Abstract
We aimed to test the hypothesis that brain large artery diameters relate to distal downstream arteriolar diameters. In a sample of 110 autopsied individuals (69% men, 76% HIV+, mean age 51), we used multilevel models to relate large artery lumen and lumen-to-wall ratio to left frontal lobe arteriolar lumen and lumen-to-wall ratio adjusting for demographics and vascular risk factors. Comparing the large artery characteristics of the whole brain did not disclose significant associations with frontal lobe arteriolar characteristics. However, restricting the comparison to large arteries upstream of the studied arterioles, demonstrated an independent association between left-sided frontal lobe arteriolar luminal diameter with large artery luminal diameters (B=1.82 ± 0.77, P=0.01) and with large artery lumen-to-wall ratio (B=0.58 ± 0.29, P=0.05). In stratified models, the point estimates in the HIV+ subsample were larger than in the HIV− subsample. These finding suggest coupling between higher proximal blood flow represented by large artery diameter and lower distal resistance represented by arteriolar dilatation. The relationship between arteriolar dilatation and brain parenchyma homeostasis should be further studied.
Keywords: dolichoectasia, hiv, arterial, cerebrovascular, cerebral hemodynamics, vascular pathology, remodeling
INTRODUCTION
Neurological manifestations of HIV infection have been a source of morbidity and mortality since the onset of the epidemic. (Morgello et al, 2002) Efficacious antiretroviral therapy has decreased many of these complications, but the prevalence and incidence of stroke remains higher than in uninfected population. (Mateen et al, 2012; Weber et al, 2013) Stroke in individuals with HIV is often devastating and it may be caused by various etiologies. (Ortiz et al, 2007) Approximately one quarter of HIV patients with brain infarcts display a form of large intracranial artery disease characterized by dilatations and elongation. (Gutierrez et al, 2015b; Gutierrez and Ortiz, 2011) The mechanisms by which these dilated vessels contribute to stroke are unclear, but may include mechanical traction of penetrating arteries due to tortuosity,(Zhang et al, 2014) dampened blood flow due to severe dilatation which may then lead to artery-to-artery emboli or in-situ thrombosis,(Drake and Peerless, 1997; Kumral et al, 2005) arterial wall hemorrhage with potential occlusion of the penetrating branches,(Nakatomi et al, 2000) or increased risk for lacunar strokes. (Pico et al, 2003) Another possible mechanism by which large artery dilatation may increase the risk of stroke is the transmission of hemodynamic stress to distal arterioles, which are the sites of brain perfusion autoregulation. (Marmarou et al, 1980)
There are established relationships between large artery diameters in basal portions of the circle of Willis and arterioles. For example, the luminal diameter of a given artery in the circle of Willis is directly proportional to the density of the capillaries in the area of the brain corresponding to that artery. (van der Zwan et al, 1993; van Laar et al, 2006) Given that brain development is the pacemaker of embryogenesis of the circle of Willis, the association between large artery diameters and brain parenchyma vascularity is interpreted as evidence of coupling between supply and demand. (Padget, 1944) The brain can further regulate this demand by dilatation or constriction of the arterioles, thus preserving a steady brain blood flow within a wide range of systemic blood pressure variations. (Brown and Thore, 2011) Arterioles also remodel in various physiological and pathological conditions, which may lead to distal changes in resistance that may theoretically stimulate remodeling of the proximal large arteries. (Brown and Thore, 2011; Cipolla et al, 2011) In this scenario, arteriolar dilatation decreases resistance to flow more proximally, and with time, may lead to proximal outward remodeling of the large arteries. Thus, one could hypothesize that brain arteriolar outward remodeling would be associated with larger proximal brain arterial diameters.
Understanding the relationship between large arterial diameters observed among individuals with HIV and arteriolar remodeling may increase the understanding of stroke physiopathology in this population, as well as in the general population.
MATERIALS AND METHODS
We studied the relationship between brain large artery characteristics and their downstream arterioles in an autopsy sample from the Manhattan HIV Brain Bank (MHBB; U24MH100931); the cases were chosen for this study if they had been used in two prior and independent analyses of large artery characteristics (the Brain Arterial Remodeling Study) and a separate analysis of risks for cerebral small vessel disease. (Morgello et al, 2014) Individuals in the MHBB are followed prospectively and agree to donate their brain for research purposes upon death via a written anatomical gift consent approved by the Mount Sinai Institutional Review Board. Clinical information is obtained during ante-mortem visits; where prospective assessment is not available, this information is obtained via post-mortem medical record review. Hypertension, diabetes, dyslipidemia, smoking, cocaine use, and Hepatitis C infection are defined by self-report and/or chart documentation, laboratory evidence supporting these diagnoses, or by self-reported or chart-extracted use of medications. Further details of the methods use in this study may be found elsewhere. (Morgello et al, 2002)
Brain large artery preparation
Brain large arteries forming the circle of Willis and the vertebrobasilar system were removed from the formalin-fixed brains or hemi-brains. Each artery was dissected individually and proximal and distal 5 mm segments were cut cross-sectionally to preserve the lumen, and embedded in paraffin. Embedded blocks were cut at 6 microns for staining with H&E and elastic Van Gieson (EVG). Each slice was digitalized using Olympus Soft Imaging Solutions software and a microscope with constant illumination, with a 10× objective lens and scale=0.643 μm/pixel in the Histology Shared Resource Facility of the Icahn School of Medicine at Mount Sinai. We used color-based thresholding with ImageJ software (WS Rasband, ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, imagej.nih.gov/ij/, 1997-2011) to quantify the areas of the lumen, intima, media and adventitia. From these areas, we derived the shrinkage-corrected luminal diameter and wall and layer thicknesses of each artery, as described previously. (Gutierrez et al, 2014b) We rated visually the presence of atherosclerosis (defined here categorically as the presence of an atheroma). The reliability of these measures has been reported as good to excellent. (Gutierrez et al, 2012)
Arteriolar preparation
Each formalin-fixed brain or hemi-brain was used to obtain sections from 57 pre-specified regions of interest, as previously described. (Morgello et al, 2001) For the purpose of this analysis, a section corresponding to the periventricular frontal area between the anterior and the middle cerebral artery territories (watershed area) was selected. All the observed arterioles in that section, which typically ranged from 15–20 per section, were measured. H&E sections were scanned at 200× with an Olympus VS110 virtual slide scanning system using VS-ASW software (Olympus America Inc, Center Valley, PA). Given obliquity of the arteriolar wall, we identified the longest axis first, and then in a perpendicular plane we measured the external and the internal diameters to derive the lumen, wall thickness and lumen-to-wall ratio in each arteriole (figure 1).
Figure 1.
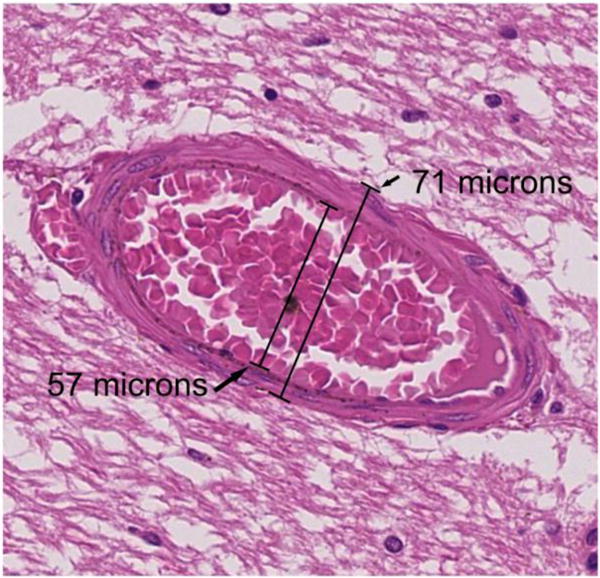
Stain: H&E. Example of an arteriole, with measurements of the lumen and interadventitial diameters.
Statistical analysis
The arteriolar luminal diameters, wall thickness and lumen-to-wall ratio were the dependent variables, and variables listed in table 1 were independent variables. We used multilevel models clustering arteries by individuals to generate beta coefficients and their standard errors to account for the lack of independence between arteries from the same individual. (Bruin, 2006) Because the parenchymal area studied in each case may have varied in the number of arterioles present, we collected the number or arterioles per case and adjusted for it in all models. We first ran a statistical model relating the large artery characteristic in the whole brain with arteriolar characteristics in the anterior circulation. Then, we only used large artery characteristics supplying the parenchymal area where the arterioles were measured (i.e. intracranial portion of the internal carotid artery, anterior cerebral artery and middle cerebral artery) to identify strengthening of the beta estimates that would support evidence of a direct mechanical relationship between large artery characteristics with their downstream arterioles. Lastly, we assessed whether the relationship between large artery characteristics with their downstream arterioles varied by HIV. A p value ≤ 0.05 was considered statistically. The statistical analysis was carried out with SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Table 1.
Characteristics of the sample at death (N=110)
| HIV+ N=84 |
HIV− N=26 |
|
|---|---|---|
| Mean age (in years, SD, median, interquartile range) | 50 (8.4, 50, 44–58) |
53 (11.8, 55, 55–81) |
| Male sex (%) | 71 | 58 |
| Ethnicity (%) | ||
| Non-Hispanic white | 20 | 39 |
| Non-Hispanic black | 50 | 27 |
| Hispanic | 30 | 34 |
| Hypertension (%) | 64 | 73 |
| Diabetes (%) | 19 | 46 |
| Dyslipidemia (%) | 23 | 19 |
| Smoking (%) | 60 | 35 |
| Hepatitis C co-infection (%) | 51 | 31 |
| cART use at death (%) | 69 | Not applicable |
| Prior opportunistic infections (%) | 80 | Not applicable |
| Mean Nadir CD4 (per mm3, SD, median, interquartile range) |
114 (71, 142, 11–172) |
Not applicable |
| Mean CD4 count at death (per mm3, SD, median, interquartile range) |
167 (82, 195, 15–268) |
Not applicable |
| CD4 count < 200% at death (%) | 67 | Not applicable |
| Viral load at death > 50 copies/ml (%) | 59 | Not applicable |
| Brain large artery unadjusted characteristics | ||
| Lumen diameter (in mm, mean ± SD) | 2.4 ± 0.8 | 2.2 ± 0.6 |
| Lumen to wall ratio | 6.9 ± 2.08 | 7.1 ± 2.0 |
| Wall thickness (in mm, mean ± SD) | 0.36 ± 0.11 | 0.32 ± 0.08 |
| Anterior frontal arteriolar unadjusted characteristics | ||
| Lumen diameter (in microns, mean ± SD) | 42.0 ± 0.33 | 31.5 ± 22.8 |
| Lumen to wall ratio | 3.36 ± 2.47 | 3.07 ± 2.13 |
| Wall thickness (in microns, mean ± SD) | 13.76 ± 8.59 | 11.00 ± 5.30 |
Abbreviations: N, number, SD, standard deviation; cART, combined antiretroviral therapy.
RESULTS
Population studied
We analyzed 110 autopsied individuals (68% men, 76% HIV+, 45% Non-Hispanic black) with a mean age of 51 ± 9 years (median 51, range 26–81). The characteristics of the sample by HIV status are described in Table 1.
On average, each case included 16 arterioles (median 15, range 5–23) and 6 large artery segments (median 6, range 2–9). The average arteriolar measures were as followed: luminal diameters 40 ± 15 microns; wall thickness 13 ± 4 and lumen-to-wall ratio 3.2 ± 1.4. The average large artery measures were: luminal diameters 2.4 ± 0.8 mm, wall thickness 0.4 ± 0.1 mm, lumen-to-wall ratio 7.0 ± 2.1. The prevalence of large artery atherosclerosis was 3.7%.
Relationship between large artery characteristics and their downstream arteriolar characteristics
Comparing the large artery characteristics of the whole brain did not disclose significant associations with frontal lobe arteriolar characteristics. However, restricting the comparison to large arteries upstream of the studied arterioles, demonstrated a positive association between large artery luminal diameters with arteriolar luminal diameter (B=1.82 ± 0.77, P=0.02, table 2). The same direction of the association was noted between large artery lumen-to-wall ratio and arteriolar luminal diameter (B=0.58 ± 0.29, P=0.05). A thicker large artery wall was also associated with larger arteriolar luminal diameters (B=8.06 ± 3.65, P=0.03) but not with arteriolar wall (−0.84 ± 0.77, P=0.27) or lumen-to-wall ratio (B=−0.56 ± 0.36, P=0.12).
Table 2.
Relationship between large artery characteristics and downstream arterioles
| Arteriolar characteristics | ||||
|---|---|---|---|---|
| Lumen diameter (in microns) B estimate ± standard error | Wall thickness (in microns) B estimate ± standard error | Lumen-to-wall ratio B estimate ± standard error | ||
| Large artery characteristics | ||||
| Lumen-to-wall ratio | All large arteries | 0.31 ± 0.40 | −0.12 ± 0.10 | 0.04 ± 0.03 |
| Ipsilateral to the arterioles | 0.58 ± 0.29† | −0.02 ± 0.04 | 0.05 ± 0.03* | |
| Lumen diameter (in mm) | All large arteries | 1.46 ± 3.88 | −1.14 ± 1.16 | 0.33 ± 0.37 |
| Ipsilateral to the arterioles | 1.82 ± 0.77† | 0.08 ± 0.11 | 0.14 ± 0.07† | |
| Wall thickness (in mm) | All large arteries | −2.92 ± 7.76 | 2.27 ± 2.31 | −0.66 ± 0.74 |
| Ipsilateral to the arterioles | 8.06 ± 3.65† | −0.84 ± 0.77 | −0.56 ± 0.36 | |
| Atherosclerosis (i.e. presence of atheroma) | All large arteries | 0.31 ± 3.41 | 1.29 ± 1.09 | −0.19 ± 0.29 |
| Ipsilateral to the arterioles | −0.09 ± 3.59 | 0.82 ± 1.12 | −0.10 ± 0.33 | |
P value < 0.10–0.05
P value <0.05
Analytic note: All models were adjusted for age, sex, race/ethnicity, hypertension, diabetes, dyslipidemia, smoking, hepatitis C, HIV, cocaine use, interadventitial size, large artery type and large artery proximal versus distal location.
Analysis by HIV status
The effects of large artery lumen diameter, wall thickness, and lumen-to-wall ratio on ipsilateral arteriolar luminal diameter and lumen-to-wall ratio varied by HIV status (table 3). In stratified models, the point estimates for the association between large artery characteristics with arteriolar luminal diameters were larger in the HIV+ subsample were larger than in the HIV− subsample. Among those with HIV only, and after adjusting for variables pertinent to HIV, there an even more significant association between ipsilateral large artery characteristics and the downstream arteriolar luminal diameter and lumen-to-wall ratio (table 2, model 2).
Table 3.
Models stratified by HIV status
| Arteriolar characteristics | ||||||
|---|---|---|---|---|---|---|
| Lumen diameter (in microns) B estimate ± standard error | Lumen-to-wall ratio B estimate ± standard error | |||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||
| Ipsilateral large artery characteristics | HIV − | HIV + | HIV + | HIV− | HIV + | HIV + |
| Lumen-to-wall ratio | 0.05 ± 0.20 | 0.69 ± 0.35† | 0.37 ± 0.18† | 0.01 ± 0.02 | 0.06 ± 0.03* | 0.04 ± 0.01‡ |
| Lumen diameter (in mm) | 0.39 ± 0.65 | 2.03 ± 0.89 | 1.56 ± 0.51‡ | 0.10 ± 0.07 | 0.14 ± 0.09 | 0.14 ± 0.04‡ |
| Wall thickness (in mm) | 3.63 ± 4.51 | 8.54 ± 4.17† | 9.15 ± 3.52† | 0.92 ± 0.49* | 0.50 ± 0.40 | 0.75 ± 0.32† |
P value < 0.10–0.05
P value <0.05
P value < 0.01
Analytic note:
Model 1 adjusted for age, sex, race/ethnicity, hypertension, diabetes, dyslipidemia, smoking, hepatitis C, cocaine use, inter-adventitial size, large artery type and large artery proximal versus distal location. Model 2 includes model 1 plus prior opportunistic infections, use and class of antiretrovirals, nadir CD4 and CD4 counts prior to death, and viral loads > 50 copies per ml prior to death.
DISCUSSION
The arteries feeding the brain are intimately related to the integrity of the brain parenchyma, and are related to stroke mechanisms (Fisher, 1965; Sacco et al, 1995) as well as to less acute but equally important outcomes such as dementia. (Honig et al, 2005; Roher et al, 2004) Intracranial large and small artery disease are overrepresented as causes of stroke among HIV individuals compared with uninfected populations,(Gutierrez et al, 2014a; Vinikoor et al, 2013) which highlights the importance of brain arterial disease in this population. Arterioles control vital hemodynamic aspects of the brain circulation such as resistance to flow and autoregulation. (Faraci and Heistad, 1990; Haubrich et al, 2003) In our study, we found evidence that the histological characteristics of large brain arteries are related to the same histological characteristics of their downstream arterioles; a finding suggestive of a continuum that encompasses the most proximal branches of the circle of Willis down to the most distal arteriolar beds. The data presented here also suggest that emphasis on radiographically evident large artery disease underestimates the contribution of vascular disease to brain parenchymal integrity.
We confirmed the hypothesis that large artery luminal diameters relate to arteriolar luminal diameters. In principle this make physiological sense given than dilated arterioles would theoretically drop the resistance to flow, leading to greater proximal flow, and subsequent outward remodeling. (Faraci and Heistad, 1990; van der Zwan et al, 1993) However, due to the cross-sectional nature of our data, we cannot produce evidence of directionality and consequently we cannot rule out the possibility that outward remodeling occurs first in the large artery, with subsequent hemodynamic transmission to the arterioles. In the case of HIV, we have previously demonstrated that immunosuppression, defined by a lower nadir CD4 and lower CD4 counts prior to death, was associated with brain arterial dilatation in the form of dolichoectasia. (Gutierrez et al, 2015b) It is surprising that even after adjusting for HIV-related immune variables in this sample, the relationship between large artery diameters and arteriolar luminal diameter persisted, and in fact, became more significant (table 3, model 2). This suggests that chronic HIV-related immunosuppression might be associated with larger arteriolar lumen diameters via not only compromised arteriolar integrity, but also by increased proximal large artery dilation and its downstream mechanical effects of a proximally dilated artery. The association of chronic immunosuppression with apoptosis of the arterial wall economy that others and we have reported (Gibellini et al, 2012; Gutierrez et al, 2016b; Kim et al, 2003) would be a potential mechanism linking HIV infection to proximal and distal vessel dilatation. By linking brain arterial disease to arteriolar remodeling, we aimed to construct a proposed pathophysiological model that explains the heightened risk of stroke and dementia among individuals with extreme arterial phenotypes such as atherosclerosis and dolichoectasia, particularly among those with HIV. (Gutierrez et al, 2016; Gutierrez et al, 2015a; Gutierrez et al, 2016a; Gutierrez et al, 2016b; Gutierrez et al, 2016c)
The strengths of this study include the systematic and reliable characterization of large arteries and arterioles in a relatively large sample size, considering the limited access to brain specimens in large populations. However, autopsied materials are inherently biased, and are less representative of the average general population, which is a limitation. Furthermore, the low prevalence of atherosclerosis in this sample as well as the relatively small number of uninfected controls may have reduced our power to detect associations related to these characteristics. It is also uncertain if these findings would be relevant in other racial/ethnic populations or in predominantly non-Hispanic white samples.
In summary, we report evidence that large artery outward remodeling, as evidence by higher luminal diameters and higher lumen-to-wall ratio, is associated with arteriolar outward remodeling. This association is strongest among HIV individuals, and it may shed light on the relationship between large artery disease and parenchymal damage.
Figure 2.
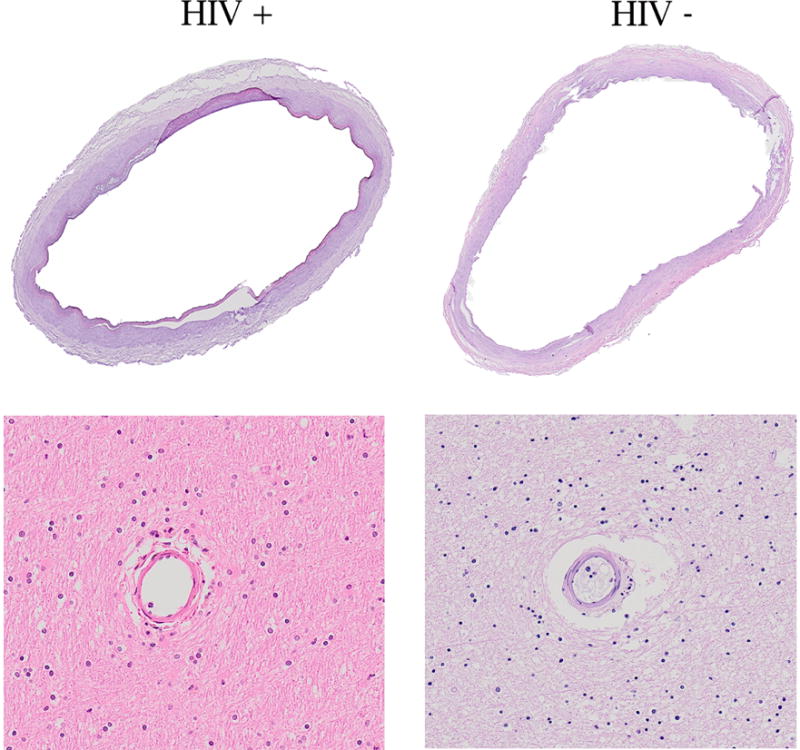
Stain: H&E. Example of dilated middle cerebral arteries and their downstream arterioles, which appeared to have a thin wall relative to their lumen.
Acknowledgments
SOURCE OF FUNDING:
-
-
AHA 13CRP14800040 and Campbell Foundation # 0003121220 (PI Jose Gutierrez)
-
-
NIH R25MH080663 and U24MH100931 (PI Susan Morgello)
Footnotes
AUTHOR CONTRIBUTION:
Jose Gutierrez: Data acquisition, statistical analysis, manuscript draft.
Jacinta Murray: Data acquisition, critical revision of the manuscript.
Christina Chon: Data acquisition, critical revision of the manuscript.
Susan Morgello: Data acquisition, manuscript draft.
DISCLOSURE/CONFLICT OF INTEREST:
None of the author reports conflicts of interest related to this manuscript.
References
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin J. Newtest: command to compute new test. UCLA: Statistical Consulting Group; 2006. [Google Scholar]
- Cipolla MJ, Sweet JG, Chan SL. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J Appl Physiol. 2011;110:329–39. doi: 10.1152/japplphysiol.01159.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141–62. doi: 10.3171/jns.1997.87.2.0141. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Fisher CM. Lacunes: Small, Deep Cerebral Infarcts. Neurology. 1965;15:774–84. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- Gibellini D, Miserocchi A, Tazzari PL, Ricci F, Clo A, Morini S, Ponti C, Pasquinelli G, Bon I, Pagliaro P, Borderi M, Re MC. Analysis of the effects of HIV-1 Tat on the survival and differentiation of vessel wall-derived mesenchymal stem cells. J Cell Biochem. 2012;113:1132–41. doi: 10.1002/jcb.23446. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Cheung HW, Bagci A, Rundek T, Alperin N, Sacco R, Elkind M, Wright C. Brain arterial diameters as biomarkers of cognitive performance: Results from the Northern Manhattan Study. Neurology. 2016;86:P2.246. [Google Scholar]
- Gutierrez J, Cheung K, Bagci A, Rundek T, Alperin N, Sacco RL, Wright CB, Elkind MS. Brain Arterial Diameters as a Risk Factor for Vascular Events. J Am Heart Assoc. 2015a;4:e002289. doi: 10.1161/JAHA.115.002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Glenn M, Isaacson RS, Marr AD, Mash D, Petito C. Thinning of the Arterial Media Layer as a Possible Preclinical Stage in HIV Vasculopathy: A Pilot Study. Stroke. 2012;43:1156–8. doi: 10.1161/STROKEAHA.111.643387. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology. 2015b;85:1139–45. doi: 10.1212/WNL.0000000000001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Honig L, Elkind MS, Mohr JP, Goldman J, Dwork AJ, Morgello S, Marshall RS. Brain arterial aging and its relationship to Alzheimer dementia. Neurology. 2016a doi: 10.1212/WNL.0000000000002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Koch S, Dong C, Casanova T, Modir R, Katsnelson M, Ortiz GA, Sacco RL, Romano JG, Rundek T. Racial and ethnic disparities in stroke subtypes: a multiethnic sample of patients with stroke. Neurol Sci. 2014a;35:577–82. doi: 10.1007/s10072-013-1561-z. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Menshawy K, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Metalloproteinases and brain arterial remodeling with and without HIV. J Infect Dis. 2016b doi: 10.1093/infdis/jiw385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Menshawy K, Gonzalez M, Goldman J, Elkind MS, Marshall R, Morgello S. Brain large artery inflammation associated with HIV and large artery remodeling. AIDS. 2016c;30:415–23. doi: 10.1097/QAD.0000000000000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Ortiz G. HIV/AIDS patients with HIV vasculopathy and VZV vasculitis: a case series. Clin Neuroradiol. 2011;21:145–51. doi: 10.1007/s00062-011-0087-0. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Rosoklija G, Murray J, Chon C, Elkind MS, Goldman J, Honig LS, Dwork AJ, Morgello S, Marshall RS. A quantitative perspective to the study of brain arterial remodeling of donors with and without HIV in the Brain Arterial Remodeling Study (BARS) Front Physiol. 2014b;5:56. doi: 10.3389/fphys.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich C, Kruska W, Diehl RR, Moller-Hartmann W, Klotzsch C. Dynamic autoregulation testing in patients with middle cerebral artery stenosis. Stroke. 2003;34:1881–5. doi: 10.1161/01.STR.0000080936.36601.34. [DOI] [PubMed] [Google Scholar]
- Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology. 2005;64:494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- Kim TA, Avraham HK, Koh YH, Jiang S, Park IW, Avraham S. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J Immunol. 2003;170:2629–37. doi: 10.4049/jimmunol.170.5.2629. [DOI] [PubMed] [Google Scholar]
- Kumral E, Kisabay A, Atac C, Kaya C, Calli C. The mechanism of ischemic stroke in patients with dolichoectatic basilar artery. Eur J Neurol. 2005;12:437–44. doi: 10.1111/j.1468-1331.2005.00993.x. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Takagi H, Shulman K. Biomechanics of brain edema and effects on local cerebral blood flow. Adv Neurol. 1980;28:345–58. [PubMed] [Google Scholar]
- Mateen FJ, Shinohara RT, Carone M, Miller EN, McArthur JC, Jacobson LP, Sacktor N, Multicenter ACSI. Neurologic disorders incidence in HIV+ vs HIV− men: Multicenter AIDS Cohort Study, 1996-2011. Neurology. 2012;79:1873–80. doi: 10.1212/WNL.0b013e318271f7b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C, Grant I, Singer EJ. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27:326–35. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Morgello S, Mahboob R, Yakoushina T, Khan S, Hague K. Autopsy findings in a human immunodeficiency virus-infected population over 2 decades: influences of gender, ethnicity, risk factors, and time. Arch Pathol Lab Med. 2002;126:182–90. doi: 10.5858/2002-126-0182-AFIAHI. [DOI] [PubMed] [Google Scholar]
- Morgello S, Murray J, Van Der Elst S, Byrd D. HCV, but not HIV, is a risk factor for cerebral small vessel disease. Neurol Neuroimmunol Neuroinflamm. 2014;1:e27. doi: 10.1212/NXI.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Segawa H, Kurata A, Shiokawa Y, Nagata K, Kamiyama H, Ueki K, Kirino T. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms : insight on the mechanism of growth. Stroke. 2000;31:896–900. doi: 10.1161/01.str.31.4.896. [DOI] [PubMed] [Google Scholar]
- Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68:1257–61. doi: 10.1212/01.wnl.0000259515.45579.1e. [DOI] [PubMed] [Google Scholar]
- Padget D. The circle of Willis: Its embryology and anatomy. In: Dandy WE, editor. Intracranial arterial aneurysms. Comstock publishing company, inc., Cornell university; Ithaca, N. Y.: 1944. pp. 67–90. [Google Scholar]
- Pico F, Labreuche J, Touboul PJ, Amarenco P. Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology. 2003;61:1736–42. doi: 10.1212/01.wnl.0000103168.14885.a8. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke. 2004;35:2623–7. doi: 10.1161/01.STR.0000143317.70478.b3. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- van der Zwan A, Hillen B, Tulleken CA, Dujovny M. A quantitative investigation of the variability of the major cerebral arterial territories. Stroke. 1993;24:1951–9. doi: 10.1161/01.str.24.12.1951. [DOI] [PubMed] [Google Scholar]
- van Laar PJ, Hendrikse J, Golay X, Lu H, van Osch MJ, van der Grond J. In vivo flow territory mapping of major brain feeding arteries. Neuroimage. 2006;29:136–44. doi: 10.1016/j.neuroimage.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and Clinical Features of Cerebrovascular Disease Among HIV-Infected Adults in the Southeastern United States. Aids Research and Human Retroviruses. 2013;29:1068–1074. doi: 10.1089/aid.2012.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, Cavassini M, Calmy A, Bernasconi E, Schmid P, Flepp M, Kowalska J, Ledergerber B, SHCS SHCS. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. Hiv Medicine. 2013;14:195–207. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- Zhang DP, Zhang SL, Zhang JW, Zhang HT, Fu SQ, Yu M, Ren YF, Ji P. Basilar artery bending length, vascular risk factors, and pontine infarction. J Neurol Sci. 2014;338:142–7. doi: 10.1016/j.jns.2013.12.037. [DOI] [PubMed] [Google Scholar]


