Abstract
Purpose
The purpose of this study was to evaluate image quality of a spectral photon-counting detector (PCD) CT system for evaluation of major arteries of the head and neck compared to conventional single-energy CT scans using energy-integrating detectors (EID).
Methods
In this IRB-approved study, sixteen asymptomatic subjects (7 men) provided informed consent and received both PCD and EID contrast-enhanced CT scans of the head and neck (mean age: 58 years, range, 46–75 years). Tube settings were (EID: 120 kVp/160 mAs vs PCD: 140 kVp/108 mAs) for all volunteers. Quantitative analysis included measurements of mean attenuation, image noise, and contrast-to-noise-ratio (CNR). Spectral PCD data was used to reconstruct virtual monoenergetic images (VMI) and iodine maps. A head phantom was used to validate iodine concentration measurements in PCD images only. Two radiologists blinded to detector type independently scored the image quality of different segments of the arteries, as well as diagnostic acceptability, image noise, and severity of artifacts of the PCD and EID images. Reproducibility was assessed with intra-class correlation coefficient. Linear mixed models that account for within-subject correlation of analyzed arterial segments were used. Linear regression and Bland-Altman analysis with 95% limits of agreement (LOAs) were used to calculate the accuracy of material decomposition.
Results
PCD image quality scores were significantly higher compared to EID image quality scores with lower image noise (P<0.01) and less image artifacts (P<0.001). PCD image noise was 9.1% lower than EID image noise (8.0±1.3 HU vs 8.8±1.5 HU, respectively, P<0.001). Arterial segments showed artifacts on EID images due to beam hardening that were not present on PCD images. On PCD images of the head phantom, there was excellent correlation (R2=0.998) between actual and calculated iodine concentrations without significant bias (bias: −0.4 mg/ml [95% LOAs: −1.1;0.4 mg/ml]). Iodine maps had 20.7% higher CNR compared to non-spectral PCD (65.2±9.0 vs 54.0±4.5, P=0.01) and VMI at 70keV showed similar CNR to non-spectral images (52.6±4.2 vs 54.0±4.5, P=0.39).
Conclusion
Photon-counting CT has the potential to improve the image quality of carotid and intracranial CT angiography compared to single-energy energy-integrating detector CT.
Keywords: Photon-counting CT, Spectral CT, CT angiography, image quality, carotid imaging, brain angiography, virtual monoenergetic images, iodine mapping, beam hardening
1. Introduction
Computed tomography angiography (CTA) of the carotid arteries is a well-established, noninvasive diagnostic tool. However, beam hardening artifacts near the base of the skull, cervical vertebrae, and dense calcified plaques can degrade CTA image quality and decrease the apparent arterial enhancement1–3. Beam hardening effect is caused by preferential absorption of lower-energy photons from the polychromatic x-ray beam passing through dense bone. This increases the mean energy of the x-ray beam4,5. Higher peak kilovoltage (kVp) settings (and therefore a smaller fraction of low-energy photons) result in reduced beam hardening artifacts. However, the contrast between soft tissue and iodine decreases with higher x-ray energy. Hence, there is a tradeoff between beam hardening and iodine-soft tissue contrast.
Conventional energy-integrating detectors (EIDs) for computed tomography use scintillating (light producing) crystals to convert incident x-ray photons into light photons. The light is detected by photodetectors and converted into an electrical current that is subsequently digitized. Energy-sensitive photon-counting detectors (PCDs) have recently been implemented for experimental computed tomography (CT) units. PCDs directly convert incident x-ray photons to electronic pulses, producing separate measurements of the number of incident photons, which equals the number of pulses and their energies6–9. Amongst other advantages, the PCDs may improve soft tissue–iodine contrast for CT imaging because high- and low-energy photons are equally weighted, whereas in EIDs the electrical signal is weighted by the energy of the incident photon. Gutjar et al. reported that the Hounsfield unit (HU) of iodine a in 140 kVp PCD scan image is similar to that of a 120 kVp EID scan10. Therefore, by using a higher x-ray tube voltage, PCD CT should effectively reduce beam hardening artifacts without compromising the soft tissue-iodine contrast. Another limitation of conventional EID carotid CTA is the overlap in HU values between iodinated contrast and dense structures such as bone and calcified plaques11. The spectral information of the PCDs can be used to calculate concentration maps of one or multiple intrinsic or injected contrast-agents in cardiac and abdomen studies12–14.
Recently a prototype whole-body PCD CT scanner has been developed based on a dual-source CT scanner with two independent EID and PCD subsystems with identical x-ray sources8,12,15. The aim of this study was to evaluate the image quality of a spectral PCD CT system compared to conventional EID CT for contrast-enhanced arterial imaging of the head and neck in human volunteers. We hypothesize that PCD CT at 140 kVp could improve the image quality by reducing beam hardening artifacts while maintaining the iodine-soft tissue contrast.
2. Materials and Methods
2.1. Ex vivo human head phantom studies
To test the accuracy of material decomposition based on PCD CT images, we inserted calibrated test tubes filled with fat, water, hydroxyapatite, different aqueous solutions of iodine (50, 10, 5, 1, 0.5, and 0.1 mg/ml) and ferrous sulfate (15, 10, and 5 mg/ml) inside an anthropomorphic head phantom made of a human skull embedded in plastic (Phantom Laboratory, Salem, NY, USA). The phantom was then filled with a gel made from a mixture of agar and sucrose to achieve HU values similar to normal brain tissue. Phantom acquisition parameters were identical to those used for the human subjects. Phantom images were reconstructed with a quantitative soft tissue kernel (Q34f) for iodine quantification.
2.2. In vivo human studies
Asymptomatic volunteers older than 45 years were prospectively enrolled at the National Institutes of Health (NIH) Clinical Center in a Health Insurance Portability and Accountability Act (HIPAA)-compliant, institutional review board-approved study. All study volunteers provided informed consent. Exclusion criteria included: pregnancy, known or possible genetic disposition to radiation induced cancer, renal insufficiency, allergy to iodinated contrast material, and CT scan within the last 12 months.
2.3. Description of the PCD CT imaging prototype
Details of this prototype scanner have been previously described8,15–17. In brief, the PCD CT subsystem is based on a second generation dual-source CT scanner (SOMATOM Definition Flash, Siemens Healthcare GmbH, Forchheim, Germany) with two independent x-ray sources at an offset of 95° in which one of the conventional EID subsystems is replaced by a cadmium telluride-based PCD. Up to 4 energy thresholds can be defined for the PCD at 1 keV increments: two “low” thresholds between 20–50 keV and two “high” thresholds between 50–90 keV. We set the energy thresholds at 25 keV and 75 keV, yielding two energy bins (low: 25–75 keV; high: 75–140 keV). The threshold setting was based on manufacturer recommendations based on expected performance of the photon counting detectors. For comparison to the EID subsystem, the 2 PCD energy bins were combined to reconstruct images containing the information of all detected photons (25–140 keV), we refer to this image as grayscale PCD image.
2.4. CT scanning protocol and image reconstruction
Both EID and PCD CT images were acquired in all subjects. Since two scans were performed after a single iodine contrast injection, the arterial iodine attenuation was likely to decrease from the first to the second CT scan. We attempted to mitigate the differences in attenuation between scans using several steps. In the first 8 subjects, PCD CT was performed prior to EID CT. In the second 8 subjects, the scan order was reversed (EID CT then PCD CT). Also, the scan delay between the two scans was set to the minimum available (6 seconds). The first scan was started 28 seconds after the injection start, to avoid the rapidly changing peak arterial enhancement in the neck that may occur prior to this. Finally, the iodine bolus (3.0 ml/sec) was slower than our normal clinical rate (5.0 ml/sec) to prolong the bolus of peak vascular enhancement. Iopamidol 370 mg/ml (Isovue-370, Bracco Diagnostics, Melville, NY), was administered intravenously followed by a 20-ml saline bolus. The iodine contrast dose was 1 ml/kg up to a maximum of 105 ml. The EID tube settings (120 kVp/160 mAs; CTDIvol = 24.4 mGy) were based on routine clinical protocols at our center. For imaging at 140 kVp, we calculated the tube current-time product that would result in the same CTDIvol as the 120 kVp acquisition. This resulted in tube settings of 140kVp/108 mAs for PCD. Estimates of CTDIvol for the PCD were approximately 10% higher than those for the EID subsystem (27.4 mGy vs 24.4 mGy)18,19. This is due to the smaller z-axis collimation of the PCD subsystem in the prototype, which increases the off-collimation radiation to the object; such off-collimation radiation does not affect the image quality but increments the total radiation dose20. Identical collimations would result in similar CTDIvol values for both systems. However, reducing the EID collimation to match the PCD would have slowed down the image acquisition and adversely affected uniform arterial contrast enhancement. Both subsystems had matching spiral pitch, which ranged between 0.5–1.5 and was individually adapted based on infusion parameters and scan range. A 0.5-second rotation time was set for both subsystems. Table 1 summarizes the scanner geometry, acquisition and reconstruction parameters.
Table 1.
Scanner and acquisition parameters of the prototype EID-PCD CT machine.
| Energy-integrating detector | Photon-counting detector | |
|---|---|---|
| Pixel size (at isocenter) | 0.5×0.6 mm2 | 0.5×0.5 mm2 |
| Collimation | 128×0.6 mm | 64×0.5 mm |
| Field of view diameter | 500 mm | 275 mm |
| Tube voltage | 120 kVp | 140 kVp |
| Tube current-time product | 160 mAs | 108 mAs |
| CTDIvol | 24.4 mGy | 27.4 mGy |
| Source to detector distance | 1085mm | |
| Source to isocenter distance | 595 mm | |
| Gantry rotation speed | 0.5 sec/rot | |
| Pitch factor | 0.5–1.5 | |
| Reconstruction diameter | 250 mm | |
| Image matrix size | 512×512 | |
| Slice thickness/increment | 1mm/1mm | |
Sinogram-affirmed iterative reconstruction (SAFIRE) strength 2 was performed with ReconCT (v13.8.5.0, Siemens Healthcare, Erlangen, Germany). Images were reconstructed with a soft tissue kernel with no iodine or bone beam hardening correction (I31f). Reconstruction FOV was fixed at 250 mm with a matrix size of 512×512 pixels and slice thickness/increment of 1 mm/1 mm for both detectors.
2.5. Image analysis
For qualitative analysis, two radiologists blinded to all parameters including the detector subsystem independently performed qualitative image analysis (author#3, with >20 years of experience, and author#2, with >10 years of experience in neuroradiology). Image quality scores were based on the European Guidelines on Quality Criteria for CT used in similar studies21. Diagnostic acceptability was scored with yes-or-no question. Image noise and artifacts were scored on a 5-point scale (minimal, mild: not interfering with diagnosis, moderate: slightly interfering with diagnosis, pronounced: interfering with diagnosis, severe: unacceptable). A 4-point image quality score (fully acceptable, probably acceptable, usable under limited conditions, unacceptable) was used for the internal carotid artery (ICA) cervical segment (C1), ICA petrous segment (C2), ICA cavernous segment (C3), ICA supraclinoid segment (C4), vertebral artery (VA) foraminal segment (V2), VA atlantic segment (V3), VA intradural segment (V4), basilar artery (BA), and the anterior, middle and posterior cerebral arteries (ACA, MCA, and PCA) (Figure 1)22. Readers used commercially available PACS software (VuePACS v.12.0.0; Carestream Health, Rochester, NY).
Figure 1.
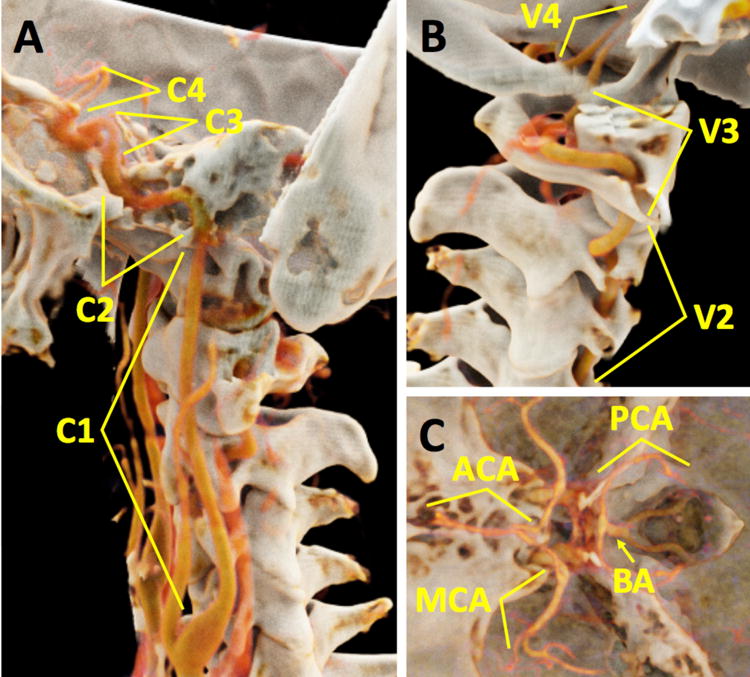
3D reconstructions in a 47-year-old male subject from a photon counting CT scan demonstrate the vessel segments that were analyzed in this study: (A) internal carotid artery (ICA) cervical segment (C1), ICA petrous segment (C2), ICA cavernous segment (C3), and ICA supraclinoid segment (C4); (B) vertebral artery (VA) foraminal segment (V2), VA atlantic segment (V3), and VA intradural segment (V4); (C) basilar artery (BA), anterior, middle and posterior cerebral arteries (ACA, MCA, and PCA). There is an intimate relationship between multiple vessel segments and surrounding bone (e.g., ICA C2, ICA C3, and VA V2). 3D volume rendering technique was performed on Cinematic Rendering v1.0 (Siemens Healthcare GmbH, Forchheim, Germany).
For quantitative analysis, regions-of-interest (ROIs) were placed bilaterally in the ICA C1, ICA C2, ICA C3, ICA C4, and MCA. The purpose of this analysis was to assess the effects of beam hardening in this challenging area of dense bone between ICA C2 and C4 (see also Figure 1). Over the relatively short distance from the ICA C1 to MCA, we expected arterial attenuation to be relatively constant. Segments ICA C1 and MCA are away from the dense bone of the skull base; hence any difference in attenuation of the most proximal and most distal segment can be attributed to timing of the arrival and departure of contrast bolus and not to beam hardening. Therefore, for the quantitative analysis, we only included subject data in which the most distal segment attenuation (i.e. MCA) was within 20 HU of the most proximal segment (i.e. ICA C1) for both detectors. We used this threshold to avoid potential bias of one detector subsystem over the other due to differences in contrast injection timing and scan duration length. Due to the difference in collimation, PCD scan duration was approximately twice as long as the EID scan. Beam hardening artifact was then quantified as the departure of HU values of the ICA C2–4 from that of ICA C1. To compare different subjects, HU values of the vessel segments of each scan were normalized to the attenuation of the proximal segment by subtracting the ICA C1 HU value from all measurements. The standard deviation (SD) of the HU values of a ROI of approximately 1 cm2 in the sternocleidomastoid muscle was used as a measure of image noise. The contrast-to-noise ratio (CNR) of the different arterial segments was calculated as the absolute difference between the mean HU values of the arterial segments and the surrounding fat divided by image noise.
2.6. Spectral data analysis
Quantitative iodine analysis of the PCD data was performed by transferring the low- and high-energy bin images to a commercially available workstation with dedicated dual-energy image processing software (Syngo, dual-energy MMWP VE61A, Siemens) using an iodine-subtraction algorithm (liver VNC, Siemens). ROIs of approximately 20 mm2 were carefully placed inside the iodine calibration vials and the iodine concentration (mg/ml) was recorded23. The software generated virtual monoenergetic images (VMI) with photon energies of 40–140 keV at 10-keV intervals for the human subjects. Based on the unique energy-dependent behavior of materials, different materials can be highlighted (e.g., iodine versus calcium)24,25.
2.7. Statistical analysis
R Statistical Software (Foundation for Statistical Computing, v.3.3.2, Vienna, Austria) was used. Continuous variables were expressed as mean±SD. The Shapiro-Wilk test was used to test normality of the data distributions. Repeated measurements analysis of variance, paired t-test and Wilcoxon-signed rank (paired) with continuity correction was used to compare continuous variables and reader quality scores. Linear mixed models that accounted for within-subject correlations were used to estimate the effects of detector subsystem, laterality, and acquisition order on HU values in the analyzed arterial segments26. The fixed-effect predictors included indicator variables for detector subsystem (EID vs PCD CT), left vs right carotid artery and scan acquisition order (first vs second). To account for the similarity of measurements within a single subject, we included a random effect for subject. Tukey test for multiple comparisons of means was used to compare the EID and PCD attenuation values for the different arterial segments using the “lme4” and “multcomp” functions in R Statistical Software27,28. The McNemar test was used to compare diagnostic acceptability of the two CT detector systems. Intra-class correlation coefficient (ICC) for inter-reader and intra-reader reproducibility was interpreted as good (ICC>0.750), fair (ICC: 0.400–0.750), or poor (ICC<0.400). A two-way model with measures of consistency was used to calculate ICC values29. Linear regression and Bland-Altman analysis with 95% limits of agreement (LOAs) was used to calculate the agreement between the true and estimated iodine concentrations of the iodine test vials30. We plotted differences against the calibrated true iodine concentrations as a gold standard method as proposed by Krouwer31, and deemed a two-tailed P<0.05 to be statistically significant.
3. Results
3.1. Head phantom, quantitative image analysis
Linear regression analysis showed an excellent correlation between true and estimated iodine concentrations in the iodine test vials inside the head phantom (R2=0.998) without significant bias in Bland-Altman analysis (bias: −0.4 mg/ml [95% LOAs: −1.1;0.4 mg/ml]) (Figure 4). In the human studies (below), vascular attenuation averaged about 240 HU. This attenuation was approximately achieved for the 10 mg/ml iodine vial. Attenuation values for the 10 mg/ml iodine calibration vial were similar for the 120 kVp EID CT images versus the 140 kVp PCD CT images (242.8±0.8 HU vs 243.6±1.7 HU, respectively, P=0.18)
Figure 4.
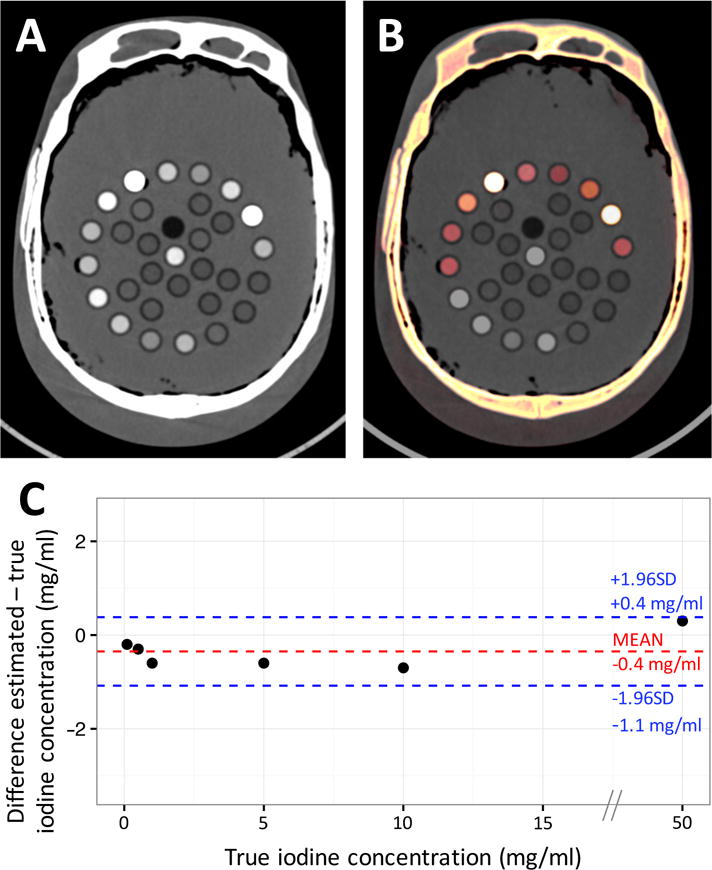
Example photon-counting detector (PCD) grayscale image (A) and iodine map overlay image (B) of the head phantom with multiple calibrated test tubes filled with fat (vegetable oil), water, hydroxyapatite, and different aqueous solutions of iodine (50, 10, 5, 1, 0.5, and 0.1 mg/ml) and ferrous sulfate (15, 10, and 5 mg/ml). (C) Bland-Altman plot shows agreement between the true iodine concentration and those estimated through PCD material decomposition. No significant bias was found with narrow 95% limits of agreement (LOAs) (bias: −0.4 mg/ml [95% LOAs: −1.1;0.4 mg/ml]).
3.2. Human studies
Sixteen study subjects were enrolled. There were 7 men and 9 women. Mean age for men, women, and all patients was 56 years (age range, 46–72 years), 60 years (age range, 48–75 years), and 58 years (age range, 46–75 years), respectively. Images of all subjects were used for qualitative analysis. Ten of 16 subjects met inclusion criteria for quantitative analysis (as specified in section 2.5: image analysis).
3.2.1 Human studies, qualitative image analysis
Image quality scores for the EID and PCD images are summarized in Figure 2. All EID and PCD images were diagnostically acceptable (P>0.99). PCD quality scores were significantly higher for the ICA C2, ICA C3, V2, V3, ACA, MCA, and PCA segments (all P<0.05) with significantly lower subjective image noise (P<0.01) and fewer image artifacts (P<0.001). Intra-reader agreement was good for all measurements (all ICC≥0.80). Inter-reader agreement was equally strong for image quality of ICA C1 (ICC:0.92), ICA C2 (ICC:0.79), ICA C3 (ICC:0.93), ICA C4 (ICC:0.78), VA V2 (ICC:0.81), VA V3 (ICC:0.91), VA V4 (ICC:0.89), BA (ICC:0.91), ACA (ICC:0.82), MCA (ICC:0.77), and PCA (ICC:0.86) vessel segments, image noise (ICC:0.91), and image artifacts (ICC:0.86) (Table 2).
Figure 2.
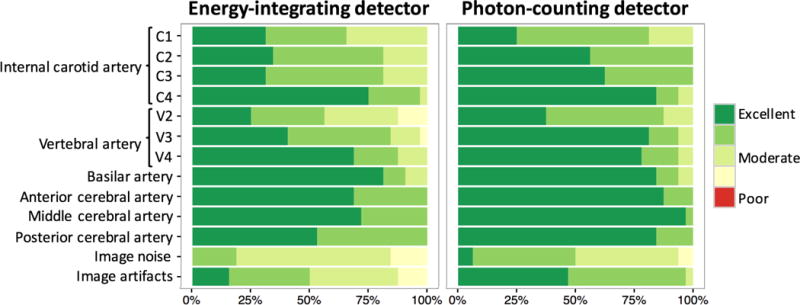
Qualitative image scores for the energy-integrating detector (EID) and photon-counting detector (PCD) images of the internal carotid artery (ICA) cervical segment (C1), ICA petrous segment (C2), ICA cavernous segment (C3), ICA supraclinoid segment (C4), vertebral artery (VA) foraminal segment (V2), VA atlantic segment (V3), VA intradural segment (V4), basilar artery (BA), anterior cerebral artery (ACA), middle cerebral artery (MCA), and posterior cerebral artery (PCA). PCD scores were significantly higher for the ICA C2, ICA C3, V2, V3, ACA, MCA, and PCA segments (all P<0.05) with significantly lower subjective image noise (P<0.01) and less image artifacts (P<0.001).
Table 2.
Inter-reader and intra-reader intra-class correlation coefficients (ICC) with 95% confidence interval (CI).
| Inter-reader | Intra-reader | |||
|---|---|---|---|---|
|
| ||||
| Parameter | ICC | 95% CI | ICC | 95% CI |
| Internal carotid artery | ||||
| cervical segment (C1) | 0.92 | 0.84–0.96 | 0.94 | 0.89–0.97 |
| petrous segment (C2) | 0.79 | 0.62–0.89 | 0.89 | 0.79–0.95 |
| cavernous segment (C3) | 0.93 | 0.86–0.96 | 0.96 | 0.92–0.98 |
| supraclinoid segment (C4) | 0.78 | 0.61–0.89 | 0.84 | 0.70–0.92 |
| Vertebral artery | ||||
| foraminal segment (V2) | 0.81 | 0.64–0.90 | 0.86 | 0.74–0.93 |
| atlantic segment (V3) | 0.91 | 0.83–0.96 | 0.91 | 0.83–0.96 |
| intradural segment (V4) | 0.89 | 0.79–0.95 | 0.88 | 0.77–0.94 |
| Basilar artery | 0.91 | 0.83–0.96 | 0.96 | 0.92–0.98 |
| Anterior cerebral artery | 0.82 | 0.67–0.91 | 0.92 | 0.84–0.96 |
| Middle cerebral artery | 0.77 | 0.58–0.88 | 0.80 | 0.63–0.90 |
| Posterior cerebral artery | 0.86 | 0.73–0.93 | 0.86 | 0.73–0.93 |
| Image noise | 0.91 | 0.82–0.95 | 0.93 | 0.87–0.97 |
| Image artifacts | 0.86 | 0.74–0.93 | 0.96 | 0.92–0.98 |
3.2.2. Human studies, quantitative image analysis
The carotid arteries were assessed in 10 subjects × 10 vessel segments = 100 paired ROIs). Mean attenuation values in the ICA C1 were similar for both detectors (234.5±78.6 HU vs 271.9±77.4 HU for the EID and PCD subsystems, respectively, P=0.06). PCD CT image noise was 9.1% lower than EID CT image noise (8.0±1.3 HU vs 8.8±1.5 HU, respectively, P<0.001). After adjusting for within-subject correlation of arterial segments, there was a significant effect of detector subsystem on arterial enhancement (P<0.001), whereas there was no significant effect of laterality (P=0.99) or scan acquisition order (P=0.15). Mean EID attenuation values in the ICA C2, ICA C3, and ICA C4 segments were 26.3 HU, 30.4 HU, and 14.6 HU lower than attenuation values in the ICA C1 segment (all P<0.001). Mean EID values in the MCA segment were not-significantly different from values in the ICA C1, indicating that the lower EID HU values observed in the ICA C2, ICA C3, and ICA C4 were not due to changes in contrast enhancement. In comparison, mean PCD attenuation values did not change significantly between the different arterial segments (all P>0.05) (Table 3 and Figure 3).
Table 3.
Effects of detector subsystem, laterality, and scan acquisition order on mean Hounsfield Unit (HU) values within the internal carotid artery (ICA) cervical segment (C1), ICA petrous segment (C2), ICA cavernous segment (C3), ICA supraclinoid segment (C4), and middle cerebral artery (MCA). Fixed-effects parameter estimates, standard errors (SEs) of the mean and corresponding P-values were computed by using the R “lme4” package by treating the subjects as clusters with repeated measurements obtained within the arterial segments within each patient. The P-values are for testing the null hypothesis that the true value of the parameter is zero versus the nonzero alternative. P-values are adjusted for multiple comparisons using Tukey contrasts. The outcomes are the differences in mean HU values of the arterial segments (see Methods).
| Fixed-effects parameter | Estimate | SE | P-value |
|---|---|---|---|
| Intercept | 0.58 | 1.82 | 0.75 |
| Detector subsystem | |||
| EID (ICA C1 vs ICA C2) | −26.26 | 2.13 | <0.001 |
| EID (ICA C1 vs ICA C3) | −30.43 | 2.13 | <0.001 |
| EID (ICA C1 vs ICA C4) | −14.62 | 2.13 | <0.001 |
| EID (ICA C1 vs MCA) | −2.44 | 2.13 | 0.98 |
| PCD (ICA C1 vs ICA C2) | −0.14 | 2.14 | 1.00 |
| PCD (ICA C1 vs ICA C3) | −2.05 | 2.14 | 0.99 |
| PCD (ICA C1 vs ICA C4) | −1.34 | 2.14 | 1.00 |
| PCD (ICA C1 vs MCA) | 1.55 | 2.14 | 1.00 |
| EID vs PCD ICA C1 | 0.28 | 2.14 | 1.00 |
| EID vs PCD ICA C2 | 26.54 | 2.14 | <0.001 |
| EID vs PCD ICA C3 | 28.38 | 2.14 | <0.001 |
| EID vs PCD ICA C4 | 13.27 | 2.14 | <0.001 |
| EID vs PCD MCA | 3.99 | 2.14 | 0.69 |
| Laterality (left vs right) | −0.02 | 0.95 | 0.99 |
| Scan acquisition order (first vs second) | −1.42 | 0.97 | 0.15 |
Figure 3.

Example energy-integrating detector (EID) (A) and photon-counting detector (PCD) (B) curved multiplanar reconstructions (MPR) of the internal carotid artery (ICA) in a 55-year-old female. Artifactual areas of low density within the ICA petrous segment (C2) which may be mistaken for pathology are seen on the EID images but no on the PCD images (arrows). (C) Graph demonstrates changes in Hounsfield Unit (HU) values (means ± standard errors) in the ICA and MCA for all subjects. HU values are normalized to the cervical ICA (C1) segment. Mean EID HU values in the ICA C2, ICA C3, and ICA C4 segments were 26.3 HU, 30.4 HU, and 14.6 HU lower than HU values in the ICA C1 segment (all P<0.001), whereas mean PCD HU values did not change significantly (all P>0.05).
3.2.3. Human studies, spectral image analysis
We performed spectral analysis of the human PCD images to calculate virtual monoenergetic images and material decomposition maps. These maps resulted in tissue highlighting of arteries versus calcified plaques or adjacent bone (Figures 5, 6). The iodine map yielded 20.7% higher CNR between the ICA C1 and the surrounding fat compared to grayscale images (mean ± standard error: 65.2±9.0 vs 54.0±4.5, respectively, P=0.01) (Figure 7). Grayscale (non-spectral) PCD CT images had arterial CNR similar to 70 keV virtual monoenergetic images (54.0±4.5 vs 52.6±4.2, respectively, P=0.39). Thus, the calculated low and high keV virtual monoenergetic images for PCD CT showed similar clinical advantages previously shown for dual-energy EID CT e.g., low keV virtual monoenergetic images showing greater iodine signal and high keV images showing less iodine signal and less calcium blooming than polyenergetic images32.
Figure 5.
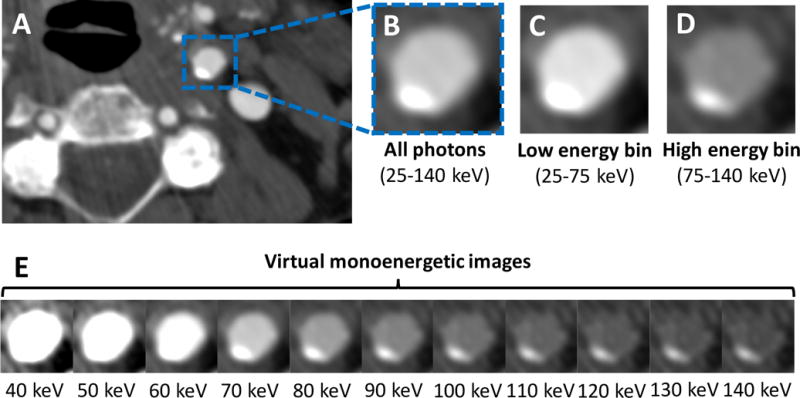
(A) Example grayscale photon-counting detector (PCD) image reconstructed from all detected photons (25–140 keV) at the level of the proximal cervical internal carotid artery (ICA C1) in a 73-year-old female demonstrates mild eccentric calcified plaque. (B) Zoomed-in image of the ICA C1 with corresponding low (C) and high (D) energy bin images. Based on the specific behavior of materials at different photon energy levels, images can be decomposed into their constituent materials (e.g., iodine versus calcium) and virtual monoenergetic images (E) can be reconstructed to enhance facilitate plaque detection [window center: 145; window width: 800].
Figure 6.
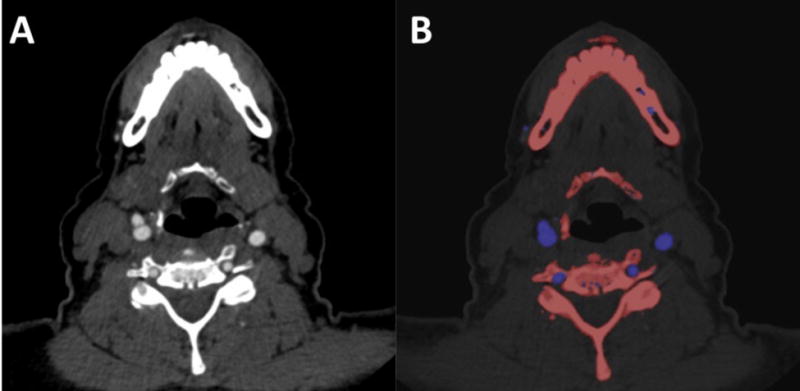
(A) Example photon-counting detector (PCD) grayscale images at the level of the cervical internal carotid artery (ICA C1) in a 75-year-old female. (B) Material classification overlay image enhances the differentiation between the arteries (iodine, blue) and the adjacent bone (calcium, red).
Figure 7.
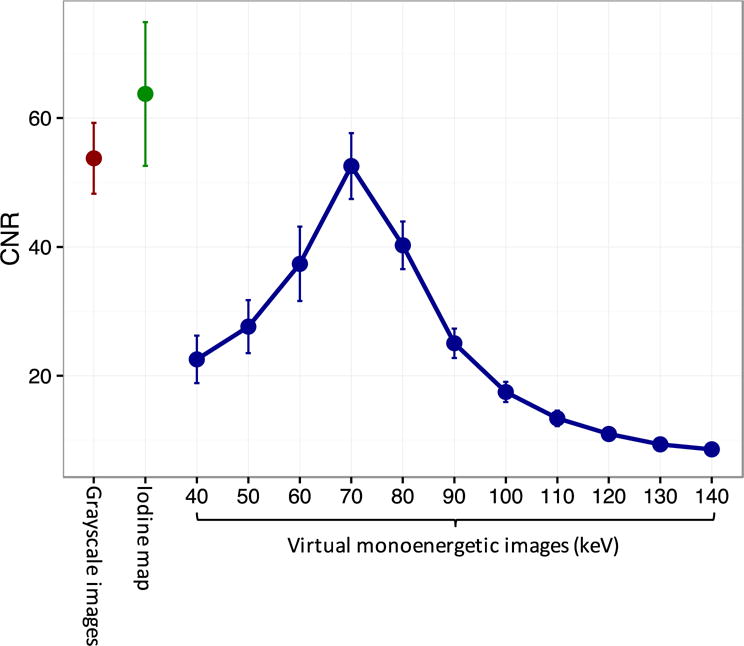
Contrast-to-noise-ratio (CNR) for differentiation between the cervical segment of the internal carotid artery (ICA C1) and the surrounding fat. Iodine map CNR was 20.7% higher than grayscale image CNR and could be used to enhance vessel differentiation and to quantify arterial enhancement (mean ± standard error: 65.2±9.0 vs 54.0±4.5, respectively, P=0.01).
4. Discussion
This study reports the first in-vivo human contrast-enhanced carotid and intracranial vessel CT scans obtained with a PCD CT scanner. Experienced readers blinded to the CT detector system identified better image quality for all vascular segments.
Quantitative measurements showed that PCD CT images had less image noise (by 9%) and had less severe beam hardening artifacts in ICA segments close to surrounding bone. Spectral imaging is also an intrinsic capability of photon counting CT33; we showed that spectral material decomposition of PCD CT images is feasible in the neck and brain for vascular imaging.
Advanced techniques for material decomposition, noise reduction, and artifact correction post-processing algorithms have been developed for EID CT systems. These post processing methods aim to overcome inherent limitations of EID technology34–36. Even state-of-the-art CT systems show artifacts from beam hardening in areas of the body with extensive bone. The C2 segment of the carotid artery is one such example (Figure 3). Incorrect attenuation values with EID CT could potentially be misinterpreted as clot or plaque in some clinical settings. For PCD CT, we used a higher x-ray tube potential than for EID CT (140 kVp vs. 120 kVp). This helped to minimize artifacts in the C2 arterial segment for PCD CT. With EID CT however, higher x-ray tube energies would be detrimental for detection of iodine contrast. Because of equal weighting of low and high energy incident photons, PCD CT images with higher tube potential showed approximately similar arterial enhancement as the low tube potential EID CT images. Further, overall image quality and CNR was better for PCD CT vascular images compared to EID CT images. Recent enhancements in noise-optimized virtual monoenergetic reconstructions may further improve CNR between iodine and surrounding tissue32,37.
In this study, we did not directly compare the spectral performance of the PCD CT scanner to a comparable dual energy EID CT. However, Leng et al. previously compared spectral performance of a similar PCD CT prototype to second- and third-generation dual-source dual-energy EID CT38. In that study, PCD CT in dual-energy mode was comparable to dual-energy EID CT. However, there are several additional points to consider in the comparison of EID CT vs PCD CT for spectral imaging: a) Lack of electronic noise in photon count signal makes PCDs more dose-efficient than EIDs. This is especially prominent in low radiation dose acquisitions8,17,19,18, 12; b) spectral information for PCDs is always available whereas dual source or fast KV switching implementation of spectral imaging require dedicated spectral mode imaging40; c) spectral imaging for PCDs can be optimized for the application, whereas dual layer EIDs have fixed spectral separation40; d) inherent registration of spectral information, which is not available for dual source EID CT; e) the potential for much more sophisticated spectral imaging, such as quad-energy acquisition. Up to four energy thresholds can be set for the prototype CT scanner in this study. Additional thresholds would allow e.g., water, gadolinium, iodine and calcium to be separated using k-edge imaging and material decomposition techniques13,14,45. f) ultra high resolution imaging (e.g., 0.25 mm or higher) is possible in PCD CTs46 due to the inherently small size of the detector elements. For EID CT, geometric constraints of the detector result in substantial radiation dose penalties as pixel size is reduced.
There are several limitations of this study. The photon-counting detector CT prototype scanner used here is an investigational device and is not yet approved by FDA. The delay time between the sequentially acquired CT scans (minimum, 6 seconds) results in lower arterial enhancement for the second CT scan compared to the first scan. Therefore, we alternated which detector subsystem was used first and used a slower injection rate (3.0 ml/sec) than our normal clinical protocols (5 ml/sec) to prolong the injection bolus. We also normalized the attenuation values to the ICA C1 segment. Since severe beam hardening is not expected in either the MCA or ICA C1 segments, we only included subjects who had similar arterial attenuation values in those segments. Also, the average arterial enhancement was about 240 HU, which is somewhat lower than what is used in clinical studies. As explained in Methods (section 2.5), this compromise was made in order to obtain comparable PCD and EID scans following a single iodine bolus injection. Nevertheless, this degree of arterial enhancement still provided high CNR (>40, Figures 3 and 7) allowing for good quality volume rendering (Figure 1) and spectral imaging (Figure 5).
In conclusion, this feasibility study demonstrates the potential of photon-counting technology to improve carotid and intracranial vessel image quality in close proximity to the human skull base and cervical vertebrae. Additionally, photon-counting CT acquires spectral information useful for virtual monoenergetic reconstructions and material decomposition.
Acknowledgments
Conflicts of Interest and Source of Funding:
This study was supported by the NIH intramural research program (ZIACL090019; ZIAEB000072; ZIANS003119), and a collaborative research and development agreement with Siemens Healthcare GmbH (Forchheim, Germany). Data inclusion and analysis was performed by authors who are not employees of or consultants for Siemens.
References
- 1.Borisch I, Boehme T, Butz B, et al. Screening for carotid injury in trauma patients: image quality of 16-detector-row computed tomography angiography. Acta radiol. 2007;48(7):798–805. doi: 10.1080/02841850701422104. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra AK, Camacho M, Ivatury RR, et al. Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: a note of caution. Ann Surg. 2007;246(4):632–643. doi: 10.1097/SLA.0b013e3181568cab. [DOI] [PubMed] [Google Scholar]
- 3.Saba L, Sanfilippo R, Pirisi R, et al. Multidetector-row CT angiography in the study of atherosclerotic carotid arteries. Neuroradiology. 2007;49(8):623–637. doi: 10.1007/s00234-007-0244-y. [DOI] [PubMed] [Google Scholar]
- 4.Brooks RA, Di Chiro G. Beam hardening in x-ray reconstructive tomography. Phys Med Biol. 1976;21(3):390. doi: 10.1088/0031-9155/21/3/004. [DOI] [PubMed] [Google Scholar]
- 5.Zatz LM, Alvarez RE. An Inaccuracy in Computed Tomography: The Energy Dependence of CT Values. Radiology. 1977;124(1):91–97. doi: 10.1148/124.1.91. [DOI] [PubMed] [Google Scholar]
- 6.Schlomka JP, Roessl E, Dorscheid R, et al. Experimental feasibility of multi-energy photon-counting K-edge imaging in pre-clinical computed tomography. Phys Med Biol. 2008;53(15):4031. doi: 10.1088/0031-9155/53/15/002. [DOI] [PubMed] [Google Scholar]
- 7.Iwanczyk JS, Nygård E, Meirav O, et al. Photon counting energy dispersive detector arrays for x-ray imaging. Nucl Sci IEEE Trans. 2009;56(3):535–542. doi: 10.1109/TNS.2009.2013709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symons R, Cork TE, Sahbaee P, et al. Low-dose lung cancer screening with photon-counting CT: a feasibility study. Phys Med Biol. 2016;62(1):202. doi: 10.1088/1361-6560/62/1/202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi K. Energy-sensitive photon counting detector-based X-ray computed tomography. Radiol Phys Technol. 2017:1–15. doi: 10.1007/s12194-017-0390-9. [DOI] [PubMed] [Google Scholar]
- 10.Gutjahr R, Halaweish AF, Yu Z, et al. Human Imaging With Photon Counting–Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Invest Radiol. 2016;51(7):421–429. doi: 10.1097/RLI.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morhard D, Fink C, Graser A, et al. Cervical and cranial computed tomographic angiography with automated bone removal: dual energy computed tomography versus standard computed tomography. Invest Radiol. 2009;44(5):293–297. doi: 10.1097/RLI.0b013e31819b6fba. [DOI] [PubMed] [Google Scholar]
- 12.Pourmorteza A, Symons R, Sandfort V, et al. Abdominal Imaging with Contrast-enhanced Photon-counting CT: First Human Experience. Radiology. 2016;279(1):239–245. doi: 10.1148/radiol.2016152601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Symons R, Cork TE, Lakshmanan MN, et al. Dual-Contrast Agent Photon-Counting Computed Tomography of the Heart: Initial Experience. Int J Cardiovasc Imaging. 2017 doi: 10.1007/s10554-017-1104-4. [DOI] [PubMed] [Google Scholar]
- 14.Symons R, Krauss B, Sahbaee P, et al. Photon‐counting CT for simultaneous imaging of multiple contrast agents in the abdomen: an in vivo study. Med Phys. 2017 doi: 10.1002/mp.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappler S, Henning A, Kreisler B, et al. Photon counting CT at elevated X-ray tube currents: contrast stability, image noise and multi-energy performance. Proc SPIE Med Imaging. 2014;9033:90331C. [Google Scholar]
- 16.Yu Z, Leng S, Jorgensen SM, et al. Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array. Phys Med Biol. 2016;61(4):1572. doi: 10.1088/0031-9155/61/4/1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Leng S, Yu Z, et al. Estimation of signal and noise for a whole-body research photon-counting CT system. J Med Imaging. 2017;4(2):23505. doi: 10.1117/1.JMI.4.2.023505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Symons R, Pourmorteza A, Sandfort V, et al. Feasibility of Dose-reduced Chest CT with Photon-counting Detectors: Initial Results in Humans. Radiology. 2017:162587. doi: 10.1148/radiol.2017162587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourmorteza A, Symons R, Reich DS, et al. Photon-counting CT of the brain: in vivo human results and image quality assessment. Am J Neuroradiol. 2017 doi: 10.3174/ajnr.A5402. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon RL. A new look at CT dose measurement: beyond CTDI. Med Phys. 2003;30(6):1272–1280. doi: 10.1118/1.1576952. [DOI] [PubMed] [Google Scholar]
- 21.Bongartz G, Golding SJ, Jurik AG, et al. European guidelines on quality criteria for computed tomography. EUR(Luxembourg) 2000 [Google Scholar]
- 22.Gibo H, Lenkey C, Rhoton AL., Jr Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55(4):560–574. doi: 10.3171/jns.1981.55.4.0560. [DOI] [PubMed] [Google Scholar]
- 23.Johnson TRC, Krauss B, Sedlmair M, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007;17(6):1510–1517. doi: 10.1007/s00330-006-0517-6. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez RE, Macovski A. Energy-selective reconstructions in x-ray computerised tomography. Phys Med Biol. 1976;21(5):733. doi: 10.1088/0031-9155/21/5/002. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi K, Iwanczyk JS. Vision 20/20: Single photon counting x-ray detectors in medical imaging. Med Phys. 2013;40(10):100901. doi: 10.1118/1.4820371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59(4):390–412. [Google Scholar]
- 27.Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. arXiv Prepr. arXiv1406.5823. 2014 [Google Scholar]
- 28.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 29.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1(1):30. [Google Scholar]
- 30.Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. [PubMed] [Google Scholar]
- 31.Krouwer JS. Why Bland–Altman plots should use X, not (Y+ X)/2 when X is a reference method. Stat Med. 2008;27(5):778–780. doi: 10.1002/sim.3086. [DOI] [PubMed] [Google Scholar]
- 32.Sandfort V, Palanisamy S, Symons R, et al. Optimized energy of spectral CT for infarct imaging: Experimental validation with human validation. J Cardiovasc Comput Tomogr. 2017 doi: 10.1016/j.jcct.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCollough CH, Leng S, Yu L, et al. Dual-and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 2015;276(3):637–653. doi: 10.1148/radiol.2015142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagawa K, George RT, Arbab-Zadeh A, et al. Characterization and correction of beam-hardening artifacts during dynamic volume CT assessment of myocardial perfusion 1. Radiology. 2010;256(1):111–118. doi: 10.1148/radiol.10091399. [DOI] [PubMed] [Google Scholar]
- 35.Lin XZ, Miao F, Li JY, et al. High-definition CT Gemstone spectral imaging of the brain: initial results of selecting optimal monochromatic image for beam-hardening artifacts and image noise reduction. J Comput Assist Tomogr. 2011;35(2):294–297. doi: 10.1097/RCT.0b013e3182058d5c. [DOI] [PubMed] [Google Scholar]
- 36.Machida H, Takeuchi H, Tanaka I, et al. Improved delineation of arteries in the posterior fossa of the brain by model-based iterative reconstruction in volume-rendered 3D CT angiography. Am J Neuroradiol. 2013;34(5):971–975. doi: 10.3174/ajnr.A3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant KL, Flohr TG, Krauss B, et al. Assessment of an advanced image-based technique to calculate virtual monoenergetic computed tomographic images from a dual-energy examination to improve contrast-to-noise ratio in examinations using iodinated contrast media. Invest Radiol. 2014;49(9):586–592. doi: 10.1097/RLI.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 38.Leng S, Zhou W, Yu Z, et al. Spectral performance of a whole-body research photon counting detector CT: quantitative accuracy in derived image sets. Phys Med Biol. 2017 doi: 10.1088/1361-6560/aa8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuhaus V, Abdullayev N, Hokamp NG, et al. Improvement of Image Quality in Unenhanced Dual-Layer CT of the Head Using Virtual Monoenergetic Images Compared With Polyenergetic Single-Energy CT. Invest Radiol. 2017;52(8):470–476. doi: 10.1097/RLI.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 40.Krauss B, Grant KL, Schmidt BT, et al. The importance of spectral separation: an assessment of dual-energy spectral separation for quantitative ability and dose efficiency. Invest Radiol. 2015;50(2):114–118. doi: 10.1097/RLI.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 41.O’Donnell T, Schoeck F, Cheheltani R, et al. SPIE Medical Imaging. International Society for Optics and Photonics; 2016. Optimal selection of thresholds for photon counting CT; pp. 97831P–97831P. [Google Scholar]
- 42.Yu Z, Leng S, Li Z, et al. Spectral prior image constrained compressed sensing (spectral PICCS) for photon-counting computed tomography. Phys Med Biol. 2016;61(18):6707. doi: 10.1088/0031-9155/61/18/6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mechlem K, Ehn S, Sellerer T, et al. Joint statistical iterative material image reconstruction for spectral computed tomography using a semi-empirical forward model. IEEE Trans Med Imaging. 2017 doi: 10.1109/TMI.2017.2726687. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt T, Barber R, Sidky EA. Spectral CT method to directly estimate basis material maps from experimental photon-counting data. IEEE Trans Med Imaging. 2017 doi: 10.1109/TMI.2017.2696338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries A, Roessl E, Kneepkens E, et al. Quantitative spectral K-edge imaging in preclinical photon-counting X-ray computed tomography. Invest Radiol. 2015;50(4):297–304. doi: 10.1097/RLI.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 46.Leng S, Gutjahr R, Ferrero A, et al. Ultra-High Spatial Resolution, Multi-Energy CT using Photon Counting Detector Technology. Proc SPIE–the Int Soc Opt Eng. 2017;10132:101320Y. doi: 10.1117/12.2255589. [DOI] [PMC free article] [PubMed] [Google Scholar]


