Abstract
Paroxetine and fluconazole have neuroprotective effects in an in-vitro model of HIV protein mediated neuronal injury. This study evaluated the safety, tolerability, and efficacy of both paroxetine and fluconazole for the treatment of HIV-associated neurocognitive disorder (HAND). A 24 week randomized double-blind, placebo-controlled 2x2 factorial design study was used. HIV+ individuals with cognitive impairment were enrolled in the 24 week trial. Participants were randomly assigned to one of four groups: 1) paroxetine 20mg/day, 2) fluconazole 100mg every 12 hours, 3) paroxetine and fluconazole or 4) placebo. Safety, tolerability, and efficacy were evaluated. 45 HIV+ individuals were enrolled. Medications were well tolerated. Compared to no paroxetine arms, HIV+ individuals receiving paroxetine showed improved NPZ8 summary scores, (mean change= 0.25 vs −0.19, p= 0.049), CalCAP sequential test reaction time (mean change = 0.34 vs −0.23, p=0.014), Trail Making Part B test performance (mean change= 0.49 vs −0.33, p=0.041), and FAS verbal fluency (mean change =0.25 vs 0.02, p= 0.020) but a decline in the Letter number sequencing test (mean change = −0.40 vs. 0.26, p=0.023). Biomarkers of cellular stress, inflammation, and neuronal damage were not affected by paroxetine. HIV+ individuals receiving fluconazole did not show a benefit in cognition and showed an increase in multiple markers of cellular stress compared to the no fluconazole arms. In conclusion, paroxetine was associated with improvement in a summary neuropsychological test measure and in several neuropsychological tests but worse performance in one neuropsychological test. Further studies of paroxetine for the treatment of HAND and to define its precise neuroprotective properties are warranted.
Keywords: paroxetine, fluconazole, HIV, cognitive impairment
HIV-associated neurocognitive disorders (HAND) continue to be an important neurological manifestation of HIV-1 infection seen in 40–50% of HIV-1 seropositive (HIV+) individuals in the era of combination antiretroviral therapy (cART) (McArthur et al, 2010). HAND is associated with persistent central nervous system (CNS) inflammation, aberrant macrophage activation, and increased CNS oxidative stress (Saylor, Nakigozi et al. 2016). An adjunctive therapy that interferes with CNS inflammatory events triggered by the HIV virus will likely play an important role in the future treatment of HAND (Epstein and Gendelman 1993). However, prior trials of adjunctive therapies have not consistently shown clinical benefit in a neurocognitive summary measure (Lampl, Boaz et al. 2007, Schifitto, Zhang et al. 2007, Uthman and Abdulmalik 2008, Schifitto, Yiannoutsos et al. 2009).
Previously, we screened more than 2000 compounds, half of which were FDA approved drugs for putative neuroprotective effects against oxidative stress-mediated neuronal injury in an in vitro model of mixed rat neuronal cultures (Steiner, Bachani et al. 2015). Selective serotonin reuptake inhibitors (SSRIs) such as paroxetine, and the anti-fungal agent, fluconazole, protected hippocampal neurons and showed a synergistic interaction (Steiner, Bachani et al 2015). Paroxetine-mediated neuroprotection was unchanged in neuronal cultures made from serotonin transporter knockout mice, suggesting that blocking serotonin reuptake at the transporter site was not necessary for the paroxetine-mediated neuroprotection (Steiner, Bachani et al. 2015). The combination of paroxetine and fluconazole protected macaques from SIV- associated neurodegeneration (Meulendyke, Queen et al. 2014)}, as measured by cerebrospinal (CSF) neurofilament protein light chain and amyloid precursor protein (APP) accumulation in axons.
Paroxetine and fluconazole have a long history of safety and efficacy for treating depression and cryptococcal meningitis respectively in HIV+ patients, but their effect on cognitive performance has not previously been evaluated in HIV+ individuals. Based upon these preliminary data, we conducted a phase I/II, randomized, double-blind, placebo-controlled study to assess the safety, tolerability, and efficacy of paroxetine and fluconazole for the treatment of HIV-associated cognitive impairment.
MATERIALS AND METHODS
Recruitment/enrollment
Enrollment of 45 individuals, 18 to 65 years of age, occurred between December, 2010 and January, 2015, in the adult outpatient clinical research unit of the Institute for Clinical and Translational Research (ICTR) at the Johns Hopkins Hospital. All participants had HIV (based on ELISA and confirmed by Western blot or plasma HIV RNA and clinical notes) and evidence of cognitive impairment. Cognitive impairment was defined as performance compared to age- and education- matched controls at least 1.0 standard deviation (SD) below average on three or more independent neuropsychological tests, or at least 2.0 SD below average on one test and at least 1.0 SD on a second test at the screening visit. Stable antiretroviral regimen was required for at least 3 months prior to screening with no plans to change the antiretroviral regimen. Once enrolled, participants were not required to stay on their baseline regimen. Participants were excluded if pregnant or breast-feeding, or if they presented with active symptomatic AIDS defining opportunistic infection within 30 days prior to entry, current systemic fungal infection, any history of CNS opportunistic infection (fungal or non-fungal), history of medical illness, chronic neurological disorder, or severe affective disorder (e.g., depression with suicidal ideation) that in the investigator’s opinion would interfere with study requirements, active illicit drug use within 3 months prior to baseline (verified by negative urine toxicology screen at screening), current use of fluconazole within one month prior to entry or SSRI (including paroxetine) within 3 months of entry, or current treatment with blood thinners. Laboratory exclusion criteria included hemoglobin ≤ 9.0 g/dl, absolute neutrophil count ≤ 500 cells/ mm3, platelet count ≤ 50,000 cells/ mm3, alanine transaminase (ALT) ≥ 2.5 times the upper limit of normal, alkaline phosphatase ≥ 3 times the upper limit of normal, and creatinine ≥ 2 times the upper limit of normal. Recruitment and enrollment were stopped upon conclusion of the funding period.
Standard Protocol Approvals, Registrations, and Patient Consents
The protocol was reviewed and approved by the Institutional Review Board and Institute for Clinical and Transitional Research at Johns Hopkins Hospital. The study is registered in clinicaltrials.gov (NCT 01354314). Informed consent was obtained from all participants or their authorized representatives.
Randomization
At baseline, participants were randomized into one of four treatment groups: 1) placebo, 2) paroxetine 20mg orally every evening per day, 3) fluconazole 100mg orally every 12 hours per day, 4) paroxetine 20mg every evening and fluconazole 100mg every 12 hours orally per day.
A block stratified randomization computer program was used to generate the random allocation sequence. A block size of four was used. Drug was dispensed by an unblinded pharmacist. The manufacturing pharmacy provided 60-count bottles with a blinded fixed label and an unblinded tear-off label, which was removed and affixed to the back of the prescription. The Investigational Drug Service protocol pharmacist generated the randomization. The unblinded Investigational Drug Service pharmacist assigned the participant to interventions according to chronological order of enrollment (i.e., receipt of entry prescription). All other study investigators remained blinded to the treatment assignment (including the research coordinator and study neurologist who enrolled participants).
Therapy and follow-up
Participants received the study drug daily for 24 weeks. Participants were re-evaluated at 4, 12 and 24 weeks after randomization. At each visit, participants were assessed for adverse clinical events. Neurological examination and safety laboratory tests including serum chemistry profiles, hematology, CD4+ T lymphocyte counts, and plasma HIV RNA levels were performed at screening, baseline, and at weeks 4, 12, and 24.
A neuropsychological test battery was performed at the screening visit, and visits at pre-entry, and at 12 and 24 weeks after entry. The battery included the Hopkins Auditory Verbal Learning test (HVLT) (Brandt 1991), Rey Complex Figure test (Reitan 1979); (Osterrieth 1944), Symbol Digit Modalities Test (Smith 1973), Trail Making test (Reitan 1958), Grooved Pegboard Test (Klove)Timed Gait test, California Computerized Assessment Package (CalCAP) Choice and Sequential reaction time test (Miller 1990), FAS verbal fluency Test (Borkowski, Benton et al. 1967) the Stroop Color Interference test (Comalli, Wapner et al. 1962), and depression symptomatology assessed with the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977). Neuropsychological test performance was summarized using the NPZ8 and the Global Deficit Scale (GDS). The NPZ8 was calculated as the mean of non-missing values at entry and 24 weeks on the following tests: Trail Making Test, parts A and B, Grooved Pegboard Test, dominant and non-dominant hand, CalCAP Choice and Sequential reaction time tests, Timed Gait Test, and Symbol Digit Modalities Test. The GDS was calculated using the methods of (Carey, Woods et al. 2004) as the mean of non-missing t-score values at entry and 24 weeks on the following tests: Timed Gait Test, Letter Number Sequence Test, Stroop Color Interference Test, Symbol Digit Modalities Test, Rey Complex Figure copy and delayed recall, CalCAP Choice and Sequential reaction time tests, HVLT total and delayed recall scores, Grooved Pegboard Test, dominant and non-dominant hand, FAS Verbal Fluency test, and Trail Making Test, parts A and B. NPZ8 and GDS measures were only considered valid if at least 2/3 of component measures were non-missing.
Functional performance was measured with the Karnofsky Performance score, and the Instrumental Activities of Daily Living at screening, baseline, week 12, and week 24, and performance based tests including the modified Medication Management test (Gandhi, Skolasky et al. 2011) and the Driving Simulation component of the Computerized Assessment of Mild Cognitive Impairment (CAMCI) tests (Rosenthal, Skolasky et al. 2013) performed at baseline and week 24. Plasma and CSF samples were collected at baseline and week 24 for biomarker analyses.
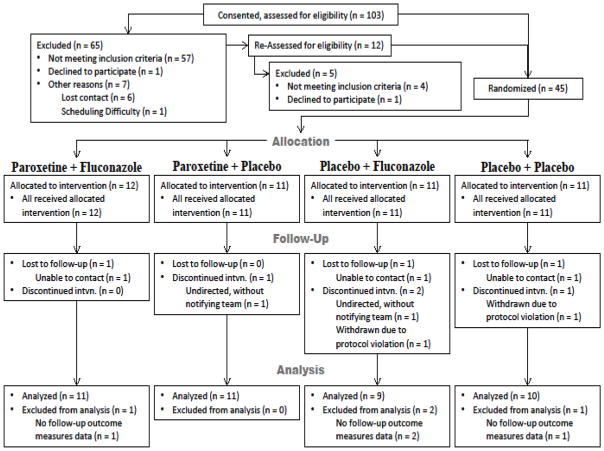
Twenty-three subjects had incomplete datasets or less than complete adherence for the 24 week study. Four subjects were excluded from analyses due to incomplete follow-up outcome measures (see Figure 1). One subject was withdrawn due to protocol violation. The remaining 18 subjects excluded from the as treated analyses were excluded because they did not consume 90% of the study drug based on pill count (see Statistical Analysis section).
Figure 1.
Subject Enrollment
Biomarker measures
Lipid markers were measured by electrospray ionization tandem mass spectrometry as described previously (Haughey, Cutler et al. 2004). Protein carbonyls were quantitated using the OxyBlot Protein Oxidation Detection Kit as described previously (Schifitto, Yiannoutsos et al. 2009). Enzyme-linked immunosorbent assays (ELISA) were used to measure plasma Neopterin (GenWay Biotech), sCD14 (R&D Systems), sCD163 (from Trillium Diagnostics) and CSF sCD14, sCD163, neurofilament protein light chain (NFL) (UmanDiagnostics), and neurofilament protein heavy chain (NFH) (BioVendor) levels according to manufacturer’s protocols.
Statistical analysis
The primary outcomes for this clinical trial evaluated safety and tolerability of paroxetine and fluconazole, and their efficacy on neurocognitive performance using the NPZ8 and CSF lipid markers of oxidative stress. Secondary outcomes evaluated the efficacy of paroxetine and/or fluconazole on other neurocognitive measures, plasma and CSF protein markers of oxidative stress, markers of macrophage/monocyte activation, inflammation, and neuronal injury, and on functional measures.
The sample size was based upon a power calculation using CSF ceramide C22:0 as the primary outcome measure to provide 80% power with a total sample size of 40 subjects.
Treatment groups were summarized according to baseline socio-demographic and clinical characteristics. Safety and tolerability were assessed using incidence (number and percent) of adverse and serious adverse events. Fisher’s exact tests were used to compare the incidence across treatment groups. Efficacy was evaluated by calculating change scores (the arithmetic difference between test measures at week 24 and baseline with negative numbers indicating a decrease in performance and positive numbers indicating an increase in performance. Assumption of normality was tested using the Shapiro-Wilk test. For continuous measures, distribution was described using median and interquartile range (25th and 75th percentile). Neuropsychological test performance measures were standardized to follow a normal distribution (z-scores); therefore, distribution was described using mean and standard deviation.
Change scores were modeled using generalized linear regression. Final models were adjusted for baseline measure, treatment effect on depressive symptoms, (i.e., 24 week change in CES-D score) and the presence of undetectable plasma HIV RNA at baseline.
Treatment assignment was governed by the randomization scheme with participants assigned to paroxetine, or fluconazole, or combination paroxetine and fluconazole, or placebo. Analyses examined the treatment effect of paroxetine (alone or in combination with fluconazole) versus no paroxetine (placebo or fluconazole alone), and fluconazole (alone or in combination with paroxetine) versus no fluconazole (placebo or paroxetine alone). Secondary analyses were conducted also to compare the three active treatment arms to the placebo arm.
Both Intention to Treat (ITT) and as-treated analyses were performed with a pre-defined statistical plan. Under the ITT framework, participants were assigned the treatment they were randomized to irrespective of treatment adherence. Participants were included in as treated analyses if they completed the trial without protocol violation and consumed (based on pill count) at least 90% of the target total doses of study medication for the trial. Type I error rate was set at 0.05. Statistical analyses were conducted using SAS Studio, version 3.6 (SAS Institute, Cary, NC).
Classification of Evidence
This interventional study provides Class II evidence for the safety, tolerability, and efficacy of paroxetine and fluconazole for the treatment of HIV-associated neurocognitive impairment.
RESULTS
Baseline characteristics
Among the 45 HIV-infected individuals with cognitive impairment enrolled, 11 were randomized to placebo, 11 to the paroxetine group, 11 to the fluconazole group, and 12 to the paroxetine and fluconazole group (Figure 1). The four treatment groups were similar with respect to age, education, race, and CD4 lymphocyte count (see Table 1A and 1B). The median (interquartile range) age for the participants enrolled was 50 (47, 55) years, 32 (71%) were male, and 36 (80%) were African-American. The median (interquartile range) CD4 lymphocyte count for all participants was 491 (389, 630) cell/mm3. The participants largely exhibited well controlled HIV replication with a plasma HIV RNA <50 copies/μl. Participants receiving paroxetine and fluconazole were more likely to be women compared to the other treatment groups in ITT analyses (p=0.020). Participants receiving paroxetine and fluconazole had a trend to be less likely to have an undetectable plasma HIV RNA compared to the other three treatment groups p=0.050) in the ITT analyses. Participants receiving paroxetine only had a lower rate of a positive hepatitis C serology, compared to the other treatment groups (p=0.010) in as treated analyses.
Table 1A.
Baseline Characteristics of the Four Treatment Groups, Intention to Treat (N=45)
| Placebo | Paroxetine | Fluconazole | Paroxetine + Fluconazole | |
|---|---|---|---|---|
|
| ||||
| n=11 | n=11 | n=11 | n=12 | |
|
| ||||
| Age-Years/median (IQR) | 49 (45, 58) | 53 (48, 59) | 51 (48, 56) | 50.5 (47.5, 53.5) |
|
| ||||
| Education-Years: Median (IQR) | 12 (12, 13) | 12 (12, 14) | 12 (12, 14) | 11.5 (10, 13.5) |
|
| ||||
| Gender (% Male) | ||||
| Male | 9 (81.1) | 9 (81.8) | 10 (90.9) | 4 (33.3) |
| Female | 1 (9.1) | 2 (18.2) | 1 (9.1) | 7 (58.3) |
| Trans | 1 (9.1) | 0 | 0 | 1 (8.3) |
|
| ||||
| Race (% African-American) | 9 (81.8) | 8 (72.7) | 10 (90.9) | 10 (83.3) |
|
| ||||
| CD4 count: Median (IQR) | 461 (359, 520) | 620 (417, 849) | 504 (408, 553) | 534.5 (341, 627) |
|
| ||||
| Plasma HIV RNA-copies/ml: Median (IQR) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,78) |
|
| ||||
| Plasma HIV RNA: % <50 copies/ml | 10 (90.9) | 9 (81.8) | 11 (100) | 7 (58.3) |
|
| ||||
| Hepatitis C serology: % positive | 8 (72.7) | 3 (27.3) | 7 (63.6) | 6 (50.0) |
|
| ||||
| CES-D Mean( IQR) | 10 (4.20) | 2 (0.6) | 18 (6.24) | 8.5 (6.5, 12.5) |
IQR, interquartile range (25th and 75th percentile)
CES-D (Center for Epidemiological Studies-Depression scale
Table 1B.
Baseline Characteristics of the Four Treatment Groups, As treated (N=22)
| Placebo | Paroxetine | Fluconazole | Paroxetine + Fluconazole | |
|---|---|---|---|---|
|
| ||||
| n=5 | n=8 | n=3 | n=6 | |
|
| ||||
| Age, years, median (IQR) | 49 (47, 61) | 51.5 (48.5, 54) | 56 (49, 57) | 50.5 (50, 53) |
|
| ||||
| Education, years, median (IQR) | 12 (12, 12) | 12 (12, 13) | 13 (12, 14) | 11.5 (10, 13.) |
|
| ||||
| Gender | ||||
| Male (%) | 3 (60) | 6 (75) | 3 (100) | 2 (33.3) |
| Female (%) | 1 (20) | 2 (25) | 0 | 3 (50) |
| Trans (%) | 1 (20) | 0 | 0 | 1 (16.7) |
|
| ||||
| Race, (% African-American) | 4 (80) | 5 (62.5) | 3 (100) | 5 (83.3) |
|
| ||||
| CD4 count, median (IQR) | 419 (359, 485) | 535.5 (413, 815.5) | 290 (269, 525) | 534.5 (389, 630) |
|
| ||||
| Plasma HIV RNA, copies/ml, median (IQR) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.64) |
|
| ||||
| Plasma HIV RNA, % <50 copies/ml | 4 (80) | 7 (87.5) | 3 (100) | 4 (66.7) |
|
| ||||
| Hepatitis C, serology % positive | 5 (100) | 1 (12.5) | 2 (66.7) | 2 (33.3) |
|
| ||||
| CES-D, median (IQR) | 13 (10.25) | 2.5 (0.5, 4.5) | 20 (18.27) | 9 (3.14) |
IQR, interquartile range (25th and 75th percentile)
CES-D Center for Epidemiological Studies-Depression scale
Safety
There were no deaths or hospitalizations reported in the study. There were no differences in the frequency of adverse events (defined as the frequency of either total adverse events or moderate/severe adverse events) between the four treatment groups. The most common side effects seen in more than one individual for each treatment group were as follows: placebo: diarrhea (n=2); paroxetine alone: sexual dysfunction (n=3), headache (n=2), insomnia (n=2), disturbing dreams (n=2); fluconazole alone: nausea (n=4), depressed mood (n=2), dry mouth (n=2); paroxetine and fluconazole: diarrhea (n=5), nausea (n=4), dizziness (n=2), dry mouth (n=2). There was no difference in the frequency of any of the above individual side effects among the four treatment groups.
Neurocognitive measures
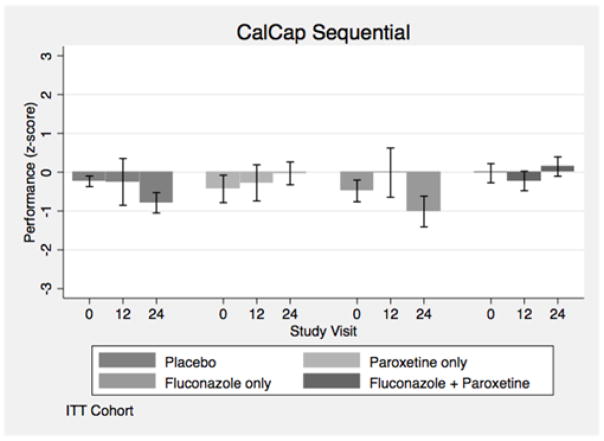
The baseline neuropsychological assessments for each of the four treatment arms are shown in Table 2 for intention-to-treat (ITT) analyses. The changes from baseline to week 24 for the ITT analyses for NP test performance are shown in Table 3. HIV+ individuals receiving paroxetine (alone or in combination with fluconazole) showed an improvement in the NPZ8 summary measure of neuropsychological test performance (Navia, Dafni et al. 1998), CalCAP sequential reaction time (Figure 2), Trail Making Part B, and FAS verbal fluency tests, but worse performance on the Letter Number Sequencing test after adjusting for NP test score, depression symptomatology, gender and baseline percent undetectable plasma HIV RNA, in ITT analyses (Table 3). HIV+ individuals receiving paroxetine showed a benefit in the CalCAP sequential reaction time test but worse performance in the Letter Number Sequencing test compared to the no paroxetine arms after adjusting for baseline NP test score, depression symptomatology, hepatitis C serostatus and baseline percent undetectable plasma HIV RNA in as treated analyses (Table 4).
Table 2.
Baseline Neuropsychological assessment, Intention to Treat (N=45)
| Placebo | Paroxetine | Fluconazole | Paroxetine + Fluconazole | |
|---|---|---|---|---|
| N=11 | N=11 | N=11 | N=12 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| CalCAP Choice Reaction time Z * | −0.72 (1.25) | −1.94 (3.01) | −0.63 (1.57) | −0.84 (1.50) |
| CalCAP Sequential Reaction Time Z * | −0.24 (0.91) | −0.43 (1.41) | −0.48 (1.25) | −0.03 (1.23) |
| Symbol Digit Z * | 1.26 (1.01) | 0.24 (1.57) | −0.20 (1.60) | 0.91 (1.06) |
| Trail Making Part A Z | 0.59 (0.83) | 0.25 (1.08) | 0.21 (1.38) | 0.67 (1.26) |
| Trail Making Part B Z | 0.47 (1.01) | 0.19 (0.87) | 0.44 (1.37) | 0.24 (1.29) |
| Timed Gait Z ** | −0.61 (1.66) | −2.31 (3.12) | −1.08 (2.75) | −0.91 (1.26) |
| Grooved Pegboard Dominant Z | 0.17 (0.95) | −0.46 (1.15) | −0.47 (1.23) | −0.12 (1.04) |
| Grooved Pegboard Non- Dominant Z | −0.27 (0.96) | −0.55 (1.25) | −0.85 (0.95) | 0.13 (1.22) |
| Hopkins Auditory Verbal Learning test Total Z | −0.80 (1.03) | −0.97 (0.94) | −0.99 (1.09) | −1.18 (1.32) |
| Hopkins Auditory Verbal Learning Test Delayed Z | −1.47 (1.30) | −1.45 (1.75) | −2.27 (1.76) | −1.60 (1.78) |
| Rey Complex Figure Copy Z * | −0.85 (0.97) | −1.08 (1.54) | −1.12 (1.49) | −1.71 (1.60) |
| Rey Complex Figure Delayed Z * | 0.00 (0.56) | −0.55 (0.85) | −0.42 (0.78) | −0.31 (0.82) |
| Stroop Color Interference Z ** | −0.36 (1.553) | −1.85 (2.05) | −2.08 (1.65) | −0.70 (1.55) |
| Letter number sequencing Z * | −0.63 (0.86) | 0.17 (0.62) | −0.3 (1.46) | −0.11 (0.73) |
| FAS verbal fluency Z * | 0.51 (1.17) | −0.10 (0.79) | 0.06 (0.59) | 0.48 (0.89) |
| NPZ8 *** | 0.13 (0.47) | −0.26 (0.71) | −0.28 (0.86) | −0.09 (0.57) |
| GDS *** | 0.49 (0.41) | 0.86 (0.51) | 0.90 (0.78) | 0.68 (0.46) |
Sample size (n) for this test: placebo n=11, paroxetine n=10, fluconazole n=11, paroxetine and fluconazole n=12
Sample size (n) for this test: placebo n=11, paroxetine n=9, fluconazole n=11, paroxetine and fluconazole n=12
Sample size (n) for this test: placebo n=10, paroxetine n=10, fluconazole n=9, paroxetine and fluconazole n=11
CalCAP-California Computerized Reaction Time test
GDS-Global deficit score
Table 3.
24 Week change of neuropsychological tests, NPZ-8, and GDS by treatment, Intention to Treat (N=45)
| No Paroxetine arms mean (SD) | Paroxetine arms mean (SD) | p value | No Fluconazole arms mean (SD) | Fluconazole arms mean (SD) | p value | |
|---|---|---|---|---|---|---|
| N=19 | N=21 | N=20 | N=20 | |||
| CalCAP Choice Reaction time change * | −0.98 (1.54) | 0.55 (2.28) | 0.092 | −0.09 (2.60) | −0.12 (1.42) | 0.756 |
| CalCAP Sequential Reaction time change * | −0.23 (0.78) | 0.34 (1.22) | 0.014 | 0.03 (1.28) | 0.16 (0.82) | 0.968 |
| Symbol Digit change | −0.01 (0.94) | 0.28 (0.87) | 0.587 | −0.06 (0.78) | 0.35 (0.99) | 0.243 |
| Trail Making Part A change | 0.08 (0.90) | 0.02 (0.81) | 0.681 | 0.05 (0.81) | 0.05 (0.90) | 0.910 |
| Trail Making Part B change | −0.33 (0.90) | 0.49 (0.98) | 0.041 | 0.27 (0.91) | −0.06 (1.11) | 0.097 |
| Timed Gait change ** | −0.28 (1.10) | −0.19 (1.04) | 0.919 | 0.07 (1.00) | −0.51 (1.05) | 0.083 |
| Grooved Pegboard Dominant change *** | 0.02 (0.95) | 0.39 (0.97) | 0.241 | 0.28 (0.99) | 0.15 (0.97) | 0.438 |
| Grooved Pegboard non-dominant change *** | −0.02 (0.71) | 0.01 (0.98) | 0.163 | 0.13 (0.83) | −0.14 (0.89) | 0.351 |
| Hopkins Auditory Verbal Learning test Total change | 0.01 (0.98) | 0.12 (0.91) | 0.590 | −0.07 (0.85) | 0.21 (1.01) | 0.475 |
| Hopkins Auditory Verbal Learning test Delayed change | 0.43 (1.29) | −0.02 (1.30) | 0.554 | 0.18 (1.36) | 0.21 (1.27) | 0.987 |
| Rey Complex Figure Copy Change | −0.45 (1.62) | 0.18 (0.97) | 0.277 | −0.04 (1.47) | −0.20 (1.23) | 0.284 |
| Rey Complex Figure Delayed change | 0.02 (0.43) | 0.34 (0.58) | 0.085 | 0.28 (0.56) | 0.10 (0.50) | 0.178 |
| Stroop Color Interference change **** | −0.05 (0.87) | −0.04 (1.32) | 0.673 | −0.16 (1.30) | 0.08 (0.88) | 0.540 |
| Letter number sequencing change | 0.26 (0.72) | −0.40 (0.74) | 0.023 | −0.11 (0.92) | −0.07 (0.55) | 0.695 |
| FAS verbal fluency change | 0.02 (0.43) | 0.25 (0.53) | 0.020 | 0.28 (0.46) | −0.01 (0.50) | 0.018 |
| CES-D | −1.95 (10.79) | 1.09 (4.99) | 0.319 | −0.71 (6.34) | 0.10 (10.01) | 0.136 |
| NPZ-8 | −0.19 (0.46) | 0.25 (0.60) | 0.049 | 0.09 (0.65) | −0.01 (0.50) | 0.476 |
| GDS***** | 0.03 (0.35) | −0.07 (0.60) | 0.536 | 0.02 (0.34) | −0.07 (0.32) | 0.515 |
Models adjusted for baseline value, gender, treatment effect on CES-D depression (with the exception of the model for changes in CES-D), and plasma HIV RNA undetectable
Sample size (n) for this test: no paroxetine n=15, paroxetine n=20, no fluconazole n=19, fluconazole n=16
Sample size (n) for this test: no paroxetine n=15, paroxetine n=18, no fluconazole n=16, fluconazole n=17
Sample size (n) for this test: no paroxetine n=18, paroxetine n=21, no fluconazole n=20, fluconazole n=19
Sample size (n) for this test: no paroxetine n=18, paroxetine n=19, no fluconazole n=19, fluconazole n=18
Higher numbers indicate worsening performance only for the GDS
Figure 2.
California Computerized Assessment Package (CalCap) Sequential reaction time test (CalCAP Sequential) Z score changes over 24 weeks stratified by treatment group in Intention-to-Treat (ITT) analyses
Table 4.
24 Week change of neuropsychological tests, NPZ-8, and GDS by treatment, As Treated (N=22)
| No Paroxetine arms mean (SD) | Paroxetine arms mean (SD) | p value | No Fluconazole arms mean (SD) | Fluconazole arms mean (SD) | p value | |
|---|---|---|---|---|---|---|
| N=8 | N=13 | N=12 | N=9 | |||
| CalCAP Choice Reaction time change * | −0.66 (1.59) | 0.85 (2.45) | 0.978 | 0.91 (2.68) | −0.54 (0.96) | 0.115 |
| CalCAP Sequential Reaction time change * | −0.35 (0.40) | 0.59 (1.23) | 0.039 | 0.30 (1.29) | 0.29 (0.83) | 0.782 |
| Symbol Digit change | −0.18 (0.87) | 0.31 (0.81) | 0.143 | 0.09 (0.93) | 0.18 (0.77) | 0.578 |
| Trail Making Part A change | 0.39 (0.94) | 0.00 (0.79) | 0.520 | 0.36 (0.76) | −0.13 (0.93) | 0.374 |
| Trail Making Part B change | −0.21 (1.02) | 0.55 (0.88) | 0.619 | 0.35 (0.74) | 0.14 (1.28) | 0.237 |
| Timed Gait change ** | −0.43 (1.20) | −0.09 (1.15) | 0.958 | −0.01 (1.13) | −0.48 (1.19) | 0.906 |
| Grooved Pegboard Dominant change | −0.34 (0.91) | 0.38 (1.15) | 0.128 | 0.28 (1.18) | −0.12 (1.01) | 0.466 |
| Grooved Pegboard non-dominant change | −0.21 (0.77) | 0.15 (1.16) | 0.484 | 0.07 (0.96) | −0.07 (1.15) | 0.847 |
| Hopkins Auditory Verbal Learning test Total change | 0.42 (0.92) | 0.18 (0.95) | 0.086 | 0.04 (0.83) | 0.57 (1.01) | 0.224 |
| Hopkins Auditory Verbal Learning test Delayed change | 0.45 (0.92) | 0.30 (0.98) | 0.833 | 0.41 (0.79) | 0.28 (1.16) | 0.378 |
| Rey Complex Figure Copy Change | 0.12 (1.11) | 0.02 (0.94) | 0.715 | 0.22 (1.00) | −0.16 (0.98) | 0.464 |
| Rey Complex Figure Delayed change | 0.17 (0.51) | 0.28 (0.41) | 0.813 | 0.27 (0.55) | 0.19 (0.26) | 0.560 |
| Stroop Color Interference change *** | 0.39 (0.71) | −0.27 (1.45) | 0.173 | −0.04 (1.59) | 0.04 (0.37) | 0.766 |
| Letter number sequencing change | 0.24 (0.83) | −0.47 (0.82) | 0.011 | −0.23 (0.93) | −0.16 (0.85) | 0.675 |
| FAS verbal fluency change | 0.16 (0.53) | 0.08 (0.33) | 0.620 | 0.23 (0.42) | −0.04 (0.33) | 0.032 |
| CES-D | −7.75 (10.28) | −0.36 (3.18) | 0.145 | −3.00 (6.70) | −3.11 (8.74) | 0.256 |
| NPZ-8 | −0.23 (0.16) | 0.35 (0.62) | 0.351 | 0.30 (0.61) | −0.10 (0.44) | 0.145 |
| GDS**** | 0.02 (0.17) | −0.12 (0.28) | 0.954 | −0.12 (0.27) | −0.01 (0.21) | 0.244 |
Models adjusted for baseline value, treatment effect on CES-D depression (with the exception of the model for changes in CES-D), hepatitis C serostatus, and plasma HIV RNA undetectable
Sample size (n) for this test: no paroxetine n=6, paroxetine n=13, no fluconazole n=12, fluconazole n=7
Sample size (n) for this test: no paroxetine n=8, paroxetine n=11, no fluconazole n=10, fluconazole n=9
Sample size (n) for this test: no paroxetine n=8, paroxetine n=12, no fluconazole n=12, fluconazole n=8
Higher numbers indicate worsening performance only for the GDS
CalCAP-California Computerized Reaction Time test
CES-D Center for Epidemiological Studies-Depression scale
GDS-Global deficit score
SD, standard deviation
HIV+ individuals receiving fluconazole (alone or in combination with paroxetine) had worse performance in the FAS verbal fluency test compared to the no fluconazole arms (placebo or paroxetine alone) in ITT analyses and as treated analyses after adjusting for similar co-variates as noted in paroxetine analyses.
In separate comparisons of paroxetine alone, fluconazole alone, or the combined paroxetine/fluconazole group to placebo, in ITT analyses the paroxetine only arm showed worse performance on the Letter Number sequencing test compared to placebo. Participants receiving paroxetine alone had improvement in NPZ8 score, CalCAP sequential reaction time, and Timed Gait, and worse performance on Trail Making Part A compared to placebo in as treated analyses. Participants receiving fluconazole alone had worse performance on global deficit score (GDS) and Trail Making Part B but better performance on Timed Gait compared to placebo in as treated analyses.
Changes in biomarkers of cellular stress, inflammation and neuronal injury over 24 weeks
Paroxetine alone compared to placebo showed a decrease in the cellular stress marker, plasma ceramide C22:0, a decrease in the chemoattractant plasma CXCL10, and an increase in CSF IL8/CXCL8 in as treated analyses (Table 5A). After adjusting for baseline level and gender, HIV+ individuals receiving fluconazole alone compared to placebo had increases in four cellular stress markers including plasma ceramides C16:0, C22:0, C24:0, and increased CSF monohexosylceramide C24:1 in addition to reduction in plasma inflammatory cytokines interleukin (IL) IL-1α, C-X-C motif chemokine 10 (CXCL10), CSF IL-6, and an increase in CSF IL8/CXCL8 (Table 5A) in as treated analyses. The combined paroxetine/fluconazole group showed increases in plasma ceramides C16:0 and C22:0, a reduction in plasma CXCL10 and an increase in CSF IL8/CXCL8 (Table 5A) in as treated analyses. Similar, but less robust changes in cellular stress and inflammatory markers were observed in the ITT group, consistent with reduced drug compliance (Table 5B). We did not observed any treatment differences in plasma or CSF levels of neopterin, soluble CD14 (sCD14), sCD163, protein carbonyls or neurofilament protein light chain (NFL) (data not shown).
Table 5A.
24 week change in plasma and cerebrospinal fluid cellular stress and inflammatory biomarkers, As Treated*
| Treatment Assignment | Placebo Median (IQR) | Paroxetine only, Median (IQR) | p value | Fluconazole only, Median (IQR) | p value | Paroxetine and Fluconazole, Median (IQR) | p value |
|---|---|---|---|---|---|---|---|
| N=4 | N=6 | N=2 | N=7 | ||||
| Cell Stress | |||||||
| Plasma ceramide C16:0 ** | −2.77 (−4.53, −1.87) | −0.16 (−1.07, 1.62) | 0.178 | 1.33 (−0.10, 2.75) | 0.016 | −1.95 (−2.62, 1.19) | 0.021 |
| Plasma ceramide | −101.34 (−150.34, | −19.61 (−36.26, | 0.020 | 2.78 (−63.71, | 0.004 | −25.91 (−104.50, | 0.026 |
| C22:0 | −13.68) | 14.84)** | 69.27) | 56.79) | |||
| Plasma ceramide C24:0 | −260.25 (−699.21, 134.64) | −428.25 (−479.72, 152.74) | 0.075 | 465.18 (−248.69, 1179.06) | 0.019 | −278.65 (−522.54, 1167.59) | 0.112 |
| CSF mono−hexosylceramide C24:1 | 83.05 (−49.45, 222.47) | 177.90 (−57.58, 225.21) | 0.476 | 553.86 (364.05, 743.67) | 0.010 | −19.27 (−122.94, 182.60) | 0.650 |
| Inflammation | |||||||
| N=3 | N=5 | N=2 | N=7 | ||||
| Plasma IL1-α | −18.93, (33.90) 194.37) | −65.82 (−90.36, −2.18) | 0.417 | −451.48 (−537.54, −365.42) | 0.004 | −2.53 (−146.45, 140.84) | 0.638 |
| N=3 | N=4 | N=2 | N=7 | ||||
| Plasma CXCL10 | 518.64 (−24.90), 846.62 | −23.58 (60.86, 16.58) | 0.005 | 66.08 (54.57, 77.59) | 0.016 | 2.31 (−163.05, 283.39) | 0.009 |
| N=3 | N=5 | N=2 | N=6 | ||||
| CSF IL-6 | −0.03 (−1.25, 2.36) | 0.72 (0.63, 1.13) | 0.734 | −5.99 (−8.00, −3.98) | 0.009 | −0.59 (−1.01, −0.19) | 0.434 |
| N=3 | N=6 | N=2 | N=3 | ||||
| CSF IL8/CXCL8 | −29.79 (−43.16–3.21) | 4.23 (−3.76, 8.25) | 0.001 | −5.48 (−7.51, 3.45) | 0.009 | 2.75 (−5.43, 5.30) | 0.011 |
IL= interleukin
CXCL= C-X-C motif chemokine
Models adjust for baseline biomarker level and gender.
Negative values indicated less cell stress or inflammation
Table 5B.
24 week change in plasma and cerebrospinal fluid cellular stress and inflammatory biomarkers, Intention to Treat*
| Treatment Assignment | Placebo Median (IQR) | Paroxetine only, Median (IQR) | p value | Fluconazole only, Median (IQR) | p value | Paroxetine and Fluconazole, Median (IQR) | p-value |
|---|---|---|---|---|---|---|---|
| Inflammation | |||||||
| N=7 | N=8 | N=5 | N=11 | ||||
| Cell Stress | |||||||
| Plasma ceramide C22:0 | −13.88 (−27.93, − 2.30) | 4.20 (−7.29, 9.44) | 0.115 | 7.69 (−0.52, 21.41) | 0.024 | 1.29 (−9.26, 6.94) | 0.117 |
| Inflammation | |||||||
| N=5 | N=7 | N=5 | N=11 | ||||
| Plasma IL-1-α | 17.75 (−18.93, 20.40) | −2.18 (−90.36, 187.85) | 0.769 | −288.85 (−365.42, −22.99** | 0.050 | −10.51 (−146.45, 87.91) | 0.615 |
| N=5 | N=6 | N=5 | N=11 | ||||
| Plasma CXCL10 | 307.02 (−24.90, 518.64) | −23.58 (−91.26, −15.97) | 0.002 | 54.57 (19.08, 77.59) | 0.001 | 2.31 (−163.05, 283.39) | 0.005 |
| N=6 | N=8 | N=5 | N=11 | ||||
| CSF CXCL10 | 125.62 322.62) (−130.07, | −32.29 (−73.02, 38.48) | 0.048 | 22.88 (13.20, 109.12) | 0.161 | −8.58 (−109.61, 13.26) | 0.063 |
IL= interleukin
CXCL= C-X-C motif chemokine
Models adjust for baseline biomarker level and gender.
Negative values indicated less cell stress or inflammation
Subjective and performance-based functional measures
There were no differences in subjective (Karnofsky Performance score, Instrumental Activities of Daily Living) and performance-based measures of function (modified Medication Management test, Driving Simulation component of the CAMCI test) between HIV+ individuals receiving paroxetine vs no paroxetine arms, or HIV+ individuals receiving fluconazole vs no fluconazole arms in as treated or ITT analyses.
DISCUSSION
In this study, paroxetine and fluconazole treatments were safe and well tolerated in individuals with HIV-associated neurocognitive impairment. Few adverse events were reported in any of the treatment groups, and no deaths reported.
Paroxetine was associated with improvement in HIV-associated neurocognitive impairment as indicated by improvement in a summary measure of neuropsychological testing performance, the NPZ8 score (in ITT analyses only), and the CalCAP sequential reaction time score (in ITT and as treated analyses), Trail Making Part B score (in ITT analyses only) (a test of executive function and psychomotor speed), and FAS verbal fluency in patients receiving paroxetine compared to those not receiving paroxetine. However, paroxetine was associated with worse performance on the Trail Making Part A and on the Letter Number Sequencing test compared to patients not receiving paroxetine, suggesting that cognitive improvement with paroxetine treatment was not consistent across all cognitive domains. Markers of cell stress, inflammation, and neuronal injury were not modulated by paroxetine treatment.
Fluconazole treatment was associated with a worsening in the FAS verbal fluency test (in ITT and as treated analyses) and increases in multiple markers of cellular stress. Of note, individuals in the fluconazole treatment group were more likely to be hepatitis C positive, and hepatic disease may have impacted the metabolism of the drug which could have contributed to higher cellular stress. However, we did adjust for differences in hepatitis C seropositivity in our model.
Combined paroxetine and fluconazole treatment was not associated with a benefit in neurocognitive, laboratory, or functional measures compared to paroxetine alone. Combined paroxetine and fluconazole treatment also was not associated with any additional adverse events. Although, preclinical studies using the SIV macaque animal model with the combination of paroxetine and fluconazole protected macaques from SIV-induced neurodegeneration as measured by CSF NFL (Meulendyke, Queen et al. 2014), we did not observe treatment-associated reductions of NFL in this human study.
The potential neuroprotective effects of paroxetine have been previously described (Steiner, Bachani et al. 2015). In addition to inhibition of biogenic amine transporters, such as the serotonin transporter, the SSRI’s are known to reduce conductance of voltage dependent anion channel (VDAC) in mitochondria, and to inhibit voltage dependent potassium channels (Choi, Hahn et al. 1999, Yeung, Millar et al. 1999, Perchenet, Hilfiger et al. 2001, Choi, Choi et al. 2003). Other potential targets of paroxetine, which may account for its neuroprotective properties include depolarization dependent calcium channels, increased proliferation of neuronal progenitor cells, increased production and release of brain derived neurotropic factor from glial cells and neural precursor cells, reduced expression of CCR5 receptors limiting entry of the virus into macrophages, and effects in the cAMP response element-binding protein (CREB) signaling pathway which is integral to long term memory formation (Steiner, Bachani et al 2015)
Although fluconazole showed protective effects in tissue culture models of HAND (8) and in combination with paroxetine in SIV (Meulendyke, Queen et al 2014), the results from the current human clinical trial suggest that while fluconazole decreased circulating levels of two inflammatory cytokines, it also increased multiple plasma and CSF ceramides suggesting increased cellular stress. The increase in cellular stress markers may account for the negative interaction of this drug with Trail Making Part A, and the Letter Number Sequencing test, and lack of beneficial effect in other cognitive function tests.
Strengths of the study include the 2X2 factorial design which allowed us to evaluate both the effects of two drugs independently compared to placebo, and the effect of combination therapy. As expected, paroxetine treatment was not associated with improvement in depression symptomatology as measured by the CES-D score. However, adjusting for depression symptomatology when evaluating changes in neuropsychological tests did not modify the outcomes suggesting that an improvement in depression symptoms was not responsible for cognitive improvement. The inclusion of a pre-entry visit and a screening visit for neuropsychological testing reduced the impact of practice effects on neurocognitive performance prior to baseline. The broad use of neurocognitive, subjective and performance-based functional measures, plasma and CSF markers of inflammation, oxidative stress, and neuronal injury allowed examination of multiple outcomes and potential pathogenic mechanisms.
Several limitations should be noted which could account for the differences in results between the in vitro experiments and non-human primate models with paroxetine and fluconazole, and the current clinical trial results. The trial was designed as a pilot study to evaluate effects on cognitive performance, and as such it had a small number of subjects in specific treatment groups. In analyses of individual neuropsychological tests, many participants had normal performance at baseline on that specific individual test (with abnormal performance at baseline in other neuropsychological tests) so that improvement with treatment would not be likely if baseline performance was normal. Co-morbid conditions such as remote effects from illicit drug or alcohol use, side effects from medications, or co-morbid conditions such as hepatitis C co-infection could have negatively influenced cognitive function in our patients.
A treatment period of 6 months for this study may have been too short a time period to see a therapeutic effect. Future phase I/II studies for the treatment of HAND should consider a one or two year duration if feasible.
It is noteworthy that our animal models did not predict the results of this study. Studies in animal models should include animals that are treated with antiretroviral medications commonly used to treat HIV+ individuals if feasible. Cognitive/motor testing should also be performed in animal models of drug candidates for HAND if feasible. Future studies evaluating the utility of animal models and false–positive and false-negative rates for these models for the treatment of HAND are warranted.
Over the past 20 years, there have been over 10 placebo-controlled trials of adjunctive agents to treat HIV-associated cognitive impairment, (Galgani, Balestra et al. 1997, Sacktor 1997, Heseltine, Goodkin et al. 1998, Sacktor 1998, Schifitto, Sacktor et al. 1999, Sacktor, Schifitto et al. 2000, Clifford, McArthur et al. 2002, Schifitto, Navia et al. 2007, Schifitto, Zhang et al. 2007, Sacktor, Miyahara et al. 2011), and none of them have shown improvement to enter clinical practice. It is notable that in our study, paroxetine is the first adjunctive agent to demonstrate neurocognitive improvement for a summary measure of neurocognitive performance (the NPZ8 score in ITT analyses) in a double-blind, placebo-controlled study for the treatment of HAND. Also, paroxetine is the first adjunctive therapy to demonstrate cognitive improvement on tests of cognitive performance in HIV+ individuals with well-controlled HIV virological replication on recent combination antiretroviral therapy regimens including integrase inhibitors. However, despite improvement in several neuropsychological tests, the effect of paroxetine for treating HIV-associated cognitive impairment was modest, as HIV+ individuals receiving paroxetine had worse performance on other neuropsychological tests, and paroxetine treatment was not associated with consistent improvement in markers of cellular stress, inflammation, neuronal injury, or functional measures.
In contrast, fluconazole was not associated with cognitive improvement in any neuropsychological tests, and was associated with an increase in inflammatory markers. Thus, our study suggests that fluconazole is unlikely to have a beneficial effect for the treatment of HAND and could be associated with neurotoxic effects.
Larger studies are needed to determine whether paroxetine may be of benefit for treating HAND, and to define whether it has neuroprotective and/or anti-inflammatory properties.
Acknowledgments
Supported by MH075673. This publication was also made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), the Johns Hopkins University Center for AIDS Research (P30A1094189) and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, CFAR, NCATS or NIH. The statistical analyses were carried out by Richard Skolasky and Michelle Mielke.
Footnotes
Drs. Sacktor, Steiner, Nath, Haughey, and McArthur contributed to the design and conceptualization of the study. Drs. Skolasky, Mielke, Munro, Wang and Mr. Moxley contributed to the analysis or interpretation of the data. All authors contributed to the drafting or revising of the manuscript for intellectual content. All authors report no disclosures.
Financial Disclosures
Drs Sacktor, Skolasky, Wang, Mielke, Munro, Haughey, and McArthur, and Mr Moxley report no financial relationship with NIH which sponsored the research. Drs Steiner and Nath are employed by NIH. All authors declare that they have no conflict of interest.
References
- Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical neuropsychologist. 1991;5:125–142. [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Choi B, Choi J, Ahn H, Kim MJ, Rhie D, Yoon S, Min D, Jo Y, Kim MS, Hahn S. Fluoxetine blocks cloned neuronal A-type K+ channels Kv1.4. Neuroreport. 2003;14:2451–2455. doi: 10.1097/00001756-200312190-00032. [DOI] [PubMed] [Google Scholar]
- Choi J, Hahn S, Rhie D, Yoon S, Jo Y, Kim M. Mechanism of fluoxetine block of cloned voltage-activated potassium channel Kv1.3. J Pharmacol Exp Ther. 1999;291:1–6. [PubMed] [Google Scholar]
- Clifford DB, McArthur JC, Schifitto G, Kieburtz K, McDermott MP, Letendre S, Cohen BA, Marder K, Ellis RJ, Marra CM. A randomized clinical trial of CPI-1189 for HIV-associated cognitive-motor impairment. Neurology. 2002;59:1568–1573. doi: 10.1212/01.wnl.0000034177.47015.da. [DOI] [PubMed] [Google Scholar]
- Comalli PE, Jr, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. The Journal of genetic psychology. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Gendelman HE. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Annals of neurology. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- Galgani S, Balestra P, Narciso P, Tozzi V, Sette P, Pau F, Visco G. Nimodipine plus zidovudine versus zidovudine alone in the treatment of HIV-1-associated cognitive deficits. AIDS (London, England) 1997;11:1520–1521. [PubMed] [Google Scholar]
- Gandhi NS, Skolasky RL, Peters KB, Moxley RTt, Creighton J, Roosa HV, Selnes OA, McArthur J, Sacktor N. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. Journal of neurovirology. 2011;17:159–165. doi: 10.1007/s13365-011-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Annals of neurology. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Heseltine PN, Goodkin K, Atkinson JH, Vitiello B, Rochon J, Heaton RK, Eaton EM, Wilkie FL, Sobel E, Brown SJ, Feaster D, Schneider L, Goldschmidts WL, Stover ES. Randomized double-blind placebo-controlled trial of peptide T for HIV-associated cognitive impairment. Archives of neurology. 1998;55:41–51. doi: 10.1001/archneur.55.1.41. [DOI] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. The Medical clinics of North America. 1963;47:1647–1658. [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Annals of neurology. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Meulendyke KA, Queen SE, Engle EL, Shirk EN, Liu J, Steiner JP, Nath A, Tarwater PM, Graham DR, Mankowski JL, Zink MC. Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. Journal of neurovirology. 2014;20:591–602. doi: 10.1007/s13365-014-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EN. California Computerized Assesment Package, (CALCAP), CALCAP. CALCAP; Los Angeles CA: 1990. [Google Scholar]
- Navia BA, Dafni U, Simpson D, Tucker T, Singer E, McArthur JC, Yiannoutsos C, Zaborski L, Lipton SA. A phase I/II trial of nimodipine for HIV-related neurologic complications. Neurology. 1998;51:221–228. doi: 10.1212/wnl.51.1.221. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe” Contribution a l'etude de la perception et de la memoire. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Perchenet L, Hilfiger L, Mizrahi J, Clement-Chomienne O. Effects of anorexinogen agents on cloned voltage-gated K(+) channel hKv1.5. J Pharmacol Exp Ther. 2001;298:1108–1119. [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reitan R. Validity of the Trail Making test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8:271–276. [Google Scholar]
- Reitan R. Laboratory, N, editor. Manual for administration of neuropsychological test batteries for adults and children. Neuropsychology Laboratory, Tucson: Neuropsychology Laboratory; 1979. [Google Scholar]
- Rosenthal LS, Skolasky RL, Moxley RT, Roosa HV, Selnes OA, Eschman A, McArthur JC, Sacktor N. A novel computerized functional assessment for human immunodeficiency virus-associated neurocognitive disorder. Journal of neurovirology. 2013;19:432–441. doi: 10.1007/s13365-013-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N. The Dana Consortium on the Therapy for HIV Dementia and Related Cognitive Disorders: a randomized, double-blind, placebo-controlled trial of deprenyl and thioctic acid in HIV-associated cognitive impairment. Neurology. 1998;50:645–651. doi: 10.1212/wnl.50.3.645. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Miyahara S, Deng L, Evans S, Schifitto G, Cohen BA, Paul R, Robertson K, Jarocki B, Scarsi K, Coombs RW, Zink MC, Nath A, Smith E, Ellis RJ, Singer E, Weihe J, McCarthy S, Hosey L, Clifford DB. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology. 2011;77:1135–1142. doi: 10.1212/WNL.0b013e31822f0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Schifitto G, McDermott MP, Marder K, McArthur JC, Kieburtz K. Transdermal selegiline in HIV-associated cognitive impairment: pilot, placebo-controlled study. Neurology. 2000;54:233–235. doi: 10.1212/wnl.54.1.233. [DOI] [PubMed] [Google Scholar]
- Sacktor NC. The Dana Consortium on the Therapy for HIV Dementia and Related Cognitive Disorders. Safety and tolerability of the antioxidant OPC-14117 in HIV-associated cognitive impairment. Neurology. 1997;49:142–146. doi: 10.1212/wnl.49.1.142. [DOI] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nature reviews. Neurology. 2016;12:309. doi: 10.1038/nrneurol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, Jarvik JG, Miller EN, Singer EJ, Ellis RJ, Kolson DL, Simpson D, Nath A, Berger J, Shriver SL, Millar LL, Colquhoun D, Lenkinski R, Gonzalez RG, Lipton SA. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS (London, England) 2007a;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Sacktor N, Marder K, McDermott M, McArthur J, Kieburtz K, Small S, Epstein LG. Randomized trial of the platelet-activating factor antagonist lexipafant in HIV-associated cognitive impairment. Neurological AIDS Research Consortium. Neurology. 1999;53:391–396. doi: 10.1212/wnl.53.2.391. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Yiannoutsos CT, Ernst T, Navia BA, Nath A, Sacktor N, Anderson C, Marra CM, Clifford DB. Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology. 2009;73:1975–1981. doi: 10.1212/WNL.0b013e3181c51a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Zhang J, Evans SR, Sacktor N, Simpson D, Millar LL, Hung VL, Miller EN, Smith E, Ellis RJ, Valcour V, Singer E, Marra CM, Kolson D, Weihe J, Remmel R, Katzenstein D, Clifford DB. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology. 2007b;69:1314–1321. doi: 10.1212/01.wnl.0000268487.78753.0f. [DOI] [PubMed] [Google Scholar]
- Smith A. Services, W.P, editor. Symbol digit modalities test. Manual. Los Angeles: 1973. [Google Scholar]
- Steiner JP, Bachani M, Wolfson-Stofko B, Lee MH, Wang T, Li G, Li W, Strayer D, Haughey NJ, Nath A. Interaction of paroxetine with mitochondrial proteins mediates neuroprotection. Neurotherapeutics. 2015;12:200–216. doi: 10.1007/s13311-014-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthman OA, Abdulmalik JO. Adjunctive therapies for AIDS dementia complex. The Cochrane database of systematic reviews. 2008:Cd006496. doi: 10.1002/14651858.CD006496.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SY, Millar JA, Mathie A. Inhibition of neuronal KV potassium currents by the antidepressant drug, fluoxetine. British journal of pharmacology. 1999;128:1609–1615. doi: 10.1038/sj.bjp.0702955. [DOI] [PMC free article] [PubMed] [Google Scholar]