Abstract
BACKGROUND/OBJECTIVES
Exposure of the normal lung tissue around the cancerous tumor during radiotherapy causes serious side effects such as pneumonitis and pulmonary fibrosis. Radioprotectors used during cancer radiotherapy could protect the patient from side effects induced by radiation injury of the normal tissue. Delphinidin has strong antioxidant properties, and it works as the driving force of a radioprotective effect by scavenging radiation-induced reactive oxygen species (ROS). However, no studies have been conducted on the radioprotective effect of delphinidin against high linear energy transfer radiation. Therefore, this study was undertaken to evaluate the radioprotective effects of delphinidin on human lung cells against a proton beam.
MATERIALS/METHODS
Normal human lung cells (HEL 299 cells) were used for in vitro experiments. The 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay assessed the cytotoxicity of delphinidin and cell viability. The expression of radiation induced cellular ROS was measured by the 2′-7′-dicholordihydrofluorescein diacetate assay. Superoxide dismutase activity assay and catalase activity assay were used for evaluating the activity of corresponding enzymes. In addition, radioprotective effects on DNA damage-induced cellular apoptosis were evaluated by Western blot assay.
RESULTS
Experimental analysis, including cell survival assay, MTT assay, and Western blot assay, revealed the radioprotective effects of delphinidin. These include restoring the activities of antioxidant enzymes of damaged cells, increase in the levels of pro-survival protein, and decrease of pro-apoptosis proteins. The results from different experiments were compatible with each to provide a substantial conclusion.
CONCLUSION
Low concentration (2.5 µM/mL) of delphinidin administration prior to radiation exposure was radioprotective against a low dose of proton beam exposure. Hence, delphinidin is a promising shielding agent against radiation, protecting the normal tissues around a cancerous tumor, which are unintentionally exposed to low doses of radiation during proton therapy.
Keywords: Delphinidin, radiation protective agent, proton therapy, reactive oxygen species
INTRODUCTION
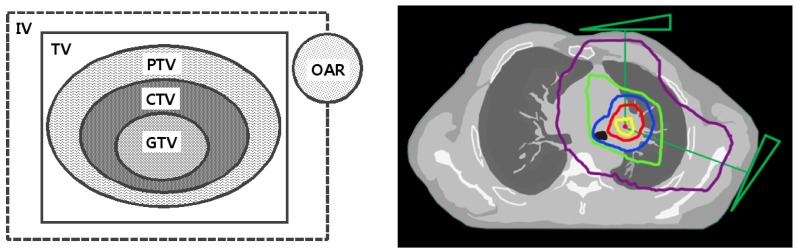
Lung cancer is globally the leading cause of death from cancer. According to the National Cancer Information Center, the number of lung cancer deaths in Korea in 2015 was 17,399, accounting for 22.63% of all deaths from cancer. The incidence of lung cancer accounts for 11.3% of all cancer cases, and is the fourth highest incidence of the Korean cancers patients. Treatments for lung cancer include surgery, chemotherapy and radiotherapy, with more than 70% of lung cancer patients receiving radiotherapy [1]. However, as shown in Fig. 1, organ at risk (OAR) is unavoidably included in irradiation volume for cancer treatment. The side effects on normal lung tissues caused by radiation exposure still remain complications that need to be solved; these include radiation pneumonitis, pulmonary fibrosis, etc. [2]. Typically, there are two methods for reducing the side effects on normal tissues. One is to reduce the radiation dose to normal tissues by optimized delivery of curative radiation to the cancer, and the other is to reduce the damage of the exposed normal tissues using radioprotective agents [3].
Fig. 1. Isodose curve for lung cancer radiotherapy.
GTV, gross tumor volume (yellow); CTV, clinical target volume (red); PTV, planning target volume (blue); TV, treated volume (green); IV, irradiation volume (purple); OAR, organs at risk. In many cases, right from the planning stage, organs at risk, which is a normal tissue, are unavoidably included in the irradiation volume for cancer treatment.
As a therapeutic technique for the optimized delivery of curative radiation to the cancer, radiotherapy using heavy charged particulate radiations, such as proton beam and carbon beam, has recently been tried. The heavy charged particulate radiations are able to localize the radiation dose within the tumor volume by the physical property called “Bragg Peak”, and are more effective than photon radiations in reducing the radiation dose to the normal cells. Nevertheless, the exposure of normal tissue around the tumor still remains a problem that requires improvement in radiotherapy. It corresponds to the exposure of highly radiosensitive tissue or critical organs around the tumor, and the normal tissue margins with microscopic disease [3]. Another way to reduce the damage of normal tissues around the tumor is to administer radioprotective agents before or during the radiation exposure. So far, amifostine is the clinically used unique radioprotector approved by the FDA [4]. However, because amifostine induces severe adverse effects such as nausea, vomiting, hypotension, nephron and neuro-toxicity, the clinical use is limited [5]. Therefore, recent studies are aiming to develop safer radioprotectors with less toxicity and more efficiency. Studies are especially being conducted on natural compounds that exist in plants or fruits [6].
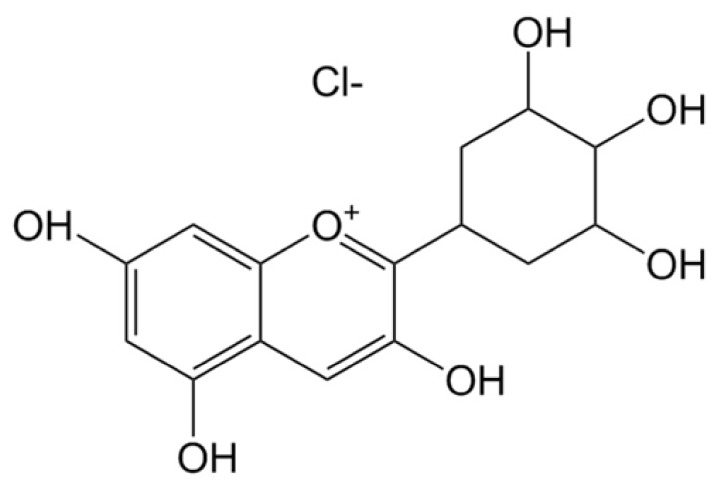
One such flavonoid, anthocyanins are pigment substances that render a bluish purple, black, or purple color to the fruit, flower, stem, and leaf of a plant. They are abundant in foods such as berries, red grapes, cherries, red potatoes and red cabbage [7,8]. In terms of functionality, anthocyanins are reported to be effective in neurological diseases, hypoglycemia, cardiac toxicity, and anti-inflammation due to their antioxidant activity [9,10,11,12,13]. Among the anthocyanins, delphinidin has the strongest antioxidant capacity because of the highest number of hydroxyl groups in the B-ring (Fig. 2) [14]. It is reported that the mechanisms associated with the antioxidant activity of anthocyanins, such as hydrogen atom transfer (HAT) or single electron transfer (SET), scavenges the radiation-induced reactive oxygen species (ROS) thereby rendering its radioprotective characteristics [15]. The radioprotective characteristics of anthocyanins against photon exposure have also been verified by researchers [16,17,18]. However, studies on the radioprotective effect of anthocyanins against proton beams have not been conducted so far. In general, the ratio of direct effect to indirect effect induced by the high linear energy transfer (LET) radiation such as a proton beam, is greater than that induced by the low LET radiation. Hence, most studies on radioprotective agents are solely interested on the protective effects against low LET radiations. However, Alan Mitteer et al. [19] reported that the proton beams produced more ROS than that produced by the same dose of photon beams. Accordingly, it is necessary to verify the protective effect of radioprotective agents against not only photon beams but also proton beams. Therefore, the present study was conducted to investigate the radio-protective effects of delphinidin in proton beam exposed-normal tissue cells.
Fig. 2. Chemical structure of delphinidin chloride (Molecular weight: 338.7 g/mol).
MATERIALS AND METHODS
Chemicals
Delphinidin chloride was purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA) and diluted to less than 1% in dimethyl sulfoxide (DMSO) and medium.
Human lung tissue cells
The HEL 299 cell line is derived from the embryonic lung tissue of a human male. They grow in a monolayer, and their morphology is similar to fibroblast cells. The cells were purchased from the Korean Culture Type Collection (KCTC; Korea) and maintained in a 5% CO2 incubator at 37℃. HEL 299 cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS).
Irradiation
Proton beam exposure was performed at the Korea Institute of Radiological and Medical Sciences using the 45 MeV MC50 cyclotron at an average dose rate of 16.8 Gy/min at room temperature. Sample cells were exposed by the proton beams with spread-out Bragg peak.
Experimental design
The experiments were conducted in four sample groups as follows:
1) Control group (CG): cells with no treatment.
2) Exposed group without pretreatment (EG): cells with 3 Gy exposure of proton beam.
3) Exposed group with low concentration of delphinidin (EG-LDp): cells cultured with 2.5 µM of delphinidin for 24 h and exposed to 3 Gy of proton beam.
4) Exposed group with high concentration of delphinidin (EG-HDp): cells cultured with 5 µM of delphinidin for 24 h and exposed to 3 Gy of proton beam.
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay
The cell viabilities of the experimental groups were determined by the MTT assay, performed as described in the reference [20]. Briefly, the HEL 299 cells were seeded at 1 × 104 cells per well in a 96 well plate and incubated for 24 h at 37℃ in a CO2 incubator. The cells were treated with delphinidin for 24 h, at concentrations ranging from 0.01–5 µM. The cells were then treated with 10 µL MTT solution in the dark for 4 h at 37℃. The formazan crystals formed within the cells were dissolved using 100 µL DMSO, and the absorbance was measured at 540 nm by a microplate reader.
Cell survival assays
Cell survival assays were performed as per the protocol mentioned in a previous article [20], with minor modifications. Briefly, cells were seeded at 1 × 104 cells per well in a 96 well plate and incubated for one day at 37℃ in a CO2 incubator. The cells were treated with 2.5 µM or 5 µM of delphinidin for 24 h, following which they were exposed to various doses of radiation (1, 3, 5, 8 and 10 Gy). After irradiation, cells were replenished with fresh media and incubated for seven day at 37℃ in a CO2 incubator. The cells were then treated with 10 µL MTT solutions in the dark for 4 h at 37℃. The absorbance was measured by the same process as described above in the MTT assay.
Measurement of reactive oxygen species (ROS)
ROS expression was measured following the procedure as described in the reference [21], with minor modifications. The cells were treated with 2.5 µM or 5 µM of delphinidin for 24 h. Next, 10 µM of 2′-7′-dicholordihydrofluorescein diacetate (DCF-DA), purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA), was added to the culture media prior to irradiation. The cells were immediately exposed to 3 Gy proton beam and incubated for 30 min. After 30 min, the cells were washed three times in PBS. The plate was read at excitation wavelength 485 nm and emission wavelength 535 nm, using the fluorescence activated cell sorter (FACS).
Superoxide dismutase (SOD) activity assay
The cells lysed with lysis buffer (ice-cold 0.1 M Tris/HCl, pH 7.4 containing 0.5% triton X-100, 5 mM β-ME and 0.1 mg/mL PMSF). The lysed cells were centrifuged at 14,000 × g for 5 min at 4℃, and the supernatants were harvested. The SOD activity was evaluated using a SOD activity assay kit (BioVision Inc. Mountain View, CA, USA) in accordance with the manufacturer's instruction [22]. The inhibition activity of SOD was determined by a colorimetric method, and the absorbance was read at 450 nm using a microplate reader.
Catalase (CAT) activity assay
The cells were lysed in cold assay buffer and centrifuged at 10,000 × g for 15 min at 4℃. The supernatants were harvested and placed in wells. The CAT activity was determined using the Catalase Activity Colorimetric assay kit (BioVision Inc. Mountain View, CA, USA) in accordance with the manufacturer's instruction [23]. The activity of CAT was measured at 570 nm using a microplate reader.
Western blot assay
Western blot assay was performed following a previously described protocol, with minor modifications [24]. The cells were exposed to 3 Gy of proton beam, and lysed in a radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, St. Louis, MO, USA). Lysates were centrifuged at 14,000 rpm for 30 min, and the supernatant was collected. The protein content was determined by use of the Bio-Rad protein assay (Bio-Rad laboratories, Hercules, CA, USA). A volume of 25 µL of cellular proteins was loaded into each well of a 10% SDS-PAGE gel; after resolution, the proteins were transferred to membrane and incubated overnight with primary antibodies against Bcl-2, Bad, PARP-1, cleaved PARP-1 and cleaved caspase-3, followed by the sequential incubation with secondary antibodies. The blots were detected by enhanced chemiluminescence.
Statistical analysis
Statistical analysis was performed using the SPSS-PC+statistics package (version 20.0). All measured data were expressed as mean ± standard deviation (SD). Statistical significance was evaluated by Duncan's multiple range test after the one-way analysis of variance (ANOVA), and was considered significant with the condition of P < 0.05.
RESULTS
Cytotoxicity of delphinidin
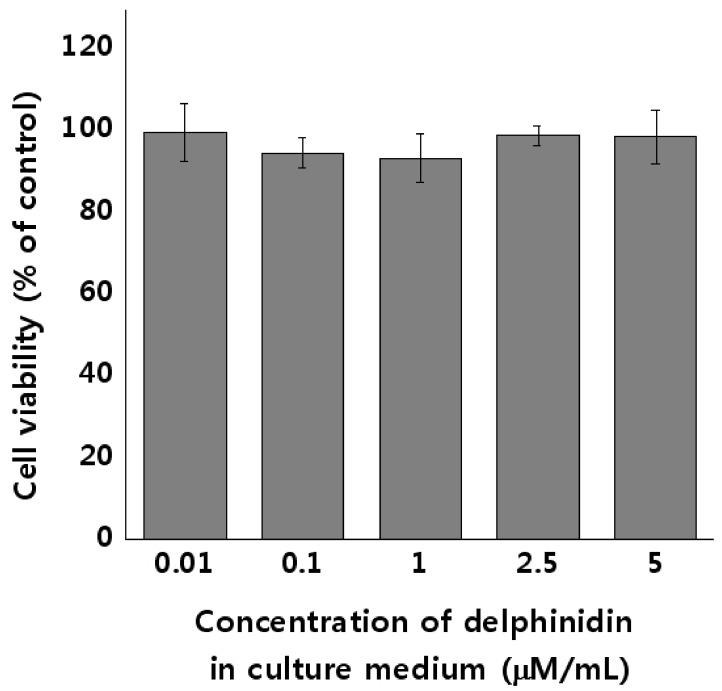
MTT assay was performed to evaluate the cytotoxicity of delphinidin in HEL 299 cells, at various concentrations ranging from 0.01 µM to 5 µM. Results are presented in Fig. 3. No significant differences in cytotoxicity were observed at concentrations less than 5 µM of delphinidin, thereby confirming that delphinidin did not affect the cell viability of HEL 299 cells at concentrations ranging from 0.01 µM to 5 µM. This experiment helped us determine the concentrations of delphinidin for the proton beam irradiation experiment: 2.5 µM for the Low delphinidin group, and 5 µM for the High delphinidin group.
Fig. 3. Cytotoxicity of delphinidin in HEL 299 cells.
The cells were treated with varying concentrations of delphinidin (0.01–5 µM/mL) in culture medium for 24 h. The cell viabilities were determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. Results are expressed as the percentages of control by means ± SD of eight independent experiments.
Cell survival assays
Cell survival assays were performed on proton-exposed HEL 299 cells to estimate the radio-protective effect of delphinidin at various radiation doses. As demonstrated in Table 1, the survival fraction of all groups decreased as the radiation dose increased. Also, the survival fractions of EG-LDp and EG-HDp were higher than that of EG at all doses. EG-LDp showed significant difference from EG at 1Gy, and EG-HDp showed significant difference from EG at all doses.
Table 1. Effects of delphinidin on the viability of the proton beam-exposed HEL 299 cells.
The measured data were expressed as mean ± SD. Values with different letters are significantly different from each other at P < 0.05 by Duncan's multiple range tests. EG, exposed group without pretreatment; EG-LDp, exposed group with low concentration of delphinidin; EG-HDp, exposed group with high concentration of delphinidin.
ROS scavenger effect of delphinidin
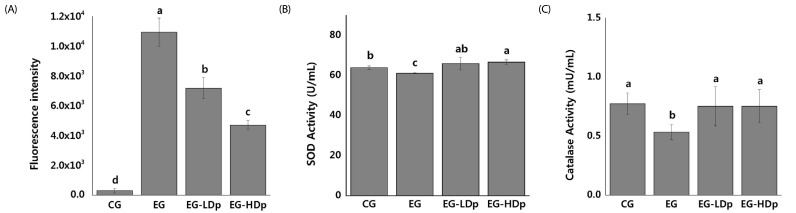
The DCF-DA assays were performed to evaluate the ROS scavenging activity of delphinidin against proton beam-induced ROS in HEL 299 cells. As demonstrated in Fig. 4 (A), radiation exposure significantly increases the amount of ROS expression compared to CG (CG: 303.45 ± 162.12, EG: 10938.59 ± 953.26, P < 0.001). However, the amount of ROS expression in EG-LDp and EG-HDp significantly decreases when compared to the EG (P < 0.001). In particular, the amount of ROS expression of EG-HDp was lower than that of EG-LDp (P < 0.01).
Fig. 4. Radioprotective effect of delphinidin against proton beam exposure.
(A) ROS scavenger effects of delphinidin in proton-exposed HEL 299 cells. ROS scavenger effect of delphinidin was measured by the 2′-7′-dicholordihydrofluorescein diacetate (DCF-DA) assay. Results are expressed as the intensity of DCF-DA fluorescence. (B) Effects of delphinidin on superoxide dismutase (SOD) activity in proton-exposed HEL 299 cells. (C) Effects of delphinidin on catalase (CAT) activity in proton-exposed HEL 299 cells. The measured data are expressed as mean ± SD. Values with different letters are significantly different from each other at P < 0.05 by Duncan's multiple range tests. CG, Control group; EG, exposed group without pretreatment; EG-LDp, exposed group with low concentration of delphinidin; EG-HDp, exposed group with high concentration of delphinidin.
SOD activity assay
SOD activity assays were performed to estimate the antioxidant activity of delphinidin against proton beam-induced oxidative stress in HEL 299 cells. As demonstrated in Fig. 4 (B), radiation exposure decreases the activity of SOD in EG, compared to CG (CG: 63.93 ± 0.83, EG: 61.11 ± 0.27, P < 0.05). However, the activity of SOD in EG-LDp and EG-HDp was increased compared to that of EG. The SOD activity of EG-LDp and EG-HDp showed statistically significant differences from EG (P < 0.05).
CAT activity assay
CAT activity assays were also performed to estimate the antioxidant activity of delphinidin against proton beam-induced oxidative stress in HEL 299 cells. The results are shown in Fig. 4 (C). Similar to the results of the SOD assay, significant decrements in CAT activity were observed in EG compared to CG (CG: 0.77 ± 0.09, EG: 0.53 ± 0.06, P < 0.05). In addition, the activity of CAT in EG-LDp and EG-HDp was significantly increased compared to EG. However, only EG-HDp showed a difference from EG with statistical significance (EG: 0.53 ± 0.06; EG-LDP: 0.75 ± 0.17, P > 0.05; EG-HDP: 0.75 ± 0.14, P < 0.05).
Western blot assay
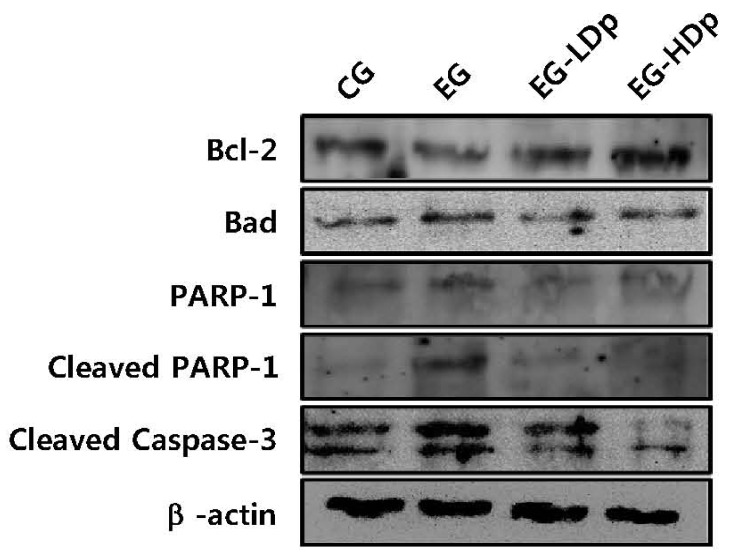
Signal proteins were quantified by western blot assay to evaluate the protective effect of delphinidin against proton beam-induced cell apoptosis in HEL 299 cells. The pro-survival regulator Bcl-2 and pro-apoptotic proteins such as Bad, PARP-1, cleaved PARP-1 and cleaved caspase-3 were measured. As shown in Fig. 5, decreased expression of Bcl-2 and increased expression of pro-apoptotic proteins were observed in EG. Whereas, in the delphinidin treated groups, increased expression of Bcl-2 and decreased expression of pro-apoptotic proteins were observed compared to EG. In particular, EG-HDp showed a greater change than EG-LDp in the expression levels of Bcl-2, Bad, and cleaved caspase-3.
Fig. 5. Effects of delphinidin on the protein expression of Bcl-2, Bad, PARP-1, cleaved PARP-1 and cleaved caspase-3 in proton-exposed HEL 299 cells.
Whole cell lysates were prepared and analyzed by western blotting using the specific antibodies Bcl-2, Bad, PARP-1, cleaved PARP-1 and cleaved caspase-3. Equal loading of protein was confirmed by stripping the blot and re-probing with β-actin antibody. Data shown here are from a representative experiment repeated three times with similar results.
DISCUSSION
Our experiments evaluated the radioprotective effect of delphinidin on normal lung tissue cells against low doses of proton beam exposure. This was achieved by evaluating (I) effects on the viabilities of the exposed human tissue cells, (II) activities of the antioxidant enzymes related to the oxidative stress of the cells, (III) effects on scavenging ROS, and (IV) quantitative/qualitative analysis on cell signal proteins related to cell apoptosis.
As presented in Fig. 3, the MTT assay showed that regardless of the concentration used, delphinidin was not cytotoxic up to 5 µM/mL in culture. Thus, the concentrations of delphinidin used in subsequent experiments were determined at 2.5 µM and 5 µM (low and high concentration, respectively). The survival fractions of lung cells at different proton beam doses were also evaluated with the different concentrations of delphinidin. As shown in Table 1, the experimental results showed that the low concentration (2.5 µM/mL) of delphinidin was radioprotective at low dose exposure, but non-radioprotective at high dose exposure. However, the higher concentration (5 µM/mL) of delphinidin was radioprotective regardless of the radiation dose. Since the radiation exposure dose is low for normal tissues but higher for the malignant cells, the radioprotective agent to be used during the radiotherapy needs to be radioprotective at a lower dose, without resorting to use a higher dose to protect normal tissues. Therefore, the low concentration of delphinidin is considered suitable for effective radioprotection during radiotherapy only for the normal tissue surrounding the cancer cells. In addition, the 50% lethal dose (LD50) of HEL 299 cells was also estimated from the achieved cell survival curve. The LD50 was about 3 Gy, and used as the reference dose in subsequent experiments for measuring activities of enzymes, expression of ROS, changes in protein expression, and DNA damage.
The radioprotective effect of delphinidin on human lung cells was further evaluated by assessing the ROS expression and activities of antioxidant enzymes at low radiation dose of proton beam. As shown in Fig. 4 (A), the ROS expression was significantly increased in radiation exposed groups without delphinidin pretreatment (EG) compared with control groups (CG). Conversely, the ROS expressions in the radiation exposed groups with delphinidin pretreatment (EG-Dp) were significantly reduced than those in the EG. Since ionizing radiations produce a number of ROS by the interaction with cellular water molecules, the overexpression of ROS is a natural phenomenon observed in irradiated cells. Therefore, the overexpression of ROS in EG is regarded as natural. The decrement in the ROS expressions after delphinidin treatment is thereby regarded as the result of radioprotective effect of delphinidin, since ROS scavenging is a characteristic property of most radioprotective agents.
The formation of excessive ROS leads to oxidative stress on essential macromolecules such as DNA, protein, and lipid containing cell membranes [25,26]. Activities of antioxidant enzymes such as super oxide dismutase (SOD) and catalase (CAT) are affected by the oxidative stress within the microcellular environments. SOD catalyzes the decomposition of toxic O2˙− by converting the O2˙- into hydrogen peroxide (H2O2) and oxygen gas (O2). CAT then catalyzes the reaction by converting 2·H2O2 to 2·H2O and O2 [27]. As shown in Fig. 4(B) and (C), due to its ROS scavenging effect, delphinidin increased the activities of SOD and CAT, which were decreased by the radiation exposure. In other words, antioxidants preferentially reduce ROS than the antioxidant enzymes and eventually balance the activity of the enzymes. The decrement in activities of antioxidant enzymes are normally observed in radiation exposed samples, and it is coincident with the results of other researches [16,28,29].
Radiation causes cellular damage by both direct and indirect interaction with intercellular molecules. Among the intercellular molecules, the damages on DNA, such as single strand break (SSB) or double strand break (DSB), are most lethal to the cell viability. Hence, cell death after proton beam-induced DNA damage in the presence of delphinidin was evaluated by measuring the pro-survival and pro-apoptosis proteins such as Bcl-2, Bad, PARP-1, cleaved PARP-1, and cleaved caspase-3. As shown in Fig. 5, proton beam exposure decreases the Bcl-2 expression, and increases the pro-apoptotic protein expressions such as Bad, PARP-1, cleaved PARP-1, and cleaved caspase-3. On the other hand, pretreatment of delphinidin retained the balance of Bcl-2 expression, and decreased the pro-apoptotic proteins. This was confirmed by the results of the protein assay, wherein pretreatment with delphinidin before radiation exposure reduces cell apoptosis.
Consequentially, it was revealed that the natural compound delphinidin is radioprotective against proton beam exposure with high LET radiation. Moreover, it was confirmed by the experimental methodologies of molecular biology that delphinidin is a safe radioprotective agent for radiotherapy to protect normal tissues surrounding the cancerous tumor, without increasing the tolerance dose of cancer cells. Hence, regular ingestion of delphinidin-rich foods such as berries, red grapes, cherries, red potatoes and red cabbage prior to radiotherapy will help reduce the side effects caused by the damage of normal tissue around the malignant tumor. This study was conducted only in a human lung cell line, which is one of the radiosensitive human cell lines. Additional studies using other radiosensitive tissues are therefore required, since human cells have different functional / physiological metabolic activities depending on their cell biological characteristics such as origin and histopathology.
Footnotes
This research was supported by the Nuclear R&D Program of the Korean Ministry of Science, ICT and Future Planning [Grant no. 2016M2B2A4912380].
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 2.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347–1356. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007;12:794–805. doi: 10.1016/j.drudis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamran MZ, Ranjan A, Kaur N, Sur S, Tandon V. Radioprotective agents: strategies and translational advances. Med Res Rev. 2016;36:461–493. doi: 10.1002/med.21386. [DOI] [PubMed] [Google Scholar]
- 6.Lim JY, Kim OK, Lee J, Lee MJ, Kang N, Hwang JK. Protective effect of the standardized green tea seed extract on UVB-induced skin photoaging in hairless mice. Nutr Res Pract. 2014;8:398–403. doi: 10.4162/nrp.2014.8.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo EY. Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines. J Nutr Health. 2013;46:503–510. [Google Scholar]
- 8.Kim SN, Kim MR, Cho SM, Kim SY, Kim JB, Cho YS. Antioxidant activities and determination of phenolic compounds isolated from oriental plums (Soldam, Oishiwase and Formosa) Nutr Res Pract. 2012;6:277–285. doi: 10.4162/nrp.2012.6.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YH, Kim DS, Woo SS, Kim HH, Lee YS, Kim HS, Ko KO, Lee SK. Antioxidant activity and cytotoxicity on human cancer cells of anthocyanin extracted from black soybean. Korean J Crop Sci. 2008;53:407–412. [Google Scholar]
- 10.Choung MG, Lim JD. Antioxidant, anticancer and immune activation of anthocyanin fraction from Rubus coreanus Miquel fruits (Bokbunja) Korean J Med Crop Sci. 2012;20:259–269. [Google Scholar]
- 11.Kim YK, Yoon HH, Lee YD, Youn DY, Ha TJ, Kim HS, Lee JH. Anthocyanin extracts from black soybean (Glycine Max L.) protect human glial cells against oxygen-glucose deprivation by promoting autophagy. Biomol Ther (Seoul) 2012;20:68–74. doi: 10.4062/biomolther.2012.20.1.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi EY. Protective effect of anthocyanin-rich bilberry (Vaccinium myrtillus L.) extract against Doxorubicin-induced cardiotoxicity [doctor's thesis] Seoul: Ewha Womans University; 2009. [Google Scholar]
- 13.Zhao JG, Yan QQ, Lu LZ, Zhang YQ. In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato. Nutr Res Pract. 2013;7:359–365. doi: 10.4162/nrp.2013.7.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson RR, Schönlau F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol®: a review. Minerva Cardioangiol. 2015;63:1–12. [PubMed] [Google Scholar]
- 15.Jhin C, Hwang KT. Prediction of radical scavenging activities of anthocyanins applying adaptive neuro-fuzzy inference system (ANFIS) with quantum chemical descriptors. Int J Mol Sci. 2014;15:14715–14727. doi: 10.3390/ijms150814715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao H, Wang Z, Ma F, Yang X, Cheng C, Yao L. Protective effect of anthocyanin from Lonicera Caerulea var. Edulis on radiation-induced damage in mice. Int J Mol Sci. 2012;13:11773–11782. doi: 10.3390/ijms130911773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan ZL, Wang ZY, Zuo LL, Tian SQ. Protective effect of anthocyanins from lingonberry on radiation-induced damages. Int J Environ Res Public Health. 2012;9:4732–4743. doi: 10.3390/ijerph9124732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Lu X, He G, Gao X, Li M, Wu J, Li Z, Wu J, Wang J, Luo C. Cytosolic protection against ultraviolet induced DNA damage by blueberry anthocyanins and anthocyanidins in hepatocarcinoma HepG2 cells. Biotechnol Lett. 2013;35:491–498. doi: 10.1007/s10529-012-1105-2. [DOI] [PubMed] [Google Scholar]
- 19.Alan Mitteer R, Wang Y, Shah J, Gordon S, Fager M, Butter PP, Kim HJ, Guardiola-Salmeron C, Carabe-Fernandez A, Fan Y. Proton beam radiation induces DNA damage and cell apoptosis in glioma stem cells through reactive oxygen species. Sci Rep. 2015;5:13961. doi: 10.1038/srep13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, Minor L. Assay guidance manual: cell viability assays [Internet] Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2013. [cited 2004 September 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK144065/ [Google Scholar]
- 21.Degli Esposti M. Measuring mitochondrial reactive oxygen species. Methods. 2002;26:335–340. doi: 10.1016/S1046-2023(02)00039-7. [DOI] [PubMed] [Google Scholar]
- 22.BioVision (USA) Superoxide dismutase (SOD) activity colorimetric assay kit [Internet] Milpitas (CA): BioVision; 2017. [cited 2017 September 30]. Available from: https://www.biovision.com/documentation/datasheets/K335.pdf. [Google Scholar]
- 23.BioVision (USA) Catalase activity colorimetric/fluorometric assay kit [Internet] Milpitas (CA): BioVision; 2017. [cited 2017 September 30]. Available from: https://www.biovision.com/documentation/datasheets/K773.pdf. [Google Scholar]
- 24.Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. 2012;4:429–434. doi: 10.4103/1947-2714.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 27.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Chiang AN, Wu HL, Yeh HI, Chu CS, Lin HC, Lee WC. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006;41:797–803. doi: 10.1007/s11745-006-5033-6. [DOI] [PubMed] [Google Scholar]
- 29.Mansour HH. Protective role of carnitine ester against radiation-induced oxidative stress in rats. Pharmacol Res. 2006;54:165–171. doi: 10.1016/j.phrs.2006.04.003. [DOI] [PubMed] [Google Scholar]