Abstract
Phenotype-based small molecule screens in zebrafish embryos and larvae have been successful in accelerating pathway and therapeutic discovery for diverse biological processes. Yet, the application of chemical screens to adult physiologies has been relatively limited due to additional demands on cost, space, and labor associated with screens in adult animals. In this study, we present a 3D printed system and methods for intermittent drug dosing that enable rapid and cost-effective chemical administration in adult zebrafish. Using prefilled screening plates, the system enables dosing of 96 fish in ∼3 min, with a 10-fold reduction in drug quantity compared to that used in previous chemical screens in adult zebrafish. We characterize water quality kinetics during immersion in the system and use these kinetics to rationally design intermittent dosing regimens that result in 100% fish survival. As a demonstration of system fidelity, we show the potential to identify two known chemical inhibitors of adult tail fin regeneration, cyclopamine and dorsomorphin. By developing methods for rapid and cost-effective chemical administration in adult zebrafish, this study expands the potential for small molecule discovery in postembryonic models of development, disease, and regeneration.
Keywords: : chemical screen, 3D printing, small molecule, adult zebrafish, dosing, chemical library
Introduction
Zebrafish are a powerful model system for human disease modeling due to their amenability to experimental approaches to therapeutic discovery that are challenging in other vertebrate systems. A prime example of such an approach is phenotype-based small molecule screening. Most commonly, zebrafish embryos or larvae are loaded into the wells of a 96-well plate (typically filled with ∼200 μL of solution/well1), compounds from chemical libraries are administered to the water (upon which they are absorbed through the skin, gills, and/or digestive tract), and the effects on phenotype are measured. Such screens have been used to study diverse processes, tissues, and organs, mostly within the first few days of development (for comprehensive reviews, see MacRae and Peterson2 and Ou et al.3).
Despite the fact that zebrafish from later stages of development (i.e., juvenile to adult) are being increasingly used to study aspects of postembryonic development,4–6 disease,7 and regeneration,8–10 chemical screens in adults have been relatively limited due to their additional demands on cost, space, and labor. For instance, adult fish require a large volume of water for housing, increasing the quantity of drug necessary to achieve an active concentration in the water. In 2010, Oppedal and Goldsmith performed the first instance of phenotype-based small molecule discovery in adult zebrafish by performing a chemical screen for modulators of adult tail fin regeneration.11 To reduce the quantity of drug required to achieve an active concentration in the water, fish were housed in 100 mL of water in 250 mL specimen cups. However, even in this reduced volume, administering compound for 3 consecutive days at 5 μM (with a 10 mM stock solution provided in a chemical library) would require 250 μL of stock solution, exceeding the quantity of drug supplied in many commercial compound libraries (100 μL of 10 mM stock solution). Since the fish were housed statically, the water and drugs were replenished daily to maintain water quality. These water exchanges can become time-consuming when conducted on a large scale. While alternative methods for drug delivery in adult zebrafish such as oral gavage12 and injection13 reduce the amount of compound needed for administration, they require multiple steps to perform (including preparation of equipment, anesthetization, individual administrations, and recovery from anesthesia) and are therefore inefficient in terms of time and throughput. Thus, there is a need for higher throughput, more cost-effective drug administration methods to increase the efficiency and scalability of chemical screening in adult zebrafish.
In this study, we present ScreenCube, a 3D printed chemical screening system that enables rapid and cost-effective chemical administration in adult zebrafish. We are able to transfer 96 fish into prefilled screening plates for dosing in ∼3 min. This low-volume dosing in the screening plates allows for a 10-fold reduction in drug quantity compared with that used in previous chemical screens in adult zebrafish. We characterize water quality kinetics during intermittent low-volume immersion, and show that these kinetics may be used to rationally design intermittent dosing regimens that result in 100% fish survival. As a showcase of system fidelity, we demonstrate the potential to identify two chemical inhibitors of adult tail fin regeneration, cyclopamine and dorsomorphin.
Materials and Methods
Zebrafish care
All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Washington. Wildtype zebrafish (Aquatic Research Organisms, Hampton, NH) were housed in 28°C water (pH 7.4, Ammonia 0–0.25 ppm, Nitrite 0, Nitrate 20–40 ppm) on a 14:10 h light:dark photoperiod using a commercial housing rack system (Aquaneering, San Diego, CA). Fish were fed commercial zebrafish diet (GEMMA Micro; Skretting, Stavanger, Norway) according to manufacturer's recommendations. All fish used for the study were mixed-sex adult animals of approximately 30–35 mm standard length. Note that in some studies adult fish were withheld from food for up to 7 days. Food restriction in adult zebrafish has been reported for up to 4 weeks.14 Although fish were not fed for the duration of the experiment, they were housed on an isolated screening system using effluent water from our main recirculating system, and thus were likely provided some nutrients from residual food in the effluent water. During the 7 days of food restriction in our studies, we observed no mortality or evidence of lethargy.
3D printing
CAD files were generated in Pro/ENGINEER (PTC, Needham, MA) or FreeCAD (http://freecadweb.org). For 3D printing, CAD designs were converted to STL files, uploaded to Makerbot 3D printing software, and printed using a Makerbot Replicator 2 desktop 3D printer in clear polylactic acid (PLA) plastic. All CAD and STL files are included as Supplementary Data S1–S10; Supplementary Data are available online at www.liebertpub.com/zeb).
Fin regeneration
Fin regeneration studies were performed as previously described.10 In brief, zebrafish were anesthetized in 0.02% MS-222 (E10521; Sigma-Aldrich, St. Louis, MO) and subjected to 50% tail fin amputation in the anteroposterior direction,15 approximated by eye, using a straight razor blade. For in vivo imaging, fish were anesthetized in MS-222 and imaged under a brightfield stereomicroscope. Percent area regrowth was calculated as described previously.10
Chemical dosing
Cyclopamine (item No. 11321; Cayman Chemical Company) and dorsomorphin (item No. 11967; Cayman Chemical Company) were dissolved in ethanol and dimethyl sulfoxide (DMSO; respectively) to form a 10 mM stock solution. For chemical dosing, screening plates were filled with system water at a volume of 10 mL/well, an appropriate volume of stock solution (10 mM) was added to each well, stirred briefly, and the inserts (with fish inside) were quickly transferred into the plates. Plates were placed in a 28.5°C incubator during dosing. Following dosing, the inserts were removed from the screening plates, quickly rinsed in a 6 L tank filled with system water to remove residual chemicals, and returned to housing. Unless otherwise noted, chemicals and water in the screening plate were replenished daily.
Water quality
Dissolved oxygen within the water of the screening plate was measured using a Pinpoint II Oxygen Monitor (American Marine, Ridgefield, CT) according to manufacturer's recommendations. Total ammonia was measured using a colorimetric test kit (API, Chalfont, PA). Volumes were ratiometrically scaled down from manufacturer's recommendations to minimize the volume of water required for sampling.
Statistical analyses
All statistical analyses were performed in Prism (GraphPad Software, La Jolla, CA). For analysis of water quality kinetics, instances of exponential decay were modeled using a standard one-phase decay equation as Y = Plateau + (Y0 − Plateau) × exp(−K × time), and comparisons of models were performed using t-tests of fitted model parameters. All other cases of water quality kinetics were modeled using linear regression, and comparisons of linear regressions were performed using analysis of covariance-based methods.16 For dose–response studies, we performed one-way analysis of variance (ANOVA) followed by Fisher's protected least significant difference (PLSD) post hoc test. Time-dependent effects of drug treatment were assessed via a two-way ANOVA with time and treatment as factors. p < 0.05 was considered statistically significant. All data are presented as mean ± standard error of the mean, unless otherwise noted.
Results
A dual-compartment system for chemical screening in adult zebrafish
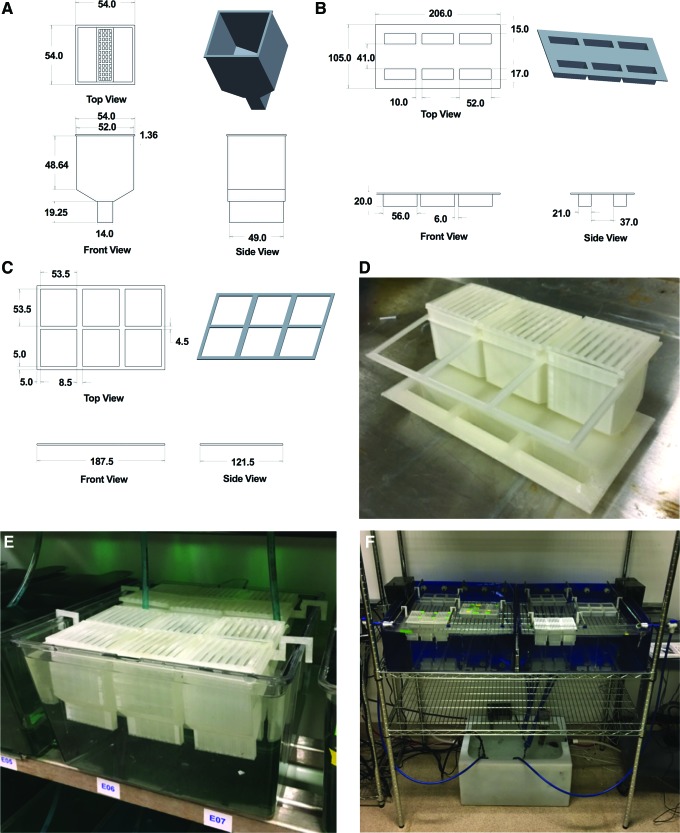
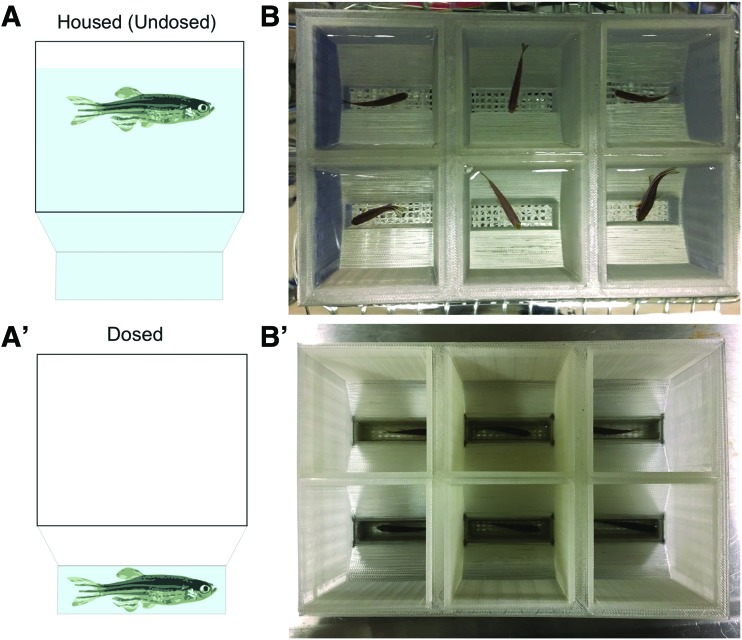
We designed ScreenCube, a multicomponent screening system (Fig. 1A–D) composed of dual-compartment housing inserts with lids, custom six-well screening plates, and insert holders. To facilitate transfer of inserts, we designed six-well insert holders that hold inserts in a 2 × 3 array. The insert holders can be used to nest inserts in either 6 L tanks (for housing on standard commercial recirculating systems) (Fig. 1E), or a dedicated recirculating screening rack (Fig. 1F). By housing fish on a recirculating system, we alleviated the need for daily water changes to maintain water quality, such as would be required if fish were housed statically in specimen cups. Each housing insert holds a single adult fish, and consists of a large-volume upper compartment that tapers into a small-volume, mesh-bottomed lower compartment. The inserts are nested in an outer tank such that the waterline is near the top of the insert, allowing for free movement of fish during normal housing (Fig. 2A, B). During chemical dosing, the inserts are removed from the outer tank, causing the fish to quickly drain into the lower compartment. The inserts are quickly placed into the six-well screening plates that have been prefilled with water (10 mL/well) plus the appropriate compound (Fig. 2A′, B′), enabling drug absorption. In general, fish drain into the lower compartment with extremely high success; in the rare occassion that a fish adheres to the side of the insert, this can be resolved by lightly tapping on the insert wall to facilitate its transfer to the bottom of the insert. During transfer of the insert into the screening well, some fish exhibit C-bends similar to those observed when fish are removed from the water via netting. Once immersed in the screening plate, the fish quickly cessate these movements.
FIG. 1.
Design of a 3D printed system for chemical screening in adult zebrafish. (A–C) Mechanical drawings for the dual-compartment housing insert (A), six-well screening plate (B), and insert holder (C). All dimensions are in mm. For mechanical drawings of insert lids and holders, see Supplementary Figure S1. (D) Image depicting system components setup for drug administration (only three housing inserts are shown to enable view of the wells). (E) Housing inserts nested within a standard 6 L tank on a commercial recirculating rack system. (F) Housing inserts nested within a custom screening rack. A benefit of a dedicated screening rack is that it reduces the potential for introducing residual compounds into the main system. Up to 16 insert arrays (each holding 8 inserts) can be housed in this system (only 6 are shown). 3D, three-dimensional. Color images available online at www.liebertpub.com/zeb
FIG. 2.
Mechanics of drug administration. (A, B) Each housing insert consists of a large-volume upper compartment that tapers into a small-volume, mesh-bottomed lower compartment. During normal housing, the inserts are nested in an outer tank such that the waterline is near the top of the insert. (A′, B′) During chemical dosing, the inserts are removed from the outer tank and quickly placed into prefilled screening plates. The fish quickly drain into the lower compartment and cessate movement following immersion. Color images available online at www.liebertpub.com/zeb
Kinetics of water quality
Due to the small volume of water within the wells of the screening plate, the duration in which zebrafish may be safely dosed during intermittent low-volume immersion is dictated by changes in water quality. Sublethal uniodized ammonia poisoning in zebrafish can occur at levels as low as 0.02 ppm.17 For the water temperature (28°C) and pH (7.2) used in this study, the percentage of total ammonia present in unionized form is ∼1%,17 suggesting that sublethal poisoning can occur at total ammonia levels of 2 ppm. The dissolved oxygen requirements of adult zebrafish have not been systematically determined.18 However, dissolved oxygen levels <3 ppm are known to be stressful to most aquatic organisms.19
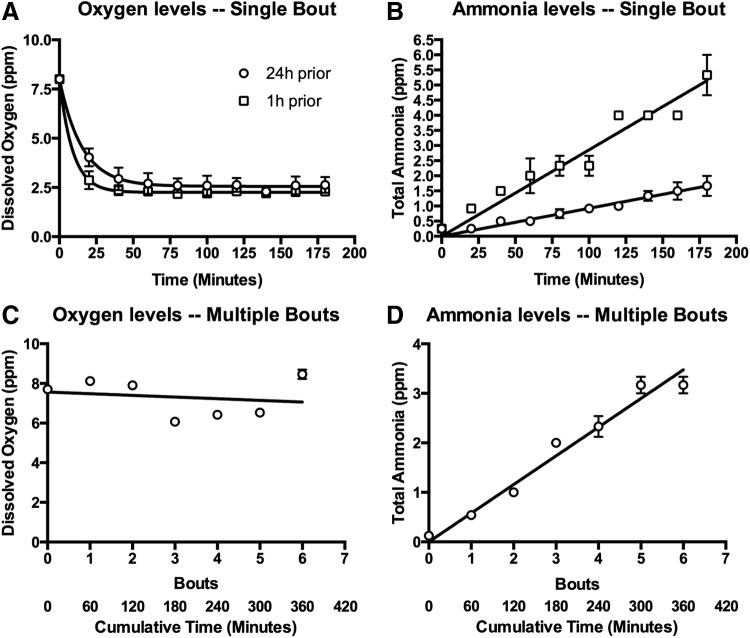
We characterized dissolved oxygen and total ammonia kinetics while fish were immersed in the screening plate wells (Fig. 3). In fish fed both 1 and 24 h before immersion, dissolved oxygen decayed exponentially, with most changes occurring in the first 20 min (Fig. 3A). When fit to a standard one-phase decay model, we observed a significantly higher decay constant and significantly lower plateau level in fish fed 1 h before experimentation compared with those fed 24 h prior (Table 1). The increased oxygen consumption in fish fed 1 h prior is consistent with the notion that oxygen consumption in fish increases following food ingestion due to increased metabolic activity.20 In regard to total ammonia, we observed a linear increase in concentration with time (Fig. 3B), with the slope significantly greater in fish fed 1 h prior compared with those fed 24 h before (Table 2). Thus, feeding of fish within an hour of dosing impacts water quality by both altering fish oxygen consumption as well as ammonia secretion.
FIG. 3.
Water quality kinetics during immersion in the screening plate. (A) Dissolved oxygen over time within a single bout of immersion. Fish were fed 24 h (black circles) or 1 h (white squares) before experimentation. (B) Total ammonia over time within a single bout of immersion. Water was sampled from the same experiment as in (A). (C) Dissolved oxygen over multiple bouts of immersion. Fish were immersed for 60 min/bout, and levels were measured before each bout. (D) Total ammonia over multiple bouts of immersion. Water was sampled from the same experiment as in (C). n = 3 for all experiments; please see Tables 1 and 2 for curve fits.
Table 1.
Exponential Decay Model Parameters Describing the Kinetics of Dissolved Oxygen Within a Single Dosing Bout
| Parameter | 24 h Prior | 1 h Prior | Comparison (p value) |
|---|---|---|---|
| Y0 (ppm) | 8.00 ± 0.21 | 8.00 ± 0.10 | >0.99 |
| Plateau (ppm) | 2.56 ± 0.08 | 2.25 ± 0.04 | 0.001 |
| K (1/min) | 0.07 ± 0.01 | 0.11 ± 0.01 | 0.0005 |
| R2 | 0.96 | 0.99 |
Table 2.
Linear Regression Model Parameters Describing the Kinetics of Total Ammonia Within a Single Dosing Bout
| Parameter | 24 h Prior | 1 h Prior | Comparison (p value |
|---|---|---|---|
| Slope (ppm/h) | 0.55 ± 0.02 | 1.71 ± 0.06 | <0.0001 |
| R2 | 0.96 | 0.99 |
Chemical libraries are typically constructed with compounds possessing a high degree of drug stability, putting forth the potential to reuse prefilled screening plates if water quality is maintained over multiple dosing bouts. Thus, we next examined changes in water quality over multiple days of immersion, reusing the same water for each bout of immersion (60 min/bout for 7 days; fish were fed 24 h before the first bout, and not fed after). We found that ammonia concentration increased linearly with the number of dosing bouts/cumulative amount of dosing time (Fig. 3D). The effective rate of total ammonia accumulation over multiple dosing bouts was 0.58 ± 0.02 ppm/h, similar to the rate of 0.55 ± 0.02 ppm/h observed in fish within a single dosing bout (i.e., in Fig. 3A). This suggests that the introduction of fish into the wells itself does not stimulate appreciable ammonia excretion. Oxygen levels before each bout of immersion were similar each day (Fig. 3C), with linear regression analysis revealing that the slope was not significantly different from zero (p = 0.68). This suggests that the water became reoxygenated to baseline levels during storage.
System validation
To test the fidelity of our system in detecting compounds affecting an adult physiology, we used tail fin regeneration as a model system, and assessed the potential to recover known pharmacological inhibitors of regrowth in this process. Cyclopamine is a hedgehog pathway antagonist that modulates smoothened activity. Quint et al. previously demonstrated that chronic administration of 10 μM cyclopamine starting at 2 days postamputation (dpa) results in impaired regrowth starting by 4 dpa, and which is sustained to 7 dpa.21 Lee et al. demonstrated that administration of 50 μM cyclopamine at 4 dpa was sufficient to reduce blastemal proliferation by 5 dpa.22
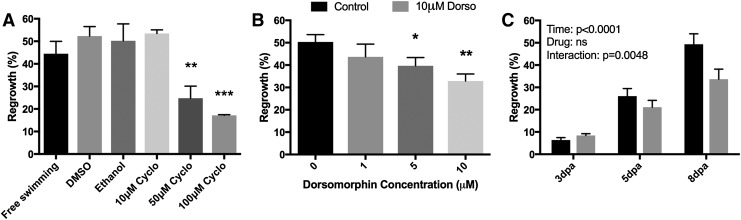
In our studies, we surmised that an immersion time of 60 min would be tolerated in fish withheld from feeding 24 h before dosing based on the fact that dissolved oxygen levels were sustained >3 mg/L for the majority of this period. We used a dosing regimen consisting of 1 h of dosing in the six-well plates per day starting on 1 dpa and ending on 7 dpa. Drug/water volumes were made fresh on each day of dosing. Reepithelialization of the amputation stump via the formation of several layers of epithelial cells is observed by 1 dpa23; by waiting to administer compounds until this time point, we minimized the potential for compound administration to occur via wound entry. We compared fin regrowth in (1) undosed free-swimming fish housed in standard 1.8 L polycarbonate tanks, (2) fish housed in the inserts and administered vehicles (DMSO or ethanol) via the six-well screening plates, and (3) fish housed in the inserts and administered different concentrations of cyclopamine (10, 50, or 100 μM) via the six-well screening plates (Fig. 4). By 8 dpa, we found that free-swimming fish housed in standard 1.8 L polycarbonate tanks had similar fin regrowth compared with DMSO- and ethanol-treated fish housed in the screening system. Fish administered cyclopamine exhibited a dose-dependent reduction in fin regrowth, with significant differences observed for 50 and 100 μM cyclopamine, but not 10 μM (Fig. 4A).
FIG. 4.
Validation of system fidelity in detecting chemical inhibitors of fin regeneration. For all studies, fish were administered drugs daily for 60 min/day starting 1 dpa. (A) Percent fin regrowth at 8 dpa in fish administered cycloplamine. n = 3 for all groups. (B) Percent fin regrowth at 8 dpa in fish administered dorsomorphin. n = 6 for all groups (C) Time-dependent effects of inhibition of fin regrowth in fish administered 10 μM dorsomorphin. n = 5–6/group. For all studies, *p < 0.05; **p < 0.01; ***p < 0.001. dpa, Days postamputation.
Next, we assessed the potential to identify a predicted small molecule inhibitor of fin regeneration representative of lead compounds encompassed in small molecule libraries. DMH1 and LDN-193189 are optimized derivatives of dorsomorphin, a synthetic small molecule inhibitor of type I bone morphogenetic protein (BMP) receptors that was previously identified in an in vivo chemical screen in embryonic zebrafish.24 Thorimbert et al. previously showed that chronically administering 10 μM of the BMP inhibitor DMH1 to adult zebrafish inhibits late- but not early-stage regrowth following amputation.25 Stewart et al. demonstrated similar inhibitory effects on late- but not early-stage regrowth following chronic administration of 5 μM of the BMP inhibitor LDN-193189.26 Due to off-target effects, the concentrations, in which dorsomorphin is active (4 μM) and lethal (20 μM) in zebrafish embryos, are close in magnitude, providing a narrow window upon which to identify effective concentrations. Using the same dosing regimen as that used for cyclopamine studies, we compared fin regrowth in fish administered 1, 5, or 10 μM dorsomorphin. By 8 dpa, fish administered dorsomorphin exhibited a dose-dependent reduction in fin regrowth, with significant differences observed for 5 and 10 μM dorsomorphin but not 1 μM (Fig. 4B). Previous studies examining the effects of dorsomorphin derivatives (DMH1 and LDN-193189) on fin regeneration indicated that drug effects were manifested at later stages of regrowth. When we assessed whether effects on regrowth observed at 8 dpa were observed at earlier time points, we found no evidence of regrowth deficits at 3 and 5 dpa in fish treated with 10 μM dorsomorphin (Fig. 4C), with a two-way ANOVA revealing significant effects of time (p < 0.0001), drug (p < 0.05), and time:drug interaction (p < 0.05).
Discussion
The application of zebrafish chemical screens in postembryonic physiologies has been limited by the lack of methods enabling rapid and cost-effective compound administration in adult animals. We have demonstrated the potential to integrate intermittent drug dosing with a dual-compartment chemical screening system to decrease the quantity of compounds required for screening by tenfold compared to previous chemical screens in adult zebrafish.11 Inserts, lids, holders, and screening plates can be fabricated inexpensively using commercial desktop 3D printers and PLA plastic. Furthermore, the system design and dimensions can be readily modified to suit zebrafish of different developmental stages, as well as other aquatic species of different body shapes and sizes. We have included all CAD and STL files as Supplementary Data. We estimate that the cost of PLA to fabricate each insert and screening plate is $1.66 and $5.24, respectively. While PLA could degrade over time, it is noteworthy that we have reused a single set of inserts and screening plates across multiple experiments over the last 24 months with no noticable signs of degradation. The congruence between active concentrations for dorsomorphin in our studies and similar compounds in other studies suggests that appreciable drug loss due to adsorbtion to the PLA surface did not occur. PLA plastic is considered both food safe and biocompatible, and we have not observed any adverse effects of PLA on fish health. However, it is important to note that toxicity studies of PLA on zebrafish health are lacking. As such, toxicity has been observed in zebrafish for other 3D printing materials,27 and we recommend the use of an isolated screening system (such as that in Fig. 1F) until effects of PLA on zebrafish are better characterized.
In designing our system, we have considered several ethical issues. First, in regard to the water volume during normal housing, the total water volume inside the housing inserts (including both the upper and bottom compartment) is 145 mL. This volume mirrors the volume of water per fish when fish are housed at a common density of seven fish per liter (143 mL/fish). Second, while the transfer of fish from the outer tank to the screening plate could generate some stress, transfer of fish in the system is much faster compared to netting, and eliminates any potential stress or behavioral changes28,29 associated with net-chasing. Finally, while there could be some decrease in fish welfare that interferes with chemical effect, our studies indicate the ability to detect chemical effects in the presence of such decreased welfare, at least for the physiology (fin regeneration) and drugs tested in this study.
We characterized the kinetics of water quality to enable rational design of regimens for intermittent low-volume drug dosing. Our studies suggest that water quality kinetics dictate the design of dosing regimens in two ways. Over a single bout of dosing, the maximum time in which a fish may be immersed in the screening plate is dictated by the decrease in dissolved oxygen, rather than accumulation of total ammonia. Specifically, for fish fed either 1 or 24 h before dosing, the time required for dissolved oxygen to decay to 3 ppm is less than the time required for total ammonia to accumulate to 2 ppm. The decay in dissolved oxygen can be altered through feeding, and is fully reversible after removal of the fish from the screening plate. In our studies, we surmised that an immersion time of 60 min would be tolerated in fish withheld from feeding 24 h before dosing based on the fact that dissolved oxygen levels were sustained >3 ppm for the majority of this period. Consistent with this notion, over the course of all experiments, we observed 100% survival in control fish and near 100% survival in drug-administered fish, with only a single mortality occurring in a fish administered the highest dose of dorsomorphin, likely due to nonspecific drug effects. Over multiple bouts of administration, due to reoxygenation, the maximum allowable times in which a drug/water volume may be reused is dictated by the accumulation in uniodized ammonia (assuming the drug remains stable during reuse). For the water temperature and pH used in this study, sublethal poisoning from uniodized ammonia can occur at total ammonia levels of 2 ppm. In our studies, the rate of increase in total ammonia was ∼0.6 ppm/h; this rate was invariant whether the fish was subjected to a single long dosing bout, or multiple shorter bouts. This suggests each volume of water/drug may be used for a total of 2/0.6 = 3.3 h of cumulative dosing time. While we used fresh drug/water for each day of treatment, our studies suggest that repeated dosing of fish within the same drug/water volume could be a viable option to further conserve drug quantities during chemical screening.
To test the fidelity of the system, we assessed the potential to identify pharmacological inhibitors of adult tail fin regeneration. We found that intermittent dosing of cyclopamine mirrored effects of chronic cyclopamine exposure reported by Quint et al. in inhibiting later stages of regrowth in a dose-dependent manner, although higher concentrations were required to achieve an active concentration. In contrast, intermittent dosing of dorsomorphin replicated dose- and time-dependent effects of two dorsomorphin analogs, LDN-193189 and DMH1, at concentrations close to those previously reported to be active during tail fin regeneration. Following fin amputation, ectopic sonic hedgehog expression leads to bone fusion through a process mediated by activation of Bmp signaling,23 suggesting that Bmp signaling in the fin may be under the influence of the Shh pathway. This relationship does not appear to be reciprocal, as inhibition of bmp does not influence Shh signaling.30 The fact that dorsomorphin phenocopied the time-dependent effects of cyclopamine in inhibiting late regrowth reinforces a potential connection between Shh and Bmp type I receptor signaling in mediating fin outgrowth and warrants further investigation in future studies.
In our studies, we only assessed a single duration of dosing (1 h/day), and it is unclear to what degree altering this duration may affect assay sensitivity. While systematic investigations of the kinetics of drug absorption in zebrafish have yet to be performed, in goldfish, it has been proposed that the dosing time necessary for the fish to absorb a quantify of drug from a drug bath of concentration [C] scales as ∼1/[C].31 In this case, dosing fish for twice as long would require half the concentration to achieve the same quantity of drug to be absorbed; conversely, dosing fish for half as long would require twice the concentration. This suggests that different dosing strategies may be used as different means to achieve the same amount of drug absorption: lower drug concentrations with a longer dosing time may be used to conserve drug quantities; higher drug concentrations with shorter dosing times may be used to increase the throughput of drug administration, although at a higher monetary cost. While dissolved oxygen decay limits the duration in which fish may be immersed within a single loading bout, it is possible that dosing fish within a high-oxygen environment may reduce or even eliminate dissolved oxygen decay and, thus, extend the range of allowable dosing times.
Some limitations of our studies should be considered. First, a concern in using the ScreenCube system is how to rinse the zebrafish/inserts to ensure that chemicals do not reenter the recirculating system. In this context, it is important to note that the adsorption forces between chemicals and the fish surface, and between chemicals and PLA plastic, can vary substantially from chemical-to-chemical. While we described a generic rinse procedure, we are unable to recommend a specific, universal strategy (e.g., a minimum number of rinses of a specific duration) to ensure that residual chemicals on the surface of the fish and/or PLA plastic (either on the insert or screening well) are removed sufficiently in all cases. In this context, we recommend testing rinse procedures for each chemical to verify safe reintroduction of fish/inserts to a recirculating system, or if this is not practical, the use of an isolated screening system as in Figure 1F. Second, while our studies of water quality kinetics suggest the potential to repeatedly use drug/water volumes over multiple dosing bouts, several practical issues need to be resolved before such strategies can be used. This includes characterizing drug stability over multiple days, potential loss of drug over multiple days as it is absorbed by the animals, and fish stress/welfare (including potential secretion of stress hormones into the water that would be concentrated in the reused drug solution). Given these issues, it is possible that the reuse of drug solutions may not be best practice in many cases. The potential complications associated with the reuse of drug solutions need to be carefully considered against possible benefits such as increased sample sizes and associated experimental power, due to lower drug costs. Finally, it is noteworthy that drug actions can differ substantially depending on whether they are administered chronically or intermittently. While chronic administration is precluded in our system, intermittent administration more closely resembles drug kinetics and bioavailability associated with intermittent oral dosing in mammals,32 and thus has potential to enhance translational value in therapeutic screening applications.
In conclusion, we have developed a system for rapid and cost-effective chemical administration in adult zebrafish, expanding the potential for small molecule discovery in postembryonic models of development, disease, and regeneration.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award number AR066061. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. R.Y.K. would also like to acknowledge support from UW Royalty Research Fund Grant A88052, the University of Washington Department of Orthopedics and Sports Medicine.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kaufman CK, White RM, Zon L. Chemical genetic screening in the zebrafish embryo. Nat Protoc 2009;4:1422–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov 2015;14:721–731 [DOI] [PubMed] [Google Scholar]
- 3.Ou HC, Santos F, Raible DW, Simon JA, Rubel EW. Drug screening for hearing loss: using the zebrafish lateral line to screen for drugs that prevent and cause hearing loss. Drug Discov Today 2010;15:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witten PE, Hansen A, Hall BK. Features of mono- and multinucleated bone resorbing cells of the zebrafish Danio rerio and their contribution to skeletal development, remodeling, and growth. J Morphol 2001;250:197–207 [DOI] [PubMed] [Google Scholar]
- 5.Mariotti M, Carnovali M, Banfi G. Danio rerio: the Janus of the bone from embryo to scale. Clin Cases Miner Bone Metab 2015;12:188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 2005;310:1782–1786 [DOI] [PubMed] [Google Scholar]
- 7.Dang M, Henderson RE, Garraway LA, Zon LI. Long-term drug administration in the adult zebrafish using oral gavage for cancer preclinical studies. Dis Model Mech 2016;9:811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J, Poss KD. Explant culture of adult zebrafish hearts for epicardial regeneration studies. Nat Protoc 2016;11:872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui SP, Dutta A, Ghosh S. Cellular response after crush injury in adult zebrafish spinal cord. Dev Dyn 2010;239:2962–2979 [DOI] [PubMed] [Google Scholar]
- 10.Recidoro AM, Roof AC, Schmitt M, Worton LE, Petrie T, Strand N, et al. Botulinum toxin induces muscle paralysis and inhibits bone regeneration in zebrafish. J Bone Miner Res 2014;29:2346–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oppedal D, Goldsmith MI. A chemical screen to identify novel inhibitors of fin regeneration in zebrafish. Zebrafish 2010;7:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zang L, Morikane D, Shimada Y, Tanaka T, Nishimura N. A novel protocol for the oral administration of test chemicals to adult zebrafish. Zebrafish 2011;8:203–210 [DOI] [PubMed] [Google Scholar]
- 13.Kinkel MD, Eames SC, Philipson LH, Prince VE. Intraperitoneal injection into adult zebrafish. J Vis Exp 2010;42:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldsmith MI, Iovine MK, O'Reilly-Pol T, Johnson SL. A developmental transition in growth control during zebrafish caudal fin development. Dev Biol 2006;296:450–457 [DOI] [PubMed] [Google Scholar]
- 15.Huang CC, Lawson ND, Weinstein BM, Johnson SL. Reg6 is required for branching morphogenesis during blood vessel regeneration in zebrafish caudal fins. Dev Biol 2003;264:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zar JH: Biostatistical Analysis. 2nd ed. Prentice-Hall, Englewood Cliffs, NJ, 1984 [Google Scholar]
- 17.Detrich HW, Westerfield M, Zon LI: Essential Zebrafish Methods: Cell and Developmental Biology. Elsevier, Amsterdam, 2009 [Google Scholar]
- 18.Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture 2007;269:1–20 [Google Scholar]
- 19.Weiner ER: Applications of Environmental Aquatic Chemistry: A Practical Guide. 3rd ed. CRC Press/Taylor & Francis Group, Boca Raton, 2013 [Google Scholar]
- 20.Jobling M. The influences of feeding on the metabolic-rate of fishes—a short review. J Fish Biol 1981;18:385–400 [Google Scholar]
- 21.Quint E, Smith A, Avaron F, Laforest L, Miles J, Gaffield W, et al. Bone patterning is altered in the regenerating zebrafish caudal fin after ectopic expression of sonic hedgehog and bmp2b or exposure to cyclopamine. Proc Natl Acad Sci U S A 2002;99:8713–8718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y, Hami D, De Val S, Kagermeier-Schenk B, Wills AA, Black BL, et al. Maintenance of blastemal proliferation by functionally diverse epidermis in regenerating zebrafish fins. Dev Biol 2009;331:270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chablais F, Jazwinska A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development 2010;137:871–879 [DOI] [PubMed] [Google Scholar]
- 24.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 2008;4:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorimbert V, Konig D, Marro J, Ruggiero F, Jazwinska A. Bone morphogenetic protein signaling promotes morphogenesis of blood vessels, wound epidermis, and actinotrichia during fin regeneration in zebrafish. Faseb J 2015;29:4299–4312 [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Gomez AW, Armstrong BE, Henner A, Stankunas K. Sequential and opposing activities of Wnt and BMP coordinate zebrafish bone regeneration. Cell Reports 2014;6:482–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdonald NP, Zhu F, Hall CJ, Reboud J, Crosier PS, Patton EE, et al. Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays. Lab Chip 2016;16:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahash K, Masuda R, Yamashita Y. Bottom feeding and net chasing improve foraging behavior in hatchery-reared Japanese flounder Paralichthys olivaceus juveniles for stocking. Fish Sci 2012;79:55–60 [Google Scholar]
- 29.Kohji Takahashi RM: Net-Chasing Training Improves the Behavioral Characteristics of Hatchery-Reared Red Sea Bream (Pagrus major) Juveniles. Canadian Journal of Fisheries and Aquatic Sciences, NRC Research Press, Ottawa, Canada, 2017 [Google Scholar]
- 30.Smith A, Avaron F, Guay D, Padhi BK, Akimenko MA. Inhibition of BMP signaling during zebrafish fin regeneration disrupts fin growth and scleroblasts differentiation and function. Dev Biol 2006;299:438–454 [DOI] [PubMed] [Google Scholar]
- 31.Nightingale CH, Gibaldi M. Kinetics of drug absorption in goldfish. J Pharm Sci 1971;60:1360–1363 [DOI] [PubMed] [Google Scholar]
- 32.Ridges S, Heaton WL, Joshi D, Choi H, Eiring A, Batchelor L, et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood 2012;119:5621–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.