Abstract
Background
Pharyngeal-provocation induced aerodigestive symptoms in infants remain an enigma. Sources of pharyngeal provocation can be anterograde as with feeding, and retrograde as in gastroesophageal reflux. We determined maturational and dose-response effects of targeted pharyngeal-stimulus on frequency, stability, and magnitude of pharyngeal and respiratory waveforms during multiple pharyngeal swallowing responses in preterm-born infants when they were of full-term postmenstrual age (PMA).
Methods
Eighteen infants (11 male) were studied longitudinally at 39.8 ± 4.8 wks PMA (time-1) and 44.1 ± 5.8 wks PMA (time-2). Infants underwent concurrent pharyngo-esophageal manometry, respiratory inductance plethysmography, and nasal airflow thermistor methods to test sensory-motor interactions between the pharynx, esophagus, and airway. Linear mixed models were used and data presented as mean ± SEM or %.
Results
Overall, responses to 250 stimuli were analyzed. Of the multiple pharyngeal swallowing responses (n=160), with maturation: a) deglutition apnea duration decreases (p<0.01), b) number of pharyngeal waveform peaks and duration decreases for initial responses (p<0.01), and subsequent responses have lesser variation and greater stability (p<0.01). With increment in stimulus volumes we noted: a) increased prevalence (%) of pharyngeal responses (p<0.05), b) increased number of pharyngeal peaks (p<0.05), yet pharyngeal frequency (Hz), variability, and stability remain unaffected (p>0.05), and c) respiratory changes were unaffected (p>0.05).
Conclusions
Initial and subsequent pharyngeal responses and respiratory rhythm interactions become more distinct with maturation. Interval oromotor experiences and volume-dependent increase in adaptive responses may be contributory. These mechanisms may be important in modulating and restoring respiratory rhythm normalcy.
Keywords: dysphagia, respiratory regulation, prematurity, deglutition, deglutition disorders
INTRODUCTION
Pharyngeal-provocation induced aerodigestive symptoms in premature infants remain an enigma. Sources of pharyngeal provocation can be anterograde as with feeding or oral secretions, and retrograde as in gastroesophageal reflux. Regardless, upon pharyngeal stimulation, bolus clearance and airway protective reflexes occur to maintain homeostasis. These regulatory reflexes modulate breathing phases and may include laryngeal chemoreflex, glottal closure, deglutition apnea, pharyngeal swallowing, and/or upper esophageal sphincter (UES) contraction to steer the bolus away from and protect the airway against aspiration (1–5). The pharyngeal and UES mechanisms to pharyngeal stimulus can be observed in Fig 1 B–C.
Figure 1. Pharyngeal provocation induced responses.
Responses are highlighted in shaded grey boxes occurring within 5 seconds of stimulus onset. A) No response is characterized by absence of esophageal or respiratory activity, B) Upper esophageal sphincter (UES) contraction is characterized by upper esophageal sphincter increase, and C) Pharyngeal swallow is characterized by pharyngeal contraction, esophageal body propagation, and deglutition apnea (grey outlined box). Subsequently, there may responses with multiple responses present as shown: D) UES contraction followed by a single pharyngeal swallow, E) UES contraction followed by multiple pharyngeal swallows (characterized by multiple pharyngeal peaks), or F) multiple pharyngeal swallow only. For the purpose of this study, multiple pharyngeal swallow responses (E and F) were analyzed for pharyngeal and respiratory rhythms.
Swallowing is a complicated process and involves multiple interactions between the foregut, respiratory, and central nervous systems (3, 4, 6–13). Sensory-motor characteristics of swallowing and central pattern generator functions have been tested in human infants and neonatal pigs during oral and pharyngeal phases of swallowing (3, 4, 14–18). We have tested these pharyngo-esophageal and respiratory reflexes in human premature neonates using micro-manometry and pharyngeal stimulation techniques (3, 4, 19), and similar methods have been applied in human adults (10, 20–22). There are differences in the characteristics of responses, in that UES contraction is more prevalent in the adult population, whereas pharyngeal swallowing is more prevalent in the neonatal population (3). Pharyngeal swallow is protective by clearing stimulus away from the pharynx and airway and in restoring normal breathing patterns. UES contraction followed by pharyngeal swallow (Fig 1 D) is another potential response (23). With increasing pharyngeal stimulus volume, there is an increase in response prevalence (%) and number of pharyngeal peaks (multiple pharyngeal swallows) (3, 19) (Fig 1 E–F). Evidence of this response has also been shown in human adults during voluntary rapid swallowing characterized by esophageal body inhibition and a terminal swallow (defined as the final pharyngeal swallow associated with esophageal body contraction and restoration of lower esophageal tone) (2, 24–27).
How multiple pharyngeal swallow sequences change during maturation in prematurely born infants is critical to the understanding of coping skills in health and disease. Although multiple studies have investigated volumetric and maturational effects on swallowing and respiratory sensory motor characteristics (3, 4, 6–8, 11–13, 19), limited data exists in regards to aerodigestive (respiratory, pharyngeal, and esophageal) rhythms in conjunction during multiple pharyngeal swallow sequence rhythms in human premature infants. Therefore, the current study was undertaken to evaluate these components that could further refine our understanding of multiple pharyngeal swallow response kinetics with particular focus on multiple pharyngeal rhythms and their influence on esophageal and respiratory characteristics. To test the hypothesis that maturation and provoking -volumes modify stimulus-response interactions and regulation of aerodigestive rhythms (with an emphasis on multiple pharyngeal swallow), our aims were to determine maturational (longitudinal changes) and volumetric effects on multiple pharyngeal swallow response rhythms (magnitude, frequency, variability, and stability) and respiratory characteristics (deglutition apnea, deglutition apnea duration, respiratory rhythm change, and respiratory rhythms change duration) in human premature infants.
MATERIALS AND METHODS
Subjects
Eighteen infants (11 male) were studied longitudinally at 39.8 ± 4.8 wks PMA (time-1) and 44.1 ± 5.8 wks PMA (time-2). To understand the physiological maturational changes with pharyngeal reflexes, we excluded infants with congenital or gastrointestinal abnormalities, and included those born preterm and were enterally/orally fed. The study protocol was approved by the ethics committee at the Institutional Research Review Board at Nationwide Children’s Hospital Research Institute, Columbus, Ohio, USA and conformed to policy guidelines and the Health Insurance Portability and Accountability Act. Informed parental consent was obtained prior to the study.
Manometry Methods and Study Protocol
As previously published, pharyngeal reflexes were evaluated using water perfusion manometry (Solar-2; Medical Measurement Systems, Dover, NH, USA) with a custom designed manometry catheter (Dentsleeve International, Mui Scientific, Ontario, Canada) with pressure channels spanning the pharynx to the stomach (3, 4, 19). Concurrent respiratory inductance plethysmography (Respitrace; Viasys, Conshohocken, Pennsylvania) and nasal airflow thermistor (Integra Life Sciences, Plainsboro, NJ, USA) were utilized to detect respiratory changes (28, 29). The manometry catheter was passed nasally in the supine infant and given adequate time for infant adaptation. To elucidate the characteristics of pharyngo-esophageal and respiratory changes, the protocol involved administration of sterile water infusions into the pharynx. Graded stimulus volume-response relationships were evaluated by physician administered volumes (0.1, 0.3, and 0.5 mL) of sterile water in triplicate as tolerated based on based on prior dose-response curves to pharyngeal stimulation (3, 19). To study the maturational physiology of pharyngeal-stimulation induced aerodigestive responses, subjects were re-evaluated when they were on exclusive oral feeds (a sign of functional maturation of safe oral feeding skills). Subjects were continuously monitored at the bedside by a physician and registered nurse for symptoms and safety.
Data Analytical Methods
Definitions of Pharyngeal Rhythm and Esophageal Body Response
Analytical methods were adapted from previous non-nutritive sucking, nutritive sucking-swallowing, and pharyngo-esophageal analysis (3–5, 14, 16, 19, 30) to characterize pharyngeal provocation induced rhythms. Based on our previous pharyngo-esophageal studies (3, 19), the following definitions were applied utilizing pharyngo-esophageal manometry waveforms to all pharyngeal stimuli administered. Prevalence (%) of primary response types to pharyngeal infusion were pharyngeal swallow (presence of a rapid pharyngeal peak ≥ 10 mmHg, within 5 seconds of pharyngeal provocation), UES contraction (UES pressure increase > 4mmHg within 5 seconds of pharyngeal provocation), or none (absence of pharyngeal swallow or UES contraction within 5 seconds of pharyngeal provocation) (3, 19). Multiple pharyngeal swallow responses were defined as any succeeding pharyngeal responses (≥ 2 pharyngeal peaks) until the terminal swallow (final pharyngeal swallow) restored respiratory and esophageal normalcy (Fig 1 E–F): The following variables were applied to only multiple pharyngeal swallow responses: 1) magnitude as number (#) and duration (sec) of pharyngeal peaks 2) frequency (Hz) as number of pharyngeal peaks divided by duration of pharyngeal peaks, 3) variability as standard deviation of pharyngeal peak to peak interval durations (sec) only if > 2 pharyngeal peaks were observed and 4) stability (only if > 2 pharyngeal peaks were observed) as standard deviation of pharyngeal peak to peak interval durations divided by the average pharyngeal peak to peak interval durations (#). Hence, stability can be interpreted similar to a coefficient of variation, i.e. lower values indicate greater stability (31). A data analytical algorithm of the above definitions can be observed (Fig 2).
Figure 2. Multiple pharyngeal swallow rhythm definitions.
Note the pharyngeal infusion stimulus along with associated multiple pharyngeal peaks. This representative example depicts the analytical method and calculations used for pharyngeal rhythm evaluation of multiple-pharyngeal swallow (A–F), along with a plot of the elapsed duration vs frequency as a marker of pharyngeal regulation.
Upon preliminary analysis of the multiple pharyngeal swallow responses, there were noticeable changes to the pharyngeal response across time until aerodigestive normalcy. Therefore, to characterize these differences we defined the composite pharyngeal response as overall pharyngeal response or the presence of pharyngeal waveforms until the terminal swallow that restored respiratory and esophageal normalcy. Within the composite pharyngeal response there were two noticeable phases observed (an initial clustered phase similar to that seen in adults as multiple rapid pharyngeal swallowing sequences, and a slower unclustered phase) (Fig 3). Thus, the initial pharyngeal response was defined as the first pharyngeal cluster until a pause in pharyngeal signaling, (Fig 3A) and the subsequent pharyngeal response (Fig 3B) was defined as the pharyngeal pressure waveforms immediately succeeding the initial response until the presence of a terminal swallow restoring respiratory and esophageal normalcy. If no pharyngeal cluster (initial pharyngeal response) occurred, this was considered as subsequent pharyngeal response. Beyond initial pharyngeal response and subsequent pharyngeal response, the prevalence (%) of initial response only, subsequent response only, and initial plus subsequent response were recorded to determine the frequency of combination responses occurring together. The initial and subsequent response phases were identified upon visual inspection by two authors (SRJ, KAH), which were analyzed and compared for prevalence, pharyngeal magnitude, frequency, variability, and stability. Prevalence of esophageal body inhibition (%) was also measured for the initial pharyngeal response. Esophageal body inhibition was defined as the absence of esophageal body waveforms (in proximal-, middle-, and distal-, esophagus) from initial pharyngeal response onset until the last pharyngeal peak in the initial pharyngeal response (Fig 3).
Figure 3. Determination of initial and subsequent pharyngeal responses and respiratory adaptation measures.
A) the initial pharyngeal response was defined as the first pharyngeal burst until a pause in pharyngeal signaling (rapid peaks >10 mmHg) B) The subsequent pharyngeal response was defined as the pharyngeal pressure waveforms immediately succeeding the initial response until the presence of a terminal swallow restoring respiratory and esophageal normalcy. Please note that the composite pharyngeal response is comprised of both initial and subsequent pharyngeal responses. The following were applied to respiratory rhythms: C) deglutition apnea or pause in breathing with initial pharyngeal swallowing response, D) respiratory response latency, E) respiratory response duration, and F) total respiratory rhythms change duration
Definitions of Respiratory Response
The following respiratory rhythm definitions were applied utilizing respiratory inductance plethysmography and nasal airflow thermistor waveforms (Fig 3). Prevalence (%) of deglutition apnea was defined as the absence of breathing associated with the initial pharyngeal swallowing response, in infants this may occur in inspiration or expiration (4, 32), and duration (sec) as airflow cessation onset to offset. Respiratory response latency (sec) was defined as duration between the onset of pharyngeal stimulus to onset of respiratory rhythm disturbance. Respiratory response duration (sec) was defined as onset to offset of respiratory rhythm disturbance. Total respiratory rhythm change duration (sec) was defined as the duration from the onset of pharyngeal stimulus to restoration of respiratory stability.
Statistical Analysis
Comparison of demographic characteristics between time-1 and time-2 was performed using paired t-tests for continuous variables and McNemar’s tests for categorical variables. Wilcoxon rank-sum test was used to compare data that was not normally distributed. Linear mixed models were used for pharyngeal and respiratory characteristics to compare a) the effect of maturation (time-1 vs time-2) while controlling for volumetric effects and b) the effect of volume (0.1, 0.3 and 0.5 mL) while controlling for maturation c) initial pharyngeal response vs. subsequent pharyngeal response within each volume while controlling for the effect of maturation, GEE models were used to compare categorical outcomes. Bonferroni method was used to adjust for multiple comparisons. To observe the change in pharyngeal peak frequency at different maturation (time-1 and time-2) and different volumes (0.3 and 0.5 mL) a generalized linear model with log-link function (PROC GENMOD) was used (http://support.sas.com/rnd/app/ets/papers/nonlinearmodels.pdf). Linear regression models were used to obtain coefficients (intercept and slope) of frequency over elapsed time for maturation (Time 1, Time 2), volume (0.3, 0.5 mL) and response type (initial, subsequent) for each individual. Boxplots were presented to summarize intercepts and slopes from these individual linear regression models. Data of the triplicate trials are presented as mean ± SEM, median (range) or %. A P-value <0.05 was considered significant. Statistical analysis was performed using SAS v. 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Clinical characteristics
Thirty-six studies from 18 preterm neonates who were able to achieve full oral feeds at Time-2 study were evaluated. Overall, subject demographics and clinical characteristics can be observed in Table 1. Clinical characteristics at time of evaluation can be observed in Table 2. At Time-2 study, 9 subjects were studied outpatient, and 9 subjects were studied inpatient. Two of the inpatient subjects were discharged the same day. The remaining 7 subjects were convalescing from lung disease and studied 4–8 weeks after Study-1 and discharged 13(8–27) days later. Of the 19 studies on nasal cannula oxygen at time of study (Time 1 or Time 2), the flow was 0.2 (0.1 – 0.7) liters per minute.
Table 1.
Subject Demographic and Clinical Characteristics
| Characteristic | N=18 Subjects |
|---|---|
| Gestational age, wks | 27 (23 – 37) |
| Birth Weight, kg | 0.8 (0.7 – 1.2) |
| APGAR at 1 minute | 4 (2 – 6) |
| APGAR at 5 minutes | 7 (5 – 8) |
| Chronic lung disease of infancy, % | 15(83) |
| Gastroesophageal reflux disease, % | 9(50) |
| History of apnea/bradycardia/desaturation, % | 8(44) |
| Length of hospital stay, days | 72 (43 – 116) |
| Weight at discharge | 3.8 (3.7 – 4.3) |
Data presented as mean ± SEM or n(%). NCPAP- nasal continuous positive airway pressure, NC- nasal cannula. Chronic lung disease of infancy was defined as oxygen requirement at 36 weeks postmenstrual age.
Table 2.
Clinical Characteristics at Time of Study
| Characteristic | Time-1 | Time-2 | P-value |
|---|---|---|---|
| Post menstrual age, wks | 39.8 ± 4.8 | 44.1 ± 5.8 | <0.01 |
| Weight, kg | 2.9 ± 0.8 | 3.8 ± 1.0 | <0.01 |
| Length, cm | 46.5 ± 4.3 | 50.8 ± 5.2 | <0.01 |
| Head circumference, cm | 32.7 ± 3.4 | 36.2 ± 2.9 | <0.01 |
| Feeding method (exclusive gavage: gavage + oral: exclusive oral), % | 17: 44: 39 | 0:0:100 | <0.01 |
| Airway maintenance method (NCPAP: NC: no support), % | 5: 56: 39 | 0: 56: 44 | 0.6 |
Data presented as mean ± SEM or %. NCPAP- nasal continuous positive airway pressure, NC- nasal cannula
Pharyngo-esophageal manometry characteristics
Over 65.5 hours of data from all 36 studies were scanned for a total of 250 administered pharyngeal stimuli (with infusion rates of 0.08±0.004 mL/sec at 0.1 mL, 0.03±0.004 mL/sec at 0.3 mL, and 0.03±0.005 mL/sec at 0.5 mL, p<0.05 vs 0.1 mL) of which 240 (96%) were analyzable. Of these analyzable responses (n=240): a) the most frequent primary response was pharyngeal swallow in 147 (61%), UES contraction in 45 (19%), and no response noted in 48 (20%). Of the 45 UES contractile responses, 38 (84%) occurred before a pharyngeal swallow response. Hence of the analyzable responses (n=240), pharyngeal swallow was present either as a primary (n=147) or secondary response (n=38) for a total of 185 (77%). Of these 185 pharyngeal swallow responses, 25 (14%) were solitary pharyngeal swallow, and 160 (86%) were multiple pharyngeal swallow sequences.
Of the 160 multiple pharyngeal swallow sequences: a) pharyngeal frequency (# pharyngeal peaks/pharyngeal peak duration) averaged 0.62 ± 0.01 Hz with median (IQR) as 0.6 (0.2 – 1.0) Hz with a minimum of 0.03 Hz and maximum of 1.85 Hz observed. b) symptoms occurred in 111(69%) with the most frequent movement/arch 90(81%) and cough 19(17%) being the most frequent. c) prevalence of any initial pharyngeal response was 144 (90%) and any subsequent pharyngeal response prevalence was 100 (62.5%), d) Prevalence of initial pharyngeal response only was 55 (34%), initial + subsequent pharyngeal response in conjunction was 89 (56%), and subsequent pharyngeal response only 14(9%), and, and 2 (1%) not analyzable). Of the initial pharyngeal responses (N=144), esophageal body inhibition occurred in 120 (83%) of initial pharyngeal responses. When esophageal body inhibition did not occur (N=24), symptoms were present in 20(83%), and d) complete esophageal body propagation occurred in 135(84%) of terminal swallows.
Maturational effect on respiratory and pharyngeal rhythms
The effect of maturation on respiratory and pharyngeal rhythms during multiple pharyngeal swallow sequences (n=160) can be observed in Table 3. Of note, deglutition apnea duration and composite number of pharyngeal peaks decrease with maturation, while stability increases. A representative of the example of maturational effect on respiratory and pharyngeal rhythms can be observed in Fig 4.
Table 3.
Effect of Maturation on Pharyngeal and Respiratory Responses during Multiple-Pharyngeal Swallowing Sequences
| Characteristic | Time-1 (n=80 stimuli) | Time-2 (n=80 stimuli) | P-value |
|---|---|---|---|
| Respiratory Response* | |||
|
| |||
| Deglutition apnea prevalence, % | 99 | 97 | 0.4 |
| Deglutition apnea duration, s | 4.7 ± 0.5 | 3.2 ± 0.5 | <0.01 |
| Respiratory rhythm change, % | 59 | 69 | 0.2 |
| Respiratory rhythm change latency, sec | 4.1 ± 0.3 | 4.0 ± 0.3 | 0.9 |
| Respiratory response duration, sec | 27.4 ± 3.0 | 22.1 ± 3.2 | 0.2 |
| Composite Pharyngeal Response | |||
|
| |||
| Pharyngeal peaks, # | 7.5 ± 0.4 | 5.1 ± 0.4 | <0.01 |
| Response duration, sec | 16.8 ± 1.6 | 10.8 ± 1.6 | <0.01 |
| Frequency, Hz | 0.7 ± 0.03 | 0.7 ± 0.03 | 0.9 |
| Peak variability, sec | 1.9 ± 0.2 | 1.4 ± 0.2 | 0.09 |
| Peak stability | 0.6 ± 0.05 | 0.4 ± 0.05 | 0.03 |
| Initial Pharyngeal Response | |||
|
| |||
| Prevalence, % | 96 | 84 | 0.08 |
| Pharyngeal peaks, # | 5.0 ± 0.4 | 3.0 ± 0.4 | <0.01 |
| Response duration, sec | 6.1 ± 0.6 | 4.3 ± 0.6 | <0.01 |
| Frequency, Hz | 1.1 ± 0.05 | 1.0 ± 0.05 | 0.2 |
| Peak variability, sec | 0.3 ± 0.04 | 0.3 ± 0.04 | 0.9 |
| Peak stability, # | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.8 |
| Subsequent Pharyngeal Response | |||
|
| |||
| Prevalence, % | 64 | 61 | 0.9 |
| Pharyngeal peaks, # | 2.4 ± 0.4 | 2.2 ± 0.4 | 0.6 |
| Response duration, sec | 17.6 ± 2.4 | 12.1 ± 2.4 | 0.05 |
| Frequency, Hz | 0.2 ± 0.03 | 0.3 ± 0.03 | 0.06 |
| Peak variability, sec | 3.8 ± 0.5 | 2.3 ± 0.4 | <0.01 |
| Peak stability, # | 0.8 ± 0.07 | 0.6 ± 0.07 | <0.01 |
Data presented as mean ± SEM, All volumes were included however P-values were calculated controlling for volume. Note: Composite pharyngeal response is comprised of initial and subsequent pharyngeal responses. Results for deglutition apnea prevalence and duration apply to only to those responses where pharyngeal swallowing response was observed, whereas other respiratory characteristics apply to the total stimuli administered.
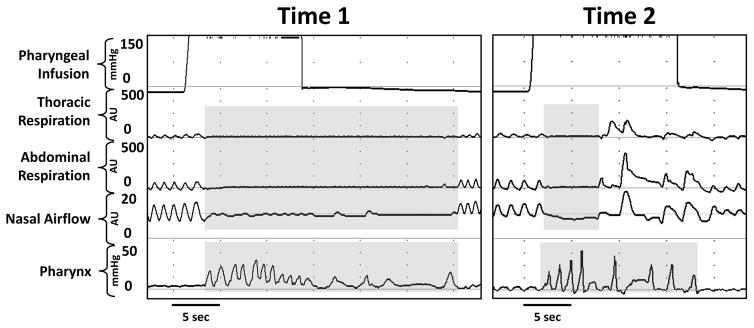
Figure 4. Maturational effect on respiratory and pharyngeal rhythms.
Note the representative recording in an infant at time-1 (younger age) exhibits increased number of pharyngeal peaks, duration, and variability between peaks, with prolonged deglutition apnea in contrast with maturation from the same infant at time-2.
Volumetric effect on pharyngeal and respiratory rhythms
The effect of pharyngeal stimulus volume on respiratory and pharyngeal rhythms during multiple pharyngeal swallow sequences can be observed in Table 4. Composite pharyngeal responses are further broken down into initial and subsequent responses (Fig 5). Note the increasing response prevalence with the 0.3 mL and 0.5 mL volumes (Fig 5A) and increasing number of pharyngeal peaks with increasing volume (Fig 5B). Additionally, for the higher volumes (0.3 mL and 0.5 mL) initial and subsequent responses differ significantly for number of pharyngeal peaks, pharyngeal response duration, pharyngeal frequency, pharyngeal variability, and pharyngeal stability (Fig 5B–F). Intercept and slope coefficients of frequency over elapsed time for maturation (Time 1, Time 2), volume (0.3, 0.5 mL), and response type (initial, subsequent) can be observed in Fig 6.
Table 4.
Effect of Pharyngeal Stimulus Volume on Respiratory and Pharyngeal Responses during Multiple-Pharyngeal Swallowing Sequences
| Characteristic | 0.1 mL (n=98 stimuli) | 0.3 mL (n=99 stimuli) | 0.5 mL (n=53 stimuli) | P-value |
|---|---|---|---|---|
| Respiratory Response | ||||
|
| ||||
| Deglutition apnea, % | 97 | 98 | 98 | 1.0 |
| Deglutition apnea duration, sec | 3.2 ± 1.2 | 4.5 ± 0.6 | 5.1 ± 0.7 | 0.4 |
| Respiratory response latency, sec | 2.6 ± 0.8 | 4.5 ± 0.4 | 4.7 ± 0.5 | 0.06 |
| Respiratory response duration, sec | 13.2 ± 6.9 | 25.0 ± 2.8 | 28.5 ± 4.3 | 0.2 |
| Respiratory Response duration, sec | 15.7 ± 7.1 | 29.5 ± 3.0 | 33.2 ± 4.5 | 0.1 |
|
| ||||
| Composite Pharyngeal Response | ||||
|
| ||||
| Prevalence, n(%) | 16(16)†‡ | 95(96) | 49(92) | <0.01 |
| Peak recruitment, # | 2.1 ± 0.6†‡ | 6.2 ± 0.4‡ | 9.0 ± 0.5† | <0.01 |
| Response duration, sec | 7.3 ± 3.0‡ | 14.5 ± 1.2 | 19.6 ± 1.7 | <0.01 |
| Frequency, Hz | 0.9 ± 0.07†‡ | 0.6 ± 0.02 | 0.5 ± 0.04 | <0.01 |
| Peak variability, sec | 2.0 ± 0.5 | 1.6 ± 0.1 | 1.4 ± 0.2 | 0.5 |
| Peak stability | 0.5 ± 0.1 | 0.5 ± 0.03 | 0.5 ± 0.04 | 0.9 |
Data presented as n(%) or mean ± SEM or,
p<0.05 vs 0.3 mL,
p<0.05 vs 0.5 mL,
Both maturations were included however P-values were calculated controlling for maturation. Results for deglutition apnea prevalence and duration apply to only to those responses where pharyngeal swallowing response was observed, whereas other respiratory characteristics apply to the total stimuli administered.
Figure 5. Volume-response relations of initial and subsequent pharyngeal rhythms.
Note an increased occurrence (%) of initial and subsequent response with higher volumes of 0.3 and 0.5 mL (Fig 5A), increasing number of pharyngeal peaks of initial and subsequent response is volume-dependent (Fig 5B), increasing initial pharyngeal response duration of bursts with increasing volumes but similar subsequent pharyngeal response durations for all volumes (Fig 5C), similar frequencies at the 0.3 and 0.5 mL higher volumes (Fig 5D), decreasing subsequent response variability with increasing volumes (Fig 5E), and similar pharyngeal stability at the 0.3 and 0.5 mL higher volumes (Fig 5F). At higher volumes of 0.3 ml and 0.5 mL, initial and subsequent responses are distinct for number of pharyngeal peaks, pharyngeal response duration, pharyngeal frequency, pharyngeal variability, and pharyngeal stability (Fig 5B–F).
Figure 6. Effects of subject maturation and stimulus volume on initial and subsequent pharyngeal responses.
For each individual, linear regression models were used to obtain coefficients (intercept and slope) of pharyngeal frequency vs elapsed time for maturation (Time 1, Time 2), stimulus volume (0.3, 0.5 mL), and response type (initial, subsequent).
DISCUSSION
Pharyngeal-respiratory interactions evoked upon modulating stimuli
In neonates who achieved full oral feeds safely, the significant findings of pharyngeal-respiratory interactions and rhythms are summarized as follows: 1) With advancing maturation: a) deglutition apnea duration decreases, b) number of pharyngeal waveform peaks and duration decreases and stability increases, 2) With graded increase in stimulus volumes: a) there is a higher prevalence of initial and subsequent responses, b) number of pharyngeal waveform peaks increases, yet pharyngeal frequency (Hz) and stability remains unaffected, c) subsequent pharyngeal response variation decreases and c) respiratory rhythms (deglutition apnea prevalence, deglutition apnea duration, respiratory response latency, and respiratory change duration) were unaffected, and 3) initial and subsequent responses are distinct. The change in these waveform patterns may be due to one or several factors: brainstem maturation, sensory and motor maturation of afferent and efferent pathway responses, or changes in central pattern generator functions. Owing to the clinical nature of this study, precise mechanisms are uncertain and developmental animal studies are needed (1, 6, 16, 30, 33).
Interpretation in the context of other studies, in adults or in animal models
In adult studies, pharyngeal stimulation tests (pharyngeal stimuli is infused in to the pharynx and the subject is instructed not to swallow, as the volume increases an irrepressible pharyngeal reflexive swallow eventually occurs) and multiple rapid swallow tests (subject is told to perform five rapid swallows) are frequently utilized to evaluate mechanisms of dysphagia (2, 10, 20, 24–27, 34). However, infants cannot perform either of these voluntary tests, therefore adaptive responses to pharyngeal stimulation are truly representative of pharyngeal reflexive swallowing in this population. Although we have studied effects of pharyngeal stimulation in infants (3, 4, 19), to our knowledge the current study is the first to evaluate specifically the characteristics of multiple pharyngeal swallow response rhythms in neonates (similar to that of multiple rapid swallow tests). In adults, normal rapid swallowing responses are characterized by esophageal body inhibition and a terminal swallow (24–27). This is most representative of the initial responses only in our population. Abnormal responses in adults may include incomplete inhibition, and/or incomplete or failed peristalsis during the terminal swallow and are potential markers of dysphagia which may lead to troublesome symptoms (24–26). In our population it appears that our initial plus subsequent responses most closely resembles the abnormal adult response. It may be more advantageous for the infant to have initial only responses as these had a lower prevalence of symptoms associated. Symptoms can occur normally and may coexist with multiple protective reflex mechanisms, and may not necessarily be a manifestation of disease. On the other hand, troublesome symptoms can be manifestation of disease. Clinical and basic science mechanisms with the generation of symptoms associated with swallowing abnormalities need further studies.
Physiological relevance and clinical implications
Achievement of aerodigestive homeostasis is critical for achieving and sustaining oral feeding ability. While initial and subsequent pharyngeal responses are distinct, it is likely that there is a dependent relationship between the two, thus contributing to aerodigestive homeostasis. Initial pharyngeal response is a rapid response to stimuli that have resulted in IX and X efferent discharge to modulate increasing pharyngeal rhythm generation, and increasing respiratory inhibitory regulation to permit airway protection, and these responses were stimulus-volume dependent. Subsequent pharyngeal response on the other hand, appears to be a response to ventilatory changes and ensures restoration of respiratory rhythm and esophageal quiescence such that peristalsis is complete (2, 33, 35–38). It should also be noted that the minimal pharyngeal stimulus volumes of sterile water were chosen for subject safety. However, this model is not necessarily equivalent to the natural feeding model originating in the oral cavity in which the infant can modify bolus extraction, swallowing attempts, and pacing over a much longer duration. Additionally, infant feeds are often comprised of breast milk or formula with varying physical and chemical characteristics (media, viscosity, taste, osmolality, etc), and positions, all of which have been shown to influence swallowing characteristics (3, 19, 39–41). Further studies are needed to more closely replicate the natural feeding model in infants.
In the current study, maturation and stimulus volume modifies respiratory and pharyngeal adaptive characteristics, similar to previous findings (3) (3, 19, 42). Clinical significance of these findings may explain the basis for feeding difficulties, and/or troublesome symptoms of apnea, bradycardia or desaturations such as may happen during feeding or during the clearance of supra-esophageal refluxate, during which bolus characteristics, widely vary. Modification of these bolus characteristics (volume, flow, chemical composition, viscosity, osmolality, etc.) along with maturation may alleviate these issues.
Limitations and future considerations
Whether some of our findings may be the result of other non-pharyngeal actions such as oromotor rhythmic tongue or jaw movement cannot be explained by our study, although is certainly plausible. Thexton et al. have shown such rhythmic oral activity in the activation of tongue, mylohyoid, and geniohyoid muscle activity (30, 43). Measurement of these areas may require different and multiple instrumentation modalities. While it has been shown that the oral, pharyngeal, and esophageal, phases of swallowing are independent of each other (2, 3), future work is needed to evaluate correlation of oromotor phases with pharyngeal and esophageal phases. Pharyngeal swallowing or UES contraction may be induced by oral, pharyngeal, esophageal, tussive, or gastroesophageal reflux stimuli (3, 5, 7, 17, 19–23, 27, 44–46). Thus, terminologies have been adopted to distinguish responses due to these specific testing conditions. For example, pharyngeal stimulus induced responses were identified as pharyngeal reflexive swallow or pharyngo-upper esophageal sphincter contractile sphincter reflex (3, 4, 20, 23). In the current study, we measure pharyngeal waveform dimensions and rhythms upon pharyngeal stimulation, which are final neuromotor markers of the antecedent activating swallowing related neural circuitry. Ideally, to assess maturational changes with pharyngo-esophageal motility, a time interval of 4–8 weeks is necessary. In some situations, when an infant has achieved full oral feeds, parents and providers hasten discharge planning. Therefore, tightly controlled demographic variables (gestational, postmenstrual, and chronological age) between time-1 and time-2 will be ideal with larger sample sizes, as this will help clarify the effects of our heterogeneous population and confounding factors that are likely to result in different results among successful feeders and unsuccessful feeders.
In summary, we evaluated the maturational changes with pharyngeal and respiratory biorhythms in infants that were able to achieve full oral feeds. From 40 to 44 weeks postmenstrual age, the coordination of respiration with swallowing matures significantly. These changes probably allow for safer swallowing with less chance of aspiration. Initial and subsequent pharyngeal responses and respiratory rhythm interactions become more distinct with maturation. Interval oromotor experiences and volume-dependent increase in adaptive responses may be contributory. Collectively, the study findings may be the result of respiratory adaptation to pharyngeal stimulus volumes and maturation of central and or peripheral neuronal sensory-motor pathways.
Acknowledgments
The authors are grateful to Brittany Durr, RN, BSN, MHA, for clinical nursing coordination and Lai Wei, PhD for statistical modeling, analysis, and verification.
FUNDING
This study is supported in part by National Institutes of Health grants 2R01DK068158 and P01DK068051.
ABBREVIATIONS
- UES
upper esophageal sphincter
Footnotes
DISCLOSURE
No conflicts of interest to declare.
LOCATION OF WORK: Nationwide Children’s Hospital, Columbus, OH, USA
CONFLICT OF INTEREST- The authors have no conflicts of interest to declare.
References
- 1.Rasch S, Sangild PT, Gregersen H, Schmidt M, Omari T, Lau C. The preterm piglet - a model in the study of oesophageal development in preterm neonates. Acta Paediatr. 2010;99:201–208. doi: 10.1111/j.1651-2227.2009.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang IM. Brain stem control of the phases of swallowing. Dysphagia. 2009;24:333–348. doi: 10.1007/s00455-009-9211-6. [DOI] [PubMed] [Google Scholar]
- 3.Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr. 2007;151:597–603. doi: 10.1016/j.jpeds.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadcherla SR, Gupta A, Wang M, Coley BD, Fernandez S, Shaker R. Definition and implications of novel pharyngo-glottal reflex in human infants using concurrent manometry ultrasonography. Am J Gastroenterol. 2009;104:2572–2582. doi: 10.1038/ajg.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol. 2007;102:2286–2293. doi: 10.1111/j.1572-0241.2007.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang IM, Dean C, Medda BK, Aslam M, Shaker R. Differential activation of medullary vagal nuclei during different phases of swallowing in the cat. Brain Res. 2004;1014:145–163. doi: 10.1016/j.brainres.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Medda BK, Lang IM, Layman R, Hogan WJ, Dodds WJ, Shaker R. Characterization and quantification of a pharyngo-UES contractile reflex in cats. Am J Physiol. 1994;267:G972–983. doi: 10.1152/ajpgi.1994.267.6.G972. [DOI] [PubMed] [Google Scholar]
- 8.Khurana A, Thach BT. Effects of upper airway stimulation on swallowing, gasping, and autoresuscitation in hypoxic mice. Journal of applied physiology. 1996;80:472–477. doi: 10.1152/jappl.1996.80.2.472. [DOI] [PubMed] [Google Scholar]
- 9.Dua K, Bardan E, Ren J, Sui Z, Shaker R. Effect of chronic and acute cigarette smoking on the pharyngoglottal closure reflex. Gut. 2002;51:771–775. doi: 10.1136/gut.51.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dua K, Bardan E, Ren J, Sui Z, Shaker R. Effect of chronic and acute cigarette smoking on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. Gut. 1998;43:537–541. doi: 10.1136/gut.43.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dua K, Surapaneni SN, Kuribayashi S, Hafeezullah M, Shaker R. Protective role of aerodigestive reflexes against aspiration: study on subjects with impaired and preserved reflexes. Gastroenterology. 2011;140:1927–1933. doi: 10.1053/j.gastro.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino T. Swallowing as a protective reflex for the upper respiratory tract. Anesthesiology. 1993;79:588–601. doi: 10.1097/00000542-199309000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Pickens DL, Schefft GL, Thach BT. Pharyngeal fluid clearance and aspiration preventive mechanisms in sleeping infants. Journal of applied physiology. 1989;66:1164–1171. doi: 10.1152/jappl.1989.66.3.1164. [DOI] [PubMed] [Google Scholar]
- 14.Barlow SM, Burch M, Venkatesan L, Harold M, Zimmerman E. Frequency Modulation and Spatiotemporal Stability of the sCPG in Preterm Infants with RDS. International journal of pediatrics. 2012;2012:581538. doi: 10.1155/2012/581538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estep M, Barlow SM, Vantipalli R, Finan D, Lee J. Non-Nutritive Suck Parameter in Preterm Infants with RDS. Journal of neonatal nursing: JNN. 2008;14:28–34. doi: 10.1016/j.jnn.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gierbolini-Norat EM, Holman SD, Ding P, Bakshi S, German RZ. Variation in the timing and frequency of sucking and swallowing over an entire feeding session in the infant pig Sus scrofa. Dysphagia. 2014;29:475–482. doi: 10.1007/s00455-014-9532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shubert TR, Sitaram S, Jadcherla SR. Effects of pacifier and taste on swallowing, esophageal motility, transit, and respiratory rhythm in human neonates. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and rate of milk delivery as determinants of swallowing in an infant model animal (Sus scrofia) Dysphagia. 2004;19:147–154. doi: 10.1007/s00455-004-0001-x. [DOI] [PubMed] [Google Scholar]
- 19.Jadcherla SR, Shubert TR, Gulati IK, Jensen PS, Wei L, Shaker R. Upper and lower esophageal sphincter kinetics are modified during maturation: effect of pharyngeal stimulus in premature infants. Pediatr Res. 2015;77:99–106. doi: 10.1038/pr.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dua K, Surapaneni SN, Kuribayashi S, Hafeezullah M, Shaker R. Pharyngeal airway protective reflexes are triggered before the maximum volume of fluid that the hypopharynx can safely hold is exceeded. Am J Physiol Gastrointest Liver Physiol. 2011;301:G197–202. doi: 10.1152/ajpgi.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dua KS, Surapaneni SN, Santharam R, Knuff D, Hofmann C, Shaker R. Effect of systemic alcohol and nicotine on airway protective reflexes. Am J Gastroenterol. 2009;104:2431–2438. doi: 10.1038/ajg.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dua KS, Bajaj JS, Rittmann T, Hofmann C, Shaker R. Safety and feasibility of evaluating airway-protective reflexes during sleep: new technique and preliminary results. Gastrointest Endosc. 2007;65:483–486. doi: 10.1016/j.gie.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Shaker R, Ren J, Xie P, Lang IM, Bardan E, Sui Z. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol. 1997;273:G854–858. doi: 10.1152/ajpgi.1997.273.4.G854. [DOI] [PubMed] [Google Scholar]
- 24.Shaker A, Stoikes N, Drapekin J, Kushnir V, Brunt LM, Gyawali CP. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol. 2013;108:1706–1712. doi: 10.1038/ajg.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushnir V, Sayuk GS, Gyawali CP. Multiple rapid swallow responses segregate achalasia subtypes on high-resolution manometry. Neurogastroenterol Motil. 2012;24:1069–e1561. doi: 10.1111/j.1365-2982.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sifrim D, Jafari J. Deglutitive inhibition, latency between swallow and esophageal contractions and primary esophageal motor disorders. Journal of neurogastroenterology and motility. 2012;18:6–12. doi: 10.5056/jnm.2012.18.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornari F, Bravi I, Penagini R, Tack J, Sifrim D. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil. 2009;21:718–e741. doi: 10.1111/j.1365-2982.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- 28.Hasenstab KA, Jadcherla SR. Respiratory events in infants presenting with apparent life threatening events: is there an explanation from esophageal motility? J Pediatr. 2014;165:250–255e251. doi: 10.1016/j.jpeds.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadcherla SR, Gupta A, Stoner E, Coley BD, Wiet GJ, Shaker R. Correlation of glottal closure using concurrent ultrasonography and nasolaryngoscopy in children: a novel approach to evaluate glottal status. Dysphagia. 2006;21:75–81. doi: 10.1007/s00455-005-9002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. Journal of applied physiology. 2007;102:587–600. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 31.Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr. 2008;97:61–67. doi: 10.1111/j.1651-2227.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev Med Child Neurol. 2006;48:589–594. doi: 10.1017/S001216220600123X. [DOI] [PubMed] [Google Scholar]
- 33.Lang IM, Medda BK, Shaker R. Differential activation of medullary vagal nuclei caused by stimulation of different esophageal mechanoreceptors. Brain Res. 2011;1368:119–133. doi: 10.1016/j.brainres.2010.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaker R, Ren J, Bardan E, Easterling C, Dua K, Xie P, Kern M. Pharyngoglottal closure reflex: characterization in healthy young, elderly and dysphagic patients with predeglutitive aspiration. Gerontology. 2003;49:12–20. doi: 10.1159/000066504. [DOI] [PubMed] [Google Scholar]
- 35.Altschuler SM. Laryngeal and respiratory protective reflexes. Am J Med. 2001;111(Suppl 8A):90S–94S. doi: 10.1016/s0002-9343(01)00862-2. [DOI] [PubMed] [Google Scholar]
- 36.Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med. 2000;108(Suppl 4a):79S–86S. doi: 10.1016/s0002-9343(99)00343-5. [DOI] [PubMed] [Google Scholar]
- 37.Broussard DL, Altschuler SM. Central integration of swallow and airway-protective reflexes. Am J Med. 2000;108(Suppl 4a):62S–67S. doi: 10.1016/s0002-9343(99)00340-x. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta JN, Saha JK, Goyal RK. Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. J Neurophysiol. 1990;64:796–812. doi: 10.1152/jn.1990.64.3.796. [DOI] [PubMed] [Google Scholar]
- 39.Dantas RO, Kern MK, Massey BT, Dodds WJ, Kahrilas PJ, Brasseur JG, Cook IJ, Lang IM. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. Am J Physiol. 1990;258:G675–681. doi: 10.1152/ajpgi.1990.258.5.G675. [DOI] [PubMed] [Google Scholar]
- 40.Dalmazo J, Aprile LR, Dantas RO. Effect of swallowed bolus viscosity and body position on esophageal transit, contraction and perception of transit. Arquivos de gastroenterologia. 2015;52:27–31. doi: 10.1590/S0004-28032015000100007. [DOI] [PubMed] [Google Scholar]
- 41.Davies AM, Koenig JS, Thach BT. Upper airway chemoreflex responses to saline and water in preterm infants. Journal of applied physiology. 1988;64:1412–1420. doi: 10.1152/jappl.1988.64.4.1412. [DOI] [PubMed] [Google Scholar]
- 42.Pickens DL, Schefft G, Thach BT. Prolonged apnea associated with upper airway protective reflexes in apnea of prematurity. Am Rev Respir Dis. 1988;137:113–118. doi: 10.1164/ajrccm/137.1.113. [DOI] [PubMed] [Google Scholar]
- 43.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol. 2009;101:1386–1393. doi: 10.1152/jn.90847.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jadcherla SR. Manometric evaluation of esophageal-protective reflexes in infants and children. Am J Med. 2003;115(Suppl 3A):157S–160S. doi: 10.1016/s0002-9343(03)00215-8. [DOI] [PubMed] [Google Scholar]
- 45.Jadcherla SR, Hasenstab KA, Shaker R, Castile RG. Mechanisms of cough provocation and cough resolution in neonates with bronchopulmonary dysplasia. Pediatr Res. 2015 doi: 10.1038/pr.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasenstab KA, Jadcherla SR. Respiratory Events in Infants Presenting with Apparent Life Threatening Events: Is There an Explanation from Esophageal Motility? J Pediatr. 2014 doi: 10.1016/j.jpeds.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]