Abstract
Purpose
There are no published data from specific regions of sub-Saharan Africa describing the clinical and pathological characteristics and molecular subtypes of invasive breast cancer by ethnic group. The purpose of this study was to investigate these characteristics among the three major ethno-cultural groupings in Kenya.
Methods
The study included women with pathologically-confirmed breast cancer seen between March 2012 and May 2015 at 11 hospitals throughout Kenya. Socio-demographic, clinical, and reproductive data were collected by questionnaire, and pathology review and immunohistochemistry were performed centrally.
Results
The 846 cases included 661 Bantus (78.1%), 143 Nilotes (16.9%), 19 Cushites (2.3%), and 23 patients of mixed ethnicity (2.7%). In analyses comparing the two major ethnic groups, Bantus were more educated, more overweight, had an older age at first birth and had a younger age at menopause than Nilotes (p<0.05 for all comparisons). In analyses restricted to definitive surgery specimens, there were no statistically significant differences in tumour characteristics or molecular subtypes, although the Nilote tumours tended to be larger (OR for ≥ 5 cm vs. < 2 cm: 3.86, 95%CI: 0.77, 19.30) and were somewhat more likely to be HER2-enriched (OR for HER2-enriched vs. Luminal A/B: 1.41, 95%CI: 0.79, 2.49).
Conclusion
This case series showed no significant differences in breast cancer tumour characteristics or molecular subtypes, but significant differences in socio-demographic characteristics and reproductive factors, among the three major ethnic groups in Kenya. We suggest further evaluation of ethnic differences in breast cancer throughout the genetically and culturally diverse populations of sub-Saharan Africa.
Keywords: Breast cancer, Kenya, Ethnic differences, Ethnicity, sub Saharan Africa
BACKGROUND
Breast cancer (BC) is the most common female malignancy worldwide, accounting for 1.7 million new cases and 521,900 deaths in 2012.[1] According to the Nairobi Population-based Cancer Registry, breast cancer is the most common malignancy in Kenyan women, accounting for 23% of all incident cases.[2]
The effect of race and ethnicity on breast cancer (BC) stage of presentation, tumor biology and treatment response has been the subject of much investigation and controversy, with numerous studies reporting that the distribution of BC molecular subtypes differs among various races and ethnic groups.
A qualitative systematic review of breast cancer demographics, size, stage, grade, histological type, extra-mammary involvement, and hormone receptor status in patients from Africa, the Middle East, Eastern Europe, Mexico, the Caribbean and South America reported that women with BC in these regions present with large aggressive tumors, and that distant metastases are frequently present at the time of diagnosis.[3] Compared with White American women, Black African women have also been reported to be younger at diagnosis,[4] to have a higher frequency of triple negative tumors,[5] and to be more likely to present with Stage III/IV disease.[6] A review of the histopathological differences between BC in African American (AA) and White Americans also reports that the former tend to have a higher nuclear grade, a higher proportion of lymph node positive tumors, and a younger age-specific incidence of triple negative tumors as compared to White Americans.[7]
Furthermore, a recent registry data review from South Africa reported that similar to the Western populations, the age distribution for breast cancer is bimodal in both Black and White South African women, however Black South African women had a younger age distribution of early onset breast cancer than all other groups studied.[8]
In Kenya, there are approximately 40–50 tribes which can be divided into 3 main ethno-cultural groupings: the Bantus who originated from West Africa after 400 AD, the Nilotic tribes who migrated from regions of the present day Sudan and Egypt around 400 AD, and the Cushitic tribes who came from northern Africa around 2000 BC (Fig 1a).
Fig 1a.
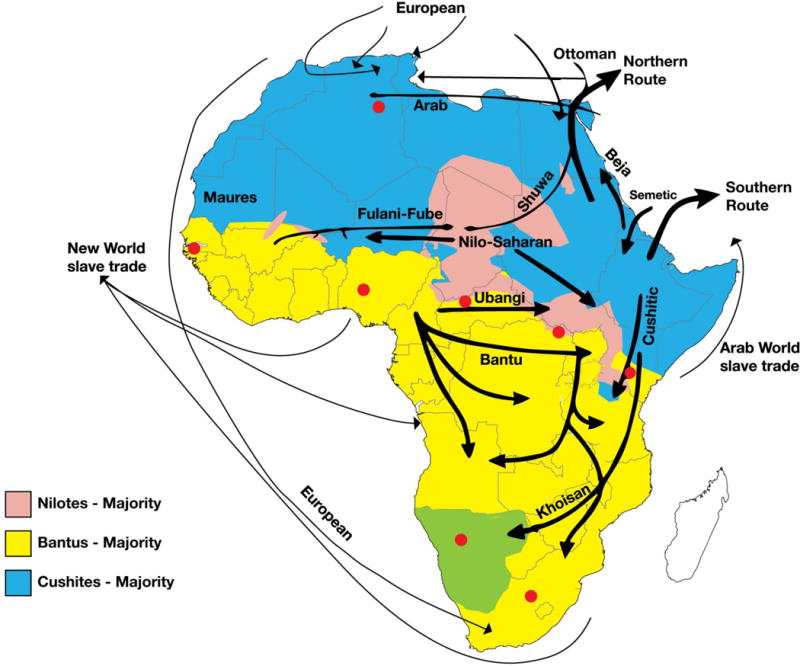
Ethinic migration patterns in Africa
The largest ethnic groups in Kenya, the Kikuyus, are of Bantu ancestry, while the Luos residing in the western part of Kenya are of Nilotic descent. The populations that inhabit the northern parts of Kenya belong to the Cushitic lineage.
The objective of this study was to investigate the differences in-patient, clinical and pathological characteristics of breast cancer in the three major ethno-cultural groupings in Kenya, given their distinct ancestral origin.
METHODS
Study design and setting
This was a Kenya-wide study involving a total of 11 health institutions (Supplementary Table 1) that recruited patients with pathologically confirmed breast cancer between March 2012 and May 2015. The study was coordinated by Aga Khan University Hospital, Nairobi, which is a 300-bed private not-for-profit hospital that provides tertiary level health care services in Kenya. The collaborating hospitals in this study are located in various parts of Kenya and their patient catchment area reflects the distribution of the total population and the three major ethnic groups (Fig. 1b).
Fig 1b.
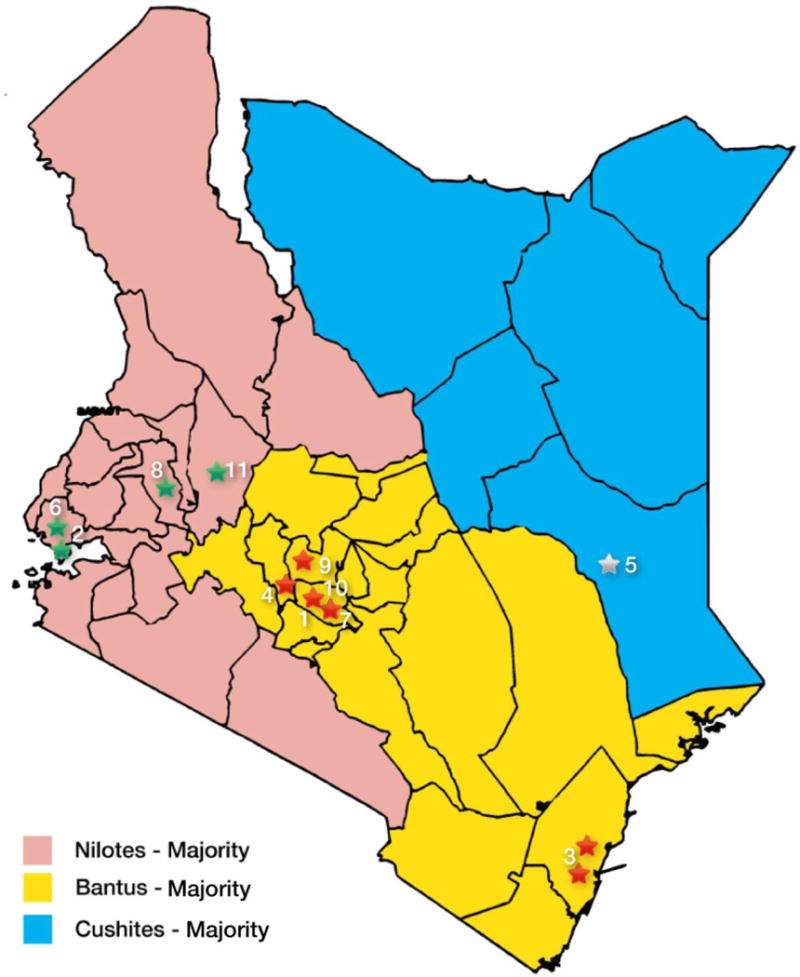
Distribution of participating study sites
Data collection
Relevant socio-demographic, reproductive, and clinical data were collected from all consenting patients using structured questionnaires and clinical data abstraction case report forms.
The ethnicity of patients was determined through self-reporting by respondents of their parents’ and maternal and paternal grandparents’ tribal affiliations. The tribes were assigned to the corresponding three major ethnic groups; Bantu, Nilotes and Cushites[9] If all maternal and paternal grandparents and parents did not belong to the same tribe, that patient’s ethnicity was categorized as mixed. Due to sparse numbers, patients reporting “mixed” ethnicity (N=23, 2.7%) were excluded from the analytic population.
All breast cancer tissues blocks were submitted to Aga Khan University Hospital, Nairobi to undergo central pathology review and immunohistochemistry. Tumor size, tumor grade, presence of lympho-vascular invasion, lymph node metastases and extra nodal extension were documented. ER/PR/HER2 status was analyzed on the Dako Automated platform as previously reported,[10] and tumors were assigned into 3 major breast cancer molecular subtypes based on immunohistochemistry: ER and/or PR positive and HER2 positive or negative (Luminal A/B), ER/PR negative and HER2 positive (HER2 enriched), and ER/PR and HER2 negative (Triple Negative).
Data entry and verification
All data were double entered. If data were missing, or needed clarification, an additional secondary review of the patient files was carried out by the data collection team. Finally, SQL scripts were prepared to facilitate data extraction.
Data Analysis
Distributions of socio-demographic and reproductive risk factors, tumor pathology characteristics, and tumor hormone receptor status were examined across the three ethnic groups (Bantu, Nilotes, and Cushite) and evaluated by Chi-square and Fisher exact tests as appropriate. Because of the small numbers of Cushite patients, these statistical tests were also rerun after restricting the analysis to the two largest ethnic groups, Bantus and Nilotes. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) for associations between Bantu or Nilote ethnicity and the tumor characteristics (tumor pathology characteristics and hormone receptor status). Risk factors associated with both ethnicity and the tumor characteristics of interest were included in multivariate logistic regression models, and the final models were determined using backwards elimination. In exploratory analyses attempting to better understand observed risk factor associations with ethnic group, we also evaluated interrelationships between select socio-demographic characteristics with tumor characteristics.
Analyses of socio-demographic, reproductive factors and hormone receptor status included all participants with relevant data. In contrast, the tumor pathology characteristics (tumor size, tumor grade, lympho-vascular invasion, lymph node metastasis, and extranodal extension) were only analyzed in the 396 (48.1%) cases that underwent definitive surgery. All analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC) and statistical significance was defined as p<0.05.
RESULTS
A total of 823 female study patients with invasive breast tumors were included in the analysis, 661 (80.3%) were Bantus, 143 (17.4%) were Nilotes, and 19 (2.3%) were Cushites. Of these 823 study participants, 427 (51.9%) were diagnosed by core biopsy only, and 396 (48.1%) had both a core biopsy and definitive surgery (lumpectomy or mastectomy). 351 (53.1%) of the Bantus, 37 (25.9%) of the Nilotes, and 8 (42.1%) of the Cushites had definitive surgery.
Sociodemographic and Behavioral Characteristics
Overall, the majority (53.9%) of women were younger than age 50 years at breast cancer diagnosis, with a median (interquartile range) age of 48 (40-57) years (Table 1). Whereas median age at diagnosis was similar for Bantus (48 years) and Nilotes (52 years), the median age at diagnosis for Cushites was much younger (36 years) (p=0.03). About half (50.2%) of the women received only primary school or no education. The most common occupations were farmer (30.8%) and employed worker (23.3%), and only 18.5% said they worked as housewives. By body mass index (BMI, kg/m2), nearly 70% of the women were overweight (39.2%) or obese (29.5%). Nearly half (42.7%) of the women were exposed to smoking, but very few of them (3.3%) reported ever having smoked or used smokeless tobacco themselves, and only 8.5% used alcohol.
Table 1.
Proportions of sociodemographic characteristics with ethnicity: All ethnicities (N=823)
| Bantu (N=661) |
Nilote (N=143) |
Cushite (N=19) |
Total (N=823) |
P values-All* | P values - Bantus and Nilotes only* | ||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||||
| Mean age at diagnosis | 49.2 | 50.8 | 42.7 | 49.3 | |||
| Median age at diagnosis (IQR) | 48 (40–57) | 52 (39–59) | 36 (32–56) | 48 (40–57) | 0.03** | 0.24** | |
| Age at diagnosis | 20–29 | 23 (3.5) | 5 (3.5) | 2 (10.5) | 30 (3.7) | 0.005 | 0.02 |
| 30–39 | 129 (19.6) | 34 (23.8) | 8 (42.1) | 171 (20.9) | |||
| 40–49 | 209 (31.8) | 27 (18.9) | 3 (15.8) | 239 (29.2) | |||
| 50+ | 296 (45.1) | 77 (53.9) | 6 (31.6) | 379 (46.3) | |||
| Missing | 4 | 0 | 0 | 4 | |||
| Highest level of education | None | 188 (28.4) | 53 (37.1) | 14 (73.7) | 255 (31.0) | 0.0002 | 0.03 |
| Primary | 132 (20) | 24 (16.8) | 2 (10.5) | 158 (19.2) | |||
| Secondary | 181 (27.4) | 25 (17.5) | 0 (0) | 206 (25.0) | |||
| Tertiary | 160 (24.2) | 41 (28.7) | 3 (15.8) | 204 (24.8) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| Place of residence | Rural | 414 (62.6) | 92 (64.3) | 1 (5.3) | 507 (61.6) | <0.0001 | 0.70 |
| Urban | 247 (37.4) | 51 (35.7) | 18 (94.7) | 316 (38.4) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| Occupation | Farmer | 224 (34) | 29 (20.3) | 0 (0) | 253 (30.8) | <0.0001 | 0.001 |
| Employed worker | 144 (21.9) | 45 (31.5) | 2 (10.5) | 191 (23.3) | |||
| Trader | 126 (19.1) | 20 (14) | 5 (26.3) | 151 (18.4) | |||
| Housewife | 103 (15.6) | 38 (26.6) | 11 (57.9) | 152 (18.5) | |||
| Casual worker | 25 (3.8) | 5 (3.5) | 0 (0) | 30 (3.7) | |||
| Other (specify) | 37 (5.6) | 6 (4.2) | 1 (5.3) | 44 (5.4) | |||
| Missing | 2 | 0 | 0 | 2 | |||
| BMI classification | Normal (<25) | 152 (29.9) | 49 (36.8) | 5 (29.4) | 206 (31.3) | 0.11 | 0.04 |
| Overweight (25.00 - 29.99) | 213 (41.9) | 40 (30.1) | 5 (29.4) | 258 (39.2) | |||
| Obese (>=30) | 143 (28.2) | 44 (33.1) | 7 (41.2) | 194 (29.5) | |||
| Missing | 153 | 10 | 2 | 165 | |||
| Alcohol Use | No | 605 (91.5) | 131 (91.6) | 17 (89.5) | 753 (91.5) | 0.95 | 0.98 |
| Yes | 56 (8.5) | 12 (8.4) | 2 (10.5) | 70 (8.5) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| Exposure to smokinga | Never exposed | 371 (56.1) | 88 (61.5) | 13 (68.4) | 472 (57.4) | 0.30 | 0.24 |
| Exposed | 290 (43.9) | 55 (38.5) | 6 (31.6) | 351 (42.7) | |||
| Missing | 0 | 0 | 0 | 0 |
p-values from chi-square test except where noted missing data were excluded from percentage calculations and statistical comparisons.
p-values from Kruskal-Wallis test.
Only 3.28% (n=27) of study participants reported ever having smoked or used smokeless tobacco. Exposure to smoking is summarized here as exposed/never exposed, where exposed is defined as personal use of tobacco as well as exposure to smoke at the workplace or home during child or adulthood.
When the analytic population was limited to Bantus and Nilotes, Bantu patients were slightly more educated (51.6% vs. 46.2% with secondary education or above) and slightly more overweight or obese (70.1% vs. 63.2%), and they were more likely to work as farmers (34.0% vs. 20.3%). Nilote patients, on the other hand, were more likely to work as employed workers (31.5% vs. 21.9%) or housewives (26.6% vs. 15.6%).
Reproductive Health Factors
In terms of reproductive health factors in the overall study population, nearly all of the participants reported having normal (age 12-14 years) or late (age ≥15 years) menarche (49.9% and 49.0%, respectively) (Table 2). Most women (67.9%) had at least three children, for most (62.2%) their age at first pregnancy was between the ages of 20 and 29 years, and the vast majority (90.1%) reported at least 15 cumulative months of breastfeeding. A little less than half (48.1%) of participants were postmenopausal at diagnosis, among whom most (57.9%) reported an age at menopause <50 years. The majority (92.2%) of patients reported no family history of breast cancer.
Table 2.
Reproductive health factors by ethnicity: All ethnicities (N=823)
| Bantu (N=661) |
Nilote (N=143) |
Cushite (N=19) |
Total (N=823) |
P values-All* | P values - Bantus and Nilotes only* | ||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||||
| Early menarche (<12) | 9 (1.4) | 0 (0) | 0 (0) | 9 (1.1) | |||
| Age at menarche | Normal menarche (12 - 14) | 305 (48.5) | 79 (56.0) | 9 (50.0) | 393 (49.9) | 0.40** | 0.12 |
| Late menarche (≥15) | 315 (50.1) | 62 (44.0) | 9 (50.0) | 386 (49.0) | |||
| Missing | 32 | 2 | 1 | 35 | |||
| Parity | Nulliparous | 28 (4.2) | 11 (7.7) | 3 (15.8) | 42 (5.1) | 0.03 | 0.10 |
| 1–2 children | 188 (28.4) | 32 (22.4) | 2 (10.5) | 222 (27.0) | |||
| >= 3 children | 445 (67.3) | 100 (69.9) | 14 (73.7) | 559 (67.9) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| Age at first pregnancy | Nulligravid | 24 (3.7) | 9 (6.3) | 2 (10.5) | 35 (4.4) | 0.0008 | 0.0037 |
| <20 years | 154 (24.0) | 50 (35.0) | 10 (52.6) | 214 (26.6) | |||
| 20 - 29 years | 424 (65.9) | 71 (49.7) | 6 (31.6) | 501 (62.2) | |||
| >= 30 years | 41 (6.4) | 13 (9.1) | 1 (5.3) | 55 (6.8) | |||
| Missing | 18 | 0 | 0 | 18 | |||
| Cumulative breastfeeding | never breastfed | 28 (4.4) | 11 (8.0) | 3 (15.8) | 42 (5.3) | 0.08** | 0.15 |
| < 15 months | 29 (4.5) | 8 (5.8) | 0 (0) | 37 (4.6) | |||
| >= 15 months | 585 (91.1) | 118 (86.1) | 16 (84.2) | 719 (90.1) | |||
| Missing | 19 | 6 | 0 | 25 | |||
| Cumulative hormonal contraception exposure | < 48 months | 175 (44.0) | 32 (52.5) | 1 (33.3) | 208 (45.0) | 0.44** | 0.21 |
| = 48 months | 223 (56.0) | 29 (47.5) | 2 (66.7) | 254 (55.0) | |||
| Missing | 263 | 82 | 16 | 361 | |||
| Menopausal status | Premenopausal | 348 (52.7) | 64 (45.1) | 14 (73.7) | 426 (51.9) | 0.04 | 0.10 |
| Postmenopausal | 312 (47.3) | 78 (54.9) | 5 (26.3) | 395 (48.1) | |||
| Missing | 1 | 1 | 0 | 2 | |||
| Age at menopause† | < 50 years | 161 (62.2) | 29 (43.9) | 1 (20.0) | 191 (57.9) | 0.004** | 0.007 |
| ≥50 years | 98 (37.8) | 37 (56.1) | 4 (80.0) | 139 (42.1) | |||
| Missing | 53 | 12 | 0 | 65 | |||
| Family history of breast cancer in first degree female relative | No | 609 (92.1) | 133 (93.0) | 17 (89.5) | 759 (92.2) | 0.85 | 0.72 |
| Yes | 52 (7.9) | 10 (7.0) | 2 (10.5) | 64 (7.8) | |||
| Missing | 0 | 0 | 0 | 0 |
p-values from chi-square test except where noted; missing data were excluded from percentage calculations and statistical comparisons.
p-values from Fisher exact test.
Numbers are calculated for post-menopausal women only.
Though based on small numbers, the Cushites seemed to present with a different reproductive profile as compared with the Bantus and Nilotes: a greater proportion of Cushites were <20 years at first pregnancy (52.6% vs. 24% (Bantus) and 35% (Nilotes); p=0.0008) and were premenopausal at diagnosis (73.7% vs. 52.7% (Bantus) and 45.1% (Nilotes); p=0.04). When reproductive health factors in Bantus and Nilotes were compared, Bantus were more likely to report older age at first pregnancy (72.3% vs. 58.8% ≥20 years) and younger age at menopause (62.2 vs. 43.9% <50 years).
Tumor pathology characteristics (among those with definitive surgery)
Overall, 31.4% of the 396 tumors with definitive surgery were ≥5cm, 59.4% were 2-<5cm, and only 9.2% were <2 cm in size at presentation. Lymphovascular invasion (LVI) was identified in 75.3% of cases, lymph node metastases in 58.8%, and extranodal extension of tumor in 39.1% (Table 3a).
Table 3a.
Tumor pathology characteristics by ethnicity (limited to patients with definitive surgery, N=396)
| Bantu (N=351) |
Nilote (N=37) |
Cushite (N=8) |
Total (N=396) |
P values-All* | P values - Bantus and Nilotes only* | ||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||||
|
|
|||||||
| Tumor size | <2 cm | 34 (9.8) | 2 (5.4) | 0 (0) | 36 (9.2) | 0.69** | 0.37 |
| 2-<5 cm | 208 (59.9) | 20 (54.1) | 5 (62.5) | 233 (59.4) | |||
| >5cm | 105 (30.3) | 15 (40.5) | 3 (37.5) | 123 (31.4) | |||
| Missing | 4 | 0 | 0 | 4 | |||
| Tumor overall grade | Grade 1 (low) | 19 (5.6) | 2 (5.6) | 1 (12.5) | 22 (5.8) | 0.34** | 0.82 |
| Grade 2 (intermediate) | 132 (39.1) | 16 (44.4) | 5 (62.5) | 153 (40.1) | |||
| Grade 3 (high) | 187 (55.3) | 18 (50.0) | 2 (25.0) | 207 (54.2) | |||
| Missing | 13 | 1 | 0 | 14 | |||
| Lymphovascular invasion | No | 90 (25.6) | 6 (16.2) | 2 (25.0) | 98 (24.8) | 0.45 | 0.21 |
| Yes | 261 (74.4) | 31 (83.8) | 6 (75.0) | 298 (75.3) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| Lymph nodes with metastasis | No | 146 (41.6) | 15 (40.5) | 2 (25.0) | 163 (41.2) | 0.73** | 0.90 |
| Yes | 205 (58.4) | 22 (59.5) | 6 (75.0) | 233 (58.8) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| Extranodal extension | No | 215 (61.3) | 23 (62.2) | 3 (37.5) | 241 (60.9) | 0.44** | 0.91 |
| Yes | 136 (38.8) | 14 (37.8) | 5 (62.5) | 155 (39.1) | |||
| Missing | 0 | 0 | 0 | 0 | |||
p-values from chi-square test except where noted; missing data were excluded from percentage calculations and statistical comparisons.
p-values from Fisher exact test.
In post hoc analyses, we found that the most highly educated patients had the smallest tumors (data not shown). Thus, in multivariate models evaluating the relation of ethnicity with tumor size, we adjusted for education level as well as age and ER status; we found that Nilotes tended to be more likely than Bantus to present with larger tumors (≥5cm vs. <2 cm), (OR: 3.86, 95% CI: 0.77, 19.30; p-trend=0.10). Continuing to compare patients from the two major ethnic groups, we additionally found that Nilotes were more likely to present with LVI (OR: 1.70, 95% CI: 0.68, 4.24), and were less likely to show extra nodal extension (OR: 0.92, 95% CI: 0.45, 1.85), although none of these associations was statistically significant (Table 3b).
Table 3b.
Association between Nilote vs. Bantu ethnicity (OR [95% CI]) and tumor pathology characteristics (limited to patients with definitive surgery, N=388)
| Tumor Size (categories) (N=379)a | Tumor grade (N=373)b | Lympho-vascular Invasion (N=385)c |
Lymph nodes w/metastasis (N=385)c |
Extranodal Extension (N=385)c | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| <2 cm (N=36) |
2-<5 cm (N=224) |
≥5cm (N=119) |
Grades 1 - 2 (low to intermediate) (N=168) |
Grade 3 (high) (N=205) |
No (N=96) |
Yes (N=289) |
No (N=160) |
Yes (N=225) |
No (N=236) |
Yes (N=149) |
||||||||
|
|
||||||||||||||||||
| N | N | OR [95% CI] | N | OR [95% CI] | p-trend | N | N | OR [95% CI] | N | N | OR [95% CI] | N | N | OR [95% CI] | N | N | OR [95% CI] | |
|
|
||||||||||||||||||
| Bantus (N=351) | 34 | 204 | ref | 104 | ref | 0.10 | 150 | 187 | ref | 90 | 258 | ref | 145 | 203 | ref | 213 | 135 | ref |
| Nilotes (N=37) | 2 | 20 | 1.92 (0.41, 8.99) | 15 | 3.86 (0.77, 19.30) | 18 | 18 | 0.76 (0.38, 1.52) | 6 | 31 | 1.7 (0.68, 4.24) | 15 | 22 | 1.01 (0.50, 2.02) | 23 | 14 | 0.92 (0.45, 1.85) | |
Adjusted for age, education level, and estrogen receptor status; 9 patients were deleted from the analysis due to missing values for tumor size, age, or estrogen receptor status.
Adjusted for age; 15 patients were deleted from the analysis due to missing values for tumor grade or age.
Adjusted for age; 3 patients were deleted from the analysis due to missing values for age.
Hormone receptor status
Overall, 68.8% of the patients were ER positive, 59.4% were PR positive, and 25.6% were HER2 positive (Table 4a). Categorized by molecular subtypes, 70.2% of patients were Luminal A or B (ER and/or PR positive and any HER2 status), 10.6% were HER2 enriched (ER and PR negative and HER2 positive), and 19.2% were triple negative (ER, PR and HER2 negative).
Table 4a.
Tumor hormone receptor status by ethnicitya
| Bantu (N=622) |
Nilote (N=136) |
Cushite (N=18) |
Total (N=776) |
P values- All* | P values - Bantus and Nilotes only* | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N (%) | N (%) | N (%) | N (%) | ||||
|
|
|||||||
| ER Status | Negative | 185 (29.7) | 50 (36.8) | 7 (38.9) | 242 (31.2) | 0.22 | 0.11 |
| Positive | 437 (70.3) | 86 (64.2) | 11 (61.1) | 534 (68.8) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| PR Status | Negative | 246 (39.6) | 61 (44.9) | 8 (44.4) | 315 (40.6) | 0.49 | 0.25 |
| Positive | 376 (60.5) | 75 (55.2) | 10 (55.6) | 461 (59.4) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| HER2 Status | Negative | 466 (74.9) | 99 (72.8) | 12 (66.7) | 577 (74.4) | 0.66 | 0.61 |
| Positive | 156 (25.1) | 37 (27.2) | 6 (33.3) | 199 (25.6) | |||
| Missing | 0 | 0 | 0 | 0 | |||
| Receptor combinations | |||||||
| ER+ and/or PR+ (Luminal type A/B) | 443 (71.2) | 90 (66.2) | 12 (66.7) | 545 (70.2) | 0.60** | 0.32 | |
| ER- and PR- and HER2 positive (HER2+) | 61 (9.8) | 19 (14.0) | 2 (11.1) | 82 (10.6) | |||
| ER- and PR- and HER2 negative (Triple Negative) | 118 (19.0) | 27 (19.9) | 4 (22.2) | 149 (19.2) | |||
p-values from chi-square test except where noted; missing data were excluded from percentage calculations and statistical comparisons.
p-values from Fisher exact test.
47 patients were excluded from analysis due to missing data for hormone receptor status: Bantu 39, Nilote 7, and Cushite 1.
In multivariate analyses evaluating the association between Bantu or Nilote ethnicity with molecular tumor subtype, Nilotes tended to be more likely to be present with ER negative tumours (36.8% versus 29.7%), although this was not statistically significant (p=0.11) and HER2 enriched tumors as compared with Luminal A/B cases, although this difference was also not statistically significant (OR: 1.41, 95% CI: 0.79, 2.49). Ethnicity was not associated with the likelihood of having a triple negative tumor (OR: 1.09, 95% CI: 0.67, 1.76) (Table 4b).
Table 4b.
Association between Nilote vs. Bantu ethnicity (OR [95% CI]) and tumor hormone receptor statusa
| Luminal A/B (comparison) | HER2+ | Triple Neg | |||
|---|---|---|---|---|---|
|
| |||||
| N=519 | N=79 | OR [95% CI] | N=142 | OR [95% CI] | |
|
|
|||||
| Bantus (N=604) | 429 | 60 | ref | 115 | ref |
| Nilotes (N=136) | 90 | 19 | 1.41 (0.79, 2.49) | 27 | 1.09 (0.67, 1.76) |
Adjusted for age and age at first birth; 64 patients were excluded from the analysis due to missing data for hormone receptor status, age or age at first birth: Bantu 57, Nilote 7.
DISCUSSION
This is the largest case series of histologically proven invasive breast cancers from East Africa that has extensive and uniform risk factor information, uniform histology review, and tumor hormone receptor status measured by immunohistochemistry in a single accredited pathology laboratory. More than eight hundred cases were enrolled over a three-year period from 11 public, faith-based and private institutions that serve a full range of the geography and population centers in Kenya.
We observed significant differences in some socio-demographic and reproductive characteristics by ethnicity. For the largest two ethnic groups, the Bantus were significantly more likely to be more educated, more overweight or obese, older at first pregnancy, and younger at menopause in comparison with the Nilotes. We found no statistically significant differences, however, in breast tumor characteristics in women from the two major ethnic groups, although the Nilotes tended to present with somewhat larger tumors and to have a slightly higher proportion of HER2-enriched tumors when compared with the Bantus. These ethnic differences are likely multifactorial in nature, reflecting variations in socioeconomic, environmental and genetic factors.
We used tumor size, tumor grade, LVI, lymph node metastasis, and extranodal extension of the tumor as biologic indicators of tumor aggressiveness. Whether the non-significantly larger tumor sizes seen among the Nilotes reflects more aggressive tumor biology or a consequence of longer delay in presentation and diagnosis is an area worthy of further exploration. In a post hoc analysis, we observed an inverse relationship between tumor size and level of education. Lack of or fewer years of education may limit a woman’s awareness of breast cancer and her knowledge of breast cancer symptoms and the importance of evaluating breast lumps, may lead to delays in presentation and diagnosis.[11] Differences in traditional beliefs and cultural practices among ethnic groups may also explain differences in time to presentation and tumor size at presentation.[12] Exploring these barriers to late presentation among the various communities warrants further study.
Numerous studies of diverse populations have suggested that socio-demographic factors are likely important for ethnic differences in breast cancer tumor size and stage at diagnosis. For example, Awadelkarim et al reported differences in tumor characteristics between Sudanese and Italian women; Sudanese women tended to present with larger tumors than their Italian counterparts (48 mm vs. 22 mm), they had a higher proportion of aggressive grade 3 tumors (68% vs. 21%), and their tumors were more likely to have nodal involvement (90% vs. 36%).[13] Additionally, a US registry-based study of the 10 largest population groups in the US, including Hispanic, White, Black, Japanese, Filipino, Chinese, Hawaiian, Korean, Vietnamese, and American Indian women diagnosed with breast cancer, reported that among the Japanese and White patients, tumors were smaller in size, had a lower tumor grade (even after adjusting for stage), and were diagnosed at an earlier stage, whereas Black and Hispanic patients were more likely than other groups to have tumors ≥2 cm in diameter, to have poorly differentiated tumors, and to be diagnosed with metastatic disease.[14] Apart from possible differential exposure to carcinogens and genetic susceptibility, the known disparities in population mammography screening levels and subsequent follow-up care may also be contributory factors.[14] In Kenya, screening for breast cancer is opportunistic and there is currently no national breast cancer-screening program in place.
Although we found suggestive differences in tumor hormone receptor and HER2 status between the Nilotes and Bantus, these differences were not statistically significant. Nilote tumors tended to be more likely to be ER negative and more likely to be HER2 positive, suggesting biologically more aggressive receptor phenotypes among the Nilotes. There are multiple reports of variation in tumor hormone receptor and HER2 status in different ethnic groups. An NCI study in the SEER registry database which examined 360,933 breast cancer cases diagnosed between 1988-2006 showed that the Asian Indian/Pakistani women had more ER/PR negative breast cancer than Caucasians (30.6% vs. 21.8%, p = 0.0095).[15] Another study reported that relative to non-Hispanic Whites, women of African, Native American, South East Asian, Mexican, South/Central American, and Puerto Rican descent living in the United States had 1.4 to 3.1-fold elevated risks of presenting with ER and PR negative breast cancer.[16] This report concluded that breast cancer tumor characteristics differ by race/ethnicity in the United States and that both biological and lifestyle factors likely contribute to these findings.[16] This hypothesis that the proportions of breast cancer molecular subtypes differ by racial/ethnic groups is also supported by recent reports that East African migrants to the US tend to have less ER negative breast cancer than migrants from West Africa.[17]
The mean and median ages of breast cancer diagnosis (49 and 48 years, respectively) for all ethnicities combined in our study population were not different from those previously reported from Kenya.[10][18] It is noteworthy that there was a markedly earlier age at diagnosis among the Cushites versus the two larger ethnic groups. Although this difference in age at presentation may be suggestive of differences in tumor biology among the ethnic groups, it could also be a chance finding due to the small number of Cushites in our patient population.
Over 60% of the Bantus and Nilotes in this study resided in rural areas, and about half reported menarche at more than 15 years of age, consistent with the findings of a Cameroonian study which reported a higher mean age at menarche for women residing in rural areas (14.3 years) as compared to those residing in urban areas (13.8 years).[19] A significantly greater proportion of Bantus reported age at menopause < 50 years compared to the Nilotes and Cushites. Interestingly, a multiethnic cohort study of non-Latina Whites, Japanese Americans, African-Americans, Native Hawaiians, and Latinas, which investigated factors influencing the timing of natural menopause among 95,704 women, found that race/ethnicity was a significant independent predictor of the timing of natural menopause. While African Americans did not differ significantly from non-Latina Whites, natural menopause occurred earlier among Latinas and later among Japanese Americans, supporting the hypothesis that the timing of natural menopause is driven by a combination of genetic, reproductive, and lifestyle factors.[20] Recently, genome-wide association studies (GWAS) have identified single-nucleotide polymorphism markers that are associated with ages at menarche[21] and menopause[22], which may aid in our understanding of the biology of menarche and menopause.
Differences in body mass were noted between Bantus and Nilotes, with a significantly higher proportion of Bantu patients being overweight or obese (BMI≥25 kg/m2). This may well reflect the dietary habits of the study population. As cited by Christensen et al,[23] a cross-sectional study in Kenya evaluating the prevalence of obesity among rural Luo (representing the Nilotes), who mainly subsist on maize, sorghum and fish, and rural Kamba (representing the Bantus), who subsist on maize, reported higher weights and BMIs in the latter. In a study of rural and urban Kenyan women, the Kamba (Bantus) had the highest Arm Muscle Area (AMA) and the highest BMI and Arm Fat Area (AFA) as compared with the Luo or the Maasai (both Nilotes), a finding which was marginally significant.[23] Additionally, there were differences related to the location of residence in the prevalence of overweight (≥BMI 25 kg/m2) women among all the rural and urban female populations (19.5% and 60.3%, respectively).[23] In our study, there was no difference in Bantu and Nilote cases with respect to rural or urban location of residence, which suggests that location of residence may not contribute to ethnic differences in BMI observed in our patient population.
Interestingly, the mean age at first pregnancy was significantly younger for the Cushite patients than for patients from the other ethnic groups. The Somali, Rendile, Oromo and Borana make up the Cushitic ethnic minority, who reside in the semi-arid and arid North Eastern region of Kenya. Traditionally within these communities, girls usually get married before the age of 20 years. As prior studies have suggested, a full-term pregnancy before the age of 20 years may have a transient cancer promoting effect as each pregnancy, including the first one, may increase the risk of early-onset breast cancer [24][25][26], so early marriages and subsequent early pregnancies could contribute to the higher proportion of early onset pre-menopausal breast cancers seen in the Cushites vs. the other two ethnic groups, although the cross-sectional nature of the study design along with the small number of Cushite cases limits our ability to draw conclusions.
The great majority of patients in all three ethnic groups reported lifetime breastfeeding of more than 15 months, and there was no significant difference in the length of cumulative breast feeding by ethnic group. These results are similar to those of a previous report of mean cumulative breast feeding in the major ethnic tribes in Kenya: 12.1 months for the Kamba (Bantus), 15.6 months for the Kikuyu (Bantus), and 17.1 months for the Luo (Nilotes), with no significant differences among these ethnic groups.[27] Based on findings from a large African American cohort study, it has been hypothesized that an early first birth without subsequent breastfeeding is associated with elevated risk of ER negative breast cancer.[28] Hence, the long lifetime duration of breastfeeding in Kenyan women of varying ethnicities may contribute to the observed similarities in the distribution of breast cancer molecular subtypes by ethnicity in our study population.
Strengths of this study include the relatively large size of this African case series; the broad national representation of the major ethnic groups in Kenya, with the majority being of Bantu ancestry (67% versus 30% Nilotes)[29]; the use of uniform questionnaires and case report forms; and the central pathology review and immunohistochemistry. Limitations of the study include the fact that it was a case series, without a control population for comparison, and the smaller representation of the Nilotes and Cushites, compared to Bantus, which limited the interpretive power of our results. Additional studies with a larger representation from each ethnic group and suitable population-based controls are warranted to further our understanding of breast cancer etiology in these populations.
CONCLUSIONS
This case series showed no significant differences in pathologic features or the molecular subtypes, but significant differences in socio-demographic characteristics and reproductive factors, of breast cancer cases in the three major ethnic groups in Kenya. These differences are probably multifactorial in origin. There were insufficient cases from the Cushite ethnic group to conclude that all three groups were clinicopathologically similar, but the available data suggested that this was the case. This study suggests the need for studying ethnic differences in breast cancer etiology and tumor characteristics throughout the genetically and culturally diverse populations of sub-Saharan Africa. Such studies will clearly enhance our overall understanding of breast cancer etiology and behavior worldwide, and may well help us develop new strategies for prevention and early diagnosis.
Supplementary Material
Acknowledgments
GSK-ERI initiative funding
Aga Khan University Administrative staff
All participating institutions
All nurses and technologists at participating sites
This study was supported in part by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the U.S. National Cancer Institute.
Footnotes
COMPETING INTERESTS
Authors have no competing interests to declare
AUTHORS’ CONTRIBUTIONS
SS, ZM and MS conceived and carried out the study; SS, ZM, PO, SG carried out the centralized laboratory work; IA, CK, TH,SM performed data management; TH, SM, CK, GLG, SF, MM, MP, analyzed data; RW, PB, RO, FWN, AJ, SVP, SV, RC, AZ, MM, BB, CM, OAS, AM, AG, JG, JK, RN, RN, IM, JOO, DOR, EBM, IA contributed to data collection and/or data management. All authors were involved in writing/reviewing the manuscript. All authors gave final approval of the submitted version of the manuscript
ETHICAL STANDARDS
Aga Khan University Hospital, Nairobi Research and Ethics Committee (REF: 2011/25) and the institutional review boards of all participating health facilities approved the study. Written informed consent was obtained from all study participants.
Contributor Information
Dr. Shahin Sayed, Department of Pathology, Aga Khan University Hospital, Nairobi.
Dr. Zahir Moloo, Department of Pathology, Aga Khan University Hospital, Nairobi.
Prof. Ronald Wasike, Department of Surgery, Aga Khan University Hospital, Nairobi.
Dr. Peter Bird, Department of Surgery, AIC Kijabe Hospital.
Dr. Raymond Oigara, Department of Surgery, St. Mary’s Mission Hospital/Kisii Referral Hospital.
Dr. Faith Wambui Njoroge, Department of Surgery, Nyeri Provincial General Hospital.
Dr. Asim Jamal, Department of Medical Oncology, Aga Khan University Hospital, Nairobi.
Dr. Satya Vara Prasad, Department of Pathology, Aga Khan Hospital, Kisumu.
Dr. Sudhir Vinayak, Department of Radiology, Aga Khan University Hospital, Nairobi.
Gretchen L. Gierach, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Sanford M. Dawsey, Division of Cancer Epidemiology and Genetic, National Cancer Institute, Bethesda, MD, USA.
Maya Palakal, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Shaoqi Fan, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Maeve Mullooly, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Dr. Rajendra Chauhan, Department of Surgery, Aga Khan University Hospital, Nairobi.
Dr. Patricia Okiro, Department of Pathology, Aga Khan University Hospital, Nairobi.
Dr. Samuel Gakinya, Department of Pathology, Aga Khan University Hospital, Nairobi.
Dr. Ancent Nzioka, Department of Pathology, AIC Kijabe Hospital.
Dr. Catherine Kyobutungi, Epidemiology, African Population And Health Research Centre (APHRC).
Shukri Mohamed, Epidemiology, African Population And Health Research Centre (APHRC).
Dr. Tilahun Haregu, Epidemiology, African Population And Health Research Centre (APHRC).
Dr. Mustafa Mussajee, Division of Surgery, Kenyatta National Hospital.
Dr. Betty Bonass, Department of Radiology, Kenyatta National Hospital.
Dr. Costa Mariwa, Department of Surgery, Aga Khan Hospital, Kisumu.
Dr. Omar Ali Sherman, Department of Pathology, Aga Khan Hospital, Mombasa.
Dr. Abdihakim Mohammed, Department of Surgery, Aga Khan Hospital, Mombasa.
Dr. Andrew Gachii, Department of Human pathology, Kenyatta National Hospital.
Dr. Joseph Githaiga, Division of Surgery, Kenyatta National Hospital.
Dr. Joseph Karanu, Department of Surgery, St. Mary’s Mission Hospital.
Dr. Robert Nyagah, Department of Surgery, St. Mary’s Mission Hospital.
Dr. Richard Njoroge, Department of Pathology, Garissa Provincial General Hospital.
Dr. Irene Muramba, Department of Pathology, Coast Provincial General Hospital.
Dr. James Obondi Otieno, Department of Surgery, New Nyanza Provincial General Hospital.
Dr. Dan Omondi Raburu, Department of Surgery, New Nyanza Hospital Provincial General.
Elizabeth B. Mwachiro, Department of Surgery, Tenwek Hospital.
Innocent Abayo, Department of Pathology, Aga Khan University Hospital, Nairobi.
Mansoor Saleh, Division of Haematology& Oncology, University of Alabama at Birmingham.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulen J, Jemal Ahmedin. Global Cancer Statistics, 2012. Ca Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Korir A, Okerosi N, Ronoh V, Mutuma G, Parkin M. Incidence of cancer in Nairobi, Kenya (2004 – 2008) Int J Cancer. 2015;2059(137):2053–9. doi: 10.1002/ijc.29674. [DOI] [PubMed] [Google Scholar]
- 3.Bhikoo R, Srinivasa S, Yu TC, Moss D, Hill AG. Systematic review of breast cancer biology in developing countries (part 1): Africa, the Middle East, Eastern Europe, Mexico, the Caribbean and South America. Cancers (Basel) 2011;3(2):2358–81. doi: 10.3390/cancers3022358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter PL, Lund MJ, Lin MG, Yuan X, Liff JM, Flagg EW, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100(12):2533–42. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 5.Der EM, Gyasi RK, Tetty Y, Edusei L, Bayor MTJM, Gyakobo M, Merajver SD, N LA. Triple Negative Breast Cancer in Ghanaian Women: The Korle Bu Teaching Hospital Experience. Breast J. 2015;21(6):627–33. doi: 10.1111/tbj.12527. [DOI] [PubMed] [Google Scholar]
- 6.Hahn KME, Bondy ML, Selvan M, Lund MJ, Liff JM, Flagg EW, et al. Factors associated with advanced disease stage at diagnosis in a population-based study of patients with newly diagnosed breast cancer. Am J Epidemiol. 2007;166(9):1035–44. doi: 10.1093/aje/kwm177. [DOI] [PubMed] [Google Scholar]
- 7.Middleton LP, Chen V, Perkins GH, Pinn V, Page D. Histopathology of breast cancer among African-American women. Cancer. 2003;97(1 Suppl):253–7. doi: 10.1002/cncr.11021. [DOI] [PubMed] [Google Scholar]
- 8.Dickens C, Pfeiffer RM, Anderson WF, Duarte R, Kellett P, Schüz J, et al. Investigation of breast cancer sub-populations in black and white women in South Africa. Breast Cancer Res Treat [Internet] 2016;160(3):531–7. doi: 10.1007/s10549-016-4019-1. [DOI] [PubMed] [Google Scholar]
- 9.Ethnic Groups of Kenya. SourceWatch [Internet] [cited 2017 Sep 2]. Available from: http://www.sourcewatch.org/index.php/Ethnic_Groups_of_Kenya.
- 10.Sayed S, Moloo Z, Wasike R, Bird P, Oigara R, Govender D, et al. Is breast cancer from Sub Saharan Africa truly receptor poor? Prevalence of ER/PR/HER2 in breast cancer from Kenya. The Breast [Internet] 2014;23(5):591–6. 1. doi: 10.1016/j.breast.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Tazhibi Mahdi, Feizi A. Awareness Levels about Breast Cancer Risk Factors, Early Warning Signs, and Screening and Therapeutic Approaches among Iranian Adult Women: A large Population Based Study Using Latent Class Analysis. Biomed Res Int. 2014;2014 doi: 10.1155/2014/306352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayed Shahin, Moloo Zahir, Ngugi Anthony, Allidina Amyn, Ndumia Rose, Mutuiri Anderson, et al. Breast Camps for Awareness and Early Diagnosis of Breast Cancer in Countries With Limited Resources: A Multidisciplinary Model From Kenya. Oncologist [Internet] 2016;21:1138–48. doi: 10.1634/theoncologist.2016-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awadelkarim KD, Arizzi C, Elamin EOM, Hamad HMA, De Blasio P, Mekki SO, et al. Pathological, clinical and prognostic characteristics of breast cancer in Central Sudan versus Northern Italy: Implications for breast cancer in Africa. Histopathology. 2008;52(4):445–56. doi: 10.1111/j.1365-2559.2008.02966.x. [DOI] [PubMed] [Google Scholar]
- 14.Miller BA, Hankey BF, Thomas TL. Impact of Sociodemographic Factors, Hormone Receptor Status, and Tumor Grade on Ethnic Differences in Tumor Stage and Size for Breast Cancer in US Women. Am J Epidemiol. 2002;155(6):534–45. doi: 10.1093/aje/155.6.534. [DOI] [PubMed] [Google Scholar]
- 15.Kakarala M, Rozek L, Cote M, Liyanage S, Brenner DE. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.–a SEER analysis. BMC Cancer. 2010;10:191. doi: 10.1186/1471-2407-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CI, Malone KE, Daling JR. Differences in Breast Cancer Hormone Receptor Status and Histology by Race and Ethnicity among Women 50 Years of Age and Older 1. 2002 Jul;11:601–7. [PubMed] [Google Scholar]
- 17.Jemal A, Fedewa S. Is the prevalence of ER-negative breast cancer in the US higher among Africa-born than US-born black women? Breast Cancer Res Treat. 2012;135(3):867–73. doi: 10.1007/s10549-012-2214-2. [DOI] [PubMed] [Google Scholar]
- 18.Bird P, Hill G, Houssami N. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann Surg Oncol. 2008;15(7):1983–8. doi: 10.1245/s10434-008-9900-7. [DOI] [PubMed] [Google Scholar]
- 19.Pasquet PA, Maguelle-Dicoum Biyong HR-A, et al. Age at menarche and urbanization in Cameroon : current status and secular trends. Ann Hum Biol. 1999;26(1):89–97. doi: 10.1080/030144699283001. [DOI] [PubMed] [Google Scholar]
- 20.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the multiethnic cohort study. Am J Epidemiol. 2008;167(11):1287–94. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 21.Elks CE, Perry JRB, Sulem P, Chasman DI, Franceschini N, He C, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet [Internet] 2010 Dec 21;42(12):1077–85. doi: 10.1038/ng.714. [cited 2017 Sep 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/21102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolk L, Perry JRB, Chasman DI, He C, Mangino M, Sulem P, et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways0. Nat Genet [Internet] 2012 Jan 22;44(3):260–8. doi: 10.1038/ng.1051. [cited 2017 Sep 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22267201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen DL, Eis J, Hansen AW, Larsson MW, Mwaniki DL, Kilonzo B, et al. Obesity and regional fat distribution in Kenyan populations: impact of ethnicity and urbanization. Ann Hum Biol [Internet] 2008;35(2):232–49. doi: 10.1080/03014460801949870. Available from: http://content.ebscohost.com/ContentServer.asp?T=P&P=AN&K=18428015&S=R&D=mnh&EbscoContent=dGJyMNLr40Sep7I4y9f3OLCmr0ueprFSr6e4SrCWxWXS&ContentCustomer=dGJyMPGvt0qvqq9NuePfgeyx63zk6gAA%5Cnhttp://search.ebscohost.com/login.aspx?direct=true&db=mnh&AN=18428015&. [DOI] [PubMed] [Google Scholar]
- 24.Osuch JR, He J. Current Knowledge and New Insights into the Effects of Reproductive and Hormonal Risk Factors in Black and White Populations. Cancer. 2000:1230–8. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1230::aid-cncr9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S, Sugiura H, Ando Y, Shiraki N, Yanagi T, Yamashita H, et al. Reproductive history and breast cancer risk. Breast Cancer. 2012;19(4):302–8. doi: 10.1007/s12282-012-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sighoko D, Ogundiran T, Ademola A, Adebamowo C, Chen L, Odedina S, et al. Breast cancer risk after full-term pregnancies among African women from Nigeria, Cameroon, and Uganda. Cancer. 2015;121(13):2237–43. doi: 10.1002/cncr.29305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson BM, Watson B. Ethnicity and Breastfeeding in Kenya Ethnicity and breastfeeding in Kenya [Internet] University of Tennessee Honors Thesis Projects. 2013 Available from: http://trace.tennessee.edu/utk_chanhonoproj/1642.
- 28.Palmer Julie R, Boggs Deborah A, Wise Lauren A, Ambrosone Christine B, Adams-Campbell LL, R L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011 Sep;20(9):1883–1891. doi: 10.1158/1055-9965.EPI-11-0465. 2011;20(9):1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenya Demographics Profile 2017 [Internet] [cited 2017 Sep 2]. Available from: https://www.indexmundi.com/kenya/demographics_profile.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


