Abstract
Introduction
Oxygenated metabolites of cholesterol (“oxysterols”) can influence carcinogenesis and contribute to resistance to endocrine therapy, an effect mostly described in vitro.
Objectives
We sought to establish a method for screening plasma levels of oxysterols in breast cancer patients, estimate their individual variability and detection limits, and provide basic information about their roles in tumor biology.
Method
Liquid-chromatography coupled with tandem mass spectrometry was used for determination of levels of 25-hydroxycholesterol, 27-hydroxycholesterol, 7α-hydroxycholesterol, and 7-ketocholesterol in plasma sample pairs from patients before and 12–24 months after surgical removal of tumors (n=24). Deuterated standards of all oxysterols were used for method validation.
Result
All oxysterols were successfully detected in patient plasma samples. A significant increase in the level of 7-ketocholesterol was observed in the samples following tumor removal and the start of therapy compared to the sampling before (p=0.002). This increase was unrelated to personal characteristics of patients, expression of estrogen receptor, or to adjuvant therapy type.
Conclusion
This study shows, for the first time, that circulating levels of oxysterols, especially 7-ketocholesterol, may reflect the presence of tumor cells in patients.
Keywords: oxysterols, breast cancer, estrogen, plasma, therapy
Introduction
Breast cancer (OMIM: 114480) is the most frequently diagnosed cancer and one of the leading causes of cancer death among women in the western world (Siegel 2016). Early diagnosis and effective therapy are prerequisite for improvement of these facts. Currently, there is no available blood-based test for prediction of breast cancer risk, which would enable preventive measures to diagnose the cancer in localized stages. Moreover, due to the heterogeneity of the disease, a significant number of patients still experience disease relapse or side effects of the treatment, and markers predicting these events and new therapy targets are urgently needed for these patients.
Oxygenated metabolites of cholesterol belong to a large group of sterols called oxysterols (Mutemberezi 2016). Oxysterols are formed in the human body or ingested in the diet, e.g., phytosterols. Despite their low tissue levels, they play important roles in the organism and, e.g., through interaction with liver X receptors, oxysterol-binding proteins, or some drug transporters, oxysterols can influence both carcinogenesis and cancer progression (Kloudova 2017).
The role of cholesterol and its metabolites in breast cancer onset and therapy outcome currently receives increasing attention due to the fact that breast cancer patients have higher cholesterol levels than healthy controls (Nelson 2013), and enzymes of cholesterol biosynthesis and the synthesis of the oxysterols 25-hydroxycholesterol and 27-hydroxycholesterol contribute to endocrine therapy (e.g., tamoxifen or aromatase inhibitors) resistance in breast cancer models in vitro (Simigdala 2016). Tamoxifen is a selective estrogen receptor modulator (SERM) and, together with its metabolites (mainly 4-hydroxytamoxifen), blocks transcriptional activation of estrogen-responsible genes in breast tumor cells (Wang 2004). In addition to its SERM activity, recent studies have shown that cholesterol metabolism is involved in the anticancer pharmacology of tamoxifen (see ref. Poirot 2012 and refs. cited therein, (Segala 2013)) and that 27-hydroxycholesterol acts as an endogenous SERM, thus possibly influencing the pathology of breast cancer (DuSell 2008). Congruently, 27-hydroxycholesterol promotes faster growth and proliferation of mouse mammary gland tumors (Nelson 2013).
Additionally, B-ring oxysterols (e.g., 7-ketocholesterol) are suspected to influence hormonal treatment efficacy because of cell death induction via interaction with the antiestrogen binding site (AEBS) (De Medina 2009) and through their binding of estrogen receptors and AEBS in tumor cells (Segala 2013, de Weille 2013).
The aim of this study was to monitor plasma levels of selected oxysterols in paired plasma samples of breast cancer patients collected before surgical removal of the tumor and in the period 12–24 months after surgery. This approach provides basic information about the suitability of the method, individual variability, and detection limits of oxysterols in circulation of patients before a larger detailed study can be performed. The study also shows the first indication of differences that may be attributed to the tumor biology.
Materials and methods
Chemicals
7-Ketocholesterol (ChemSpider ID: 82599), 7α-hydroxycholesterol (96891), 25-hydroxycholesterol (58604), and 27-hydroxycholesterol (110495) and their deuterated standards (7-ketocholesterol-d7, 7α-hydroxycholesterol-d7, 25-hydroxycholesterol-d6, and 27-hydroxycholesterol-d6) were purchased from Steraloids (Newport, RI). Methanol, isopropanol, and 2,6-di-tert-butyl-4-methylphenol (butylated hydroxytoluene) were purchased from Sigma-Aldrich (St. Louis, MO), and HPLC-grade solvents were products of Fisher Scientific (Pittsburgh, PA).
Patients
A total of 24 primary breast carcinoma patients diagnosed in the Department of Oncosurgery, Medicon and surgically treated in the Institute for the Care for Mother and Child (both in Prague, Czech Republic) during 2013 were included in the study. Blood samples were collected from each patient one day before surgical tumor removal (first sampling) and then from the same patient in the period 12–24 months after surgery (second sampling). As oxysterol levels are impacted by circadian and feeding rhythm, patients were asked to refrain from eating after 8 p.m. before the day of sampling, which was performed between 8–9 AM on the next day. There was no radiologically proven disease relapse at the time of second sample collection in any of the enrolled patients.
Blood samples were collected to one vacutainer tube with anticoagulant potassium EDTA for plasma separation. The separation was performed immediately after the blood withdrawal by centrifugation at 2,200 × g for 10 minutes at 4 °C. Then samples were aliquoted to three separate aliquots, snap frozen, and stored at −80 °C. Diagnosis of all patients was confirmed histologically according to standard diagnostic procedures (Tavassoli 2003). Hormonal receptor expression was evaluated based on a 1% cut off. Immunohistochemistry was utilized for ERBB2 (erb-b2 receptor tyrosine kinase 2) testing, 3+ scores were considered positive and 1+ as negative. In the case of 2+ scores, fluorescent in situ hybridization (FISH) was used for status confirmation. Refusal of the informed consent of the patient, preoperative chemotherapy or endocrine therapy, and lack of histological diagnosis were exclusion criteria for the study. Personal and clinical characteristics of the patients including adjuvant therapy are summarized in Tables 1 and 2.
Table 1.
Personal data of patients
| Sample ID | Age at diagnosis |
Menopausal status |
Body mass index |
Smoking | Personal history related to cholesterol and hormones |
|---|---|---|---|---|---|
| 433 | 75 | post | 25 | current smoker | hysterectomy |
| 434 | 60 | post | 26 | never smoker | hysterectomy |
| 435 | 48 | pre | 23 | never smoker | hypercholesterolemia |
| 438 | 54 | pre | 22 | never smoker | none |
| 440 | 58 | post | 25 | current smoker | none |
| 443 | 75 | post | 27 | never smoker | hypercholesterolemia, hormonal replacement therapy |
| 445 | 43 | pre | 22 | never smoker | none |
| 452 | 62 | post | 24 | never smoker | hormonal replacement therapy |
| 453 | 56 | peri | 23 | ex-smoker | none |
| 456 | 54 | pre | 19 | current smoker | none |
| 463 | 55 | post | 23 | current smoker | none |
| 473 | 41 | pre | 20 | ex-smoker | hormonal replacement therapy |
| 476 | 60 | post | 22 | never smoker | hypercholesterolemia, hypothyroidism, hysterectomy |
| 500 | 71 | post | 28 | never smoker | none |
| 504 | 61 | post | 26 | N/A | hypercholesterolemia, hypothyroidism |
| 505 | 57 | post | 22 | never smoker | none |
| 523 | 39 | pre | 19 | ex-smoker | hypothyroidism |
| 535 | 60 | post | 31 | never smoker | none |
| 590 | 36 | pre | 22 | never smoker | none |
| 599 | 82 | post | 26 | never smoker | none |
| 600 | 59 | post | 25 | current smoker | none |
| 606 | 37 | pre | 21 | N/A | none |
| 611 | 57 | post | 26 | never smoker | none |
| 614 | 70 | post | 24 | never smoker | hypercholesterolemia, hysterectomy |
N/A: not available
Table 2.
Clinical data of patients
| Sample ID |
Surgery date (day/month/year)a |
Histology | Grade | Lymph node metastasis |
Stage | ER expression |
PR expression |
ERBB2 expression |
Ki67 expression |
Adjuvant therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| 433 | 30/01/2013 (21) | lobular | 1 | absent | IIA | positive | positive | negative | 5% | TAM, AI |
| 434 | 01/02/2013 (21) | ductal | 3 | absent | Tis | positive | positive | negative | 20% | TAM, AI |
| 435 | 01/02/2013 (21) | ductal | 1 | absent | IA | positive | positive | negative | 10% | TAM, AI |
| 438 | 06/02/2013 (25) | ductal | 3 | present | IIB | negative | positive | negative | 30% | TAM |
| 440 | 08/02/2013 (21) | tubular | 1 | absent | IA | positive | positive | negative | 5% | AI |
| 443 | 13/02/2013 (21) | ductal | 3 | absent | IA | negative | negative | negative | 60% | none |
| 445 | 13/02/2013 (23) | ductal | 3 | absent | IIA | negative | negative | negative | 40% | AC, PCT |
| 452 | 22/02/2013 (23) | tubular | 1 | absent | I | positive | positive | negative | 10% | TAM |
| 453 | 27/02/2013 (24) | ductal | 1 | absent | I | positive | positive | negative | 5% | TAM |
| 456 | 01/03/2013 (20) | ductal | 1 | absent | I | positive | positive | negative | 20% | TAM |
| 463 | 13/03/2013 (24) | ductal | 1 | present | IIA | positive | positive | negative | 10% | EC, TAM |
| 473 | 22/03/2013 (20) | ductal | 3 | absent | I | negative | negative | positive | 30% | EC |
| 476 | 22/03/2013 (20) | ductal | 2 | absent | I | positive | positive | negative | 15% | TAM, AI |
| 500 | 03/05/2013 (18) | ductal | 3 | absent | I | positive | positive | negative | 30% | TAM, AI |
| 504 | 10/05/2013 (20) | tubular | 2 | absent | I | positive | positive | negative | 15% | TAM |
| 505 | 15/05/2013 (20) | ductal | 1 | absent | I | positive | positive | negative | 5% | TAM |
| 523 | 23/06/2013 (19) | ductal | 3 | absent | I | negative | negative | negative | 50% | EC |
| 535 | 10/07/2013 (16) | ductal | 2 | absent | IIA | positive | positive | negative | 20% | EC, TAM, AI |
| 590 | 23/09/2013 (14) | ductal | 3 | present | IIA | positive | positive | positive | 20% | EC, TXT, HER, TAM |
| 599 | 25/10/2013 (13) | mucinous | 1 | absent | IIA | positive | positive | negative | 15% | TAM |
| 600 | 01/11/2013 (15) | ductal | 3 | present | IIA | positive | positive | negative | 30% | EC, PCT, AI |
| 606 | 20/11/2013 (12) | ductal | 3 | absent | IA | negative | negative | negative | 60% | none |
| 611 | 29/11/2013 (14) | ductal | 2 | present | IIA | positive | positive | negative | 10% | EC, TAM |
| 614 | 13/12/2013 (11) | ductal | 1 | present | IIA | positive | positive | negative | 3% | TAM |
Sampling interval in months in parentheses
ER=estrogen receptor, PR=progesterone receptor, ERBB2=erb-b2 receptor tyrosine kinase 2, Ki67=proliferation-related Ki-67 antigen
All patients were informed about the study and those who agreed and signed an informed consent participated in the study. The study protocol was approved by the Ethical Commission of the National Institute of Public Health in Prague. The methods were carried out in accordance with guidelines approved by the above Ethical Commission.
Extraction of oxysterols from plasma samples
The method described by Helmschrodt (2013) was adopted for extraction of oxysterols from plasma. Briefly, thawed plasma samples were centrifuged at 3,200 × g for 5 minutes and 80 µl of plasma was added to vials containing 80 µl of a methanolic solution of a mixture of internal standards (400 pg of the deuterated version of each oxysterol) or blank (methanol without oxysterols). Then 1.44 ml of a methanol/isopropanol (1:1, v/v) mixture was added, and the samples were thoroughly mixed using a vortex device for 1 min and centrifuged for 10 min at 12,500 × g. Supernatants were transferred into amber glass vials and evaporated to dryness at room temperature under a stream of nitrogen in the dark. Vials with dried extracts were filled with argon gas, sealed, and stored at −80 °C until analysis. On the day of analysis, extracts were reconstituted with 80 µl of a methanol/water mixture (3:1, v/v) and centrifuged prior to analysis for 5 min at 3,220 × g. All solutions contained 0.05% (v/v) butylated hydroxytoluene to prevent autoxidation (Helmschrodt 2013).
Measurement of oxysterols
Dissolved extracts were subjected to the positive-ion APCI LC/MS/MS analysis as previously described (Shinkyo 2011). Briefly, oxysterols were resolved by UPLC (50 °C) on an Acquity BEH octadecylsilane (C18) column (1.7 µm; 1 mm × 100 mm) (Waters) using solvent mixtures of H2O/CH3OH/CH3CN (30:3.5:66.5, v/v/v), holding for 1 min, then programmed to CH3OH/CH3CN (5:95, v/v) over 4 min with a linear gradient at a flow rate of 0.16 ml/min and quantitated by positive-ion APCI/MS/MS-SRM monitoring of product ions: m/z 367.2 (25-hydroxycholesterol), m/z 367.2 (27-hydroxycholesterol), m/z 367.2 (7α-hydroxycholesterol), m/z 383.2 (7-ketocholesterol), m/z 385.2 (25-hydroxycholesterol), m/z 385.2 (27-hydroxycholesterol), m/z 385.2 (7α-hydroxycholesterol), m/z 401.2 (7-ketocholesterol), respectively, and m/z 373.2 (d6-25-hydroxycholesterol), m/z 373.2 (d6-27-hydroxycholesterol), m/z 374.2 (d7-7α-hydroxycholesterol), m/z 390.2 (d7-7-ketocholesterol), m/z 391.2 (d6-25-hydroxycholesterol), m/z 391.2 (d6-27-hydroxycholesterol), m/z 392.2 (d7-7α-hydroxycholesterol), and m/z 408.2 (d7-7-ketocholesterol). The injection volume (onto the column) was 20 µl. Quantitation was done using an isotope ratio method. The limit of quantitation (LOQ) for oxysterols in human plasma was 0.05 ng per sample based on calibration curves for each oxysterol.
Data analysis
Raw data were first processed in order to identify outliers and deviations from normal distribution by the Shapiro-Wilk test. Differences between the compared groups of patients were evaluated by the paired Wilcoxon test and the independent Mann-Whitney or Spearman test. Comparisons including and excluding outliers have been performed. A p-value of less than 0.05 was considered statistically significant. Analyses were conducted by the statistical program SPSS v16.0 (SPSS, Chicago, IL).
Results
Characteristics of patients
A total of 24 patients with both samplings and complete clinical follow up data were included in the study. Another 24 patients were collected for method establishment and individual variability estimate, but paired samples or complete data follow up were not available (data not shown). Eight patients were pre-menopausal and the rest were peri- or post-menopausal. Patients from the study set were mostly diagnosed with ductal adenocarcinoma histology at locally advanced stage I or II. One patient had an in situ tumor, and in five patients the tumor had spread into axillary lymph nodes. The majority of patients (17/24) received adjuvant therapy containing tamoxifen and eight patients had only tamoxifen before the day of second sample collection. All personal and clinical data are summarized in Tables 1 and 2.
Levels of oxysterols in breast cancer patients–comparison of paired samples before and after tumor removal
Oxysterols were analyzed in plasma samples of the 24 patients with both samplings available. The individual and mean ± S.D. plasma levels of oxysterols in these patients are presented in Table 3. Data were further processed by non-parametric tests due to the diversion of majority of data from the normal distribution (p<0.05 by the Shapiro-Wilk test). Analysis by the independent Mann-Whitney test showed that the level of 27-hydroxycholesterol was significantly lower and that the level of 7-ketocholesterol was higher in the second sampling compared to the first sampling (p=0.034 and 0.013, respectively, Table 4). Analysis by the paired Wilcoxon test showed non-significant results for both oxysterols as well as for others (Table 4).
Table 3.
Individual levels of oxysterols in patients with two samplings
| 25-hydroxycholesterol | 27-hydroxycholesterol | 7α-hydroxycholesterol | 7-ketocholesterol | |||||
|---|---|---|---|---|---|---|---|---|
| Sample ID | F | S | F | S | F | S | F | S |
| 433 | 0.6 | 1.0 | 32.1 | 24.1 | 4.5 | 7.4 | 9.0 | 10.4 |
| 434 | 5.5 | BLQ | 36.2 | 27.0 | 8.4 | 5.0 | 12.1 | 10.2 |
| 435 | BLQ | 1.7 | 33.7 | 16.6 | 4.5 | BLQ | 5.9 | 8.4 |
| 438 | BLQ | 0.6 | 37.2 | 30.1 | 4.4 | 3.7 | 5.6 | 8.2 |
| 440 | 0.5 | 0.7 | 31.2 | 36.2 | 4.0 | 5.1 | 5.6 | 8.6 |
| 443 | BLQ | 1.4 | 34.9 | 24.9 | 3.7 | 4.9 | 5.9 | 8.7 |
| 445 | 0.9 | 0.9 | 39.7 | 21.9 | 2.5 | 4.6 | 5.1 | 6.5 |
| 452 | 1.5 | 1.4 | 33.6 | 27.9 | 3.4 | 4.5 | 8.5 | 7.7 |
| 453 | 1.1 | BLQ | 24.0 | 32.1 | 6.7 | 5.0 | 9.6 | 8.9 |
| 456 | BLQ | 1.0 | 28.0 | 35.0 | 4.4 | 7.7 | 6.1 | 11.1 |
| 463 | 1.2 | 0.6 | 34.6 | 24.4 | 3.7 | 3.0 | 6.9 | 6.2 |
| 473 | 0.9 | BLQ | 40.1 | 17.6 | 2.7 | 2.6 | 4.9 | 5.2 |
| 476 | BLQ | 1.2 | 30.4 | 30.4 | 2.5 | 2.2 | 10.9 | 5.1 |
| 500 | 1.0 | 1.5 | 29.7 | 14.6 | 6.7 | 10.9 | 27.6 | 16.1 |
| 504 | BLQ | 0.6 | 31.2 | 22.7 | 5.7 | 5.6 | 22.5 | 10.0 |
| 505 | 0.9 | 2.0 | 23.2 | 33.7 | 2.9 | 5.7 | 6.2 | 7.6 |
| 523 | 1.0 | 0.5 | 42.6 | 2.2 | 3.5 | 5.0 | 2.9 | 8.2 |
| 535 | 0.6 | 1.6 | 24.0 | 31.5 | 5.6 | 2.9 | 6.4 | 6.6 |
| 590 | 1.6 | 1.1 | 32.9 | 29.1 | 2.7 | 4.7 | 5.7 | 10.7 |
| 599 | BLQ | 1.2 | 22.9 | 25.0 | 4.5 | 5.4 | 5.4 | 10.5 |
| 600 | 1.5 | 1.5 | 31.9 | 42.0 | 3.5 | 6.0 | 8.1 | 9.2 |
| 606 | BLQ | 1.4 | 22.5 | 30.0 | 3.2 | 5.5 | 5.0 | 7.4 |
| 611 | 1.1 | 1.9 | 31.5 | 39.7 | 3.6 | 3.4 | 4.4 | 12.1 |
| 614 | 1.5 | 1.7 | 31.5 | 23.2 | 5.6 | 4.4 | 5.9 | 9.6 |
| Mean | 1.3 | 1.2 | 31.7 | 26.8 | 4.3 | 5.0 | 8.2 | 8.9 |
| S.D. | 1.2 | 0.4 | 5.5 | 8.6 | 1.5 | 1.9 | 5.7 | 2.4 |
All levels in ng/ml of plasma. BLQ=below limit of quantification, S.D.=standard deviation, F=first sampling, S=second sampling
Table 4.
Differences in plasma levels of oxysterols between both samplings
| All data | Independent Mann-Whitney test | Paired Wilcoxon test | ||
|---|---|---|---|---|
| Number of patients F/S | p-value | Number of patients F/S | p-value | |
| 25-hydroxycholesterol | 16/21 | 0.488 | 13/13 | 0.285 |
| 27-hydroxycholesterol | 24/24 | 0.034 | 24/24 | 0.075 |
| 7α-hydroxycholesterol | 24/23 | 0.105 | 23/23 | 0.104 |
| 7-ketocholesterol | 24/24 | 0.013 | 24/24 | 0.086 |
| Data without outliers | ||||
| 25-hydroxycholesterol | 15/21 | 0.287 | 13/13 | 0.285 |
| 27-hydroxycholesterol | 24/23 | 0.053 | 23/23 | 0.127 |
| 7α-hydroxycholesterol | 23/22 | 0.088 | 21/21 | 0.068 |
| 7-ketocholesterol | 22/23 | 0.002 | 22/22 | 0.007 |
For individual variability and average levels see Table 3. F/S=first/second sampling. Significant results in bold.
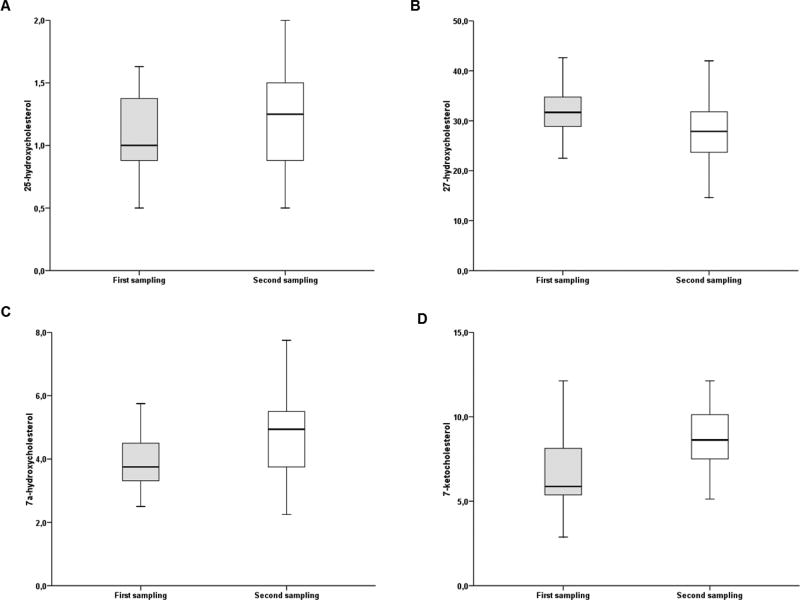
When analyzing individual values, several extreme values (outliers) were noted. Thus, separate analyses without these outliers were performed as well (for value distribution see Fig. 1). One outlier with 5.5 ng/ml (closest value 1.6) of 25-hydroxycholesterol from the first sampling, one with 2.2 ng/ml (14.6) of 27-hydroxycholesterol from the second sampling, one with 8.4 ng/ml of 7α-hydroxycholesterol (6.7 from the first sampling) and one with 10.9 ng/ml (7.7 from the second sampling), two with 27.6 and 22.5 ng/ml 7-ketocholesterol (14.6 from the first sampling) and one with 16.1 ng/ml (12.1 from the second sampling) were removed from the analysis. The samples to these outliers were not further processed in the paired analyses. Sample pairs with oxysterol levels below the limit of quantitation in one or both samplings were also removed from subsequent paired analyses. Analysis by the independent Mann-Whitney test again showed that samples from the second sampling contained significantly higher 7-ketocholesterol levels compared to the corresponding first samplings (p=0.007). However, the previously observed difference for 27-hydroxycholesterol levels was non-significant (p=0.053), as well as differences for 25-hydroxycholesterol and 7α-hydroxycholesterol. These results were also confirmed by analyses using a paired Wilcoxon test (Table 4).
Fig. 1.
Distribution of levels of oxysterols in patients before and after tumor removal with all outliers removed
A, 25-hydroxycholesterol; B, 27-hydroxycholesterol; C, 7α-hydroxycholesterol; D, 7-ketocholesterol. All levels are expressed as ng/ml of plasma.
In analyses stratified according to personal data of patients (see Table 1), significantly higher levels of 7-ketocholesterol in the first sampling of patients with post or perimenopausal status compared to premenopausal ones were found, but no such association was seen in the second sampling (Table 5A). Similarly, associations of age, body mass index, or smoking status with level of individual oxysterols observed in one of the samplings were not consistent with the other sampling. Age significantly correlated with body mass index of patients (p=0.001, r2=0.776). Interestingly, the 27-hydroxycholesterol level was significantly higher in the first sampling of patients with positive personal history of hypercholesterolemia, hypothyroidism, hysterectomy, or hormonal replacement therapy compared to patients without such conditions. On the other hand, in the second sampling of these patients opposite association was found (i.e., higher level in patients with negative history) (Table 5A).
Table 5.
Differences in plasma levels of oxysterols in stratified analyses
| Analyses stratified by personal characteristics of patients (Table 1) | |||||
|---|---|---|---|---|---|
| First sampling | Characteristics | Numbers of patients |
Oxysterol concentrationa | p-value | |
| negatived | positived | ||||
| 25-hydroxycholesterol | None | - | - | - | - |
| 27-hydroxycholesterol | History | 14/10 | 29.5 ± 5.6 | 34.7 ± 4.0 | 0.030b |
| 7α-hydroxycholesterol | Age | 23 | r2=0.488 | 0.018c | |
| Body mass index | 23 | r2=0.570 | 0.004c | ||
| 7-ketocholesterol | Menopause | 8/14 | 5.2 ± 1.0 | 7.5 ± 2.3 | 0.009b |
| Age | 22 | r2=0.497 | 0.019c | ||
| Second sampling | |||||
| 25-hydroxycholesterol | Smoking | 13/6 | 1.4 ± 0.4 | 0.9 ± 0.4 | 0.022b |
| 27-hydroxycholesterol | History | 14/9 | 30.4 ± 7.2 | 23.8 ± 4.5 | 0.020b |
| 7α-hydroxycholesterol | None | - | - | - | - |
| 7-ketocholesterol | None | - | - | - | - |
| Analyses stratified by clinical characteristics of patients (Table 2) | |||||
|---|---|---|---|---|---|
| First sampling | Characteristics | Numbers of patients |
Oxysterol concentrationa | p-value | |
| ER+/TAM+ | ER−/TAM− | ||||
| 25-hydroxycholesterol | None | - | - | - | - |
| 27-hydroxycholesterol | ER expression | 18/6 | 30.2 ± 4.1 | 36.2 ± 7.2 | 0.020b |
| 7α-hydroxycholesterol | TAM therapy | 16/7 | 4.5 ± 1.3 | 3.3 ± 0.5 | 0.035b |
| 7-ketocholesterol | ER expression | 16/6 | 7.3 ± 2.2 | 4.9 ± 1.1 | 0.006b |
| TAM therapy | 15/7 | 7.2 ± 2.2 | 5.4 ± 1.6 | 0.024b | |
| Second sampling | |||||
| All oxysterols | None | - | - | - | - |
Mean ± standard deviation in ng/ml of plasma
Significance by the Mann-Whitney test
Significance by the Spearman rho test
Negative versus positive means groups of patients coded as never smokers versus current or ex smokers (smoking), premenopausal versus post or perimenopausal (menopausal status) and patients with negative versus positive personal history related to cholesterol and hormones. Personal history of hypercholesterolemia, hypothyroidism, hysterectomy or hormonal replacement therapy was coded as positive. Significant results in bold. Associations observed in both samplings are in grey.
ER=expression of estrogen receptor. TAM= therapy by tamoxifen
Further analyses based on clinical data (Table 2) have been conducted. Patients treated with tamoxifen had significantly higher plasma levels of 7-ketocholesterol in the second sampling compared to the corresponding first sampling (n=15/16, first/second sampling without outliers, p=0.034 by the Mann-Whitney test). The rest of oxysterols did not differ between samplings of tamoxifen-treated patients. Analysis of a group of patients expressing the estrogen receptor also showed significantly higher 7-ketocholesterol levels in the second sampling compared to the corresponding first sampling (n=16/17, p=0.022 by the Mann-Whitney test).
We then performed analysis of differences in levels of oxysterols between groups of patients stratified by estrogen receptor expression and tamoxifen-based therapy. Patients with tumors expressing estrogen receptor had significantly higher levels of 7-ketocholesterol and lower levels of 27-hydroxycholesterol within the first sampling than those without expression (p=0.020 and 0.006, respectively, Table 5B). Patients treated with tamoxifen had higher levels of both 7-ketocholesterol and 7α-hydroxycholesterol in the first sampling than those treated by other regimens (p=0.035 and 0.024, respectively). These differences were non-significant in analyses of the second sampling (Table 5B).
Discussion
The lack of reliable predictive and prognostic biomarkers for prevention, diagnosis, and therapy of human cancer and the need for non-invasive diagnostics has emerged in a number of studies addressing this problem using circulating levels of various biomarkers, e.g., cell-free DNA, microRNA, exosomes, peptides, and metabolites. Except numerous studies using genomic and proteomic tools, lipidomics also represents a highly promising approach. The present exploratory study followed the question of feasibility of detection of selected oxysterols in plasma of breast cancer patients. We also asked whether tumor removal causes detectable perturbations in levels of oxysterols that could then be used, e.g., for estimation of the disease risk or monitoring of its recurrence.
Some oxysterols are considered to be tumor promoters (Nelson 2013, Silvente-Poirot 2014) and estrogen receptor agonists (see ref. Griffiths 2016 for review) and thus their role in breast cancer is currently attracting considerable interest. Although human plasma levels of oxysterols have already been assessed by various authors (reviewed, e.g., in Mutemberezi 2016), there is currently only one very recent study reporting differences in levels of various oxysterols in plasma of breast cancer patients (Dalenc 2016). Thus, our study adds novel information essential for this evolving area of research.
The above cited study (Dalenc 2016) has shown that levels of oxysterols—including 25-hydroxycholesterol, 27-hydroxycholesterol, 7α-hydroxycholesterol, and 7-ketocholesterol—can be reliably detected in patients plasma by high-performance gas chromatography coupled to mass spectrometry (GC–MS). The present study used a different technique for oxysterol level detection, liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS). The assay method may be the source of variations in oxysterols between the studies, e.g., the observed median of 27-hydroxycholesterol was between 55.6–58.7 ng/ml in the previous study and 27.9–31.7 in the present one. On the other hand, the mean levels of the rest of oxysterols we analyzed are comparable to other published data in humans (Mutemberezi 2016).
Our study was designed to compare levels of oxysterols before and after tumor removal. Hence, we provide the first evidence showing a significant rise of 7-ketocholesterol levels in the plasma of breast cancer patients after surgical removal of tumors. This change was found both in all patients and in a subgroup with estrogen receptor-expressing tumors treated with tamoxifen, suggesting the general nature of this phenomenon. Dalenc (2016) compared patients on day 0 and 28 (n=27) after the beginning of endocrine therapy with tamoxifen or aromatase inhibitors and observed a number of significant changes between both samplings, but none for 7-ketocholesterol and 27-hydroxycholesterol.
The present study differs from the previous one also in the fact that we followed breast cancer patients in the early stage I or II of the disease while Dalenc (2016) observed significantly higher levels of 25-hydroxycholesterol and 6-oxo-cholestan-3β,5α-diol in metastatic patients compared with adjuvant-treated (i.e., clinically considered as tumor-free) patients. Together with our data, these results indirectly support the view that levels of oxysterols may vary not just as a result of the treatment but also reflect the presence of tumor cells in the patients’ bodies. It remains to be determined whether such variations provide a driving force for the tumor growth and its metastatic spread or are a consequence of tumorigenesis.
Furthermore, we observed that 7-ketocholesterol levels were significantly higher in the second sampling compared to the first sampling of the subgroup of patients with tumors expressing estrogen receptor, as well as in patients treated with tamoxifen-based regimens. On the other hand, although levels of some oxysterols varied according to estrogen receptor expression or tamoxifen-based therapy in the first sampling, they did not differ in the second sampling again, suggesting that the tumor presence plays a key role.
Taken together, the rise of 7-ketocholesterol levels after tumor removal, which is not relevant to estrogen expression or tamoxifen therapy, suggests that it may be connected with tumor growth, independent of estrogenic activity. 7-Ketocholesterol was recently suggested as a potential diagnostic and predictive biomarker of Niemann-Pick disease (Boenzi 2016). It was also shown to be a cytotoxic and sensitizing agent in multidrug resistant chronic myeloid leukemia cell lines in vitro (Rosa Fernandes 2017), but there is no report about its role in human cancer onset or progression in vivo.
From a mechanistic point of view, 7-ketocholesterol (together with other 7-oxysterols) is a ligand of nuclear liver X receptors (LXRA and LXRB) (Janowski 1999, Aye 2011) and an agonist of RAR-related orphan receptors (RORA and RORC) (Wang 2010), potentially modulating the mammalian clock. LXRs are considered to exert an anti-proliferative role through reduction of cellular cholesterol levels needed for tumor cell growth, and some studies suggest their roles in both cancer development (reviewed in (Kuzu 2016)) and potential as therapeutic targets. Very little is known about the role of RORs in human cancer, except that high RORC expression correlated with longer progression-free survival of breast cancer patients (Cadenas 2014) and RORA was shown to enhance proliferation through up-regulation of the cytochrome P450 19A1 aromatase in an estrogen receptor-expressing model of human breast cancer, MCF7 cells (Odawara 2009). These contradictory roles of RORs, together with the pleiotropic effects of LXRs, need to be further studied and explained.
Recently 25- and 27-hydroxycholesterol have been reported to increase the expression of estrogen receptor-controlled trefoil factor 1 (TFF1) gene in a panel of breast cancer model cell lines in vitro, suggesting that these oxysterols may be involved in endocrine therapy resistance (Simigdala 2016). Although our study did not find significant variations in levels of these oxysterols in patients treated by endocrine therapy in their second sampling (in vivo), we admit that samplings over a much longer period and evaluation of survival of patients are necessary for rigorous assessment of the utility of these oxysterols for monitoring of resistance.
Bearing in mind the potential influence of personal characteristics as age, body mass index, smoking status, or history of steroid homeostasis-related conditions on circulating levels of oxysterols, we also addressed the role of these factors. We have not observed consistent trend towards modulation of 7-ketocholesterol levels by these confounders. Interestingly, the level of 27-hydroxycholesterol was modulated by body mass index of patients in both samplings and thus, it should be further considered as a potential confounder in future studies.
Taken together, the present study shows that the assessment of 7-ketocholesterol, 7α-hydroxycholesterol, 25-hydroxycholesterol, and 27-hydroxycholesterol in plasma samples of breast cancer patients is feasible using liquid-chromatograpraphy-mass spectrometry. Most importantly, the above data suggest that 7-ketocholesterol levels rise after tumor removal. Further studies are necessary to assess the reproducibility of this observation and justify a more detailed search for the mechanism behind this phenomenon before clinical utilization, e.g., estimation of disease risk, monitoring of disease recurrence, or employment of therapeutic interventions can be considered.
Acknowledgments
This work was supported by the Czech Science Foundation (grant no. 13-25222J), the Ministry of Health of the Czech Republic, (project no. 17-28470A), the National Sustainability Program I (NPU I) provided by the Ministry of Education Youth and Sports of the Czech Republic, (grant no. LO1503), and National Institutes of Health (grant no. R01 GM118122). The authors state that there are no interests to declare. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Requirements
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- Aye IL, Waddell BJ, Mark PJ, Keelan JA. Oxysterols inhibit differentiation and fusion of term primary trophoblasts by activating liver X receptors. Placenta. 2011;32:183–191. doi: 10.1016/j.placenta.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Boenzi S, Deodato F, Taurisano R, Goffredo BM, Rizzo C, Dionisi-Vici C. Evaluation of plasma cholestane-3β,5α,6β-triol and 7-ketocholesterol in inherited disorders related to cholesterol metabolism. J Lipid Res. 2016;57:361–367. doi: 10.1194/jlr.M061978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas C, van de Sandt L, Edlund K, Lohr M, Hellwig B, Marchan R, Schmidt M, Rahnenführer J, Oster H, Hengstler JG. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle. 2014;13:3282–3291. doi: 10.4161/15384101.2014.954454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalenc F, Iuliano L, Filleron T, Zerbinati C, Voisin M, Arellano C, Chatelut E, Marquet P, Samadi M, Roché H, Poirot M, Silvente-Poirot S. Circulating oxysterol metabolites as potential new surrogate markers in patients with hormone receptor-positive breast cancer: Results of the OXYTAM study. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.06.010. [DOI] [PubMed] [Google Scholar]
- De Medina P, Payré B, Boubekeur N, Bertrand-Michel J, Tercé F, Silvente-Poirot S, Poirot M. Ligands of the antiestrogen-binding site induce active cell death and autophagy in human breast cancer cells through the modulation of cholesterol metabolism. Cell Death Differ. 2009;16:1372–1384. doi: 10.1038/cdd.2009.62. [DOI] [PubMed] [Google Scholar]
- de Weille J, Fabre C, Bakalara N. Oxysterols in cancer cell proliferation and death. Biochem Pharmacol. 2013;86:154–160. doi: 10.1016/j.bcp.2013.02.029. [DOI] [PubMed] [Google Scholar]
- DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-Hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths WJ, Abdel-Khalik J, Hearn T, Yutuc E, Morgan AH, Wang Y. Current trends in oxysterol research. Biochem Soc Trans. 2016;44:652–658. doi: 10.1042/BST20150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmschrodt C, Becker S, Schröter J, Hecht M, Aust G, Thiery J, Ceglarek U. Fast LC-MS/MS analysis of free oxysterols derived from reactive oxygen species in human plasma and carotid plaque. Clin. Chim. Acta. 2013;425:3–8. doi: 10.1016/j.cca.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. U. S. A. 1999;96:266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloudova A, Guengerich FP, Soucek P. The role of oxysterols in human cancer. Trends Endocrinol. Metab. 2017 doi: 10.1016/j.tem.2017.03.002. pii: S1043-2760(17)30038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzu OF, Noory MA, Robertson GP. The role of cholesterol in cancer. Cancer Res. 2016;76:2063–2070. doi: 10.1158/0008-5472.CAN-15-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutemberezi V, Guillemot-Legris O, Muccioli GG. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016;64:152–169. doi: 10.1016/j.plipres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odawara H, Iwasaki T, Horiguchi J, Rokutanda N, Hirooka K, Miyazaki W, Koibuchi Y, Shimokawa N, Iino Y, Takeyoshi I, Koibuchi N. Activation of aromatase expression by retinoic acid receptor-related orphan receptor (ROR) α in breast cancer cells, identification of a novel ROR response element. J. Biol. Chem. 2009;284:17711–17719. doi: 10.1074/jbc.M109.009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot M, Silvente-Poirot S, Weichselbaum RR. Cholesterol metabolism and resistance to tamoxifen. Curr. Opin. Pharmacol. 2012;12:683–689. doi: 10.1016/j.coph.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Rosa Fernandes L, Stern AC, Cavaglieri RC, Nogueira FC, Domont G, Palmisano G, Bydlowski SP. 7-Ketocholesterol overcomes drug resistance in chronic myeloid leukemia cell lines beyond MDR1 mechanism. J. Proteomics. 2017;151:12–23. doi: 10.1016/j.jprot.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Segala G, de Medina P, Iuliano L, Zerbinati C, Paillasse MR, Noguer E, Dalenc F, Payré B, Jordan VC, Record M, Silvente-Poirot S, Poirot M. 5,6-Epoxycholesterols contribute to the anticancer pharmacology of tamoxifen in breast cancer cells. Biochem. Pharmacol. 2013;86:175–189. doi: 10.1016/j.bcp.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Shinkyo R, Xu L, Tallman KA, Cheng Q, Porter NA, Guengerich FP. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 2011;286:33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Silvente-Poirot S, Poirot M, Cancer Cholesterol and cancer: in the balance. Science. 2014;343:1445–1446. doi: 10.1126/science.1252787. [DOI] [PubMed] [Google Scholar]
- Simigdala N, Gao Q, Pancholi S, Roberg-Larsen H, Zvelebil M, Ribas R, Folkerd E, Thompson A, Bhamra A, Dowsett M, Martin LA. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016;18:58. doi: 10.1186/s13058-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli FA, Devilee P, editors. Pathology and Genetics: Tumours of the Breast and Female Genital Organs. Vol. 4. IARC; Lyon: 2003. [Google Scholar]
- Wang DY, Fulthorpe R, Liss SN, Edwards EA. Identification of estrogen-responsive genes by complementary deoxyribonucleic acid microarray and characterization of a novel early estrogen-induced gene, EEIG1. Mol. Endocrinol. 2004;18:402–411. doi: 10.1210/me.2003-0202. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RD, Stayrook KR, Zhang X, Novick S, Chalmers MJ, Griffin PR, Burris TP. Modulation of retinoic acid receptor-related orphan receptor alpha and gamma activity by 7-oxygenated sterol ligands. J. Biol. Chem. 2010;285:5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]