Abstract
Background
Women who initiate antiretroviral therapy (ART) during pregnancy are reported to have lower risk of preterm birth compared to those who enter pregnancy care already receiving ART. We hypothesize this association can be largely attributed to selection bias.
Methods
We simulated a cohort of 1000 preconceptional, HIV-infected women, where half were randomly allocated to receive immediate ART and half to delay ART until their presentation for pregnancy care. Gestational age at delivery was drawn from population data unrelated to randomization group (i.e., the true effect of delayed ART was null). Outcomes of interest were preterm birth (<37 weeks), very preterm birth (<32 weeks), and extreme preterm birth (<28 weeks). We analyzed outcomes in two ways: (1) a prospectively enrolled clinical trial, where all women were considered (the intent-to-treat (ITT) analysis); and (2) an observational study, where women who deliver before initiating ART were excluded (the naïve analysis). We explored the impact of later ART initiation and gestational age measurement error on our findings.
Results
Preconception ART initiation was not associated with preterm birth in ITT analyses. Risk ratios (RR) for the effect of preconception ART initiation were RR=1.10 (preterm), RR=1.41 (very preterm), and RR=5.01 (extreme preterm) in naïve analyses. Selection bias increased in the naïve analysis with advancing gestational age at ART initiation and with introduction of gestational age measurement error.
Conclusions
Analyses of preterm birth that compare a preconception exposure to one that occurs in pregnancy are at risk of selection bias.
Keywords: Antiretroviral therapy, HIV, Selection bias, Preterm birth
Introduction
Increasing availability of combination antiretroviral therapy (ART) has transformed the global human immunodeficiency virus (HIV) pandemic. Perhaps nowhere is this more evident than in the antenatal clinic, where lifelong ART is now the worldwide standard of pregnancy care for HIV-infected pregnant women.1 As these programmatic successes have dramatically improved maternal health and reduced mother-to-child HIV transmission in many settings, new concerns have emerged regarding antiretroviral drug exposure and adverse birth outcomes, especially preterm birth (usually defined as birth before 37 weeks completed gestation) and its close correlate, low birthweight.2,3
Timing of ART initiation with respect to gestation is frequently reported as a risk factor for preterm birth and low birth weight. Some observational studies suggest that ART initiated before conception (or very early in pregnancy) may cause a higher risk of preterm birth than ART started later in pregnancy. The European Collaborative Study and the Swiss Mother and Child HIV Cohort Study found that women who initiated ART preconception or in the first trimester had 2.17 (95% confidence interval (CI): 1.03, 4.58) times the odds of preterm birth compared to women who initiated in the 2nd or 3rd trimester.4 Similar results indicating a higher risk with early initiation have since been reported in Brazil,5 Europe,6–8 Botswana,9 Nigeria,10 Tanzania,11 and the U.S.3,12 A recent meta-analysis of studies before 2016 found that women who initiated ART before conception were more likely to deliver preterm (RR=1.20; 95% CI:1.01, 1.44) or very preterm (RR=1.53; 95% CI: 1.22, 1.92) compared to women initiating during pregnancy.13 In contrast, few studies reported a null effect of timing14,15 or an increased risk of preterm birth with the initiation of treatment later during pregnancy compared to preconception.16,17 All evidence to date is based on observational studies, and randomized evidence is not forthcoming.
That preconception ART would be associated with a higher risk of preterm birth seems at odds with biology. Untreated HIV infection is characterized by systemic inflammation and immune activation and confers an increased risk of preterm birth.18 Although preterm birth is a complex syndrome with several causes, the most common phenotype is inflammatory,19 where local or systemic inflammation trigger labor prematurely. Control of viral replication with ART dramatically reduces inflammation and immune activation over time. Thus, preconception ART would be expected to reduce, not potentiate, the risk of preterm birth.
We hypothesized that reports of an increased risk of preterm birth associated with preconception ART initiation largely arose from selection bias. In earlier studies, which have all been observational, women who experience a preterm birth before starting ART are systematically excluded from analyses.13 Exclusion of these women may result in a falsely lowered risk of preterm birth among those women who remain, thus inflating the risk ratio for preterm birth when compared to those who initiate before conception. If women were randomized to timing of treatment and followed to delivery as in an intent-to-treat (ITT) analysis of a randomized trial, the randomization and ITT analysis would protect against this selection bias. We further hypothesized that the selection bias would be more pronounced in the following circumstances (a) settings where women initiate treatment later in gestation, (b) settings where ultrasound dating is not routine and thus gestational age measurements are imprecise, and (c) analyses that define preterm birth with criteria earlier in gestation (e.g., <32 weeks, <28 weeks). Here we use simulated data to explore and quantify the extent of selection bias reasonably possible in the effect of immediate versus delayed (during pregnancy) ART initiation on preterm birth in HIV-infected women.
Methods
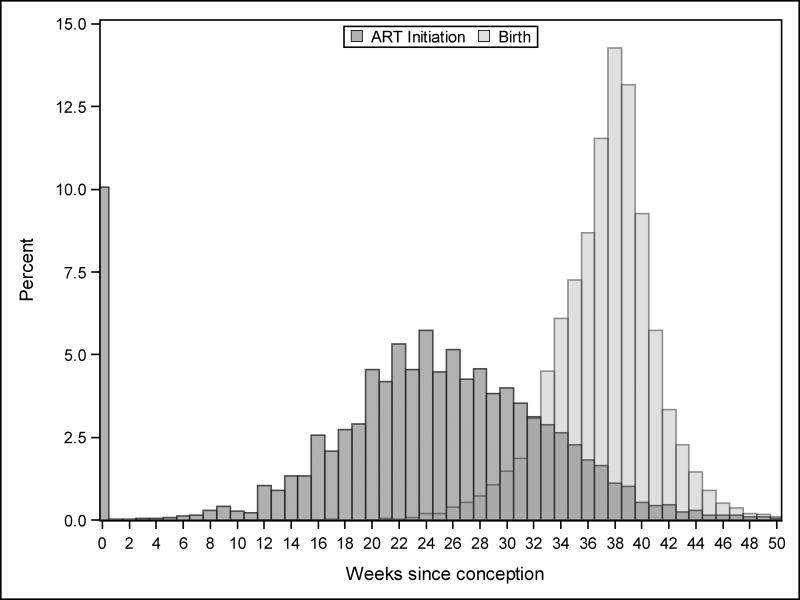
Research in this topic area aims to answer the following question: Should HIV-infected women who intend pregnancy in the near future start ART immediately or wait until they become pregnant? The specific interest is in whether such a strategy would result in a reduction in the risk of preterm birth. We are not interested in the effect of ART initiation on fecundability or on early pregnancy loss/miscarriage (although these questions are also interesting and potentially relevant). Toward this end, we simulated a cohort of 1,000 HIV-infected women recruited into a trial before conception.20 A sample size of 1,000 was chosen to be similar in size to earlier observational studies. Half of the cohort was randomized to immediate (preconception) ART and the other half was randomized to delay treatment initiation until pregnancy. Although under real-world conditions, we would expect approximately 80% of participants to become pregnant within the first 6 months and more than 90% within 1 year,21 we assumed for purposes of this exercise that all women would become pregnant and carry their pregnancy to viability. The outcomes of interest were preterm birth, very preterm birth, and extreme preterm birth, which were defined as birth before 37, 32, and 28 completed gestational weeks, respectively.22 Two critical timing measures were necessary for this simulation: gestational age at ART start and gestational age at delivery. To simulate these dates, we drew from multinomial distributions based on data from the Zambia Electronic Perinatal Registration System (ZEPRS) (Figure 1).23 ZEPRS includes 12,324 deliveries to HIV-infected women initiating ART in pregnancy or the early postpartum period between 2006 and 2012. Dates of initiation of treatment and delivery were assigned in our simulated data without respect to randomized group such that the true causal effect of delayed ART is null, and the true risk ratio is therefore one.
Figure 1.
Distributions of gestational age from the Zambia Electronic Perinatal Registration System (ZEPRS).
In an ITT analysis, preterm birth rates were compared between those women randomized to immediate preconception ART and those randomized to delayed ART during pregnancy. In an alternate naïve analysis mimicking the observational evidence base, all women randomized to immediate preconception ART were compared to the subset of women randomized to delayed ART during pregnancy whose delivery occurred after ART was initiated (i.e., women who delivered before ART start were excluded). Groups were compared using the risk ratio and 95% confidence interval, as estimated with a log-binomial model fit by maximum likelihood. An average of 200 simulations was taken for all estimates. We also calculated the confidence-interval coverage proportion as the percentage of the 200 confidence intervals that contained the true parameter value (ideally 95%).
We then explored scenarios shifting the gestational age at ART initiation in the cohort from 1 to 10 weeks later than the mean of 26 weeks estimated gestational age observed in the Zambia registry data. Next, we introduced measurement error in gestational age dating to our original scenario by adding a random error to estimated gestational age at birth from a uniform distribution ranging from −2 to 2 weeks and from −5 to 5 weeks. Finally, for all sets of assumptions, we evaluated the effect of using definitions of preterm birth with criteria limits earlier in the gestation (<37 weeks, <34 weeks, < 28 weeks). SAS version 9.3 (SAS Institute, Cary, NC) was used for all analyses.
Results
In the ITT analysis, 34.7% of women who started ART preconception had a preterm birth, 6.6% had a very preterm birth, and 1.5% had an extreme preterm birth, similar to 35.1% preterm, 6.7% very preterm, and 1.6% extremely preterm in those who initiated during pregnancy. As expected, preterm birth (RR=0.99; 95% CI: 0.84, 1.17), very preterm birth (RR=0.98; 95% CI: 0.61, 1.57), and extreme preterm birth (RR=0.95; 95% CI: 0.32, 2.79) were not associated with starting ART preconception (Table 1).
Table 1.
Average percentage with a preterm birth and very preterm birth by timing of treatment initiation and average risk ratio (RR) with average 95% confidence interval (CI) in the intent to treat versus naive analysis.a
| Intent to treat analysis | Naïve analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Preterm Category |
Exposure category | N | % | Average RR (95% CI) |
Coverage interval |
N | % | Average RR (95% CI) |
Coverage interval |
| <37 weeks | Preconception | 174 | 34.7 | 0.99 (0.84,1.17) | 0.95 | 174 | 34.7 | 1.10 (0.92,1.32) | 0.85 |
| During pregnancy | 175 | 35.1 | 1 | 1 | 144 | 31.7 | 1 | 1 | |
| Excluded women | - | - | - | - | 31 | 69.8 | - | - | |
|
| |||||||||
| <32 weeks | Preconception | 33 | 6.6 | 0.98 (0.61,1.57) | 0.97 | 33 | 6.6 | 1.41 (0.82,2.42) | 0.77 |
| During pregnancy | 34 | 6.7 | 1 | 21 | 4.7 | 1 | 1 | ||
| Excluded women | - | - | - | - | 12 | 27.3 | - | - | |
|
| |||||||||
| <28 weeks | Preconception | 8 | 1.5 | 0.95 (0.32,2.79) | 0.96 | 8 | 1.5 | 5.01 (1.35,18.54) | 0.89 |
| During pregnancy | 8 | 1.6 | 1 | 4 | 0.8 | 1 | 1 | ||
| Excluded women | - | - | - | - | 4 | 9.3 | - | - | |
ITT: Preconception N=502, during pregnancy 498; Naïve analysis: Preconception N=502, during pregnancy N=454, excluded N=44; ITT indicates intent to treat.
In the naïve analysis, which excluded women who delivered before initiating ART, 34.7% of women on preconception ART had a preterm birth compared to 31.7% in those who initiated during pregnancy. A large proportion (69.8%) of those who were excluded delivered preterm and removing them from this arm of the trial artificially lowered the proportion among those who remained. Thus, in the naïve analysis, the risk of preterm birth in women on preconception ART was 1.10 times the risk of preterm birth in women who initiated later during pregnancy (95% CI 0.92, 1.32). This bias of the effect estimate was stronger under the definition of very preterm birth (RR=1.41; 95% CI: 0.82, 2.42) and extreme preterm birth (RR=5.01; 95% CI: 1.35, 18.54) comparing preconception initiation to initiation during pregnancy.
Shifting the estimated gestational age when women initiated treatment did not change the risk ratio for the association between preconception initiation and preterm birth in the ITT analysis, irrespective of the gestational age criterion used to define preterm birth (Table 2). However, in the naive analysis, bias in the risk ratio increased as women initiated treatment later during pregnancy. The risk ratios for the effect of preconception initiation on preterm birth (RR=1.54; 95% CI: 1.21, 1.97) and very preterm birth (RR=3.74; 95% CI: 1.43, 9.78) were higher when women initiated treatment 10 weeks later than the mean of 26 weeks observed in the Zambia registry. The risk ratio for extreme preterm birth also increased dramatically with later treatment initiation, so much so as to become unstable (RR>50; Table 2). The risk ratio was inflated at later gestational ages because shifting the ART initiation to an average of 36 weeks practically eliminates the chance of a preterm birth among the post-conception ART initiators, especially among those with an extreme preterm birth.
Table 2.
Average risk ratio (RR) and average 95% confidence interval (CI) for the association between preconception initiation versus initiation during pregnancy, shifting EGA at ART initiation
| Intent to treat analysis |
Naive analysis | Intent to treat analysis |
Naive analysis | Intent to treat analysis |
Naive analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <37 weeks | <32 weeks | <28 weeks | ||||||||||
| Mean EGA at ART initiation |
RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| 26 | 0.99 | (0.84,1.17) | 1.10 | (0.92,1.32) | 0.98 | (0.61,1.57) | 1.41 | (0.82,2.42) | 0.95 | (0.32,2.79) | 5.01 | (1.35,18.54) |
|
| ||||||||||||
| 27 | 0.99 | (0.84,1.18) | 1.12 | (0.94,1.35) | 1.00 | (0.62,1.6) | 1.49 | (0.86,2.6) | 0.99 | (0.33,2.96) | 3.66 | (0.86,15.54) |
| 28 | 0.99 | (0.84,1.18) | 1.15 | (0.95,1.38) | 1.02 | (0.64,1.64) | 1.63 | (0.92,2.88) | 0.96 | (0.32,2.84) | 5.11 | (1.15,22.81) |
| 29 | 1.01 | (0.85,1.2) | 1.19 | (0.98,1.44) | 1.02 | (0.64,1.63) | 1.76 | (0.98,3.18) | 0.98 | (0.36,2.79) | 18.79 | (0.06,16.3) |
| 30 | 0.99 | (0.84,1.18) | 1.20 | (0.99,1.46) | 1.00 | (0.62,1.6) | 1.81 | (0.99,3.32) | 1.09 | (0.38,3.15) | 53.74 | (12.53,230.54) |
| 31 | 1.00 | (0.84,1.18) | 1.25 | (1.02,1.53) | 0.98 | (0.61,1.56) | 2.01 | (1.05,3.84) | 0.99 | (0.34,2.86) | >50a | |
| 32 | 1.00 | (0.84,1.18) | 1.29 | (1.05,1.59) | 1.03 | (0.64,1.64) | 2.33 | (1.17,4.63) | 1.03 | (0.36,2.97) | >50a | |
| 33 | 1.00 | (0.84,1.19) | 1.34 | (1.08,1.66) | 0.99 | (0.62,1.58) | 2.40 | (1.17,4.92) | 1.03 | (0.36,2.96) | >50a | |
| 34 | 0.99 | (0.83,1.17) | 1.37 | (1.1,1.71) | 0.99 | (0.62,1.58) | 2.81 | (1.28,6.18) | 0.99 | (0.34,2.92) | >50a | |
| 35 | 1.01 | (0.85,1.2) | 1.47 | (1.16,1.85) | 1.02 | (0.64,1.65) | 3.23 | (1.36,7.67) | 1.04 | (0.35,3.07) | >50a | |
| 36 | 1.01 | (0.85,1.19) | 1.54 | (1.21,1.97) | 0.99 | (0.62,1.58) | 3.74 | (1.43,9.78) | 1.00 | (0.34,2.92) | >50a | |
Sample sizes are too small to show stable estimates because the number of preterm pregnancies is practically eliminated
Finally, in the ITT analysis, adding measurement error to the gestational age dating at birth again did not change the association between preconception ART and preterm birth (Table 3). However, the percentage of preterm, very preterm, and extreme preterm birth increased overall when adding ±2 and ± 5 weeks random error. In the naive analysis, risk of preterm birth was higher among women who started preconception ART compared to later during pregnancy when adding up to 2 weeks (RR=1.11; 95% CI: 0.96, 1.31) and 5 weeks measurement error (RR=1.12; 95% CI: 0.96, 1.32). The risk ratio for the effect of preconception initiation on very preterm and extreme preterm birth also increased as more measurement error was added to gestational age dating. As measurement error increases, the standard errors decrease, owing to the increased number of preterm births.
Table 3.
Average percentage with a preterm birth and very preterm birth by timing of treatment initiation and the average risk ratio (RR) with average 95% confidence interval (CI) in the intent to treat versus naive analysis, adding measurement error
| Intent to treat analysis | Naïve analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preterm Category | Measurement Error |
Category | N | % | Average RR (95% CI) |
Coverage interval |
N | % | Average RR (95% CI) |
Coverage interval |
| <37 weeks | +− 2 Weeks | Preconception | 186 | 37.0 | 0.99 (0.85,1.17) | 0.97 | 186 | 36.0 | 1.11 (0.96,1.31) | 0.81 |
| During pregnancy | 185 | 37.2 | 1 | 154 | 34.0 | 1 | ||||
| Excluded women | - | - | - | 31 | 69.9 | |||||
|
| ||||||||||
| +− 5 Weeks | Preconception | 211 | 42.0 | 1.00(0.87,1.16) | 0.96 | 211 | 42.0 | 1.12 (0.96,1.32) | 0.70 | |
| During pregnancy | 209 | 41.9 | 1 | 1 | 179 | 39.4 | 1 | 1 | ||
| Excluded women | - | - | - | - | 30 | 67.6 | - | - | ||
|
| ||||||||||
| <32 weeks | +− 2 Weeks | Preconception | 38 | 7.5 | 0.99 (0.64,1.54) | 0.94 | 38 | 7.5 | 1.42 (0.86,2.35) | 0.73 |
| During pregnancy | 38 | 7.6 | 1 | 25 | 5.5 | |||||
| Excluded women | - | 13 | 23.4 | |||||||
|
| ||||||||||
| +− 5 Weeks | Preconception | 56 | 11.1 | 1.00 (0.70,1.42) | 0.95 | 56 | 11.1 | 1.42 (0.94,2.13) | 0.60 | |
| During pregnancy | 56 | 11.2 | 1 | 1 | 42 | 9.2 | 1 | 1 | ||
| Excluded women | - | - | - | - | 14 | 32.0 | - | |||
|
| ||||||||||
| <28 weeks | +− 2 Weeks | Preconception | 9 | 1.7 | 0.96 (0.36,2.58) | 0.97 | 9 | 1.7 | 2.17 (0.79,5.98) | 0.81 |
| During pregnancy | 9 | 1.8 | 1 | 4 | 1.0 | 1 | ||||
| Excluded women | - | - | - | 4 | 25.0 | - | ||||
|
| ||||||||||
| +− 5 Weeks | Preconception | 14 | 2.8 | 1.00 (0.47,2.14) | 0.94 | 14 | 2.8 | 2.17 (0.79,5.98) | 0.69 | |
| During pregnancy | 14 | 2.8 | 1 | 8 | 1.8 | 1 | ||||
| Excluded women | - | - | - | - | 6 | 12.7 | - | - | ||
Discussion
This study provides a reasonable explanation for the counterintuitive finding in earlier reports that preconceptional ART is associated with a higher risk of preterm birth than in those newly initiating ART in pregnancy. Our simulation quantifies the extent to which exclusion of women who deliver before initiating ART selectively lowers the risk of preterm birth among those who remain. Because this selection applies only to women who initiate treatment during pregnancy it makes continuation of preconceptional ART look worse by comparison. The effect of this selection bias is magnified as the average gestational age at treatment initiation advances, with increasingly severe definitions of preterm birth, and with increased measurement error in gestational age dating. Typically, when nondifferential measurement error is introduced, it is expected to produce bias toward the null. However, under our naive analysis with selection bias, increased measurement error increased the number of preterm births and the number who are excluded because they deliver before ART initiation.
Our results are compatible with earlier studies showing an elevated risk of preterm birth with preconception initiation. The magnitude of the association of preconception initiation with preterm birth was half that of the recent meta-analysis (pooled RR=1.20; 95% CI: 1.01, 1.44).13 The meta-analysis also reported a higher association in low and middle-income countries (pooled RR=1.41; 95% CI: 1.22, 1.63) compared to high-income countries (pooled RR=0.89; 95% CI: 0.54, 1.47).13 These results are compatible with our theory that the bias will be higher in settings where women present to care later in gestation and where pregnancy dating relies on patient report of the last menstrual period rather than ultrasound biometry. Additionally, there was a higher risk ratio for the effect of preconception initiation on very preterm birth (pooled RR=1.53; 95% CI: 1.22, 1.92)13, which is similar to our results. When we used a criterion for preterm birth earlier in gestation, a greater proportion of preterm births in the cohort are systematically removed, and the risk ratio is further inflated. To address selection bias, researchers can randomize women to ART, define exposures prospectively, or use methods to address selection bias, such as inverse probability weighting.24
While an epidemiologist who has been sensitized to biases in observational studies might recognize this selection bias immediately, the wider community of health and medical scientists may not, as evidenced by publication of these papers in prestigious journals without discussion of this possible bias. We chose to explore this likely selection bias using simulation so that we could quantify the size of the bias using realistic input data, alternatively one could quantify the bias analytically. Our simulation shows the magnitude of bias under the null where there is no association between ART initiation and preterm birth. However, it is possible that there is also some effect of ART initiation on preterm birth operating in addition to this selection bias. If there were a harmful effect of preconception ART initiation on preterm birth we would expect the magnitude of the association to be larger than what is observed in our simulation, essentially the composite of the effect and the bias. If there were a protective effect of preconception ART initiation on preterm birth we would expect the risk ratio to appear closer to the null. The association reported in the recent meta-analysis (pooled RR=1.20; 95% CI: 1.01, 1.44) suggests that there may be a harmful effect of delayed ART operating in addition to the illustrated selection bias.13
In addition to the case of ART initiation timing and preterm birth, this selection bias will apply in all naive analyses of time-related exposures with preterm birth outcomes. For example, in studies looking at timing of prenatal care visits or doses of malaria prophylaxis as an exposure in relation to preterm birth outcomes.25,26 When using a time-dependent exposure, there will always be women who are excluded because they deliver prematurely, before they can receive the timed exposure later in pregnancy. Therefore, the risk of preterm birth with later exposure will be falsely lowered and earlier exposure will appear to have a higher risk or later exposure will appear protective. For example, women with more prenatal care visits will have a lower risk of preterm birth compared to those fewer visits because women who have already had a preterm birth in the later category will be excluded.26 Further, the bias will apply in all studies in which the exposure and outcome are both contingent on survival in time. For example, when looking at age of first childbirth and survival to age 90, women who do not survive to have later births cannot survive to age 90 and therefore, selection bias is introduced.27
Lastly, in naïve analyses of observational studies looking at the association between ART initiation and subsequent outcomes, the timing of ART initiation is often defined retrospectively. Essentially, these studies are asking the question “What is the effect of having previously initiated ART at the time of pregnancy compared to initiating it now or shortly thereafter?” Since one cannot retrospectively initiate ART, the implications of this question are unclear. By re-conceptualizing the study as an RCT, we are able to more clearly answer the question “Should I initiate ART before I become pregnant?,” which is far more relevant in terms of developing an intervention.
Our simulation uses data from a cohort of pregnant women in Zambia, where gestational age dating is not confirmed by ultrasound and is not always accurate. Although estimates from our simulated cohort depend on the accuracy of these parameters, we have illustrated how the selection bias would be further affected by changing parameters in our study including the accuracy of gestational age dating and average timing of treatment initiation. The selection bias described may exist in similar settings but the magnitude of the bias should be assessed using empirical data from different settings and by reporting median gestational age at ART initiation, which is rarely provided in current studies.
In addition, there may be confounding in naive analyses of observational data that could also explain the association between timing of ART initiation and preterm birth. Confounding is not considered in our simulation The selection bias in our simulation is a result of conditioning on a collider (i.e., women who start ART late and deliver early are excluded).28 If we were able to measure and control variables that are causes of delivering before initiating ART we could reduce the selection bias. Lastly, our simulation assumes women become pregnant, enter antenatal care, and carry a pregnancy to viability. While it is possible that fecundity and miscarriage may be related to preconception initiation of ART, evidence is currently lacking to establish a clear association13 and only women who become pregnant and do not have a miscarriage can have a preterm birth. Presentation for antenatal care may also be related to timing of ART initiation and to preterm birth but the intention of our trial is to mimic a randomized trial in which women would have to present for care to be put on treatment.
We have illustrated that the apparent effect of preconception ART initiation on preterm birth may be due to a selection bias in naive analyses of observational studies. The selection bias increases with lower thresholds of prematurity, when women initiate treatment later in pregnancy, and with measurement error in gestational age dating. The magnitude of the association in current studies suggests that there may be a slight although reduced effect of preconception ART initiation on preterm birth, or that the association could be explained by the selection bias given the characteristics of the study populations. In the absence of randomized evidence, it is critical that researchers report the median gestational age at ART initiation to assess the potential for this bias in their studies and/or apply analytic strategies for dealing with the selection bias.
Supplementary Material
Acknowledgments
Global Women's Health Division at the University of North Carolina, Chapel Hill. We would also like to thank the women and staff who made the Zambia Electronic Perinatal Record System possible.
Source of Funding: This work was supported by grant T32 5T32AI007001 from National Institutes of Health.
Footnotes
Conflicts of Interest: None declared
Code for replication and creation of the simulated dataset is available as a supplementary file.
References
- 1.Zambia consolidated guidelines for the treatment and prevention of HIV infection. Lusaka, Zambia: Zambia Ministry of Health and Ministry of Community Development, Mother and Child Health; 2014. Zambia Ministry of Health and Ministry of Community Development MaCH. [Google Scholar]
- 2.Short C-ES, Taylor GP. Antiretroviral therapy and preterm birth in HIV-infected women. Expert review of anti-infective therapy. 2014;12(3):293–306. doi: 10.1586/14787210.2014.885837. [DOI] [PubMed] [Google Scholar]
- 3.Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS (London, England) 2007;21(5):607–615. doi: 10.1097/QAD.0b013e32802ef2f6. [DOI] [PubMed] [Google Scholar]
- 4.European Collaborative S, Swiss M, Child HIVCS. Combination antiretroviral therapy and duration of pregnancy. Aids. 2000;14(18):2913–2920. doi: 10.1097/00002030-200012220-00013. [DOI] [PubMed] [Google Scholar]
- 5.Machado ES, Hofer CB, Costa TT, et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sexually transmitted infections. 2009;85(2):82–87. doi: 10.1136/sti.2008.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibiude J, Warszawski J, Tubiana R, et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(9):1348–1360. doi: 10.1093/cid/cis198. [DOI] [PubMed] [Google Scholar]
- 7.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. Aids. 2004;18(17):2337–2339. doi: 10.1097/00002030-200411190-00019. [DOI] [PubMed] [Google Scholar]
- 8.Boer K, Nellen JF, Patel D, et al. The AmRo study: pregnancy outcome in HIV-1-infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG: An International Journal of Obstetrics & Gynaecology. 2007;114(2):148–155. doi: 10.1111/j.1471-0528.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. The Journal of infectious diseases. 2012;206(11):1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezechi OC, Gab-Okafor CV, Oladele DA, et al. Pregnancy, obstetric and neonatal outcomes in HIV positive Nigerian women. African journal of reproductive health. 2013;17(3):160–168. [PubMed] [Google Scholar]
- 11.Li N, Sando MM, Spiegelman D, et al. Antiretroviral Therapy in Relation to Birth Outcomes among HIV-infected Women: A Cohort Study. The Journal of infectious diseases. 2016;213(7):1057–1064. doi: 10.1093/infdis/jiv389. [DOI] [PubMed] [Google Scholar]
- 12.Watts DH, Williams PL, Kacanek D, et al. Combination antiretroviral use and preterm birth. The Journal of infectious diseases. 2013;207(4):612–621. doi: 10.1093/infdis/jis728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV. 2016 doi: 10.1016/S2352-3018(16)30195-3. [DOI] [PubMed] [Google Scholar]
- 14.Rudin C, Spaenhauer A, Keiser O, et al. Antiretroviral therapy during pregnancy and premature birth: analysis of Swiss data. HIV medicine. 2011;12(4):228–235. doi: 10.1111/j.1468-1293.2010.00876.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin F, Taylor GP. Increased rates of preterm delivery are associated with the initiation of highly active antiretrovial therapy during pregnancy: a single-center cohort study. The Journal of infectious diseases. 2007;196(4):558–561. doi: 10.1086/519848. [DOI] [PubMed] [Google Scholar]
- 16.Lopez M, Figueras F, Hernandez S, et al. Association of HIV infection with spontaneous and iatrogenic preterm delivery: effect of HAART. Aids. 2012;26(1):37–43. doi: 10.1097/QAD.0b013e32834db300. [DOI] [PubMed] [Google Scholar]
- 17.Short CE, Douglas M, Smith JH, Taylor GP. Preterm delivery risk in women initiating antiretroviral therapy to prevent HIV mother-to-child transmission. HIV medicine. 2014;15(4):233–238. doi: 10.1111/hiv.12083. [DOI] [PubMed] [Google Scholar]
- 18.Brocklehurst P. The Association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 105:836–848. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 19.Smid MC, Stringer EM, Stringer JS. A Worldwide Epidemic: The Problem and Challenges of Preterm Birth in Low- and Middle-Income Countries. American journal of perinatology. 2016;33(3):276–289. doi: 10.1055/s-0035-1571199. [DOI] [PubMed] [Google Scholar]
- 20.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Human reproduction. 2003;18(9):1959–1966. doi: 10.1093/humrep/deg366. [DOI] [PubMed] [Google Scholar]
- 22.WHO. Preterm birth. [Accessed December, 2016];2015 http://www.who.int/mediacentre/factsheets/fs363/en/
- 23.Chi BH, Vwalika B, Killam WP, et al. Implementation of the Zambia electronic perinatal record system for comprehensive prenatal and delivery care. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011;113(2):131–136. doi: 10.1016/j.ijgo.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernan MA. When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. Int J Biostat. 2010;6(2) doi: 10.2202/1557-4679.1212. Article 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marie CD, Stoner BV, Smid Marcela, Kumwenda Andrew, Stringer Elizabeth, Benjamin H. Chi aJSAS. Dosage of Sulfadoxine–Pyrimethamine and Risk of Low Birth Weight in a Cohort of Zambian Pregnant Women in a Low Malaria Prevalence Region. Am. J. Trop. Med. Hyg. 2017;91(1):170–177. doi: 10.4269/ajtmh.16-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vintzileos AM, Ananth CV, Smulian JC, Scorza WE, Knuppel RA. The impact of prenatal care in the United States on preterm births in the presence and absence of antenatal high-risk conditions. Am J Obstet Gynecol. 2002;187(5):1254–1257. doi: 10.1067/mob.2002.127140. [DOI] [PubMed] [Google Scholar]
- 27.Shadyab AH, Gass ML, Stefanick ML, et al. Maternal Age at Childbirth and Parity as Predictors of Longevity Among Women in the United States: The Women's Health Initiative. Am J Public Health. 2017;107(1):113–119. doi: 10.2105/AJPH.2016.303503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.