Secondary central nervous system lymphoma (SCNSL) is characterized by the infiltration of the central nervous system (CNS) with concomitant or recurrent systemic lymphoma.1 The incidence of CNS dissemination varies widely by lymphoma histology, ranging from 5% in patients with diffuse large B-cell lymphoma (DLBCL) to 30% in those diagnosed with Burkitt’s lymphoma.1,2 Treatment of SCNSL usually relies on high doses of methotrexate, but relapses are frequent and salvage strategies are scarce.3 SCNSL has a dismal prognosis, with an overall survival (OS) of less than 6 months.1–3
Exportin 1 (XPO-1) mediates the nuclear export of many regulatory proteins including tumor suppressors, and it is overexpressed in many cancers including hematological malignancies.4,5 XPO-1 overexpression leads to inactivation of tumor suppressor proteins by their translocation outside the nucleous to the cytoplasm resulting in genomic survelliance evasion and apoptosis escape of cancer cells.5 Selinexor (KTP-330) is a selective inhibitor of nuclear export (SINE) that specifically inactivates XPO-1, promoting retention of tumor suppressor proteins into the nucleous where they can restore their anti-tumor functions.6 Anti-tumor activity of selinexor in DLBCL has recently been demonstrated in phase I trials.7,8 Moreover, selinexor has shown excellent brain penetration and encouraging efficacy in patients with recurrent glioblastoma.9,10 Promising results in pre-clinical models of primary CNS lymphoma (PCNSL) have been reported as well.11 The efficacy of selinexor in patients with SCNSL or PCNSL has not yet been demonstrated.
Herein, we describe a patient with a DLBCL who developed an isolated CNS relapse. The patient was salvaged with selinexor, obtaining a prolonged control of the disease.
A 55-year-old male, physician, was diagnosed with a DLBCL stage II-A IPI 0 in February 2011. Immunohistochemical analysis of the tumor showed a germinal center-like phenotype (CD10 positive, BCL6 positive, MUM-1 positive), with Ki67 90%, lack of BCL2 expression, and weak expression of MYC. FISH analysis excluded the presence of MYC, BCL2 or BCL6 rearrangements. The patient was treated with six courses of R-CHOP achieving a complete remission (CR). Three years later, he relapsed with the same histology and phenotype as the diagnosis. He was salvaged with three courses of R-ESHAP, attaining a second CR that was subsequently consolidated with an autologous stem cell transplantation conditioned with BEAM. Seven months later, he was admitted due to dysphagia and dysphonia caused by cranial nerve palsy. Cytologic and flow cytometric analysis of the cerebrospinal fluid (CSF) revealed the presence of clonal CD20 and CD10 lymphocytes. In addition, PET scan showed a hypermetabolic occipital brain lesion without evidence of systemic lymphoma. The patient did not respond to the salvage treatment with high dose intravenous methotrexate (3gr/m2) plus intrathecal methotrexate, and was therefore enrolled in a clinical trial with the oral pleiotropic pathway modifier CC-122 (NCT01421524). After nine cycles, he presented with left peripheral facial paralysis. Cranial MRI showed two lesions, one in the left lenticular nucleus (14×15×16 mm) and another one in the right cerebellar hemisphere (19×16×13 mm). Abnormal lymphocytes in CSF were detected again by flow cytometry (0.75 cells/μL). PET scan did not show evidence of systemic lymphoma involvement. In June 2016, the patient started selinexor on a compassionate use progam at a dose of 60 mg day, twice a week (days 1 and 3), orally. After one month of treatment, the MRI showed a reduction of the left lenticular nucleus mass of more than 50%, along with complete resolution of the right cerebellar lesion and normalization of the CSF documented by flow citometry. After 5 months of selinexor, the patient remained asymptomatic and the MRI showed a complete resolution of the brain tumors. During the treatment with selinexor, the patient developed grade 3 fatigue and grade 2 anorexia that persisted despite low-dose dexamethasone and appetite stimulant megestrol acetat. Although these symptomps were momentarily mitigated with dose reduction to 20 mg twice weekly, selinexor was finally stopped in February 2017. Three weeks after selinexor discontinuation, the patient presented with impared vision, the MRI confirming progression of the lymphoma with optic nerve and chiasm infiltration. In addition, PET scan showed laterocervical nodal involvement. Selinexor could not be restarted due to poor general condition along with persistence of fatigue and anorexia. Therefore, palliative treatment was subsequently initiated.
Figure 1.
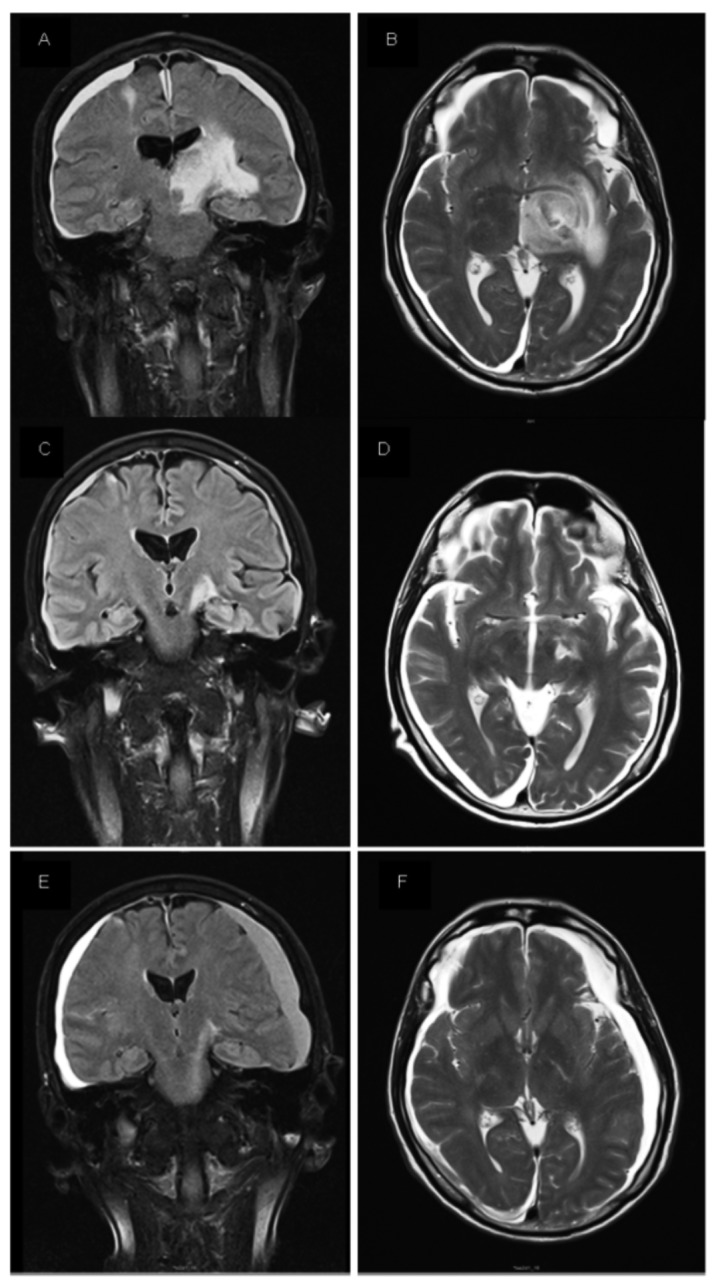
Magnetic nuclear resonance images. (A) T1 coronal, post-gadolinium MRI image before starting selinexor. (B) T2 axial, MRI image before starting selinexor. (C,D) axial and coronal images 1 month after starting treatment with selinexor. (E,F) axial and coronal images 6 months after treatment with selinexor.
Patients with systemic DLBCL relapsing or progressing in the CNS have a dismal outcome, with an OS inferior to 6 months.1,2 This poor outcome is due largely to the lack of effective treatments that can cross the blood brain barrier. Recently, preliminary results of studies using novel drugs such as ibrutinib or lenalidomide have shown promising activity in patients with relapsed/refractory PCNSL with initial responses observed in 56% – 75% of patients. Despite these encouraging results, responses were short-lasting with a median progression-free survival (PFS) of 7–8 months.12–14
The orally bioavailable selective inhibitor of nuclear export selinexor has shown significant anti-tumor activity in patients with systemic relapsed or refractory DLBCL. In a phase I trial, overall response rate of 32% and CR rate of 10% were observed among 41 patients with relapsed or refractory DLBCL treated with selinexor. Moreover, objective responses were correlated with improved PFS and OS.8 In addition, selinexor has demonstrated excellent brain penetration and promising results in both pre-clinical and clinical models of glioblastoma.9,10 Recently, a phase 2 study in patients with recurrent glioblastoma showed 17% of PR and 33% of stable disease in 12 patients, which confirms its activity in brain tumors.9 Finally, our group studied the effect of selinexor in a pre-clinical model of PCNSL using an intracerebral xenograft murine model. In this model, selinexor showed excellent penetration in CNS, reduced tumor growth and significantly increased the survival of mice.11
To our knowledge, and after a thorough review of literature, this is the first time that this drug has been used in patients with SCNSL. In the patient herein presented, selinexor demonstrated efficacy with a significant reduction of the brain tumor lesions after the first month of treatment, resulting in a rapid control of the neurological symptoms and a significant improvement of the general condition of the patient. Moreover, despite reducing the dose of selinexor due to toxicity, improvement of response was observed in the following weeks and was maintained after 7 months of the onset of selinexor. The most common adverse events of selinexor reported in phase I/II trials were nausea, fatigue and anorexia.7–9 Our patient presented grade 3 fatigue and grade 2 anorexia which were not controlled with corticosteroids and appetite stimulants resulting in two dose reductions and eventually in treatment discontinuation. Based on our experience, prophylactic appetite stimulants and nutritional counseling should be recommended before starting selinexor to prevent treatment discontinuation.
CNS involvement in patients with DLBCL is a fatal complication associated with poor prognosis. Selinexor has proven CNS penetration and here, for the first time, has shown activity in a patient with SCNSL. This clinical observation should be properly evaluated in prospective clinical trials aimed to evaluate the efficacy of selinexor in SCNSL and PCNSL patients.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Fletcher CD, Kahl BS. Central nervous system involvement in diffuse large B-cell lymphoma: an analysis of risks and prevention strategies in the post-rituximab era. Leuk Lymphoma. 2014;55(10):2228–2240. [DOI] [PubMed] [Google Scholar]
- 2.Hill QA, Owen RG. CNS proophylaxis in lymphoma. Who to target and what therapy to use. Blood Rev. 2006;20(6):319–332. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein JL, Gupta NK, Mannis GN, Lamarre AK, Treseler P. How I treat CNS lymphomas. Blood. 2013;122(14):2318–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004;4(2):106–117. [DOI] [PubMed] [Google Scholar]
- 5.Tai YT, Landesman Y, Acharya C, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28(1):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lain S, Xirodimas D, Lane DP. Accumulating active p53 in the nucleus by inhibition of nuclear export: a novel strategy to promote the p53 tumor suppressor function. Exp Cell Res. 1999;253(2):315–324. [DOI] [PubMed] [Google Scholar]
- 7.Abdul Razak AR, Mau-Soeresen M, Gabrail NY, et al. Fist-in-class, first in human phase I study of Selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol. 2016;34(34):4142–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuruvilla J, Savona M, Baz R, et al. Selective inhibibition of nuclear export with selinexor in patientes with non-Hodgkin’s lymphoma. Blood. 2017;129(24):3175–3183. [DOI] [PubMed] [Google Scholar]
- 9.Lassen UN, Soerensen MM, Kung AL, et al. A phase 2 study on efficacy, safety and intratumoral pharmacokinetics of oral selinexor (KPT-330) in patients with recurrent glioblastoma. J Clin Oncol. 2016;34 (suppl; abstr 2077). [Google Scholar]
- 10.Green AL, Ramkissoon SH, McCauley D, et al. Preclinical antitumor efficacy of selective exportin 1 inhibitors in glioblastoma. Neuro Oncol. 2015;17(5):697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crespo M, Carabia J, Jiménez I, et al. XPO1 inhibition by Selinexor synergizes with BCR inhibition, blocks tumor growth and prolongs survival in a bioluminescent animal model of primary central nervous system lymphoma. [abstract]. Blood. 2016;128(22):463. [Google Scholar]
- 12.Choquet S, Houillier C, Bijou F, et al. Ibrutinib monotherapy in relapse or refractory primary CNS lymphoma (PCNSL) and primary vitreo-retinal lymphoma (PVRL). Result of the interim analysis of the iLOC phase II study from the Lysa and the French LOC network.[abstract]. Blood. 2016;128(22):784. [Google Scholar]
- 13.Grommes C, Pastore A, Gavrilovic I, et al. Single-agent ibrutinib in recurrent/refractory central nervous system lymphoma.[abstract]. Blood. 2016;128(22):783.27301863 [Google Scholar]
- 14.Ghesquieres H, Houillier C, Chinot O, et al. Rituximab-Lenalidomide (REVRI) in relapse or refractory primary central nervous system (PCNSL) or vitreo retinal lymphoma (PVRL): results of a “proof of concept” phase II study of the French LOC network.[abstract]. Blood. 2016;128(22):785. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


