Abstract
Significance: Scarring of the skin from burns, surgery, and injury constitutes a major burden on the healthcare system. Patients affected by major scars, particularly children, suffer from long-term functional and psychological problems.
Recent Advances: Scarring in humans is the end result of the wound healing process, which has evolved to rapidly repair injuries. Wound healing and scar formation are well described on the cellular and molecular levels, but truly effective molecular or cell-based antiscarring treatments still do not exist. Recent discoveries have clarified the role of skin stem cells and fibroblasts in the regeneration of injuries and formation of scar.
Critical Issues: It will be important to show that new advances in the stem cell and fibroblast biology of scarring can be translated into therapies that prevent and reduce scarring in humans without major side effects.
Future Directions: Novel therapies involving the use of purified human cells as well as agents that target specific cells and modulate the immune response to injury are currently undergoing testing. In the basic science realm, researchers continue to refine our understanding of the role that particular cell types play in the development of scar.

Michael T. Longaker, MD, MBA
Scope and Significance
This review begins with an overview of how human skin wounds heal, with an emphasis on the formation of scar as the end result of wound healing. This is followed by a review of recent developments in the cellular biology of wound healing and scarring, particularly related to stem cells and fibroblasts. Finally, traditional and novel treatments intended to prevent and reduce scarring are described. This review will be of interest to clinicians and basic scientists who wish to better understand the processes of wound healing and scarring as well as the treatment options available.
Translational Relevance
A great deal has been learned about the cellular and molecular pathways that contribute to scar formation. At the same time, clinicians have understood for centuries that certain techniques allow wounds to heal faster with less scarring. Despite the enormity of the clinical problem presented by scarring, there has been a failure to translate the basic science of scarring into improved scarring treatments that take advantage of specific molecular and cellular targets. However, in the field, there is optimism that recent advances will bridge the gap between the basic science and the clinical treatment of scarring.
Clinical Relevance
Scarring of the skin affects millions of patients and in some, particularly children and burn victims, causes a great deal of suffering. Scar prevention mainly consists of the principles of wound closure without tension and avoiding infection and wound breakdown. Various treatments for established scars exist, with varying levels of effectiveness. Burn scars are particularly challenging, and treatment is largely supportive with the option for revision surgery to remove scarred skin. A novel drug-based treatment that makes use of a specific molecular or cellular target in the scarring pathway would revolutionize the treatment of scars and would have the potential to improve the lives of many patients.
Overview
Biomedical and societal burden of scars
Scarring of the skin, typically from burns or surgery, places an enormous burden on individual patients and on society. Children are particularly affected and can suffer from long-term physical dysfunction1,2 and psychological harm3–5 from the scars that result from major burns and surgery. In the United States, 500,000 patients per year are treated for burns, many of which leave scars and painful contractures that require major surgery.6,7 Up to $7.5 billion is spent annually on treatment of burns in the United States,8 and much of this cost is related to treatment of the resulting scar and contracture. An estimated 100 million patients per year acquire scars from surgery in the developed world.9 Patients with visible scars, particularly on the face, suffer from social stigma and psychological trauma.9,10 As discussed later, there are many treatments for scarring available, and there is an estimated $12 billion annual market in the United States for scar treatment.11
Discussion
Keloids and hypertrophic scars
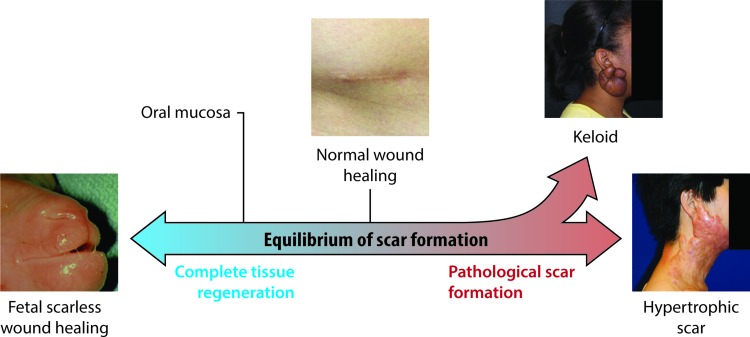
There is a spectrum of scar formation, with scarless regeneration on one end, “normal” scar formation in the center, and pathological scar formation, including hypertrophic and keloid scarring, on the other end (Fig. 1). Keloid and hypertrophic scarring contribute to much of the morbidity of scarring after surgery. Hypertrophic scar can be defined as a scar forming after injury that is larger or more raised than usual, or that results in contracture. Hypertrophic scar is more likely to occur after infection of the wound, closure of the wound with excessive tension, or with position of the wound in areas of skin with high natural tension (such as the shoulders, neck, and sternum).12 Keloid scars, on the contrary, represent an abnormally exuberant scarring response that extends beyond the borders of the original injury. Keloids cause symptoms of pruritus and hyperesthesia and tend to recur after excision, as opposed to hypertrophic scars that may not recur if the scar if revised appropriately.13 While hypertrophic scars often flatten over several years, keloid scars typically do not regress.13 Histologically, hypertrophic and keloid scars both contain abnormal amounts of dermal collagen, but hypertrophic scars consist of mainly type III collagen fibers arranged parallel with the skin surface, while keloids contain disorganized type I and III collagen.14 While hypertrophic scar contains little elastin, the deep dermal layer of keloid scar actually contains more elastin than normal skin.15
Figure 1.
Spectrum of cutaneous scar formation. Illustration of the various possible endpoints of scar formation. Left, a fetal lamb that healed a prior lip wound with no scar whatsoever. Middle, a normal and well-healed appendectomy scar. Upper right, a keloid scar in the classic ear lobe location. Lower right, hypertrophic scar resulting from a scald burn. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
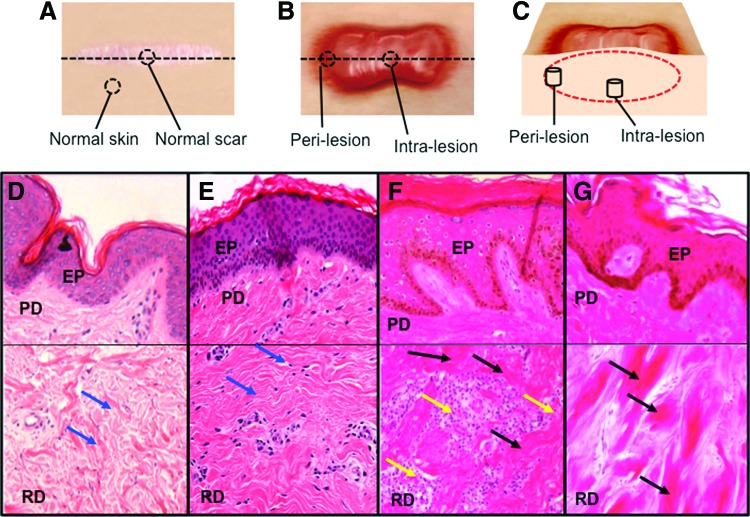
Histological features of keloid that distinguish it from normal skin and hypertrophic scar include whorls and nodules of thick, hyalinized collagen bundles, known as keloidal collagen, and tongue-like projections of scar tissue that advance underneath the surrounding normal epidermis16 (Fig. 2). Compared with hypertrophic scar, keloid scar has relatively nonflattened overlying epidermis and nonfibrotic papillary dermis.17 Keloid scar is seen in all ethnicities but is more common in dark skinned individuals and Asians, and there is often a family history of keloid formation, both of which suggest a genetic predisposition.12 There are cases in which it is difficult for the clinician to determine if a scar represents hypertrophic scar or keloid, and there is no consensus whether keloid is simply an extreme form of hypertrophic scarring or whether it is a distinct clinical entity.
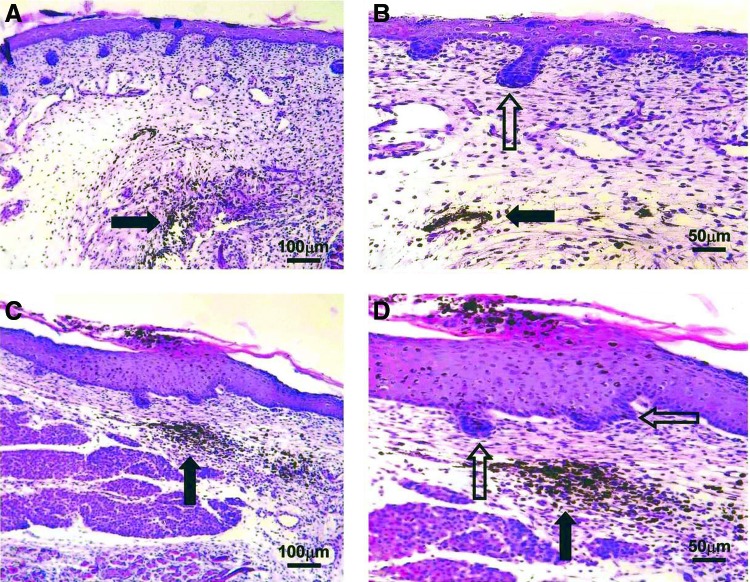
Figure 2.
Dermal biopsy locations from healthy controls and keloid patients with corresponding histology. (A) Transverse view of biopsy locations from normal dermal scar tissue and adjacent normal dermal (nonwounded) skin from which in vitro primary cell cultures were subsequently established. (B) Transverse view of marginal perilesional and reticular dermal intralesional biopsy sites from the keloid scar. (C) Cross section of keloid scar indicating depth of perilesional and intralesional biopsies. (D) Representative H&E staining of tissue section from normal skin indicating organized wavy deposition of collagen (blue arrows). (E) Representative H&E staining of tissue section from a normal scar. (F) Representative H&E staining of a perilesional keloid tissue section indicating a thickened EP with increased cell infiltration (yellow arrow) and deposition of hyalinized collagen bundles in the RD (black arrow). (G) Representative H&E staining of an intralesional keloid tissue section indicating thick compact hyalinized collagen bundle deposition in the RD (black arrow). All the H&E micrographs (D–G) were taken at 200 magnifications. Reprinted with permission from Ashcroft et al.16 EP, epidermis; PD, papillary dermis; H&E, hematoxylin and eosin; RD, reticular dermis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Mechanisms of contracture
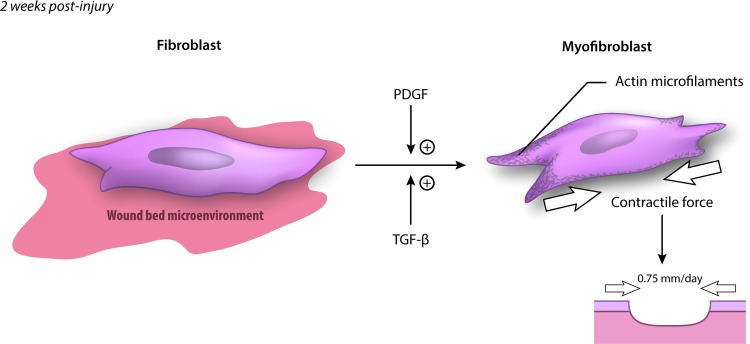
Much of the functional morbidity of major hypertrophic scars, particularly burn scars, is due to contracture of the scar across joints.18,19 Myofibroblasts are the principle cell responsible for scar contraction.20,21 These cells arise from differentiation of fibroblasts at around 1–2 weeks after injury and express smooth muscle actin22,23 (Fig. 3). This transformation is promoted by platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β).21,24,25 The contractile force exerted by myofibroblasts allows unsutured wound edges in a human to move toward each other at up to 0.75 mm per day.26,27
Figure 3.
Transformation of the fibroblast to a myofibroblast. PDGF and TGF-β signaling promotes transformation of fibroblasts to myofibroblasts, which contribute to wound contraction and are characterized by expression of α-smooth muscle actin. The contractile force provided by myofibroblasts can cause wound edges to move toward each other by 0.75 mm per day. PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-beta. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In normal scar, wound contraction is an important part of healing, but myofibroblasts apoptose after epithelialization is complete and contraction does not continue.28 However, in hypertrophic scar, myofibroblasts persist in the wound after epithelialization and cause ongoing contraction, leading to painful and functionally limiting skin contractures.29 The cause of the persistence of contraction in hypertrophic scar is not well understood, but there is evidence that continuous tension across a wound promotes continued production and activity of myofibroblasts.30 This matches the observation that scar contractures are most common over joints and mobile skin31 and may be related to the finding that mechanical stress downregulates proapoptotic genes in fibroblasts.32
Normal wound healing
The production of pathological scar takes place during the process of wound healing, so much of the research into scarring has focused on understanding the cellular and molecular processes involved in normal wound healing. Although a great deal has been learned, there are still important unanswered questions. In particular, the observation that wounds in the early gestation fetus and in the oral mucosa of mammals heal without scar has prompted the question of how to achieve regenerative, scarless wound healing in adult human wounds.
Wound healing in adult humans
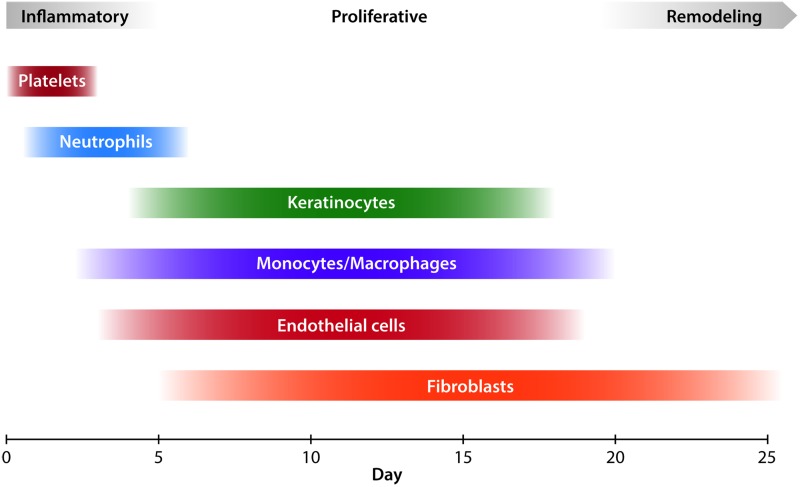
The microscopic process of wound healing is classically described in three overlapping stages: inflammation, proliferation, and remodeling33 (Fig. 4). After the initial injury occurs, a platelet and fibrin clot forms in the injured space, which provides hemostasis and induces a complex cellular and chemical inflammatory response.34 Activated platelets release several chemical mediators, including PDGF.25 Severed nerves also provide signals that increase inflammation in the injured area.35 Thrombin and complement as well as chemical mediators released by resident mast cells cause local capillaries to vasodilate and increase permeability. This augments local blood flow and facilitates migration of inflammatory cells.34 Neutrophils are typically the first cells to migrate into the fibrin matrix that forms the structure of the clot.26 They are attracted by many cytokines and growth factors, including PDGF and interleukin 8 (IL-8), generated by the clot.25 Neutrophils phagocytose cellular debris and bacteria, as well as foreign material, and are usually removed by physical sloughing or by being phagocytosed themselves by macrophages.23 Neutrophils also release a number of factors that further the inflammatory reaction, including IL-1, IL-6, and tumor necrosis factor-α (TNF-α).25
Figure 4.
Classical stages of wound healing with key cellular players. Platelets are the first agents to arrive and contribute to hemostasis. During the inflammatory phase, neutrophils clean up the wound by phagocytosing debris and bacteria. Macrophages arrive after neutrophils and reside for longer, performing phagocytosis as well. During the proliferative phase, keratinocytes migrate onto the wound surface to re-epithelialize the wound, while endothelial cells reconstruct blood vessels. Beginning in the proliferative phase and extending indefinitely in the remodeling phase, fibroblasts lay down collagen and contribute to scar formation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Neutrophils peak in number at 24–48 h, and after this, monocytes become the dominant inflammatory cell in the wound.26 Monocytes are attracted to the wound by fragments of extracellular matrix (ECM) protein, TGF-β, and monocyte chemoattractant protein-1 (MCP-1) and on arrival transform into macrophages.34 Macrophages perform further phagocytosis of dead cells and bacteria, and also release growth factors such as PDGF and vascular endothelial growth factor (VEGF), which promote the process of creating granulation tissue.23
Surprisingly, there is recent experimental evidence that challenges the classical view that neutrophils and macrophages are essential for wound healing. Martin et al. showed that PU.1 null mice lacking both neutrophils and macrophages were able to heal wounds as quickly as wild-type mice and with less fibrosis.36 Phagocytic clearance of debris was slower than in wild-type mice, but “stand in” fibroblasts were able to perform phagocytosis of dead cells and debris.
Other cells that participate in the inflammatory phase of wound healing are T lymphocytes, fibrocytes (circulating fibroblast-like cells), and eosinophils. T cells play a largely regulatory role through the release of cytokines and growth factors. Fibrocytes are recently discovered circulating cells that produce collagen and secrete cytokines and growth factors. Eosinophils are less well studied in wound healing but appear to contribute to epithelialization by secreting TGF-α.34
The inflammatory phase of wound healing serves mainly to clear bacteria and debris from the wound and to prepare the wound environment for repair. At about 72 h after injury, the inflammatory phase winds down and the wound transitions into the proliferative phase. The purpose of the proliferative phase, which occurs from 2 to 10 days after injury, is to construct granulation tissue to fill the defect caused by the wound.33
Fibroblasts are the major cellular agent in the proliferative phase. Their function is to produce collagen to provide structural integrity to the new tissue. Fibroblasts derived from different genetic lineages appear to serve different roles in the formation of normal skin architecture.37 In the healing wound, they provide contractile force to minimize the wound surface area.26 Through production of collagen, fibroblasts are also responsible for scar formation. As discussed later, recent evidence indicates that fibroblasts derived from different genetic lineages based on the transcription factor Engrailed-1 have different roles in scarring.38
Fibroblasts are stimulated to migrate into the wound by PDGF, fibroblast growth factor (FGF), nerve growth factor (NGF), TGF-β, connective tissue growth factor (CTGF), and cysteine-rich 61 (Cyr61).25 PDGF is especially important as it stimulates fibroblast migration as well as ECM production and differentiation into myofibroblasts. Furthermore, exogenous PDGF may be useful to enhance healing in chronic ulcers. Epidermal growth factor (EGF) appears to increase wound fibroblast activity as well.25 Fibroblasts travel along connective tissue fibers in the wound, aided by interactions between cell surface integrins and ECM components such as fibrin, vitronectin, fibronectin, and hyaluronic acid.34 As fibroblasts and other cells navigate the wound environment, they secrete matrix metalloproteinases (MMPs) to clear their path of debris.39 In normal wound healing, most fibroblasts apoptosis after they have laid down adequate ECM.40 Fibroblasts also express cytokines, such as Neu differentiation factor (NDF), which influence other wound healing processes, including keratinocyte migration.25
In addition to production of ECM, the other critical process in the production of granulation tissue is angiogenesis. Beginning 2 days after injury, endothelial cells in local uninjured capillaries temporarily break down their basement membrane, migrate into the wound, and form tubules that mature into new capillaries.41 MMPs play a critical role in angiogenesis by providing a clear path through ECM for the migration of endothelial cells.42 At the same time, fibrils of ECM protein provide tracks on which the migrating endothelial cells move.34 The concentration of blood vessels in granulation tissue, also called “proud flesh,” may be as much as three times higher than that of normal tissue, accounting for the beefy red appearance of the tissue. Later on, many of the capillaries recede through apoptosis of the endothelial cells.43 Signals that promote angiogenesis of the wound include lactic acid and hypoxia, as well as VEGF, FGF, angiopoietin, and TGF-β.41,44
The process of epithelialization of the wound begins just hours after injury and continues during the inflammatory and proliferative phases. Keratinocytes from the edge of the wound and from local hair follicles migrate onto the wound surface. They advance across the wound, leaving a population of proliferating keratinocytes behind, and continue until they reach the keratinocytes of the opposite wound edge. Advancing keratinocytes are also capable of debriding the wound surface by phagocytosing dead cells and debris.34,45
As discussed earlier, fibroblasts in the wound transform into myofibroblasts from 1 to 2 weeks after injury.23 The transcription factor myocardin-related transcription factor-A appears to be critical for myofibroblast differentiation.46 Expression of smooth muscle actin allows the myofibroblasts to cause wound contraction, bringing wound edges closer together and decreasing wound surface area.26
By 2–3 weeks after injury, the tissue defect has been replaced with granulation tissue and has been covered by new epithelial cells. The wound tissue now consists of a relatively acellular mass of disorganized collagen and other ECM proteins, contains no dermal appendages such as hair follicles or sweat glands, and is covered with the epithelium. The surface of the wound has contracted, causing the base of the wound to be wider than the surface. Many of the fibroblasts, macrophages, and endothelial cells that entered the wound space earlier now apoptosis or exit the wound.33 At this point, the process of remodeling begins and can last for months to over a year. During this time, type III collagen and proteoglycans are replaced with type I collagen, and the orientation of collagen fibrils becomes more organized.27 Corresponding with this rearrangement of collagen, the tensile strength of the wound increases from 20% at 3 weeks after injury to 70–80% at 6 weeks, but never reaches the full strength of uninjured tissue.47 Over time, the rearrangement of collagen fibers causes the scar to become less thick and firm. The scar also becomes less red, as many of the initially formed capillaries regress.34
Scarless wound healing
There are several natural scenarios in which animals are able to heal wounds scarlessly. The differences between these processes and scar-forming healing are important areas for wound healing research and may yield insight into strategies to reduce human scarring.
Fetal wound healing
Burrington reported in 1971 that surgical incisions placed on a fetal lamb healed rapidly with little to no scar formation.48 The same phenomenon has been demonstrated experimentally in many animal models, including rabbit,49 rat,50 and mouse.51 Later, an experimental model for human fetal tissue healing developed by Lorenz et al. also demonstrated scarless healing.52 Healed fetal skin is nearly identical to uninjured tissue, with normal-appearing collagen, epidermis, and epidermal appendages, while healed adult skin contains disorganized collagen bundles, flattened epidermis, and no epidermal appendages (hair follicles, sebaceous glands, and sweat glands)53 (Fig. 5).
Figure 5.
Histology of the scarless fetal wound. E16 fetal wounds (hematoxylin and eosin stain). Black arrows indicate India ink tattoo made at the time of wounding to demonstrate scarless wound location. Healed wounds (above, left and below, left) at 72 h (100×). The epidermal appendage (developing hair follicles) pattern shows numerous appendages directly in the healed wound. Magnified views of the same wounds (above, right and below, right) showing epidermal appendages (open arrows) within the wound site (200×). No inflammatory infiltrate is present. Reprinted with permission from Beanes et al.53 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Initially, it was presumed that the intrauterine environment was responsible for the absence of scarring, but subsequent experiments showed that the scarless phenotype is intrinsic to the tissue, regardless of the external environment in which the healing takes place.52,54 Generally, the transition from scarless to scarring healing occurs in humans at 24 weeks of gestation and in mice on gestational day 18.5 (where birth is day 22).55 However, the size of the wound also plays a role, with larger wounds healing with scar earlier in gestation and smaller wounds able to heal scarlessly later.56
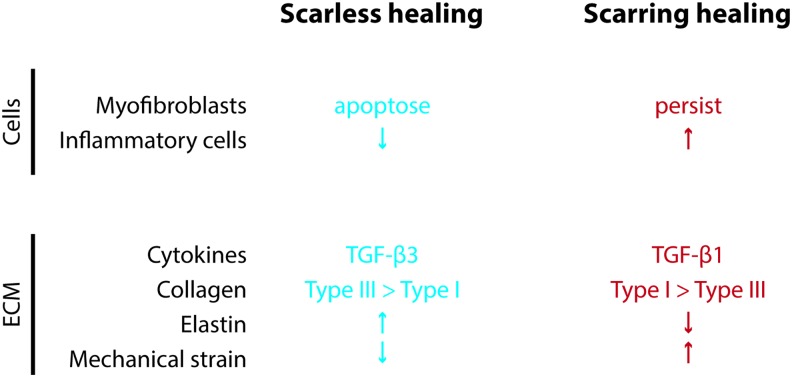
There are several differences between the fetal and adult wound that may contribute to the ability to heal without scar (Fig. 6). The early stage of adult healing is characterized by an inflammatory reaction with migration of neutrophils and macrophages, while the early healing fetal wound contains few of these cells. Several cytokines, including IL-6 and IL-8, are elevated in adult healing and low in fetal healing, while IL-10 is higher in fetal healing. TGF-β1 and TGF-β2 concentrations are higher in the adult wound, while TGF-β3 is higher in the fetal wound. Fibroblasts produce ECM at a higher rate in the fetal wound, and the ratio of type III to type I collagen is higher in the fetal wound than in the adult. The amount of hyaluronic acid in the ECM is high in the fetal wound and low in the adult wound. Myofibroblasts, which are found in the adult wound and are upregulated by mechanical tension,30 are absent in the fetal wound.57,58
Figure 6.
Differences between scarless and scarring wound healing processes. At a cellular and molecular level, there are several differences between fetal and adult wound healing that may contribute to the scarless versus scarring phenotype. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Oral mucosa
Even in the adult human, the oral mucosa is able to heal after injury with little scar, resembling the regenerative healing of fetal skin.59 Even though the oral mucosal wound progresses through the same stages of wound healing as a skin wound, there is less inflammatory response at the beginning, and the overall rate of healing is higher.60 The presence of saliva accelerates wound healing in mouse skin,61 and sialectomized mice that were allowed to lick their wound healed more slowly than controls, suggesting that the absence of normal saliva inhibited healing.62 Extraoral tissue transplanted into the oral cavity remains histologically distinct from mucosal tissue63 and produces scar tissue.64 In addition, recent experiments showed that dermal fibroblasts possessing a “scarring phenotype” transplanted into the oral mucosa produce more scar-like connective tissue compared with oral mucosal fibroblasts transplanted into the dermis.38 Together, these results strongly suggest that factors intrinsic to cells residing in the oral mucosa account for much of the reduced scarring seen in that tissue.
Stem cells in human wound healing
Recently, it has become clear that stem cells from many sources may contribute to skin regeneration (Fig. 7). This is a promising avenue of research because effective stem cell-based therapies for wound healing could also be extended to the regeneration of injured tissue in other organ systems, such as the heart, lungs, and liver.
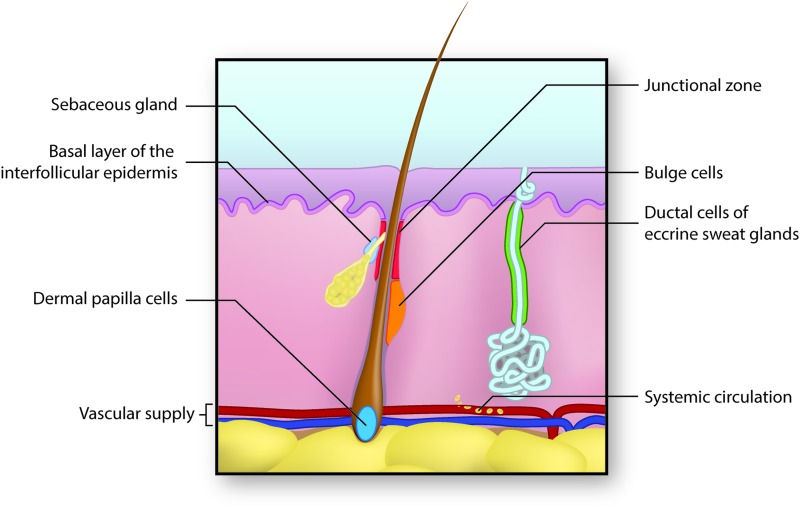
Figure 7.
Sources of stem cells for skin regeneration. Stem cells from several locations may contribute to the regeneration of skin after injury. Epidermal stem cells residing in the basal layer of the interfollicular dermis repopulate the epidermis under normal condition and after injury.65,66 Cells of the dermal papilla can direct the formation of new hair follicles in uninjured skin.67–69 Cells from the hair follicle bulge region repopulate the hair follicle itself normally and can help to repopulate the epidermis after injury.70 The hair follicle junctional zone contains cells with distinct lineages that contribute to hair follicle and epidermal regeneration.65,71,72 Cells of the sebaceous gland primarily regenerate the gland itself,74 while cells of the eccrine sweat gland duct may contribute to epidermal repair after injury.75 Finally, mesenchymal stem cells arising from the bone marrow and circulating in blood may migrate into injured skin and assist in regeneration.76–78 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Under normal circumstances, cells in the basal layer of the interfollicular epidermis proliferate to provide new cells to populate the epidermal surface. The nature of the cells involved in this proliferation is incompletely understood. According to the traditional Epidermal Proliferating Unit (EPU) model, a single multipotent stem cell infrequently divides to produce shorter lived transiently amplifying cells. These undergo several rounds of proliferation before terminally differentiating and migrating to the epidermal surface directly above the stem cell. This vertical unit comprises the EPU.65,66 Recently, the validity of the EPU model has been challenged, and a more complex committed progenitor model has been proposed. In this stochastic model, a proliferating basal stem cell divides to give rise to two more basal stem cells, two differentiated progeny, or one of each.65,66 The differentiated cells then migrate to the epidermal surface, while the basal stem cells remain in place and continue to undergo division.
Stem cells expressing CD133 residing in the dermal papilla (DP) are necessary for the formation of hair follicles in the developing embryo. Different populations of DP stem cells characterized by positivity or negativity for Sox2 influence the type of hair follicle that develops.67 In normal postnatal skin, DP stem cells can interact with epidermal cells to form new hair follicles.67,68 Higgins et al. recently showed that DP cells cultured into microspheroids using the hanging drop culture technique are able to form hair follicles de novo.69 These findings raise the possibility of using cultured DP cells to restore hair growth in alopecia.
Within the hair follicle, there are several populations of stem cells that occupy distinct regions of the hair follicle and display unique cell surface markers. The first to be discovered were those residing in the bulge region of the hair follicle. These bulge stem cells divide infrequently and produce differentiated cells to populate the hair follicle itself, and after injury can contribute cells to epidermal repair.70
The junctional zone of the hair follicle contains multiple populations of multipotent stem cells with different roles.65 Those expressing the protein Lrig1 contribute to infundibulum and sebaceous gland cell turnover and occasionally to interfollicular epidermis repair.71 Those expressing Lgr6 and Gli1 contribute to epidermal and sebaceous gland cell replacement.72 There is evidence that the identity of these hair follicle stem cells is not fixed and that they may switch surface markers as they travel through the hair follicle.73 Lgr5- and Lrt15-expressing stem cells from the secondary hair germ at the base of the hair follicle contribute to hair follicle repair but apparently not to epidermal repair.65
Within the sebaceous gland, there are unipotent progenitor cells identified by the protein Blimp1, which produce differentiated sebocytes.74 Also, the eccrine sweat gland duct region contains a population of unipotent progenitor cells that primarily not only maintain the cellular population of the sweat gland duct but can also participate in epidermal wound repair.75
A recent exciting finding is that circulating bone marrow cells may contribute to skin wound healing. Several groups have shown that bone marrow-derived mesenchymal stem cells can differentiate into epidermal cells and participate in skin wound healing.76–78
Scarring
Scar formation is the natural consequence of large or deep wounds in adult mammals. As discussed earlier, the burden of pathological scarring on patients and the healthcare system is enormous, and as a result, a great deal of research has been conducted to improve treatments for scarring. Despite this work, a truly effective cellular or molecular treatment for scarring remains elusive.
Characteristics of scar
A mature cutaneous scar consists of a large amount of collagen, 80–90% of it type I collagen and the rest type III.27 In fact, 50% of the protein in scar tissue is collagen.79 Collagen in scar tissue is arranged in bundles parallel to the skin surface, while the collagen in normal skin is arranged in a nonparallel “basket-weave” orientation80 (Fig. 8). The basement membrane of the epidermis that develops over scar tissue is flatter than normal because it does not contains the rete pegs that normally penetrate the dermis.27 In addition, cutaneous scar does not contain dermal appendages such as hair follicles and sebaceous glands, and the stem cells that typically inhabit these structures are also absent34,57 (Fig. 9).
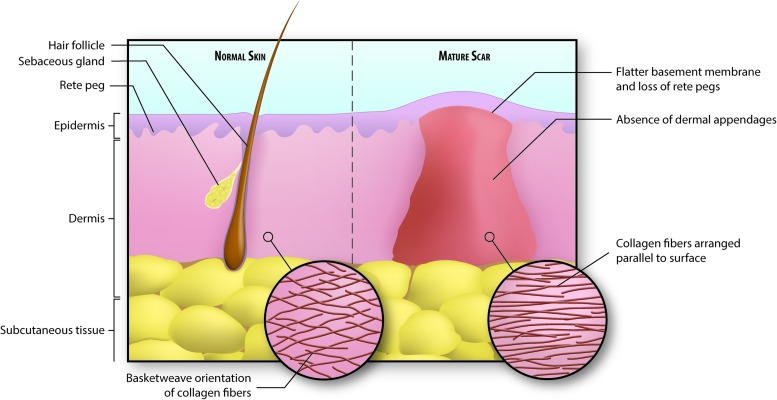
Figure 8.
Illustration of normal versus scarred skin. Collagen in normal skin is arranged in a basket-weave pattern, whereas scar collagen is arranged in parallel fibers. This, in addition to the lack of elastic fibers, contributes to the stiffness of scar tissue. Also, there is a notable absence of dermal appendages, including hair follicles, sebaceous glands, and sweat glands, in scarred skin. Finally, the basement membrane separating epidermis from dermis is flatter in scarred skin and does not contain rete pegs that normally extend down into the dermis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
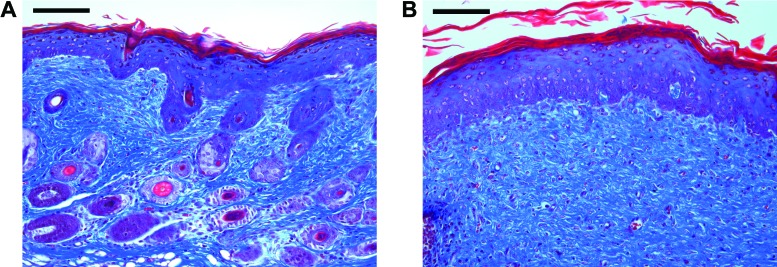
Figure 9.
Histology of normal and scarred skin. Trichrome stain of uninjured (a) and scarred (b) adult mouse skin. Hair follicles are plentiful in normal skin but absent in scarred skin. The scarred dermis is a relatively acellular expanse of collagen. Scale bar, 100 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
After the scar matures, fibroblasts decrease in number, which, along with the absence of dermal appendages, results in a dermal layer containing few cells.34 The ECM of scar tissue contains less elastin than normal skin, contributing to the lack of elasticity seen in scar tissue.15 Scar tissue tends to be raised above the skin surface and hyperpigmented, although these traits may improve over time as the scar matures.34 Finally, the activity of fibroblasts and myofibroblasts can result in contractures that cause pain and restrict movement, particularly when the scar is located over a joint.20
Basic science research
Insights into the cellular and molecular mechanisms of wound healing will hopefully lead to more effective treatment for pathological scars, as well as for other forms of fibrosis.81 In this study, we review several recent developments in the science of wound healing that may provide meaningful targets for new wound healing therapies.
Fibrosis
Our group has shown that in dorsal mouse skin, fibroblasts of a lineage defined by the gene Engrailed 1 and expressing the cell surface marker CD26 are responsible for the vast majority of scar collagen production. Furthermore, inhibition of the activity of these fibroblasts in vivo allowed wound healing with less scar, and remarkably, slowed melanoma growth.38,82 These findings raise the exciting possibility of reducing scarring by targeting the specific cells responsible for the production of scar collagen (Fig. 10).
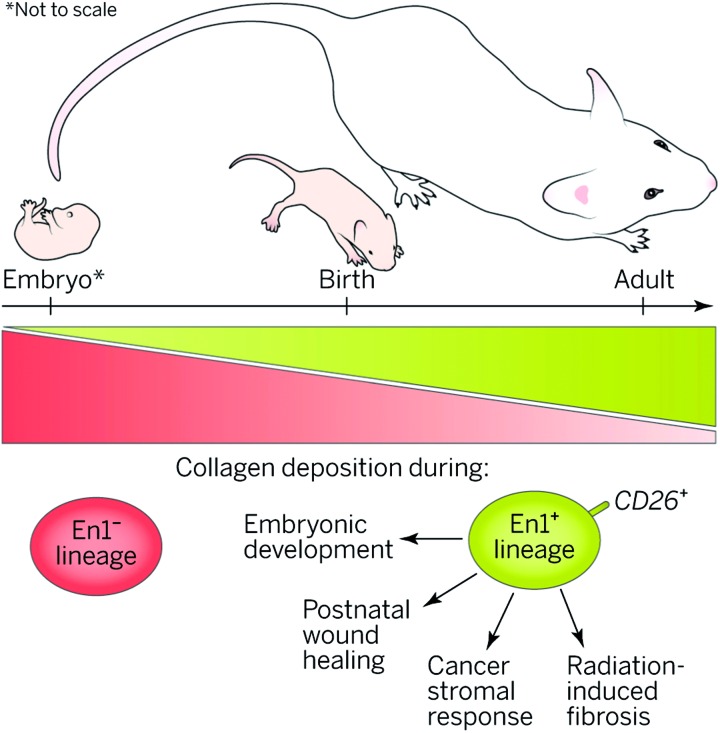
Figure 10.
Scar potential. A specific fibroblast lineage in dorsal skin increasingly populates skin with age and is responsible for extracellular matrix production in multiple developmental and pathophysiological scenarios. Reprinted with permission from Sennett and Rendl.82 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Another recent breakthrough in the field of fibrosis relates to mechanotransduction, the process by which mechanical strain on a wound contributes to the formation of fibrosis. Wong et al. showed that the production of skin fibrosis in response to mechanical strain is mediated, in part, by a pathway consisting of focal adhesion kinase (FAK), extracellular-related kinase (ERK), and MCP-1.83 Small molecule inhibition of FAK allowed for attenuated scar formation through decreased MCP-1 signaling and diminished migration of inflammatory cells.
Stem cells
In 2009, Biernaskie et al. determined that a population of Sox2-expressing cells isolated from the hair follicle are able to migrate into a hair follicle niche and induce hair follicle morphogenesis, can go on to reconstitute dermal cells, and can maintain these abilities after several cycles of division. These attributes suggest that these cells may represent dermal stem cells, which had previously gone unidentified.84 This finding suggests that it may be possible to use dermal stem cells to encourage regeneration by repopulating the dermal appendages that are absent in scar tissue.
Mesenchymal stem cells, which are produced in organs other than the skin, are a potential source of cell-based therapy for wounds. Several groups have shown that mesenchymal stem cells migrate to injured skin and participate in wound repair.76–78 For example, Tamai et al. showed in 2011 that epithelial cells bearing PDGF receptor-α and originating in bone marrow migrated to skin graft sites and contributed to epidermal repair for up to 5 months.77 This work may contribute to the development of therapies in which the patient's own stem cells are isolated for use in regenerating injured tissue.
Lin et al. showed that mouse wounds treated with a combination of the CXCR4 blocking agent AMD3100 and the calcineurin inhibitor tacrolimus experienced greater CD133 progenitor cell migration as well as increased wound epithelialization, hair follicle regeneration, and decreased scar formation. Based on these findings, the authors argue that the combination of AMD3100 and tacrolimus recruited CD133 stem cells to the wound, which were then able to participate in regeneration of the wound.85 These findings raise the possibility of a strategy in which endogenous stem cells are recruited to a wound pharmacologically, rather than by physical transplantation.
Hair follicles
Gay et al. showed that overexpression of FGF9, normally secreted by γδ T cells, increased the generation of hair follicles after wounding. This effect depended, in part, on increased Wnt expression by local fibroblasts.86 This suggests that FGF9 could have therapeutic value in human wound healing, in which the absence of hair follicles in scar tissue is an obstacle for regenerative healing.
Driskell et al. made an important advance in our understanding of wound healing with the discovery that two separate lineages of fibroblasts are responsible for formation of the upper and lower layers of the dermis, respectively. The upper population is required for the formation of hair follicles but is not recruited to the healing wound until later in the healing process, after epithelialization is complete. Activation of β-catenin caused accelerated expansion of the upper dermal lineage with a resulting increase in the formation of hair follicles.37 Based on this result, it is possible that β-catenin signaling could be exploited to accelerate upper dermis development and promote hair follicle generation in human wounds, which could reduce scar formation and allow for more regenerative healing.
Transforming growth factor-beta
TGF-β is a family of growth factors and its three isoforms, TGF-β1, 2, and 3, are involved in many steps in wound healing.87 The TGF-β pathway is a promising target for the modulation of the scarring response. Fibroblasts isolated from hypertrophic scar express higher levels of TGF-β1 and TFG-β receptor, suggesting that an exaggerated TGF-β feedback loop may contribute to the hypertrophic scar phenotype.88,89 Building on these results, Wang et al. inhibited expression of the fibroblast TGF-β receptor and found that this decreased fibroblast production of ECM as well as scar tissue formation in vivo.90 Numerous other studies have shown that inhibition of the TGF-β pathway alters the scaring process in vitro or in animal models,91–94 but it remains to be seen whether these results can be translated into clinical practice.
Three-dimensional lattices
While most in vitro studies are conducted in traditional two-dimensional cell cultures, the development of three-dimensional environments has allowed for a closer simulation of the in vivo environment. For example, the fibroblast-populated collagen lattice was developed in 1979 as a skin substitute95 but more recently has been used to study wound contraction, cell-ECM interactions, and the effects of stretch on cells.96–98
Traditional and current treatments
Dressings
Strategies traditionally used by surgeons to reduce scar formation include placement of incisions along Langer's lines, placement of deep sutures to bring skin edges closer together, and placement of dressings that offload tension from the wound.99
Simple paper tape, when applied to healing cesarean section wounds for 12 weeks after surgery, reduced scar formation and decreased the probability of developing hypertrophic scar.100
Silicone gel sheets (SGS) have been used for the treatment of scarring. A commonly proposed mechanism of action is improved hydration of the stratum corneum.101 SGS have been evaluated for treatment of prevention of hypertrophic and keloid scar in up to fifteen controlled trials, which were analyzed in a Cochrane review. Four studies compared SGS with no treatment, and the other eleven compared SGS with alternate treatments, including nonsilicone sheets, laser therapy, and steroid injection. After pooling results, the Cochrane group found no evidence that SGS are superior to alternate treatments or to no treatment in preventing or treating hypertrophic and keloid scars.102
The embrace device (Neodyne Biosciences, Inc., Menlo Park, CA) is a novel adhesive silicone sheet that is applied to a healing wound and continuously offloads tension. In a recent randomized controlled trial, patients undergoing elective abdominoplasty received the embrace device on one side of the scar, and the other side of the scar was allowed to receive any postoperative and antiscarring care of the surgeon's choice. The embrace device significantly improved the appearance of the treated side compared with the control side after 12 months.103 In a later study in patients undergoing scar revision surgery, the embrace device was applied to one side of the revision wound several days after the surgery and was left for up to 12 weeks. The other side of the incision was treated according to the surgeon's preference. After 6 months of follow-up, the side of the wound treated with the embrace device had a better significant appearance than the control side.104
Topical treatments
Various topical treatments have been tested for their ability to reduce scar formation. MEBO (moist exposed burn ointment, Julphar, Gulf Pharmaceutical Industries, United Arab Emirates), a commercial formula containing herbal extracts, was applied to facial wounds closed primarily with suture and was compared to an antibiotic solution and no topical treatment. The wounds treated with MEBO had better appearance than the other groups at 6 months.105
The popular over-the-counter product Mederma Skin Care gel (Merz Pharmaceuticals, Greensboro, NC, USA), whose active ingredient is an onion extract, provided no clinical benefit in scarring in a rabbit ear wound model.106 There was also no benefit seen with Mederma in a trial involving human patients following Mohs microsurgery.107
The drug imiquimod modulates the inflammatory response in wound healing and stimulates interferon activity.101 Several noncontrolled studies evaluated the use of imiquimod cream after surgical excision of keloids and reported a wide range of recurrence rates (0–100%).108–111 In one study, all keloids recurred within 4 weeks of stopping imiquimod therapy, suggesting that its effect may be only temporary.111
Surgical revision
Hypertrophic scars or normal scars in sensitive locations can often be surgically excised with improved healing of the resulting wound. Careful planning of the surgery is key to prevent reformation of a scar of similar or greater severity. It is also critical to identify keloid scars, as these will often recur after simple surgical excision.13 Surgeons often prefer to wait from several months to over a year for the existing scar to mature before considering surgical revision.101 The most straightforward technique is excision of the scarred skin with linear closure of the wound. This technique is successful only if the surgeon can prevent the condition, such as excessive tension across the wound or infection, that caused the scar to form originally. Z-plasty is a technique that may be useful if the wound is not aligned with Langer's tension lines, because it changes the orientation of the wound. This technique has the added benefit of adding length to the wound, so it is particularly useful for revising contracted scars caused by burns. W-plasty and geometric broken-line closure are similar techniques that change the orientation of the incision, but do not add length to the wound.101,112
Injection treatments
Corticosteroid injection has been the mainstay therapy for keloid scar and can be considered for hypertrophic scars as a second-line treatment. The mechanism of action is thought to relate to reduced fibroblast collagen production as well as inhibition of inflammation.101 Several noncontrolled studies have shown that the majority of patients experience improvement of the keloid without recurrence after steroid injection.113–116 In one controlled study, patients with keloid scars underwent surgical excision and were then randomized to corticosteroid injection (16 mg triamcinolone on postoperative days 0, 7, 21, and 35) or radiation therapy (single dose of 700 or 1,000 cGy). After 30 months of follow-up, no significant difference was found between the treatment arms, and only 22% of patients experienced recurrence.117
Botulinum toxin (Botox, Allergan, Irvine, CA) was associated with improved scar appearance compared with placebo after injection adjacent to excisional wounds on the foreheads of macaque monkeys.118 However, in a trial in which humans presenting to an emergency department with forehead wounds received either botulinum toxin or placebo at the time of suture repair, botulinum toxin was not associated with improved scar appearance in three out of four visual scales.119
Intralesional injection treatments with interferon120–123 and fluorouracil124,125 have been reported with varying levels of efficacy. Injected bleomycin was effective at causing flattening of keloid scars in two noncontrolled studies.126,127
Pressure therapy
Pressure garments are a commonly used conservative therapeutic option for scars and are particularly popular for large burn scars. A proposed mechanism of action involves mild hypoxia of the scar tissue caused by compression of local blood vessels.101 Unfortunately, the evidence for the effectiveness of pressure garments is limited. A recent meta-analysis analyzed six high-quality randomized trials examining the use of pressure garments in patients with large burn scars. Three studies randomized individual patients to either wear the pressure garments or not, two used garments applied to only one of two burned extremities, and the last applied the garment to only part of a burn scar. In the individual studies as well as in the pooled analysis, there was no significant difference between pressure garments and no treatment.128 In addition, the garments can be uncomfortable and expensive, and patient compliance is often poor.129 These results call into question the routine use of pressure garments for burn scars.
Laser
Many different types of lasers have been used to improve scar appearance, with varying rates of success. It appears that different wavelengths of light may act via different mechanisms.101 For example, the 585 nm pulsed dye laser improved scar appearance in a study where only part of the scar was treated with laser and the other part remained untreated and served as a control.130 Proposed mechanisms of action of the 585 nm laser include reduction of TGF-β expression,131 thermolysis of small vessels,132 and rearrangement of collagen fibers.133 1,064 nm Nd-YAG and 1,550 nm Fraxel lasers have also shown some efficacy in improving scar appearance.101
Dermabrasion
Dermabrasion utilizes a mechanical rotating diamond-surface fraise or wire brush that is used to sand off the epidermis and superficial dermal layer of a scar. The bare surface then undergoes reepithelialization and ideally the resulting healed skin is improved in appearance compared with the original scar.101 One trial used dermabrasion to treat only one side of fifteen scars, using the untreated side as a control. At 6 months, the treated side had a superior appearance to the untreated side in 80% of patients. In the remaining 20%, though, the treated side appeared worse than the untreated side at 6 months, raising concern that the procedure could be harmful in some patients.134
Radiation
Radiation is occasionally used for treatment of keloid scars but is ineffective when used alone.101 Instead, radiation is often used following surgical excision of the scar, with reported recurrence rates under 20%.117,135
Cryotherapy
Cryotherapy involves the application of a cold source to the scar, typically liquid nitrogen. The mechanism of action likely involves occlusion of the microcirculation.133 One trial randomized patients with keloid to treatment with cryotherapy or triamcinolone injection and found that most lesions responded minimally to either treatment, and that there was minimal difference between the two treatments.136 A semicontrolled study showed that combined treatment with corticosteroid injection and cryotherapy was superior to either treatment alone.137
Emerging and novel treatments for scar
Several novel agents have been tested for efficacy in treating scarring and other fibrotic conditions in humans, but the results have largely been disappointing (Table 1). Metelimumab, a monoclonal antibody against TGF-β1, showed no efficacy compared with placebo in the treatment of systemic sclerosis.138 Imatinib mesylate, an inhibitor of PDGF and TGF-β, was similarly ineffective in the treatment of scleroderma.139 Human recombinant TGF-β3 failed to meet endpoints in a phase III trial on human scarring, and the results of this study were not published.140 Similarly, a trial evaluating the use of dermal fibroblasts for burn scars was terminated early, and results have not been made available.141 EXC 001, an antisense nucleotide that inhibits CTGF, showed promise in several phase II trials.142 The current status of this drug has not been publicly announced, and there are no ongoing clinical trials registered with clinicaltrials.gov. RXI 109, an RNAi-based inhibitor of CTGF, has completed two phase I trials and is currently undergoing two phase II trials.143,144
Table 1.
Emerging and investigational pharmaceutical scarring treatments
| Agent | Setting | Mechanism | Preliminary results |
|---|---|---|---|
| Metelimumab136 | Systemic sclerosis | Monoclonal antibody against TGF-β1 | No improvement compared with placebo |
| Imatinib mesylate137 | Scleroderma | Inhibitor of PDGF and TGF-β | No improvement compared with placebo |
| Human recombinant TGF-β 3138 | Skin scarring after breast reduction surgery | Modulation of dermal and epidermal cell migration | Failed to meet endpoint in phase III trial |
| Dermal fibroblasts139 | Burn scars | Unclear | Phase I/II trial terminated early, results unknown |
| EXC 001140 | Skin scarring after abdominoplasty | Antisense RNA inhibitor of CTGF | Promising phase II results, no current ongoing trials |
| RXI 109141,142 | Scar revision surgery | RNAi inhibition of CTGF | In phase II trial |
CTGF, connective tissue growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-beta.
Summary
Cutaneous scarring can be a significant source of morbidity for patients and places a tremendous burden on the healthcare system. We have made important advances in our understanding in the processes of wound healing and scarring. Many surgical and pharmaceutical options exist for treating and preventing scarring, but none is completely effective at preventing scarring, particularly in patients with the most severe injuries. Dressings that offload mechanical tension, including the embrace device, have been shown to reduce the severity of scar formation. However, there is a dire need for new therapies that can bring relief to patients with debilitating large burn scars, symptomatic keloid and hypertrophic scars, and for those with conspicuous scars in aesthetically sensitive locations. Novel therapeutics based on precise inhibition of elements of the wound healing pathway have been a disappointment in human clinical trials thus far, but there is hope that continued work in this field will yield new insights into wound healing and will allow for the development of more effective treatments.
Take-Home Messages.
• Scarring of the skin is a major problem for patients and for the healthcare system.
• Wound healing is an evolved and orchestrated process that repairs injury rapidly but ultimately results in scar.
• Scar tissue consists mainly of collagen and is formed by fibroblasts during the proliferative phase of wound healing.
• Wounds in mammalian fetuses and in the oral mucosa can heal virtually without scar under some conditions.
• The propensity for a wound to heal without scar likely involves environmental conditions such as mechanical strain and also depends on the presence or absence of fibroblasts with a scarring phenotype.
• Stem cells from multiple locations within the skin contribute to the regeneration of skin after injury.
• Closure of an incision or laceration with little tension is critical for scar minimization, and certain tension-offloading dressings further reduce scar formation.
• Several therapeutic techniques can improve the appearance of scar that has already formed.
• Many pharmaceutical agents have been tested for the prevention and reduction of scarring but no completely effective therapy exists.
Abbreviations and Acronyms
- CTGF
connective tissue growth factor
- Cyr-61
cysteine-rich 61
- DP
dermal papilla
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EPU
epidermal proliferating unit
- ERK
extracellular-related kinase
- FAK
focal adhesion kinase
- FGF
fibroblast growth factor
- IL
interleukin
- MCP-1
monocyte chemoattractant protein-1
- MEBO
moist exposed burn ointment
- MMP
matrix metalloproteinase
- NDF
Neu differentiation factor
- NGF
nerve growth factor
- PDGF
platelet-derived growth factor
- SGS
silicone gel sheet
- TGF-β
transforming growth factor-beta
- TNF-α
tumor necrosis factor-alpha
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
C.D.M. was supported by the American College of Surgeons (ACS) Resident Research Scholarship. M.S.H. was supported by the California Institute for Regenerative Medicine (CIRM) Clinical Fellow training grant TG2-01159. L.A.B. was supported by the Howard Hughes Medical Institute Medical Research Fellowship. M.S.H., H.P.L., and M.T.L. were supported by the American Society of Maxillofacial Surgeons (ASMS)/Maxillofacial Surgeons Foundation (MSF) Research Grant Award. H.P.L. was supported by NIH grant R01 GM087609, a gift from Ingrid Lai and Bill Shu in honor of Anthony Shu, the Hagey Laboratory for Pediatric Regenerative Medicine and the Oak Foundation. M.T.L. was supported by the Gunn/Olivier fund and the Hagey Laboratory for Pediatric Regenerative Medicine.
Author Disclosure and Ghostwriting
M.T.L. is a cofounder of and owns stock in Neodyne Biosciences, LLC, a commercial stage startup company that produces the embrace device. No other competing financial interests exist. The content of this article was entirely written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Clement D. Marshall, MD, is a general surgery resident at Stanford and a postdoctoral research fellow in the Hagey Laboratory for Pediatric Regenerative Medicine at Stanford. Michael S. Hu, MD, MPH, MS, is a general surgery resident at the University of Hawaii and a postdoctoral research fellow in the Hagey Laboratory for Pediatric Regenerative Medicine at Stanford. Tripp Leavitt, BS, BA, is a medical student at Boston University and a researcher in the Hagey Laboratory for Pediatric Regenerative Medicine at Stanford. Leandra A. Barnes, BS, is a medical student at Stanford and a researcher in the Hagey Laboratory for Pediatric Regenerative Medicine at Stanford. H. Peter Lorenz, MD, is Professor of Plastic Surgery and Chief of Pediatric Plastic Surgery at Stanford. Michael T. Longaker, MD, MBA, is the Deane P. and Louise Mitchell Professor of Surgery at Stanford University School of Medicine. He is the Director of the Hagey Laboratory for Pediatric Regenerative Medicine and the Co-Director of the Institute of Stem Cell Biology and Regenerative Medicine.
References
- 1.Sheridan RL, et al. Long-term outcome of children surviving massive burns. JAMA 2000;283:69–73 [DOI] [PubMed] [Google Scholar]
- 2.Esselman PC. Burn rehabilitation: an overview. Arch Phys Med Rehabil 2007;88:S3–S6 [DOI] [PubMed] [Google Scholar]
- 3.Robert R, et al. Disfiguring burn scars and adolescent self-esteem. Burns 1999;25:581–585 [DOI] [PubMed] [Google Scholar]
- 4.Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol 2003;4:245–272 [DOI] [PubMed] [Google Scholar]
- 5.Thomas CR, et al. Personality disorders in young adult survivors of pediatric burn injury. J Pers Disord 2012;26:255–266 [DOI] [PubMed] [Google Scholar]
- 6.Asuku ME, Ibrahim A, Ijekeye FO. Post-burn axillary contractures in pediatric patients: a retrospective survey of management and outcome. Burns 2008;34:1190–1195 [DOI] [PubMed] [Google Scholar]
- 7.Egeland B, More S, Buchman SR, Cederna PS. Management of difficult pediatric facial burns: reconstruction of burn-related lower eyelid ectropion and perioral contractures. J Craniofac Surg 2008;19:960–969 [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein E, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. Oxford: Oxford University Press, 2006:xiii, 187 [Google Scholar]
- 9.Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthetic Surg 2008;61:1049–1058 [DOI] [PubMed] [Google Scholar]
- 10.Hunt O, Burden D, Hepper P. Stevenson M. Johnston C. Self-reports of psychosocial functioning among children and young adults with cleft lip and palate. Cleft Palate Craniofac J 2006;43:598–605 [DOI] [PubMed] [Google Scholar]
- 11.Sen CK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 2011;17:113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg 2006;8:362–368 [DOI] [PubMed] [Google Scholar]
- 14.Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr 2006;18:396–402 [DOI] [PubMed] [Google Scholar]
- 15.Amadeu TP, Braune AS, Porto LC, Desmouliere A. Costa AM. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen 2004;12:169–174 [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft KJ, Syed F, Bayat A. Site-specific keloid fibroblasts alter the behaviour of normal skin and normal scar fibroblasts through paracrine signalling. PloS One 2013;8:e75600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Yang CC, Chao SC, Wong TW. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol 2004;26:379–384 [DOI] [PubMed] [Google Scholar]
- 18.Esselman PC, Thombs BD, Magyar-Russell G, Fauerbach JA. Burn rehabilitation: state of the science. Am J Phys Med Rehabil 2006;85:383–413 [DOI] [PubMed] [Google Scholar]
- 19.Leblebici B, et al. Quality of life after burn injury: the impact of joint contracture. J Burn Care Res 2006;27:864–868 [DOI] [PubMed] [Google Scholar]
- 20.Shin D, Minn KW. The effect of myofibroblast on contracture of hypertrophic scar. Plast Reconstr Surg 113:633–640 [DOI] [PubMed] [Google Scholar]
- 21.Kwan P, Hori K, Ding J, Tredget EE. Scar and contracture: biological principles. Hand Clin 2009;25:511–528 [DOI] [PubMed] [Google Scholar]
- 22.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 1999;250:273–283 [DOI] [PubMed] [Google Scholar]
- 23.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 24.Nedelec B, Shankowsky H, Scott PG, Ghahary A. Tredget EE. Myofibroblasts and apoptosis in human hypertrophic scars: the effect of interferon-alpha2b. Surgery 2001;130:798–808 [DOI] [PubMed] [Google Scholar]
- 25.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870 [DOI] [PubMed] [Google Scholar]
- 26.Lawrence WT. Physiology of the acute wound. Clin Plast Surg 1998;25:321–340 [PubMed] [Google Scholar]
- 27.Monaco JL, Lawrence WT. Acute wound healing an overview. Clin Plast Surg 2003;30:1–12 [DOI] [PubMed] [Google Scholar]
- 28.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 2005;13:7–12 [DOI] [PubMed] [Google Scholar]
- 29.Ehrlich HP, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 1994;145:105–113 [PMC free article] [PubMed] [Google Scholar]
- 30.Junker JP, Kratz C, Tollback A, Kratz G. Mechanical tension stimulates the transdifferentiation of fibroblasts into myofibroblasts in human burn scars. Burns 2008;34:942–946 [DOI] [PubMed] [Google Scholar]
- 31.Nedelec B, Ghahary A, Scott PG, Tredget EE. Control of wound contraction. Basic and clinical features. Hand Clin 2000;16:289–302 [PubMed] [Google Scholar]
- 32.Derderian CA, et al. Mechanical strain alters gene expression in an in vitro model of hypertrophic scarring. Ann Plast Surg 2005;55:69–75; discussion 75 [DOI] [PubMed] [Google Scholar]
- 33.Gurtner GC, Werner S. Barrandon Y. Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 34.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg 2005;31:674–686; discussion 686 [DOI] [PubMed] [Google Scholar]
- 35.Kim LR, Whelpdale K, Zurowski M, Pomeranz B. Sympathetic denervation impairs epidermal healing in cutaneous wounds. Wound Repair Regen 1998;6:194–201 [DOI] [PubMed] [Google Scholar]
- 36.Martin P, et al. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol 2003;13:1122–1128 [DOI] [PubMed] [Google Scholar]
- 37.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013;504:277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinkevich Y, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015;348:aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hieta N, Impola U, Lopez-Otin C, Saarialho-Kere U, Kahari VM. Matrix metalloproteinase-19 expression in dermal wounds and by fibroblasts in culture. J Invest Dermatol 2003;121:997–1004 [DOI] [PubMed] [Google Scholar]
- 40.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995;146:56–66 [PMC free article] [PubMed] [Google Scholar]
- 41.Li J. Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 2003;60:107–114 [DOI] [PubMed] [Google Scholar]
- 42.Raza SL, Cornelius LA. Matrix metalloproteinases: pro- and anti-angiogenic activities. J Invest Dermatol Symp Proc 2000;5:47–54 [DOI] [PubMed] [Google Scholar]
- 43.DiPietro LA. Angiogenesis and scar formation in healing wounds. Curr Opin Rheumatol 2013;25:87–91 [DOI] [PubMed] [Google Scholar]
- 44.Detmar M, et al. Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J Invest Dermatol 1997;108:263–268 [DOI] [PubMed] [Google Scholar]
- 45.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol 2003;121:219–230 [DOI] [PubMed] [Google Scholar]
- 46.Velasquez LS, et al. Activation of MRTF-A-dependent gene expression with a small molecule promotes myofibroblast differentiation and wound healing. Proc Natl Acad Sci U S A 2013;110:16850–16855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levenson SM, et al. The healing of rat skin wounds. Ann Surg 1965;161:293–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burrington JD. Wound healing in the fetal lamb. J Pediatr Surg 1971;6:523–528 [DOI] [PubMed] [Google Scholar]
- 49.Somasundaram K, Prathap K. Intra-uterine healing of skin wounds in rabbit foetuses. J Pathol 1970;100:81–86 [DOI] [PubMed] [Google Scholar]
- 50.Goss AN. Intra-uterine healing of fetal rat oral mucosal, skin and cartilage wounds. J Oral Pathol 1977;6:35–43 [DOI] [PubMed] [Google Scholar]
- 51.Whitby DJ. Ferguson MW. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 1991;112:651–668 [DOI] [PubMed] [Google Scholar]
- 52.Lorenz HP, et al. Scarless wound repair: a human fetal skin model. Development 1992;114:253–259 [DOI] [PubMed] [Google Scholar]
- 53.Beanes SR, et al. Confocal microscopic analysis of scarless repair in the fetal rat: defining the transition. Plast Reconstr Surg 2002;109:160–170 [DOI] [PubMed] [Google Scholar]
- 54.Longaker MT, et al. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg 1994;219:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colwell AS, Krummel TM, Longaker MT, Lorenz HP. An in vivo mouse excisional wound model of scarless healing. Plast Reconstr Surg 2006;117:2292–2296 [DOI] [PubMed] [Google Scholar]
- 56.Cass DL, et al. Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg 1997;32:411–415 [DOI] [PubMed] [Google Scholar]
- 57.Hu MS, et al. Tissue engineering and regenerative repair in wound healing. Ann Biomed Eng 2014;42:1494–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walmsley GG, et al. Scarless wound healing: chasing the holy grail. Plast Reconstr Surg 2015;135:907–917 [DOI] [PubMed] [Google Scholar]
- 59.Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res 2003;82:621–626 [DOI] [PubMed] [Google Scholar]
- 60.Wong JW, et al. Wound healing in oral mucosa results in reduced scar formation as compared with skin: evidence from the red Duroc pig model and humans. Wound Repair Regen 2009;17:717–729 [DOI] [PubMed] [Google Scholar]
- 61.Hutson JM, Niall M, Evans D, Fowler R. Effect of salivary glands on wound contraction in mice. Nature 1979;279:793–795 [DOI] [PubMed] [Google Scholar]
- 62.Bodner L, Knyszynski A, Adler-Kunin S, Danon D. The effect of selective desalivation on wound healing in mice. Exp Gerontol 1991;26:357–363 [DOI] [PubMed] [Google Scholar]
- 63.Bussi M, Valente G, Curato MP, Carlevato MT, Cortesina G. Is transposed skin transformed in major head and neck mucosal reconstruction? Acta Oto-laryngol 1995;115:348–351 [DOI] [PubMed] [Google Scholar]
- 64.Reilly JS, Behringer WH, Trocki I. Intraoral keloid: complication of forehead flap. Otolaryngol Head Neck Surg 1980;88:139–141 [DOI] [PubMed] [Google Scholar]
- 65.Plikus MV, et al. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol 2012;23:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones PH, Simons BD, Watt FM. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell 2007;1:371–381 [DOI] [PubMed] [Google Scholar]
- 67.Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci 2011;124:1179–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 1984;311:560–562 [DOI] [PubMed] [Google Scholar]
- 69.Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci U S A 2013;110:19679–19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005;11:1351–1354 [DOI] [PubMed] [Google Scholar]
- 71.Jensen KB, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 2009;4:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snippert HJ, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010;327:1385–1389 [DOI] [PubMed] [Google Scholar]
- 73.Petersson M, et al. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J 2011;30:3004–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 2006;126:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu CP, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 2012;150:136–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–2659 [DOI] [PubMed] [Google Scholar]
- 77.Tamai K, et al. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A 2011;108:609–6614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sasaki M, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581–2587 [DOI] [PubMed] [Google Scholar]
- 79.Nimni ME. Collagen: Its structure and function in normal and pathological connective tissues. Semin Arthritis Rheum 1974;4:95–150 [DOI] [PubMed] [Google Scholar]
- 80.van Zuijlen PP, et al. Collagen morphology in human skin and scar tissue: no adaptations in response to mechanical loading at joints. Burns 2003;29:423–431 [DOI] [PubMed] [Google Scholar]
- 81.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol 2014;32:795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sennett R. Rendl M. Developmental biology. A scar is born: origins of fibrotic skin tissue. Science 2015;348:284–285 [DOI] [PubMed] [Google Scholar]
- 83.Wong VW, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 2012;18:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Biernaskie J, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 2009;5:610–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin Q, et al. Pharmacological mobilization of endogenous stem cells significantly promotes skin regeneration after full-thickness excision: the synergistic activity of AMD3100 and tacrolimus. J Invest Dermatol 2014;134:2458–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gay D, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med 2013;19:916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma 2012;2:18–28 [PMC free article] [PubMed] [Google Scholar]
- 88.Wang R, et al. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen 2000;8:128–137 [DOI] [PubMed] [Google Scholar]
- 89.Schmid P, Itin P, Cherry G, Bi C, Cox DA. Enhanced expression of transforming growth factor-beta type I and type II receptors in wound granulation tissue and hypertrophic scar. Am J Pathol 1998;152:485–493 [PMC free article] [PubMed] [Google Scholar]
- 90.Wang YW, et al. siRNA-targeting transforming growth factor-beta type I receptor reduces wound scarring and extracellular matrix deposition of scar tissue. J Invest Dermatol 2014;134:2016–2025 [DOI] [PubMed] [Google Scholar]
- 91.Fan DL, Zhao WJ, Wang YX, Han SY, Guo S. Oxymatrine inhibits collagen synthesis in keloid fibroblasts via inhibition of transforming growth factor-beta1/Smad signaling pathway. Int J Dermatol 2012;51:463–472 [DOI] [PubMed] [Google Scholar]
- 92.Wang Z, et al. Inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J Plast Reconstr Aesthetic Surg 2007;60:1193–1199 [DOI] [PubMed] [Google Scholar]
- 93.Zhang Z, et al. Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns 2007;33:634–641 [DOI] [PubMed] [Google Scholar]
- 94.Kopp J, et al. Abrogation of transforming growth factor-beta signaling by SMAD7 inhibits collagen gel contraction of human dermal fibroblasts. J Biol Chem 2005;280:21570–21576 [DOI] [PubMed] [Google Scholar]
- 95.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 1979;76:1274–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen 2008;16:472–479 [DOI] [PubMed] [Google Scholar]
- 97.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Ann Rev Cell Dev Biol 2010;26:335–361 [DOI] [PubMed] [Google Scholar]
- 98.Throm Quinlan AM, Sierad LN, Capulli AK, Firstenberg LE, Billiar KL. Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PloS One 2011;6:e23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen MA, Davidson TM. Scar management: prevention and treatment strategies. Curr Opin Otolaryngol Head Neck Surg 2005;13:242–247 [DOI] [PubMed] [Google Scholar]
- 100.Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd M. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines. Plast Reconstr Surg 116:1648–1656; discussion 2005;1657–1648 [DOI] [PubMed] [Google Scholar]
- 101.Thomas JR, Somenek M. Scar revision review. Arch Facial Plast Surg 2012;14: 162–174 [DOI] [PubMed] [Google Scholar]
- 102.O'Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2006;CD003826. [DOI] [PubMed] [Google Scholar]
- 103.Longaker MT, et al. A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation. Plast Reconstr Surg 2014;134:536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lim AF, et al. The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr Surg 2014;133:398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atiyeh BS, Amm CA, El Musa KA. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthetic Plast Surg 2003;27:411–417 [DOI] [PubMed] [Google Scholar]
- 106.Saulis AS, Mogford JH, Mustoe TA. Effect of Mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg 110:177–183; discussion 2002;184–176 [DOI] [PubMed] [Google Scholar]
- 107.Jackson BA, Shelton AJ. Pilot study evaluating topical onion extract as treatment for postsurgical scars. Dermatol Surg 1999;25:267–269 [DOI] [PubMed] [Google Scholar]
- 108.Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol 2002;47:S209–S211 [DOI] [PubMed] [Google Scholar]
- 109.Martin-Garcia RF, Busquets AC. Postsurgical use of imiquimod 5% cream in the prevention of earlobe keloid recurrences: results of an open-label, pilot study. Dermatol Surg 2005;31:1394–1398 [DOI] [PubMed] [Google Scholar]
- 110.Cacao FM, Tanaka V, Messina MC. Failure of imiquimod 5% cream to prevent recurrence of surgically excised trunk keloids. Dermatol Surg 2009;35:629–633 [DOI] [PubMed] [Google Scholar]
- 111.Malhotra AK, Gupta S, Khaitan BK, Sharma VK. Imiquimod 5% cream for the prevention of recurrence after excision of presternal keloids. Dermatology 2007;215:63–65 [DOI] [PubMed] [Google Scholar]
- 112.Shockley WW. Scar revision techniques: z-plasty, w-plasty, and geometric broken line closure. Facial Plast Surg Clin North Am 2011;19:455–463 [DOI] [PubMed] [Google Scholar]
- 113.Ardehali B, et al. Objective assessment of keloid scars with three-dimensional imaging: quantifying response to intralesional steroid therapy. Plast Reconstr Surg 2007;119:556–561 [DOI] [PubMed] [Google Scholar]
- 114.Chowdri NA, Masarat M, Mattoo A, Darzi MA. Keloids and hypertrophic scars: results with intraoperative and serial postoperative corticosteroid injection therapy. Aust N Z J Surg 1999;69:655–659 [DOI] [PubMed] [Google Scholar]
- 115.Darzi MA, Chowdri NA, Kaul SK, Khan M. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg 1992;45:374–379 [DOI] [PubMed] [Google Scholar]
- 116.Kiil J. Keloids treated with topical injections of triamcinolone acetonide (kenalog). Immediate and long-term results. Scand J Plast Reconstr Surg 1977;11:169–172 [DOI] [PubMed] [Google Scholar]
- 117.Sclafani AP, Gordon L, Chadha M, Romo T., 3rd. Prevention of earlobe keloid recurrence with postoperative corticosteroid injections versus radiation therapy: a randomized, prospective study and review of the literature. Dermatol Surg 1996;22:569–574 [DOI] [PubMed] [Google Scholar]
- 118.Gassner HG, Sherris DA, Otley CC. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 2000;105:1948–1953; discussion 1954–1945 [DOI] [PubMed] [Google Scholar]
- 119.Ziade M, et al. Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthetic Surg 2013;66:209–214 [DOI] [PubMed] [Google Scholar]
- 120.Berman B, Flores F. Recurrence rates of excised keloids treated with postoperative triamcinolone acetonide injections or interferon alfa-2b injections. J Am Acad Dermatol 1997;37:755–757 [DOI] [PubMed] [Google Scholar]
- 121.Conejo-Mir JS, Corbi R, Linares M. Carbon dioxide laser ablation associated with interferon alfa-2b injections reduces the recurrence of keloids. J Am Acad Dermatol 1998;39:1039–1040 [DOI] [PubMed] [Google Scholar]
- 122.Granstein RD, et al. A controlled trial of intralesional recombinant interferon-gamma in the treatment of keloidal scarring. Clinical and histologic findings. Arch Dermatol 1990;126:1295–1302 [PubMed] [Google Scholar]
- 123.Wong TW, Chiu HC, Yip KM. Intralesional interferon alpha-2b has no effect in the treatment of keloids. Br J Dermatol 1994;130:683–685 [DOI] [PubMed] [Google Scholar]
- 124.Fitzpatrick RE. Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol Surg 1999;25:224–232 [DOI] [PubMed] [Google Scholar]
- 125.Uppal RS, et al. The effects of a single dose of 5-fluorouracil on keloid scars: a clinical trial of timed wound irrigation after extralesional excision. Plast Reconstr Surg 2001;108:1218–1224 [DOI] [PubMed] [Google Scholar]
- 126.Saray Y, Gulec AT. Treatment of keloids and hypertrophic scars with dermojet injections of bleomycin: a preliminary study. Int J Dermatol 2005;44:777–784 [DOI] [PubMed] [Google Scholar]
- 127.Aggarwal H, Saxena A, Lubana PS, Mathur RK, Jain DK. Treatment of keloids and hypertrophic scars using bleom. J Cosmet Dermatol 2008;7:43–49 [DOI] [PubMed] [Google Scholar]
- 128.Anzarut A, Olson J, Singh P, Rowe BH, Tredget EE. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthetic Surg 2009;62:77–84 [DOI] [PubMed] [Google Scholar]
- 129.Macintyre L, Baird M. Pressure garments for use in the treatment of hypertrophic scars—a review of the problems associated with their use. Burns 2006;32:10–15 [DOI] [PubMed] [Google Scholar]
- 130.Nouri K, et al. Comparison of the effects of short- and long-pulse durations when using a 585-nm pulsed dye laser in the treatment of new surgical scars. Lasers Med Sci 2010;25:121–126 [DOI] [PubMed] [Google Scholar]
- 131.Kuo YR, et al. Flashlamp pulsed dye laser (PDL) suppression of keloid proliferation through down-regulation of TGF-beta1 expression and extracellular matrix expression. Lasers Surg Med 2004;34:104–108 [DOI] [PubMed] [Google Scholar]
- 132.Reiken SR, et al. Control of hypertrophic scar growth using selective photothermolysis. Lasers Surg Med 1997;21:7–12 [DOI] [PubMed] [Google Scholar]
- 133.Alster TS, Nanni CA. Pulsed dye laser treatment of hypertrophic burn scars. Plast Reconstr Surg 1998;102:2190–2195 [DOI] [PubMed] [Google Scholar]
- 134.Poulos E, Taylor C, Solish N. Effectiveness of dermasanding (manual dermabrasion) on the appearance of surgical scars: a prospective, randomized, blinded study. J Am Acad Dermatol 2003;48:897–900 [DOI] [PubMed] [Google Scholar]
- 135.Ragoowansi R, Cornes PG, Glees JP, Powell BW, Moss AL. Ear-lobe keloids: treatment by a protocol of surgical excision and immediate postoperative adjuvant radiotherapy. Br J Plast Surg 2001;54:504–508 [DOI] [PubMed] [Google Scholar]
- 136.Layton AM, Yip J, Cunliffe WJ. A comparison of intralesional triamcinolone and cryosurgery in the treatment of acne keloids. Br J Dermatol 1994;130:498–501 [DOI] [PubMed] [Google Scholar]
- 137.Yosipovitch G, Widijanti Sugeng M, Goon A, Chan YH, Goh CL. A comparison of the combined effect of cryotherapy and corticosteroid injections versus corticosteroids and cryotherapy alone on keloids: a controlled study. J Dermatol Treat 2001;12:87–90 [DOI] [PubMed] [Google Scholar]
- 138.Denton CP, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 2007;56:323–333 [DOI] [PubMed] [Google Scholar]
- 139.Prey S, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol 2012;167:1138–1144 [DOI] [PubMed] [Google Scholar]
- 140.“Juvista (Avotermin) in Breast Reduction Surgery Scars - Full Text View - clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT00432328 (last accessed February4, 2016)
- 141.“A Clinical Study of Allogeneic Human Dermal Fibroblasts for Remodeling Scar Contractures.” https://clinicaltrials.gov/ct2/show/study/NCT01564407 (last accessed August6, 2015)
- 142.Galiano RD. An Antisense Oligonucleotide (EXC 001) Targeting Connective Tissue Growth Factor Reduces Skin Scarring Associated with Abdominoplasty and Reduces CTGF Expression. Plast Reconstr Surg 2011;128:121441845 [Google Scholar]
- 143.“A Study to Evaluate the Effectiveness and Safety of RXI 109 on the Outcome of Revised Hypertrophic Scars.” https://clinicaltrials.gov/ct2/show/NCT02246465 (last accessed August6, 2015)
- 144.“A Study to Evaluate the Effectiveness and Safety of RXI 109 on the Outcome of Scar Revision Surgery in Healthy Adults.” https://clinicaltrials.gov/ct2/show/NCT02030275 (last accessed August6, 2015)