Abstract
Few therapeutic options are available for patients with aplastic anemia who are ineligible for transplantation or refractory to immunosuppressive therapy. Eltrombopag was recently shown to produce trilineage responses in refractory patients. However, the effects of real-life use of this drug remain unknown. This retrospective study (2012–2016) was conducted by the French Reference Center for Aplastic Anemia on patients with relapsed/refractory aplastic anemia, and patients ineligible for antithymocyte globulin or transplantation, who received eltrombopag for at least 2 months. Forty-six patients with aplastic anemia were given eltrombopag without prior antithymocyte globulin treatment (n=11) or after antithymocyte globulin administration (n=35) in a relapsed/refractory setting. Eltrombopag (median daily dose 150 mg) was introduced 17 months (range, 8–50) after the diagnosis of aplastic anemia. At last followup, 49% were still receiving treatment, 9% had stopped due to a robust response, 2% due to toxicity and 40% due to eltrombopag failure. Before eltrombopag treatment, all patients received regular transfusions. The overall rates of red blood cell and platelet transfusion independence were 7%, 33%, 46% and 46% at 1, 3, 6 months and last follow-up. Responses were slower to develop in antithymocyte treatment-naïve patients. In patients achieving transfusion independence, hemoglobin concentration and platelet counts improved by 3 g/dL (interquartile range, 1.4–4.5) and 42×109/L (interquartile range, 11–100), respectively. Response in at least one lineage (according to National Institutes of Health criteria) was observed in 64% of antithymocyte treatment-naïve and 74% of relapsed/refractory patients, while trilineage improvement was observed in 27% and 34%, respectively. We found high rates of hematologic improvement and transfusion independence in refractory aplastic anemia patients but also in patients ineligible for antithymocyte globulin receiving first-line treatment. In conclusion, elderly patients unfit for antithymocyte globulin therapy may benefit from eltrombopag.
Introduction
Aplastic anemia (AA) is caused by the destruction of hematopoietic stem cells, leading to pancytopenia. Rapid front-line bone marrow transplantation with an HLA-identical sibling donor can lead to excellent outcomes.1–4 However, most patients cannot undergo such a procedure because of the absence of a sibling donor or because of their age and/or co-morbidities. Immunosuppressive therapy with horse antithymocyte globulin (ATG) plus cyclosporine A (CsA) is considered to be the standard treatment in this situation, producing an overall hematologic response rate of 60–70%.5–7 Nevertheless, few therapeutic options are currently open to patients with AA who fail to achieve a hematologic response or those who relapse after this therapy and are ineligible for allogeneic stem cell transplantation. Moreover, in elderly patients who cannot receive ATG, the chance of obtaining a hematologic response with CsA alone is low.8,9 In these latter cases, complications including infections,10 bleeding and anemia may occur and lead to significantly poorer quality of life, recurrent transfusions, hospital admissions, secondary hemochromatosis, and death.
It has recently been reported that eltrombopag, a non-peptide thrombopoietin mimetic oral drug which binds to the transmembrane domain of the MPL receptor, can induce trilineage response in patients with refractory AA.11 In a single center phase 2 trial, hematologic improvement was obtained in 17 out of 43 patients who had previously failed to benefit from one or several courses of ATG.12
However, the effects of real-life use of this drug remain largely unknown, as the risks and benefits have not yet been independently assessed, while data for five patients achieving a robust response to eltrombopag may yet be updated.12 In France, physicians have access to eltrombopag through a compassionate use program. We used this program to assess the indications for and the safety and efficacy of eltrombopag in a large number of AA patients in France who received eltrombopag in the case of refractory AA, but also as a first-line treatment for patients considered unfit to receive ATG.
Methods
Identifying cases
This retrospective study (2012–2016) was conducted in 15 centers. A survey to identify patients receiving eltrombopag in France in the setting of AA was created on behalf of the French Reference Center for Aplastic Anemia. For the purposes of this study, we screened files for all patients referred to the center, and sent three waves of e-mails to more than 100 specialized physicians (Online Supplementary Material). The study was conducted in accordance with the Declaration of Helsinki. The institutional review board of the national AA center approved the study, and anonymous data collection was declared to the appropriate authorities. In accordance with French law, written informed consent was not required for this retrospective, non-interventional study, as patients had provided a non-opposition statement.
Population
Patients with a diagnosis of AA confirmed by a bone marrow biopsy, irrespective of their age or the primary etiology, were eligible for inclusion if they received at least 2 months of treatment with eltrombopag, regardless of their indication for treatment. Patients who received eltrombopag in a relapsed or refractory setting (defined by occurring at least 6 months after initial ATG treatment) were considered to be relapsed/refractory, irrespective of the number of previous ATG courses. Unfit patients who had not received at least one course of ATG before eltrombopag were considered to be receiving first-line treatment, even if they had previously been administered CsA as a stand-alone therapy.
Patients with moderate AA requiring blood transfusion less frequently than every 4 weeks were not included. Furthermore, prior diagnoses of myelodysplastic syndrome, acute leukemia or immune thrombocytopenia were considered to be exclusion criteria. Patients who received eltrombopag in the setting of thrombocytopenia after stem cell transplantation were also excluded.
Procedures and definitions
The minimal initial diagnostic panel for AA required for this study consisted of a complete blood count, a bone marrow biopsy, bone marrow karyotype analysis and assessment of a paroxysmal nocturnal hemoglobinuria (PNH) clone (Online Supplementary Methods)
Given the retrospective nature of this study, dose adaptations for adverse events or insufficient responses were not formally defined. However, the recommendations of the reference center were shared with all centers once eltrombopag was approved for treatment in France (July 1, 2012). Centers were therefore advised to start treatment at the dose of 75 mg/day for 2 weeks and then increase the dose to 150 mg/day if no clinical or biological adverse events occurred.5,11 In the absence of any hematologic response or adverse event, some clinicians used higher doses, of up to 225 mg/day, after 3 months. When eltrombopag was used with rabbit ATG, the eltrombopag was started on day 15 after the ATG treatment.
Hematologic improvements were assessed using the National Institutes of Health (NIH) response criteria. Thus, a platelet response was defined by a platelet count increase of 20×109/L above baseline, or stable platelet counts with transfusion independence for at least 8 weeks; an erythroid lineage response was defined by a hemoglobin increase of >1.5 g/dL or a reduction in >4 units of transfused red blood cells (RBC) for 8 consecutive weeks; and a leukocyte response was defined as an absolute neutrophil increase of 100% or an absolute neutrophil count increase >0.5×109/L.12 A robust response was defined as platelets >50×109/L, hemoglobin >10 g/dL, and neutrophils >1×109/L for longer than 8 weeks without transfusion support.12 Not achieving at least one NIH criterion during eltrombopag treatment was considered to be treatment failure.
Statistical analysis
The data were recorded as percentages for discrete variables and medians with interquartile ranges (IQR) for continuous variables. A Fisher exact test and Wilcoxon non-parametric rank sum test were used, respectively, to compare these two types of variables. Follow-up and survival were reported since the start of eltrombopag treatment. Kaplan-Meier plots were constructed to estimate overall survival. All statistical tests were two-sided, with P values ≤0.05 indicating statistical significance. Statistical analyses were performed using R 2.14.0 (http://www.R-project.org/) packages.
Results
Patients
Forty-six patients were identified for this study. We separated the subjects into two different cohorts according to the rationale for having initiated eltrombopag treatment and principal clinical situations.
Eleven ATG-naïve patients (cohort A) were given eltrombopag because they were considered ineligible for ATG treatment. For nine of these patients, the reason for ATG ineligibility was their age of over 65 years. One patient had dyskeratosis congenita with severe portal hypertension and hepatic dysfunction, while another, a 48-year old woman, had severe ischemic myocardial disease, complicated by a post-anoxia encephalopathy. Of these 11 patients, five had previously failed to benefit from treatment with CsA alone, four had been treated with androgens, while two further ATG-naïve patients had received eltrombopag as first-line therapy.
Thirty-five patients (cohort B) received eltrombopag in the setting of refractory or relapsed disease, defined by the persistence or reappearance of transfusion dependency or a neutrophil count <0.5×109/L at least 6 months after treatment with ATG. The first ATG treatment used was horse ATG in 66% of cases and rabbit ATG in the other 33%. Thirty percent of these patients had initially responded before relapsing, and 70% were primary refractory to this treatment. Not all patients were candidates for allogeneic stem cell transplantation at the time of eltrombopag initiation, either because of the lack of a suitable donor and/or age or comorbidities. The median age was 53 years old (IQR, 26–63) and 48% of patients had already received one cycle of ATG. Thirty-seven percent had previously received two cycles, including eight patients with primary refractory disease who were given eltrombopag in combination with the second course of ATG (eltrombopag was started between day 15 and day 45 after the rabbit ATG). The remaining 11% of patients in this cohort received three cycles of ATG prior to eltrombopag.
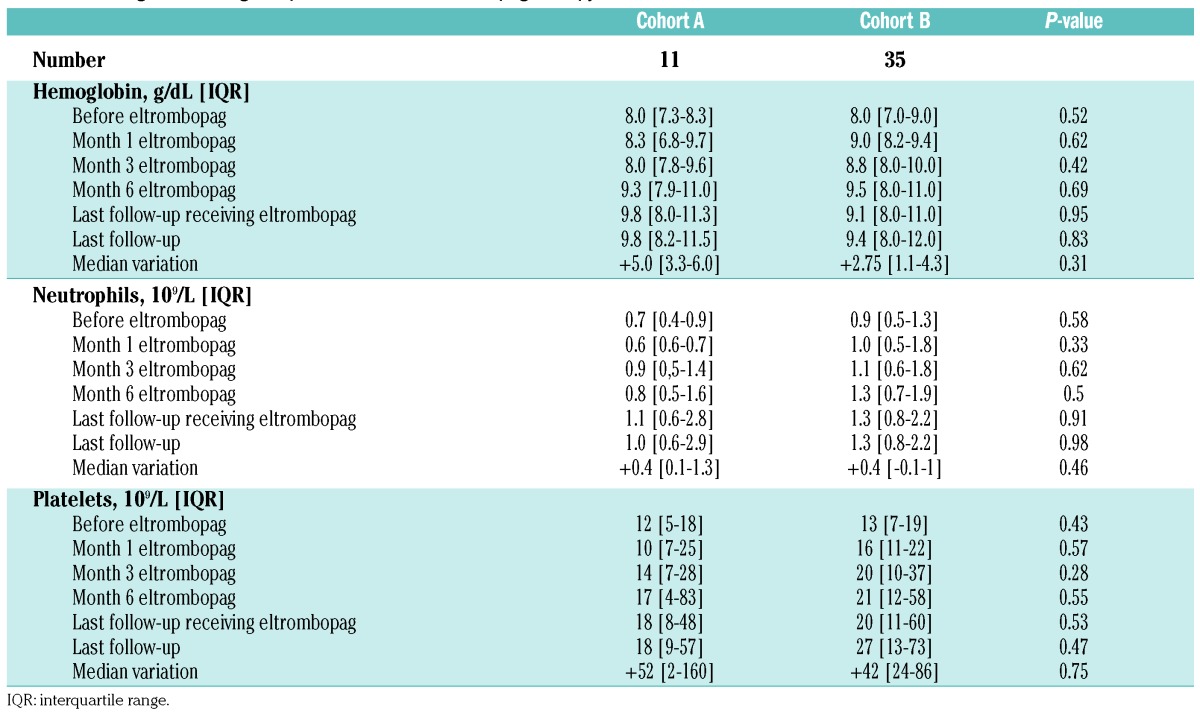
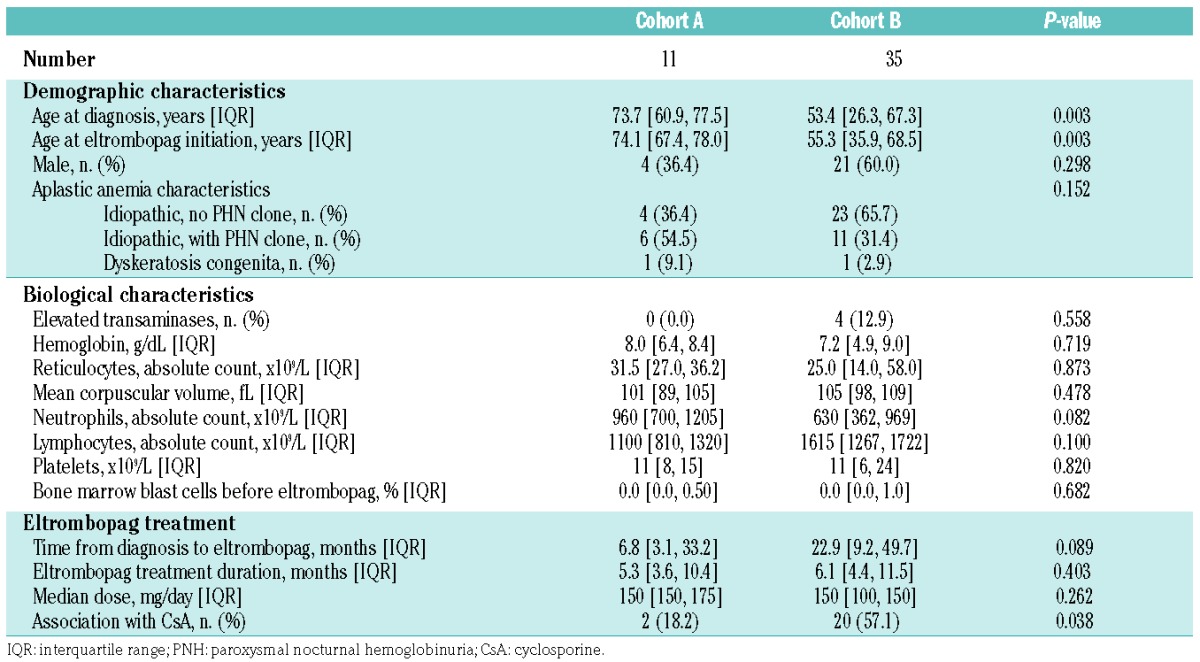
Table 1 shows the main characteristics of the patients divided into the two cohorts described above. At the time of AA diagnosis, a PNH clone was detected in 37% of all patients. One patient in each cohort was found to have germinal mutations consistent with dyskeratosis congenita, including a patient with no extra-hematologic phenotype who received one ATG course prior to genetic testing.
Table 1.
Main baseline characteristics of the patients divided by cohort (A and B).
Eltrombopag treatment
Cohort A
The median time from the diagnosis of AA to the start of eltrombopag treatment was 7 months (IQR, 3–33). The patients received eltrombopag for a median of 5 months (range, 3–20). The median eltrombopag dose prescribed was 150 mg once a day, and CsA was associated with eltrombopag in two patients (18%) from this cohort.
Cohort B
The median time from the diagnosis of AA to starting eltrombopag treatment was 23 months (IQR, 9–50). Overall, these patients received eltrombopag for a median of 6 months (range, 2–39). Those who had a hematologic response received treatment for a median of 8 months (IQR, 5–18), compared with 5 months (IQR, 4–6) in cases of treatment failure. The median eltrombopag dose prescribed was 150 mg once a day, and CsA was associated with eltrombopag in 20 patients (57%) from this cohort.
Hematologic evolution and transfusion dependency
Before starting eltrombopag treatment, all patients in both cohorts were transfusion-dependent. In cohort A (n=11), all patients were RBC transfusion-dependent, requiring a median number of three packed RBC units (IQR, 2–4) per month, and ten of the 11 patients were dependent on platelet transfusions, being given a median number of two units (IQR, 1.5–3.5) of platelet concentrates (0.5×1011× kg) per month. In cohort B, 34/35 patients were RBC transfusion-dependent, requiring a median number of four packed RBC units (IQR, 2–4) per month, and 33/35 patients were dependent on platelet transfusions, being given a median number of three units (IQR, 2–4) of platelet concentrates (0.5×1011× kg) per month.
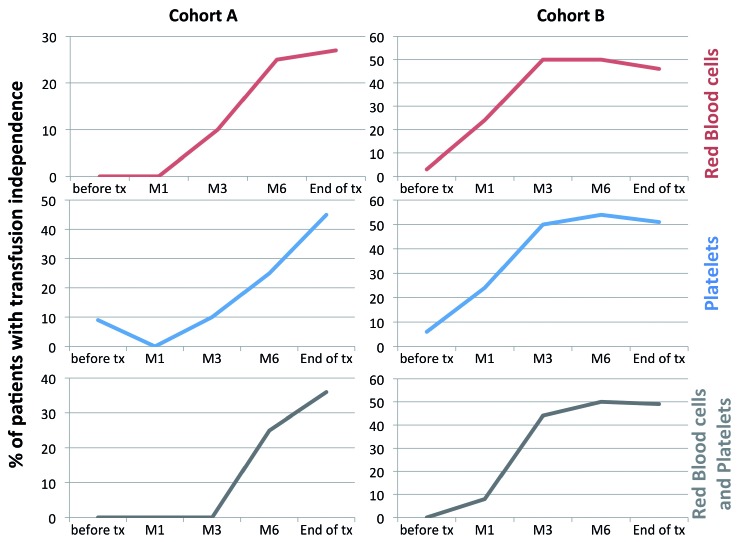
Figure 1 shows the kinetics and proportions of patients achieving transfusion independence (RBC and platelets) in both cohorts. At last follow up, we confirmed transfusion independence (both RBC and platelets) in 36% and 49% of cohort A and B patients, respectively. It is worth noting that patients treated with eltrombopag first-line seemed to respond more slowly, with no responders during the first 3 months of treatment, compared with 44% in cohort B (P=0.02). In patients achieving transfusion independence, the increased hemoglobin level was 5 g/dL (IQR, 3.3–6) and 2.75 g/dL (IQR, 1.15–4.03) in cohorts A and B, respectively (P=0.3). Neutrophil and platelet counts also improved significantly in both cohorts (Table 2).
Figure 1.
Rate (percentage) of transfusion independence before and after eltrombopag treatment by cohort and blood product type.
Table 2.
Trilineage hematologic improvement after eltrombopag therapy.
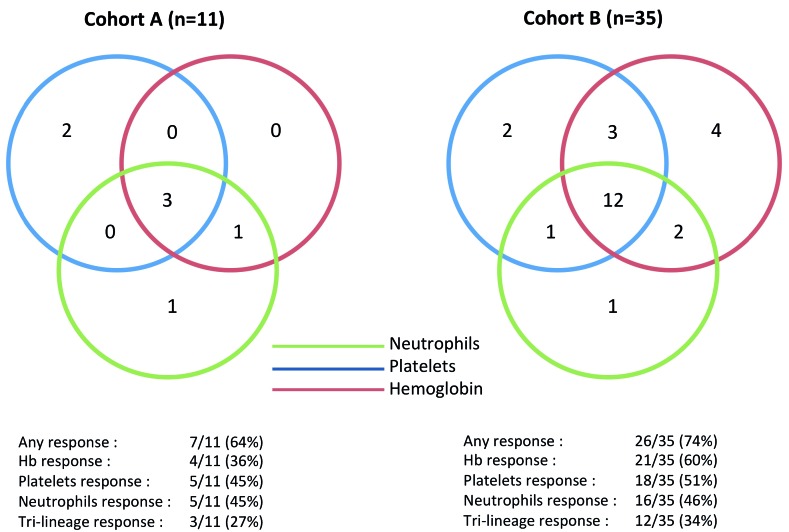
Hematologic improvements were also assessed for each lineage using NIH criteria (Figure 2). According to these criteria, a response was observed in at least one lineage in 64% and 74% of cohorts A and B, respectively, including trilineage responses in 27% and 34% of cohorts A and B, respectively. Furthermore, a robust hematologic response12 was observed in three patients from cohort A and seven patients from cohort B.
Figure 2.
Hematologic responses for each cell lineage in accordance with National Institutes of Health criteria.
Among the patients with refractory AA, the median eltrombopag dose in responders (i.e., patients who had a response in at least one lineage according to NIH criteria) was 150 mg/day (IQR, 100–150), which was not different from that in non-responders. In cohort A patients, the median eltrombopag dose in responders was 150 mg/day (IQR, 131–162), which was not different from that in non-responders. Hematologic responses were observed in two out of four and three out of three patients whose daily eltrombopag dose was increased above 150 mg, to a maximum of 300 mg/day, in refractory patients and patients treated with eltrombopag first-line, respectively.
At last follow-up, 22 patients (49%) were still receiving eltrombopag treatment, four (9%) eventually stopped after gradual tapering due to a robust hematologic response, one (2%) due to limited toxicity, and 18 (40%) due to eltrombopag failure. There were no significant differences in these proportions between the two cohorts. The four patients who were weaned off eltrombopag all remained in hematologic response 27, 24, 12 and 7 months after eltrombopag withdrawal.
We also conducted a separate analysis on the eight patients with primary refractory AA who received eltrombopag in combination with a second course of ATG and CsA. These patients were 49.2 years old (IQR, 33.8–55.5) and received eltrombopag at a median of 21 days after rabbit ATG therapy (having all previously received horse ATG). Remarkably, all eight patients achieved transfusion independence (7/8 at 3 months), despite the fact that they had received a median of three packed RBC units/month and three platelet units/month before treatment. At 6 months, the median hemoglobin level was 11 g/dL, the median platelet count 85×109/L, and the median neutrophil count 1.6×109/L.
In cohort B, four patients with a median age of 25 years (IQR, 19–37) underwent hematopoietic stem cell transplantation with an alternative donor (2 HLA mismatched donors and 2 cord blood transplants) at a median time of 8 months (IQR, 6–11) after eltrombopag failure. One patient eventually died of Epstein-Barr virus-associated post-transplant lymphoproliferative disease 4 months after cord blood transplantation. The other patients were alive at last follow-up, more than 2 years after transplantation. No patient in the first-line cohort was considered for hematopoietic stem cell transplantation.
Of particular note, neither of the two patients with dyskeratosis congenita experienced a hematologic response.
Finally, we failed to identify any baseline factor associated with the occurrence of transfusion independence or hematologic improvement in this cohort.
Safety analysis and clonal evolution
We retrospectively recorded potential toxicities of treatment among patients who received eltrombopag. The median follow-up after starting eltrombopag was 9 and 13 months for cohorts A and B, respectively. Table 3 reports the adverse events recorded in both cohorts. Most of the events were related to bone marrow dysfunction, with infections (mainly febrile neutropenia) and hemorrhages reported in a total of 13 and six patients, respectively. Thirteen patients developed elevated transaminase levels (grade 1, n=9; grade 2, n=2; grade 3, n=2) between 1.5 and 8 times the upper limit of normal without hepatic dysfunction, and one patient developed grade 2 hyperbilirubinemia. One patient had grade 2 insomnia, and one patient developed a localized lung cancer that required surgery. No thrombotic events or thrombocytosis (maximum platelet count: 250×109/L) were observed in either cohort. Among patients who had their PNH clone size evaluated after eltrombopag treatment, we found a non-statistically significant increase in size in 45%, which is consistent with the natural history of refractory AA. There was no statistically significant difference between responders and non-responders (43% versus 50%; P=NS).
Table 3.
Adverse events and outcomes by treatment cohort during the follow-up observation time.
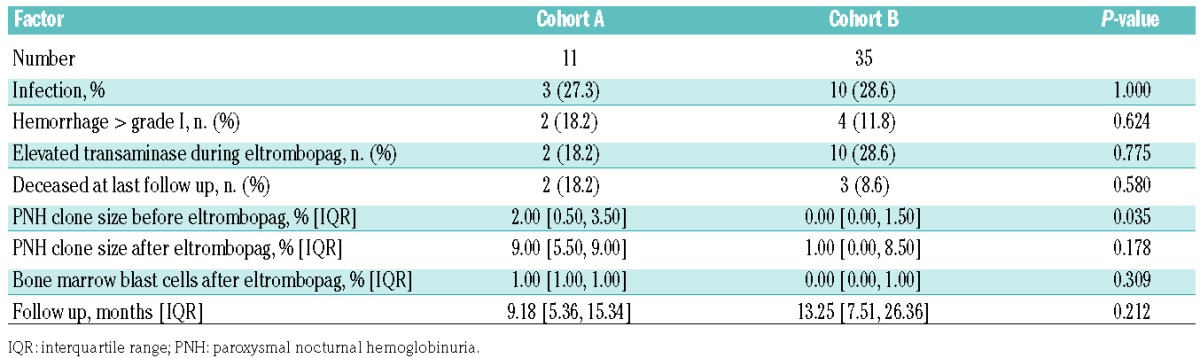
The median follow-up was 9 months (IQR, 5–15) in cohort A and 13 months (IQR, 7.5–26) in cohort B. We recorded six deaths, all of which occurred in non-responding patients. Deaths were caused by cerebral hemorrhage in two thrombocytopenic patients and acute myeloid leukemia in one patient: another patient had a sudden death, probably caused by a pulmonary embolism occurring 6 months after eltrombopag had been discontinued, one patient died of septic shock, and one died following a cord blood transplant.
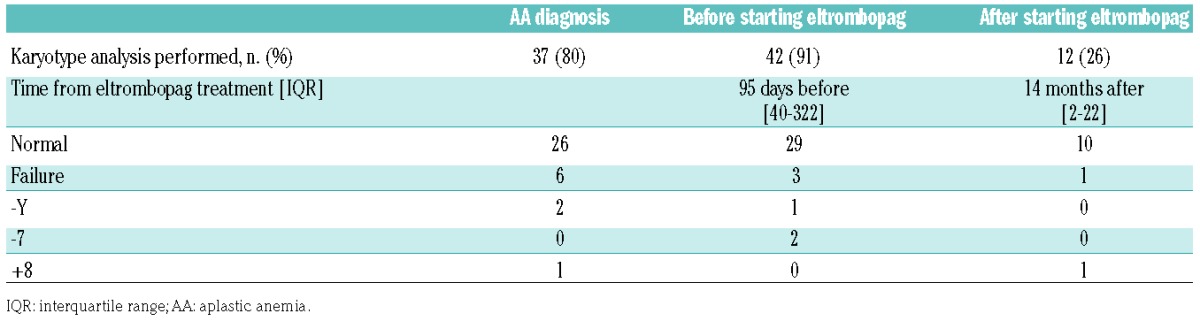
While 42 (91%) of patients had a bone marrow karyotype analysis at a median of 95 days before eltrombopag was introduced, only 12 patients (26% of the whole study population) had a subsequent karyotype analysis after eltrombopag had been started, to evaluate the risk of clonal evolution, despite an overall exposure to eltrombopag of 428 patient-months. The post-treatment karyotype analysis was conducted at a median of 14 months (IQR, 2–22) after eltrombopag had been started. Trisomy 8 was identified in one patient during eltrombopag treatment, with no myelodysplastic marrow morphology, but this abnormality had already been present at the time of diagnosis of the AA. The two patients with monosomy 7 before starting eltrombopag treatment did not undergo new karyotype analysis after treatment initiation (1 had a complete response with no evolution after 2 years of follow-up, while the other developed acute myeloid leukemia and eventually died). Online Supplementary Table S1 provides details of all karyotype analyses performed on the entire study population.
Discussion
Recently, eltrombopag has been reported to induce clinically significant increases in blood counts and/or decreases in transfusion requirements in 40% of patients with refractory AA, and some of these patients achieved multi-lineage responses.11,12 However, to date, little use has been made of this drug in refractory AA patients, with available prospective data for 43 patients from the NIH12 and data from another single center study that included ten patients from China.13 On behalf of the French Reference Center for Aplastic Anemia, we retrospectively collected data on all patients who received eltrombopag between 2012 and 2016. Forty-six patients were identified who had been given eltrombopag for relapsed/refractory AA after ATG treatment (n=35) or as first-line therapy (n=11). We confirmed the overall efficacy of eltrombopag which pro duced a 46% rate of red blood cell and platelet transfusion independence. Hematologic improvement in more than one lineage, according to NIH criteria, was observed in 74% of relapsed/refractory patients but also in 64% of ATG-naïve patients, while trilineage improvement was observed in 27% and 34%, respectively.
Table 4.
Summary of bone marrow karyotype analysis before and after eltrombopag treatment.
The standard treatment for patients who do not have an HLA–identical sibling donor is the association of horse ATG plus CsA, although 30% show primary refractoriness to this treatment,4,7,14 and 30% of initial responders relapse after this first therapy. In this situation, transplantation from a well-matched unrelated donor may be considered in younger patients (under 30 years of age) in the first year after the diagnosis of AA,15 while results with mismatched unrelated donors are currently not as favorable.4 A significant number of patients overall will therefore be considered to be refractory and possibly exposed to infectious complications and intensive transfusion support, mainly complicated by hemosiderosis and alloimmunization.1 Until recently, the standard second-line treatment for patients without a histocompatible donor was a second course of immunosuppression using rabbit, horse or anti-CD52 antibodies, which produces an overall response rate of 30%.7,16 This latter treatment requires extensive hospitalization and careful, close follow-up because of initial worsening of transfusion requirements and induced immune deficiency arising from T-cell depletion.6 Eltrombopag is an oral thrombopoietin mimetic that is easy to use in an outpatient context. In this study, we confirmed an overall hematologic response of 71%, with almost half of these responders achieving trilineage improvement. The only alternative, in this situation, would be androgens such as oxymetholone, which were used to treat AA before the emergence of ATG plus CsA, and are, in fact, still used to treat certain patients with constitutional AA in a relapsed/refractory setting. However, data available regarding idiopathic AA are very scarce, especially in relapsed/refractory patients.17 Furthermore, side effects are commonplace after prolonged exposure to androgens.8 In our experience, eltrombopag was very well tolerated, with mild hepatic dysfunction in only one patient who stopped taking the drug because of a rise in transaminase levels. On the whole, our experience has led us to prefer the use of eltrombopag in this situation.
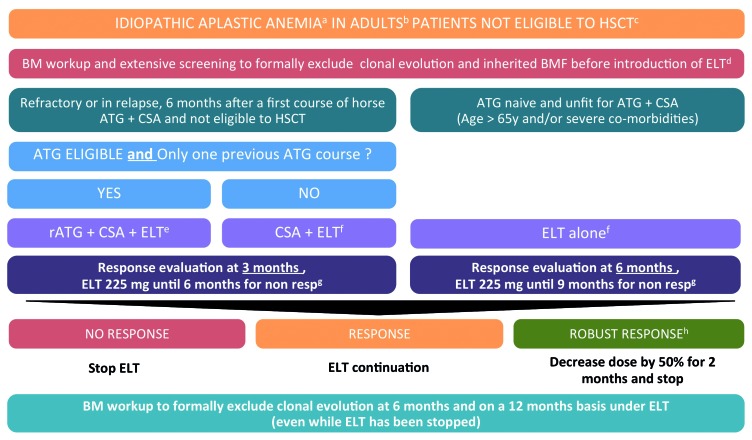
As already mentioned, 11 patients were given eltrombopag as first-line treatment alone (n=9) or in association with CsA (n=2). These patients were not eligible for conventional ATG plus CsA treatment because of their age (median 74 years, versus 53 years for patients with refrac tory) or comorbidities (notably kidney dysfunction, which precludes the use of CsA). In this subgroup, we observed a 40% rate of hematologic improvement and transfusion independence at 6 months, with no excess of mortality compared to that of younger patients. In this particular population, treatment options are limited most of the time and include growth factors, transfusion support and antibiotics. While requiring confirmation through prospective control trials, eltrombopag alone might be a reasonable option in these specific patients. Of note, in this group, responses seemed to be slower, as no response was observed during the first 3 months of treatment. Eltrombopag should not, therefore, be discontinued too early in this specific setting, and we propose a treatment algorithm based on the findings of this study in Figure 3.
Figure 3.
French guidelines for the use of eltrombopag in patients with aplastic anemia (2017). (a) All patients should be screened at diagnosis for (i) an inherited bone marrow failure regardless of their family history and clinical findings, (ii) clonal evolution. (b) Data for children and in the literature are insufficient at present to do anything more than generate hypotheses, and should not be applied in patients < 18 years old. (c) Patients with aplastic anemia are considered to be eligible for transplantation as a second-line treatment in case of refractory status after first-line immunosuppressive therapy, excellent health status and (i) if a matched sibling donor is available (for patients who have been offered immunosuppressive therapy first line because of age > 40 years) or (ii) if a matched unrelated donor is available for patients aged 30 years and under. Regarding the age limits, stated cutoff ages are recommendations and are therefore open to debate in accordance with institution and patient specificities. (d) For refractory patients, a careful reassessment of the diagnosis – to exclude a clonal evolution such as myelodysplastic syndrome or constitutional bone marrow failure – is mandatory. (d) In patients over 65–70 years old or patients with severe comorbidities (cardiac and/or renal failure), the use of ATG may be responsible for inadequate toxicity. (e) Rabbit antithymoglobulin: 3.75 mg/kg continuous intravenous administration over 12 h from day 1 to day 5, Cyclosporine A: 5 mg/kg/d from day 1 in order to achieve residual dosage of between 200 and 400 ng/mL. Start the treatment orally. Cyclosporine should not be withdrawn prematurely before 6 months unless toxicity grade >2 occurs. Eltrombopag 75 mg per day for 2 weeks, and thereafter increased to 150 mg per day from day 14 and as soon as transaminases < 2 times upper limit of normal (ULN) and bilirubin < 1,5 ULN for a minimum of 3 months. Eltrombopag should be interrupted in case of transaminases > 3N. (f) Eltrombopag should be initiated at 75 mg per day for 2 weeks in a fasting patient and thereafter increased to 150 mg per day if no clinical or biological toxicity is identified. The dosage should be halved for subjects of fully (both parents) East Asian heritage (i.e. Japanese, Chinese, Taiwanese and Korean) because plasma eltrombopag AUC(0-τ) concentrations have been found to be approximately 80% higher in healthy Japanese subjects than in non-Japanese healthy subjects (predominantly Caucasian). (g) The starting date for evaluation is the first day at 150 mg. In patients not responding at 150 mg per day, the dosage may be carefully increased, up to 225 mg per day. These patients should be monitored closely for adverse events (abdominal pain, diarrhea, cataract). (h) A robust response is considered for patients with Hemoglobin>10g/dL, neutrophils 1×109/L and platelets more than 50×109/L. HSCT: hematopoietic stem cell transplantation; ATG: anti-thymocyte globulins; hATG: horse anti-thymocyte globulins; rATG: rabbit anti-thymocyte globulins; CSA: cyclosporin A; ELT: eltrombopag; resp: responders; BM: bone marrow; BMF: bone marrow failure.
Regarding refractory patients, our results are in line with those published previously.11,12 Initial studies defined the optimal dose of eltrombopag as 150 mg/day for hematologic response at 3 to 4 months.11,12 In this compassionate use program, we recommended that French centers using eltrombopag in 35 relapsed/refractory patients took into account the published NIH studies.11,12 Following this treatment plan, 43% of patients responded at 3 months and 50% at 6 months, which illustrates that the optimal time to appreciate the efficacy of this treatment is about 6 months overall. Of note, only three out of 21 evaluated patients responded to the higher dose of eltrombopag of 225 mg/day, illustrating the minimal benefit of increasing dosage in Caucasian patients. Importantly, four patients fulfilled the criteria for robust response;12 in these patients eltrombopag was then tapered and discontinued after a median of 14 months. All four patients then maintained stable blood counts, with a median medication-free follow-up of 18 months. Of particular note, two patients were also included in our study because of genetically proven dyskeratosis congenita with no response.
The mechanism by which eltrombopag acts in the setting of bone marrow failure remains largely unclear. Among the nine patients in our study who received eltrombopag alone as a first-line treatment because they were ineligible for standard treatment, five responded of whom three had a trilineage response. This suggests that abrogation of immune attack may not be necessary for a response to eltrombopag in patients with idiopathic AA, which is in line with reported observed responses to eltrombopag in patients with moderate AA not previously treated with immunosuppression.12 It is thus likely that eltrombopag acts directly to stimulate the proliferation of small numbers of residual stem-progenitor cells in patients with AA. However, outstanding results published very recently regarding responses in treatment-naïve AA patients in a phase 2 trial investigating the association of ATG plus CsA plus eltrombopag18 highlighted the benefit of decreasing the intensity of the immune attack to improve responses to eltrombopag. Two ongoing prospective phase 3 trials have randomized the addition of eltrombopag to standard therapy (Figure 3). The RACE study is comparing horse ATG plus CsA with or without eltrombopag as front-line therapy for patients with severe AA (EudraCT: 2014-000363-40), while the EMAA study is comparing the use of CsA with or without eltrombopag as front-line therapy for patients with moderate AA (EudraCT: 2014-000147-19).
We did not identify any predictors of response in our study. However, higher reticulocyte counts in refractory patients,12 as well as longer telomere length and younger age in treatment-naïve patients18 have been reported to be associated with better responses, although these factors are also a reflection of the stem cell pool. Collectively, this suggests that a critical mass of stem cells is required for bone marrow recovery, and that this is better if the immune response has been abated. While the exact role of eltrombopag in AA is still being investigated, enough evidence exists to suggest that it directly stimulates hematopoietic stem and progenitor cells, which could theoretically affect the emergence of abnormal clones, as has already been retrospectively reported with the use of hematopoietic growth factor in AA.19,20 In two prospective studies examining both refractory12 and treatment-naïve patients,18 no evidence of a higher rate of clonal evolution was identified after comparison with historical controls treated in the same institution. Unfortunately, patients in our study were not examined serially, so any potential clonal evolution of karyotypic aberrations could not be extensively and systematically defined. Regarding the potential risk of clonal evolution associated with the use of eltrombopag alone, randomized studies with careful serial evaluation of clonal evolution, including karyotype, fluorescence in situ hybridization analysis and molecular studies, will enable the risks associated with eltrombopag in this setting to be assessed more accurately. We detected a non-significant increase in the size of the PNH clone after eltrombopag treatment, especially in patients who had already received immunosuppressive therapy. There are no data in the literature suggestimg a similar finding with eltrombopag; indeed, no firm conclusions can be drawn from this observation.
Our work has both strengths and limitations. The latter are mostly due to its retrospective nature and limited numbers of patients. The strengths include the systematic enrollment of all AA patients who received eltrombopag in France between 2012 and 2016 through the French Reference Center for Aplastic Anemia. This provides a clear picture of our current practice for refractory patients but also on first-line monotherapy for patients who are not eligible for standard immunosuppression.
In conclusion, eltrombopag has shown convincing efficacy in the majority of refractory AA patients both in this analysis and in previously published studies.11,12 We also confirmed that eltrombopag was able to restore trilineage hematopoiesis in half of the responders. Responses were identified up to 6 months after treatment with an overall acceptable toxicity profile. This report, based on real-life clinical practice, also provides three novel findings that require further investigation: (i) eltrombopag monotherapy may benefit older patients considered to be unfit for ATG; (ii) in patients with a first relapse or who are refractory after one cycle of ATG, high response rates may be achieved with eltrombopag when combined with a second course of ATG plus CsA treatment; and (iii) the optimal dose of eltrombopag merits further investigation, as it would appear that some patients might respond to a dose higher than 150 mg/day. In brief, the encouraging overall results now need to be confirmed through prospective controlled trials.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Antonio Risitano for constructive intellectual input and advice and Mr David Williams for editorial assistance.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/2/212
References
- 1.Marsh JCW, Kulasekararaj AG. Management of the refractory aplastic anemia patient: what are the options? Blood. 2013;122(22):3561–3567. [DOI] [PubMed] [Google Scholar]
- 2.Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT* SAA Working Party. Br J Haematol. 1988;70(2):177–182. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida N, Kobayashi R, Yabe H, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica. 2014;99(12):1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peffault de Latour R. Transplantation for bone marrow failure: current issues. Hematology Am Soc Hematol Educ Program. 2016;2016(1):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006;72–77. [DOI] [PubMed] [Google Scholar]
- 7.Scheinberg P. Aplastic anemia: therapeutic updates in immunosuppression and transplantation. ASH Education Program Book 2012;2012(1):292–300. [DOI] [PubMed] [Google Scholar]
- 8.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120(6):1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh J, Schrezenmeier H, Marin P, et al. Prospective randomized multicenter study comparing cyclosporin alone versus the combination of antithymocyte globulin and cyclosporin for treatment of patients with nonsevere aplastic anemia: a report from the European Blood and Marrow Transplant (EBMT) Severe Aplastic Anaemia Working Party. Blood. 1999;93(7):2191–2195. [PubMed] [Google Scholar]
- 10.Torres HA, Bodey GP, Rolston KVI, Kantarjian HM, Raad II, Kontoyiannis DP. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98(1):86–93. [DOI] [PubMed] [Google Scholar]
- 11.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123(12):1818–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill H, Leung GMK, Lopes D, Kwong Y-L. The thrombopoietin mimetics eltrombopag and romiplostim in the treatment of refractory aplastic anaemia. Br J Haematol. 2017;176(6):991–994. [DOI] [PubMed] [Google Scholar]
- 14.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devillier R, Dalle J-H, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the EBMT Severe Aplastic Anemia Working Party. Haematologica. 2016;101(7):884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Wu CO, Young NS. Activity of alemtuzumab monotherapy in treatment-naive, relapsed, and refractory severe acquired aplastic anemia. Blood. 2012;119(2):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuhjo T, Yamazaki H, Omine M, Nakao S. Danazol therapy for aplastic anemia refractory to immunosuppressive therapy. Am J Hematol. 2008;83(5):387–389. [DOI] [PubMed] [Google Scholar]
- 18.Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima S, Ohara A, Tsuchida M, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002;100(3):786–790. [DOI] [PubMed] [Google Scholar]
- 20.Socie G, Mary J-Y, Schrezenmeier H, et al. Granulocyte-stimulating factor and severe aplastic anemia: a survey by the European Group for Blood and Marrow Transplantation (EBMT). Blood. 2007;109(7):2794–2796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.