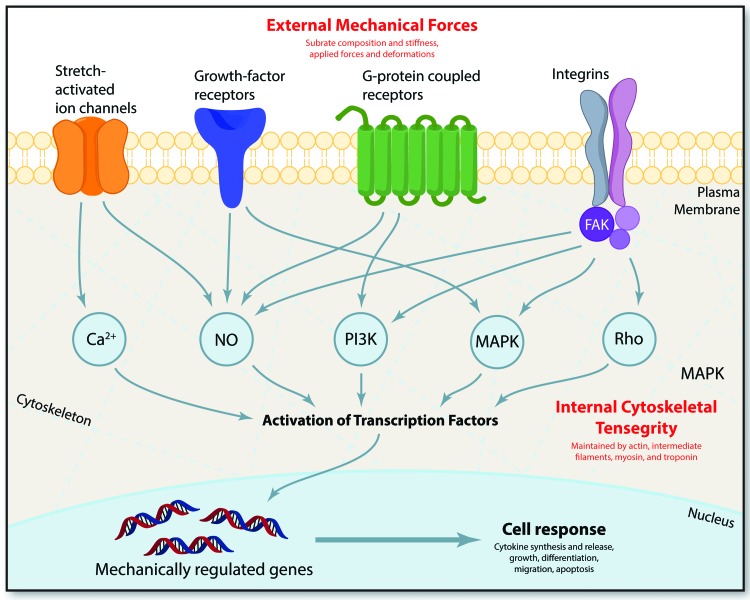
In addition to tension, cells experience and respond to compressive, shear, and osmotic forces,37–53 as illustrated in Fig. 1. It has been shown that mechanical properties play an important role in proliferation and differentiation of stem cells. For example, mechanical properties of hydrogels influence mesenchymal stem cell (MSC) differentiation. Specifically, local degradability was found to be necessary for human MSC (hMSC) spreading and traction responses that direct cell fate. The authors also showed that introduction of nondegradable crosslinks via delayed secondary crosslinking could switch hMSC cell fate from osteogenic to adipogenic. This was observed by histological as well as biochemical staining.54 Another study has shown that hMSCs possess mechanical memory, allowing them to maintain predisposition toward a certain cell fate. This phenomenon is dose dependent and reversible within a time window of 3 days in culture.55 There have been several publications that demonstrate how skin responds to biomechanical cues and which biomechanical signal mediators are important in mechanotransduction.56–62 The known intracellular mechanisms involved in mechanotransduction are summarized in Fig. 2. Advancements in the past decade pertaining to the exploration of mechanical forces on the individual cell and system levels can be attributed to developments in nanotechnology, fluorescence energy transfer-based mechanosensors, atomic force microscopy, traction force microscopy, and magnetic twist cytometry.63–67
Figure 1.
Schema of mechanical forces acting on a cell and resulting cellular responses. An illustration of the types of mechanical forces to which cells respond. Far left, a cell adhering to the ECM via adhesion molecules such as integrins. Four types of physical stimuli are depicted: tension (stretching in a plane perpendicular to the cell cross-section), compression (pushing inward), shear (stretching in a plane parallel to the cell cross-section), and osmotic (internal pressure maintaining the turgor of a cell, preventing it from collapsing on itself). The stimuli are color-coded as follows: tension (red), compression (orange), shear (green), and osmotic (blue). These stimuli are transmitted to the cell via mechanoreceptors, such as integrins, ion channels, growth factor receptors, and G-protein coupled receptors. Mechanoreceptors trigger various cellular responses, as depicted and cited earlier. ECM, extracellular matrix. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 2.
Intracellular mechanisms involved in mechanotransduction. External mechanical forces are transmitted across the cell membrane by mechanoreceptors, resulting in the activation of various intracellular signaling pathways. Such mechanoreceptors include stretch-activated ion channels, growth-factor receptors, G-protein coupled receptors, and integrins. In fibroblasts and keratinocytes, two of the key mechanosensitive cells in the skin, mechanical signals transmitted via integrins activate focal adhesion complexes containing FAK. Downstream biochemical pathways, such as calcium regulated targets, nitric oxide (NO) targets, phosphoinositol-3-kinase (PI3K) targets, mitogen-associated protein kinases (MAPKs), and Rho GTPases, all synergize to activate transcription factors that translocate into the nucleus and activate mechanically regulated genes. Adapted and used with permission from Wong et al. (2011).61 FAK, focal adhesion kinase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Fibroblasts have been extensively studied in biomechanical wound models, and physical forces are known to influence the expression of ECM genes and inflammatory genes involved in scar formation.68–70 Fibroblasts grown in mechanically loaded three-dimensional collagen lattices resembling connective tissue develop dendritic extensions that enable them to migrate and remodel their matrices.71–74 Simply subjecting fibroblasts to microdeformations caused by suction, as one would observe with vacuum-assisted wound closure, results in increased fibroblast proliferation and up-regulation of typical genes expressed by fibroblasts (e.g., type 1 collagen alpha 1, fibroblast growth factor 2, TGF-β1).75 Using an in vitro model to investigate the effects of cyclically stretching cells in culture, increased tension was also demonstrated to promote human fibroblast proliferation and mechanical strengthening.76 Cyclically stretched fibroblasts exhibited increased migration speed and distance when compared with unstretched cells. This induced the cells to align themselves perpendicularly to the vector of applied mechanical force and was associated with reduced apoptosis via mechanisms related to the integrin (ITG) and Wingless-type mechanotransduction pathways.77 Mechanical stretching also induces phosphorylation and activation of protein kinase B (Akt) in keratinocytes in vitro,78 providing support for the concept that keratinocytes are mechanosensitive and can modulate their intracellular signaling pathways in response to mechanical deformations in the environment.
Aarabi et al. were first to show the impact of mechanical signal transduction on cutaneous wound healing in vivo. They demonstrated that the addition of mechanical stress in the early phases of wound healing induces hypertrophic scarring by inhibiting Akt-dependent cellular apoptosis.24 In subsequent studies, microarray analysis of scars in an established mouse model has shown that focal adhesion kinase (FAK), a tyrosine kinase without a receptor protein, is critical in cell mechanotransduction. Conditional knock-out of FAK in fibroblasts revealed that FAK is necessary for the stimulation of chemokine signaling and collagen production in response to mechanical stimulation in vivo.79 This further suggests that the fibroblast is critical for mechanosensing and transduction in the wound environment. Paradoxically, deletion of FAK signaling in keratinocytes had a different phenotype with atrophic dermis, indicating the complexity of epithelial-to-mesenchymal signaling that occurs during wound healing.80 However, it is important to note that the significant differences between properties of mouse skin and those of human skin may limit our ability to translate these findings clinically.81 Large animal studies have been conducted in the pig, which possesses skin similar to that of humans. Mechanical stress has been shown to regulate collagen fibril thickness, fibrosis, microvascular blood flow, inflammatory response via neuropeptide release, and numbers of myofibroblasts.2,36,82–86 Given evidence that mechanical forces influence wound healing, targeting these forces has the potential to reduce scar formation.
Mechanomodulation and emerging mechanotherapies
A variety of techniques utilize mechanical offloading to limit fibrosis and minimize scarring. As described earlier, increased mechanical tension plays a major role in the development of scar tissue through a variety of biochemical signals. Prior studies have demonstrated that direct modification of these biochemical signals results in reduced adult skin scarring.87–92 For example, neutralization of TGF-β1/2 and/or addition of TGF-β3,88 downregulation of connexin 43 (Cx43) protein levels,89 and exogenous application of angiotensin peptides87 have all been shown to reduce scar formation in adult skin. However, these studies are validated primarily in animal models. Here, we discuss three different mechanical offloading techniques used in humans: silicone gel sheets, paper tape, and embrace advanced scar therapy.
Silicone gel sheets
The silicone gel sheet is a common therapy for scar management, despite limited insight to its mechanism and evidence supporting its clinical efficacy. Silicone gel sheets reduce tensile stresses in the wound environment,93 but some studies propose that silicone gel sheets minimize scarring via hydration of the stratum corneum, ultimately mediating cytokine signaling pathways that have downstream effects on fibroblasts and keratinocytes.94–96 Other studies demonstrated that silicone gel sheet treatment decreases TGF-β197 and TGF-β2 expression in fibroblasts.98 TGF-β1 is the predominant TGF-β isoform in the skin and is a known profibrotic cytokine.10,99,100 By decreasing TGF-β1 (and TGF-β2) expression, silicone gel sheet treatment may confer a more scarless fetal repair phenotype than scarring adult repair phenotype.23,24 In a 30-patient study with various scar types (superficial, hypertrophic, and keloid), silicone gel was applied to scars within 10 days after wound closure for ∼6 months. This resulted in improvements in scar appearance, and considerably fewer scars were characterized as hypertrophic or keloid at the 6-month follow-up.101 In a 20-patient study focusing on the evolution of evolving hypertrophic and keloid scars, wearing a silicone gel sheet dressing for at least 12 h a day for 8–12 weeks led to a reduction in scar size in 85% of patients.102 Though similar studies showed statistically significant differences between silicone gel sheet treated and untreated groups, a recent Cochrane review of 20 trials evaluating the efficacy of silicone gel sheet treatment to reduce the incidence of hypertrophic/keloid scarring, reduce scar thickness, and ameliorate scar color revealed that current studies on the topic are of poor quality and are susceptible to bias.103 Ultimately, better evidence supporting use of silicone gel sheets to reduce scar formation will be required before their routine use can be recommended.
Paper tape
Paper tape has been reported to reduce scarring through its ability to reduce wound tension.104,105 In a randomized, controlled trial testing the efficacy of paper tape in preventing hypertrophic scarring, intradermal scar volumes were assessed in 39 patients with cesarean section surgical incisions that traversed Langer's skin tension lines. Paper tape significantly reduced scar volume, and the odds of developing a hypertrophic scar was 13.6 times greater in patients receiving no postoperative intervention than those treated with paper tape.104 In a blinded study with 195 patients, taping elliptical torso wounds for 12 weeks improved scar appearance at 6 months. The authors postulate that applying paper tape perpendicular to the wound edges reduced mechanical tension, thereby minimizing scar formation.105 Similarly, photographic analysis revealed reduced hypertrophic scarring after treatment with microporous paper tape in a rabbit ear model.106 Recently, attempts have been made to improve the action of tapes, yielding products such as Dynaclose (mediGroup Australia Pty Ltd., Melbourne, Victoria, Australia), a hybrid of silicone elastomer and paper tape, and Steri-Strip S (3M, St. Paul, MN).107,108
Embrace Advanced Scar Therapy
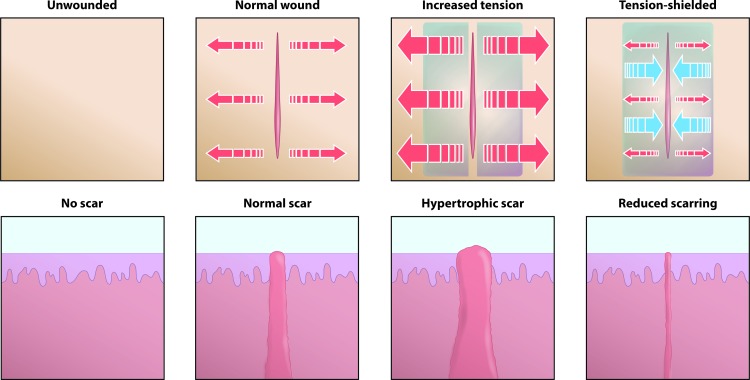
Embrace Advanced Scar Therapy is a silicone sheet-based polymer dressing device that was developed to harness the potential for mechanomodulation to improve wound-healing outcomes.2,109 This device was first tested on a hypertrophic-like scar model in the red Duroc pig, which is known as a robust model for studying human-like hypertrophic scarring.2,109,110 The Embrace device was designed to apply compressive forces to incision sites, thereby off-loading tension and shielding the incisions from stress, as illustrated in Fig. 3.
Figure 3.
Mechanical tension around cutaneous wound impacts scar formation. Human skin is always under tension. When injured, that tension causes the wound to splay open. This results in a typical scar. Greater tension induces increased scar formation in the form of a hypertrophic scar. Conversely, tension-shielding decreases scar formation. This phenomenon is the basis for the development of the Embrace Advanced Scar Therapy, a tension-shielding silicone sheet-based polymer dressing device. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In both large animal studies and early human clinical trials, the incisions off-loaded by the device exhibited significantly improved scar appearance based on blinded ratings using a validated visual analogue scale. In addition, histological analysis revealed a recapitulation of unwounded epithelial architecture when compared with wounds subjected to physiological levels of mechanical stress.2,109
In the pivotal (Phase III) randomized clinical trial, Embrace reduced scarring in abdominoplasty incisions.111 Of the 67 subjects enrolled, 36 completed the 12-month study and were included in the final analysis (two patients were exited before treatment due to body mass index out of range or missing the treatment window; 13 withdrew early due to irritation or rash, one due to a wound-site infection, and four for miscellaneous reasons; 11 subjects completed treatment but did not complete the required 12-month follow-up). Four to 8 days after abdominoplasty surgery, one half of the subject's wound was treated (randomized) with the Embrace device and the other half was treated (randomized) with the operating physician's optimal treatment method. Removal and reapplication of the Embrace device was repeated weekly for up to 13 visits, and photographic evaluation was performed at 6 and 12 months postoperation. The Embrace device significantly improved scar appearance (p = 0.027) according to visual analogue scale scores.111 Using the Patient and Observer Scar Assessment Scale, both subjects and investigators concluded that Embrace treated scars displayed a significantly improved appearance (p = 0.02 and p < 0.001, respectively).111 As each patient served as their own control, the rate at which subjects exited the study likely had little effect on the final outcome. However, these results could be skewed if the 11 subjects who did not complete the required 12-month follow-up did so because they did not believe that the Embrace device improved scar appearance. This study represents the first level I evidence in scar reduction after surgery to the authors' knowledge.
The clinical trial that followed was a prospective, randomized study assessing the therapeutic ability of the Embrace device to improve aesthetic outcomes after scar revision. Twelve patients underwent scar revision, and the Embrace device was applied to one side of the closed incision 1 to 4 days postoperatively. The standard treatment (Steri-Strips alone, Steri-Strips plus Mederma cream, or no treatment) side of the closed incision served as the control. Ten patients completed the study, and four independent surgeons evaluated their 6-month postrevision scar images. The Embrace device significantly improved scar appearance (p < 0.005), and 100% of patients were either “satisfied” or “very satisfied” with the minimized scarring.112
Summary
In the past decade, there have been major advancements in our understanding of scarless wound healing and the role of mechanotransduction in scar formation. In conjunction with the mounting clinical and financial burdens of scarring, these developments inspire innovative therapies to address wound healing. Basic science research gives insight to the role of mechanical forces in wound healing, whereas clinical trials support reducing tension in the wound environment to minimize scar formation. Future studies should more precisely define the specific molecular mechanisms by which different therapies that reduce tension in the wound environment minimize scar formation. However, there are limitations to the use of tension-shielding therapies for scar reduction. The etiology of keloid formation is complex, and some parts of the body (e.g., the ear) are susceptible to keloid formation with minor injuries to the skin and in the absence of increased tension at the site of injury. In addition, the therapies described are not applicable in cases where severe scarring covers large parts of the body, as is often observed with burn patients. Though compression garment therapy is often used in these cases, there are limited objective data that support the use of compression garment therapy to reduce scarring.113,114 Thus, we propose that studies address how mechanical loading (pressure) therapies, in addition to tension offloading therapies, can be used to reduce scar formation. We also propose the further study of skin regeneration in the adult African spiny mouse and the development of novel therapies that modulate stretch-activated ion channels, growth factor receptors, G-protein coupled receptors, ITGs, and their downstream targets to minimize scar formation. There is hope that continued advancements will produce more effective mechanotherapies for patients who are affected by scarring.
Take-Home Messages.
• Cutaneous wound healing occurs in three phases (inflammation, new tissue formation, and remodeling), all of which are affected by mechanical forces.
• There are differences in the mechanical forces and cellular processes involved in adult wound healing and scarless fetal wound healing.
• Fibroblasts are particularly sensitive to mechanical forces in the wound environment.
• Minimizing mechanical forces in the wound environment improves wound healing and reduces scar formation. This is the basis of emerging mechanotherapies.
Abbreviations and Acronyms
- Akt
protein kinase B
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- hMSC
human mesenchymal stem cell
- ITG
integrin
- MSC
mesenchymal stem cell
- TGFβ
transforming growth factor β
Acknowledgment and Funding Sources
This work was supported by the Howard Hughes Medical Institute.
Author Disclosure and Ghostwriting
G.C.G. and M.T.L. are co-founders of, and own stock in, Neodyne Biosciences, a commercial-stage startup company that produces Embrace Advanced Scar Therapy. The other authors are researchers in the laboratory of M.T.L. and have no other conflicts of interest to declare. The authors listed expressly wrote the content of this article. No ghostwriters were used to write this article.
About the Authors
Leandra A. Barnes, BA, is a medical student at the Stanford University and a Howard Hughes Medical Institute Medical Research Fellow studying wound healing. Clement D. Marshall, MD, is a general surgery resident and a postdoctoral research fellow at Stanford University. He is interested in wound healing, fibrosis, and abdominal adhesions. Tripp Leavitt, BA, BS, is a medical student at Boston University studying wound healing. Michael S. Hu, MD, MPH, MS, is a surgical resident and postdoctoral fellow studying wound healing. Alessandra L. Moore, MD, is a general surgery resident at Brigham and Women's Hospital and a postdoctoral research fellow at Stanford University. Jennifer G. Gonzalez is a research assistant studying wound healing. Michael T. Longaker, MD, MBA, is Professor of Surgery and Bioengineering at Stanford University and the Director of the Program in Regenerative Medicine. His research experience includes wound healing, tissue engineering, and developmental/stem cell biology. Geoffrey C. Gurtner, MD, is a Johnson and Johnson Distinguished Professor of Surgery, Professor of Bioengineering, and Vice Chairman for Research in the Department of Surgery at Stanford University. His laboratory studies the human response to injury for the promotion of tissue repair and regeneration.
References
- 1.Burrington JD. Wound healing in the fetal lamb. J Pediatr Surg 1971;6:523–528 [DOI] [PubMed] [Google Scholar]
- 2.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg 2011;254:217–225 [DOI] [PubMed] [Google Scholar]
- 3.Hassel JC, Roberg B, Kreuter A, Voigtlander V, Rammelsberg P, Hassel AJ. Treatment of ear keloids by compression, using a modified oyster-splint technique. Dermatol Surg 2007;33:208–212 [DOI] [PubMed] [Google Scholar]
- 4.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009;10:75–82 [DOI] [PubMed] [Google Scholar]
- 5.Wilson AM. Use of botulinum toxin type A to prevent widening of facial scars. Plast Reconstr Surg 2006;117:1758–1766; discussion 1767–1768. [DOI] [PubMed] [Google Scholar]
- 6.Reports NRAI: ARRA IMPACT REPORT: Wound Healing. http://report.nih.gov/recovery/impactreports/ViewReport.aspx?Id=59&page=Result&RC=8&sI=7&sS=&sCS=&sKey=&sOrder=ASC&sSort=false (last accessed February9, 2015)
- 7.Aarabi S, Longaker MT, Gurtner GC. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med 2007;4:e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 9.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg 2005;31:674–686; discussion 686. [DOI] [PubMed] [Google Scholar]
- 10.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 11.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005;15:599–607 [DOI] [PubMed] [Google Scholar]
- 12.Martin P, D'Souza D, Martin J, et al. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol 2003;13:1122–1128 [DOI] [PubMed] [Google Scholar]
- 13.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 2007;127:998–1008 [DOI] [PubMed] [Google Scholar]
- 14.Levenson SM, Geever EF, Crowley LV, Oates JF, 3rd, Berard CW, Rosen H. The healing of rat skin wounds. Ann Surg 1965;161:293–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med 1988;187:493–497 [DOI] [PubMed] [Google Scholar]
- 16.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 1998;176:26S–38S [DOI] [PubMed] [Google Scholar]
- 17.Lovvorn HN, 3rd, Cheung DT, Nimni ME, Perelman N, Estes JM, Adzick NS. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg 1999;34:218–223 [DOI] [PubMed] [Google Scholar]
- 18.Longaker MT, Whitby DJ, Adzick NS, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg 1990;25:63–68; discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz HP, Whitby DJ, Longaker MT, Adzick NS. Fetal wound healing. The ontogeny of scar formation in the non-human primate. Ann Surg 1993;217:391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol 1991;147:207–215 [DOI] [PubMed] [Google Scholar]
- 21.Krummel TM, Michna BA, Thomas BL, et al. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg 1988;23:647–652 [DOI] [PubMed] [Google Scholar]
- 22.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 1986;83:4167–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo DD, Zimmermann AS, Nauta A, Longaker MT, Lorenz HP. Scarless fetal skin wound healing update. Birth defects research. Birth Defects Res C Embryo Today. 2012;96:237–247 [DOI] [PubMed] [Google Scholar]
- 24.Aarabi S, Bhatt KA, Shi Y, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 2007;21:3250–3261 [DOI] [PubMed] [Google Scholar]
- 25.Colwell AS, Longaker MT, Lorenz HP. Mammalian fetal organ regeneration. Adv Biochem Eng Biotechnol 2005;93:83–100 [DOI] [PubMed] [Google Scholar]
- 26.Longaker MT, Whitby DJ, Jennings RW, et al. Fetal diaphragmatic wounds heal with scar formation. J Surg Res 1991;50:375–385 [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Wysocki A, Warburton D, Tuan TL. Wound healing in development. Birth Defects Res C Embryo Today 2012;96:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012;489:561–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature 1992;360:179–183 [DOI] [PubMed] [Google Scholar]
- 30.Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol 1996;135:1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyczalkowski MA, Varner VD, Taber LA. Computational and experimental study of the mechanics of embryonic wound healing. J Mech Behav Biomed 2013;28:125–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci 2004;359:777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langer K. Anatomy and physiology of skin .1. Cleavability of cutis. Brit J Plast Surg 1978;31:3–8342028 [Google Scholar]
- 34.Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol 2003;9:3–23 [DOI] [PubMed] [Google Scholar]
- 35.Meyer M, McGrouther DA. A study relating wound tension to scar morphology in the pre-sternal scar using Langers technique. Br J Plast Surg 1991;44:291–294 [DOI] [PubMed] [Google Scholar]
- 36.Wray RC. Force required for wound closure and scar appearance. Plast Reconstr Surg 1983;72:380–382 [DOI] [PubMed] [Google Scholar]
- 37.Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol 2001;155:1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Kim M, Hu YL, et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem 1997;272:30455–30462 [DOI] [PubMed] [Google Scholar]
- 39.Okuda M, Takahashi M, Suero J, et al. Shear stress stimulation of p130(cas) tyrosine phosphorylation requires calcium-dependent c-Src activation. J Biol Chem 1999;274:26803–26809 [DOI] [PubMed] [Google Scholar]
- 40.Wilson E, Sudhir K, Ives HE. Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J Clin Invest 1995;96:2364–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sai X, Naruse K, Sokabe M. Activation of pp60(src) is critical for stretch-induced orienting response in fibroblasts. J Cell Sci 1999;112(Pt 9):1365–1373 [DOI] [PubMed] [Google Scholar]
- 42.Kuipers AJ, Middelbeek J, van Leeuwen FN. Mechanoregulation of cytoskeletal dynamics by TRP channels. Eur J Cell Biol 2012;91:834–846 [DOI] [PubMed] [Google Scholar]
- 43.Thodeti CK, Matthews B, Ravi A, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 2009;104:1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tejada MA, Stople K, Hammami Bomholtz S, Meinild AK, Poulsen AN, Klaerke DA. Cell volume changes regulate slick (Slo2.1), but not slack (Slo2.2) K+ channels. PLoS One 2014;9:e110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tschumperlin DJ, Dai G, Maly IV, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature 2004;429:83–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han O, Li GD, Sumpio BE, Basson MD. Strain induces Caco-2 intestinal epithelial proliferation and differentiation via PKC and tyrosine kinase signals. Am J Physiol 1998;275:G534–G541 [DOI] [PubMed] [Google Scholar]
- 47.Balestreire EM, Apodaca G. Apical epidermal growth factor receptor signaling: regulation of stretch-dependent exocytosis in bladder umbrella cells. Mol Biol Cell 2007;18:1312–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graness A, Cicha I, Goppelt-Struebe M. Contribution of Src-FAK signaling to the induction of connective tissue growth factor in renal fibroblasts. Kidney Int 2006;69:1341–1349 [DOI] [PubMed] [Google Scholar]
- 49.Salcedo R, Resau JH, Halverson D, et al. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J 2000;14:2055–2064 [DOI] [PubMed] [Google Scholar]
- 50.Zeng Y, Sun HR, Yu C, et al. CXCR1 and CXCR2 are novel mechano-sensors mediating laminar shear stress-induced endothelial cell migration. Cytokine 2011;53:42–51 [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Nannuru KC, Sadanandam A, Varney ML, Singh RK. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br J Cancer 2009;100:1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension 2003;41:903–911 [DOI] [PubMed] [Google Scholar]
- 53.Gasparski AN, Beningo KA. Mechanoreception at the cell membrane: more than the integrins. Arch Biochem Biophys 2015;586:20–26 [DOI] [PubMed] [Google Scholar]
- 54.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 2013;12:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater 2014;13:645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das T, Safferling K, Rausch S, Grabe N, Boehm H, Spatz JP. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol 2015;17:276–287 [DOI] [PubMed] [Google Scholar]
- 57.Kenny FN, Connelly JT. Integrin-mediated adhesion and mechano-sensing in cutaneous wound healing. Cell Tissue Res 2015;360:571–582 [DOI] [PubMed] [Google Scholar]
- 58.Takada H, Furuya K, Sokabe M. Mechanosensitive ATP release from hemichannels and Ca(2)(+) influx through TRPC6 accelerate wound closure in keratinocytes. J Cell Sci 2014;127:4159–4171 [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Zhang Y, Zhang N, Wang C, Herrler T, Li Q. An updated review of mechanotransduction in skin disorders: transcriptional regulators, ion channels, and microRNAs. Cell Mol Life Sci 2015;72:2091–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong VW, Longaker MT, Gurtner GC. Soft tissue mechanotransduction in wound healing and fibrosis. Semin Cell Dev Biol 2012;23:981–986 [DOI] [PubMed] [Google Scholar]
- 61.Wong VW, Akaishi S, Longaker MT, Gurtner GC. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol 2011;131:2186–2196 [DOI] [PubMed] [Google Scholar]
- 62.Januszyk M, Wong VW, Bhatt KA, et al. Mechanical offloading of incisional wounds is associated with transcriptional downregulation of inflammatory pathways in a large animal model. Organogenesis 2014;10:186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delanoe-Ayari H, Rieu JP, Sano M. 4D traction force microscopy reveals asymmetric cortical forces in migrating Dictyostelium cells. Phys Rev Lett 2010;105:248103. [DOI] [PubMed] [Google Scholar]
- 64.Liu B, Kim TJ, Wang Y. Live cell imaging of mechanotransduction. J R Soc Interface 2010;7(Suppl 3):S365–S375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sen S, Kumar S. Combining mechanical and optical approaches to dissect cellular mechanobiology. J Biomech 2010;43:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JH, Lin JS. Cell traction force and measurement methods. Biomech Model Mechanobiol 2007;6:361–371 [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Wang N. FRET and mechanobiology. Integr Biol 2009;1:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiquet M, Tunc-Civelek V, Sarasa-Renedo A. Gene regulation by mechanotransduction in fibroblasts. Appl Physiol Nutr Metab 2007;32:967–973 [DOI] [PubMed] [Google Scholar]
- 69.Eckes B, Zweers MC, Zhang ZG, et al. Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. J Invest Dermatol Symp Proc 2006;11:66–72 [DOI] [PubMed] [Google Scholar]
- 70.Kadi A, Fawzi-Grancher S, Lakisic G, Stoltz JF, Muller S. Effect of cyclic stretching and TGF-beta on the SMAD pathway in fibroblasts. Biomed Mater Eng 2008;18:S77–S86 [PubMed] [Google Scholar]
- 71.Miron-Mendoza M, Seemann J, Grinnell F. Collagen fibril flow and tissue translocation coupled to fibroblast migration in 3D collagen matrices. Mol Biol Cell 2008;19:2051–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhee S, Grinnell F. Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev 2007;59:1299–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tamariz E, Grinnell F. Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell 2002;13:3915–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol 2010;26:335–361 [DOI] [PubMed] [Google Scholar]
- 75.Lu F, Ogawa R, Nguyen DT, et al. Microdeformation of three-dimensional cultured fibroblasts induces gene expression and morphological changes. Ann Plast Surg 2011;66:296–300 [DOI] [PubMed] [Google Scholar]
- 76.Webb K, Hitchcock RW, Smeal RM, Li W, Gray SD, Tresco PA. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J Biomech 2006;39:1136–1144 [DOI] [PubMed] [Google Scholar]
- 77.Huang C, Miyazaki K, Akaishi S, Watanabe A, Hyakusoku H, Ogawa R. Biological effects of cellular stretch on human dermal fibroblasts. J Plast Reconstr Aesthet Surg 2013;66:e351–e361 [DOI] [PubMed] [Google Scholar]
- 78.Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K. Activation of Akt by mechanical stretching in human epidermal keratinocytes. Exp Dermatol 2006;15:356–361 [DOI] [PubMed] [Google Scholar]
- 79.Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 2012;18:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong VW, Garg RK, Sorkin M, et al. Loss of keratinocyte focal adhesion kinase stimulates dermal proteolysis through upregulation of MMP9 in wound healing. Ann Surg 2014;260:1138–1146 [DOI] [PubMed] [Google Scholar]
- 81.Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011;2011:969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borgquist O, Ingemansson R, Malmsjo M. Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from −10 to −175 mmHg. Plast Reconstr Surg 2010;125:502–509 [DOI] [PubMed] [Google Scholar]
- 83.Malmsjo M, Ingemansson R, Martin R, Huddleston E. Negative-pressure wound therapy using gauze or open-cell polyurethane foam: similar early effects on pressure transduction and tissue contraction in an experimental porcine wound model. Wound Repair Regen 2009;17:200–205 [DOI] [PubMed] [Google Scholar]
- 84.Malmsjo M, Ingemansson R, Martin R, Huddleston E. Wound edge microvascular blood flow: effects of negative pressure wound therapy using gauze or polyurethane foam. Ann Plast Surg 2009;63:676–681 [DOI] [PubMed] [Google Scholar]
- 85.Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–562 [DOI] [PubMed] [Google Scholar]
- 86.Sanders JE, Mitchell SB, Wang YN, Wu K. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng 2002;49:1626–1631 [DOI] [PubMed] [Google Scholar]
- 87.Rodgers KE, Ellefson DD, Espinoza T, Roda N, Maldonado S, Dizerega GS. Effect of NorLeu3-A(1–7) on scar formation over time after full-thickness incision injury in the rat. Wound Repair Regen 2005;13:309–317 [DOI] [PubMed] [Google Scholar]
- 88.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995;108(Pt 3):985–1002 [DOI] [PubMed] [Google Scholar]
- 89.Qiu C, Coutinho P, Frank S, et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol 2003;13:1697–1703 [DOI] [PubMed] [Google Scholar]
- 90.Iocono JA, Ehrlich HP, Keefer KA, Krummel TM. Hyaluronan induces scarless repair in mouse limb organ culture. J Pediatr Surg 1998;33:564–567 [DOI] [PubMed] [Google Scholar]
- 91.Ha X, Li Y, Lao M, Yuan B, Wu CT. Effect of human hepatocyte growth factor on promoting wound healing and preventing scar formation by adenovirus-mediated gene transfer. Chin Med J (Engl) 2003;116:1029–1033 [PubMed] [Google Scholar]
- 92.Fish PV, Allan GA, Bailey S, et al. Potent and selective nonpeptidic inhibitors of procollagen C-proteinase. J Med Chem 2007;50:3442–3456 [DOI] [PubMed] [Google Scholar]
- 93.Akaishi S, Akimoto M, Hyakusoku H, Ogawa R. The tensile reduction effects of silicone gel sheeting. Plast Reconstr Surg 2010;126:109e–111e [DOI] [PubMed] [Google Scholar]
- 94.Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthet Plast Surg 2008;32:82–92 [DOI] [PubMed] [Google Scholar]
- 95.Quinn KJ. Silicone gel in scar treatment. Burns Incl Therm Inj 1987;13(Suppl):S33–S40 [DOI] [PubMed] [Google Scholar]
- 96.Chang CC, Kuo YF, Chiu HC, Lee JL, Wong TW, Jee SH. Hydration, not silicone, modulates the effects of keratinocytes on fibroblasts. J Surg Res 1995;59:705–711 [DOI] [PubMed] [Google Scholar]
- 97.Choi J, Lee EH, Park SW, Chang H. Regulation of transforming growth factor beta1, platelet-derived growth factor, and basic fibroblast growth factor by silicone gel sheeting in early-stage scarring. Arch Plast Surg 2015;42:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuhn MA, Moffit MR, Smith PD, et al. Silicone sheeting decreases fibroblast activity and downregulates TGFbeta2 in hypertrophic scar model. Int J Surg Invest 2001;2:467–474 [PubMed] [Google Scholar]
- 99.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994;331:1286–1292 [DOI] [PubMed] [Google Scholar]
- 100.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest 1992;90:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg 2009;2:104–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fulton JE., Jr Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatol Surg 1995;21:947–951 [DOI] [PubMed] [Google Scholar]
- 103.O'Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013:CD003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd M. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines. Plast Reconstr Surg 2005;116:1648–1656; discussion 1657–8. [DOI] [PubMed] [Google Scholar]
- 105.Rosengren H, Askew DA, Heal C, Buettner PG, Humphreys WO, Semmens LA. Does taping torso scars following dermatologic surgery improve scar appearance? Dermatol Pract Concept 2013;3:75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tollefson TT, Kamangar F, Aminpour S, Lee A, Durbin-Johnson B, Tinling S. Comparison of effectiveness of silicone gel sheeting with microporous paper tape in the prevention of hypertrophic scarring in a rabbit model. Arch Facial Plast Surg 2012;14:45–51 [DOI] [PubMed] [Google Scholar]
- 107.Doumit J, Vale R, Kanigsberg N. Dynaclose tape: a practical alternative to punch biopsy site closure. J Cutan Med Surg 2013;17:62–65 [DOI] [PubMed] [Google Scholar]
- 108.van de Gevel DF, Hamad MA, Elenbaas TW, Ostertag JU, Schonberger JP. Is the use of Steri-StripTM S for wound closure after coronary artery bypass grafting better than intracuticular suture? Interact Cardiovasc Thorac Surg 2010;10:561–564 [DOI] [PubMed] [Google Scholar]
- 109.Wong VW, Beasley B, Zepeda J, et al. A mechanomodulatory device to minimize incisional scar formation. Adv Wound Care 2013;2:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harunari N, Zhu KQ, Armendariz RT, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns 2006;32:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Longaker MT, Rohrich RJ, Greenberg L, et al. A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation. Plast Reconstr Surg 2014;134:536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lim AF, Weintraub J, Kaplan EN, et al. The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr Surg 2014;133:398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Atiyeh BS, El Khatib AM, Dibo SA. Pressure garment therapy (PGT) of burn scars: evidence-based efficacy. Ann Burns Fire Disasters 2013;26:205–212 [PMC free article] [PubMed] [Google Scholar]
- 114.Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg 2014;72:S198–S201 [DOI] [PubMed] [Google Scholar]