Abstract
Development of neutralizing antibodies against therapeutic Factor VIII (FVIII) is the most serious complication of the treatment of hemophilia A. There is growing evidence to show the multifactorial origin of the anti-FVIII immune response, combining both genetic and environmental factors. While a role for the complement system on innate as well as adaptive immunity has been documented, the implication of complement activation on the onset of the anti-FVIII immune response is unknown. Here, using in vitro assays for FVIII endocytosis by human monocyte-derived dendritic cells and presentation to T cells, as well as in vivo complement depletion in FVIII-deficient mice, we show a novel role for complement C3 in enhancing the immune response against therapeutic FVIII. In vitro, complement C3 and its cleavage product C3b enhanced FVIII endocytosis by dendritic cells and presentation to a FVIII-specific CD4+ T-cell hybridoma. The C1 domain of FVIII had previously been shown to play an important role in FVIII endocytosis, and alanine substitutions of the K2092, F2093 and R2090 C1 residues drastically reduce FVIII uptake in vitro. Interestingly, complement activation rescued the endocytosis of the FVIII C1 domain triple mutant. In a mouse model of severe hemophilia A, transient complement C3 depletion by humanized cobra venom factor, which does not generate anaphylatoxin C5a, significantly reduced the primary anti-FVIII immune response, but did not affect anti-FVIII recall immune responses. Taken together, our results suggest an important adjuvant role for the complement cascade in the initiation of the immune response to therapeutic FVIII.
Introduction
Hemophilia A is a rare X-linked inherited hemorrhagic disorder consecutive to the absence of functional pro-coagulant Factor VIII (FVIII). Prevention or treatment of bleeding consists of replacement therapy with exogenous FVIII. However, up to 30% of the patients develop antibodies of IgG isotype that neutralize the procoagulant activity of therapeutic FVIII, and are referred to as FVIII inhibitors.1 The immune response that develops against therapeutic FVIII is thought to be a classical T-cell dependent immune response to a foreign glycoprotein antigen. Thus, the antibody response and the nature of the implicated T cells have been extensively characterized.2 The initiation of a naïve T-cell-dependent immune response requires that the antigen-presenting cells (APCs) endocytose the target antigen. The APCs also need to receive appropriate maturation signals, referred to as “danger signals”, in order to mature and present the processed antigen to CD4+ T cells in an immunogenic context. In the case of the immune response to therapeutic FVIII, the nature of the danger signals that adjuvant the anti-FVIII immune response and permit activation of naïve FVIII-specific CD4+ T cells is not known. A role for bleeding and inflammation in the development of FVIII inhibitor has been proposed by several authors.3–5
The in vitro study of FVIII endocytosis has consistently been performed using serum-free medium or medium containing heat-inactivated serum,6–10 thus ignoring a potential role for the complement system in the observed mechanism. The complement system plays a major role in the development of immune responses.11 It is an integral part of the innate and adaptive host defense. Complement activation occurs through different pathways: the classical pathway is triggered by C1q binding to immune complexes, the lectin pathway is triggered by the binding of mannose binding lectin to mannose residues on pathogens, and the alternative pathway is spontaneously and continuously activated at a low rate (i.e. spontaneous C3 tick-over).12,13 Inappropriate complement triggering is pathogenic and has been associated with autoimmune reactions.14
In the present work, we investigated the role of the complement system in the initiation and development of the anti-FVIII immune response. We demonstrate that transient depletion of complement using humanized cobra venom factor (hCVF) dampens the intensity of the primary anti-FVIII immune response in FVIII-deficient mice. We propose that initiation of the anti-FVIII immune response involves, at least in part, facilitation of FVIII endocytosis by C3 and its activation fragment C3b.
Methods
Antibodies and reagents
Full length FVIII was either a kind gift from CSL-Behring (Helixate® NexGen, Marburg, Germany) or from Baxter (Recombinate®, Maurepas, France). Recombinant human A disintegrin and metalloprotease with thrombospondin type I repeats-13 (ADAMTS-13) was a kind gift from Baxter. Complement human proteins Factor B, Factor D, C3, C3b and C3-depleted serum were purchased from Complement Technology (Comptech, TX, USA) and Merck Millipore (Merck Chemicals Ltd., Nottingham, UK). Human serum was obtained from AB blood type healthy donors. Antibodies against CD1a, CD3, CD14, CD40, CD83, CD86, HLA-DR, CD206, low density lipoprotein receptor-related protein (LRP, CD91), CD209, CD68 and APC-labeled Annexin V were purchased from BD Pharmingen (San Jose, CA, USA). Antibody against CD20 was purchased from eBiosciences (San Diego, CA, USA).
The biotinylated monoclonal mouse anti-human FVIII antibody GMA-8015 and sheep polyclonal anti-human FVIII (SAF8C) were from Green Mountain Antibodies (Burlington, VT, USA) and Affinity Biological (Ancaster, Canada). The monoclonal anti-human FVIII antibody 77IP52H7 was a kind gift from LFB (Les Ulis, France). The biotinylated monoclonal mouse anti-human ADAMTS-13 antibody (20A5) and polyclonal goat anti-mouse C3b/iC3b (clone A209) were from Clinisciences (Nanterre, France) and Quidel (San Diego, USA), respectively.
Generation and production of recombinant wild-type or mutated FVIII, and of humanized cobra venom factor
The wild-type human B-domain-deleted (BDD) FVIII (FVIIIHSQ) and the R2090A-K2092A-F2093A FVIII mutant (FVIIIC1) were generated and purified as described previously.15,16 Preparation of the plasmid expressing HC3-1496, and expression and purification of HC3-1496 were performed as described previously for the preparation of pMB-HC3-1348.17 Details are provided in the Online Supplementary Appendix.
Generation of human immature monocyte-derived dendritic cells and monocyte-derived macrophages
Monocytes were isolated from the blood of healthy donors using anti-CD14+ magnetic microbeads (Miltenyi Biotec, Paris, France). Monocytes were incubated in RPMI-1640 (Lonza, Verviers, Belgium) supplemented with GM-CSF (1000 IU/106 cells) and IL-4 (500 IU/106 cells) (Miltenyi Biotec) for five days to generate immature monocyte-derived dendritic cells (MO-DCs). The immature status was confirmed by flow cytometry (LSR II, BD Biosciences, Le Pont au Claix, France) with a CD1a, CD14, CD80, CD86, CD83, CD40 and HLA-DR staining. To differentiate monocytes into macrophages (MO-Φ), cells were plated in RPMI-1640 supplemented with M-CSF (ImmunoTools, Friesoythe, Germany, 100 ng/106 cells) for five days. Macrophage phenotype was confirmed by flow cytometry with CD68 staining.
Isolation of circulating human blood dendritic cells
Circulating dendritic cells (DCs) were isolated from the blood of healthy donors using the Blood Dendritic Cell Isolation Kit II (Miltenyi Biotec). Purity was assessed as more than 80% by flow cytometry using exclusion staining based on CD3, CD14 and CD20.
Uptake of antigens by immature MO-DCs
FVIII (50 nM, Helixate®), FVIIIHSQ (50 nM), FVIIIC1 (50 nM), ADAMTS-13 (50 nM) conjugated to Dylight 633 (Thermo Scientific, Courtaboeuf, France) or human Factor IX (50 nM, Benefix, Pfizer, France) were incubated with 20% normal serum (NS), heat-inactivated (56°C for 30 min) serum (HIS) or C3-depleted serum (ΔC3) in RPMI-1640 at 37°C for 1 hour (h). When indicated, FVIII or Dylight 633-labeled ADAMTS-13 were incubated with C3 (250 μg/mL), Factor B (50 μg/mL) and Factor D (1 μg/mL) or with C3b (250 μg/mL) in 20 mM HEPES 150 mM NaCl 10 mM MgCl2 for 1 h at 37°C. Samples were then incubated with 5-day-old immature MO-DCs (2.105 cells/100 μL) for 2 h at 4°C or 37°C. Cells were washed with ice-cold phosphate buffer saline, fixed with 4% paraformaldehyde (Sigma-Aldrich). In the case of FVIII, cells were permeabilized using 0.5% saponin (Sigma-Aldrich) and stained with an FITC-conjugated monoclonal mouse anti-human FVIII IgG (77IP52H7). Cells were analyzed by flow cytometry and data were computed using the BD FACS Diva software (v.6.1, BD Biosciences). The uptake was quantified as the difference in the median fluorescence intensities between 37°C and 4°C (ΔMFI37°C–4°C) to exclude the signal generated by the binding of FVIII to the cell surface.
Stimulation of an FVIII-specific HLA-DRB1*0101-restricted mouse CD4+ T-cell hybridoma
Activation of the HLA-DRB1*0101-restricted mouse CD4+ T-cell hybridoma specific for human FVIII (1G8-A2), was assessed as described previously.18 FVIII (10 nM) was incubated with 20% NS, HIS or ΔC3 serum in RPMI-1640 supplemented with 2 mM CaCl2 and 10 mM MgCl2 for 1 h at 37°C. Samples were then incubated with 5-day-old MO-DCs (104 cells/condition) generated from healthy donors with the DRB1*0101 haplotype, for 2 h at 37°C to allow FVIII uptake. MO-DCs were then washed, suspended in X-VIVO15 medium, and co-cultured with 105 1G8-A2 CD4+ T cells for 18 h at 37°C. Controls included T cells incubated alone, or incubated with MO-DCs in the presence of concanavalin A (2 μg/mL, Sigma-Diagnostics, MO, USA) or in the absence of FVIII. Levels of interleukine-2 secreted by T cells were assessed using BD OptEIA™ mouse IL-2 ELISA set (BD Biosciences).
FVIII binding to immobilized C3b by ELISA
C3b was coated on ELISA plates at 10 nM in sodium bicarbonate buffer (pH 9.4) overnight at 4°C. Wells were blocked in PBS-5% bovine serum albumin (BSA). FVIII was diluted in blocking buffer and incubated on C3b-coated wells for 1 h at 37°C. Bound FVIII was revealed using a biotinylated monoclonal mouse anti-human FVIII antibody GMA-8015, streptavidin-HRP (R&D Systems, Lille, France) and the HRP o-phenylenediamine dihydrochloride substrate (OPD, Sigma-Aldrich, St. Louis, MO, USA). As a control, bound ADAMTS-13 was revealed using the biotinylated mouse anti-human ADAMTS-13 antibody, 20A5.
Co-localization of FVIII and C3b by immunofluorescence
FVIII (50 nM, Helixate®) or buffer alone were incubated in the presence of 20% HIS or NS for 1 h at 37°C in RPMI-1640. The mixture was added on MO-DCs (250,000 cells) for 2 h at 37°C. After washing with ice-cold PBS, cells were fixed using 3.7% paraformaldehyde and permeabilized. FVIII and C3b/iC3b were recognized after incubation with anti-human FVIII SAF8C (2 μg/mL) and a polyclonal anti-mouse C3b/iC3b (10 μg/mL) antibody for 30 minutes (min) at room temperature under agitation followed by the addition of the secondary A647-conjugated donkey anti-sheep IgG and A488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, USA), respectively. After washing, nuclei were stained with Hoecht®33342 (Sigma-Aldrich) and dendritic cells were plated onto poly-L-lysine coated slides using cytospin centrifugation (5 min at 1500 rpm), and mounted in ProLong Diamond Anti-Fade Mountant (Molecular Probes). Images were acquired with LSM 710 confocal microscope (Zeiss, Oberkochen, Germany).
Animals and administration of FVIII
Exon 16 FVIII-deficient mice on the C57Bl/6 background (a gift from Prof. H.H. Kazazian, University of Pennsylvania School of Medicine, Philadelphia, PA, USA) were 8-15 weeks old. Mice were injected intravenously with 1 μg human recombinant FVIII (Helixate®) once a week for four weeks. Blood was collected five days after the last FVIII administration. Plasma was isolated and kept at −80°C until use.
In vivo complement blockade
Complement was depleted in FVIII-deficient mice by intraperitoneal injection of 20 μg of hCVF. Importantly, hCVF does not cleave C5.19 C3 levels in plasma were measured by sandwich ELISA, using a polyclonal goat anti-mouse C3 antibody (MP Biomedicals, Illkirch, France) to capture C3 and a biotinylated polyclonal goat anti-mouse C3 antibody, followed by streptavidin-HRP and OPD substrate, to reveal bound C3. hCVF administration occurred 6 h prior to FVIII administration.
Titration of anti-FVIII IgG and FVIII inhibitors
ELISA plates (Nunc, Roskilde, Denmark) were coated with FVIII (1 μg/mL, Recombinate®) overnight at 4°C. After blocking with PBS-1% BSA, plasma was incubated for 1 h at 37°C. Bound IgG were revealed using an HRP-coupled polyclonal goat anti-mouse IgG antibody (Southern Biotech, Anaheim, CA, USA) and the OPD substrate. Absorbance was read at 492 nm. The monoclonal mouse FVIII heavy chain-specific IgG mAb6 (a gift from Dr J.M. Saint-Remy, Katholieke Universiteit Leuven, Leuven, Belgium) was used as a standard. FVIII inhibitors were measured by incubating heat-inactivated mouse plasma with human standard plasma (Siemens Healthcare Diagnostics, Marburg, Germany) for 2 h at 37°C. The residual FVIII pro-coagulant activity was measured using a chromogenic assay (Siemens Healthcare Diagnostics). Results are expressed in Bethesda Units (BU/mL) that correspond to the reciprocal dilution of the mouse plasma that yields 50% residual FVIII activity.
Ethical considerations
Mice were handled in agreement with French ethical authorities (authorization ns. 02058.04 and 8275.02). Ethical committee permission was obtained for the use of buffy bags from healthy donors to isolate monocytes.
Results
Complement in FVIII endocytosis by MO-DCs and blood DCs but not MO-Φ
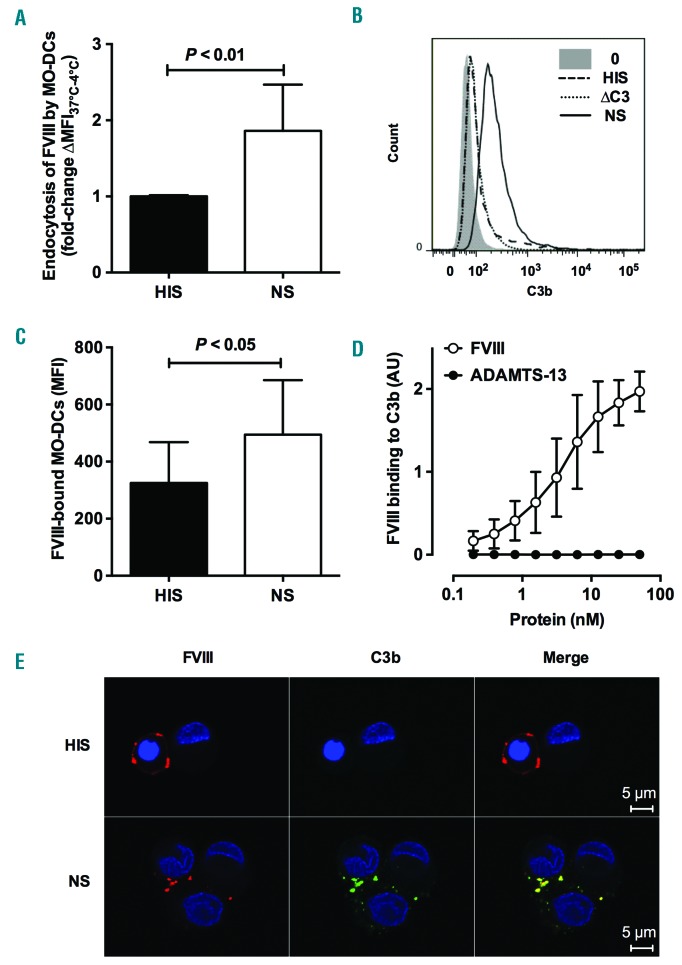
We first assessed FVIII endocytosis by MO-DCs using flow cytometry. Incubation of FVIII with immature human MO-DCs in the presence of normal serum (NS) resulted in a 1.86±0.61-fold increase in FVIII uptake as compared to incubation in the presence of heat-inactivated serum (HIS) (P<0.01) (Figure 1A). The deposition of C3b at the cell surface upon incubation of immature MO-DCs in the presence of NS at 37°C (Figure 1B) confirmed complement activation. No deposition of C3b occurred when cells were incubated in the presence of C3-depleted serum. Opsonization of the cells by C3b was associated with an increased binding of FVIII to MO-DCs, as assessed by incubation at 4°C (P<0.05) (Figure 1C). Interestingly, a direct binding of FVIII to immobilized C3b was observed by ELISA (Figure 1D), while ADAMTS-13, used as a control antigen, failed to bind to C3b. In line with this, immunofluorescence confirmed the co-localization of FVIII and C3b on MO-DCs (Figure 1E and Online Supplementary Figure S2). Taken together, the data suggest a role for C3b in facilitating FVIII capture and internalization by MO-DCs. Of note, incubation with NS did not significantly modify the expression of LRP (CD91) and CD206 (two endocytic receptors for FVIII) or DC-SIGN (CD209) (Online Supplementary Figure S1).
Figure 1.
Complement enhances Factor VIII (FVIII) endocytosis by human monocyte-derived dendritic cells (MO-DCs). (A, C and E) FVIII (50 nM) was incubated in 20% normal serum (NS) or in 20% heat-inactivated serum (HIS) for 1 hour (h) at 37°C. Mixtures were added on 5-day-old immature MO-DCs (2.105 cells in RPMI-1640) for 2 h at 4°C or 37°C. Mean fluorescence intensity (MFI) was measured by flow cytometry using an FITC-coupled anti-FVIII antibody. (A) MFI values measured at 4°C were subtracted from values measured at 37°C, in order to eliminate the signal due to the binding to the cell surface. Results represent the fold change of ∆MFI37°C–4°C measured for each condition versus ∆MFI37°C–4°C obtained in the presence of HIS. Data are shown as means±Standard Deviation (SD) of fold change of MFI measured in the case of NS versus that measured in the case of HIS (n=6). Dead cells were excluded using fixable viability dye. (B) MO-DCs were incubated alone (gray area), with NS (full line curve), HIS (broken line curve) or C3-deficient plasma (∆C3, dotted line curve) for 1 h at 37°C. Surface-bound C3b was detected by flow cytometry using an anti-C3b antibody. Results are shown as histograms. (C) FVIII bound at the surface of the cells was detected by flow cytometry following incubation of FVIII with the cells at 4°C. (D) The binding of FVIII and ADAMTS-13 to immobilized C3b (10 nM) was measured by ELISA using specific anti-FVIII or anti-ADAMTS-13 IgG. The results are expressed in optical density (OD) measured at 492 nm. (C and D) Mean±SD of at least 3 independent experiments. Statistical significance was assessed using the double-sided nonparametric Mann-Whitney test (A) or paired Wilcoxon test (C). (E) The co-localization of FVIII (red) and C3b (green) was assessed by immunofluorescence, using polyclonal anti-human FVIII and anti-C3b/iC3b antibodies and the appropriate fluorescent-labeled secondary antibodies. Images were acquired by confocal microscopy. Control images obtained in the absence of FVIII are shown in Online Supplementary Figure S1.
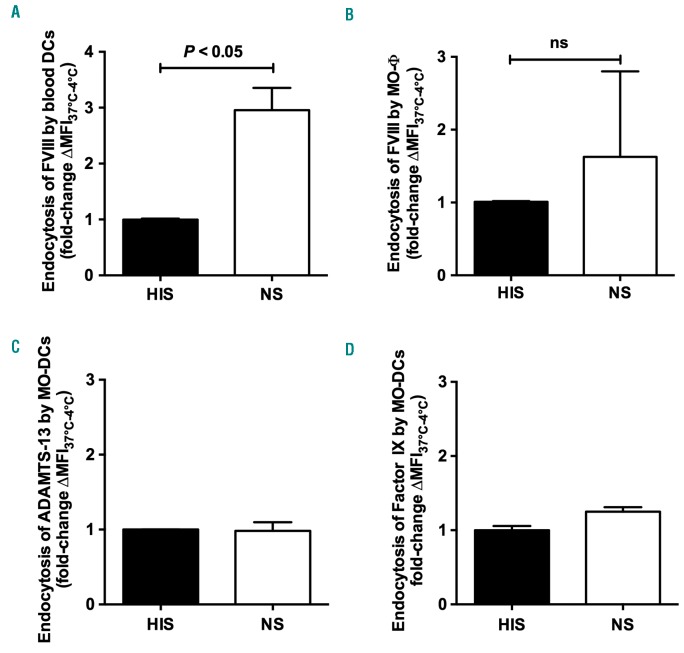
Incubation of FVIII with purified circulating blood DCs from healthy donors in the presence of NS yielded a 3-fold increase in FVIII uptake (P<0.05) (Figure 2A), while it had only a marginal effect on FVIII uptake in the case of MO-Φ (Figure 2B). Interestingly, the presence of complement did not alter the endocytosis by MO-DCs of human recombinant ADAMTS-13 and Factor IX, used as control antigens (Figure 2C and D).
Figure 2.
Complement enhances FVIII endocytosis by human professional antigen-presenting cells. (A and B) FVIII (50 nM) Dylight 633-conjugated ADAMTS-13 (50 nM, panel C) or human recombinant FITC-labeled Factor IX (50 nM, panel D) were incubated in 20% normal serum (NS) or in 20% heat-inactivated serum (HIS) for 1 hour (h) at 37°C. Mixtures were added on purified blood dendritic cells (DCs) (A), 5-day-old MO-Φ (B) or 5-day-old immature human monocyte-derived dendritic cells (MO-DCs) (2.105 cells in RPMI-1640, panels C and D), for 2 h at 4°C or 37°C. Mean fluorescence intensity (MFI) was measured by flow cytometry using an FITC-coupled anti-FVIII antibody in the case of FVIII, or directly in the case of labeled ADAMTS-13 and Factor IX. MFI values measured at 4°C were subtracted from values measured at 37°C in order to eliminate the signal due to the binding to the cell surface. Results represent the fold change of ∆MFI37°C–4°C measured for each condition versus ∆MFI37°C–4°C obtained in the presence of HIS. Graphs depict mean±Standard Deviation (SD) of at least 3 independent experiments. Dead cells were excluded. Statistical significance was assessed using the double-sided non-parametric Mann-Whitney test. ns: not significant.
Complement C3 participates in FVIII endocytosis by MO-DCs leading to presentation to CD4+ T cells
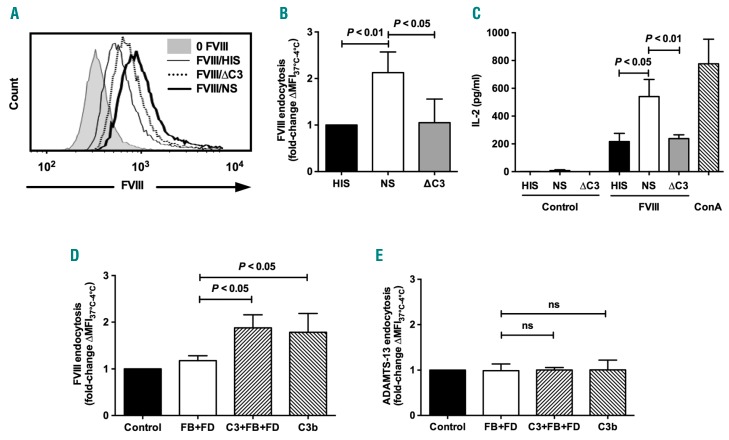
Because the complement C3 molecule is the central component of the complement cascade, and because FVIII directly binds to C3b in ELISA and co-localizes with C3b on the cells, we assessed the involvement of C3 in FVIII endocytosis by dendritic cells. Levels of FVIII endocytosis by MO-DCs were similar in the presence of C3-depleted serum (ΔC3) and in the presence of HIS (Figure 3A and B). Importantly, incubation of MO-DCs with HIS, NS or ΔC3 lead to similar degrees of cell surface expression of phosphatidylserine, a ligand for activated FVIII at the surface of activated platelets20 (Online Supplementary Figure S3A). Differences in FVIII endocytosis were thus not related to different levels of phosphatidylserine expression (i.e. apoptosis) on MO-DCs.
Figure 3.
Complement C3 enhances Factor VIII (FVIII) endocytosis by dendritic cells and presentation to CD4+ T cells. (A and B) FVIII (50 nM) was incubated for 1 hour (h) at 37°C in 20% normal serum (NS), heat-inactivated serum (HIS) or C3-depleted serum (ΔC3). Mixtures were added on immature human monocyte-derived dendritic cells (MO-DCs) (2.105 cells in RPMI-1640) for 2 h at 4°C or 37°C. FVIII was then detected with an FITC-coupled anti-FVIII antibody. Mean fluorescence intensity (MFI) values measured at 4°C were subtracted from values measured at 37°C. (A) Histograms from FVIII-positive cells incubated with HIS (solid line), NS (bold line) and ΔC3 (dashed line). The gray histogram depicts the fluorescence of cells incubated alone. (B) Fold-change of ∆MFI37°C–4°C measured for each condition versus ∆MFI37°C–4°C obtained in the presence of HIS. (C) DCs were differentiated from monocytes of healthy donors with the HLA-DRB1*0101 haplotype. Five-day-old immature MO-DCs (104 cells in X-VIVO15) were incubated alone (Control), with concanavalin A (2 μg/mL, ConA) or with 10 nM FVIII in the presence of 20% normal serum (NS), heat-inactivated serum (HIS) or C3-depleted serum (ΔC3) for 2 h at 37°C. Cells were washed and incubated with an FVIII-specific HLA-DRB1*0101-restricted mouse CD4+ T-cell hybridoma for 18 h at 37°C. Culture supernatants were collected and analyzed for IL-2 secretion. (D and E) FVIII (50 nM, panel D) or Dylight 633-conjugated ADAMTS-13 (50 nM, panel E) were incubated alone (Control), in the presence of Factor B (FB, 50 μg/mL) and Factor D (FD, 1 μg/mL) with or without C3 (250 μg/mL), or in the presence of C3b (250 μg/mL) for 1 h at 37°C. Samples were then incubated with 5-day-old immature MO-DCs (2.105 cells in X-VIVO15) for 2 h at 4°C or 37°C. MFI was measured by flow cytometry using an FITC-coupled anti-FVIII antibody in the case of FVIII or directly in the case of labeled ADAMTS-13. MFI values measured at 4°C were subtracted from values measured at 37°C. Results represent the fold change of ∆MFI37°C–4°C measured for each condition versus ΔMFI MFI37°C–4°C measured with FVIII alone (Control). All results are expressed as mean±Standard Deviation (SD) and are representative of at least 5 independent experiments. Statistical significance was assessed using the double-sided non-parametric Mann-Whitney test. ns: non-significant.
To confirm that the reduced FVIII endocytosis observed upon C3 depletion results in a reduced presentation to CD4+ T lymphocytes, we assessed the activation of a CD4+ T-cell hybridoma (1G8-A2) by MO-DCs incubated with FVIII in the presence of the different serum preparations. Importantly, the use of the T-cell hybridoma provides an opportunity to work with FVIII concentrations closer to those reached in patients with replacement therapy. The activation of 1G8-A2, assessed by the secretion of interleukine-2 (IL-2),18 was significantly higher when FVIII was incubated with MO-DCs in the presence of NS (541±123 pg/mL of IL-2) (Figure 3C) as compared to HIS (217±132 pg/mL; P≤0.05) or to ΔC3 serum (238±61 pg/mL; P≤0.01). There was no activation of 1G8-A2 when MO-DCs were incubated with the different serum preparations in the absence of FVIII. Of note, incubation of immature human MO-DCs with HIS, NS or ΔC3 serum did not induce DC maturation in vitro, as assessed by the surface expression of co-stimulatory CD40, CD80, CD86, and maturation CD83 molecules, and of HLA-DR (Online Supplementary Figure S4).
We then evaluated the role of C3b in FVIII endocytosis by MO-DCs in the absence of serum proteins. We co-incubated FVIII with either purified C3b or with an artificially reconstituted C3 convertase containing C3, Factor B and Factor D. The reconstituted C3 convertase leads to the generation of C3b as confirmed by western blot (data not shown). Samples were then added to MO-DCs and FVIII endocytosis was monitored by flow cytometry (Figure 3D). Co-incubation of immature MO-DCs with the C3 convertase or with C3b did not induce detectable apoptosis as assessed by the surface expression of phosphatidylserine (Online Supplementary Figure S3B). FVIII endocytosis was significantly higher when FVIII was incubated in the presence of the whole C3 convertase (1.88±0.28-fold increase) or C3b (1.78±0.40-fold increase) than when FVIII was incubated with Factors B and D alone (P<0.05). Consistent with the fact that ADAMTS-13 did not bind to C3b, incubation of ADAMTS-13 with the C3 convertase or with C3b did not result in increased endocytosis by MO-DCs (Figure 3E).
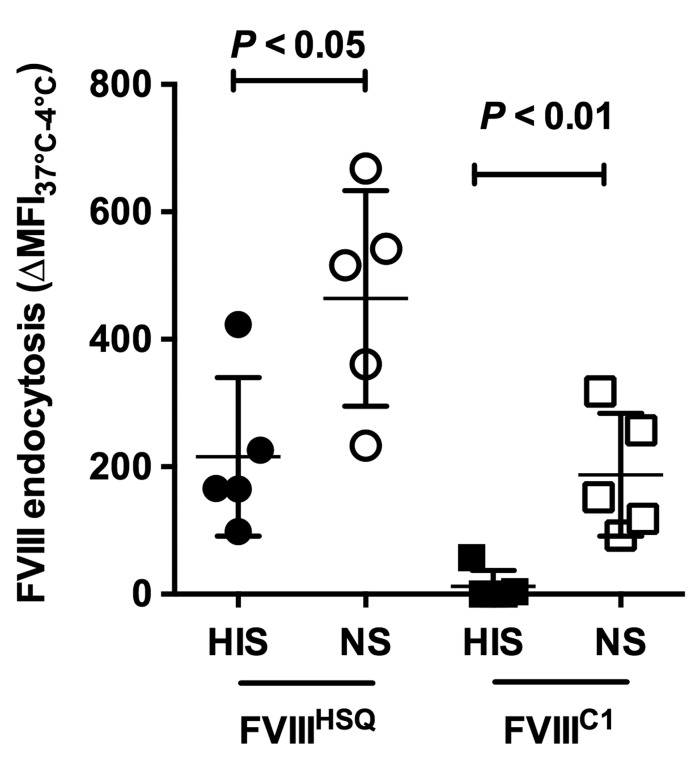
We then compared the endocytosis by MO-DCs of a recombinant B-domain-deleted FVIII (FVIIIHSQ) to that of a triple R2090A/F2092A/K2093A FVIII mutant (FVIIIC1) in the absence and presence of complement activation. As previously reported using serum-free medium,9 FVIIIC1 was not endocytosed by MO-DCs when incubated in HIS (Figure 4). In contrast, in the presence of NS, FVIIIC1 was endocytosed to levels similar to those reached with FVII-IHSQ incubated in HIS. Taken together, the data confirm a role for complement C3 in FVIII endocytosis by MO-DCs and presentation to T cells, independently of known endocytic receptors and critical FVIII residues.
Figure 4.
(Left). Complement rescues the endocytosis of a triple Factor VIII (FVIII) FVIIIC1 mutant by dendritic cells. Recombinant wild-type (FVIIIHSQ) and mutated (FVIIIC1) B-domain-deleted FVIII (50 nM) were incubated for 1 hour (h) at 37°C in 20% normal serum (NS) or heat-inactivated serum (HIS). Mixtures were added on immature monocyte-derived dendritic cells (MO-DCs) (2.105 cells in RPMI-1640) for 2 h at 4°C or 37°C. FVIII was then detected with an FITC-coupled anti-FVIII antibody directed to the A2 domain of FVIII. Mean fluorescence intensity (MFI) values measured at 4°C were subtracted from values measured at 37°C. Horizontal bars depict mean±Standard Deviation (SD) of 5 independent experiments. Statistical significance was assessed using the double-sided non-parametric Mann-Whitney test.
Complement depletion decreases the primary immune response against FVIII in FVIII-deficient mice
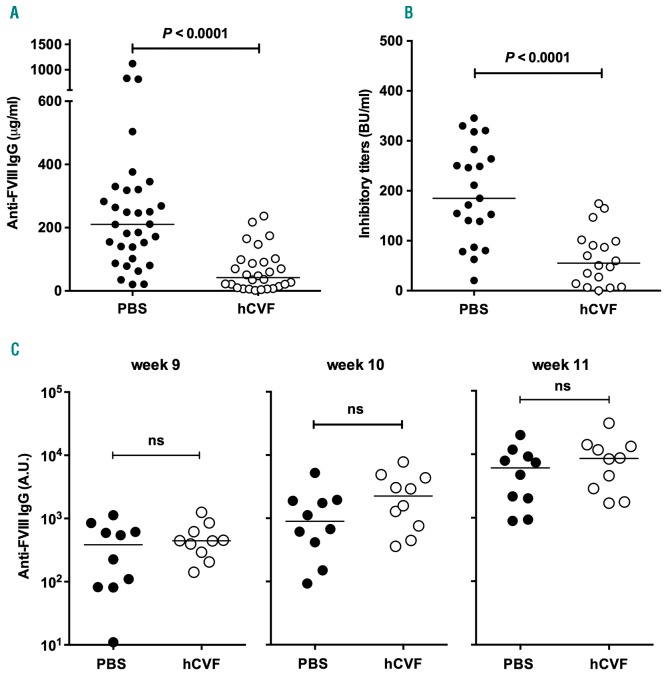
Naïve FVIII-deficient mice were treated with hCVF prior to each administration of FVIII, in order to transiently deplete the complement C3 molecule in the blood. Injection of hCVF was followed by a 90% or more decrease of circulating C3 levels that lasted for at least 5 h (Online Supplementary Figure S5). The efficacy of hCVF-mediated C3 depletion was retained following repeated weekly administration to the same mice, indicating the absence of induction of a neutralizing immune response against hCVF (data not shown). Of note, hCVF lacks C5-convertase activity and hence does not generate the potent pro-inflammatory C5a anaphylatoxin.19,21 FVIII-deficient mice treated with hCVF prior to FVIII infusion demonstrated significantly reduced levels of anti-FVIII IgG titers in plasma, as compared to the PBS control group [(5±67 vs. 267±245 μg/mL mAb6-equivalent, respectively, mean±Standard Deviation (SD); P<0.0001] (Figure 5A), as well as reduced inhibitory titers (66±55 vs. 195±97 BU/mL), respectively; depletion decreases the primary immune response against FVIII in FVIII-deficient mice, P<0.0001) (Figure 5B). Of note, depletion of C3 using hCVF did not significantly affect the half-life of FVIII in FVIII-deficient mice (Online Supplementary Figure S6). In contrast, administration of hCVF to primed FVIII-deficient mice did not have any effect on the recall anti-FVIII immune response (Figure 5C and Online Supplementary Figure S7).
Figure 5.
Complement modulates the primary immune response to therapeutic Factor VIII (FVIII) in vivo. (A and B) FVIII (1 μg/mouse) was injected intravenously to naïve FVIII-deficient mice 6 hours (h) after an intraperitoneal injection of hCVF (20 μg) or phosphate buffered saline (PBS), once a week for four weeks. At day 28, blood was collected. (C) Naïve FVIII-deficient mice were injected once a week for four weeks with FVIII (1 μg/mouse). At week 9, FVIII-primed mice were treated with either 20 μg hCVF or PBS, and 6 h later with FVIII. Blood was collected before hCVF/PBS injection and at weeks 10 and 11 (see Online Supplementary Figure S7 for the protocol). Anti-FVIII IgG titers in plasma were assessed by ELISA (panels A and C, using the monoclonal mouse anti-FVIII antibody mAb6 as a standard) and FVIII inhibitory titers by chromogenic assay [panel B, expressed in Bethesda Units (BU) per milliliter]. Results are representative of 3 independent experiments. In all panels, horizontal bars represent medians, and statistical difference weas assessed using the double-sided non-parametric Mann-Whitney test. ns: non-significant.
Discussion
Complement C3 has been shown to mediate antigen uptake by professional APCs and presentation to CD4+ and CD8+ T cells.22 Therefore, we investigated the role of complement in the endocytosis and presentation of FVIII by human APCs in vitro. The use of heat-treated serum, wherein the complement system is inactivated, was associated with baseline levels of FVIII endocytosis by MO-DCs, as previously described.7,23,24 Similar levels of endocytosis were observed when the serum was immune depleted from the C3 component. In contrast, the use of normal serum that allows activation of complement leads to an increased uptake of FVIII in the case of both MO-DCs and circulating blood DCs. In line with these data, normal serum enhanced presentation of FVIII to an FVIII-specific T-cell hybridoma, as compared to serum lacking active C3. Since heating of serum may affect proteins other than complement, we reconstituted the C3-convertase in vitro using purified proteins. In vitro reconstitution of the C3-convertase, or addition of the C3-activation fragment C3b, in the absence of other complement molecules was sufficient to restore close to maximal levels of FVIII endocytosis. In agreement with in vitro data, transient depletion of component C3 in naïve FVIII-deficient mice using hCVF resulted in a drastically reduced production of antibodies to exogenous FVIII.
Since the complement system is not expected to preferentially affect the specific immune response towards a particular antigen, we investigated the effect of complement on the endocytosis of ADAMTS-13. Human recombinant ADAMTS-13 was used as a control antigen for human recombinant FVIII, with the rationale that: i) recombinant ADAMTS-13 is being developed as a drug for replacement therapy to be used in patients with congenital thrombotic thrombocytopenic purpura (TTP), which is reminiscent of the development of recombinant FVIII for hemophilia A patients; ii) ADAMTS-13, like FVIII, is endocytosed by MO-DCs in a CD206-dependent manner;24 and, iii) as is seen in the case of FVIII, autoantibodies to endogenous ADAMTS-13 may develop, thus leading to acquired TTP. In contrast to the results obtained in the case of FVIII, ADAMTS-13 did not bind to C3b, and in vitro complement activation or addition of C3b had no effect on the endocytosis of ADAMTS-13 by MO-DCs. The data suggest a selective role of complement in immune responses towards some glycoproteins, including FVIII.
The effect of complement on endocytosis was dependent on the cell type (i.e. blood DCs as well as MO-DCs, but not MO-Φ) and on the antigen (i.e. FVIII, but not ADAMTS-13 or Factor IX), suggesting the involvement of particular endocytic pathway(s) in complement activation. It is not likely that activation of complement induces expression of endocytic receptors for FVIII at the surface of the cells due to the short incubation time used in our study. Accordingly, activation of complement on MO-DCs did not alter the surface expression of LRP (CD91) and CD206, which are known endocytic receptors for FVIII.7,25,26 Particular FVIII moieties have been implicated in its uptake by APCs. Charged residues in the C1 and C2 domains of FVIII were recently shown to play critical roles in the endocytosis process. Masking of the R2090, F2092 and K2093 residues of the C1 domain by the monoclonal antibody Km33, or of the R2215 and R2220 residues of the C2 domain by the monoclonal antibody BO2C11, or mutation towards alanine residues, independently abrogate FVIII uptake by MO-DCs.9,27,28 Importantly, the experiments leading to the identification of the latter pathways of FVIII uptake had been performed systematically in the presence of serum-free medium, and therefore did not allow assessment of whether complement has a role in the endocytic process. The fact that complement activation rescues, at least in part, the internalization of the triple FVIIIC1 mutant reveals the existence of novel endocytic pathways for FVIII, independently of charged residues in C1.27 Together, the deposition of C3b at the surface of MO-DCs upon complement activation and the binding of FVIII to immobilized C3b in ELISA, suggest that FVIII interacts with the C3b deposited at the cell surface; this would in turn allow the recruitment of those as yet unidentified endocytic receptors (Figure 6).
Figure 6.
Endocytosis pathways for Factor VIII (FVIII) in the absence or presence of complement activation. (A) In the absence of complement activation (i.e. use of decomplemented serum or of serum-free medium in cell culture), FVIII is endocytosed by three known routes: key residues in the C1 and in the C2 domain interact with yet unknown endocytic receptors,27,28 while high mannose-ending glycans at position 2118 on the C1 domain interact with the mannose-sensitive receptor CD206 (MR).7 Of note, alteration of either of these structures (e.g. mutation of key residues in either the C1 domain or the C2 domain) leads to a more than 80% reduction in FVIII uptake, suggesting that these three endocytic pathways are interdependent. (B) In the presence of complement activation, C3b deposition on dendritic cells is associated with an increase in FVIII binding and internalization by dendritic cells (Figure 1A and C). Importantly, complement activation partially restores the uptake of the C1 FVIII mutant. The data suggest that complement engages yet unidentified endocytic pathways rather than those at play in the absence of complement activation.
The question as to the origin of complement C3 activation at the time of FVIII administration in vivo remains. Injection of FVIII into FVIII-deficient mice did not lead to detectable complement activation. Conversely, immune responses to FVIII developed in FVIII-deficient mice without any overt sign of spontaneous hemorrhage or inflammation,29 and this was also seen in wild-type mice. However, the anti-FVIII immune response was drastically reduced in hemophilic mice upon exhaustion of C3 using hCVF. Under physiological conditions, a small fraction of the C3 molecules is hydrolyzed to C3(H2O), exposing new binding sites. The Factor B protease then binds C3(H2O) and is cleaved by Factor D, generating an initial C3 convertase, C3(H2O)Bb, that activates complement by cleaving C3 into its active fragments, C3a and C3b.30,31 The continuously generated C3b indiscriminately binds to host cells and pathogens and initiates a set of cascade reactions.12,13 Importantly, the C3(H2O)Bb convertase is more resistant to inactivation by Factor H and Factor I than is the C3bBb convertase.12 Taken together, these data suggest that such a spontaneous tick-over of C3, leading to permanent C3b generation, is sufficient to facilitate FVIII uptake by professional APCs, thus setting the stage for the initiation of the anti-FVIII immune response in vivo. More generally, our data illustrate a novel biological role of C3 tick-over in the recognition of some non-particulate foreign antigens. In addition, the immune response to FVIII might be modulated by the cross-talks between the complement and coagulation cascades32 or by the influence of von Willebrand factor on complement activation.33–35
Obviously, part of the anti-FVIII immune response does not depend on C3b and on activation of the complement cascade, as is suggested by the fact that: i) FVIII demonstrates substantial levels of endocytosis in the absence of complement activation; and ii) FVIII-deficient mice still develop anti-FVIII IgG when treated with hCVF. However, our results do highlight the existence of a new endocytic route for FVIII which is independent of the endocytosis mediated by the C1 domain of the molecule,27 and which should lead to the identification of the endocytic receptors involved in vivo. Future studies should be conducted to decipher the contribution of each of the different pathways of complement activation in the inhibitory anti-FVIII immune response, and should also investigate whether temporary C3 depletion with hCVF is a promising therapeutic strategy to prevent the development of anti-FVIII antibody in patients with hemophilia A.
Supplementary Material
Acknowledgments
We also would like to thank the staff from the Centre d’Imagerie Cellulaire et Cytométrie platform and Centre d’Expérimentation Fonctionnelle for assistance (Centre de Recherche des Cordeliers, Paris).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/2/352
Funding
This study was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Université Pierre et Marie Curie (UPMC) Paris 6, and by a grant from Pfizer (Aspire Haemophilia Research award 2016 WI212828). MI and IP were recipients of fellowships from Ministère de l’Enseignement Supérieur et de la Recherche. LG was the recipient of a ‘poste d’accueil INSERM’ fellowship. FVIIIHSQ in the ReNeo plasmid and BHK-M cells were kind gifts from Prof. Pete Lollar (Aflac Cancer and Blood Disorders Center, Department of Pediatrics, Emory University, Atlanta, GA, USA). Recombinant ADAMTS-13 and FVIII were kind gifts from Baxter Innovations GmbH (Vienna, Austria), and CSL-Behring (Marburg, Germany), respectively.
References
- 1.Ehrenforth S, Kreuz W, Scharrer I, et al. Incidence of development of factor VIII and factor IX inhibitors in haemophiliacs. Lancet. 1992;339(8793):594–598. [DOI] [PubMed] [Google Scholar]
- 2.Lacroix-Desmazes S, Navarrete AM, Andre S, Bayry J, Kaveri SV, Dasgupta S. Dynamics of factor VIII interactions determine its immunologic fate in hemophilia A. Blood. 2008;112(2):240–249. [DOI] [PubMed] [Google Scholar]
- 3.Skupsky J, Zhang AH, Su Y, Scott DW. A role for thrombin in the initiation of the immune response to therapeutic factor VIII. Blood. 2009;114(21):4741–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimitrov JD, Dasgupta S, Navarrete AM, et al. Induction of heme oxygenase-1 in factor VIII-deficient mice reduces the immune response to therapeutic factor VIII. Blood. 2010;115(13):2682–2685. [DOI] [PubMed] [Google Scholar]
- 5.Kurnik K, Auerswald G, Kreuz W. Inhibitors and prophylaxis in paediatric haemophilia patients: focus on the German experience. Thromb Res. 2014;134 (Suppl 1):S27–32. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Nunez L, Dienava-Verdoold I, Herczenik E, Mertens K, Meijer AB. Shear stress is required for the endocytic uptake of the factor VIII-von Willebrand factor complex by macrophages. J Thromb Haemost. 2012;10(9):1929–1937. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta S, Navarrete AM, Bayry J, et al. A role for exposed mannosylations in presentation of human therapeutic self-proteins to CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2007;104(21):8965–8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta S, Repesse Y, Bayry J, et al. VWF protects FVIII from endocytosis by dendritic cells and subsequent presentation to immune effectors. Blood. 2007;109(2):610–612. [DOI] [PubMed] [Google Scholar]
- 9.Herczenik E, van Haren SD, Wroblewska A, et al. Uptake of blood coagulation factor VIII by dendritic cells is mediated via its C1 domain. J Allergy Clin Immunol. 2012; 129(2):501–509. [DOI] [PubMed] [Google Scholar]
- 10.Sorvillo N, Hartholt RB, Bloem E, et al. von Willebrand factor binds to the surface of dendritic cells and modulates peptide presentation of factor VIII. Haematologica. 2016;101(3):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merle N, Church S, Fremeaux-Bacchi V, Roumenina L. Complement system part I -molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bexborn F, Andersson PO, Chen H, Nilsson B, Ekdahl KN. The tick-over theory revisited: formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb). Mol Immunol. 2008; 45(8):2370–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zewde N, Gorham RD, Jr, Dorado A, Morikis D. Quantitative Modeling of the Alternative Pathway of the Complement System. PLoS One. 2016;11(3):e0152337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballanti E, Perricone C, Greco E, et al. Complement and autoimmunity. Immunol Res. 2013;56(2–3):477–491. [DOI] [PubMed] [Google Scholar]
- 15.Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods Enzymol. 1993; 217:270–279. [DOI] [PubMed] [Google Scholar]
- 16.Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P. High level expression of recombinant porcine coagulation factor VIII. J Biol Chem. 2002;277(41):38345–38349. [DOI] [PubMed] [Google Scholar]
- 17.Fritzinger DC, Hew BE, Thorne M, et al. Functional characterization of human C3/cobra venom factor hybrid proteins for therapeutic complement depletion. Dev Comp Immunol. 2009;33(1):105–116. [DOI] [PubMed] [Google Scholar]
- 18.Delignat S, Repesse Y, Gilardin L, et al. Predictive immunogenicity of Refacto AF. Haemophilia. 2014;20(4):486–492. [DOI] [PubMed] [Google Scholar]
- 19.Vogel CW, Finnegan PW, Fritzinger DC. Humanized cobra venom factor: structure, activity, and therapeutic efficacy in preclinical disease models. Mol Immunol. 2014;61(2):191–203. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert GE, Furie BC, Furie B. Binding of human factor VIII to phospholipid vesicles. J Biol Chem. 1990;265(2):815–822. [PubMed] [Google Scholar]
- 21.Vogel CW, Fritzinger DC. Cobra venom factor: Structure, function, and humanization for therapeutic complement depletion. Toxicon. 2010;56(7):1198–1222. [DOI] [PubMed] [Google Scholar]
- 22.Jacquier-Sarlin MR, Gabert FM, Villiers MB, Colomb MG. Modulation of antigen processing and presentation by covalently linked complement C3b fragment. Immunology. 1995;84(1):164–170. [PMC free article] [PubMed] [Google Scholar]
- 23.Repesse Y, Dasgupta S, Navarrete AM, Delignat S, Kaveri SV, Lacroix-Desmazes S. Mannose-sensitive receptors mediate the uptake of factor VIII therapeutics by human dendritic cells. J Allergy Clin Immunol. 2012;129(4):1172–1173. [DOI] [PubMed] [Google Scholar]
- 24.Sorvillo N, Pos W, van den Berg LM, et al. The macrophage mannose receptor promotes uptake of ADAMTS13 by dendritic cells. Blood. 2012;119(16):3828–3835. [DOI] [PubMed] [Google Scholar]
- 25.Lenting P, Neels J, van den Berg B, et al. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. J Biol Chem. 1999;274:23734–23739. [DOI] [PubMed] [Google Scholar]
- 26.Saenko E, Yakhyaev A, Mikhailenko I, Strickland D, Sarafanov A. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. J Biol Chem. 1999;274:37685–37692. [DOI] [PubMed] [Google Scholar]
- 27.Wroblewska A, van Haren SD, Herczenik E, et al. Modification of an exposed loop in the C1 domain reduces immune responses to factor VIII in hemophilia A mice. Blood. 2012;119(22):5294–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gangadharan B, Ing M, Delignat S, et al. The C1 and C2 domains of blood coagulation factor VIII mediate its endocytosis by dendritic cells. Haematologica. 2017; 102(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi L, Sarkar R, Naas T, et al. Further characterization of factor VIII-deficient mice created by gene targeting: RNA and protein studies. Blood. 1996;88:3446–3450. [PubMed] [Google Scholar]
- 30.Lachmann PJ, Halbwachs L. The influence of C3b inactivator (KAF) concentration on the ability of serum to support complement activation. Clin Exp Immunol. 1975; 21(1):109–114. [PMC free article] [PubMed] [Google Scholar]
- 31.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981; 154(3):856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley JH, Conway EM. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res. 2016;118(9):1392–1408. [DOI] [PubMed] [Google Scholar]
- 33.Rayes J, Roumenina LT, Dimitrov JD, et al. The interaction between factor H and VWF increases factor H cofactor activity and regulates VWF prothrombotic status. Blood. 2014;123(1):121–125. [DOI] [PubMed] [Google Scholar]
- 34.Feng S, Liang X, Kroll MH, Chung DW, Afshar-Kharghan V. von Willebrand factor is a cofactor in complement regulation. Blood. 2015;125(6):1034–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noone DG, Riedl M, Pluthero FG, et al. Von Willebrand factor regulates complement on endothelial cells. Kidney Int. 2016; 90(1):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.